Abstract
Defining the cellular electrophysiological mechanisms for ventricular tachyarrhythmias is difficult, given the wide array of potential mechanisms, ranging from abnormal automaticity to various types of reentry and kk activity. The degree of difficulty is increased further by the fact that any particular mechanism may be influenced by the evolving ionic and anatomic environments associated with many forms of heart disease. Consequently, static measures of a single electrophysiological characteristic are unlikely to be useful in establishing mechanisms. Rather, the dynamics of the electrophysiological triggers and substrates that predispose to arrhythmia development need to be considered. Moreover, the dynamics need to be considered in the context of a system, one that displays certain predictable behaviors, but also one that may contain seemingly stochastic elements. It also is essential to recognize that even the predictable behaviors of this complex nonlinear system are subject to small changes in the state of the system at any given time. Here we briefly review some of the short-, medium-, and long-term alterations of the electrophysiological substrate that accompany myocardial disease and their potential impact on the initiation and maintenance of ventricular arrhythmias. We also provide examples of cases in which small changes in the electrophysiological substrate can result in rather large differences in arrhythmia outcome. These results suggest that an interrogation of cardiac electrical dynamics is required to provide a meaningful assessment of the immediate risk for arrhythmia development and for evaluating the effects of putative antiarrhythmic interventions.
Keywords: alternans, dynamics, spiral waves, ventricle
this review article is part of a collection on Electrophysiology and Excitation-Contraction Coupling. Other articles appearing in this collection, as well as a full archive of all Review collections, can be found online at http://ajpheart.physiology.org/.
Introduction
Sudden cardiac death secondary to the development of ventricular tachyarrhythmias, in particular ventricular fibrillation (VF), has for decades been a leading cause of death in the United States and other developed nations (1). Despite intensive research by multiple groups of investigators, the mechanisms for the induction of VF and for its maintenance have not been clearly defined, with the result that currently there are no reliable means of preventing VF in patients with the most prevalent forms of heart disease. Moreover, the only clinically useful method of terminating VF is the delivery of a large electrical shock.
Information accumulated to date indicates that multiple mechanisms, ranging from abnormal automaticity, triggered activity, and various forms of reentry, are capable of provoking ventricular arrhythmias (6, 43, 68, 105, 128, 148, 158, 201, 209). Several of these mechanisms can be attributed to specific abnormalities of cellular electrophysiology, such as mutations in a single ion channel, transporter, or pump (14, 172). However, the vast majority of ventricular rhythm disturbances appear to be multifactorial in origin and associated with various combinations of derangements in ion channel distribution and function, intracellular ion dynamics, cardiac innervation, and metabolic and signaling pathways, as well as in gross and microscopic anatomical features [for reviews, see (3, 12, 97, 108, 110)].
It has also become apparent that arrhythmia development is not solely a function of a static set of electrophysiological properties but also depends on changes that occur over multiple time scales ranging from seconds to months. Beat-to-beat changes in electrophysiological properties occur over seconds during irregular or rapid cardiac activation and appear to be important determinants of the propensity to develop and sustain arrhythmias, particularly as they relate to the development of dynamical heterogeneity of refractoriness (23, 83, 84, 186). On a longer time scale of minutes to hours, key electrical properties such as action potential duration (APD) are importantly influenced by an accumulation and dissipation of short-term cardiac memory, as occurs during prolonged pacing (30, 71, 79, 106). At a still longer time scale of days to months, the heart can remodel, both structurally and functionally, in response to various stressors (3, 146, 196). Along with the presence of multiple time scales, the nonlinearity of cardiac tissue (67, 86, 117) can result in complex dynamics from such properties as multistability (53, 68, 103, 150, 188, 199) and dependence on initial conditions (34, 42, 68).
It seems possible that the combination of short, intermediate, and long time scales and nonlinear properties governs the evolution of cardiac dynamics and may account for some of the features of arrhythmia development that up to now have defied explanation. Accordingly, this review focuses on the importance of certain aspects of these temporal and nonlinear dynamics in the initiation and perpetuation of ventricular tachyarrhythmias. With regard to the temporal dynamics, we segregate the discussion according to those that occur over short (beat-to-beat to seconds), intermediate (minutes to hours), and long (days to months) time scales, recognizing that these classifications, although useful for organizing the discussion, are somewhat arbitrary and that some of the dynamics may evolve over a continuum of time. In addition to reviewing the relevant literature, we provide preliminary evidence for a somewhat different perspective on arrhythmia development and propose corresponding future directions for research.
Triggers and substrates.
Mechanisms for the development of ventricular arrhythmias traditionally have been considered in the context of triggers and substrates (107), where particular characteristics of each are required to produce a permissive environment for the initiation and maintenance of sustained arrhythmias. In that regard, the normal heart can be easily fibrillated by the application of high-frequency stimuli (36) or even by contact with a 9-V battery (56). However, normal hearts are subjected to few, if any, arrhythmogenic triggers [outside of unusual events such as inadvertent electrical shock or commotio cordis (8)]. Consequently, symptomatic ventricular arrhythmias are rare in individuals with normal cardiac function. On the other hand, sequences of potential triggers (e.g., premature ventricular complexes) that ordinarily would be benign in an otherwise normal heart may precipitate more sustained rhythm disturbances in infarcted or myopathic hearts. Thus, understanding the relationship between trigger and substrate at any given moment is essential for predicting when (or if) an arrhythmia is imminent.
Nonlinear dynamics and the heart.
One of the signature features of nonlinear systems is that they exhibit sensitivity to initial conditions, where small changes in the initial state of the system can lead to very different outcomes over time (98, 198). Rather extreme examples of this phenomenon are common in the physics and mathematical literature (e.g., the “butterfly effect”) and may contribute to the apparently chaotic fluctuations in the behaviors of various biological systems (87). However, fluctuations in heart rate and blood pressure, for example, typically are not abrupt. From a functional standpoint, the lack of sharp transitions in cardiac rhythm makes sense, in that such changes would be counterproductive, given that the heart requires a balance between electrical and mechanical systole and diastole to optimize the relationship between filling and ejection and to maintain optimal excitation-contraction coupling. Nevertheless, certain functions of the heart, notably restitution of APD, are known to exhibit a sensitive dependence on initial conditions. (34, 42, 68, 103, 150, 188). Perhaps these properties and other nonlinear effects, such as multistability, where a system may oscillate between two quasistable states, underlie the seemingly unpredictable emergence of some ventricular tachyarrhythmias. Although this idea is intuitively attractive, it is, at least for the time being, insufficiently validated. That said, we will review some of the existing evidence for it in the following sections.
Role of Cardiac Dynamics in the Generation of Ventricular Arrhythmias
Evolution of dynamics over short times scales.
Although several hypotheses regarding cellular electrophysiological mechanisms for VF have been proposed [for reviews, see (68, 105)], substantial evidence has accumulated over the past few years to suggest that fibrillation is a state of spatiotemporal chaos consisting of the perpetual nucleation and disintegration of spiral waves (38, 90, 224), often in association with a period-doubling bifurcation of local electrical properties (115, 158, 219). Nucleation of the initiating spiral wave pair is caused by local conduction block (wave break) secondary to spatial heterogeneity of refractoriness in the ventricle (105, 127, 158, 222). Until recently, spatial heterogeneity was thought to result solely from regional variations of intrinsic cellular electrical properties [e.g., (192, 230)]. However, it is now appreciated that purely dynamical heterogeneity can be sufficient to cause conduction block (68, 115, 177, 217, 219).
For the case of pacing at a fixed cycle length, the period-doubling bifurcation implicated in the transition to conduction block is manifest as alternans, a beat-to-beat long-short alternation in the duration of the cardiac action potential (147). Previous investigators have hypothesized that alternans can be accounted for by a simple unidimensional return map called the APD restitution function (44, 58, 60, 91, 125, 147). The combination of a steeply sloped APD restitution function and a monotonically increasing conduction velocity (CV) restitution function has been shown to be sufficient to produce dynamical conduction block during sustained pacing at a short cycle length (78, 174, 217). This combination of restitution functions promotes the development of so-called “discordant alternans,” in which the alternations in APD are out of phase with one another at different locations in the tissue (162). However, when the APD restitution relation is considered as a function of more than one variable (e.g., to account for memory), its steepness is neither necessary nor sufficient for the development of alternans under certain circumstances, as has been observed experimentally (19, 92) and verified numerically (41, 55, 68, 113, 206).
The cellular mechanism for APD alternans is complex, but Ca2+ cycling appears to be an important determinant in many cases (48, 76, 89, 195). Moreover, blockade of L-type Ca2+ current has been shown to reduce the slope of the APD restitution relation and convert VF into monomorphic ventricular tachycardia in vitro (181, 226) and to suppress the induction of VF in vivo (83).
Although constant pacing studies may provide a generic mechanism for wave break and the onset of ventricular tachycardia and fibrillation, it is unlikely that the conditions used to demonstrate this phenomenon experimentally apply to the clinical situation, where the induction of ventricular tachyarrhythmias is typically associated with the interruption of normal cardiac rhythm by several premature beats. In addition, APD dynamics during rapid sustained pacing at a constant cycle length rely on the steady-state APD restitution relation, where APD is solely a function of the preceding diastolic interval. More realistic descriptions of APD restitution require consideration of multiple rate-dependent effects, as characterized by restitution portraits (113) and by determination of restitution kinetics during random or cyclical pacing protocols (46, 225). Marked differences in APD dynamics derived from the steady-state restitution relation and the restitution relations determined after abrupt changes in pacing intervals raise questions as to whether studies conducted during constant rapid pacing are relevant to the development of clinical ventricular tachyarrhythmias.
To address this issue, studies were recently conducted to determine whether dynamic heterogeneity and conduction block occur in one-dimensional cardiac fibers in which pacing at a slow rate was interrupted by one to four premature stimuli at variable intervals (77). This protocol simulated the interruption of sinus rhythm by one to four premature ventricular complexes, a situation that can lead to the onset of VF clinically. Computer modeling studies indicated that a short-long-short-short coupling interval pattern of premature stimuli induced marked spatial dispersion of repolarization and conduction block at some distance from the stimulus site, as predicted by interactions between APD and CV restitution (58, 77, 152, 217). Thus the dynamical mechanism for the development of block was the same as for the development of discordant alternans during sustained rapid pacing. Other premature stimulus interval patterns, such as short-long-long-short, also created conduction block, with each family of such patterns depending on both the steepness and the shape of the APD and CV restitution functions (77, 152). The predictions generated by the computer models subsequently were validated in vivo in normal dogs (83) and in dogs with an inherited predisposition to the development of polymorphic ventricular tachycardia and fibrillation (84).
These results are consistent with other studies that have examined related aspects of this problem. For example, Watanabe et al. (217) demonstrated that multiple beats at one site and a single premature beat at a different location from the pacing site are both sufficient to cause spatial heterogeneity in the form of discordant alternans, and Qu et al. (175, 176) studied how preexisting gradients in refractoriness can interact with one or more premature stimuli to produce conduction block. Moreover, Comtois et al. (52) have demonstrated that two properly timed stimuli following the passage of a propagating wave can produce unidirectional block and spiral wave reentry with a large window of vulnerability. Other studies have demonstrated that the development of conduction block does not necessarily require steep APD restitution, in that interactions between regions of ventricular myocardium having different APD restitution relations may be sufficient, as has been shown both numerically (16, 25, 50) and experimentally (69).
Taken together, these studies provide a rationale for the development of conduction block and reentry following certain patterns of premature stimuli (but not others) and indicate that heterogeneity of refractoriness is a function of not only preexisting or intrinsic heterogeneity but dynamical heterogeneity as well and that the latter can fluctuate on a beat-to-beat basis.
