Abstract
Pulmonary vessel constriction results from an imbalance between vasodilator and vasoconstrictor factors released by the endothelium including nitric oxide, endothelin, prostanoids, and reactive oxygen species (ROS). ROS, generated by a variety of enzymatic sources (such as mitochondria and NADPH oxidases, a.k.a. Nox), appear to play a pivotal role in vascular homeostasis, whereas elevated levels effect vascular disease. The pulmonary circulation is very sensitive to changes in the partial pressure of oxygen and differs from the systemic circulation in its response to this change. In fact, the pulmonary vessels contract in response to low oxygen tension, whereas systemic vessels dilate. Growing evidence suggests that ROS production and ROS-related pathways may be key factors that underlie this differential response to oxygen tension. A major emphasis of our laboratory is the role of Nox isozymes in cardiovascular disease. In this review, we will focus our attention on the role of Nox-derived ROS in the control of pulmonary vascular tone.
Keywords: reactive oxygen species, vascular tone
this article is part of a collection on Hypertension and Novel Modulators of Vascular Tone. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Introduction
Since their discovery, reactive oxygen species (ROS) have aroused increasing interest because of evidence that they regulate important physiological and pathophysiological conditions. Under physiological conditions, the redox environment of vascular tissue is maintained in an overall reduced state via an interplay and balance between oxidants (reactive oxygen and nitrogen species) and antioxidant systems. Major ROS produced in the pulmonary vasculature are, among others, superoxide (O2·−), hydrogen peroxide (H2O2), hydroxyl radical (HO·), and hydroperoxyl radical (HO2·), and the most important reactive nitrogen species emerge as nitric oxide (NO), nitrogen dioxide (NO2), dinitrogen trioxide (N2O3), and peroxynitrite (Fig. 1). In this review, we decided to focus our attention on the ROS effects on pulmonary vascular tone. For a complete overview about reactive nitrogen species and their pathophysiological role, we refer the reader to an excellent review on the topic (167). Endogenous antioxidant systems include enzymes such as superoxide dismutase [SOD, which dismutes superoxide anion (O2·−) into H2O2], catalase (which converts H2O2 to water), peroxiredoxins (which catabolize H2O2 to water), glutathione peroxidase, peroxiredoxin, heme oxygenase, glutaredoxin and thioredoxin (which reduce the disulfide bridges of various target proteins), and nonenzymatic agents such as glutathione, β-carotene, retinol, vitamin C, and vitamin E (109). When endogenous antioxidant systems are inadequate or overwhelmed by ROS generation, increased ROS steady-state levels initiate multiple pathologies including diabetes (53, 74, 146), hypertension (40, 96, 125, 169), atherosclerosis (44, 76, 159), and hypoxia/reperfusion injury (89, 95, 190). In the lungs and cardiovascular system, principal sources of ROS include mitochondrial electron transport, xanthine oxidase, cytochrome P-450, cyclooxygenases, uncoupled NO synthase, lipoxygenases, and the NADPH oxidase (Nox) family of proteins (5). It is known that these different sources of ROS modulate each other through mechanisms of negative feedback and feed-forward processes. Dysregulation in these processes contribute to the development of diseases. For example, ROS produced from Nox can stimulate ROS production by the other sources, and vice versa, increased levels of mitochondria-derived ROS lead to Nox activation. In this review, we will focus our attention in analyzing the effect of the Nox-derived ROS. In the pulmonary system, Nox-derived ROS production is involved in diseases such as pulmonary hypertension (57, 103, 120), fibrosis (4, 41, 110), chronic granulomatous disease (144, 154), acute lung injury and acute respiratory distress syndrome (81, 175, 178), emphysema and asthma (1, 88, 126, 141, 156, 165, 174), chronic obstructive pulmonary disease (45, 129, 130), and lung cancer (72, 107) (Table 1). On the other hand, in the pulmonary vasculature, Nox-derived ROS contribute to the maintenance of vascular tone and regulate important processes such as cytoskeletal organization, cell migration, cell growth, proliferation, differentiation, and apoptosis (71). These seemingly contradictory phenotypes can be explained by the current theory that gradation and spatial orientation of ROS levels elicit distinct outcomes; that is, when levels of ROS exceed basal, distinct physiological and pathophysiological outcomes ensue, depending on the degree and delivery of ROS to effector pathways.
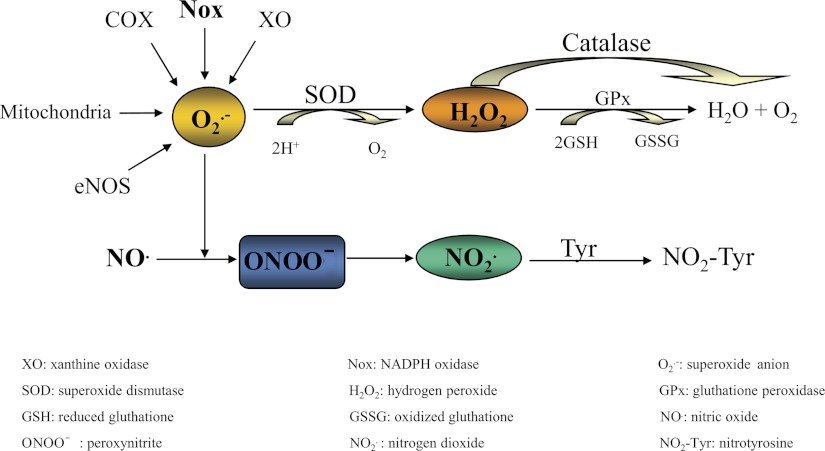
Fig. 1.
Simplified schematic illustration of common forms of reactive oxygen and nitrogen species. O2·− derived from different sources such as XO, Nox enzymes, mithocondria, uncoupled endothelial NO synthase (eNOS) and cyclooxygenase (COX) is dismuted to H2O2 by SOD. H2O2 can be converted to H2O by catalase or GPx. O2·− can also react with NO to generate the reactive nitrogen species ONOO− that could be converted into NO2·, which reacts with protein tyrosine residues to generate NO2-Tyr. This reaction can lead to a decrease in the bioavailability of NO, leading to endothelial dysfunction.
Table 1.
Involvement of Nox isoforms in lung disease
| Nox Isoform | Localization | Pulmonary Disease Associated | References |
|---|---|---|---|
| Nox2, Nox4 | Pulmonary artery endothelial cells | PAH, ALI/ARDS | (51, 56, 70, 90, 105, 156) |
| Nox4 | Pulmonary artery smooth muscle cells | PAH | (51, 105) |
| Nox4 | Myofibroblasts | Pulmonary fibrosis | (3, 36, 95) |
| Nox2 | Macrophages/neutrophils | Chronic granulomatous disease | (125, 135) |
| Nox3 | Endothelial cells of airways | Emphysema | (1, 122, 143) |
| DUOX1 and -2 | Airway epithelial cells | COPD and asthma | (66, 166) |
| DUOX1 and -2 | Lung carcinoma cells | Lung cancer | (93) |
Multiple studies demonstrate the involvement of various NADPH oxidase (Nox) isoforms in the development and progression of different pulmonary diseases, such as pulmonary arterial hypertension (PAH), acute lung injury (ALI), pulmonary fibrosis, chronic granulomatous disease, emphysema, obstructive lung disorders [chronic obstructive pulmonary disease (COPD)], asthma, and lung cancer. ARDS, acute respiratory distress syndrome; DUOX, dual oxidase.
Nox Enzymes
The structure and function of Nox was first described in neutrophils. The phagocytic Nox is a multisubunit complex comprised of the flavocytochrome b558, which is composed of two subunits, p22phox and Nox2 (previously known as gp91phox), as well as four cytoplasmic subunits, namely, p47phox (organizer subunit), p67phox (activator subunit), p40phox,, and the small G protein, Rac. In resting phagocytes, this multienzyme complex is unassembled and inactive. Activation involves the phosphorylation and translocation of the cytoplasmic subunits of Nox to the membrane-integrated catalytic subunits, leading to the production of O2·−. Phosphorylation and activation of essential organizing subunit p47phox initiates this process. To date, seven isoforms of the catalytic Nox subunit have been identified in humans and classified into three groups based on their basic structure (20) (Fig. 2). The first group is comprised of Nox1, Nox3, and Nox4, which share similar structural organization with Nox2. The second group includes dual oxidases 1 and 2 (DUOX1 and DUOX2), which possess an extracellular peroxidase-like domain and intracellular EF hand-type Ca2+-binding pockets involved in exquisite activation of these oxidases. The main product detected by DUOX1 and -2 is H2O2, but it remains controversial whether these enzymes produce O2·−, which rapidly dismutes to H2O2 or whether H2O2 is the main product. Third, Nox5 possesses an extra amino-terminal calmodulin-like domain that contains four calcium binding EF hands, in addition to the Nox2-like catalytic core. To date, four splice variants of Nox5 (Nox5α, Nox5β, Nox5γ, and Nox5δ) and a truncated variant (Nox5ε) have been identified in humans (16, 35). The different isoforms of the Nox family vary in their tissue expression, subcellular distribution, and mechanisms of activation and appear to be involved in distinct physiological functions in most cases (19).
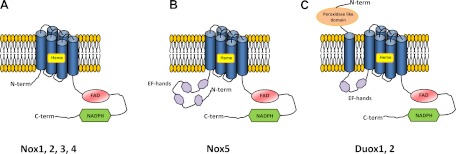
Fig. 2.
Schematic structures of distinct identifying domains in Nox isoform catalytic subunits. A: Nox2 and Nox2-like isoforms (Nox1, 3, and 4) present a basic structure of 6 transmembrane domains containing two heme groups and COOH-terminal FAD and NADPH domains. B: in addition to those domains, Nox5 possesses Ca2+-binding EF motifs in the NH2-terminal region. C: dual oxidasess, in addition to Ca2+-binding EF motifs, possess an NH2-terminal peroxidase-like domain.
