Abstract
QT-RR hysteresis is characterized by longer QT intervals at a given RR interval while heart rates are increasing during exercise and shorter QT intervals at the same RR interval while heart rates are decreasing during recovery. It has been attributed to a lagging QT response to different directional changes in RR interval during exercise and recovery. Twenty control subjects (8 males, age 51 ± 6 yr), 16 subjects with type 2 diabetes (12 males, age 56 ± 8 yr), 71 subjects with coronary artery disease (CAD) and preserved left ventricular ejection fraction (LVEF) (≥50%) (51 males, age 59 ± 12 yr), and 17 CAD subjects with depressed LVEF (<50%) (13 males, age 57 ± 10 yr) underwent two 16-min exercise tests followed by recovery. In session 2, parasympathetic blockade with atropine (0.04 mg/kg) was achieved at end exercise. QT-RR hysteresis was quantified as: 1) the area bounded by the QT-RR relationships for exercise and recovery in the range of the minimum RR interval at peak exercise to the minimum RR interval + 100 ms and 2) the difference in QT interval duration between exercise and recovery at the minimum RR interval achieved during peak exercise plus 50 ms (ΔQT). The effect of parasympathetic blockade was assessed by substituting the QT-RR relationship after parasympathetic blockade. QT-RR hysteresis was positive in all groups at baseline and reversed by parasympathetic blockade (P < 0.01). We conclude that QT-RR hysteresis is not caused by different directional changes in RR interval during exercise and recovery. Instead, it is predominantly mediated by differential autonomic nervous system effects as the heart rate increases during exercise vs. as it decreases during recovery.
Keywords: nervous system, autonomic; exercise; electrophysiology
a hysteresis effect in the QT-RR relationship during exercise and recovery has been previously described (35). This phenomenon is characterized by longer QT intervals at a given RR interval while heart rates are increasing (RR intervals decreasing) during exercise and shorter QT intervals at the same RR interval when heart rates are decreasing (RR intervals increasing) during recovery. Evaluation of this QT-RR relationship during exercise and recovery has shown clinical value in certain situations, such as differentiating patients with long QT syndrome from patients with borderline QT duration (22) and as a marker of myocardial ischemia (26, 40). However, the mechanism of QT-RR hysteresis is not understood. The prevailing understanding attributes the phenomenon to a lag of the QT interval when adapting to the change in RR interval when decreasing (exercise) vs. increasing (recovery) (2, 11, 25, 35, 37). Others have speculated that alterations in autonomic tone contribute to differences in repolarization in exercise and recovery (23, 35). Specifically, late exercise is characterized by sympathoexcitation and minimal parsympathetic effects. In contrast, early recovery is characterized by rapid parasympathetic reactivation with persistent, but declining, sympathoexcitation.
We have previously shown that parasympathetic blockade shifts the QT-RR relationship in recovery upward, so that the QT is longer for any given RR interval in recovery in the presence vs. absence of parasympathetic blockade (42). In this study, we therefore evaluated QT-RR hysteresis in subjects who underwent an exercise protocol with and without parasympathetic blockade to test the hypothesis that the rapid recovery of parasympathetic tone that occurs early in recovery is a significant contributor to QT-RR hysteresis. We also examined whether QT-RR hysteresis is greater in normal subjects than in populations in which reduced parasympathetic tone has been reported, specifically coronary artery disease (CAD) and diabetes mellitus (DM).
METHODS
Study population.
Three groups of subjects were studied. Group one consisted of normal subjects without DM or CAD. Group two consisted of subjects with type 2 DM with no known cardiovascular disease. Group three consisted of subjects with CAD defined by a history of myocardial infarction or a known 50% or greater stenosis in one or more coronary arteries. This group was subdivided into two subgroups with preserved left ventricular ejection fraction (LVEF ≥50%) and depressed LVEF (<50%). Details of the study population have been described previously (44). This study was approved by the Northwestern University Institutional Research Board. All subjects were evaluated at the Clinical Research Unit of Northwestern Memorial Hospital (Chicago, IL). Informed consent was obtained from each subject at study initiation.
Study protocol.
Exercise stress tests were performed on a stationary bicycle (SciFit Pro II, Tulsa, OK) on two visits separated by at least 72 h as previously described (44). All recordings were made with subjects seated on the bicycle. Briefly, 12-lead electrocardiographic data were recorded on a commercially available system (Quest Exercise Stress System; Burdick, Deerfield, WI). After a 5-min rest period, 5 min of rest electrocardiogram (ECG) were recorded. On the first visit, subjects underwent a 16-min submaximal exercise protocol with an initial workload of 50 watts followed by increases to 75 watts at 4 min and 100 watts at 6 min as tolerated. ECG recording was continued for at least 30 min of recovery. On the second visit, an identical exercise protocol was performed, but atropine was administered intravenously in four doses of 0.01 mg/kg each every 30 s starting at the 12th min of exercise. Thus the final 2 min of exercise as well as the entire recovery period occurred under parasympathetic blockade (16).
ECG analysis.
Electrocardiographic analysis was performed with custom MATLAB (Mathworks, Natick, MA) software. QRS complexes were detected using a template-matching algorithm with the maximum negative slope used as the fiducial point, from which RR intervals were calculated. One RR interval preceding and two RR intervals following premature atrial or ventricular complexes were excluded from analysis. Baseline wander was linearly corrected, referenced to the QRS onsets of each complex. For the measurement of QT intervals, the T wave offsets for each lead were identified using the intersection of the tangent of the maximum descending slope of the T wave with the baseline. For each beat, the median T wave offset of the 12 leads was chosen as the representative value for that beat. Median T wave offset was used to exclude outliers and has been shown to provide accurate QT interval data (45). A seven-beat median filter was applied to the resulting QT intervals to account for outliers. All tracings were reviewed and overread as necessary.