Although the destabilizing effects of an APD restitution curve that decreases monotonically with the pacing period have received the most attention, other types of APD restitution curves can also be associated with chaotic behavior and spiral wave breakup. For example, biphasic APD restitution curves, which contain a range of pacing periods over which APD increases as the period is decreased (negative slope region), have been shown to yield complex dynamics for one-dimensional maps (216), spatiotemporal chaos for one-dimensional rings (178), and spiral wave instability (68, 161). It is not necessary in this case for the absolute value of the slope of the APD restitution curve to exceed one for spiral wave breakup to result; the altered morphology of the restitution curve can lead to complex dynamics and breakup even when the curve is not steeply sloped over any range of periods (68). Biphasic APD restitution curves have been observed experimentally in different cardiac tissue preparations (80, 100, 200), although it has been suggested (68) that biphasic curves may result only when restitution curves are measured using a particular protocol, namely, the S1-S2 protocol (80, 122).
Similarly, a CV restitution curve that is not monotonically decreasing with the pacing period also can be associated with complex dynamics. An increase in CV as the period is decreased is also known as supernormal conduction and is associated with supernormal excitability (33, 45, 62, 63). The existence of supernormal CV restitution curves has been questioned (74); however, nonmonotonic CV has been observed in cell cultures (129). Along with chaotic dynamics in one-dimensional maps (45), increasing CV with decreasing period has been shown in simulations to lead to the bunching of propagating waves, which in turn can produce conduction block and generation of reentry (68, 85).
Factors other than APD and CV restitution may also vary over short time scales and account for spiral wave breakup. For example, the trajectories followed by spiral waves can affect their behavior. Meandering or drift of a spiral wave can produce a Doppler effect, in which the movement of the spiral wave affects the period at which the tissue is stimulated in a spatially dependent manner. Such an effect has been observed in cardiac tissue (57, 64, 163) and has been shown numerically to be capable of producing spiral wave breakup (20, 21, 68). Spiral wave trajectories can also play a role in the breakup of spiral waves when structural features such as the periodic boundary conditions of the ventricles are included. If the reentry follows a hypermeandering trajectory whose length is comparable to the length of the tissue, periodic boundaries can lead to wavefront-waveback interactions that produce localized conduction block and nucleation of additional reentrant waves (70, 73).
Spiral wave breakup can also result over short time scales from a negative tension property of the medium associated the stability of three-dimensional scroll waves. Specifically, tension in this setting describes the propensity of the organizing center of a scroll wave, called a filament, to remain straight if perturbed from an initially straight state. Scroll waves initiated in tissue with normal filament tension will quickly become straight again if perturbed, whereas scroll waves in tissue with negative filament tension will amplify small perturbations, resulting in long, complicated filaments that are prone to breakup into multiple scroll waves (29, 68). Although healthy cardiac tissue is in the normal filament tension regime, some types of heart disease, including ischemia, are associated with electrophysiological modifications that modulate the filament tension to the negative regime, so that this mechanism of breakup may be associated with such pathophysiological conditions.
Anatomical structure also may interact with and destabilize reentrant waves. For example, the intrinsic transmural rotation of fibers from the epicardium to the endocardium (rotational anisotropy) (197, 205) has been shown numerically to produce an instability in the presence of meandering scroll waves that can give rise to complex dynamics and breakup of waves (65, 66, 73, 179). Although other destabilizing factors such as steep APD restitution may exacerbate the effect of fiber rotation (177), the instability caused by fiber rotation requires only a minimum tissue thickness and fiber rotation rate in combination with a sufficiently meandering tip trajectory (65, 66, 68, 73, 179). Even without considering such factors as fiber rotation, the complex geometry of realistic cardiac tissue has been shown in simulations to be capable of producing spiral and scroll wave breakup (40, 184).
Evolution of dynamics over intermediate times scales.
Initial conditions and the resulting dynamics may also change over intermediate time scales, such as following periods of sustained pacing, during which memory may accumulate (30, 71, 75, 79, 106, 151, 206, 218). Typically, accumulation of memory flattens APD restitution, an effect that results from augmented rate-dependent activation of the delayed rectifier (75, 101, 102) and by increased inward rectifier (221).
Although the flattening of APD restitution resulting from sustained pacing at rapid rates is expected to reduce the probability of conduction block and reentry initiation, rapid pacing also promotes the transition from concordant to discordant APD alternans (41, 78, 162, 217), which would be expected to facilitate the development of reentry, secondary to the development of marked dispersion of APD and refractoriness. Consequently, arrhythmia initiation under these circumstances requires a rather specific interplay between APD alternans and memory. It should be noted that steep APD restitution is neither necessary nor sufficient for the development of alternans; along with memory, electrotonic effects arising from intercellular coupling together with CV restitution can interact to either suppress alternans when the APD restitution curve is steep or to produce alternans when the APD restitution curve is relatively flat (41, 55, 58, 151, 206).
Changes in triggering mechanisms for ventricular arrhythmias also may occur secondary to the rate and duration of pacing, with delayed afterdepolarization (DAD)-induced triggered activity typically being promoted by sustained rapid pacing and early afterdepolarization (EAD)-induced triggered activity being facilitated by slow pacing (223). Given that induction of triggered activity is rare in normal myocardium, the arrhythmogenic effects of sustained pacing at fast- or slow-pacing rates also will depend on the type and severity of underlying myocardial disease, as discussed in more detail in the following section.
Evolution of dynamics over prolonged times scales.
Initial conditions may evolve over rather long time scales as the result of disease-induced electrophysiological and anatomical remodeling [for recent reviews, see (3, 97, 108, 110, 146)]. Several forms of myocardial disease have been shown to produce changes in ionic currents (density and type) and intracellular concentrations of various ions (Na+, K+, Ca2+), secondary to changes in ionic currents, as well as alterations in exchangers, pumps and the Ca2+-sequestering capacities of the sarcoplasmic reticulum (SR), nucleus, and mitochondria. Although the specific changes in individual ionic currents vary in different experimental models and clinical manifestations of heart failure, patterns of alterations are apparent, as discussed briefly in the following sections, which emphasize those changes that occur in nonischemic heart disease. Ultimately, alterations in ionic currents, in association with abnormalities of cell coupling and intracellular Ca2+ dynamics, conspire to create a permissive substrate for arrhythmia development, as well as an increased incidence of arrhythmogenic triggers.
RESTING MEMBRANE POTENTIAL: INWARD RECTIFIER K+ CURRENT.
Inward rectifier K+ current (IK1) is significantly reduced at negative voltages in terminal human heart failure (28) and in rapid pacing-induced heart failure in the dog (112, 133), but not in the rabbit (187, 207). Reduction of IK1 in the canine model is not accompanied by changes in the steady-state level of Kir2.1 mRNA, suggesting that the reduced current results from post-translational alterations of the channel protein (185). Kir2.1 and 2.2 knockout (234) and Kir2.1 dominant negative overexpressing mice (143) exhibited evidence of reduced IK1 in the form of prolonged APDs, but as in most other models, there were no significant changes in resting membrane potential.
ACTION POTENTIAL UPSTROKE: NA+ CURRENT.
It has been well established that Na+ current (INa) is reduced in the setting of a subacute canine myocardial infarction (173). Myocytes isolated from the infarct border zone exhibit reduced INa current density, accelerated inactivation, and slowed recovery from inactivation. These effects are manifest as reduced upstroke velocity and slow conduction, effects that resolve as the infarct heals (210). It might be expected that reduction of INa would contribute to slow conduction in other forms of myocardial disease [e.g., (6, 130)], but the direct recordings of INa available to date indicate that INa is unchanged in human heart failure (112, 189).
EARLY REPOLARIZATION: TRANSIENT OUTWARD K+ CURRENT.
Reduction of the transient outward K+ current (Ito) occurs in a wide variety of heart disease, ranging from Chagas' disease to chronic heart failure (28, 81, 111, 112, 144, 154, 155, 208). Although a reduction in current density occurs under all of these conditions, changes in current kinetics vary from no change (28) to rather profound changes (154, 155).
In human heart failure, reduced Ito current is associated with reduced steady-state levels of Kv4 mRNA (7, 111, 144, 235). Studies in experimental models indicate that the downregulation of Ito expression in heart failure may be mediated by the Ca2+/calmodulin-dependent protein kinase II (CaMKII) and calcineurin/nuclear factor of activated T-cells signaling cascades (157, 228).
ACTION POTENTIAL PLATEAU: CA2+ CURRENT AND LATE NA+ CURRENT.
Changes in L-type Ca2+ current (ICa-L) density in diseased ventricles are variable, depending on the type of disease and its severity. In the early stages of heart failure marked by hypertrophy, ICa-L may be increased (31, 37), whereas in the failing heart it may be unchanged (27, 112) or decreased (97, 166, 182). Studies of ICa-L density in human heart failure suggest that current density is held near normal levels by increased phosphorylation, despite the fact that the response to β-adrenergic receptor-mediated phosphorylation is attenuated (39, 153). In that regard, single channel studies in failing myocytes suggest that phosphorylation may offset a reduction in channel number by increasing open channel probability (193).
The molecular bases for changes in ICa-L density in failing ventricles are incompletely understood, as reflected by reports of variable subunit mRNA expression (202) and isoform switching of both α1C- (231) and β-subunits (104). A role for the latter is suggested by studies in which knockdown of the β-subunit reduced ICa-L density and the development of hypertrophy in an experimental model, with minimal negative inotropic effects (49).
With respect to late INa during the action potential plateau, several studies have indicated that this current is increased in human and canine heart failure (209, 211), possibly resulting from a slowing of inactivation kinetics and a shift of the voltage dependence of steady-state inactivation, as mediated by intracellular Ca2+/CaMKII signaling (140, 214).
TERMINAL REPOLARIZATION: DELAYED (IKR AND IKS) AND INWARD (IK1) RECTIFIER K+ CURRENTS.
Reduced IK density, slower activation, and faster deactivation kinetics have been observed in hypertrophied feline ventricles (82). Downregulation of both IKr and IKs have been reported in a rabbit model of rapid ventricular pacing-induced heart failure (207, 208), whereas IKs but not IKr was downregulated in all layers of the left ventricular myocardium in a canine model of tachycardia-induced heart failure (133). The molecular basis for IK downregulation in heart failure remains unclear. Studies of mRNA levels of the genes encoding the α-subunits for the rapidly human ether-a-go-go gene (HERG) and slowly voltage-gated K+ channel long Q-T mutant (KvLQT1) activating components of IK found no statistical difference between normal and failing canine hearts (185). Reduced IK1 density in heart failure also may contribute to a prolongation of APD (133, 148, 169, 185).
CA2+ TRANSIENTS.
The initiation and termination of the Ca2+ transient in cardiac myocytes is a complex interplay between Ca2+-induced Ca2+ release from the SR via the ryanodine receptor (RyR2), reuptake of Ca2+ into the SR by the sarco(endo)plasmic reticulum Ca2+-ATPase 2 (SERCA2a), and extrusion of Ca2+ via the Na+/Ca2+ exchanger (NCX). Each of these components of intracellular Ca2+ dynamics is altered during heart failure (3, 95, 108, 110, 142).
First and foremost, the amplitude of the Ca2+ transient and its rate of decay are reduced (149). The reduction in the amplitude reflects a reduction in the density of RyR2 (15, 180), resulting in less Ca2+ release from the SR, whereas the prolongation of the transient is caused, in part, by a reduction in the density and uptake rate of SERCA2a (149, 190, 191). NCX density and function are upregulated in heart failure (17, 26, 99), perhaps as a compensatory mechanism to counteract the reduction in SR Ca2+ uptake.