Nox1 is highly expressed in the colon epithelium, and to date two splicing isoforms have been described as NOH-1S and NOH-1L (15), also named Nox1-L and Nox1-S (78). Nox1 requires the presence of two cytosolic subunits: the p47phox homolog, Nox organizer subunit 1 (NOXO1) and the p67phox homolog, Nox activator subunit 1 (NOXA1). Four isoforms of NOXO1 have been described in humans (NOXO1α, NOXO1β, NOXO1γ, NOXO1δ) (36, 172). Despite the structural similarities among regulatory subunits of Nox2 and Nox1, different functional aspects are involved in the regulation of enzyme activity. For example, NOXO1 is localized to caveolae with Nox1 and p22phox, whereas p47phox localizes in the cytosol of resting cells. A hallmark characteristic of the Nox1 system is the lack of an autoinhibitory domain in NOXO1 (compared with p47phox in the Nox2 system), which is invoked to explain the unique preassembled state of Nox1.
Nox3 is principally expressed in the inner ear and less notably in fetal brain and kidney tissues (14) and seems to generate low levels of O2·− in a cytosolic subunit-independent manner and high levels of O2·− in the presence of activator and organizer subunits (p47phox and p67phox, NOXO1 and NOXA1) (14).
In contrast to other Nox isoforms, Nox4, which is highly expressed in the kidney, endothelial cells, and pulmonary artery smooth muscle cells (PASMCs), does not require cytosolic subunits p47phox and p67phox or their homologues for activation and thus appears to be constitutively active or regulated by heretofore undefined cytosolic subunits. Intriguingly, Lyle and colleagues (108) showed that the polymerase δ-interacting protein-2 associates with p22phox to activate Nox4.
Nox5 is highly abundant in the spleen and testis (16) and is less abundant in vascular tissue, cells of the gastrointestinal tract, reproductive system, and fetal organs (16, 35, 97). The regulation of Nox5 activity appears not to require the presence of cytosolic subunits that are recognized as necessary for other Nox isoforms (16). Nox5 has also been shown to bind p22phox, but the significance of this is unclear because changes in the expression level of p22phox, or dominant negative constructs of p22phox, do not affect Nox5 activity (21, 91). The elevation of intracellular calcium promotes the occupation of the calcium-binding EF-hand domains of Nox5. This leads to conformational changes among the domains that trigger O2·− production (16).
DUOX1 and DUOX2 were originally identified and cloned from the epithelium of the thyroid gland (31, 43, 49). DUOXs are widely expressed in epithelial tissues, including epithelia of the airways (61, 66, 155), alveoli, intestinal tract (52, 65), salivary glands (65), and prostate (177). When DUOX2 is partially glycosylated, it is located to the endoplasmic reticulum (ER) and generates O2·−, whereas when it is fully glycosylated, it is transported to the plasma membrane and generates H2O2, (7, 69, 196). DUOX proteins require the presence of two maturation factors, DUOX activator 1 and 2 (DUOXA1 and DUOXA2), which allow proper DUOX processing and trafficking to the membrane (69). Only the combination of the DUOXs with their corresponding DUOXAs renders the complex active and allows exit from the ER (68). In experiments with transfection of DUOX2 into PCCl3 cells and Cos7 cells, an enhanced generation of H2O2 in response to Ca2+ has been shown (42, 152). Moreover, site-directed mutation of the predicted Ca2+-binding glutamate residues to glutamine in the EF hands of DUOX1 (E839Q and E875Q) or DUOX2 (E843Q and E879Q) resulted in an almost complete loss of function (152). In addition, NOXA1 was recently shown to interact with and inhibit DUOX1 in a Ca2+-dependent fashion, possibly involving the COOH-terminal region of DUOX1 (133). The levels of expression of DUOX1 and DUOX2 are selectively upregulated by inflammatory cytokines (IL-4 and IL-13) and interferon-γ, respectively (77).
Expression of Nox Isoforms in the Lung
In total lung tissue, mRNA for Nox1, Nox2, Nox4, DUOX1, DUOX2, p22phox as well as the cytosolic subunits NOXA1, NOXO1, p47phox, p40phox, and p67phox are expressed (51, 65, 120) (Table 2). In this section, we will discuss Nox isoform expression across the different sections of the lung tissue and in the different layers of the pulmonary vasculature.
Table 2.
Expression and function of Nox isoforms in lung tissue
| Nox Isoform | Distribution | Function | References |
|---|---|---|---|
| DUOX1 and -2 | Epithelial airway | Host defense, mucin production, cellular migration and differentiation | (60, 139, 176) |
| Nox2, Nox4 | Pulmonary endothelial cells | Control of vascular tone, vascular cell growth and remodeling, angiogenesis, proinflammatory cytokine production | (24, 104, 118) |
| Nox4 | Pulmonary smooth muscle cells | Differentiation and hypoxia-induced proliferation | (32,144) |
| Nox4 | Myofibroblasts | Differentiation | (42) |
Distinct isoforms of Nox (especially Nox2, Nox4, DUOX1, and DUOX2) are expressed in lung and participate in multiple physiological and pathological processes such as control of vascular tone, cell growth, and differentiation.
Airway epithelium and alveolar cells.
The primary sources of ROS production in airway epithelium are DUOX1 (66, 77, 98, 155) and DUOX2 (61, 77, 155). However, the expression of other Nox isoforms, Nox1, Nox2, and their regulatory proteins, has been reported (98). mRNA levels of DUOX1 and DUOX2 are regulated by cytokines. Reportedly, IL-4 and IL-13 (produced by Th2) induce a moderate increase of DUOX1 mRNA, whereas interferon-γ (from Th1) markedly increases mRNA for DUOX2, perhaps suggesting a constitutive role of DUOX1 in noninflamed airways and an inducible role of DUOX2 in infection and inflammation (77). Other Nox enzyme functions in airway epithelia include host defense (65), response to mechanical stress (34), regulation of matrix metalloproteinase-12 (MMP-12), MMP gene expression (98, 135), production of epithelial mucins (158), induction of cell death (199), regulation of lung development, epithelial cell differentiation (59, 77), and epithelial integrity (93, 188).
In type I pneumocytes, Nox2, p22phox, p67phox, p47phox, and p40phox subunits, as well as Rac1, are present at substantially higher levels than in type II cells (168) in which ROS production is reportedly derived primarily from mitochondria (143). In type II cells DUOX1 is expressed (there is no significant expression of DUOX2), but the functions of this Nox isoform in alveolar cells remains unknown (58).
Pulmonary vasculature.
In the pulmonary vasculature, Nox isozymes are activated by a wide range of stimuli including G protein-coupled receptor agonists, angiotensin II, thrombin, endothelin, serotonin, thromboxane A2 (38, 105), cytokines [tumor necrosis factor-α (TNF-α) and transforming growth factor-β (TGF-β)] (166), mechanical forces, and shear stress. Proposed functions of Nox-derived ROS include NF-κβ activation (25), MAPK activation (136), cell proliferation (119), and potassium channel regulation in response to changes in the oxygen concentration (9, 186).
Pulmonary endothelium.
Generally speaking, endothelial cells express Nox1, Nox2, and Nox4 (and Nox5 in human cells) and their cofactors (70, 173). In human pulmonary artery endothelial cells and human lung microvascular endothelial cells, mRNA levels for Nox4 are much higher compared with mRNA for Nox2 (27, 139). Interestingly, in these cells, Nox4 silencing induces Nox2 mRNA upregulation, and in Nox2−/− mice mRNA Nox4 upregulation was described, suggesting that a compensatory regulation induced by their respective deficiency exists for Nox2 and Nox4 mRNA expression (138). Nox endothelial activity is increased in response to pulmonary ischemia (6) and hyperoxia (136), is associated with activation of ATP-sensitive K+ channels, and results in stimulated endothelial cell proliferation and NO production (119, 198). The roles of endothelial Noxs include control of vascular tone, vascular cell growth, angiogenesis, and inflammation. Studies suggest a role for both H2O2 (119) and O2·− (79) in response to general pulmonary endothelial injury.
PASMCs.
In PASMCs, both Nox1 and Nox4 are expressed, whereas Nox4 seems to be the most abundant isoform (24, 120, 139). Nox4 expression in PASMCs can be induced by several stimuli including hypoxia/ischemia, shear stress, and ER stress (19, 97, 120). Nox4 seems to be required for the expression of smooth muscle differentiation markers and maintenance of smooth muscle actin-based stress fibers as suggested by the correlation between the decrease of Nox4 expression and the loss of smooth muscle differentiation markers, such as smooth α-actin and myosin heavy chain, in multiple passages of freshly isolated PASMCs (37, 166). Both Nox1 and Nox4 induce proliferation of PASMCs (120). In particular, it has been shown, that Nox4 is involved in this process through induction by TGF-β1 (166).
Fibroblasts.
In lung fibroblasts, p47phox, p67phox, p22phox, Nox1, and Nox4 are expressed but not Nox2 (47), and Nox4 expression dominates Nox1. Oxidase-derived H2O2 generation is observed in response to TGF-β (170), irradiation with α-particles (127), rhinovirus infection (47), and hypoxia (99). Nox4 is upregulated by hypoxia, inflammatory mediators, and fibrotic stimuli, such as TNF-α and TGF-β, contributing to fibroblast activation and transdifferentiation. The consequences of Nox4-induced ROS by TGF-β in pulmonary fibroblasts include IL-8 upregulation (47) and induction of epithelial cell death through a paracrine mechanism (176). The TGF-β-induced Nox4-derived ROS production in human fibroblasts seems to be extracellular, in contrast to what is observed in human PASMCs in which the activation of Nox4 by TGF-β leads to intracellular ROS production (166, 176). Experiments using siRNA-mediated knockdown of Nox4 demonstrated that Nox4 contributed to the increase in ROS generation under hypoxic conditions, stimulated proliferation, and inhibited apoptosis in pulmonary fibroblasts (99).
