Abstract
During pregnancy, reduced vascular responses to constrictors contribute to decreased uterine and total vascular resistance. Thromboxane A2 (TxA2) is a potent vasoconstrictor that exerts its actions via diverse signaling pathways, and its biosynthesis increases in preeclampsia. In this study, we hypothesized that maternal vascular responses to TxA2 will be attenuated via Rho kinase, PKC, p38 MAPK, and ERK1/2 signaling pathways. Isolated ring segments of uterine and small mesenteric arteries from late pregnant (19–21 days) and virgin rats were suspended in a myograph, and isometric force was measured. Pregnancy did not affect uterine and mesenteric artery responses to the TxA2 analog U-46619 (10−9–10−5 M), but transduction signals associated with these contractions were different between pregnant and nonpregnant rats. Inhibition of Rho kinase (10−6 M Y-27632) reduced sensitivity to U-46619 in virgin uterine vessels but did not inhibit these contractions in pregnant uterine arteries and had no effect on mesenteric vessels. Treatment of arterial segments with a PKC inhibitor (10−6 M bisindolylmaleimide I) reduced U-46619-induced contractions in virgin uterine and mesenteric arteries and in pregnant mesenteric arteries. Pregnant uterine arteries, however, were unresponsive to PKC inhibition. Inhibition of ERK1/2 (10−5 M PD-98059) and p38 MAPK (10−5 M SB-203580) reduced U46619-induced contractions in nonpregnant vessels and in pregnant uterine and mesenteric vessels. These data suggest that normal pregnancy does not affect uterine and mesenteric contractile responses to TxA2 but reduces the contribution of Rho kinase and PKC signaling pathways to these contractions in the uterine vasculature. In contrast, the role of ERK1/2 and p38 MAPK in U-46619-induced uterine contractions remains unchanged with pregnancy. TxA2-associated transduction signals and its regulators might present potential targets for the development of new treatments for preeclampsia and other pregnancy-associated vascular diseases.
Keywords: gestation, prostanoids, vascular reactivity, thromboxane A2 prostanoid receptor
normal pregnancy is associated with profound adaptive responses in the maternal cardiovascular system. Systemic hemodynamic changes during gestation include an increase in cardiac output, a reduction in total vascular resistance, and an expansion of plasma volume (6, 18, 39). Being responsible for adequate perfusion of the maternal-fetal interface, the uterine vasculature undergoes dramatic changes during pregnancy to ensure continuous blood supply and nutrients to the growing uterus and fetus. Substantial remodeling (5) and sympathetic denervation (35) of the uterine arteries, enhanced uterine vasodilatation (7, 49), and reduced vascular tone (44) enable the uterine vasculature to act as a low-resistance shunt to accommodate a 20-fold increase in uterine blood flow during gestation.
The mechanisms for the reduced vascular tone seen in pregnancy may involve a decreased role of endogenous vasoconstrictors and/or an enhanced contribution of endogenous vasodilators. The vast majority of studies on uterine adaptations to pregnancy have focused on endothelial function, whereas less is known about the changes in contractile responses of the uterine vasculature in response to gestation. Ex vivo experiments have demonstrated a diminished response of the uterine artery but not the carotid artery of pregnant guinea pigs to thromboxane A2 (TxA2) analogs (43), suggesting that pregnancy reduces contractile responses to TxA2 in a regional bed-dependent manner. Furthermore, in vivo studies have shown that the acute administration of the TxA2 analog U-46619 increases the systemic hypertensive effect in rats (22) and rabbits (27) during gestation, indicating that TxA2 may have the characteristics of a mediator of blood pressure increases during pregnancy. Indeed, women with preeclampsia (37) and animals models of pregnancy-induced hypertension (47) have shown elevated levels of TxA2 metabolites.
TxA2 is a metabolite of arachidonic acid metabolism that mediates a number of cellular responses including platelet aggregation and vasoconstriction (34). Alterations in TxA2 biosynthesis and actions have been reported in a series of pathophysiological conditions such as myocardial ischemia, asthma, hypertension, and pregnancy-induced hypertension (8, 9, 30, 37). Moreover, TxA2 modulates the vasoconstrictor effects of other agonists, including ANG II and endothelin-1, which are suspected mediators of chronic hypertension and pregnancy-associated hypertension (17). TxA2 exerts its action via stimulation of the TxA2 prostanoid (TP) receptor, a member of the G protein-coupled receptor superfamily (34). TP receptors are known to mediate cellular responses by activating Ca2+ signaling (33) and various kinase pathways such as Rho kinase (46), PKC, and MAPKs (2, 11, 32). In the rat caudal artery, TxA2-induced contraction involves stimulation of Rho-mediating activation of Ca2+ entry and Ca2+ sensitization (46), and in the rat mesenteric artery, inhibition of PKC and p38 MAPK attenuated contractile responses to U-46619 (2). Data on the role of ERK1/2 in U-46619-induced contractions are not consistent among vessel types and species (2, 50).
Studies in women, ewes, and rats have reported that normal pregnancy alters the levels of protein expression and/or activity of Rho kinase, PKC, and ERK1/2 in the uterine and other vascular beds (19, 42, 50). The role of p38 MAPK in vascular contractility during pregnancy has not been previously investigated. Given that these kinase-signaling pathways are involved in TxA2-induced vascular contractions and that these contractions are reduced in the uterine artery of pregnant animals, we tested the hypothesis that normal pregnancy reduces uterine contractile responses to TxA2 via reductions in Rho kinase-, PKC-, ERK1/2-, and p38 MAPK-dependent pathways. To test our hypotheses, we used ex vivo experiments to assess uterine artery responses to U-46619 in the presence and absence of various kinase inhibitors.
MATERIALS AND METHODS
Animals and breeding.
Female virgin Sprague-Dawley rats (198–244 g, 12 wk of age) were used in this study (Harlan Laboratories, Indianapolis, IN). Rats were housed in a temperature- and humidity-controlled environment under 12:12-h light-dark cycles and were provided with tap water and standard laboratory rodent chow ad libitum. Females were paired with fertile males (1:2 male-to-female ratio), and the morning on which spermatozoa were found in vaginal smears was considered day 1 of pregnancy. Pregnant dams were housed separately and monitored daily for body weight changes. Experiments were performed on days 19–21 of gestation (term: day 22). Age-matched virgin rats served as controls at various times throughout their estrous cycle. All procedures were performed in accordance with the Guiding Principles in the Care and Use of Animals and were approved by the Georgia Health Sciences University Committee on the Use of Animals in Research and Education.
Arterial isolation and functional experiments.