In addition to being downregulated during heart failure, RyR2 receptors are “leaky,” secondary to hyperphosphorylation of RyR by protein kinase A (141) or by CaMKII (2, 54, 123, 135, 139) or possibly by redox modification (204). Excessive SR Ca2+ may increase diastolic Ca2+ concentration, and generate spontaneous Ca2+ waves and delayed afterdepolarization-induced triggered activity.
Although altered Ca2+ dynamics are likely to contribute importantly to the development of ventricular tachyarrhythmias, particularly in the setting of heart failure, the exact nature of that contribution has yet to be established (94, 109, 132, 138, 227). As discussed briefly below, Ca2+ participates in the development of arrhythmogenic triggers (EAD and DAD), as well as permissive substrates for arrhythmia initiation and perpetuation. In particular, a complex interplay between voltage and Ca2+ alternans may lead to multiple arrhythmogenic scenarios, as reviewed recently by Weiss et al. (220).
CONNEXINS.
The density, distribution, and molecular properties of the predominant cardiac gap junction protein connexin 43 (Cx43) are altered in various forms of myocardial disease (6, 35, 167, 232). Cx43 typically is downregulated and is redistributed from the intercalated disk to the cell border, a process known as lateralization (4, 6, 114, 126, 165). In rapid pacing-induced heart failure, Cx43 downregulation is associated with an overall reduction in Cx43 mRNA, yet a hypophosphorylated component of the protein is actually more abundant than in control myocardium (4, 6). Other studies have supported the concept that changes in gap junction function and concomitant alterations of cell coupling and CV may not be solely functions of gap junction density but also may relate to Cx43 localization, colocalization with other gap junction proteins, trafficking, and phosphorylation status (22, 61, 96, 194, 233).
Alterations in intercellular coupling are known to contribute to the development and maintenance of reentrant waves in cardiac tissue (32, 57b, 93, 121). Although a reduction of coupling in experimental settings is unlikely to occur homogeneously and may be considered as promoting wave break by increasing tissue heterogeneity, uniform reduced coupling has been shown in numerical simulations to give rise to the breakup of reentrant waves, especially in the setting of rotational anisotropy (10, 68, 118–120, 159, 160).
ARRHYTHMOGENIC TRIGGERS AND SUBSTRATES.
The predominant electrophysiological changes that accompany heart failure-induced remodeling are slowing of conduction and prolongation of APD (3, 5, 146). Prolongation of APD typically is heterogeneous (5, 88), amplifying the intrinsic APD dispersion of the normal heart, including base-to-apex (51, 57c) and transmural gradients (13, 213), where the magnitude of the latter is still a matter of some debate.
The electrophysiological alterations associated with heart failure promote the development of arrhythmia triggers. Specifically, the combination of prolonged APD, abnormal Ca2+ cycling, and increased late Na+ current is conducive to the generation of EAD-induced triggered activity (133, 148, 209, 229). In addition, increased SR Ca2+ leak and increased NCX promotes DAD-induced triggered activity (169, 212).
Afterdepolarizations provide a potential triggering mechanism for arrhythmias both in the setting of bradycardia (EADs) and tachycardia (DADs), although the clinical manifestations of these phenomena have yet to be demonstrated conclusively. Triggered activity also may be the underlying mechanism for sustained tachycardias in the setting of ischemic cardiomyopathy and dilated or hypertrophic cardiomyopathy, where in many cases the arrhythmias appear to be generated by a focal, as opposed to reentrant, mechanism (11, 116, 168, 170, 171).
With respect to arrhythmogenic substrates, the combination of heterogeneously prolonged repolarization and slowed conduction in diseased hearts is expected to facilitate the initiation and maintenance of reentrant arrhythmias. The requirements for reentrant excitation—unidirectional conduction block, slow conduction, and reexcitation of the previously blocked region—are satisfied by dispersion of refractoriness secondary to heterogeneous APD prolongation and by slow conduction secondary to decreased INa and connexin lateralization and hyperphosphorylation, as discussed above. Dispersion of refractoriness may be exacerbated in some forms of myocardial disease secondary to alterations of APD restitution (124, 145, 156). Reentrant arrhythmias are most commonly observed in the setting of a healed myocardial infarction (35, 57a, 164), but evidence of reentry also has been observed in models of nonischemic heart failure (5, 6, 131).
A systems-based perspective on arrhythmia development.
As is evident from the foregoing, the changes in cellular electrophysiology that accompany myocardial disease are many and varied, impacting virtually every aspect of ventricular electrical activity (and an equally long list of alterations in mechanical activity could easily be generated). Consequently, it does not seem reasonable to expect a single root cause for ventricular arrhythmias, the identification and eradication of which would return the system to normal function. The cardiovascular system reacts to disease as a system, often in counterintuitive or even counterproductive ways, such as by remodeling the atria in response to paroxysmal atrial fibrillation to promote more sustained fibrillation. Understanding the adaptations of the cardiovascular system to disease and their consequences for arrhythmia development might best be accomplished using a systems-based approach.
By a systems-based approach, we mean an approach similar to that used in systems biology, where various known inputs are delivered to the system (a.k.a. a “black box”) and the resulting output characteristics of the system are measured. The properties of the system are then derived from the differences between the inputs and outputs. If the system is sufficiently well characterized using this method, its behavior can be understood without a detailed knowledge of its component parts. Moreover, if something (but not everything) is known about the system, control theory can be applied to predictably alter its behavior (47).
The effectiveness of a purely systems-based approach begins to break down, however, if one wants to develop specific therapies for arrhythmias, pharmacological therapy in particular. Whereas knowing that a given behavior of the system depends on the properties of the “slow variable” in a simple computer model can be very useful for defining the dynamics of the system, it is not very useful for defining therapy, in that there are no “slow variable”-specific drugs. It is necessary to know what ionic current (or, more likely, currents) underlies the slow variable and then design drugs to bind to the relevant ion channels with the appropriate affinities and kinetics. Given the apparently infinite number of potential interactions between the large number of ion channels, transporters, pumps, second-messenger systems, etc., that determine cardiac electrical activity and the equally large number of alterations of those determinants with various forms of heart disease, the chances of developing an effective antiarrhythmic therapy based on a reductionist approach seem small compared with what might be achieved using a systems-based approach. However, that conjecture remains to be validated.
Examples of different arrhythmia outcomes depending on initial conditions.
As discussed above, the characteristics of a given ventricular arrhythmia may importantly depend on the electrophysiological state of the ventricles at the time of arrhythmia initiation, where small changes in the initial state can lead to large differences in outcome. To illustrate that point, we present in this section examples of different arrhythmia outcomes following perturbations to the initial state of isolated perfused rabbit hearts and a mathematical model of the canine ventricle.
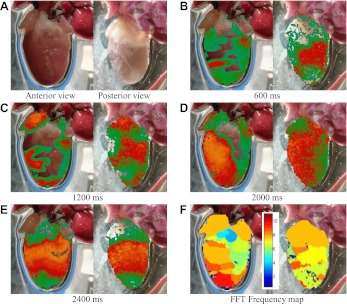
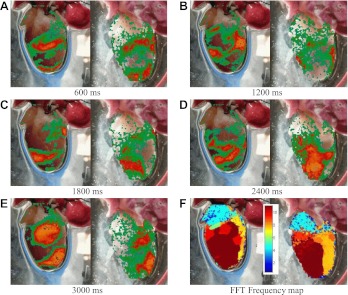
Figure 1 shows an example of nonsustained fibrillation initiated in rabbit ventricles using a rapid pacing protocol following a training period of pacing for 5 min at 500 ms. Although immediately after initiation multiple waves are present in the tissue, the propagation pattern in the ventricles soon becomes more regular, eventually leaving a single long-wavelength wave that cannot fit in the tissue and extinguishes itself. Despite the apparent difficulty of inducing sustained fibrillation in rabbit ventricles (19, 136), it is possible to initiate sustained fibrillation in rabbit ventricles by pacing at a faster rate before inducing an arrhythmia. Figure 2 shows an example of sustained fibrillation initiated in the same preparation as that shown in Fig. 1 using the same rapid pacing protocol, but in this case following a training period of pacing at 250 ms for 5 min. In this case, the difference in pacing history between the two episodes affected the initial state of the system so as to allow fibrillation to be sustained following more rapid pacing. For both instances of fibrillation, the dominant frequency is between 8 and 10 Hz and the frequencies appear more spatially uniform in the sustained case, although the average frequency is also somewhat higher in that scenario. Results were replicated in five different preparations.
Fig. 1.
Nonsustained ventricular fibrillation in rabbit ventricles initiated by rapid pacing at a period of 80 ms. A: anterior and posterior views. B–E: optical signal during nonsustained fibrillation; color represents voltage. Frames show anterior and posterior views at 4 different times (600, 1,200, 2,000, and 2,400 ms). F: spatial frequency domain showing a maximum frequency of about 10 Hz. Color bar indicates frequency in hertz. Hearts were Langendorff perfused with oxygenated Tyrode solution at 37°C with 10 μM blebbistatin.
Fig. 2.
Sustained ventricular fibrillation in rabbit ventricles initiated by rapid pacing at a period of 80 ms preceded by a conditioning period (15 min) of fast pacing at 250 ms in the same heart as shown in Fig. 1. A–E: optical signal during sustained fibrillation; color represents voltage. Frames show anterior and posterior views at 5 different times (600, 1,200, 1,800, 2,400, and 3,000 ms). F: spatial frequency domain showing a maximum frequency of about 10 Hz. When compared with the nonsustained VF episode shown in Fig. 1, a larger fraction of the tissue exhibits the maximum frequency. Color bar indicates frequency in hertz.
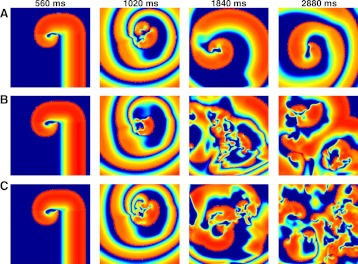
A comparable sensitivity to initial conditions has been observed in computer simulations as well. For the model of ten Tusscher et al. (203), it was previously observed that although a spiral wave remains stable when initiated in a domain conditioned by pacing at a relatively slow period of 1,000 ms, the same initiation protocol gives rise to sustained breakup when the tissue had been previously paced at a period of 300 ms (34). As shown in Fig. 3, the model of Fox et al. (76) exhibits similar behavior, with a stable spiral wave resulting in tissue paced for 30 s at a period of 500 ms and sustained breakup resulting after pacing for 30 s at periods of 300 ms and 220 ms, with the shorter period producing more complicated behavior with a larger number of waves having shorter wavelengths. The dynamics of a reentrant wave may also depend on the timing of a premature beat used to initiate it, especially when the reentrant wave follows a hypermeandering trajectory (42).
Fig. 3.
Stable and unstable spiral waves in a computer simulation of the FMG mathematical model. A: initiation of a spiral wave with transient breakup that results in a stable spiral wave. The spiral ultimately follows a complex hypermeandering trajectory. B: sustained spiral wave breakup initiated following tissue preconditioning by pacing at 300 ms for 30 s. C: sustained spiral wave breakup initiated following tissue preconditioning at 220 ms for 30 s, resulting in more waves with shorter wavelengths. The domain in all cases is 30 cm × 30 cm, and the spatial resolution is 0.0125 cm.