ROS-Induced Regulation of Pulmonary Vascular Tone
Nox-derived ROS (both O2·− and H2O2) play an important role as mediators and signaling molecules capable of activating multiple pathways involved in the control of pulmonary vascular tone, cell proliferation and apoptosis, inflammation, and fibrosis. One of the potential mechanisms by which ROS are able to regulate pulmonary vessel tone is via the control of the cellular redox potential (87). The pulmonary vascular responses to exogenously added ROS are heterogeneous because O2·− or H2O2 can lead to contractile or relaxant responses. This heterogeneity might be explained by the nature (H2O2 or O2·−) and dose of ROS applied on the existence of a previous contractile tone and on the nature of the preconstrictor used.
O2·− induces constriction of pulmonary artery, triggering Rho-kinase-mediated Ca2+ sensitization (92), and is involved in hypoxia-induced vasoconstriction, as suggested by the fact that inhibitors of SOD, such as diethyldithiocarbamate or triethylenetetramine, produce larger potentiation of the hypoxic contraction (2, 101) and that EC-SOD overexpression in the lung ameliorates monocrotaline-induced pulmonary hypertension (PH) in rats (90).
More controversial is the effect of H2O2 on the regulation of pulmonary vascular tone. Studies provide evidence that H2O2 constricts both isolated perfused rat lung (28) and isolated, perfused rabbit lung (157), and also isolated rat and rabbit pulmonary arteries (86, 132, 137, 145, 160). These findings are substantiated by our finding in systemic vessels (11, 12). The possible mechanisms involved in H2O2-induced vasoconstriction are stimulation of serine esterase with subsequent activation of phospholipase A2 and production of arachidonic acid products (33, 87, 160), activation of PKC, and activation of tyrosine kinases in smooth muscle and endothelium (32, 128, 195, 197, 200). H2O2 is also responsible for the elevation of intracellular calcium concentration ([Ca2+]i) in PASMC during hypoxia (150, 183), and multiple similarities have been drawn between H2O2-induced vasoconstriction and hypoxic pulmonary vasoconstriction. In contrast, it has been shown that H2O2 produces concentration-dependent relaxation of precontracted isolated bovine arteries by a mechanism independent of the endothelium or prostaglandin mediators but related to soluble guanylate cyclase and cGMP (30). A similar H2O2-induced vasodilatation is described in isolated perfused rabbit lungs (29). Finally, in arteries stimulated with PGF2α or phenylephrine, a biphasic response is observed (64, 106, 113). Taken together, the variable effects of H2O2 could be tone dependent and related to the dose of H2O2 applied; it seems that low doses of H2O2 are involved in physiological vasodilatory control of pulmonary tone, whereas high or supraphysiological doses of H2O2 are implicated in pathophysiological response leading to vasoconstriction and pulmonary hypertension (87).
In addition, it has been shown that ROS controls vascular tone via the release of or interaction with other vasoactive factors. For example, ROS interacts with cyclooxygenase (COX) (140). The relationship between ROS and COX is complex because COX is a potential source of O2·− in the pulmonary circulation (140), and it has been shown that thromboxane A2 stimulates Nox-derived ROS (38); conversely, ROS can stimulate COX activity. Another way for ROS to control vascular tone is its reaction with NO (the most powerful vasodilator in endothelial cells). O2·− can react with NO in a diffusion-limited manner to form peroxynitrite, which could also be converted into nitrogen dioxide and nitrotyrosine. These reactions cause a decrease in NO bioavailability that leads to impaired vascular relaxation, upregulation of proinflammatory and prothrombotic molecules and endothelial dysfunction in pulmonary arteries.
Hypoxic Pulmonary Vasoconstriction
The physiological response to short-term hypoxia is a reversible contraction of the PASMC, a compensatory physiological response that serves to redirect blood to better ventilated areas of the lung and preserve proper ventilation of the systemic circulation (54). The response of the pulmonary circulation to a decrease in partial pressure of oxygen is discordant with the response of the systemic circulation in which arterial smooth muscle cells (SMCs) relax in response to hypoxia maintaining a fairly constant blood flow to important organs and tissues. This suggests that oxygen-sensing mechanisms in vascular smooth muscle in each circulatory system are different. Despite many recent studies, the mechanism involved in hypoxic pulmonary vasoconstriction (HPV) is, to date, not fully understood. The redox theory of HPV involves a redox-based O2 sensor regulating the activity of effector proteins, and there is strong evidence indicating a role of ROS as signaling intermediates in this response (8, 118). The redox theory, initially proposed by Weir and colleagues (118), describe a hypoxia-induced decrease in ROS production leading to potassium channel inhibition, membrane depolarization, and cell contraction. Hypoxia has also been reported to decrease intracellular concentration of ROS ([ROS]i) in isolated rat lungs and PASMCs (9, 116, 117), as well as in microsome-enriched fractions of calf pulmonary arteries (121, 124). Michelakis et al. (116) has shown that hypoxia decreases H2O2 in rat PASMCs but not in renal (systemic) artery SMCs. In addition, hypoxia has been found to decrease H2O2 levels in both cultured human pulmonary and coronary (systemic) artery SMCs (114). Conversely, Waypa et al. (182) showed that hypoxia produces an increase in ROS production in cultured rat pulmonary and renal artery SMCs, and other studies (149, 150) indicate that hypoxia causes a large increase in ROS and Nox activity in freshly isolated mouse PASMCs but not in mesenteric SMCs. Although still a matter of debate, the concept that a paradoxical increase in ROS arises during hypoxia is gaining traction (179). Many studies have revealed that hypoxia increases intracellular ROS concentrations ([ROS]i) in isolated rabbit and goat lungs, rat and dog pulmonary arteries, and cultured calf, dog, and rat PASMCs (26, 62, 84, 102, 112, 134, 180–182). In accordance with these findings, studies using a novel, redox-sensitive, ratiometric fluorescent protein sensor (RoGFP) (181, 182) and a developed, genetically encoded, specific ROS biosensor HyPer (22) reveal that hypoxia augments ROS signals in PASMCs. The explanation for these potentially odd results could be the use of different experimental conditions, but also recent studies suggest that these contrary hypotheses may hold true. That is, hypoxia causes a decrease in ROS generation in the mitochondrial matrix compartment, whereas it increases ROS production in the mitochondrial intermembrane space, which diffuses to the cytosol (182). This can lead to the activation of Nox proteins and a further increase in ROS levels (150).
One of the mechanisms by which hypoxia-derived ROS may lead to pulmonary artery vasoconstriction involves inhibition of KV channels (principally Kv 1.5 and Kv 2.1), depolarization, activation of Ca2+ voltage-dependent L-type channels, and an increase [Ca2+]i in PASMCs (8). In support of this notion, many research groups have reported that exogenous H2O2, similar to hypoxia, inhibits KV (38), induces an increase in [Ca2+]i in PASMCs (183) (100), and vasoconstriction in pulmonary arteries (28, 86, 151, 157, 160, 189, 194).
Role of Nox-derived ROS in HPV.
Many studies show that HPV is biphasic with an acute phase occurring in seconds/minutes and a sustained phase developing over several minutes to hours. Nox and mitochondria are the major sources of intracellular ROS generation mediating hypoxic responses in PASMCs (185, 191), and they are interdependent at least in acute HPV. The first evidence of a role for Nox in HPV came from experiments in which iodonium compounds or apocynin (both nonspecific Nox inhibitors) attenuates the hypoxic increase in [ROS]i (112, 123), inhibits KV currents (39, 184), increases [Ca2+]i, and induces contraction of PASMCs (112) and HPV in isolated lungs and pulmonary arteries (63, 73, 122, 123, 185, 186). Experiments using other Nox inhibitors, such as 4-(2-aminoethyl)bensenesulfonyl fluoride in isolated rabbit lung (186) and cadmium sulfate in isolated rat pulmonary artery further support the role of Nox in HPV. Although, Nox2−/− mice show normal or reduced hypoxic responses (10, 103); in p47phox−/− mice, acute but not sustained HPV is inhibited (185), suggesting that a Nox isoform other than Nox2 using p47phox contributes to the acute phase of HPV. Recently, a role for Nox4 in HPV has been shown by Ahmad et al. (3). They showed that Nox4-derived H2O2 maintains a basal relaxation under normoxic condition, which is removed by hypoxia leading to acute HPV. Increasing Nox4 expression with TGF-β was also observed to enhance HPV (192). Wolin et al. (75, 192) suggest that the mechanism of HPV in isolated bovine pulmonary arteries originates from the ability of pulmonary arteries to maintain increased smooth muscle levels of cytosolic NADPH, which sustain Nox4-derived H2O2 production. Moreover, Diebold et al. (48) showed that hypoxia induces the upregulation of Nox4 mRNA though hypoxia-inducible factor-1α (HIF-1α), which stimulates the synthesis of matrix metalloproteinases (MMPs), activation of MAPKs, transactivation of growth factor receptors, and signaling molecules and transcription factors involved in the proliferation of PASMCs and angiogenesis (109). In human lung adenocarcinoma A549 cells, an upregulation of Nox1 mRNA and protein occurred during hypoxia, accompanied by the activation of HIF-1-dependent target gene expression (heme oxygenase-1 mRNA, hypoxia-responsive element reporter gene activity), followed by enhanced ROS generation. HIF-1 induction is inhibited by diphenylene iodonium (DPI) and catalase, suggesting that hypoxic upregulation of Nox1 and subsequently augmented ROS generation may activate HIF-1-dependent pathways (67). Rathore et al. (150) recently revealed that hypoxia markedly increases Nox activity in PASMCs through activation of protein kinase C ε (PKCε) (150). Previous findings from the same group showed that the hypoxic activation of PKCε is secondary to increasing mitochondrial ROS (149), suggesting that hypoxia may enhance mitochondrial ROS generation, which activates PKCε and then augments Nox activity to cause further generation of intracellular ROS. Furthermore, several inhibitors of PKC isoforms inhibit HPV (17), and studies show the role for PKCζ in the activation of Nox induced by hypoxia (62). ROS also can activate a number of PKCs, and PKCα is involved in H2O2-induced contraction in pulmonary arteries (145). Whether various PKCs are simultaneously required remains unknown.