Hysteresis and heart rate recovery computation.
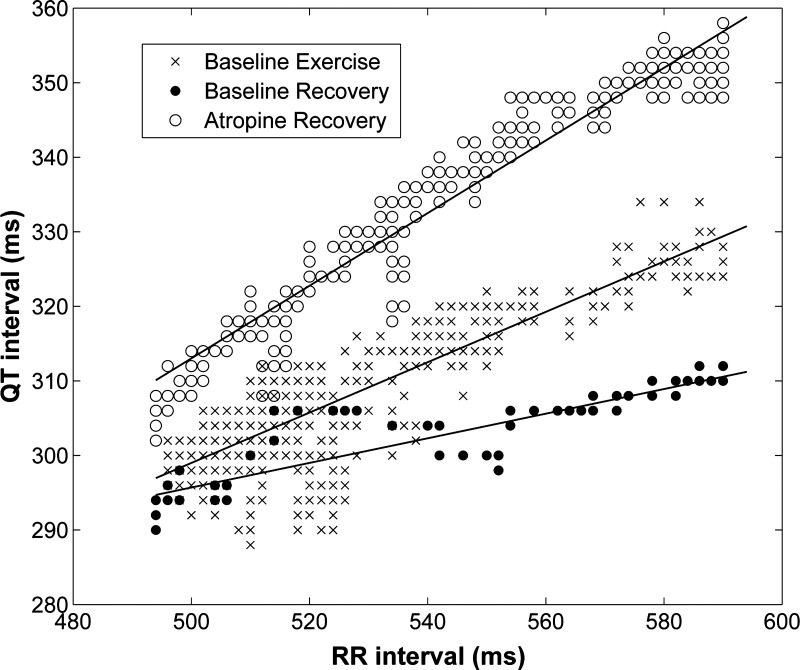
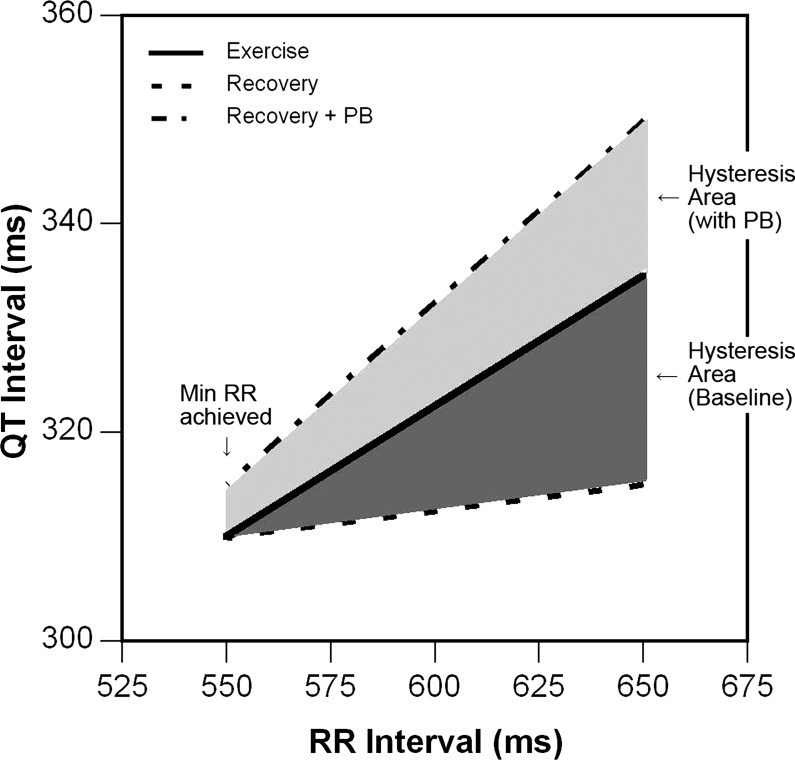
A representative example of QT-RR relationships for exercise and recovery (before and after parasympathetic blockade) is shown in Fig. 1. The method used to quantify QT-RR hysteresis is shown in Fig. 2. This method calculates the area between the QT-RR plots for exercise and recovery over a range of RR intervals.
Fig. 1.
Example of QT-RR relationships for the baseline exercise test during exercise and during recovery from the exercise test with parasympathetic blockade. For any given RR interval, the QT interval is longest for recovery with parasympathetic blockade and shortest for baseline recovery.
Fig. 2.
Demonstration of the calculation of hysteresis area. Hysteresis area is calculated as the area between the exercise and recovery curves, and is represented above as dark gray in the physiological study (positive area because exercise curve is above recovery curve) and as light gray with parasympathetic blockade (negative area because recovery curve is above exercise curve). PB, parasympathetic blockade.
The QT-RR interval plots in exercise and recovery were modeled by linear regression analysis, performed over a specified range of RR intervals. This range was defined by the peak heart rate (minimum RR interval) at end exercise up to the RR interval constituting two-thirds of the total change in RR interval during recovery. Three sets of linear modeling of the QT-RR relationship were derived for each subject: 1) during exercise; 2) during recovery; and 3) during recovery after parasympathetic blockade. As shown in Fig. 2, the shaded area between the exercise and recovery QT-RR relationships defines the extent of QT-RR hysteresis. If this is mediated by differential parasympathetic effects between exercise and recovery, the recovery QT-RR relationship after parasympathetic blockade would be shifted upward. Thus, quantifying the area between the exercise QT-RR relationship and the QT-RR relationship during recovery, with and without parasympathetic blockade, will allow delineation of the role of parasympathetic effects on hysteresis.