Conclusion
Given the dynamical nature of cardiac arrhythmia initiation and perpetuation, the likelihood of identifying a therapeutic “magic bullet” along the lines of penicillin for infection or insulin for diabetes seems remote (59, 215). In addition, the apparent dependence of arrhythmia development on initial conditions is likely to confound predictions of arrhythmia risk based on static measures of electrophysiological properties. Consequently, for the near-term arrhythmia therapy is likely to be dominated by brute-force approaches such as ablation and high-voltage defibrillation defibrillation [although lower-energy alternatives may be on the horizon (9, 72, 134, 137)]. It should be noted that even the draconian therapies are not uniformly effective and that their efficacy depends on the state of the myocardium at the time of delivery (e.g., the efficacy of defibrillation varies depending on how soon after the onset of fibrillation the shocks are delivered and on the disease-dependent state of the myocardium). Even with substantial improvements in electrical and pharmacological therapy, however, an elimination of most lethal arrhythmias will not occur unless and until the root causes of those arrhythmias—heart failure and coronary artery disease—-are eradicated. Nevertheless, a successful antiarrhythmic therapy has clear benefits with respect to quality of life and longevity and should continue to be pursued vigorously.
GRANTS
This work was supported by National Science Foundation Grant 1028261 (to E. M. Cherry and F. H. Fenton) and by National Heart, Lung, and Blood Institute Grants HL-075515 and HL-073644 (both to R. F. Gilmour, Jr.). This research also was supported in part by National Science Foundation Grant TG-IBN050000N through TeraGrid resources provided by Purdue University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.M.C., F.H.F., and R.F.G. conception and design of research; E.M.C., F.H.F., and R.F.G. drafted manuscript; E.M.C., F.H.F., and R.F.G. edited and revised manuscript; E.M.C., F.H.F., and R.F.G. approved final version of manuscript; F.H.F. and E.M.C. performed experiments; F.H.F. and E.M.C. analyzed data; F.H.F. interpreted results of experiments; F.H.F. and E.M.C. prepared figures.
REFERENCES
- 1. Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J, on behalf of the American Heart Association Statistics Committee, and Stroke Statistics Subcommittee Heart Disease and Stroke Statistics—2010 Update: A Report From the American Heart Association. Circulation 121: e46–e215, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res 97: 1314–1322, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Aiba T, Tomaselli GF. Electrical remodeling in the failing heart. Curr Opin Cardiol 25: 29–36, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akar FG, Nass RD, Hahn S, Cingolani E, Shah M, Hesketh GG, DiSilvestre D, Tunin RS, Kass DA, Tomaselli GF. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am J Physiol Heart Circ Physiol 293: H1223–H1230, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Akar FG, Rosenbaum DS. Transmural electrophysiological heterogeneities underlying arrhythmogenesis in heart failure. Circ Res 93: 638–645, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Akar FG, Spragg DD, Tunin RS, Kass DA, Tomaselli GF. Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circ Res 95: 717–725, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Akar FG, Wu RC, Juang GJ, Tian Y, Burysek M, Disilvestre D, Xiong W, Armoundas AA, Tomaselli GF. Molecular mechanisms underlying K+ current downregulation in canine tachycardia-induced heart failure. Am J Physiol Heart Circ Physiol 288: H2887–H2896, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Alsheikh-Ali AA, Madias C, Supran S, Link MS. Marked variability in susceptibility to ventricular fibrillation in an experimental commotio cordis model. Circulation 122: 2499–2504, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Ambrosi CM, Ripplinger CM, Efimov IR, Fedorov VV. Termination of sustained atrial flutter and fibrillation using low-voltage multiple-shock therapy. Heart Rhythm 8: 101–108, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anderson AR, Sleeman BD. Wave front propagation and its failure in coupled systems of discrete bistable cells modelled by FitzHugh-Nagumo dynamics. Int J Bifurcation Chaos 5: 63–74, 1995 [Google Scholar]
- 11. Anderson KP, Walker R, Urie P, Ershler PR, Lux RL, Karwandee SV. Myocardial electrical propagation in patients with idiopathic dilated cardiomyopathy. J Clin Invest 92: 122–140, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anderson ME. Multiple downstream proarrhythmic targets for calmodulin kinase II: moving beyond an ion channel-centric focus. Cardiovasc Res 73: 657–666, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Antzelevitch C, Sicouri S, Litovsky SH, Lukas A, Krishnan SC, Di Diego JM, Gintant GA, Liu DW. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ Res 69: 1427–1449, 1991 [DOI] [PubMed] [Google Scholar]
- 14. Antzelevitch C. Role of spatial dispersion of repolarization in inherited and acquired sudden cardiac death syndromes. Am J Physiol Heart Circ Physiol 293: H2024–H2038, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arai M, Alpert NR, MacLennan DH, Barton P, Periasamy M. Alterations in sarcoplasmic reticulum gene expression in human heart failure. A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res 72: 463–469, 1993 [DOI] [PubMed] [Google Scholar]
- 16. Arce H, Xu A, Gonzalez H, Guevara MR. Alternans and higher-order rhythms in an ionic model of a sheet of ischemic ventricular muscle. Chaos 10: 411–426, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Armoundas AA, Hobai IA, Tomaselli GF, Winslow RL, O'Rourke B. Role of sodium-calcium exchanger in modulating the action potential of ventricular myocytes from normal and failing hearts. Circ Res 93: 46–53, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Banville I, Gray RA. Effect of action potential duration and conduction velocity restitution and their spatial dispersion on alternans and the stability of arrhythmias. J Cardiovasc Electrophysiol 13: 1141–1149, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Bär M, Eiswirth M. Turbulence due to spiral breakup in a continuous excitable medium. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics 48: R1635–R1637, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Bär M, Hildebrand M, Eiswirth M, Falcke M, Engel H, Neufeld M. Chemical turbulence and standing waves in a surface reaction model: the influence of global coupling and wave instabilities. Chaos 4: 499–508, 1994 [DOI] [PubMed] [Google Scholar]
- 22. Barker RJ, Price RL, Gourdie RG. Increased association of ZO-1 with connexin43 during remodeling of cardiac gap junctions. Circ Res 90: 317–324, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Berger RD, Kasper EK, Baughman KL, Marban E, Calkins H, Tomaselli GF. Beat-to-beat QT interval variability: novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation 96: 1557–1565, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Bernus O, Zemlin CW, Zaritsky RM, Mironov SF, Pertsov AM. Alternating conduction in the ischaemic border zone as precursor of reentrant arrhythmias: a simulation study. Europace 7, Suppl 2: 93–104, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Bers DM, Pogwizd SM, Schlotthauer K. Upregulated Na/Ca exchange is involved in both contractile dysfunction and arrhythmogenesis in heart failure. Basic Res Cardiol 97, Suppl 1: I36–I42, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Beuckelmann DJ, Näbauer M, Erdmann E. Characteristics of calcium-current in isolated human ventricular myocytes from patients with terminal heart failure. J Mol Cell Cardiol 23: 929–937, 1991 [DOI] [PubMed] [Google Scholar]
- 28. Beuckelmann DJ, Näbauer M, Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res 73: 379–385, 1993 [DOI] [PubMed] [Google Scholar]
- 29. Biktashev VN, Holden AV, Zhang H. Tension of organizing filaments of scroll waves. Philos Trans R Soc Lond A 347: 611–630, 1994 [Google Scholar]
- 30. Boyett MR, Jewell BR. Analysis of the effects of changes in rate and rhythm upon electrical activity in the heart. Prog Biophys Mol Biol 36: 1–52, 1980 [DOI] [PubMed] [Google Scholar]
- 31. Brooksby P, Levi AJ, Jones JV. The electrophysiological characteristics of hypertrophied ventricular myocytes from the spontaneously hypertensive rat. J Hypertens 11: 611–622, 1993 [DOI] [PubMed] [Google Scholar]
- 32. Bub G, Shrier A, Glass L. Spiral wave generation in heterogeneous excitable media. Phys Rev Lett 88: 058101, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Buchanan JW, Jr, Saito T, Gettes LS. The effects of antiarrhythmic drugs, stimulation frequency, and potassium-induced resting membrane potential changes on conduction velocity and dV/dtmax in guinea pig myocardium. Circ Res 56: 696–703, 1985 [DOI] [PubMed] [Google Scholar]
- 34. Bueno-Orovio A, Cherry EM, Fenton FH. Minimal model for human ventricular action potentials in tissue. J Theor Biol 253: 544–560, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Cabo C, Yao J, Boyden PA, Chen S, Hussain W, Duffy HS, Ciaccio EJ, Peters NS, Wit AL. Heterogeneous gap junction remodeling in reentrant circuits in the epicardial border zone of the healing canine infarct. Cardiovasc Res 72: 241–249, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Casaleggio A, Gramatikov B, Thakor N. Dynamic differences between ventricular fibrillation types induced in human patients by different types of stimulation. Comput Cardiol (September 7, 1997). doi: 10.1109/CIC.1997.647837 [Google Scholar]
- 37. Cerbai E, Barbieri M, Li Q, Mugelli A. Ionic basis of action potential prolongation of hypertrophied cardiac myocytes isolated from hypertensive rats of different ages. Cardiovasc Res 28: 1180–1187, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Chen PS, Wolf PD, Dixon EG, Danieley ND, Frazier DW, Smith WM, Ideker RE. Mechanism of ventricular vulnerability to single premature stimuli in open-chest dogs. Circ Res 62: 1191–1209, 1988 [DOI] [PubMed] [Google Scholar]
- 39. Chen X, Piacentino V, 3rd, Furukawa S, Goldman B, Margulies KB, Houser SR. L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ Res 91: 517–524, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Cherry EM, Evans SJ. Properties of two human atrial cell models in tissue: restitution, memory, propagation, and reentry. J Theor Biol 254: 674–90, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cherry EM, Fenton FH. Suppression of alternans and conduction blocks despite steep APD restitution: electrotonic, memory, and conduction velocity restitution effects. Am J Physiol Heart Circ Physiol 286: H2332–H2341, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Cherry EM, Fenton FH. A tale of two dogs: analyzing two models of canine ventricular electrophysiology. Am J Physiol Heart Circ Physiol 292: H43–H55, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Cherry EM, Fenton FH. Visualization of spiral and scroll waves in simulated and experimental cardiac tissue. New J Phys 10: 125016, 2008 [Google Scholar]
- 44. Chialvo DR, Gilmour RF, Jr, Jalife J. Low dimensional chaos in cardiac tissue. Nature 343: 653–657, 1990 [DOI] [PubMed] [Google Scholar]
- 45. Chialvo DR, Michaels DC, Jalife J. Supernormal excitability as a mechanism of chaotic dynamics of activation in cardiac Purkinje fibers. Circ Res 66: 525–545, 1990 [DOI] [PubMed] [Google Scholar]
- 46. Choi BR, Liu T, Salama G. Adaptation of cardiac action potential durations to stimulation history with random diastolic intervals. J Cardiovasc Electrophysiol 15: 1188–1197, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Christini DJ, Riccio ML, Culianu CA, Fox JJ, Karma A, Gilmour RF., Jr Control of electrical alternans in canine cardiac Purkinje fibers. Phys Rev Lett 96: 104101, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chudin E, Goldhaber J, Garfinkel A, Weiss J, Kogan B. Intracellular Ca2+ dynamics and the stability of ventricular tachycardia. Biophys J 77: 2930–2941, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cingolani E, Ramirez Correa GA, Kizana E, Murata M, Cho HC, Marbán E. Gene therapy to inhibit the calcium channel beta subunit: physiological consequences and pathophysiological effects in models of cardiac hypertrophy. Circ Res 101: 166–175, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Clayton RH, Taggart P. Regional differences in APD restitution can initiate wavebreak and re-entry in cardiac tissue: a computational study. Biomed Eng Online 4: 54, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cohen I, Giles W, Noble D. Cellular basis for the T wave of the electrocardiogram. Nature 262: 657–661, 1976 [DOI] [PubMed] [Google Scholar]
- 52. Comtois P, Vinet A, Nattel S. Wave block formation in homogeneous excitable media following premature excitations: dependence on restitution relations. Phys Rev E Stat Nonlin Soft Matter Phys 72: 031919, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Comtois P, Vinet A. Multistability of reentrant rhythms in an ionic model of a two-dimensional annulus of cardiac tissue. Phys Rev E Stat Nonlin Soft Matter Phys 72: 051927, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Curran J, Hinton MJ, Ríos E, Bers DM, Shannon TR. Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res 100: 391–398, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Cytrynbaum E, Keener JP. Stability conditions for traveling pulse: modifying the restitution hypothesis. Chaos 12: 788–799, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Darwin Awards. 1999. http://www.darwinawards.com/darwin/darwin1999-50.html.