In all experiments, rats were anesthetized with isoflurane via a nose cone for surgical procedures (initially with 5% and then maintained at 2.5% in 100% oxygen) and euthanized by thoracotomy and exsanguination via cardiac puncture. Euthanasia of feti was accomplished as soon as possible after removal from the dam via decapitation. The uterine horns and small intestine with the attached vasculature were rapidly excised and placed in ice-cold physiological salt solution of the following composition (in mM): 130 NaCl, 4.7 KCl, 14.9 NaHCO3, 5.5 dextrose, 1.18 KH2PO4, 1.17 MgSO4, 1.6 CaCl2, and 0.026 EDTA. The uterine and second-order mesenteric arteries were carefully isolated by dissection of fat and connective tissue. The uterine artery of one uterine horn was detached from the uterus, frozen immediately in liquid nitrogen, and stored in −80°C until being used for Western blots. Similarly, the mesenteric vasculature was cleaned of fat and connective tissue, detached from the intestine body, and frozen in liquid nitrogen for further analysis. The main uterine artery of the other uterine horn and small mesenteric arteries were cut into 2-mm rings and used for isometric tension experiments. Each ring was mounted on an isometric wire myograph system (Danish MyoTech, Aarhus, Denmark) using two 40-μm wires and allowed to equilibrate for 30–45 min before any tension was applied. Vascular rings were stretched to an optimum resting tension of 1.8 mN (virgin uterine artery and virgin and pregnant mesenteric arteries) or 2.0 mN (pregnant uterine artery) and allowed to equilibrate for 45 min in a tissue bath filled with 5 ml physiological salt solution continuously gassed with 95% O2-5% CO2 at 37°C. Optimum resting tension had been previously determined via a length-tension curve. Arterial integrity was assessed by contracting the vascular segments with a depolarizing concentration of KCl (120 mM) and, subsequently, with phenylephrine (PE; 3 × 10−6 M) followed by relaxation with ACh (3 × 10−6 M). Cumulative response curves to the TxA2 analog U-46619 (10−9–10−5 M) were performed alone or after a 30-min incubation with SQ-29548 (selective TP antagonist, 10−6 M) (2, 46), Y-27632 (Rho kinase inhibitor, 10−6 M) (1, 29), bisindolylmaleimide I (BIM; PKC inhibitor that shows high selectivity for PKC α-, β1-, β2-, γ-, δ-, and ε-isozymes, 10−6 M) (23), PD-98059 (specific inhibitor of the MEK/ERK1/2 pathway, 10−5 M) (12, 29), and SB-203580 (specific inhibitor of p38 MAPK, 10−5 M) (15).
Western blot analysis.
Frozen tissues were homogenized in ice-cold lysis buffer that contained 100 mM Na3VO4, 100 mM PMSF, and 1% proteinase inhibitor cocktail (P8340, Sigma, St. Louis, MO) in T-Per tissue protein extraction solution (no. 78510, ThermoScientific, Rockford, IL). The homogenate was centrifuged at 10,000 g for 15 min at 4°C, and the supernatant was collected. Protein concentration in the supernatant was measured by a bicinchoninic acid assay (ThermoScientific). Equal amounts of protein (uterine artery: 5 μg and mesenteric artery: 15 μg) were loaded on SDS-polyacrylamide gels (10%), which were then electrophoresed at 80 V for 1 h followed by 100 V for 1 h at room temperature and transferred at 110 V for 1 h and 30 min at 4°C to nitrocellulose membranes. The blotting amount of protein was checked by Ponceau S solution (Sigma). Before being immunoblotted, membranes were incubated in blocking solution (Tris-buffered saline-Tween-5% skim dry milk) for 30 min at room temperature. Immunoblots were incubated with primary antibodies overnight at 4°C, washed, and incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Results from mesenteric arteries were normalized by β-actin expression. However, β-actin was different between uterine virgin and pregnant arteries (data not shown), and, therefore, the results from uterine arteries were normalized by GAPDH expression. β-Actin and GAPDH protein levels were determined after stripping the membrane and probing with anti-β-actin or anti-GAPDH antibody with horseradish peroxidase-conjugated secondary antibody. Primary antibodies were as follows: mouse anti-Rho (21 kDa, 1:500), mouse anti-Rho-associated kinase (ROCK) I (160 kDa, 1:1,000), anti-ROCK II (180 kDa, 1:1,000), mouse anti-PKC-α (82 kDa, 1:1,000), mouse anti-PKC-ε (90 kDa, 1:1,000), rabbit anti-phosphorylated (p-)p44/42 MAPK (p-ERK1/2; 42 and 44 kDa, 1:1,000), rabbit anti- p44/42 MAPK (ERK1/2; 42 and 44 kDa, 1:1,000), rabbit anti-p-p38 MAPK (43 kDa, 1:1,000), rabbit anti-p38 MAPK (43 kDa, 1:1,000), mouse anti-β-actin (42 kDa, 1:15,000), and rabbit anti-GAPDH (37 kDa, 1:5,000). Immunoreactive bands were visualized with the enhanced chemiluminescence detection system and quantified using Alpha Imager software (Alpha Innotech, San Leandro, CA).
Data analysis.
For arterial contractions, contractile force was recorded as changes in the displacement from baseline (in mN). Concentration-response curves were fitted using nonlinear regression analysis as follows: Y = Emax/1 + 10e(logEC50 − X)slope, where X is the logarithm of the agonist concentration, Y is the response obtained, Emax is the maximum response to agonist, and EC50 is the concentration of agonist producing 50% of Emax. Agonist potencies are expressed in pD2 (negative logarithm of EC50). Arterial contractions are expressed in mN. Expressing these data as the percent maximum contraction to 120 mM KCl did not change the outcomes for group comparisons and effects of inhibitors (data not shown). For Western blot analysis, each value was normalized to the loading control (β-actin or GAPDH) from the same blot. For ERK1/2 and p38 MAPK phosphorylation, ratios were calculated for the optical density of p-ERK1/2, total ERK1/2, p-p38 MAPK, and total p38 MAPK over that of the corresponding β-actin (mesenteric artery) and GAPDH (uterine artery) value.
Statistical analysis.
An independent t-test was used to determine group differences in maternal and fetal physical parameters and protein expression, and the Mann-Whitney test was used to examine the effect of various inhibitors on Emax and pD2. Western blot data were not normally distributed and were log transformed (log10) before parametric statistics were used for group comparisons. Two-way ANOVA with repeated measures followed by a Bonferroni post hoc test was used to determine the effects of various inhibitors on concentration-response curves. Data are presented as means ± SE; n represents the number of animals used in each experiment. All statistical tests were performed with Graph Pad Prism (version 5.0, Graph Pad Software, San Diego, CA). The significance level of all tests was set at α = 0.05.
Chemicals and drugs.
PE, ACh, and antibodies against β-actin and GAPDH were obtained from Sigma Chemical, and U-46619, SQ-29548, and BIM were obtained from Cayman Chemical (Ann Arbor, MI). Antibodies against PKC-α, PKC-ε, Rho, ROCK I, and ROCK II were purchased from BD Transduction Laboratories (BD Biosciences, San Jose, CA), and antibodies against ERK1/2, p-ERK1/2 (Thr202/Tyr204), p38 MAPK, and p-p38 MAPK (Thr180/Tyr182) were purchased from Cell Signaling Technology (Beverly, MA). PD-98059 and SB-203580 were purchased from Calbiochem (San Diego, CA), and Y-27632 was obtained from Tocris Bioscience (Ellisville, MO). Stock solutions were prepared in deionized water or DMSO. Control solutions containing vehicle levels of DMSO were used through the experimental protocols.
RESULTS
Maternal and fetal physical parameters.