The hysteresis area was computed as the area between the two modeled curves over the first 100 ms of RR interval recovery. Based on the previously established QT-RR hysteresis relationship (i.e., the QT interval in recovery was shorter than during exercise for any given RR interval), it was expected that the regression line for recovery would be below the regression line for exercise. In this situation, the area was considered positive. The area was considered negative if the modeled recovery line was above the exercise line (i.e., the QT interval in recovery was longer than during exercise for any given RR interval). Subjects in whom an R2 value of >0.5 for all three regression lines was not obtained were excluded from analysis.
Hysteresis was also quantified as the difference in QT interval duration (ΔQT) between exercise and recovery at a predetermined RR interval, the minimum RR interval achieved during peak exercise plus 50 ms. ΔQT was defined as the QT interval duration during exercise at this predetermined RR interval minus the QT interval duration during recovery at this predetermined RR interval, derived from the regression equations determined as above. The effect of parasympathetic blockade on ΔQT was assessed by substituting the QT interval duration during recovery after parasympathetic blockade at this predetermined RR interval.
Heart rate recovery (HRR) was defined as peak exercise heart rate minus heart rate at 1 min of recovery during the baseline study.
Statistics.
All data are presented as means ± SD. Continuous variables were compared by use of ANOVA. A P value of <0.05 was considered statistically significant. Because early HRR is a marker of parasympathetic reactivation (13, 14, 19, 42), linear regression analysis was performed to assess for a relationship between QT-RR hysteresis area and HRR.
RESULTS
Clinical characteristics.
There were 20 subjects in the control group (8 males, age 51 ± 6 yr), 16 subjects in the type 2 DM group (12 males, age 56 ± 8 yr, hemoglobin A1c 6.4 ± 0.7%), and 88 subjects in the CAD group (64 males, age 58 ± 12 yr). The CAD group was subdivided by LVEF, with 71 having preserved LVEF (51 males, age 59 ± 12 yr, LVEF 60 ± 5%) and 17 having depressed LVEF (13 males, age 57 ± 10 yr, LVEF 36 ± 7%). Of the 124 subjects studied, 110 (88.7%) had R2 values >0.5 for all three analyses of the QT-RR relationship. Thus, 14 subjects were excluded from analysis of QT-RR measures. Mean R2 values were 0.86 ± 0.10. There were no significant differences in age, sex, body mass index, or LVEF between included and excluded subjects.
Subject characteristics are listed in Table 1. β-Adrenergic blockers were commonly used in subjects with CAD but none of the control and DM subjects. Average body mass index did not differ among the groups.
Table 1.
Baseline characteristics
| Control | Type 2 DM | CAD with Preserved LVEF | CAD with Depressed LVEF | P | |
|---|---|---|---|---|---|
| No. | 20 | 16 | 71 | 17 | |
| Age, yr | 51 ± 6 | 56 ± 8 | 59 ± 12 | 57 ± 10 | 0.03 |
| Male, % | 40.0 | 75.0 | 71.8 | 76.5 | 0.03 |
| Caucasian, % | 60.0 | 75.0 | 80.3 | 82.4 | 0.27 |
| Ejection fraction, % | 60 ± 5 | 36 ± 7 | <0.01 | ||
| WMA, % | 12.7 | 88.2 | <0.01 | ||
| Hemoglobin A1c, % | 6.4 ± 0.7 | ||||
| Hypertension, % | 0.0 | 25.0 | 52.1 | 41.2 | <0.01 |
| Hyperlipidemia, % | 10.0 | 81.3 | 73.2 | 76.5 | <0.01 |
| Smoker, % | 15.0 | 12.5 | 11.3 | 23.5 | 0.63 |
| Medications, % | |||||
| β-Blocker | 0.0 | 0.0 | 80.3 | 100.0 | <0.01 |
| CCB | 0.0 | 0.0 | 16.9 | 0.0 | 0.02 |
| Aspirin | 0.0 | 56.3 | 95.8 | 88.2 | <0.01 |
| Clopidogrel | 0.0 | 0.0 | 19.7 | 47.1 | <0.01 |
| Statin | 5.0 | 81.3 | 97.2 | 82.4 | <0.01 |
| ACE inhibitor | 0.0 | 25.0 | 50.7 | 64.7 | <0.01 |
| ARB | 0.0 | 6.3 | 2.8 | 5.9 | 0.68 |
| Diuretic | 0.0 | 6.3 | 15.5 | 11.8 | 0.24 |
| Nitrate | 0.0 | 0.0 | 5.6 | 11.8 | 0.30 |
| Insulin | 0.0 | 0.0 | 0.0 | 0.0 | |
| Metformin | 0.0 | 87.5 | 0.0 | 0.0 | |
| TZD | 0.0 | 37.5 | 0.0 | 0.0 | |
| Sulfonylurea | 0.0 | 25.0 | 0.0 | 0.0 | |
| Meglitinide | 0.0 | 12.5 | 0.0 | 0.0 | |
| Exenatide | 0.0 | 6.3 | 0.0 | 0.0 | |
| Sitagliptin | 0.0 | 6.3 | 0.0 | 0.0 | |
| BMI | 27 ± 3 | 28 ± 3 | 28 ± 3 | 28 ± 3 | 0.32 |
Values are means ± SD.
DM, diabetes mellitus; CAD, coronary artery disease; LVEF, left ventricular ejection fraction; WMA, wall motion abnormalities; CCB, calcium channel blocker; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; TZD, thiazolidinedione.