- 57. Davidenko JM, Pertsov AV, Salomonsz R, Baxter W, Jalife J. Stationary and drifting spiral waves of excitation in isolated cardiac muscle. Nature 355: 349–351, 1992 [DOI] [PubMed] [Google Scholar]
- 57a. De Bakker JM, van Capelle FJ, Janse MJ, Wilde AA, Coronel R, Becker AE, Dingemans KP, van Hemel NM, Hauer RN. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation 77: 589–606, 1988 [DOI] [PubMed] [Google Scholar]
- 57b. Del Rio CL, McConnell PI, Kukielka M, Dzwonczyk R, Clymer BD, Howie MB, Billman GE. Electrotonic remodeling following myocardial infarction in dogs susceptible and resistant to sudden cardiac death. J Appl Physiol 104: 386–393, 2008 [DOI] [PubMed] [Google Scholar]
- 57c. Di Bernardo D, Murray A. Explaining the T-wave shape in the ECG. Nature 403: 40, 2000 [DOI] [PubMed] [Google Scholar]
- 58. Echebarria B, Karma A. Instability and spatiotemporal dynamics of alternans in paced cardiac tissue. Phys Rev Lett 88: 208101, 2002 [DOI] [PubMed] [Google Scholar]
- 59. Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL, Huther ML, Richardson DW, the Investigators CAST Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med 324: 781–788, 1991 [DOI] [PubMed] [Google Scholar]
- 60. Elharrar V, Surawicz B. Cycle length effect on restitution of action potential duration in dog cardiac fibers. Am J Physiol Heart Circ Physiol 244: H782–H792, 1983 [DOI] [PubMed] [Google Scholar]
- 61. Emdad L, Uzzaman M, Takagishi Y, Honjo H, Uchida T, Severs NJ, Kodama I, Murata Y. Gap junction remodeling in hypertrophied left ventricles of aortic-banded rats: prevention by angiotensin II type 1 receptor blockade. J Mol Cell Cardiol 33: 219–231, 2001 [DOI] [PubMed] [Google Scholar]
- 62. Endresen K, Amlie JP, Forfang K, Simonsen S, Jensen O. Monophasic action potentials in patients with coronary artery disease: reproducibility and electrical restitution and conduction at different stimulation rates. Cardiovasc Res 21: 696–702, 1987 [DOI] [PubMed] [Google Scholar]
- 63. Endresen K, Amlie JP. Electrical restitution and conduction intervals of ventricular premature beats in man: influence of heart rate. Pacing Clin Electrophysiol 12: 1347–1354, 1989 [DOI] [PubMed] [Google Scholar]
- 64. Fast VG, Pertsov AM. Drift of vortex in the myocardium. Biofizika 35: 478–482, 1990 [PubMed] [Google Scholar]
- 65. Fenton F, Karma A. Fiber-rotation-induced vortex turbulence in thick myocardium. Phys Rev Lett 81: 481–484, 1998 [Google Scholar]
- 66. Fenton F, Karma A. Vortex dynamics in three-dimensional continuous myocardium with fiber rotation: Filament instability and fibrillation. Chaos 8: 20–47, 1998 [DOI] [PubMed] [Google Scholar]
- 67. Fenton FH, Cherry EM, Glass L. Cardiac arrhythmia. Scholarpedia 3: 1665, 2008 [Google Scholar]
- 68. Fenton FH, Cherry EM, Hastings HM, Evans SJ. Multiple mechanisms of spiral wave breakup in a model of cardiac electrical activity. Chaos 12: 852–892, 2002 [DOI] [PubMed] [Google Scholar]
- 69. Fenton FH, Cherry EM, Kornreich BG. Termination of equine atrial fibrillation by quinidine: an optical mapping study. J Vet Cardiol 10: 87–103, 2008 [DOI] [PubMed] [Google Scholar]
- 70. Fenton FH, Evan SJ, Hastings HM, Karma A. Transition from ventricular tachycardia to ventricular fibrillation as a function of tissue characteristics in a computer model. Europace 1: D126, 2000 [Google Scholar]
- 71. Fenton FH, Evans SJ, Hastings HM. Memory in an excitable medium: a mechanism for spiral wave breakup in the low-excitability limit. Phys Rev Lett 83: 3964, 1999 [Google Scholar]
- 72. Fenton FH, Luther S, Cherry EM, Otani NF, Krinsky V, Pumir A, Bodenschatz E, Gilmour RF., Jr Termination of atrial fibrillation using pulsed low-energy far-field stimulation. Circulation 120: 467–476, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fenton FH. Theoretical investigation of spiral and scroll wave instabilities underlying cardiac fibrillation (PhD thesis). Boston, MA: Northeastern Univ., 1999 [Google Scholar]
- 74. Fisch C. Electrocardiographic manifestations of exit block and supernormal and concealed conduction. In: Cardiac Electrophysiology From Cell to Bedside, edited by Zipes D, Jalife J. Philadelphia: Saunders, 2000, p. 685–690 [Google Scholar]
- 75. Fox JJ, Bodenschatz E, Gilmour RF., Jr Period-doubling instability and memory in cardiac tissue. Phys Rev Lett 89: 138101, 2002 [DOI] [PubMed] [Google Scholar]
- 76. Fox JJ, McHarg JL, Gilmour RF. Ionic mechanism of electrical alternans. Am J Physiol Heart Circ Physiol 282: H516–H530, 2002 [DOI] [PubMed] [Google Scholar]
- 77. Fox JJ, Riccio ML, Drury P, Werthman A, Gilmour RF. Dynamic mechanism for conduction block in heart tissue. New J Phys 5: 101.1–101.4, 2003 [Google Scholar]
- 78. Fox JJ, Riccio ML, Hua F, Bodenschatz E, Gilmour RF., Jr Spatiotemporal transition to conduction block in canine ventricle. Circ Res 90: 289–296, 2002 [DOI] [PubMed] [Google Scholar]
- 79. Franz MR, Schaefer J, Schöttler M, Seed WA, Noble MI. Electrical and mechanical restitution of the human heart at different rates of stimulation. Circ Res 53: 815–822, 1983 [DOI] [PubMed] [Google Scholar]
- 80. Franz MR, Swerdlow CD, Liem LB, Schaefer J. Cycle length dependence of human action potential duration in vivo. Effects of single extrastimuli, sudden sustained rate acceleration and deceleration, and different steady-state frequencies. J Clin Invest 82: 972–979, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Freeman LC, Pacioretty LM, Moise NS, Kass RS, Gilmour RF., Jr Decreased density of Ito in left ventricular myocytes from German shepherd dogs with inherited arrhythmias. J Cardiovasc Electrophysiol 8: 872–883, 1997 [DOI] [PubMed] [Google Scholar]
- 82. Furukawa T, Bassett AL, Furukawa N, Kimura S, Myerburg RJ. The ionic mechanism of reperfusion-induced early afterdepolarizations in feline left ventricular hypertrophy. J Clin Invest 91: 1521–1531, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gelzer AR, Koller ML, Otani NF, Fox JJ, Enyeart MW, Hooker GJ, Riccio ML, Bartoli CR, Gilmour RF., Jr Dynamic mechanism for initiation of ventricular fibrillation in vivo. Circulation 118: 1123–1129, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gelzer A, Otani N, Koller M, Enyeart M, Moise N, Gilmour R. Dynamically-induced spatial dispersion of repolarization and the development of VF in an animal model of sudden death. Comput Cardiol 36: 309–312, 2009 [PMC free article] [PubMed] [Google Scholar]
- 85. Giaquinta A, Boccaletti S, Arecchi FT. Superexcitability induced spiral breakup in excitable systems. Int J Bifurcation Chaos 6: 1753–1759, 1996 [Google Scholar]
- 86. Glass L, Mackey MC. From clocks to chaos: the rhythms of life. Princeton, NJ: Princeton Univ. Press, 1988 [Google Scholar]
- 87. Glass L. Synchronization and rhythmic processes in physiology. Nature 410: 277–284, 2001 [DOI] [PubMed] [Google Scholar]
- 88. Glukhov AV, Fedorov VV, Lou Q, Ravikumar VK, Kalish PW, Schuessler RB, Moazami N, Efimov IR. Transmural dispersion of repolarization in failing and nonfailing human ventricle. Circ Res 106: 981–991, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Goldhaber JI, Motter C, Duong TK, Weiss JN. Cellular basis of action potential duration alteranans: role of the L-type calcium current and intracellular calcium cycling. Circulation 106: 228, 2002 [Google Scholar]
- 90. Gray RA, Pertsov AM, Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature 392: 75–78, 1998 [DOI] [PubMed] [Google Scholar]
- 91. Guevara MR, Ward G, Shrier A, Glass L. Electrical alternans and period-doubling bifurcations. Comput Cardiol 11: 167–170, 1984 [Google Scholar]
- 92. Hall GM, Bahar S, Gauthier DJ. The prevalence of rate-dependent dynamics in cardiac tissue. Phys Rev Lett 82: 2995–2998, 1999 [Google Scholar]
- 93. Harper JR, Jr, Johnson TA, Engle CL, Martin DG, Fleet W, Gettes LS. Effect of rate on changes in conduction velocity and extracellular potassium concentration during acute ischemia in the in situ pig heart. J Cardiovasc Electrophysiol 4: 661–671, 1993 [DOI] [PubMed] [Google Scholar]
- 94. Harzheim D, Movassagh M, Foo RSY, Ritter O, Tashfeen A, Conway SJ, Bootman MD, Roderick HL. Increased InsP3Rs in the junctional sarcoplasmic reticulum augment Ca2+ transients and arrhythmias associated with cardiac hypertrophy. Proc Natl Acad Sci USA 106: 11406–11411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hasenfuss G, Meyer M, Schillinger W, Preuss M, Pieske B, Just H. Calcium handling proteins in the failing human heart. Basic Res Cardiol 92, Suppl 1: 87–93, 1997 [DOI] [PubMed] [Google Scholar]
- 96. Hesketh GG, Shah MH, Kass DA, Van Eyk JE, Tomaselli GF. Gap junction internalization and autophagic degradation in the failing heart. Circulation 118: S340, 2008 [Google Scholar]
- 97. Hill JA. Electrical remodeling in cardiac hypertrophy. Trends Cardiovasc Med 13: 316–322, 2003 [DOI] [PubMed] [Google Scholar]
- 98. Hirsch MW, Smale S, Devaney RL. Differential equations, dynamical systems and an introduction to chaos. San Diego: Elsevier, 2004 [Google Scholar]
- 99. Hobai IA, O'Rourke B. Enhanced Ca2+-activated Na+-Ca2+ exchange activity in canine pacing-induced heart failure. Circ Res 87: 690–698, 2000 [DOI] [PubMed] [Google Scholar]
- 100. Horner SM, Dick DJ, Murphy CF, Lab MJ. Cycle length dependence of the electrophysiological effects of increased load on the myocardium. Circulation 94: 1131–1136, 1996 [DOI] [PubMed] [Google Scholar]
- 101. Hua F, Gilmour RF., Jr Contribution of IKr to rate-dependent action potential dynamics in canine endocardium. Circ Res 94: 810–819, 2004 [DOI] [PubMed] [Google Scholar]
- 102. Hua F, Johns DC, Gilmour RF., Jr Suppression of electrical alternans by overexpression of HERG in canine ventricular myocytes. Am J Physiol Heart Circ Physiol 286: H2342–H2351, 2004 [DOI] [PubMed] [Google Scholar]