At the time of the experiment, pregnant dams (days 19–21 of gestation) were heavier than age-matched virgin rats, had smaller heart weights (expressed relative to body weight), larger uteri weights, and lower nonfasted blood glucose levels (P < 0.05). Table 1 shows maternal and fetal physical characteristics.
Table 1.
Maternal and fetal physical parameters
| Virgin Rats | Pregnant Rats | |
|---|---|---|
| Body weight, g | 251 ± 3 | 333 ± 9* |
| Organ weights, %body wt | ||
| Left ventricle | 0.199 ± 0.004 | 0.169 ± 0.004* |
| Right ventricle | 0.055 ± 0.002 | 0.049 ± 0.002* |
| Uterus | 0.191 ± 0.013 | 1.047 ± 0.047* |
| Blood glucose, mg/dl | 134 ± 7 | 99 ± 2* |
| Placental weight, g | 0.49 ± 0.02 | |
| Fetal weight, g | 2.11 ± 0.17 | |
| Litter size | 12.7 ± 0.9 |
Values are means ± SE; n = 8–11 virgin rats and 8–12 pregnant rats.
P < 0.05 vs. virgin rats.
U-46619-induced contractions in pregnant rat uterine and mesenteric arteries.
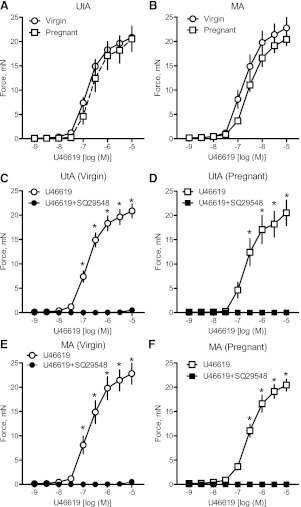
Cumulative administration of U-46619 (10−9–10−5 M) induced concentration-dependent contractions in uterine (Fig. 1A) and mesenteric (Fig. 1B) arterial segments in both virgin and pregnant rats. In some arterial segments, contractions to U-46619 were repeated to assess the effect of U-46619 on TP sensitization. Vascular responses did not desensitize after repeated concentration-response curves to U-46619 (data not shown). Uterine and mesenteric artery responses to U-46619 were similar between groups (Fig. 1, A and B, and Tables 2 and 3).Treatment with the highly selective TP antagonist SQ-29548 completely abolished U-46619-induced contractions in virgin uterine (Fig. 1C), pregnant uterine (Fig. 1D), virgin mesenteric (Fig. 1E), and pregnant mesenteric (Fig. 1F) arteries, indicating that U-46619 exclusively activates TP receptors in uterine and mesenteric vascular beds in virgin and pregnant rats. Inhibition of the TP receptor did not affect contractile responses to other vasoconstrictors, such as endothelin-1 and PE (data not shown).
Fig. 1.
Uterine artery (UtA) and mesenteric artery (MA) responses to U-46619 in pregnant and virgin rats. A and B: cumulative concentration-response curves to U-46619 (10−9–10−5 M) in UtAs (A) and MAs (B) from virgin (n = 11) and pregnant (n = 12) rats. C–F: treatment with the thromboxane A2 prostanoid receptor antagonist SQ-29548 (10−6 M) blocked U-46619-induced contractions in virgin uterine (C; n = 5), pregnant uterine (D; n = 7), virgin mesenteric (E; n = 6), and pregnant mesenteric (F; n = 7) arteries. Data are means ± SE. *P < 0.05 vs. virgin arteries as determined by two-way repeated-measures ANOVA.
Table 2.
Emax values for U-46619-induced contractions in uterine and mesenteric arteries from virgin and pregnant rats
| Virgin Emax, mN |
Pregnant Emax, mN |
|||
|---|---|---|---|---|
| Means ± SE | n | Means ± SE | n | |
| Uterine artery | ||||
| Untreated | 20.86 ± 1.45 | 11 | 20.54 ± 2.62 | 12 |
| Y-27632 (10−6 M) | 17.84 ± 1.35 | 11 | 22.62 ± 1.34 | 12 |
| BIM (10−6 M) | 17.41 ± 1.63 | 11 | 18.70 ± 2.08 | 12 |
| PD-98059 (10−5 M) | 14.74 ± 1.78* | 11 | 15.45 ± 1.84 | 12 |
| SB-203580 (10−5 M) | 13.58 ± 1.74* | 5 | 20.75 ± 0.95 | 5 |
| Mesenteric artery | ||||
| Untreated | 22.79 ± 2.17 | 11 | 20.45 ± 1.25 | 12 |
| Y-27632 (10−6 M) | 18.52 ± 1.53 | 11 | 18.82 ± 2.21 | 12 |
| BIM (10−6 M) | 16.42 ± 0.87* | 11 | 13.67 ± 2.04* | 12 |
| PD-98059 (10−5 M) | 17.28 ± 1.82* | 11 | 10.88 ± 1.78* | 12 |
| SB-203580 (10−5 M) | 10.66 ± 2.20* | 5 | 10.93 ± 2.44* | 5 |
Emax, maximal response; BIM, bisindolylmaleimide I;
P < 0.05 vs. the corresponding untreated group.
Table 3.
pD2 values for U-46619-induced contractions in uterine and mesenteric arteries from Virgin and Pregnant rats
| Virgin pD2 |
Pregnant pD2 |
|||
|---|---|---|---|---|
| Means ± SE | n | Means ± SE | n | |
| Uterine artery | ||||
| Untreated | 6.86 ± 0.09 | 11 | 6.41 ± 0.22 | 12 |
| Y-27632 (10−6 M) | 6.27 ± 0.21* | 11 | 6.29 ± 0.14 | 12 |
| BIM (10−6 M) | 6.42 ± 0.15* | 11 | 6.41 ± 0.21 | 12 |
| PD-98059 (10−5 M) | 6.38 ± 0.16* | 11 | 6.04 ± 0.25 | 12 |
| SB-203580 (10−5 M) | 6.20 ± 0.13* | 5 | 5.46 ± 0.29* | 5 |
| Mesenteric artery | ||||
| Untreated | 6.70 ± 0.11 | 11 | 6.43 ± 0.13 | 12 |
| Y-27632 (10−6 M) | 6.63 ± 0.14 | 11 | 6.38 ± 0.11 | 12 |
| BIM (10−6 M) | 6.54 ± 0.12 | 11 | 6.33 ± 0.14 | 12 |
| PD-98059 (10−5 M) | 6.44 ± 0.10 | 11 | 5.80 ± 0.11* | 12 |
| SB-203580 (10−5 M) | 6.02 ± 0.15* | 5 | 5.84 ± 0.17* | 5 |
pD2 values are the negative logEC50. Values are mean ± SE (number of animals).
P < 0.05 versus corresponding Untreated group.
Pregnancy diminishes the contribution of the RhoA/Rho kinase signaling pathway in the uterine artery contractile response to U-46619.