Role of Nox-derived ROS in pulmonary vascular remodeling.
Chronic hypoxia induces irreversible change in vascular remodeling characterized by medial and adventitial thickening of the muscular and elastic vessels and muscularization of previously nonmuscular more distal small vessels. This leads to a thickening of the vessel wall and reduction of the lumen area, increased vascular resistance, and thus development of pulmonary hypertension and right ventricular hypertrophy. Distinct segments of the pulmonary artery appear to contribute to this process. Endothelial cells from the intima exhibit atypical proliferation and participate in the formation of plexiform lesions. Nox2, Nox4, and p22phox are pivotal for proliferation of pulmonary endothelial cells (18, 142); SMCs from the medial layer exhibit hyperplasia and enhanced contractility (50). Both Nox1 and Nox4 are involved in the proliferation of SMCs (115, 120, 166). In particular, Nox4 is known to be induced by TGF-β and has been related to the TGF-β-dependent proliferation of SMCs in the pulmonary artery (82). In contrast to the mechanisms of TGF-β1 signaling observed in normoxic human PASMCs (166), the hypoxic release of TGF-β1 increases insulin-like growth factor binding protein (IGFBP-3) expression through phosphatidylinositol 3-kinase (PI3K) signaling with subsequent serine/threonine kinase (Akt) phosphorylation and further Nox4 activation. Moreover, IGFBP-3 increases Nox4 gene expression, resulting in PASMC proliferation (82).
The adventitial layer is thickened via hypoxia-induced proliferation of adventitial fibroblasts (153, 164). Furthermore, transdifferentiation of adventitial fibroblasts into myofibroblasts or SMCs appears to be critically involved in this process (162). Several studies demonstrate a role of Nox4 in the pathogenesis of hypoxia-induced pulmonary arterial remodeling and pulmonary hypertension, as well as idiopathic pulmonary arterial hypertension (IPAH) (120). In adventitial fibroblasts, a higher level of Nox4 mRNA expression than Nox1 is observed under normal conditions and this expression is significantly upregulated under hypoxic conditions (99). Consistent with this, a significant increase of Nox4 mRNA expression was observed under hypoxic conditions in adventitial fibroblasts from the lungs of patients with IPAH compared with healthy donors (99). Silencing of Nox4 causes reduction of H2O2 levels under normoxic and hypoxic conditions, suppression of the hypoxia-induced ROS increase, a decrease in adventitial fibroblast proliferation, and enhanced apoptosis (99).
Pulmonary Arterial Hypertension
Pulmonary arterial hypertension (PAH) is characterized by vascular remodeling and enhanced constrictor vasoreactivity. In PAH, there is a progressive decrease in arterial lumen and an increase of pulmonary arterial pressure that ultimately leads to right ventricular failure and death (148). Three mechanisms principally contribute to the decrease of the arterial lumen: the increase in contractility of the pulmonary resistance arteries, the proliferation and remodeling of pulmonary endothelial SMCs and fibroblasts, and the thrombosis. Accumulating evidence indicates that ROS contributes to all three mechanisms mentioned above, which are involved in the pathogenesis of PAH. Liu et al. (103) show that a disruption of the murine Nox2 gene completely prevents chronic hypoxia-induced PAH and vascular remodeling. In addition to studies supporting a role for Nox2 in PAH in response to hypoxia (103), it has been demonstrated that PAH is also characterized by the induction of Nox4, perhaps in response to an initial activation of Nox2 within the pulmonary endothelium, which is responsible for increased ROS generation and SMC proliferation (120). Hypoxia-dependent development of PAH in mice is linked to increased Nox4 expression in PASMCs (120), suggesting a role for Nox4 in the vascular remodeling associated with hypoxia-induced PAH. As mentioned above, hypoxia increases the expression of TGF-β (85) and Nox4 expression (166); Nox4 has also been shown to be critical for hypoxia-inducible factor 2α (HIF-2α) expression and transcriptional activation in renal carcinoma cells (111). This suggests that in hypoxic vascular remodeling, a mechanism involving HIF-2α, TGF-β, and Nox4 is operant.
One of the early events contributing to the pathophysiology of all forms of PAH is endothelial dysfunction (148). A role for Nox2 in hypoxia-induced endothelial dysfunction involving intrapulmonary arteries has been established. In addition, ROS generation from Nox-independent sources, xanthine oxidase (83), or uncoupled endothelial NO synthase (eNOS) (94) may also contribute to hypoxia-induced vascular dysfunction and PAH.
Several strategies designed to diminish ROS levels, through scavenging ROS or through the inhibition of the sources of ROS, have been proposed as potential therapeutic treatments for PAH. For example, treatment with recombinant human SOD is able to enhance ROS clearance, which leads to enhanced eNOS expression and function in animal models (neonatal lambs) of persistent pulmonary hypertension of the newborn (PPHN) (55). Interestingly, Kamezaki et al. (90) recently reported that gene transfer of extracellular SOD (EC-SOD) protects rats against monocrotaline-induced PH, suggesting that extracellular O2·− is important in the disease process. Manipulation of EC-SOD in models of vascular and lung diseases confirm a role for extracellular O2·− in the pathogenesis of a number of disease models associated with fibrosis and tissue remodeling (13, 23, 56, 60, 147). Using wild-type (WT) and transgenic mice overexpressing human EC-SOD in the lung, Nozik-Grayck et al. (131) show a protective effect of lung EC-SOD overexpression on chronic hypoxic-induced PAH and vascular remodeling. This protective effect is suggested to involve the suppression of redox-sensitive transcription factor early growth response-1 (Egr-1) pathway (131). In addition, catalase and SOD mimetics have proven beneficial in PH (46). Moreover, inhibition of xanthine oxidase (83) or eNOS (193) has shown beneficial effects in rat neonates with PH and in caveolin-1 knockout mice with PH, respectively. Also, soluble guanylate cyclase activators have yielded beneficial effects in preclinical studies in different animal models of PAH (163). Finally, in the clinic, use of phosphodiesterase-5 inhibitors has also been reported to reduce ROS levels (80).
Conclusion
For nearly two decades, Nox-derived ROS have been described as critical mediators of systemic vascular hypertension. While ROS vasoconstrictor and vasodilator effects in pulmonary vessels have been studied for at least as long, interest in the role of Nox in the complex orchestration of events leading to pulmonary hypertension has burgeoned in recent years. Nox isoforms appear to subserve a pivotal role in lung signaling, leading to marked changes in pulmonary airway, pneumocyte, and vascular cell phenotypes, including proliferation, hypertrophy, and apoptosis, resulting in acute lung injury, emphysema, and pulmonary hypertension among other pathologies. Evidence has emerged for a highly influential role of Nox in pulmonary hypertension; however, much of these data are either associative or are gleaned from in vitro studies. Studies designed to modulate Nox isoforms in a pulmonary vascular cell-specific manner will be needed to draw more definitive conclusions. Even less well studied is the direct or indirect contribution Nox isoforms play in right ventricular failure, the major cause of death in patients with PH. As past drug therapy has been focused solely on various pulmonary vascular targets and has largely been disappointing, emphasis should be placed on targeting individual or combinations of Nox in both the pulmonary vasculature and the right ventricle. For this reason, major priority is now being given to the discovery of specific Nox small molecule and peptidic inhibitors and therapies targeting these drugs to the pulmonary vasculature and right ventricle.