- 103. Huang X, Qian Y, Zhang X, Hu G. Hysteresis and bistability in periodically paced cardiac tissue. Phys Rev E Stat Nonlin Soft Matter Phys 81: 051903, 2010 [DOI] [PubMed] [Google Scholar]
- 104. Hullin R, Khan IFY, Wirtz S, Mohacsi P, Varadi G, Schwartz A, Herzig S. Cardiac L-type calcium channel beta-subunits expressed in human heart have differential effects on single channel characteristics. J Biol Chem 278: 21623–21630, 2003 [DOI] [PubMed] [Google Scholar]
- 105. Jalife J. Ventricular fibrillation: mechanisms of initiation and maintenance. Annu Rev Physiol 62: 25–50, 2000 [DOI] [PubMed] [Google Scholar]
- 106. Janse MJ, Sosunov EA, Coronel R, Opthof T, Anyukhovsky EP, de Bakker JMT, Plotnikov AN, Shlapakova IN, Danilo P, Jr, Tijssen JGP, Rosen MR. Repolarization gradients in the canine left ventricle before and after induction of short-term cardiac memory. Circulation 112: 1711–1718, 2005 [DOI] [PubMed] [Google Scholar]
- 107. Janse MJ, Wit AL. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev 69: 1049–1169, 1989 [DOI] [PubMed] [Google Scholar]
- 108. Janse MJ. Electrophysiological changes in heart failure and their relationship to arrhythmogenesis. Cardiovasc Res 61: 208–217, 2004 [DOI] [PubMed] [Google Scholar]
- 109. Jiang MT, Lokuta AJ, Farrell EF, Wolff MR, Haworth RA, Valdivia HH. Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circ Res 91: 1015–1022, 2002 [DOI] [PubMed] [Google Scholar]
- 110. Jin H, Lyon AR, Akar FG. Arrhythmia mechanisms in the failing heart. Pacing Clin Electrophysiol 31: 1048–1056, 2008 [DOI] [PubMed] [Google Scholar]
- 111. Kääb S, Dixon J, Duc J, Ashen D, Näbauer M, Beuckelmann DJ, Steinbeck G, McKinnon D, Tomaselli GF. Molecular basis of transient outward potassium current downregulation in human heart failure: a decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation 98: 1383–1393, 1998 [DOI] [PubMed] [Google Scholar]
- 112. Kääb S, Nuss HB, Chiamvimonvat N, O'Rourke B, Pak PH, Kass DA, Marban E, Tomaselli GF. Ionic mechanism of action potential prolongation in ventricular myocytes from dogs with pacing-induced heart failure. Circ Res 78: 262–273, 1996 [DOI] [PubMed] [Google Scholar]
- 113. Kalb SS, Dobrovolny HM, Tolkacheva EG, Idriss SF, Krassowska W, Gauthier DJ. The restitution portrait: a new method for investigating rate-dependent restitution. J Cardiovasc Electrophysiol 15: 698–709, 2004 [DOI] [PubMed] [Google Scholar]
- 114. Kalcheva N, Qu J, Sandeep N, Garcia L, Zhang J, Wang Z, Lampe PD, Suadicani SO, Spray DC, Fishman GI. Gap junction remodeling and cardiac arrhythmogenesis in a murine model of oculodentodigital dysplasia. Proc Natl Acad Sci USA 104: 20512–20516, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Karma A. Electrical alternans and spiral wave breakup in cardiac tissue. Chaos 4: 461–472, 1994 [DOI] [PubMed] [Google Scholar]
- 116. Kawara T, Derksen R, de Groot JR, Coronel R, Tasseron S, Linnenbank AC, Hauer RN, Kirkels H, Janse MJ, de Bakker JM. Activation delay after premature stimulation in chronically diseased human myocardium relates to the architecture of interstitial fibrosis. Circulation 104: 3069–3075, 2001 [DOI] [PubMed] [Google Scholar]
- 117. Keener JP, Sneyd J. Mathematical Physiology: Cellular Physiology. New York, NY: Springer, 2009 [Google Scholar]
- 118. Keener JP. Propagation and its failure in coupled systems of discrete excitable cells. SIAM J Appl Math 47: 556–572, 1987 [Google Scholar]
- 119. Keener JP. On the formation of circulating patterns of excitation in ansiotropic excitable media. J Math Biol 26: 41–56, 1988 [DOI] [PubMed] [Google Scholar]
- 120. Keener JP. The effects of discrete gap junction coupling on propagation in myocardium. J Theor Biol 148: 49–82, 1991 [DOI] [PubMed] [Google Scholar]
- 121. Kléber AG, Riegger CB, Janse MJ. Electrical uncoupling and increase of extracellular resistance after induction of ischemia in isolated, arterially perfused rabbit papillary muscle. Circ Res 61: 271–279, 1987 [DOI] [PubMed] [Google Scholar]
- 122. Kobayashi Y, Peters W, Khan SS, Mandel WJ, Karagueuzian HS. Cellular mechanisms of differential action potential duration restitution in canine ventricular muscle cells during single versus double premature stimuli. Circulation 86: 955–967, 1992 [DOI] [PubMed] [Google Scholar]
- 123. Kohlhaas M, Zhang T, Seidler T, Zibrova D, Dybkova N, Steen A, Wagner S, Chen L, Brown JH, Bers DM, Maier LS. Increased sarcoplasmic reticulum calcium leak but unaltered contractility by acute CaMKII overexpression in isolated rabbit cardiac myocytes. Circ Res 98: 235–244, 2006 [DOI] [PubMed] [Google Scholar]
- 124. Koller ML, Maier SKG, Gelzer AR, Bauer WR, Meesmann M, Gilmour RF., Jr Altered dynamics of action potential restitution and alternans in humans with structural heart disease. Circulation 112: 1542–1548, 2005 [DOI] [PubMed] [Google Scholar]
- 125. Koller ML, Riccio ML, Gilmour RF., Jr Dynamic restitution of action potential duration during electrical alternans and ventricular fibrillation. Am J Physiol Heart Circ Physiol 275: H1635–H1642, 1998 [DOI] [PubMed] [Google Scholar]
- 126. Kostin S, Dammer S, Hein S, Klovekorn WP, Bauer EP, Schaper J. Connexin 43 expression and distribution in compensated and decompensated cardiac hypertrophy in patients with aortic stenosis. Cardiovasc Res 62: 426–436, 2004 [DOI] [PubMed] [Google Scholar]
- 127. Krinsky VI. Self-Organization, Autowaves and Structures Far from Equilibrium. Berlin: Springer, 1984 [Google Scholar]
- 128. Kurian TK, Efimov IR. Mechanisms of fibrillation: neurogenic or myogenic? Reentrant or focal? Multiple or single? Still puzzling after 160 years of inquiry. J Cardiovasc Electrophysiol 21: 1274–1275, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kwon O, Kim TY, Lee KJ. Period-2 spiral waves supported by nonmonotonic wave dispersion. Phys Rev E Stat Nonlin Soft Matter Phys 82: 046213, 2010 [DOI] [PubMed] [Google Scholar]
- 130. Laurita KR, Chuck ET, Yang T, Dong WQ, Kuryshev YA, Brittenham GM, Rosenbaum DS, Brown AM. Optical mapping reveals conduction slowing and impulse block in iron-overload cardiomyopathy. J Lab Clin Med 142: 83–89, 2003 [DOI] [PubMed] [Google Scholar]
- 131. Laurita KR, Katra R, Wible B, Wan X, Koo MH. Transmural heterogeneity of calcium handling in canine. Circ Res 92: 668–675, 2003 [DOI] [PubMed] [Google Scholar]
- 132. Lehnart SE, Terrenoire C, Reiken S, Wehrens XHT, Song LS, Tillman EJ, Mancarella S, Coromilas J, Lederer WJ, Kass RS, Marks AR. Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. Proc Natl Acad Sci USA 103: 7906–7910, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Li GR, Lau CP, Ducharme A, Tardif JC, Nattel S. Transmural action potential and ionic current remodeling in ventricles of failing canine hearts. Am J Physiol Heart Circ Physiol 283: H1031–H1041, 2002 [DOI] [PubMed] [Google Scholar]
- 134. Li W, Ripplinger CM, Lou Q, Efimov IR. Multiple monophasic shocks improve electrotherapy of ventricular tachycardia in a rabbit model of chronic infarction. Heart Rhythm 6: 1020–1027, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, Dalton ND, Peterson KL, Chen J, Bers D, Heller Brown J. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest 119: 1230–1240, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Lou Q, Li W, Efimov IR. The role of dynamic instability and wavelength in arrhythmia maintenance as revealed by panoramic imaging with blebbistatin vs. 2,3-butanedione monoxime. Am J Physiol Heart Circ Physiol 302: H262–H269, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Luther S, Fenton FH, Kornreich BG, Squires A, Bittihn P, Hornung D, Zabel M, Flanders J, Gladuli A, Campoy L, Cherry EM, Luther G, Hasenfuss G, Krinsky VI, Pumir A, Gilmour RF, Jr, Bodenschatz E. Low-energy control of electrical turbulence in the heart. Nature 475: 235–239, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. MacDonnell SM, García-Rivas G, Scherman JA, Kubo H, Chen X, Valdivia H, Houser SR. Adrenergic regulation of cardiac contractility does not involve phosphorylation of the cardiac ryanodine receptor at serine 2808. Circ Res 102: e65–e72, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res 92: 904–911, 2003 [DOI] [PubMed] [Google Scholar]
- 140. Maltsev VA, Reznikov V, Undrovinas NA, Sabbah HN, Undrovinas A. Modulation of late sodium current by Ca2+, calmodulin, and CaMKII in normal and failing dog cardiomyocytes: similarities and differences. Am J Physiol Heart Circ Physiol 294: H1597–H1608, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101: 365–376, 2000 [DOI] [PubMed] [Google Scholar]
- 142. Mattiello JA, Margulies KB, Jeevanandam V, Houser SR. Contribution of reverse-mode sodium-calcium exchange to contractions in failing human left ventricular myocytes. Cardiovasc Res 37: 424–431, 1998 [DOI] [PubMed] [Google Scholar]
- 143. McLerie M, Lopatin AN, Lopatin A. Dominant-negative suppression of I(K1) in the mouse heart leads to altered cardiac excitability. J Mol Cell Cardiol 35: 367–378, 2003 [DOI] [PubMed] [Google Scholar]
- 144. Näbauer M, Beuckelmann DJ, Erdmann E. Characteristics of transient outward current in human ventricular myocytes from patients with terminal heart failure. Circ Res 73: 386–394, 1993 [DOI] [PubMed] [Google Scholar]
- 145. Nash MP, Bradley CP, Sutton PM, Clayton RH, Kallis P, Hayward MP, Paterson DJ, Taggart P. Whole heart action potential duration restitution properties in cardiac patients: a combined clinical and modelling study. Exp Physiol 91: 339–354, 2006 [DOI] [PubMed] [Google Scholar]
- 146. Nattel S, Maguy A, Le Bouter S, Yeh YH. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev 87: 425–456, 2007 [DOI] [PubMed] [Google Scholar]