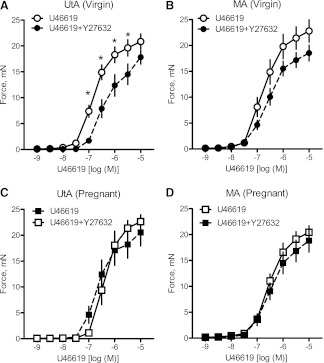
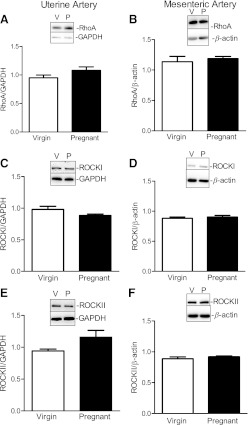
The Rho kinase inhibitor Y-27632 (10−6 M) significantly reduced virgin uterine (Fig. 2A and Table 3) but not mesenteric (Fig. 2B) artery submaximal contractile responses to U-46619 (i.e., 3 × 10−5, 10−6, 3 × 10−6, and 10−7 M for uterine arteries). Treatment with Y-27632 did not have any effect on pregnant uterine (Fig. 2C) and mesenteric (Fig. 2D) artery responses to U-46619. These data suggest that the Rho kinase signaling pathway contributes to uterine but not to mesenteric contractile responses to U-46619 in virgin rats and that pregnancy diminishes the role of Rho signaling in U-46619-induced uterine artery contractions. To determine whether the alterations in Rho kinase signal seen in rat pregnancy were due to a decrease in the expression of RhoA and/or Rho kinase in the uterine artery, we used Western blot analysis to measure the expressions of these proteins. Western blot analysis revealed that there were no group differences for any of the vessels in RhoA (Fig. 3, A and B), ROCK I (Fig. 3, C and D), and ROCK II (Fig. 3, E and F). The above data suggest that the diminishing effects of pregnancy on the contribution of Rho signaling to U-46619-induced uterine contractions are not due to a reduction in the basal expression of RhoA and Rho kinase.
Fig. 2.
Inhibition of the RhoA/Rho kinase signal does not reduce uterine and mesenteric contractile responses to U-46619 in pregnant rats. A–D: effects of the Rho kinase inhibitor Y-27632 (10−6 M) on cumulative concentration-response curves to U-46619 (10−9–10−5 M) in virgin uterine (A; n = 11), virgin mesenteric (B; n = 11), pregnant uterine (C; n = 12), and pregnant mesenteric (D; n = 12) arteries. Data are means ± SE. *P < 0.05 vs. U-46619 alone as determined by two-way repeated-measures ANOVA.
Fig. 3.
Pregnancy does not change the expression of RhoA, Rho-associated kinase (ROCK) I, and ROCK II of nonstimulated UtAs and MAs. A–F: representative Western blots (insets) and corresponding densitometric analysis showing the expression of uterine (A) and mesenteric (B) RhoA (21 kDa), uterine (C) and mesenteric (D) ROCK I (160 kDa), and uterine (E) and mesenteric (F) ROCK II (180 kDa) arteries from virgin (V) and pregnant (P) rats. Results from uterine tissue were normalized to GAPDH (37 kDa), and results from mesenteric tissue were normalized to β-actin (42 kDa). Densitometric data are expressed as means ± SE; n = 4–7.
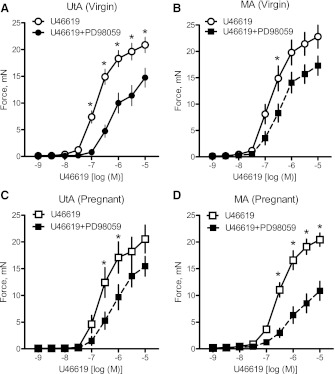
Pregnancy reduces the contribution of PKC signaling to U-46619-induced contractions in the uterine artery but not in the mesenteric artery.
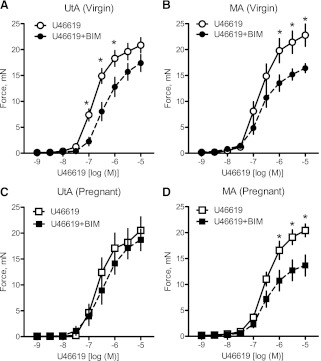
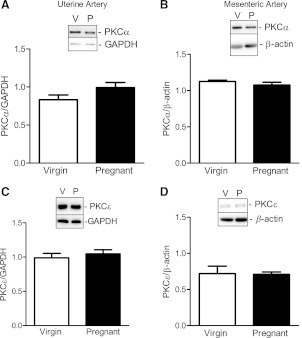
Treatment with the PKC inhibitor BIM (10−6 M) significantly decreased virgin uterine (Fig. 4A) and virgin and pregnant mesenteric (Fig. 4, B and D, and Table 2) artery responses to U-46619. In contrast, treatment with BIM had no effect on pregnant uterine (Fig. 4C) artery contractile responses to U-46619. Taken together, these data suggest that activation of the PKC signal significantly contributes to contractile responses to U-46619 in virgin uterine and mesenteric arteries as well as in pregnant mesenteric arteries, but in the uterine artery of pregnant rats this contribution is diminished. To determine whether changes in the PKC signaling pathway with pregnancy can be attributed to a reduction in PKC expression, we measured the expression of two PKC isoforms. There were no significant differences between pregnant and virgin rats in the expression of PKC-α (Fig. 5, A and B) and PKC-ε (Fig. 5, C and D) in uterine and mesenteric arteries. These data suggest that the reduction in the contribution of the PKC signaling pathway to U-46619-induced uterine artery contractions cannot be explained by a reduction in basal levels of PKC-α or PKC-ε expression.
Fig. 4.
Inhibition of the PKC signal does not reduce uterine contractile responses to U-46619 in pregnant rats. A–D: effects of the PKC inhibitor bisindolylmaleimide I (BIM; 10−6 M) on cumulative concentration-response curves to U-46619 (10−9–10−5 M) in virgin uterine (A; n = 11), virgin mesenteric (B; n = 11), pregnant uterine (C; n = 12), and pregnant mesenteric (D; n = 12) arteries. Data are means ± SE. *P < 0.05 vs. U-46619 alone as determined by two-way repeated-measures ANOVA.
Fig. 5.
Pregnancy does not change the expression of PKC-α and PKC-ε in nonstimulated UtAs and MAs. A–D: representative Western blots (insets) and corresponding densitometric analysis showing the expression of uterine (A) and mesenteric (B) PKC-α (82 kDa) and uterine (C) and mesenteric (D) PKC-ε (90 kDa) in arteries from virgin and pregnant rats. Results from uterine tissue were normalized to GAPDH (37 kDa), and results from mesenteric tissue were normalized to β-actin (42 kDa). Densitometric data are expressed as means ± SE; n = 4–7.
ERK1/2 and p38 MAPK signaling pathways play a significant role in U-46619-induced contractions in pregnant uterine and mesenteric arteries.