GRANTS
This work was support by National Heart, Lung, and Blood Institute (NHLBI) Grant R01-HL-079207 and by the Ri.MED Foundation. H. C. Champion is supported by NHLBI Grants PO1-HL-103455 and UO1-HL-108642-01 and grants from the Vascular Medicine Institute, the Institute for Transfusion Medicine, and the Hemophilia Center of Western Pennsylvania.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.F. drafted manuscript; H.C.C. and P.J.P. edited and revised manuscript; P.J.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Imad Al Ghouleh and Sheila Frizzell for critical reviews of the manuscript
REFERENCES
- 1. Abdala-Valencia H, Earwood J, Bansal S, Jansen M, Babcock G, Garvy B, Wills-Karp M, Cook-Mills JM. Nonhematopoietic NADPH oxidase regulation of lung eosinophilia and airway hyperresponsiveness in experimentally induced asthma. Am J Physiol Lung Cell Mol Physiol 292: L1111–L1125, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abdalla S, Will JA. Potentiation of the hypoxic contraction of guinea-pig isolated pulmonary arteries by two inhibitors of superoxide dismutase. Gen Pharmacol 26: 785–792, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Ahmad M, Kelly MR, Zhao X, Kandhi S, Wolin MS. Roles for Nox4 in the contractile response of bovine pulmonary arteries to hypoxia. Am J Physiol Heart Circ Physiol 298: H1879–H1888, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akasaki T, Ohya Y, Kuroda J, Eto K, Abe I, Sumimoto H, Iida M. Increased expression of gp91phox homologues of NAD(P)H oxidase in the aortic media during chronic hypertension: involvement of the renin-angiotensin system. Hypertens Res 29: 813–820, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM, Kelley EE, Bauer PM, Darley-Usmar V, Shiva S, Cifuentes-Pagano E, Freeman BA, Gladwin MT, Pagano PJ. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med 51: 1271–1288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Al-Mehdi AB, Zhao G, Dodia C, Tozawa K, Costa K, Muzykantov V, Ross C, Blecha F, Dinauer M, Fisher AB. Endothelial NADPH oxidase as the source of oxidants in lungs exposed to ischemia or high K+. Circ Res 83: 730–737, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Ameziane-El-Hassani R, Morand S, Boucher JL, Frapart YM, Apostolou D, Agnandji D, Gnidehou S, Ohayon R, Noel-Hudson MS, Francon J, Lalaoui K, Virion A, Dupuy C. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem 280: 30046–30054, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Archer S, Michelakis E. The mechanism(s) of hypoxic pulmonary vasoconstriction: potassium channels, redox O2 sensors, and controversies. News Physiol Sci 17: 131–137, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Archer SL, Huang J, Henry T, Peterson D, Weir EK. A redox-based O2 sensor in rat pulmonary vasculature. Circ Res 73: 1100–1112, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Archer SL, Reeve HL, Michelakis E, Puttagunta L, Waite R, Nelson DP, Dinauer MC, Weir EK. O2 sensing is preserved in mice lacking the gp91 phox subunit of NADPH oxidase. Proc Natl Acad Sci USA 96: 7944–7949, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ardanaz N, Beierwaltes WH, Pagano PJ. Comparison of H2O2-induced vasoconstriction in the abdominal aorta and mesenteric artery of the mouse. Vascul Pharmacol 47: 288–294, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Ardanaz N, Beierwaltes WH, Pagano PJ. Distinct hydrogen peroxide-induced constriction in multiple mouse arteries: potential influence of vascular polarization. Pharmacol Rep 60: 61–67, 2008 [PubMed] [Google Scholar]
- 13. Auten RL, O'Reilly MA, Oury TD, Nozik-Grayck E, Whorton MH. Transgenic extracellular superoxide dismutase protects postnatal alveolar epithelial proliferation and development during hyperoxia. Am J Physiol Lung Cell Mol Physiol 290: L32–L40, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem 279: 46065–46072, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Banfi B, Maturana A, Jaconi S, Arnaudeau S, Laforge T, Sinha B, Ligeti E, Demaurex N, Krause KH. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science 287: 138–142, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, Krause KH. A Ca2+-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem 276: 37594–37601, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Barman SA. Effect of protein kinase C inhibition on hypoxic pulmonary vasoconstriction. Am J Physiol Lung Cell Mol Physiol 280: L888–L895, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Bayraktutan U, Blayney L, Shah AM. Molecular characterization and localization of the NAD(P)H oxidase components gp91-phox and p22-phox in endothelial cells. Arterioscler Thromb Vasc Biol 20: 1903–1911, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Bedard K, Lardy B, Krause KH. NOX family NADPH oxidases: not just in mammals. Biochimie 89: 1107–1112, 2007 [DOI] [PubMed] [Google Scholar]
- 21. BelAiba RS, Djordjevic T, Petry A, Diemer K, Bonello S, Banfi B, Hess J, Pogrebniak A, Bickel C, Gorlach A. NOX5 variants are functionally active in endothelial cells. Free Radic Biol Med 42: 446–459, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods 3: 281–286, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Bowler RP, Barnes PJ, Crapo JD. The role of oxidative stress in chronic obstructive pulmonary disease. COPD 1: 255–277, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Brandes RP, Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res 65: 16–27, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Brar SS, Kennedy TP, Sturrock AB, Huecksteadt TP, Quinn MT, Murphy TM, Chitano P, Hoidal JR. NADPH oxidase promotes NF-κB activation and proliferation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 282: L782–L795, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, Black SM. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res 92: 683–691, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Brueckl C, Kaestle S, Kerem A, Habazettl H, Krombach F, Kuppe H, Kuebler WM. Hyperoxia-induced reactive oxygen species formation in pulmonary capillary endothelial cells in situ. Am J Respir Cell Mol Biol 34: 453–463, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Burghuber OC, Strife R, Zirolli J, Mathias MM, Murphy RC, Reeves JT, Voelkel NF. Hydrogen peroxide induced pulmonary vasoconstriction in isolated rat lungs is attenuated by U60,257, a leucotriene synthesis blocker. Wien Klin Wochenschr 98: 117–119, 1986 [PubMed] [Google Scholar]
- 29. Burke-Wolin T, Abate CJ, Wolin MS, Gurtner GH. Hydrogen peroxide-induced pulmonary vasodilation: role of guanosine 3′,5′-cyclic monophosphate. Am J Physiol Lung Cell Mol Physiol 261: L393–L398, 1991 [DOI] [PubMed] [Google Scholar]
- 30. Burke TM, Wolin MS. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am J Physiol Heart Circ Physiol 252: H721–H732, 1987 [DOI] [PubMed] [Google Scholar]
- 31. Caillou B, Dupuy C, Lacroix L, Nocera M, Talbot M, Ohayon R, Deme D, Bidart JM, Schlumberger M, Virion A. Expression of reduced nicotinamide adenine dinucleotide phosphate oxidase (ThoX, LNOX, Duox) genes and proteins in human thyroid tissues. J Clin Endocrinol Metab 86: 3351–3358, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Chakraborti S, Batabyal SK, Chakraborti T. Role of hydroxyl radical in the stimulation of arachidonic acid release caused by H2O2 in pulmonary smooth muscle cells: protective effect of anion channel blocker. Mol Cell Biochem 146: 91–98, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Chakraborti S, Batabyal SK, Dutta G, Michael JR. Role of serine esterase in hydrogen peroxide-mediated activation of phospholipase A2 in rabbit pulmonary arterial smooth muscle cells. Indian J Biochem Biophys 29: 477–481, 1992 [PubMed] [Google Scholar]
- 34. Chapman KE, Sinclair SE, Zhuang D, Hassid A, Desai LP, Waters CM. Cyclic mechanical strain increases reactive oxygen species production in pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol 289: L834–L841, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269: 131–140, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Cheng G, Lambeth JD. Alternative mRNA splice forms of NOXO1: differential tissue expression and regulation of Nox1 and Nox3. Gene 356: 118–126, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol 27: 42–48, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cogolludo A, Frazziano G, Cobeno L, Moreno L, Lodi F, Villamor E, Tamargo J, Perez-Vizcaino F. Role of reactive oxygen species in Kv channel inhibition and vasoconstriction induced by TP receptor activation in rat pulmonary arteries. Ann NY Acad Sci 1091: 41–51, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Cogolludo A, Moreno L, Frazziano G, Moral-Sanz J, Menendez C, Castaneda J, Gonzalez C, Villamor E, Perez-Vizcaino F. Activation of neutral sphingomyelinase is involved in acute hypoxic pulmonary vasoconstriction. Cardiovasc Res 82: 296–302, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cruzado MC, Risler NR, Miatello RM, Yao G, Schiffrin EL, Touyz RM. Vascular smooth muscle cell NAD(P)H oxidase activity during the development of hypertension: Effect of angiotensin II and role of insulinlike growth factor-1 receptor transactivation. Am J Hypertens 18: 81–87, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 97: 900–907, 2005 [DOI] [PubMed] [Google Scholar]
- 42. De Deken X, Wang D, Dumont JE, Miot F. Characterization of ThOX proteins as components of the thyroid H2O2-generating system. Exp Cell Res 273: 187–196, 2002 [DOI] [PubMed] [Google Scholar]
- 43. De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem 275: 23227–23233, 2000 [DOI] [PubMed] [Google Scholar]