- 147. Nolasco JB, Dahlen RW. A graphic method for the study of alternation in cardiac action potentials. J Appl Physiol 25: 191–196, 1968 [DOI] [PubMed] [Google Scholar]
- 148. Nuss HB, Kääb S, Kass DA, Tomaselli GF, Marbán E. Cellular basis of ventricular arrhythmias and abnormal automaticity in heart failure. Am J Physiol Heart Circ Physiol 277: H80–H91, 1999 [DOI] [PubMed] [Google Scholar]
- 149. O'Rourke B, Kass DA, Tomaselli GF, Kääb S, Tunin R, Marbán E. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, I: experimental studies. Circ Res 84: 562–570, 1999 [DOI] [PubMed] [Google Scholar]
- 150. Oliver RA, Henriquez CS, Krassowska W. Bistability and correlation with arrhythmogenesis in a model of the right atrium. Ann Biomed Eng 33: 577–589, 2005 [DOI] [PubMed] [Google Scholar]
- 151. Otani NF, Gilmour RF. Memory models for the electrical properties of local cardiac systems. J Theor Biol 187: 409–436, 1997 [DOI] [PubMed] [Google Scholar]
- 152. Otani NF. Theory of action potential wave block at-a-distance in the heart. Phys Rev E Stat Nonlin Soft Matter Phys 75: 021910, 2007 [DOI] [PubMed] [Google Scholar]
- 153. Ouadid H, Albat B, Nargeot J. Calcium currents in diseased human cardiac cells. J Cardiovasc Pharmacol 25: 282–291, 1995 [DOI] [PubMed] [Google Scholar]
- 154. Pacioretty LM, Barr SC, Han WP, Gilmour RF., Jr Reduction of the transient outward potassium current in a canine model of Chagas' disease. Am J Physiol Heart Circ Physiol 268: H1258–H1264, 1995 [DOI] [PubMed] [Google Scholar]
- 155. Pacioretty LM, Cooper BJ, Gilmour RF., Jr Reduction of the transient outward potassium current in canine X-linked muscular dystrophy. Circulation 90: 1350–1356, 1994 [DOI] [PubMed] [Google Scholar]
- 156. Pak HN, Hong SJ, Hwang GS, Lee HS, Park SW, Ahn JC, Moo Ro Y, Kim YH. Spatial dispersion of action potential duration restitution kinetics is associated with induction of ventricular tachycardia/fibrillation in humans. J Cardiovasc Electrophysiol 15: 1357–1363, 2004 [DOI] [PubMed] [Google Scholar]
- 157. Panama BK, Latour-Villamil D, Farman GP, Zhao D, Bolz SS, Kirshenbaum LA, Backx PH. Nuclear factor kappaB downregulates the transient outward potassium current I(to,f) through control of KChIP2 expression. Circ Res 108: 537–543, 2011 [DOI] [PubMed] [Google Scholar]
- 158. Panfilov A, Pertsov A. Ventricular fibrillation: evolution of the multiple-wavelet hypothesis. Philos Trans R Soc Lond A 359: 1315–1325, 2001 [Google Scholar]
- 159. Panfilov AV, Keener JP. Generation of reentry in anisotropic myocardium. J Cardiovasc Electrophysiol 4: 412–421, 1993 [DOI] [PubMed] [Google Scholar]
- 160. Panfilov AV, Keener JP. Re-entry in three-dimensional Fitzhugh-Nagumo medium with rotational anisotropy. Physica D 84: 545–552, 1995 [Google Scholar]
- 161. Panfilov AV, Zemlin CW. Wave propagation in an excitable medium with a negatively sloped restitution curve. Chaos 12: 800–806, 2002 [DOI] [PubMed] [Google Scholar]
- 162. Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation 99: 1385–1394, 1999 [DOI] [PubMed] [Google Scholar]
- 163. Pertsov AM, Davidenko JM, Salomonsz R, Baxter WT, Jalife J. Spiral waves of excitation underlie reentrant activity in isolated cardiac muscle. Circ Res 72: 631–650, 1993 [DOI] [PubMed] [Google Scholar]
- 164. Peters NS, Coromilas J, Severs NJ, Wit AL. Disturbed connexin43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation 95: 988–996, 1997 [DOI] [PubMed] [Google Scholar]
- 165. Peters NS, Green CR, Poole-Wilson PA, Severs NJ. Reduced content of connexin43 gap junctions in ventricular myocardium from hypertrophied and ischemic human hearts. Circulation 88: 864–875, 1993 [DOI] [PubMed] [Google Scholar]
- 166. Pitt GS, Dun W, Boyden PA. Remodeled cardiac calcium channels. J Mol Cell Cardiol 41: 373–388, 2006 [DOI] [PubMed] [Google Scholar]
- 167. Poelzing S, Rosenbaum DS. Altered connexin43 expression produces arrhythmia substrate in heart failure. Am J Physiol Heart Circ Physiol 287: H1762–H1770, 2004 [DOI] [PubMed] [Google Scholar]
- 168. Pogwizd SM, McKenzie JP, Cain ME. Mechanisms underlying spontaneous and induced ventricular arrhythmias in patients with idiopathic dilated cardiomyopathy. Circulation 98: 2404–2414, 1998 [DOI] [PubMed] [Google Scholar]
- 169. Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res 88: 1159–1167, 2001 [DOI] [PubMed] [Google Scholar]
- 170. Pogwizd SM. Focal mechanisms underlying ventricular tachycardia during prolonged ischemic cardiomyopathy. Circulation 90: 1441–1458, 1994 [DOI] [PubMed] [Google Scholar]
- 171. Pogwizd SM. Nonreentrant mechanisms underlying spontaneous ventricular arrhythmias in a model of nonischemic heart failure in rabbits. Circulation 92: 1034–1048, 1995 [DOI] [PubMed] [Google Scholar]
- 172. Priori SG. The fifteen years of discoveries that shaped molecular electrophysiology: time for appraisal. Circ Res 107: 451–456, 2010 [DOI] [PubMed] [Google Scholar]
- 173. Pu J, Boyden PA. Alterations of Na+ currents in myocytes from epicardial border zone of the infarcted heart. A possible ionic mechanism for reduced excitability and postrepolarization refractoriness. Circ Res 81: 110–119, 1997 [DOI] [PubMed] [Google Scholar]
- 174. Qu Z, Garfinkel A, Chen PS, Weiss JN. Mechanisms of discordant alternans and induction of reentry in simulated cardiac tissue. Circulation 102: 1664–1670, 2000 [DOI] [PubMed] [Google Scholar]
- 175. Qu Z, Garfinkel A, Weiss JN. Vulnerable window for conduction block in a one-dimensional cable of cardiac cells, 1: single extrasystoles. Biophys J 91: 793–804, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Qu Z, Garfinkel A, Weiss JN. Vulnerable window for conduction block in a one-dimensional cable of cardiac cells, 2: multiple extrasystoles. Biophys J 91: 805–815, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Qu Z, Kil J, Xie F, Garfinkel A, Weiss JN. Scroll wave dynamics in a three-dimensional cardiac tissue model: roles of restitution, thickness, and fiber rotation. Biophys J 78: 2761–2775, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Qu Z, Weiss JN, Garfinkel A. Spatiotemporal chaos in a simulated ring of cardiac cells. Phys Rev Lett 78: 1387, 1997 [Google Scholar]
- 179. Rappel WJ. Filament instability and rotational tissue anisotropy: a numerical study using detailed cardiac models. Chaos 11: 71–80, 2001 [DOI] [PubMed] [Google Scholar]
- 180. Reiken S, Gaburjakova M, Guatimosim S, Gomez AM, D'Armiento J, Burkhoff D, Wang J, Vassort G, Lederer WJ, Marks AR. Protein kinase A phosphorylation of the cardiac calcium release channel (ryanodine receptor) in normal and failing hearts. Role of phosphatases and response to isoproterenol. J Biol Chem 278: 444–453, 2003 [DOI] [PubMed] [Google Scholar]
- 181. Riccio ML, Koller ML, Gilmour RF. Electrical restitution and spatiotemporal organization during ventricular fibrillation. Circ Res 84: 955–963, 1999 [DOI] [PubMed] [Google Scholar]
- 182. Richard S, Leclercq F, Lemaire S, Piot C, Nargeot J. Ca2+ currents in compensated hypertrophy and heart failure. Cardiovasc Res 37: 300–311, 1998 [DOI] [PubMed] [Google Scholar]
- 184. Rogers JM. Wave front fragmentation due to ventricular geometry in a model of the rabbit heart. Chaos 12: 779–787, 2002 [DOI] [PubMed] [Google Scholar]
- 185. Rose J, Armoundas AA, Tian Y, DiSilvestre D, Burysek M, Halperin V, O'Rourke B, Kass DA, Marbán E, Tomaselli GF. Molecular correlates of altered expression of potassium currents in failing rabbit myocardium. Am J Physiol Heart Circ Physiol 288: H2077–H2087, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Rosenbaum DS, Kaplan DT, Kanai A, Jackson L, Garan H, Cohen RJ, Salama G. Repolarization inhomogeneities in ventricular myocardium change dynamically with abrupt cycle length shortening. Circulation 84: 1333–1345, 1991 [DOI] [PubMed] [Google Scholar]
- 187. Rozanski GJ, Xu Z, Whitney RT, Murakami H, Zucker IH. Electrophysiology of rabbit ventricular myocytes following sustained rapid ventricular pacing. J Mol Cell Cardiol 29: 721–732, 1997 [DOI] [PubMed] [Google Scholar]
- 188. Sachdeva G, Kalyanasundaram K, Krishnan J, Chakravarthy VS. Bistable dynamics of cardiac cell models coupled by dynamic gap junctions linked to cardiac memory. Biol Cybern 102: 109–121, 2010 [DOI] [PubMed] [Google Scholar]
- 189. Sakakibara Y, Furukawa T, Singer DH, Jia H, Backer CL, Arentzen CE, Wasserstrom JA. Sodium current in isolated human ventricular myocytes. Am J Physiol Heart Circ Physiol 265: H1301–H1309, 1993 [DOI] [PubMed] [Google Scholar]
- 190. Sakata S, Lebeche D, Sakata N, Sakata Y, Chemaly ER, Liang LF, Tsuji T, Takewa Y, del Monte F, Peluso R, Zsebo K, Jeong D, Park WJ, Kawase Y, Hajjar RJ. Restoration of mechanical and energetic function in failing aortic-banded rat hearts by gene transfer of calcium cycling proteins. J Mol Cell Cardiol 42: 852–861, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Sakata S, Liang L, Sakata N, Sakata Y, Chemaly ER, Lebeche D, Takewa Y, Chen J, Park WJ, Kawase Y, Hajjar RJ. Preservation of mechanical and energetic function after adenoviral gene transfer in normal rat hearts. Clin Exp Pharmacol Physiol 34: 1300–1306, 2007 [DOI] [PubMed] [Google Scholar]
- 192. Sampson KJ, Henriquez CS. Simulation and prediction of functional block in the presence of structural and ionic heterogeneity. Am J Physiol Heart Circ Physiol 281: H2597–H2603, 2001 [DOI] [PubMed] [Google Scholar]
- 193. Schröder F, Handrock R, Beuckelmann DJ, Hirt S, Hullin R, Priebe L, Schwinger RH, Weil J, Herzig S. Increased availability and open probability of single L-type calcium channels from failing compared with nonfailing human ventricle. Circulation 98: 969–976, 1998 [DOI] [PubMed] [Google Scholar]