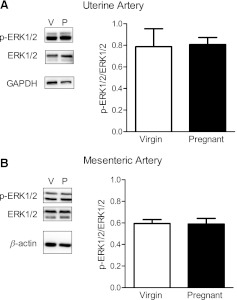
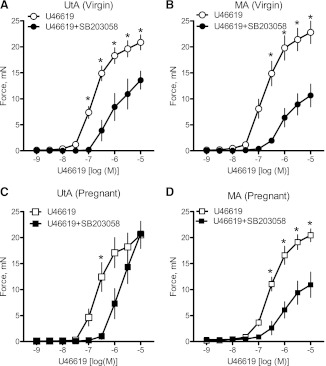
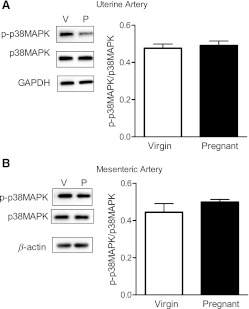
Inhibition of the ERK1/2 signaling pathway via treatment with PD-98059 (10−5 M) attenuated the contractile response to U-46619 in virgin uterine (Fig. 6A and Table 3) and mesenteric (Fig. 6B) arteries, and this effect remained unaltered in pregnant uterine and mesenteric arteries (Fig. 6, C and D, and Tables 2 and 3). The magnitude of the effect of ERK1/2 inhibition on U-46619-induced mesenteric contractions was greater in pregnant rats compared with virgin rats (virgin rats: 24% vs. pregnant rats: 47% decrease in Emax). Total and phosphorylated ERK1/2 expressions were similar between groups (data not shown), and there were no differences in p-ERK1/2/total ERK1/2 (Fig. 7, A and B). SB-203580 also reduced U-46619-induced contractions in virgin uterine (Fig. 8A and Tables 2 and 3) and mesenteric (Fig. 8B and Tables 2 and 3) arteries. In the pregnant uterine artery, the U46619-induced contraction was rightward shifted by SB-203580 (Fig. 8C and Table 3), whereas SB-203580 reduced U-46619-induced contractions in pregnant mesenteric arteries (Fig. 8D and Tables 2 and 3). These data suggest that activation of the ERK1/2 and p38 MAPK pathways contributes to U-46619-induced contractions in virgin and pregnant uterine and mesenteric arteries. Total and phosphorylated p38 MAPK expressions were similar between groups (data not shown), and there were no differences in p-p38 MAPK/total p38 MAPK (Fig. 9, A and B).
Fig. 6.
Inhibition of the ERK1/2 signal does not reduce uterine contractile responses to U-46619 in pregnant rats. A–D: effects of the ERK1/2 inhibitor PD-98059 (10−5 M) on cumulative concentration-response curves to U-46619 (10−9–10−5 M) in virgin uterine (A; n = 11), virgin mesenteric (B; n = 11), pregnant uterine (C; n = 12), and pregnant mesenteric (D; n = 12) arteries. Data are means ± SE. *P < 0.05 vs. U-46619 alone as determined by two-way repeated-measures ANOVA.
Fig. 7.
Pregnancy does not change the expression of ERK1/2 in nonstimulated UtAs and MAs. Left: representative Western blots showing the expression of phosphorylated (p-)p44/42 MAPK (p-ERK1/2; 42 and 44 kDa) and total ERK1/2 in UtAs (A) and MAs (B) from virgin and pregnant rats. Right: ratios of the optical density of p-ERK1/2 over that of the corresponding total ERK1/2 were calculated for UtAs (A) and MAs (B) from virgin and pregnant rats. Results from uterine tissue were normalized to GAPDH (37 kDa), and results from mesenteric tissue were normalized to β-actin (42 kDa). Densitometric data are expressed as means ± SE; n = 4–7.
Fig. 8.
Inhibition of p38 MAPK reduces uterine artery sensitivity to U-46619 in pregnant and virgin rats. A–D: effects of the p38 MAPK inhibitor SB-203580 (10−5 M) on cumulative concentration-response curves to U-46619 (10−9–10−5 M) in virgin uterine (A), virgin mesenteric (B), pregnant uterine (C), and pregnant mesenteric (D) arteries. Data are expressed as means ± SE. *P < 0.05 vs. U-46619 alone as determined by two-way repeated-measures ANOVA.
Fig. 9.
Pregnancy does not change the expression of p38 MAPK in nonstimulated UtAs and MAs. Left: representative Western blots showing the expression of p-p38 MAPK (40 kDa) and total p38 MAPK in UtAs (A) and MAs (B) from virgin and pregnant rats. Right: ratios of the optical density of p-p38 MAPK over that of the corresponding total p38 MAPK were calculated for UtAs (A) and MAs (B) from virgin and pregnant rats. Results from uterine arteries were normalized to GAPDH (37 kDa), and results from mesenteric arteries were normalized to β-actin (42 kDa). Densitometric data are expressed as means ± SE; n = 6.
DISCUSSION
The novel finding of this study is that normal pregnancy reduces the contribution of RhoA/Rho kinase and PKC signaling pathways to U-46619-induced contractions in uterine arteries while the role of ERK1/2 and p38 MAPK in these contractions is preserved in pregnancy. The effects of gestation on TxA2-associated transduction signals were vascular bed specific as they were not evident in mesenteric vessels from pregnant rats. In our study, U-46619-evoked contractions were completely blocked by the TP receptor antagonist SQ-29548, indicating that these contractions were mediated solely by the TP receptor.
TxA2 is an unstable arachidonic acid metabolite that exerts various physiological actions, including vascular smooth muscle contraction (33). During normal pregnancy, the production of TxA2 increases gradually and reaches its maximum concentrations after delivery (37). The enhanced biosynthesis of TxA2 in late gestation contributes to the generalized hypercoagulable state that characterizes pregnancy (16, 40). As stated, in addition to its effect on platelet activation, TxA2 exhibits contractile potency toward vascular smooth muscle. Studies in isolated uterine and carotid arteries from guinea pigs have shown that during pregnancy, sensitivity to TxA2 is reduced in uterine arteries but not in carotid arteries (43). Furthermore, pregnant rabbits show reduced contractile responses to U-46619 in pulmonary arteries (26) and skeletal muscle arterioles (41). In contrast to these findings, our results demonstrate that contractile responses to TxA2 do not change in uterine and mesenteric arteries from pregnant rats.
Despite the lack of difference in vascular responsiveness to TxA2 between virgin and pregnant rats, we found that pregnancy modifies TxA2-associated transduction signals. TxA2 exerts its contractile action via stimulation of the TP receptor, which results in the activation of signal transduction pathways through G protein coupling (33). Our data suggest that pregnant and nonpregnant uterine arteries achieve the same contractile response to TP receptor stimulation via different signaling pathways, whereas mesenteric artery responses remain unchanged with pregnancy. Previous studies on the mechanisms of activation of vascular smooth muscle contraction by U-46619 demonstrated the existence of tissue-specific TxA2-mediated signaling pathways for the regulation of vascular smooth muscle contractions. Consequently, stimulation of TP receptors has been shown to elicit the activation of different kinase-dependent pathways, including Rho kinase, PKC, ERK1/2, and p38 MAPK, in a tissue-dependent manner. Wilson et al. (46) reported that U-46619-induced contraction of rat caudal smooth muscle involved RhoA-mediated increases in Ca2+ sensitization. Rho kinase inhibitors Y-27632 and H-1152 blocked this contraction, suggesting that the downstream target of RhoA activation after TP stimulation is Rho kinase. In our study, inhibition of Rho kinase by Y-27632 reduced the contractile response to U-46619 in uterine arteries but not in mesenteric arteries from nonpregnant rats, indicating that the RhoA/Rho kinase signaling pathway is involved in U-46619-associated contractions in uterine arteries but not in mesenteric arteries from female rats. In contrast, uterine arteries from pregnant rats did not respond to inhibition of Rho kinase, demonstrating that pregnancy attenuates the role of the RhoA/Rho kinase pathway in the uterine vasculature. Our findings show that pregnancy-dependent changes in the role of the Rho/Rho kinase signal in U-46619-induced uterine contractions cannot be ascribed to changes in basal expression levels of RhoA, ROCK I, and ROCK II, as the expression of these proteins did not differ between pregnant and virgin rats.