Resting heart rates were 64 ± 9, 73 ± 12, 64 ± 9, and 68 ± 12 beats/min (P = 0.02), and peak heart rates achieved during exercise were 127 ± 13, 134 ± 22, 114 ± 15, and 120 ± 15 beats/min (P < 0.01) for control, DM, CAD with preserved LVEF, and CAD with depressed LVEF groups, respectively (P = 0.02). Peak workloads achieved were 91 ± 19, 90 ± 13, 86 ± 20, and 80 ± 19 W for control, DM, CAD with preserved LVEF, and CAD with depressed LVEF groups, respectively (P = 0.40).
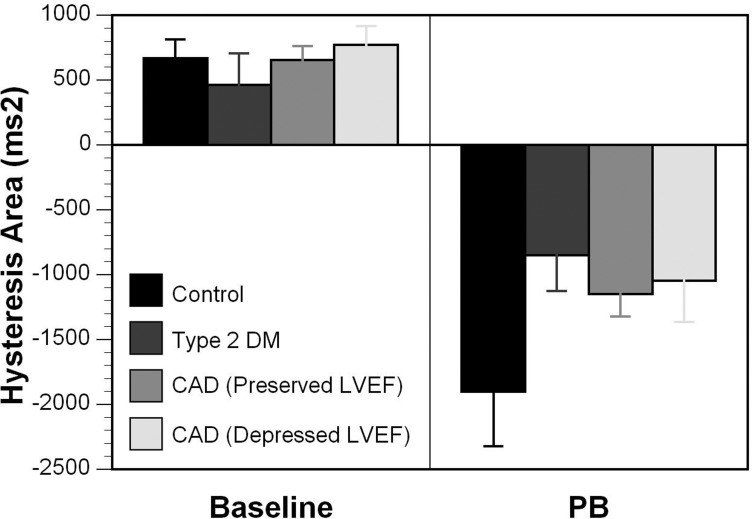
Hysteresis area.
Figure 3 shows the QT-RR hysteresis area. QT-RR hysteresis area was 688.9 ± 625.9 ms2 in the control group, 460.2 ± 841.4 ms2 in the type 2 diabetic group, and 674.8 ± 806.1 ms2 in the CAD group in the baseline study. There was no significant difference in hysteresis area among the groups, nor between CAD subjects with preserved vs. depressed LVEF. With parasympathetic blockade, positive hysteresis area was reversed in all groups (−1,902.1 ± 1,844.7 ms2 in the control group, −853.3 ± 944.6 ms2 in the type 2 diabetic group, and −1,132.6 ± 1,341.3 ms2 in the CAD group). The QT-RR hysteresis area was not significantly different across groups either with or without parasympathetic blockade (P = 0.20).
Fig. 3.
Hysteresis area shown for each group during the baseline exercise study and after parasympathetic blockade. DM, diabetes mellitus; CAD, coronary artery disease; LVEF, left ventricular ejection fraction.
QT-RR hysteresis area was positive in all three groups without parasympathetic blockade, demonstrating that for any given RR interval, the QT interval was shorter during recovery than during exercise. These areas became negative after parasympathetic blockade, demonstrating that parasympathetic blockade reversed hysteresis (P < 0.0001).
Hysteresis-ΔQT interval.
For all groups, the QT interval obtained during exercise at the predetermined RR interval of peak exercise RR interval plus 50 ms was longer than the QT interval during recovery at the same RR interval, whereas the QT interval during recovery with parasympathetic blockade was longer than both of these intervals (P < 0.0001). ΔQT intervals without parasympathetic blockade were 6.8 ± 6.5, 4.6 ± 8.3, 6.6 ± 8.6, and 7.9 ± 5.6 ms in the control, DM, CAD with preserved LVEF, and CAD with reduced LVEF groups, respectively. After parasympathetic blockade, ΔQT intervals were −19.1 ± 18.5, −8.5 ± 9.3, −11.6 ± 13.7, and −10.4 ± 12.5 ms in the control, DM, CAD with preserved LVEF, and CAD with reduced LVEF groups, respectively.
Heart rate recovery.
HRR was 23.0 ± 6.2, 10.9 ± 4.5, and 14.2 ± 6.3 beats/min for control, DM, and CAD groups, respectively. HRR was significantly greater in the control group compared with the CAD and DM groups (P < 0.0001). There was no significant difference in HRR between CAD and DM groups.
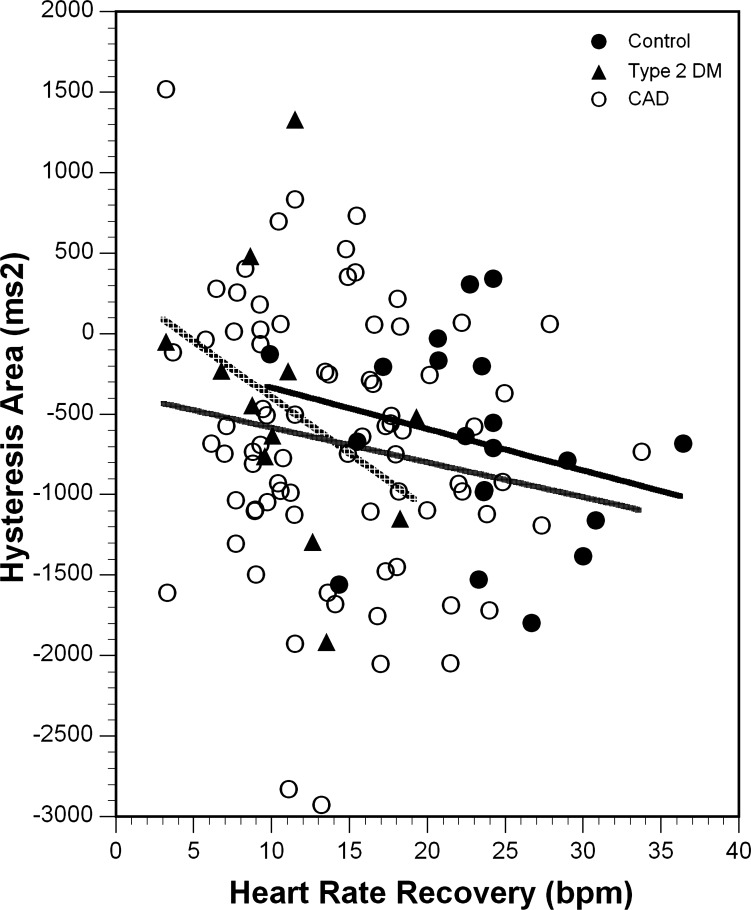
HRR was compared with hysteresis area in the absence of parasympathetic blockade. There was no significant relationship between HRR and hysteresis area, with R2 values of 0.07, 0.14, and 0.03 for the control, DM, and CAD groups, respectively (Fig. 4).
