CASE PRESENTATION
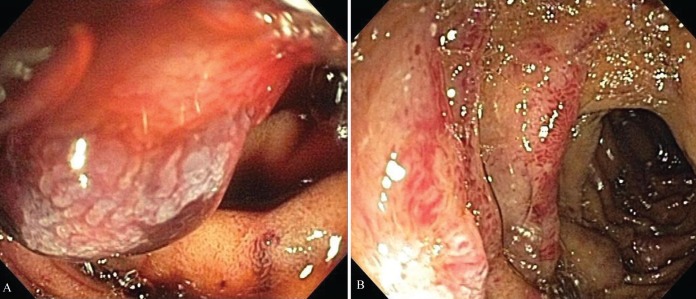
An 81-year-old Jamaican woman presented with melena and severe anemia, and a hemoglobin level of 47 g/L (normal range 115 g/L to 160 g/L). A gastroscopy and colonoscopy were negative for any lesions. Duodenal biopsies were normal. A subsequent computed tomography scan of the abdomen with contrast showed diffuse thickening from the jejunum to the distal small bowel and a small area of active bleeding in the mid small bowel. A technetium-99m red blood cell scan demonstrated active bleeding in the proximal jejunum. Push enteroscopy (120 cm to 150 cm below incisor) revealed mild diffuse friability starting from the proximal jejunum, as well as erosions, nodularities, polypoid protrusions and valvulae conniventes thickening in the remaining jejunum (Figures 1A and 1B). Jejunal biopsies showed extensive amyloid deposition in the lamina propria and submucosa, with Congo red stain demonstrating apple-green birefringence under polarized light (Figure 2), and hematoxylin counterstain revealing amorphous, salmon-pink, sparsely cellular hyaline material (Figure 3). Serum protein electrophoresis, immunofixation and a serum-free light chain assay confirmed the presence of monoclonal immunoglobulin G lambda paraprotein. The patient was transfused with packed red blood cells as needed (average one unit per day), and treated with bortezomib and dexamethasone for primary amyloidosis. By day 17 of hospitalization, the bleeding had resolved and the patient was subsequently discharged without any further bleeding episodes.
Figure 1).
A and B Endoscopic views of amyloidosis showing a markedly abnormal jejunum with edema, erythema, friability, polypoid protrusions and valvulae conniventes thickening
Figure 2).
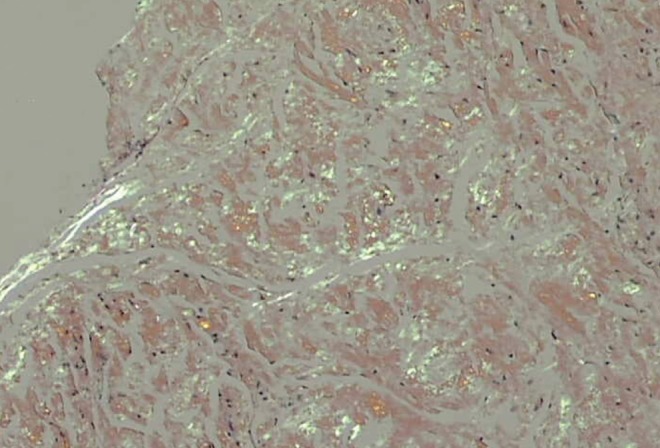
Jejunal biopsy demonstrating apple-green birefringence of amyloid deposits under polarized light (Congo red stain, original magnification ×100)
Figure 3).
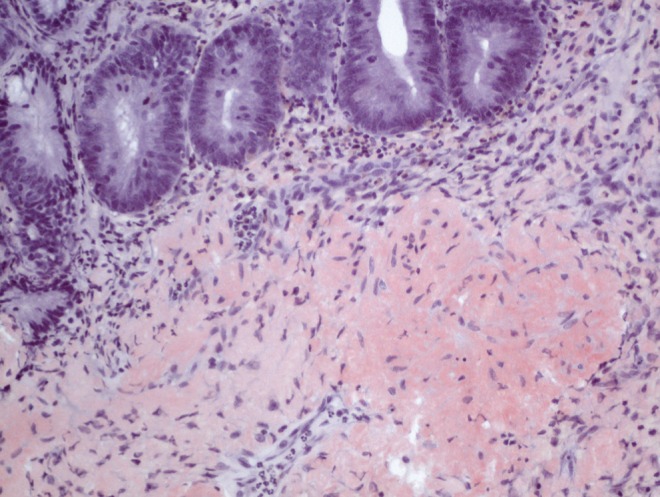
Jejunal specimen revealing extensive amyloid deposition of amorphous, salmon-pink, sparsely cellular, hyaline material within the submucosa and focally within the lamina propria (hematoxylin counterstain, original magnification ×200)
DISCUSSION
Amyloidosis is a multisystem disease characterized by extracellular deposition of abnormal protein fibrils in various tissues and organs. The protein fibrils are derived from monoclonal immunoglobulin light chains in primary amyloidosis (AL), acute phase reactant serum amyloid A in secondary amyloidosis (AA), and beta2-microglobulin in dialysis-related amyloidosis (Aβ2m). Clinical manifestations in amyloidosis are variable, but the heart and kidneys are most commonly involved. Gastrointestinal symptoms due to bleeding, dysmotility and malabsorption are present in up to 60% of AA (1), but in only 1% of AL (2). The diagnosis of amyloidosis can only be confirmed by tissue biopsy because endoscopic and radiologic findings are nonspecific. Therapy is directed at the underlying cause in AL (plasma cell dyscrasia) and AA (infection, inflammation and malignancy), whereas treatment in Aβ2m involves alteration of dialysis mode or consideration of renal transplantation (3).