The TxA2-TP receptor interaction is coupled to increased production of diacylglycerol, which activates PKC (33). Both TP stimulation and direct activation of PKC (via phorbol esters) cause sustained contraction of vascular smooth muscle with modest increases in intracellular Ca2+, indicating that TxA2 and PKC have a robust effect on Ca2+ sensitization of the contractile apparatus. Evidence has shown that PKC is involved in U-46619-induced contractions of porcine coronary arteries and small mesenteric arteries from male rats (2), while others have reported no involvement of PKC in contractile responses to U-46619 in the rat caudal artery (46). These data suggest that the involvement of PKC in TxA2-associated contractions is tissue specific. Our findings demonstrate that PKC is involved in uterine and mesenteric arterial contractions in response to U-46619 in female nonpregnant rats. Most importantly, we found that pregnancy attenuates the role of PKC in TP-associated contractions in the rat uterine artery. These findings are in agreement with previous investigations that showed reduced phorbol 12,13-dibutyrate (PKC activator)-induced contractions in the uterine artery from pregnant ewes (51) and the thoracic aorta from pregnant rats (19). Our results extend these data by showing that pregnancy does not regulate the role of PKC in U-46619-induced contractions in rat mesenteric arteries.
The differential effect of PKC on U-46619-induced contractions in uterine versus mesenteric arteries from pregnant rats and pregnant versus virgin uterine arteries may be due to the differential expression of PKC isozymes. PKC is not a simple enzyme but rather a family of several isoforms with different enzymatic properties, substrates, and functions in various blood vessels and species. Zhang et al. (51) showed that expression levels of PKC-β and PKC-ζ are increased, whereas PKC-α and PKC-ε are reduced in uterine arteries from pregnant compared with nonpregnant ewes. Reductions in PKC-α and PKC activity have been also found in the pregnant rat aorta (19). In this study, we used BIM, a PKC inhibitor that shows high selectivity for PKC α-, β1-, β2-, γ-, δ-, and ε-isozymes (23). Therefore, we conclude that the TxA2-associated PKC signal related to the activation of any of these enzymes is suppressed in rat uterine pregnant arteries, whereas the contribution of other PKC isozymes (i.e., PKC-ζ) to these contractions needs further investigation. In addition, we measured basal levels of expression of two PKC isoforms, PKC-α and PKC-ε, because they have been previously implicated in vascular contractions through increasing Ca2+ sensitivity. We found no differences in the expression of these isozymes between groups, indicating that pregnancy-induced adaptations of PKC involvement in contractile responses to U-46619 cannot be explained by alterations in basal expression levels of PKC-α and PKC-ε. Other isozymes that inhibit Ca2+ mobilization, such as PKC-β and PKC-ζ, may be involved in these adaptations.
In addition to Rho kinase and PKC, studies have implicated ERK1/2 in agonist-induced cellular actions, such as vascular smooth muscle cell proliferation and hypertrophy (11, 32). Conflicting results, however, have been obtained on the role of ERK1/2 in vascular smooth muscle contraction. Accordingly, inhibition of ERK1/2 had no effect on agonist-mediated contractions in the swine carotid artery (14) but reduced ANG II-induced contractions in rat mesenteric arteries (28). Furthermore, ERK1/2 played an important role in the ovine uterine artery contractile response to PE but not to KCl and serotonin (50) and had no role in TxA2-induced contractions in perfused and pressurized mesenteric arteries from male rats (2). Taken together, these data suggest that the role of ERK1/2 in vascular smooth muscle contractions is agonist dependent and is reduced in TxA2-induced contractions in resistance vessels from male rats. To the best of our knowledge, we are the first to demonstrate that ERK1/2 is involved in TxA2-induced contractions in both uterine and mesenteric arteries from female nonpregnant rats. Most importantly, our results demonstrate that the role of ERK1/2 in rat uterine artery contractile responses to U-46619 is preserved during pregnancy. Our findings are partially in agreement with Zhang et al. (51), who reported that inhibition of ERK1/2 by PD-98059 reduced PE-mediated uterine artery contractions in pregnant ewes but not in nonpregnant ewes, suggesting that, in ewes, pregnancy potentiates the role of ERK1/2 in uterine artery contractions. The discrepancy between Zhang et al.'s and our findings with regard to the role of ERK1/2 in uterine contractility in the nonpregnant state suggests that the role of ERK1/2 in vascular smooth muscle contraction varies based on the agonist used.
The present finding that inhibition of Rho kinase, PKC, and ERK1/2 reduced, but did not completely abolish, U-46619-induced contractions in uterine and mesenteric arteries from nonpregnant rats suggests the involvement of additional signaling pathways in these contractions. Bolla et al. (2) showed that p38 MAPK inhibition with SB-203580 inhibited U-46619-evoked contractions in small mesenteric arteries from male rats. Furthermore, these contractions were associated with increased phosphorylation of p38 MAPK, which was inhibited by SB-203580. The involvement of p38 MAPK in endothelin-1- and ANG II-induced contractions has been previously reported in conduit and small resistance vessels (31, 36). In this study, we demonstrated, for the first time, that p38 MAPK contributes to U-46619-induced uterine and mesenteric contractions in female rats and that pregnancy does not affect the role of this kinase in TxA2-induced contractions.
The present study characterized signal transduction pathways after TP receptor stimulation and demonstrated that some of these pathways (i.e., RhoA/Rho kinase, PKC associated with α-, β1-, β2-, γ-, δ-, and ε-isozymes) are suppressed in the uterine vasculature during pregnancy. Since pregnancy had no effect on the basal protein expression of key enzymes associated with these pathways, we suggest that the regulation of these enzymes is altered in late pregnancy. Our pharmacological interventions were performed in endothelium-intact vessels, and previous studies have shown that endothelium-derived relaxing factors (EDRFs) have a regulatory role upon the activity of the downstream target proteins of TP receptor activation (38). For example, our laboratory (4) and that of others (38) have reported that nitric oxide inhibits RhoA/Rho kinase signaling. Therefore, it is possible that pregnancy-induced increases in EDRFs, such as nitric oxide (48) and endothelium-derived hyperpolarizing factor (13), promote TP receptor desensitization and/or directly inhibit RhoA/Rho kinase and PKC signaling pathways (45). Conversely, diminished production of these factors, which is a characteristic of maternal vascular dysfunction in pregnancy complications, would lead to a potentiation of these signals and, subsequently, to enhanced uterine artery contractility.