- 44. De Keulenaer GW, Alexander RW, Ushio-Fukai M, Ishizaka N, Griendling KK. Tumour necrosis factor à activates a p22phox-based NADH oxidase in vascular smooth muscle. Biochem J 329: 653–657, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dekhuijzen PN, Aben KK, Dekker I, Aarts LP, Wielders PL, van Herwaarden CL, Bast A. Increased exhalation of hydrogen peroxide in patients with stable and unstable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 154: 813–816, 1996 [DOI] [PubMed] [Google Scholar]
- 46. Delannoy E, Courtois A, Freund-Michel V, Leblais V, Marthan R, Muller B. Hypoxia-induced hyperreactivity of pulmonary arteries: role of cyclooxygenase-2, isoprostanes, and thromboxane receptors. Cardiovasc Res 85: 582–592, 2010 [DOI] [PubMed] [Google Scholar]
- 47. Dhaunsi GS, Paintlia MK, Kaur J, Turner RB. NADPH oxidase in human lung fibroblasts. J Biomed Sci 11: 617–622, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Diebold I, Petry A, Hess J, Gorlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol Biol Cell 21: 2087–2096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dupuy C, Ohayon R, Valent A, Noel-Hudson MS, Deme D, Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cdnas. J Biol Chem 274: 37265–37269, 1999 [DOI] [PubMed] [Google Scholar]
- 50. Durmowicz AG, Stenmark KR. Mechanisms of structural remodeling in chronic pulmonary hypertension. Pediatr Rev 20: e91–e102, 1999 [PubMed] [Google Scholar]
- 51. Edens WA, Sharling L, Cheng G, Shapira R, Kinkade JM, Lee T, Edens HA, Tang X, Sullards C, Flaherty DB, Benian GM, Lambeth JD. Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J Cell Biol 154: 879–891, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. El Hassani RA, Benfares N, Caillou B, Talbot M, Sabourin JC, Belotte V, Morand S, Gnidehou S, Agnandji D, Ohayon R, Kaniewski J, Noel-Hudson MS, Bidart JM, Schlumberger M, Virion A, Dupuy C. Dual oxidase2 is expressed all along the digestive tract. Am J Physiol Gastrointest Liver Physiol 288: G933–G942, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Erdos B, Snipes JA, Miller AW, Busija DW. Cerebrovascular dysfunction in Zucker obese rats is mediated by oxidative stress and protein kinase C. Diabetes 53: 1352–1359, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Euler US von, Liljestrand G. Observations on the pulmonary arterial blood pressure in the cat. Acta Physiol Scand 12: 301–320, 1946 [Google Scholar]
- 55. Farrow KN, Lakshminrusimha S, Reda WJ, Wedgwood S, Czech L, Gugino SF, Davis JM, Russell JA, Steinhorn RH. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L979–L987, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fattman CL, Chang LY, Termin TA, Petersen L, Enghild JJ, Oury TD. Enhanced bleomycin-induced pulmonary damage in mice lacking extracellular superoxide dismutase. Free Radic Biol Med 35: 763–771, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Fike CD, Slaughter JC, Kaplowitz MR, Zhang Y, Aschner JL. Reactive oxygen species from NADPH oxidase contribute to altered pulmonary vascular responses in piglets with chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L881–L888, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fischer H. Mechanisms and function of DUOX in epithelia of the lung. Antioxid Redox Signal 11: 2453–2465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fischer H, Gonzales LK, Kolla V, Schwarzer C, Miot F, Illek B, Ballard PL. Developmental regulation of DUOX1 expression and function in human fetal lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 292: L1506–L1514, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Folz RJ, Abushamaa AM, Suliman HB. Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. J Clin Invest 103: 1055–1066, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Forteza R, Salathe M, Miot F, Conner GE. Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am J Respir Cell Mol Biol 32: 462–469, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Frazziano G, Moreno L, Moral-Sanz J, Menendez C, Escolano L, Gonzalez C, Villamor E, Alvarez-Sala JL, Cogolludo AL, Perez-Vizcaino F. Neutral sphingomyelinase, NADPH oxidase and reactive oxygen species. Role in acute hypoxic pulmonary vasoconstriction. J Cell Physiol 226: 2633–2640, 2011 [DOI] [PubMed] [Google Scholar]
- 63. Fuchs B, Sommer N, Dietrich A, Schermuly RT, Ghofrani HA, Grimminger F, Seeger W, Gudermann T, Weissmann N. Redox signaling and reactive oxygen species in hypoxic pulmonary vasoconstriction. Respir Physiol Neurobiol 174: 282–291, 2010 [DOI] [PubMed] [Google Scholar]
- 64. Gao YJ, Hirota S, Zhang DW, Janssen LJ, Lee RM. Mechanisms of hydrogen-peroxide-induced biphasic response in rat mesenteric artery. Br J Pharmacol 138: 1085–1092, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Geiszt M, Lekstrom K, Witta J, Leto TL. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J Biol Chem 278: 20006–20012, 2003 [DOI] [PubMed] [Google Scholar]
- 66. Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J 17: 1502–1504, 2003 [DOI] [PubMed] [Google Scholar]
- 67. Goyal P, Weissmann N, Grimminger F, Hegel C, Bader L, Rose F, Fink L, Ghofrani HA, Schermuly RT, Schmidt HH, Seeger W, Hanze J. Upregulation of NAD(P)H oxidase 1 in hypoxia activates hypoxia-inducible factor 1 via increase in reactive oxygen species. Free Radic Biol Med 36: 1279–1288, 2004 [DOI] [PubMed] [Google Scholar]
- 68. Grasberger H, De Deken X, Miot F, Pohlenz J, Refetoff S. Missense mutations of dual oxidase 2 (DUOX2) implicated in congenital hypothyroidism have impaired trafficking in cells reconstituted with DUOX2 maturation factor. Mol Endocrinol 21: 1408–1421, 2007 [DOI] [PubMed] [Google Scholar]
- 69. Grasberger H, Refetoff S. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J Biol Chem 281: 18269–18272, 2006 [DOI] [PubMed] [Google Scholar]
- 70. Griendling KK. Novel NAD(P)H oxidases in the cardiovascular system. Heart 90: 491–493, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Griendling KK, Ushio-Fukai M. Reactive oxygen species as mediators of angiotensin II signaling. Regul Pept 91: 21–27, 2000 [DOI] [PubMed] [Google Scholar]
- 72. Griffith B, Pendyala S, Hecker L, Lee PJ, Natarajan V, Thannickal VJ. NOX enzymes and pulmonary disease. Antioxid Redox Signal 11: 2505–2516, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Grimminger F, Wahn H, Kramer HJ, Stevens J, Mayer K, Walmrath D, Seeger W. Differential influence of arachidonic vs. eicosapentaenoic acid on experimental pulmonary hypertension. Am J Physiol Heart Circ Physiol 268: H2252–H2259, 1995 [DOI] [PubMed] [Google Scholar]
- 74. Gupta A, Tripathi AK, Tripathi RL, Madhu SV, Banerjee BD. Advanced glycosylated end products-mediated activation of polymorphonuclear neutrophils in diabetes mellitus and associated oxidative stress. Indian J Biochem Biophys 44: 373–378, 2007 [PubMed] [Google Scholar]
- 75. Gupte RS, Rawat DK, Chettimada S, Cioffi DL, Wolin MS, Gerthoffer WT, McMurtry IF, Gupte SA. Activation of glucose-6-phosphate dehydrogenase promotes acute hypoxic pulmonary artery contraction. J Biol Chem 285: 19561–19571, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Haddad P, Dussault S, Groleau J, Turgeon J, Maingrette F, Rivard A. Nox2-derived reactive oxygen species contribute to hypercholesterolemia-induced inhibition of neovascularization: effects on endothelial progenitor cells and mature endothelial cells. Atherosclerosis 217: 340–349, 2011 [DOI] [PubMed] [Google Scholar]
- 77. Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H, Wu R. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett 579: 4911–4917, 2005 [DOI] [PubMed] [Google Scholar]
- 78. Harper RW, Xu C, Soucek K, Setiadi H, Eiserich JP. A reappraisal of the genomic organization of human Nox1 and its splice variants. Arch Biochem Biophys 435: 323–330, 2005 [DOI] [PubMed] [Google Scholar]
- 79. Hawkins BJ, Madesh M, Kirkpatrick CJ, Fisher AB. Superoxide flux in endothelial cells via the chloride channel-3 mediates intracellular signaling. Mol Biol Cell 18: 2002–2012, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hemnes AR, Zaiman A, Champion HC. PDE5A inhibition attenuates bleomycin-induced pulmonary fibrosis and pulmonary hypertension through inhibition of ROS generation and RhoA/Rho kinase activation. Am J Physiol Lung Cell Mol Physiol 294: L24–L33, 2008 [DOI] [PubMed] [Google Scholar]
- 81. Hoidal JR, Xu P, Huecksteadt T, Sanders KA, Pfeffer K, Sturrock AB. Lung injury and oxidoreductases. Environ Health Perspect 106, Suppl 5: 1235–1239, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, Hoidal J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-β1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol 296: L489–L499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jankov RP, Kantores C, Pan J, Belik J. Contribution of xanthine oxidase-derived superoxide to chronic hypoxic pulmonary hypertension in neonatal rats. Am J Physiol Lung Cell Mol Physiol 294: L233–L245, 2008 [DOI] [PubMed] [Google Scholar]
- 84. Jernigan NL, Walker BR, Resta TC. Endothelium-derived reactive oxygen species and endothelin-1 attenuate NO-dependent pulmonary vasodilation following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 287: L801–L808, 2004 [DOI] [PubMed] [Google Scholar]
- 85. Jiang Y, Dai A, Li Q, Hu R. Hypoxia induces transforming growth factor-beta1 gene expression in the pulmonary artery of rats via hypoxia-inducible factor-1alpha. Acta Biochim Biophys Sin (Shanghai) 39: 73–80, 2007 [DOI] [PubMed] [Google Scholar]
- 86. Jin N, Rhoades RA. Activation of tyrosine kinases in H2O2-induced contraction in pulmonary artery. Am J Physiol Heart Circ Physiol 272: H2686–H2692, 1997 [DOI] [PubMed] [Google Scholar]
- 87. Jones RD, Morice AH. Hydrogen peroxide—an intracellular signal in the pulmonary circulation: involvement in hypoxic pulmonary vasoconstriction. Pharmacol Ther 88: 153–161, 2000 [DOI] [PubMed] [Google Scholar]