Severe gastrointestinal bleeding rarely occurs as the sole or initial manifestation of systemic amyloidosis (4). Mechanisms by which hemorrhage occurs in gastrointestinal amyloidosis have been postulated, including intestinal ischemia, necrotic ulceration, mucosal tearing, vascular fragility, abnormal platelet aggregation, and factor IX and X deficiencies (5,6). In addition to supportive care for bleeding, treatment must be tailored to the specific type of amyloidosis, as determined by endoscopy and histology. Endoscopy classically shows polypoid protrusions and valvulae conniventes thickening in AL; a coarse mucosal pattern with a fine granular appearance in AA; and intestinal dilation in Aβ2m (7). Histology typically reveals amyloid deposition in muscularis mucosae, submucosa and muscularis propria in AL; infiltration of lamina propria and myenteric plexus in AA; and involvement of muscularis propria in Aβ2m (7,8). Knowledge of the characteristic endoscopic appearance and histological findings of gastrointestinal amyloidosis is essential in making a timely and accurate diagnosis.
The Canadian Journal of Gastroenterology is now considering a limited number of submissions for IMAGE OF THE MONTH. These are based on endoscopic, histological, radiological and/or patient images, which must be anonymous with no identifying features visible. The patient must consent to publication and the consent must be submitted with the manuscript. All manuscripts should be practical and relevant to clinical practice, and not simply a case report of an esoteric condition. The text should be brief, structured as CASE PRESENTATION and DISCUSSION, and not more than 700 words in length. A maximum of three images can be submitted and the number of references should not exceed five. The submission may be edited by our editorial team.
REFERENCES
- 1.Okuda Y, Takasugi K, Oyama T, Onuma M, Oyama H. Amyloidosis in rheumatoid arthritis – clinical study of 124 histologically proven cases. Ryumachi. 1994;34:939. [PubMed] [Google Scholar]
- 2.Menke DM, Kyle RA, Fleming CR, Wolfe JT, 3rd, Kurtin PJ, Oldenburg WA. Symptomatic gastric amyloidosis in patients with primary systemic amyloidosis. Mayo Clin Proc. 1993;68:763. doi: 10.1016/s0025-6196(12)60634-x. [DOI] [PubMed] [Google Scholar]
- 3.Gorevic PD, Schur PH, Romain PL. An overview of amyloidosis. In: Basow DS, editor. UpToDate. Waltham: 2011. UpToDate. [Google Scholar]
- 4.Jarnum S. Gastrointestinal hemorrhage and protein loss in primary amyloidosis. Gut. 1965;6:14–8. doi: 10.1136/gut.6.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang HS, Myung SJ, Yang SK, et al. Massive small bowel bleeding in a patient with amyloidosis. Gastrointest Endosc. 2004;59:126–9. doi: 10.1016/s0016-5107(03)02352-6. [DOI] [PubMed] [Google Scholar]
- 6.Kaiserling E, Kröber S. Massive intestinal hemorrhage associated with intestinal amyloidosis. An investigation of underlying pathologic processes. Gen Diagn Pathol. 1995;141:147–54. [PubMed] [Google Scholar]
- 7.Tada S, Iida M, Yao T, et al. Endoscopic features in amyloidosis of the small intestine: Clinical and morphologic differences between chemical types of amyloid protein. Gastrointest Endosc. 1994;40:45–50. doi: 10.1016/s0016-5107(94)70008-7. [DOI] [PubMed] [Google Scholar]
- 8.Tada S, Iida M, Yao T, Kitamoto T, Yao T, Fujishima M. Intestinal pseudo-obstruction in patients with amyloidosis: Clinicopathologic differences between chemical types of amyloid protein. Gut. 1993;34:1412–7. doi: 10.1136/gut.34.10.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]