Fig. 4.
Relationship between hysteresis area and heart rate recovery. Each group is represented by a different symbol, and the associated linear regression analysis is plotted. The black trendline represents the control group, the gray trendline represents the CAD group, and the dashed trendline represents the DM group. There was no significant relationship for any of the groups (control R2 = 0.002, P = 0.84; type 2 DM R2 = 0.138, P = 0.23; CAD R2 = 0.029, P = 0.13). bpm, beats/min.
DISCUSSION
QT-RR hysteresis is the phenomenon characterized by longer QT intervals at a given RR interval while heart rates are increasing during exercise and shorter QT intervals at the same RR interval when heart rates are decreasing during recovery. This study demonstrates that parasympathetic blockade reverses the expected QT-RR hysteresis that would be due to directional changes in the RR interval, suggesting that differential parasympathetic inputs during exercise and recovery are responsible in large part for this phenomenon. Furthermore, there was no difference in this phenomenon among controls, subjects with CAD with and without depressed LVEF, or subjects with type 2 DM, suggesting that parasympathetic effects on the QT interval are preserved in these subjects.
Hysteresis is a phenomenon in which the value of a physical property (i.e., the QT interval) lags behind changes in the property that affects it (the RR interval). Specifically in relation to the QT interval, the observation of “hysteresis” has been thought to represent a memory effect in the QT interval duration, such that the QT interval duration is affected by the direction of heart rate change (6, 11, 18, 25, 28, 35, 37), that is, at a given RR interval, the QT will be longer if the preceding RR intervals were longer and shorter if the preceding RR intervals were shorter. In addition, the rate of change in the RR interval may also affect the QT interval, with longer time periods required for the QT to adjust to rapid changes in rate. The QT-RR hysteresis observed in the setting of exercise has been thought to be due to differing directions of heart rate change during exercise (increasing HR) and recovery (decreasing HR). However, our study demonstrates that the predominant factor in determining QT-RR hysteresis is differential autonomic effects in exercise and recovery, not heart rate memory. During recovery with parasympathetic blockade, heart rates were decreasing as is typical of recovery in the physiological state (44). Nevertheless, the QT intervals during recovery with parasympathetic blockade were longer than the corresponding QT intervals during exercise, suggesting that the differing QT intervals are not primarily due to the memory effect of heart rate change but were due to the differing autonomic states in exercise and recovery. Furthermore, although the rate of change in the RR interval after atropine was less than the rate of change in the RR interval in the absence of atropine, this might affect the magnitude of the hysteresis effect, but would not completely reverse it as noted in this study. Therefore, there is no classical “QT-RR hysteresis.” It appears that the direction and rate of heart rate change do not have the predominant influence on the QT interval duration in the setting of exercise and recovery, but instead QT-RR hysteresis is an epiphenomenon caused by the differing autonomic states.
The QT interval is a complex physiological property that is influenced by a number of factors. These factors include the heart rate at which the QT interval is measured and the direction and rate of heart rate change. The interplay between the sympathetic and parasympathetic effects on the ventricular myocardium is another important factor that determines the duration of the ventricular action potential and therefore the QT interval. Sympathetic activation occurs during exercise and persists long into recovery after only moderate exercise (44). Our study demonstrates that blockade of the parasympathetic nervous system effects on the QT interval during recovery reverses the direction of the QT-RR hysteresis relationship.
Many studies have assessed the effect of changes in rate on repolarization. When the paced cycle length is altered, the QT interval or action potential duration does not adapt immediately but instead adapts over time, with the amount of time required to reach equilibrium dependent on the magnitude of change in cycle length, autonomic state, and intrinsic factors that differ between subjects (2, 11, 25, 37, 38). In one study, fewer than 10 beats was found to be sufficient to achieve a new stable action potential duration after abrupt rate change (11). Bazett's formula for correcting the QT interval for heart rate has been disputed in the setting of changing heart rates, since the QT interval adapts over time, not instantaneously (24). The influence of changing RR intervals can be appreciated in the setting of atrial fibrillation. Ehlert et al. (8) used three QT prediction formulas based on multiple prior RR intervals in patients with atrial fibrillation and demonstrated that a weighted average of five RR intervals was predictive of sinus rhythm QT duration, suggesting that this rate adaptation occurs rapidly. QT-RR dynamics vary among individuals, with reported lag adaptation times of 3–215 s when pacing rates are abruptly changed (25, 31). It is important to consider that the changes in RR interval during exercise and recovery are gradual and not as abrupt as in these studies.