Since suppression of TxA2-associated signal transduction was seen only in the uterine vascular bed, we suggest that local factors (i.e., placenta-derived endocrine factors) contribute to these pregnancy-associated vascular adaptations. A role of sex steroids in modulating U-46619-induced contractions during gestation has been previously proposed (41). Female rats exhibit greater contractile responses to U-46619 compared with males (25), and exogenous estrogen potentiates vascular reactivity to TxA2 via increased TP receptor expression (24). In our study, however, TP receptor expression in uterine vessels did not differ between pregnant and nonpregnant rats (data not shown), indicating that endogenous estrogen levels do not affect TP receptor expression and the downstream pathway(s) of TP activation are important controlling TP-mediated contraction during pregnancy, as mentioned above. It has been reported, however, that estrogen and progesterone block Ca2+ influx (10) in smooth muscle and inhibit agonist- and GTPγS-induced Ca2+ sensitization by increasing the expression of Rnd proteins, which inhibit the RhoA/Rho kinase signaling pathway (3, 21). The role of Rnd proteins in TxA2-associated signal transduction in the uterine vasculature needs further investigation.
In conclusion, the present study provides evidence that normal pregnancy suppresses the role of RhoA/Rho kinase and PKC signals in TxA2-induced contractions in the rat uterine artery, whereas the contribution of ERK1/2 and p38 MAPK to these contractions is preserved. Given that pregnancy regulates TxA2-associated signal transduction specifically in the uterine (vs. the mesenteric) vasculature, it is reasonable to suggest that alterations in the regulation of the downstream target proteins of TP receptor activation would compromise uterine artery reactivity, which could contribute to insufficient nutrient supply to the fetus and intrauterine growth restriction. TxA2-associated transduction signals and its regulators might present potential targets for the development of new treatments for preeclampsia and other pregnancy-associated vascular diseases complicated with intrauterine growth restriction.
GRANTS
This work was supported in part by National Institutes of Health Grants R01-HL-071138, R01-DK-083685, and T32-HL-066993-09, the Naito Foundation of Japan, the Society for Women's Health Research, and the Heart and Stroke Foundation of Canada.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.G., J.L.H., T.M., and R.C.W. conception and design of research; S.G. and J.L.H. performed experiments; S.G. and T.M. analyzed data; S.G., J.L.H., T.M., and R.C.W. interpreted results of experiments; S.G. prepared figures; S.G. drafted manuscript; S.G., J.L.H., T.M., and R.C.W. edited and revised manuscript; S.G., J.L.H., T.M., and R.C.W. approved final version of manuscript.
REFERENCES
- 1.Allahdadi KJ, Hannan JL, Ergul A, Tostes RC, Webb RC. Internal pudendal artery from type 2 diabetic female rats demonstrate elevated endothelin-1-mediated constriction. J Sex Med 8: 2472–2483, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolla M, Matrougui K, Loufrani L, Maclouf J, Levy B, Levy-Toledano S, Habib A, Henrion D. p38 mitogen-activated protein kinase activation is required for thromboxane- induced contraction in perfused and pressurized rat mesenteric resistance arteries. J Vasc Res 39: 353–360, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Cario-Toumaniantz C, Reillaudoux G, Sauzeau V, Heutte F, Vaillant N, Finet M, Chardin P, Loirand G, Pacaud P. Modulation of RhoA-Rho kinase-mediated Ca2+ sensitization of rabbit myometrium during pregnancy–role of Rnd3. J Physiol 552: 403–413, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chitaley K, Webb RC. Nitric oxide induces dilation of rat aorta via inhibition of rho-kinase signaling. Hypertension 39: 438–442, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Cipolla M, Osol G. Hypertrophic and hyperplastic effects of pregnancy on the rat uterine arterial wall. Am J Obstet Gynecol 171: 805–811, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Clark SL, Cotton DB, Lee W, Bishop C, Hill T, Southwick J, Pivarnik J, Spillman T, DeVore GR, Phelan J, et al. Central hemodynamic assessment of normal term pregnancy. Am J Obstet Gynecol 161: 1439–1442, 1989 [DOI] [PubMed] [Google Scholar]
- 7.Cooke CL, Davidge ST. Pregnancy-induced alterations of vascular function in mouse mesenteric and uterine arteries. Biol Reprod 68: 1072–1077, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Devillier P, Bessard G. Thromboxane A2 and related prostaglandins in airways. Fundam Clin Pharmacol 11: 2–18, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Dorn GW, 2nd, Liel N, Trask JL, Mais DE, Assey ME, Halushka PV. Increased platelet thromboxane A2/prostaglandin H2 receptors in patients with acute myocardial infarction. Circulation 81: 212–218, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Fomin VP, Cox BE, Word RA. Effect of progesterone on intracellular Ca2+ homeostasis in human myometrial smooth muscle cells. Am J Physiol Cell Physiol 276: C379–C385, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Gao Y, Tang S, Zhou S, Ware JA. The thromboxane A2 receptor activates mitogen-activated protein kinase via protein kinase C-dependent Gi coupling and Src-dependent phosphorylation of the epidermal growth factor receptor. J Pharmacol Exp Ther 296: 426–433, 2001 [PubMed] [Google Scholar]
- 12.Giachini FR, Sullivan JC, Lima VV, Carneiro FS, Fortes ZB, Pollock DM, Carvalho MH, Webb RC, Tostes RC. Extracellular signal-regulated kinase 1/2 activation, via downregulation of mitogen-activated protein kinase phosphatase 1, mediates sex differences in desoxycorticosterone acetate-salt hypertension vascular reactivity. Hypertension 55: 172–179, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gokina NI, Kuzina OY, Vance AM. Augmented EDHF signaling in rat uteroplacental vasculature during late pregnancy. Am J Physiol Heart Circ Physiol 299: H1642–H1652, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorenne I, Su X, Moreland RS. Inhibition of p42 and p44 MAP kinase does not alter smooth muscle contraction in swine carotid artery. Am J Physiol Heart Circ Physiol 275: H131–H138, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265: 808–811, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Hayashi M, Inoue T, Hoshimoto K, Hirabayashi H, Negishi H, Ohkura T. The levels of five markers of hemostasis and endothelial status at different stages of normotensive pregnancy. Acta Obstet Gynecol Scand 81: 208–213, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Howarth SR, Vallance P, Wilson CA. Role of thromboxane A2 in the vasoconstrictor response to endothelin-1, angiotensin II and 5-hydroxytryptamine in human placental vessels. Placenta 16: 679–689, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Johnson RL, Gilbert M, Meschia G, Battaglia FC. Cardiac output distribution and uteroplacental blood flow in the pregnant rabbit: a comparative study. Am J Obstet Gynecol 151: 682–686, 1985 [DOI] [PubMed] [Google Scholar]