- 88. Joseph BZ, Routes JM, Borish L. Activities of superoxide dismutases and NADPH oxidase in neutrophils obtained from asthmatic and normal donors. Inflammation 17: 361–370, 1993 [DOI] [PubMed] [Google Scholar]
- 89. Kahles T, Kohnen A, Heumueller S, Rappert A, Bechmann I, Liebner S, Wittko IM, Neumann-Haefelin T, Steinmetz H, Schroeder K, Brandes RP. NADPH oxidase Nox1 contributes to ischemic injury in experimental stroke in mice. Neurobiol Dis 40: 185–192, 2010 [DOI] [PubMed] [Google Scholar]
- 90. Kamezaki F, Tasaki H, Yamashita K, Tsutsui M, Koide S, Nakata S, Tanimoto A, Okazaki M, Sasaguri Y, Adachi T, Otsuji Y. Gene transfer of extracellular superoxide dismutase ameliorates pulmonary hypertension in rats. Am J Respir Crit Care Med 177: 219–226, 2008 [DOI] [PubMed] [Google Scholar]
- 91. Kawahara T, Ritsick D, Cheng G, Lambeth JD. Point mutations in the proline-rich region of p22 phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation. J Biol Chem 280: 31859–31869, 2005 [DOI] [PubMed] [Google Scholar]
- 92. Knock GA, Snetkov VA, Shaifta Y, Connolly M, Drndarski S, Noah A, Pourmahram GE, Becker S, Aaronson PI, Ward JP. Superoxide constricts rat pulmonary arteries via Rho-kinase-mediated Ca2+ sensitization. Free Radic Biol Med 46: 633–642, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Koff JL, Shao MX, Kim S, Ueki IF, Nadel JA. Pseudomonas lipopolysaccharide accelerates wound repair via activation of a novel epithelial cell signaling cascade. J Immunol 177: 8693–8700, 2006 [DOI] [PubMed] [Google Scholar]
- 94. Konduri GG, Bakhutashvili I, Eis A, Pritchard K., Jr Oxidant stress from uncoupled nitric oxide synthase impairs vasodilation in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol 292: H1812–H1820, 2007 [DOI] [PubMed] [Google Scholar]
- 95. Korge P, Ping P, Weiss JN. Reactive oxygen species production in energized cardiac mitochondria during hypoxia/reoxygenation: modulation by nitric oxide. Circ Res 103: 873–880, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lacy F, Kailasam MT, O'Connor DT, Schmid-Schönbein GW, Parmer RJ. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. Hypertension 36: 878–884, 2000 [DOI] [PubMed] [Google Scholar]
- 97. Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med 43: 319–331, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lavigne MC, Eppihimer MJ. Cigarette smoke condensate induces MMP-12 gene expression in airway-like epithelia. Biochem Biophys Res Commun 330: 194–203, 2005 [DOI] [PubMed] [Google Scholar]
- 99. Li S, Tabar SS, Malec V, Eul BG, Klepetko W, Weissmann N, Grimminger F, Seeger W, Rose F, Hanze J. NOX4 regulates ROS levels under normoxic and hypoxic conditions, triggers proliferation, and inhibits apoptosis in pulmonary artery adventitial fibroblasts. Antioxid Redox Signal 10: 1687–1698, 2008 [DOI] [PubMed] [Google Scholar]
- 100. Lin MJ, Yang XR, Cao YN, Sham JS. Hydrogen peroxide-induced Ca2+ mobilization in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 292: L1598–L1608, 2007 [DOI] [PubMed] [Google Scholar]
- 101. Liu J, Yang F, Yang XP, Jankowski M, Pagano PJ. NAD(P)H oxidase mediates angiotensin II-induced vascular macrophage infiltration and medial hypertrophy. Arterioscler Thromb Vasc Biol 23: 776–782, 2003 [DOI] [PubMed] [Google Scholar]
- 102. Liu JQ, Sham JS, Shimoda LA, Kuppusamy P, Sylvester JT. Hypoxic constriction and reactive oxygen species in porcine distal pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 285: L322–L333, 2003 [DOI] [PubMed] [Google Scholar]
- 103. Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 290: L2–L10, 2006 [DOI] [PubMed] [Google Scholar]
- 105. Liu Y, Suzuki YJ, Day RM, Fanburg BL. Rho kinase-induced nuclear translocation of ERK1/ERK2 in smooth muscle cell mitogenesis caused by serotonin. Circ Res 95: 579–586, 2004 [DOI] [PubMed] [Google Scholar]
- 106. Lucchesi PA, Belmadani S, Matrougui K. Hydrogen peroxide acts as both vasodilator and vasoconstrictor in the control of perfused mouse mesenteric resistance arteries. J Hypertens 23: 571–579, 2005 [DOI] [PubMed] [Google Scholar]
- 107. Luxen S, Belinsky SA, Knaus UG. Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res 68: 1037–1045, 2008 [DOI] [PubMed] [Google Scholar]
- 108. Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res 105: 249–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Manea A. NADPH oxidase-derived reactive oxygen species: involvement in vascular physiology and pathology. Cell Tissue Res 342: 325–339, 2010 [DOI] [PubMed] [Google Scholar]
- 110. Manoury B, Nenan S, Leclerc O, Guenon I, Boichot E, Planquois JM, Bertrand CP, Lagente V. The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis. Respir Res 6: 11, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Maranchie JK, Zhan Y. Nox4 is critical for hypoxia-inducible factor 2-alpha transcriptional activity in von Hippel-Lindau-deficient renal cell carcinoma. Cancer Res 65: 9190–9193, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Marshall C, Mamary AJ, Verhoeven AJ, Marshall BE. Pulmonary artery NADPH-oxidase is activated in hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol 15: 633–644, 1996 [DOI] [PubMed] [Google Scholar]
- 113. Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest 106: 1521–1530, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Mehta JP, Campian JL, Guardiola J, Cabrera JA, Weir EK, Eaton JW. Generation of oxidants by hypoxic human pulmonary and coronary smooth-muscle cells. Chest 133: 1410–1414, 2008 [DOI] [PubMed] [Google Scholar]
- 115. Menshikov M, Plekhanova O, Cai H, Chalupsky K, Parfyonova Y, Bashtrikov P, Tkachuk V, Berk BC. Urokinase plasminogen activator stimulates vascular smooth muscle cell proliferation via redox-dependent pathways. Arterioscler Thromb Vasc Biol 26: 801–807, 2006 [DOI] [PubMed] [Google Scholar]
- 116. Michelakis ED, Hampl V, Nsair A, Wu X, Harry G, Haromy A, Gurtu R, Archer SL. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circ Res 90: 1307–1315, 2002 [DOI] [PubMed] [Google Scholar]
- 117. Michelakis ED, Rebeyka I, Wu X, Nsair A, Thebaud B, Hashimoto K, Dyck JR, Haromy A, Harry G, Barr A, Archer SL. O2 sensing in the human ductus arteriosus: regulation of voltage-gated K+ channels in smooth muscle cells by a mitochondrial redox sensor. Circ Res 91: 478–486, 2002 [DOI] [PubMed] [Google Scholar]
- 118. Michelakis ED, Thebaud B, Weir EK, Archer SL. Hypoxic pulmonary vasoconstriction: redox regulation of O2-sensitive K+ channels by a mitochondrial O2-sensor in resistance artery smooth muscle cells. J Mol Cell Cardiol 37: 1119–1136, 2004 [DOI] [PubMed] [Google Scholar]
- 119. Milovanova T, Chatterjee S, Manevich Y, Kotelnikova I, Debolt K, Madesh M, Moore JS, Fisher AB. Lung endothelial cell proliferation with decreased shear stress is mediated by reactive oxygen species. Am J Physiol Cell Physiol 290: C66–C76, 2006 [DOI] [PubMed] [Google Scholar]
- 120. Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH, Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res 101: 258–267, 2007 [DOI] [PubMed] [Google Scholar]
- 121. Mohazzab KM, Kaminski PM, Wolin MS. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. Am J Physiol Heart Circ Physiol 266: H2568–H2572, 1994 [DOI] [PubMed] [Google Scholar]
- 122. Mohazzab KM, Wolin MS. Sites of superoxide anion production detected by lucigenin in calf pulmonary artery smooth muscle. Am J Physiol Lung Cell Mol Physiol 267: L815–L822, 1994 [DOI] [PubMed] [Google Scholar]
- 123. Mohazzab KM, Fayngersh RP, Kaminski PM, Wolin MS. Potential role of NADH oxidoreductase-derived reactive O2 species in calf pulmonary arterial Po2-elicited responses. Am J Physiol Lung Cell Mol Physiol 269: L637–L644, 1995 [DOI] [PubMed] [Google Scholar]
- 124. Mohazzab KM, Wolin MS. Sites of superoxide anion production detected by lucigenin in calf pulmonary artery smooth muscle. Am J Physiol Lung Cell Mol Physiol 267: L815–L822, 1994 [DOI] [PubMed] [Google Scholar]
- 125. Murdoch CE, Alom-Ruiz SP, Wang M, Zhang M, Walker S, Yu B, Brewer A, Shah AM. Role of endothelial Nox2 NADPH oxidase in angiotensin II-induced hypertension and vasomotor dysfunction. Basic Res Cardiol 106: 527–538, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Nadeem A, Raj HG, Chhabra SK. Increased oxidative stress in acute exacerbations of asthma. J Asthma 42: 45–50, 2005 [DOI] [PubMed] [Google Scholar]
- 127. Narayanan PK, Goodwin EH, Lehnert BE. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res 57: 3963–3971, 1997 [PubMed] [Google Scholar]
- 128. Niwa Y, Ozaki Y, Kanoh T, Akamatsu H, Kurisaka M. Role of cytokines, tyrosine kinase, and protein kinase C on production of superoxide and induction of scavenging enzymes in human leukocytes. Clin Immunol Immunopathol 79: 303–313, 1996 [DOI] [PubMed] [Google Scholar]
- 129. Nowak D, Antczak A, Krol M, Pietras T, Shariati B, Bialasiewicz P, Jeczkowski K, Kula P. Increased content of hydrogen peroxide in the expired breath of cigarette smokers. Eur Respir J 9: 652–657, 1996 [DOI] [PubMed] [Google Scholar]
- 130. Nowak D, Kasielski M, Pietras T, Bialasiewicz P, Antczak A. Cigarette smoking does not increase hydrogen peroxide levels in expired breath condensate of patients with stable COPD. Monaldi Arch Chest Dis 53: 268–273, 1998 [PubMed] [Google Scholar]
- 131. Nozik-Grayck E, Suliman HB, Majka S, Albietz J, VanRheen Z, Roush K, Stenmark KR. Lung EC-SOD overexpression attenuates hypoxic induction of Egr-1 and chronic hypoxic pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol 295: L422–L430, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Oeckler RA, Arcuino E, Ahmad M, Olson SC, Wolin MS. Cytosolic NADH redox and thiol oxidation regulate pulmonary arterial force through ERK MAP kinase. Am J Physiol Lung Cell Mol Physiol 288: L1017–L1025, 2005 [DOI] [PubMed] [Google Scholar]