While difficult to study independently given the strong dependence of the QT interval on the RR interval, it has been shown that the QT interval duration and ventricular refractory periods are strongly influenced by parasympathetic and sympathetic autonomic nervous system inputs (21, 29). In the resting state, several studies have attempted to determine the effects of the autonomic nervous system on QT duration by pacing at specific cycle lengths and modulating the autonomic nervous system inputs through selective autonomic blockade (1, 3, 4). These studies suggest that, at rest, a time of minimal sympathetic activation, there is little contribution of the sympathetic nervous system on the intrinsic QT duration. Several studies have evaluated the effect of β-adrenergic blockade in the setting of elevated heart rate achieved with atrial pacing. These studies did not show an influence of β-adrenergic blockade on QT interval duration (1, 4). However, elevation of heart rate with atrial pacing does not replicate the complex autonomic milieu of exercise, precluding any conclusions regarding the effects of the sympathetic nervous system on the intrinsic QT duration.
Evaluating the effects of the sympathetic nervous system on the intrinsic QT duration during exercise is complicated by the simultaneous changes in autonomic tone and heart rate, both of which may affect the QT interval. During exercise, there is early withdrawal of the parasympathetic inputs followed by activation of the sympathetic nervous system (9, 10, 17, 33, 34). During recovery, there is early parasympathetic reactivation and concurrent withdrawal of sympathoexcitation (14, 36, 42). Multiple studies (7, 17–19, 32, 34, 42) have evaluated the effects of exercise on the QT interval, demonstrating that the QT interval shortens with exercise. The use of rate correction formulas to isolate the effects of sympathoexcitation (independent of rate) yields inconsistent results (32, 34). In contrast, evaluation of the QT-RR relationship with selective autonomic blockade has demonstrated that, in the immediate postexercise recovery period, sympathetic effects lengthen the QT interval and parasympathetic effects counteract the sympathetically mediated increase in QT interval (34, 42). Specifically, during recovery, activation of the parasympathetic nervous system serves to shorten the QT interval (42).
Given the differential autonomic states at the end of exercise and beginning of recovery, it would be expected that, for any given RR interval near end-exercise, the QT interval would be longer during exercise (heightened sympathetic tone and minimal parasympathetic tone) than during recovery (continued sympathoexcitation but with enhanced parasympathetic tone). As such, the autonomic milieu is predominantly responsible for the hysteresis effect. Our demonstration of reversal of the QT-RR hysteresis relationship with parasympathetic blockade provides strong support to the body of evidence that suggests that parasympathetic inputs on repolarization shorten the QT duration, particularly in the setting of sympathoexcitation.
HRR is a parameter that has been shown to be highly reflective of early parasympathetic reactivation after exercise (13, 14, 19, 42). The lack of a correlation between HRR and QT-RR hysteresis area suggests that parasympathetic and sympathetic effects on the sinus node differ from their effects on ventricular repolarization. In this study, CAD and DM subjects, despite having diminished HRR after exercise, did not have decreased QT-RR hysteresis, suggesting that, although evidence of parasympathetic dysfunction was present, there was sufficient parasympathetic effect on the ventricular myocardium to shorten the QT interval.
Multiple studies have demonstrated that patients with CAD, particularly those with depressed LVEF (20, 43), and patients with DM (27, 39, 41) have low heart rate variability. In addition, these patients have diminished HRR after exercise, another sign of diminished parasympathetic effect (5, 12, 15, 30). Although our CAD and DM subjects manifested low HRR, it is notable that the parasympathetic effects on the QT-RR hysteresis were preserved.
Limitations.
Limitations of the study include the fact that, as atropine accelerates the heart rate during recovery, the RR interval range achieved during recovery with parasympathetic blockade does not completely overlap the range noted during exercise. Thus, the linear regressions after parasympathetic blockade are extrapolated to the required RR range noted during exercise. However, the duration of this extrapolation was not large (average overlap of curves was 119.3 ± 74.0 ms, with QT-RR hysteresis area computed over 100 ms). Additionally, the exercise protocol did not target maximal exertion and achieved only modest elevations in heart rate. Because the duration and intensity of exercise likely affect the autonomic conditions of late exercise and early recovery, it is likely that this may be another factor that affects the QT-RR hysteresis. Differences in exercise intensity (maximum achieved heart rate) may also affect the values obtained among the different study groups. Nevertheless, the qualitative reversal of the classic QT-RR hysteresis by parasympathetic blockade in this experimental protocol confirms that differential autonomic effects in exercise and recovery are present even with submaximal exercise and account for the hysteresis effect.
In addition, as noted above, the selection criteria for this study may have identified a “healthier” group of subjects with DM and CAD. It is notable that these subjects who were well-treated medically and accustomed to exercise did not manifest large abnormalities in QT-RR hysteresis compared with control subjects; milder abnormalities cannot be ruled out. Finally, although atropine was used to provide parasympathetic blockade, we cannot rule out other effects of atropine that may have led to these findings.
In conclusion, this study demonstrates that the hysteresis effect is driven in large part by differential parasympathetic inputs during exercise and recovery. It appears that in selected subjects with CAD, with and without impaired left ventricular function, and subjects with type 2 DM, there is adequate parasympathetic effect to create a significant QT-RR hysteresis effect.
GRANTS
This work was supported by Grant 1RO1HL-70179-01A2 from the National Heart, Lung, and Blood Institute.
DISCLOSURES
No conflict of interest is declared.