- 19.Kanashiro CA, Cockrell KL, Alexander BT, Granger JP, Khalil RA. Pregnancy-associated reduction in vascular protein kinase C activity rebounds during inhibition of NO synthesis. Am J Physiol Regul Integr Comp Physiol 278: R295–R303, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Katoue MG, Khan I, Oriowo MA. Pregnancy-induced modulation of calcium mobilization and down-regulation of Rho-kinase expression contribute to attenuated vasopressin-induced contraction of the rat aorta. Vascul Pharmacol 44: 170–176, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Kim YS, Kim B, Karaki H, Hori M, Ozaki H. Up-regulation of Rnd1 during pregnancy serves as a negative-feedback control for Ca2+ sensitization of contractile elements in rat myometrium. Biochem Biophys Res Commun 311: 972–978, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Kriston T, Venuto RC, Baylis C, Losonczy G. Hemodynamic and renal effects of U-46619, a TXA2/PGH2 analog, in late-pregnant rats. Am J Physiol Regul Integr Comp Physiol 276: R831–R837, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Kutz C, Paintz M, Glusa E. Inhibition of thrombin-induced contractile responses by protein kinase inhibitors in porcine pulmonary arteries. Exp Toxicol Pathol 50: 497–500, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Li M, Kuo L, Stallone JN. Estrogen potentiates constrictor prostanoid function in female rat aorta by upregulation of cyclooxygenase-2 and thromboxane pathway expression. Am J Physiol Heart Circ Physiol 294: H2444–H2455, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Li M, Stallone JN. Estrogen potentiates vasopressin-induced contraction of female rat aorta by enhancing cyclooxygenase-2 and thromboxane function. Am J Physiol Heart Circ Physiol 289: H1542–H1550, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Losonczy G, Brown G, Mucha I, Klocke R, Muller V, Merkely B, Tornoci L, Rosivall L, Venuto R. Gestational resistance to the pulmonary vasoconstrictor effect of the TxA2 mimetic U-46619: possible mechanism. Am J Physiol Regul Integr Comp Physiol 272: R1734–R1739, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Losonczy G, Singh JP, Schoenl M, Mucha I, Venuto RC. Pregnancy enhances the pressor response to thromboxane analogues in rabbits. Am J Physiol Regul Integr Comp Physiol 269: R720–R725, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Matrougui K, Tanko LB, Loufrani L, Gorny D, Levy BI, Tedgui A, Henrion D. Involvement of Rho-kinase and the actin filament network in angiotensin II-induced contraction and extracellular signal-regulated kinase activity in intact rat mesenteric resistance arteries. Arterioscler Thromb Vasc Biol 21: 1288–1293, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto T, Kobayashi T, Ishida K, Taguchi K, Kamata K. Enhancement of mesenteric artery contraction to 5-HT depends on Rho kinase and Src kinase pathways in the ob/ob mouse model of type 2 diabetes. Br J Pharmacol 160: 1092–1104, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meagher EA, FitzGerald GA. Disordered eicosanoid formation in pregnancy-induced hypertension. Circulation 88: 1324–1333, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Meloche S, Landry J, Huot J, Houle F, Marceau F, Giasson E. p38 MAP kinase pathway regulates angiotensin II-induced contraction of rat vascular smooth muscle. Am J Physiol Heart Circ Physiol 279: H741–H751, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Miggin SM, Kinsella BT. Regulation of extracellular signal-regulated kinase cascades by alpha- and beta-isoforms of the human thromboxane A2 receptor. Mol Pharmacol 61: 817–831, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Nakahata N. Thromboxane A2: physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacol Ther 118: 18–35, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev 79: 1193–1226, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Nelson SH, Steinsland OS, Johnson RL, Suresh MS, Gifford A, Ehardt JS. Pregnancy-induced alterations of neurogenic constriction and dilation of human uterine artery. Am J Physiol Heart Circ Physiol 268: H1694–H1701, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Ohanian J, Cunliffe P, Ceppi E, Alder A, Heerkens E, Ohanian V. Activation of p38 mitogen-activated protein kinases by endothelin and noradrenaline in small arteries, regulation by calcium influx and tyrosine kinases, and their role in contraction. Arterioscler Thromb Vasc Biol 21: 1921–1927, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Perneby C, Vahter M, Akesson A, Bremme K, Hjemdahl P. Thromboxane metabolite excretion during pregnancy–influence of preeclampsia and aspirin treatment. Thromb Res 127: 605–606, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, Chardin P, Pacaud P, Loirand G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem 275: 21722–21729, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Sibai BM, Frangieh A. Maternal adaptation to pregnancy. Curr Opin Obstet Gynecol 7: 420–426, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Stirling Y, Woolf L, North WR, Seghatchian MJ, Meade TW. Haemostasis in normal pregnancy. Thromb Haemost 52: 176–182, 1984 [PubMed] [Google Scholar]
- 41.Ungvari Z, Brown G, Venuto R, Koller A, Losonczy G. Increased NO-mediated and reduced TxA2-dependent responses in skeletal muscle arterioles in pregnancy. Hypertens Pregnancy 21: 135–146, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Wareing M, O'Hara M, Seghier F, Baker PN, Taggart MJ. The involvement of Rho-associated kinases in agonist-dependent contractions of human maternal and placental arteries at term gestation. Am J Obstet Gynecol 193: 815–824, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Weiner CP, Thompson LP, Liu KZ, Herrig JE. Endothelium-derived relaxing factor and indomethacin-sensitive contracting factor alter arterial contractile responses to thromboxane during pregnancy. Am J Obstet Gynecol 166: 1171–1181, 1992 [DOI] [PubMed] [Google Scholar]
- 44.Wight E, Kung CF, Moreau P, Takase H, Bersinger NA, Luscher TF. Aging, serum estradiol levels, and pregnancy differentially affect vascular reactivity of the rat uterine artery. J Soc Gynecol Investig 7: 106–113, 2000 [PubMed] [Google Scholar]
- 45.Wikstrom K, Kavanagh DJ, Reid HM, Kinsella BT. Differential regulation of RhoA-mediated signaling by the TPα and TPβ isoforms of the human thromboxane A2 receptor: independent modulation of TPα signaling by prostacyclin and nitric oxide. Cell Signal 20: 1497–1512, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson DP, Susnjar M, Kiss E, Sutherland C, Walsh MP. Thromboxane A2-induced contraction of rat caudal arterial smooth muscle involves activation of Ca2+ entry and Ca2+ sensitization: Rho-associated kinase-mediated phosphorylation of MYPT1 at Thr-855, but not Thr-697. Biochem J 389: 763–774, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woods LL. Importance of prostaglandins in hypertension during reduced uteroplacental perfusion pressure. Am J Physiol Regul Integr Comp Physiol 257: R1558–R1561, 1989 [DOI] [PubMed] [Google Scholar]
- 48.Xiao D, Liu Y, Pearce WJ, Zhang L. Endothelial nitric oxide release in isolated perfused ovine uterine arteries: effect of pregnancy. Eur J Pharmacol 367: 223–230, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Xiao D, Pearce WJ, Zhang L. Pregnancy enhances endothelium-dependent relaxation of ovine uterine artery: role of NO and intracellular Ca2+. Am J Physiol Heart Circ Physiol 281: H183–H190, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Xiao D, Zhang L. ERK MAP kinases regulate smooth muscle contraction in ovine uterine artery: effect of pregnancy. Am J Physiol Heart Circ Physiol 282: H292–H300, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Zhang H, Xiao D, Longo LD, Zhang L. Regulation of α1-adrenoceptor-mediated contractions of uterine arteries by PKC: effect of pregnancy. Am J Physiol Heart Circ Physiol 291: H2282–H2289, 2006 [DOI] [PubMed] [Google Scholar]