- 194. Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell 128: 547–560, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Shiferaw Y, Watanabe MA, Garfinkel A, Weiss JN, Karma A. Model of intracellular calcium cycling in ventricular myocytes. Biophys J 85: 3666–3686, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Shvilkin A, Danilo P, Wang J, Burkhoff D, Anyukhovsky EP, Sosunov EA, Hara M, Rosen MR. Evolution and resolution of long-term cardiac memory. Circulation 97: 1810–1817, 1998 [DOI] [PubMed] [Google Scholar]
- 197. Streeter DD., Jr Gross morphology and fiber geometry in the heart. In: Handbook of Physiology, The Cardiovascular System. The Heart. Bethesda, MD: Am. Physiol. Soc., 1979, sect. 2, vol. I, chapt. 4, p. 61–112 [Google Scholar]
- 198. Strogatz SH. Nonlinear Dynamics and Chaos: with Applications to Physics, Biology, Chemistry, and Engineering. Cambridge, MA: Da Capo Press, 1994 [Google Scholar]
- 199. Surovyatkina E, Noble D, Gavaghan D, Sher A. Multistability property in cardiac ionic models of mammalian and human ventricular cells. Prog Biophys Mol Biol 103: 131–141, 2010 [DOI] [PubMed] [Google Scholar]
- 200. Szigligeti P, Bányász T, Magyar J, Szigeti G, Papp Z, Varró A, Nánási PP. Intracellular calcium and electrical restitution in mammalian cardiac cells. Acta Physiol Scand 163: 139–147, 1998 [DOI] [PubMed] [Google Scholar]
- 201. Tabereaux PB, Walcott GP, Rogers JM, Kim J, Dosdall DJ, Robertson PG, Killingsworth CR, Smith WM, Ideker RE. Activation patterns of Purkinje fibers during long-duration ventricular fibrillation in an isolated canine heart model. Circulation 116: 1113–1119, 2007 [DOI] [PubMed] [Google Scholar]
- 202. Takahashi T, Allen PD, Lacro RV, Marks AR, Dennis AR, Schoen FJ, Grossman W, Marsh JD, Izumo S. Expression of dihydropyridine receptor (Ca2+ channel) and calsequestrin genes in the myocardium of patients with end-stage heart failure. J Clin Invest 90: 927–935, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Ten Tusscher KH, Noble D, Noble PJ, Panfilov AV. A model for human ventricular tissue. Am J Physiol Heart Circ Physiol 286: H1573–H1589, 2004 [DOI] [PubMed] [Google Scholar]
- 204. Terentyev D, Györke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, de Blanco EC, Khanna S, Sen CK, Cardounel AJ, Carnes CA, Györke S. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res 103: 1466–1472, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Thomas CE. The muscular architecture of the ventricles of hog and dog hearts. Am J Anat 101: 17–57, 1957 [DOI] [PubMed] [Google Scholar]
- 206. Tolkacheva EG, Schaeffer DG, Gauthier DJ, Krassowska W. Condition for alternans and stability of the 1:1 response pattern in a “memory” model of paced cardiac dynamics. Phys Rev E Stat Nonlin Soft Matter Phys 67: 031904, 2003 [DOI] [PubMed] [Google Scholar]
- 207. Tsuji Y, Opthof T, Kamiya K, Yasui K, Liu W, Lu Z, Kodama I. Pacing-induced heart failure causes a reduction of delayed rectifier potassium currents along with decreases in calcium and transient outward currents in rabbit ventricle. Cardiovasc Res 48: 300–309, 2000 [DOI] [PubMed] [Google Scholar]
- 208. Tsuji Y, Zicha S, Qi XY, Kodama I, Nattel S. Potassium channel subunit remodeling in rabbits exposed to long-term bradycardia or tachycardia: discrete arrhythmogenic consequences related to differential delayed-rectifier changes. Circulation 113: 345–355, 2006 [DOI] [PubMed] [Google Scholar]
- 209. Undrovinas AI, Maltsev VA, Sabbah HN. Repolarization abnormalities in cardiomyocytes of dogs with chronic heart failure: role of sustained inward current. Cell Mol Life Sci 55: 494–505, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Ursell PC, Gardner PI, Albala A, Fenoglio JJ, Jr, Wit AL. Structural and electrophysiological changes in the epicardial border zone of canine myocardial infarcts during infarct healing. Circ Res 56: 436–451, 1985 [DOI] [PubMed] [Google Scholar]
- 211. Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, Kamp TJ, Makielski JC. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol 38: 475–483, 2005 [DOI] [PubMed] [Google Scholar]
- 212. Vermeulen JT, McGuire MA, Opthof T, Coronel R, de Bakker JM, Klöpping C, Janse MJ. Triggered activity and automaticity in ventricular trabeculae of failing human and rabbit hearts. Cardiovasc Res 28: 1547–1554, 1994 [DOI] [PubMed] [Google Scholar]
- 213. Voss F, Opthof T, Marker J, Bauer A, Katus HA, Becker R. There is no transmural heterogeneity in an index of action potential duration in the canine left ventricle. Heart Rhythm 6: 1028–1034, 2009 [DOI] [PubMed] [Google Scholar]
- 214. Wagner S, Dybkova N, Rasenack ECL, Jacobshagen C, Fabritz L, Kirchhof P, Maier SKG, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest 116: 3127–3138, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Waldo AL, Camm AJ, deRuyter H, Friedman PL, MacNeil DJ, Pauls JF, Pitt B, Pratt CM, Schwartz PJ, Veltri EP. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol. Lancet 348: 7–12, 1996 [DOI] [PubMed] [Google Scholar]
- 216. Watanabe M, Otani NF, Gilmour RF., Jr Biphasic restitution of action potential duration and complex dynamics in ventricular myocardium. Circ Res 76: 915–921, 1995 [DOI] [PubMed] [Google Scholar]
- 217. Watanabe MA, Fenton FH, Evans SJ, Hastings HM, Karma A. Mechanisms for discordant alternans. J Cardiovasc Electrophysiol 12: 196–206, 2001 [DOI] [PubMed] [Google Scholar]
- 218. Watanabe MA, Koller ML. Mathematical analysis of dynamics of cardiac memory and accommodation: theory and experiment. Am J Physiol Heart Circ Physiol 282: H1534–H1547, 2002 [DOI] [PubMed] [Google Scholar]
- 219. Weiss JN, Chen PS, Qu Z, Karagueuzian HS, Lin SF, Garfinkel A. Electrical restitution and cardiac fibrillation. J Cardiovasc Electrophysiol 13: 292–295, 2002 [DOI] [PubMed] [Google Scholar]
- 220. Weiss JN, Nivala M, Garfinkel A, Qu Z. Alternans and arrhythmias: from cell to heart. Circ Res 108: 98–112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Williams BA, Dickenson DR, Beatch GN. Kinetics of rate-dependent shortening of action potential duration in guinea-pig ventricle; effects of IK1 and IKr blockade. Br J Pharmacol 126: 1426–1436, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Winfree AT. Evolving perspectives during 12 years of electrical turbulence. Chaos 8: 1, 1998 [DOI] [PubMed] [Google Scholar]
- 223. Wit AL, Rosen MR. Afterdepolarizations and triggered activity: distinction from automaticity as an arrhythmogenic mechanism. In: The Heart and Cardiovascular System, edited by Fozzard HA, Haber E, Jennings RB, Katz AM. New York, NY: Raven Press, 1986, p. 1449–1490 [Google Scholar]
- 224. Witkowski FX, Leon LJ, Penkoske PA, Giles WR, Spano ML, Ditto WL, Winfree AT. Spatiotemporal evolution of ventricular fibrillation. Nature 392: 78–82, 1998 [DOI] [PubMed] [Google Scholar]
- 225. Wu R, Patwardhan A. Restitution of action potential duration during sequential changes in diastolic intervals shows multimodal behavior. Circ Res 94: 634–641, 2004 [DOI] [PubMed] [Google Scholar]
- 226. Wu TJ, Lin SF, Weiss JN, Ting CT, Chen PS. Two types of ventricular fibrillation in isolated rabbit hearts: importance of excitability and action potential duration restitution. Circulation 106: 1859–1866, 2002 [DOI] [PubMed] [Google Scholar]
- 227. Xiao B, Jiang MT, Zhao M, Yang D, Sutherland C, Lai FA, Walsh MP, Warltier DC, Cheng H, Chen SRW. Characterization of a novel PKA phosphorylation site, serine-2030, reveals no PKA hyperphosphorylation of the cardiac ryanodine receptor in canine heart failure. Circ Res 96: 847–855, 2005 [DOI] [PubMed] [Google Scholar]
- 228. Xiao L, Coutu P, Villeneuve LR, Tadevosyan A, Maguy A, Le Bouter S, Allen BG, Nattel S. Mechanisms underlying rate-dependent remodeling of transient outward potassium current in canine ventricular myocytes. Circ Res 103: 733–742, 2008 [DOI] [PubMed] [Google Scholar]
- 229. Yan GX, Rials SJ, Wu Y, Liu T, Xu X, Marinchak RA, Kowey PR. Ventricular hypertrophy amplifies transmural repolarization dispersion and induces early afterdepolarization. Am J Physiol Heart Circ Physiol 281: H1968–H1975, 2001 [DOI] [PubMed] [Google Scholar]
- 230. Yan GX, Shimizu W, Antzelevitch C. Characteristics and distribution of M cells in arterially perfused canine left ventricular wedge preparations. Circulation 98: 1921–1927, 1998 [DOI] [PubMed] [Google Scholar]
- 231. Yang Y, Chen X, Margulies K, Jeevanandam V, Pollack P, Bailey BA, Houser SR. L-type Ca2+ channel alpha 1c subunit isoform switching in failing human ventricular myocardium. J Mol Cell Cardiol 32: 973–984, 2000 [DOI] [PubMed] [Google Scholar]
- 232. Yao JA, Hussain W, Patel P, Peters NS, Boyden PA, Wit AL. Remodeling of gap junctional channel function in epicardial border zone of healing canine infarcts. Circ Res 92: 437–443, 2003 [DOI] [PubMed] [Google Scholar]
- 233. Yoshioka J, Prince RN, Huang H, Perkins SB, Cruz FU, MacGillivray C, Lauffenburger DA, Lee RT. Cardiomyocyte hypertrophy and degradation of connexin43 through spatially restricted autocrine/paracrine heparin-binding EGF. Proc Natl Acad Sci USA 102: 10622–10627, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Zaritsky JJ, Eckman DM, Wellman GC, Nelson MT, Schwarz TL. Targeted disruption of Kir2.1 and Kir22 genes reveals the essential role of the inwardly rectifying K+ current in K+-mediated vasodilation. Circ Res 87: 160–166, 2000 [DOI] [PubMed] [Google Scholar]
- 235. Zicha S, Xiao L, Stafford S, Cha TJ, Han W, Varro A, Nattel S. Transmural expression of transient outward potassium current subunits in normal and failing canine and human hearts. J Physiol 561: 735–748, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]