AUTHOR CONTRIBUTIONS
Author contributions: D.J.P., A.B.C., D.W.B., and J.J.G. performed experiments; D.J.P., J.N., A.B.C., D.W.B., and J.J.G. analyzed data; D.J.P., J.N., A.B.C., D.W.B., and J.J.G. interpreted results of experiments; D.J.P., J.N., and J.J.G. prepared figures; D.J.P., J.N., and J.J.G. drafted manuscript; D.J.P., J.N., and J.J.G. edited and revised manuscript; D.J.P., J.N., A.B.C., D.W.B., and J.J.G. approved final version of manuscript; A.B.C. and J.J.G. conception and design of research.
ACKNOWLEDGMENTS
This research was presented, in part, at the Young Investigator Award Competition at the 61st Annual Scientific Sessions of the American College of Cardiology.
REFERENCES
- 1.Ahnve S, Vallin H. Influence of heart rate and inhibition of autonomic tone on the QT interval. Circulation 65: 435–439, 1982 [DOI] [PubMed] [Google Scholar]
- 2.Attwell D, Cohen I, Eisner DA. The effects of heart rate on the action potential of guinea-pig and human ventricular muscle. J Physiol 313: 439–461, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Browne KF, Zipes DP, Heger JJ, Prystowsky EN. Influence of the autonomic nervous system on the Q-T interval in man. Am J Cardiol 50: 1099–1103, 1982 [DOI] [PubMed] [Google Scholar]
- 4.Cappato R, Alboni P, Pedroni P, Gilli G, Antonioli GE. Sympathetic and vagal influences on rate-dependent changes of QT interval in healthy subjects. Am J Cardiol 68: 1188–1193, 1991 [DOI] [PubMed] [Google Scholar]
- 5.Carnethon MR, Jacobs DR, Jr, Sidney S, Liu K. Influence of autonomic nervous system dysfunction on the development of type 2 diabetes: the CARDIA study. Diabetes Care 26: 3035–3041, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Chauhan VS, Krahn AD, Walker BD, Klein GJ, Skanes AC, Yee R. Sex differences in QTc interval and QT dispersion: dynamics during exercise and recovery in healthy subjects. Am Heart J 144: 858–864, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Chicos AB, Kannankeril PJ, Kadish AH, Goldberger JJ. Parasympathetic effects on cardiac electrophysiology during exercise and recovery in patients with left ventricular dysfunction. Am J Physiol Heart Circ Physiol 297: H743–H749, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehlert FA, Goldberger JJ, Rosenthal JE, Kadish AH. Relation between QT and RR intervals during exercise testing in atrial fibrillation. Am J Cardiol 70: 332–338, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Ekblom B, Goldbarg AN, Kilbom A, Astrand PO. Effects of atropine and propranolol on the oxygen transport system during exercise in man. Scand J Clin Lab Invest 30: 35–42, 1972 [DOI] [PubMed] [Google Scholar]
- 10.Fagraeus L, Linnarsson D. Autonomic origin of heart rate fluctuations at the onset of muscular exercise. J Appl Physiol 40: 679–682, 1976 [DOI] [PubMed] [Google Scholar]
- 11.Franz MR, Swerdlow CD, Liem LB, Schaefer J. Cycle length dependence of human action potential duration in vivo. Effects of single extrastimuli, sudden sustained rate acceleration and deceleration, and different steady-state frequencies. J Clin Invest 82: 972–979, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georgoulias P, Orfanakis A, Demakopoulos N, Xaplanteris P, Mortzos G, Vardas P, Karkavitsas N. Abnormal heart rate recovery immediately after treadmill testing: correlation with clinical, exercise testing, and myocardial perfusion parameters. J Nucl Cardiol 10: 498–505, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Goldberger JJ, Le FK, Lahiri M, Kannankeril PJ, Ng J, Kadish AH. Assessment of parasympathetic reactivation after exercise. Am J Physiol Heart Circ Physiol 290: H2446–H2452, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Imai K, Sato H, Hori M, Kusuoka H, Ozaki H, Yokoyama H, Takeda H, Inoue M, Kamada T. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol 24: 1529–1535, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Jae SY, Carnethon MR, Heffernan KS, Choi YH, Lee MK, Park WH, Fernhall B. Slow heart rate recovery after exercise is associated with carotid atherosclerosis. Atherosclerosis 196: 256–261, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Jose AD. Effect of combined sympathetic and parasympathetic blockade on heart rate and cardiac function in man. Am J Cardiol 18: 476–478, 1966 [DOI] [PubMed] [Google Scholar]
- 17.Kannankeril PJ, Goldberger JJ. Parasympathetic effects on cardiac electrophysiology during exercise and recovery. Am J Physiol Heart Circ Physiol 282: H2091–H2098, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Kannankeril PJ, Harris PA, Norris KJ, Warsy I, Smith PD, Roden DM. Rate-independent QT shortening during exercise in healthy subjects: terminal repolarization does not shorten with exercise. J Cardiovasc Electrophysiol 19: 1284–1288, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kannankeril PJ, Le FK, Kadish AH, Goldberger JJ. Parasympathetic effects on heart rate recovery after exercise. J Investig Med 52: 394–401, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Kleiger RE, Miller JP, Bigger JT, Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 59: 256–262, 1987 [DOI] [PubMed] [Google Scholar]
- 21.Kolman BS, Verrier RL, Lown B. Effect of vagus nerve stimulation upon excitability of the canine ventricle. Role of sympathetic-parasympathetic interactions. Am J Cardiol 37: 1041–1045, 1976 [DOI] [PubMed] [Google Scholar]
- 22.Krahn AD, Klein GJ, Yee R. Hysteresis of the RT interval with exercise: a new marker for the long-QT syndrome? Circulation 96: 1551–1556, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Krahn AD, Yee R, Chauhan V, Skanes AC, Wang J, Hegele RA, Klein GJ. Beta blockers normalize QT hysteresis in long QT syndrome. Am Heart J 143: 528–534, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Lang CC, Flapan AD, Neilson JM. The impact of QT lag compensation on dynamic assessment of ventricular repolarization: reproducibility and the impact of lead selection. Pacing Clin Electrophysiol 24: 366–373, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Lau CP, Freedman AR, Fleming S, Malik M, Camm AJ, Ward DE. Hysteresis of the ventricular paced QT interval in response to abrupt changes in pacing rate. Cardiovasc Res 22: 67–72, 1988 [DOI] [PubMed] [Google Scholar]
- 26.Lauer MS, Pothier CE, Chernyak YB, Brunken R, Lieber M, Apperson-Hansen C, Starobin JM. Exercise-induced QT/R-R-interval hysteresis as a predictor of myocardial ischemia. J Electrocardiol 39: 315–323, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Liao D, Cai J, Brancati FL, Folsom A, Barnes RW, Tyroler HA, Heiss G. Association of vagal tone with serum insulin, glucose, and diabetes mellitus–The ARIC Study. Diabetes Res Clin Pract 30: 211–221, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Malik M, Hnatkova K, Novotny T, Schmidt G. Subject-specific profiles of QT/RR hysteresis. Am J Physiol Heart Circ Physiol 295: H2356–H2363, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Morady F, Kou WH, Nelson SD, de Buitleir M, Schmaltz S, Kadish AH, Toivonen LK, Kushner JA. Accentuated antagonism between beta-adrenergic and vagal effects on ventricular refractoriness in humans. Circulation 77: 289–297, 1988 [DOI] [PubMed] [Google Scholar]
- 30.Panzer C, Lauer MS, Brieke A, Blackstone E, Hoogwerf B. Association of fasting plasma glucose with heart rate recovery in healthy adults: a population-based study. Diabetes 51: 803–807, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Pueyo E, Smetana P, Laguna P, Malik M. Estimation of the QT/RR hysteresis lag. J Electrocardiol Suppl 36: 187–190, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Rajappan K, O'Connell C, Sheridan DJ. Changes in QT interval with exercise in elite male rowers and controls. Int J Cardiol 87: 217–222, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Robinson BF, Epstein SE, Beiser GD, Braunwald E. Control of heart rate by the autonomic nervous system. Studies in man on the interrelation between baroreceptor mechanisms and exercise. Circ Res 19: 400–411, 1966 [DOI] [PubMed] [Google Scholar]
- 34.Sarma JS, Venkataraman K, Samant DR, Gadgil UG. Effect of propranolol on the QT intervals of normal individuals during exercise: a new method for studying interventions. Br Heart J 60: 434–439, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarma JS, Venkataraman SK, Samant DR, Gadgil U. Hysteresis in the human RR-QT relationship during exercise and recovery. Pacing Clin Electrophysiol 10: 485–491, 1987 [DOI] [PubMed] [Google Scholar]
- 36.Savin WM, Davidson DM, Haskell WL. Autonomic contribution to heart rate recovery from exercise in humans. J Appl Physiol 53: 1572–1575, 1982 [DOI] [PubMed] [Google Scholar]
- 37.Seed WA, Noble MI, Oldershaw P, Wanless RB, Drake-Holland AJ, Redwood D, Pugh S, Mills C. Relation of human cardiac action potential duration to the interval between beats: implications for the validity of rate corrected QT interval (QTc). Br Heart J 57: 32–37, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seethala S, Shusterman V, Saba S, Mularski S, Nemec J. Effect of beta-adrenergic stimulation on QT interval accommodation. Heart Rhythm 8: 263–270, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Singh JP, Larson MG, O'Donnell CJ, Wilson PF, Tsuji H, Lloyd-Jones DM, Levy D. Association of hyperglycemia with reduced heart rate variability (The Framingham Heart Study). Am J Cardiol 86: 309–312, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Starobin JM, Cascio WE, Goldfarb AH, Varadarajan V, Starobin AJ, Danford CP, Johnson TA. Identifying coronary artery flow reduction and ischemia using quasistationary QT/RR-interval hysteresis measurements. J Electrocardiol 40: S91–S96, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Stein PK, Barzilay JI, Domitrovich PP, Chaves PM, Gottdiener JS, Heckbert SR, Kronmal RA. The relationship of heart rate and heart rate variability to non-diabetic fasting glucose levels and the metabolic syndrome: the Cardiovascular Health Study. Diabet Med 24: 855–863, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Sundaram S, Carnethon M, Polito K, Kadish AH, Goldberger JJ. Autonomic effects on QT-RR interval dynamics after exercise. Am J Physiol Heart Circ Physiol 294: H490–H497, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Van Hoogenhuyze D, Weinstein N, Martin GJ, Weiss JS, Schaad JW, Sahyouni XN, Fintel D, Remme WJ, Singer DH. Reproducibility and relation to mean heart rate of heart rate variability in normal subjects and in patients with congestive heart failure secondary to coronary artery disease. Am J Cardiol 68: 1668–1676, 1991 [DOI] [PubMed] [Google Scholar]
- 44.Wang NC, Chicos A, Banthia S, Bergner DW, Lahiri MK, Ng J, Subacius H, Kadish AH, Goldberger JJ. Persistent sympathoexcitation long after submaximal exercise in subjects with and without coronary artery disease. Am J Physiol Heart Circ Physiol 301: H912–H920, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou SH, Helfenbein ED, Lindauer JM, Gregg RE, Feild DQ. Philips QT interval measurement algorithms for diagnostic, ambulatory, and patient monitoring ECG applications. Ann Noninvasive Electrocardiol, 14 Suppl 1: S3–S8, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]