- 133. Pacquelet S, Lehmann M, Luxen S, Regazzoni K, Frausto M, Noack D, Knaus UG. Inhibitory action of NoxA1 on dual oxidase activity in airway cells. J Biol Chem 283: 24649–24658, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Paddenberg R, Goldenberg A, Faulhammer P, Braun-Dullaeus RC, Kummer W. Mitochondrial complex II is essential for hypoxia-induced ROS generation and vasoconstriction in the pulmonary vasculature. Adv Exp Med Biol 536: 163–169, 2003 [DOI] [PubMed] [Google Scholar]
- 135. Papaiahgari S, Kleeberger SR, Cho HY, Kalvakolanu DV, Reddy SP. NADPH oxidase and ERK signaling regulates hyperoxia-induced Nrf2-ARE transcriptional response in pulmonary epithelial cells. J Biol Chem 279: 42302–42312, 2004 [DOI] [PubMed] [Google Scholar]
- 136. Parinandi NL, Kleinberg MA, Usatyuk PV, Cummings RJ, Pennathur A, Cardounel AJ, Zweier JL, Garcia JG, Natarajan V. Hyperoxia-induced NAD(P)H oxidase activation and regulation by MAP kinases in human lung endothelial cells. Am J Physiol Lung Cell Mol Physiol 284: L26–L38, 2003 [DOI] [PubMed] [Google Scholar]
- 137. Pelaez NJ, Braun TR, Paul RJ, Meiss RA, Packer CS. H2O2 mediates Ca2+ and MLC20 phosphorylation-independent contraction in intact and permeabilized vascular muscle. Am J Physiol Heart Circ Physiol 279: H1185–H1193, 2000 [DOI] [PubMed] [Google Scholar]
- 138. Pendyala S, Gorshkova IA, Usatyuk PV, He D, Pennathur A, Lambeth JD, Thannickal VJ, Natarajan V. Role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid Redox Signal 11: 747–764, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Pendyala S, Usatyuk PV, Gorshkova IA, Garcia JG, Natarajan V. Regulation of NADPH oxidase in vascular endothelium: the role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid Redox Signal 11: 841–860, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Perez-Vizcaino F, Lopez-Lopez JG, Santiago R, Cogolludo A, Zaragoza-Arnaez F, Moreno L, Alonso MJ, Salaices M, Tamargo J. Postnatal maturation in nitric oxide-induced pulmonary artery relaxation involving cyclooxygenase-1 activity. Am J Physiol Lung Cell Mol Physiol 283: L839–L848, 2002 [DOI] [PubMed] [Google Scholar]
- 141. Peters EA, Hiltermann JT, Stolk J. Effect of apocynin on ozone-induced airway hyperresponsiveness to methacholine in asthmatics. Free Radic Biol Med 31: 1442–1447, 2001 [DOI] [PubMed] [Google Scholar]
- 142. Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, Gorlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal 8: 1473–1484, 2006 [DOI] [PubMed] [Google Scholar]
- 143. Piotrowski WJ, Marczak J, Dinsdale D, Kurmanowska Z, Tarasow Y, Komos J, Nowak D. Release of hydrogen peroxide by rat type II pneumocytes in the prolonged culture. Toxicol In Vitro 14: 85–93, 2000 [DOI] [PubMed] [Google Scholar]
- 144. Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet 9: 202–209, 1995 [DOI] [PubMed] [Google Scholar]
- 145. Pourmahram GE, Snetkov VA, Shaifta Y, Drndarski S, Knock GA, Aaronson PI, Ward JP. Constriction of pulmonary artery by peroxide: role of Ca2+ release and PKC. Free Radic Biol Med 45: 1468–1476, 2008 [DOI] [PubMed] [Google Scholar]
- 146. Pricci F, Leto G, Amadio L, Iacobini C, Cordone S, Catalano S, Zicari A, Sorcini M, DiMario U, Pugliese G. Oxidative stress in diabetes-induced endothelial dysfunction involvement of nitric oxide and protein kinase C. Free Radic Biol Med 35: 683–694, 2003 [DOI] [PubMed] [Google Scholar]
- 147. Rabbani ZN, Anscher MS, Folz RJ, Archer E, Huang H, Chen L, Golson ML, Samulski TS, Dewhirst MW, Vujaskovic Z. Overexpression of extracellular superoxide dismutase reduces acute radiation induced lung toxicity. BMC Cancer 5: 59, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 118: 2372–2379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Rathore R, Zheng YM, Li XQ, Wang QS, Liu QH, Ginnan R, Singer HA, Ho YS, Wang YX. Mitochondrial ROS-PKCepsilon signaling axis is uniquely involved in hypoxic increase in [Ca2+]i in pulmonary artery smooth muscle cells. Biochem Biophys Res Commun 351: 784–790, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med 45: 1223–1231, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Rhoades RA, Jin N, Packer CS. Signal transduction in hypoxic pulmonary vasoconstriction (HPV). Prog Clin Biol Res 327: 565–573, 1990 [PubMed] [Google Scholar]
- 152. Rigutto S, Hoste C, Grasberger H, Milenkovic M, Communi D, Dumont JE, Corvilain B, Miot F, De Deken X. Activation of dual oxidases Duox1 and Duox2: differential regulation mediated by camp-dependent protein kinase and protein kinase C-dependent phosphorylation. J Biol Chem 284: 6725–6734, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Rose F, Grimminger F, Appel J, Heller M, Pies V, Weissmann N, Fink L, Schmidt S, Krick S, Camenisch G, Gassmann M, Seeger W, Hanze J. Hypoxic pulmonary artery fibroblasts trigger proliferation of vascular smooth muscle cells: role of hypoxia-inducible transcription factors. FASEB J 16: 1660–1661, 2002 [DOI] [PubMed] [Google Scholar]
- 154. Sadikot RT, Zeng H, Yull FE, Li B, ChengDsKernodle DS, Jansen ED, Contag CH, Segal BH, Holland SM, Blackwell TS, Christman JW. p47phox deficiency impairs NF-κB activation and host defense in Pseudomonas pneumonia. J Immunol 172: 1801–1808, 2004 [DOI] [PubMed] [Google Scholar]
- 155. Schwarzer C, Machen TE, Illek B, Fischer H. NADPH oxidase-dependent acid production in airway epithelial cells. J Biol Chem 279: 36454–36461, 2004 [DOI] [PubMed] [Google Scholar]
- 156. Sedgwick JB, Geiger KM, Busse WW. Superoxide generation by hypodense eosinophils from patients with asthma. Am Rev Respir Dis 142: 120–125, 1990 [DOI] [PubMed] [Google Scholar]
- 157. Seeger W, Suttorp N, Schmidt F, Neuhof H. The glutathione redox cycle as a defense system against hydrogen-peroxide-induced prostanoid formation and vasoconstriction in rabbit lungs. Am Rev Respir Dis 133: 1029–1036, 1986 [DOI] [PubMed] [Google Scholar]
- 158. Shao MX, Nadel JA. Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci USA 102: 767–772, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Sheehan AL, Carrell S, Johnson B, Stanic B, Banfi B, Miller FJ., Jr Role for Nox1 NADPH oxidase in atherosclerosis. Atherosclerosis 216: 321–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Sheehan DW, Giese EC, Gugino SF, Russell JA. Characterization and mechanisms of H2O2-induced contractions of pulmonary arteries. Am J Physiol Heart Circ Physiol 264: H1542–H1547, 1993 [DOI] [PubMed] [Google Scholar]
- 162. Short M, Nemenoff RA, Zawada WM, Stenmark KR, Das M. Hypoxia induces differentiation of pulmonary artery adventitial fibroblasts into myofibroblasts. Am J Physiol Cell Physiol 286: C416–C425, 2004 [DOI] [PubMed] [Google Scholar]
- 163. Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 123: 2263–2273, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Stenmark KR, Mecham RP. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annu Rev Physiol 59: 89–144, 1997 [DOI] [PubMed] [Google Scholar]
- 165. Stolk J, Hiltermann TJN, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol 11: 95–102, 1994 [DOI] [PubMed] [Google Scholar]
- 166. Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. Transforming growth factor-β1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 290: L661–L673, 2006 [DOI] [PubMed] [Google Scholar]
- 167. Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov 6: 662–680, 2007 [DOI] [PubMed] [Google Scholar]
- 168. Takemura Y, Goodson P, Bao HF, Jain L, Helms MN. Rac1-mediated NADPH oxidase release of O2− regulates epithelial sodium channel activity in the alveolar epithelium. Am J Physiol Lung Cell Mol Physiol 298: L509–L520, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Tanito M, Nakamura H, Kwon YW, Teratani A, Masutani H, Shioji K, Kishimoto C, Ohira A, Horie R, Yodoi J. Enhanced oxidative stress and impaired thioredoxin expression in spontaneously hypertensive rats. Antioxid Redox Signal 6: 89–97, 2004 [DOI] [PubMed] [Google Scholar]
- 170. Thannickal VJ, Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem 270: 30334–30338, 1995 [DOI] [PubMed] [Google Scholar]
- 172. Ueyama T, Lekstrom K, Tsujibe S, Saito N, Leto TL. Subcellular localization and function of alternatively spliced Noxo1 isoforms. Free Radic Biol Med 42: 180–190, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc Res 71: 226–235, 2006 [DOI] [PubMed] [Google Scholar]
- 174. Vachier I, Damon M, LeDoucen C, dePaulet AC, Chanez P, Michel FB, Godard P. Increased oxygen species generation in blood monocytes of asthmatic patients. Am Rev Respir Dis 146: 1161–1166, 1992 [DOI] [PubMed] [Google Scholar]
- 175. Vejrazka M, Micek R, Stipek S. Apocynin inhibits NADPH oxidase in phagocytes but stimulates ROS production in non-phagocytic cells. Biochim Biophys Acta 1722: 143–147, 2005 [DOI] [PubMed] [Google Scholar]
- 176. Waghray M, Cui Z, Horowitz JC, Subramanian IM, Martinez FJ, Toews GB, Thannickal VJ. Hydrogen peroxide is a diffusable paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J 19: 854–856, 2005 [DOI] [PubMed] [Google Scholar]
- 177. Wang D, De Deken X, Milenkovic M, Song Y, Pirson I, Dumont JE, Miot F. Identification of a novel partner of duox: EFP1, a thioredoxin-related protein. J Biol Chem 280: 3096–3103, 2005 [DOI] [PubMed] [Google Scholar]
- 178. Wang W, Suzuki Y, Tanigaki T, Rank DR, Raffin TA. Effect of the NADPH oxidase inhibitor apocynin on septic lung injury in guinea pigs. Am J Respir Crit Care Med 150: 1449–1452, 1994 [DOI] [PubMed] [Google Scholar]
- 179. Ward JP, McMurtry IF. Mechanisms of hypoxic pulmonary vasoconstriction and their roles in pulmonary hypertension: new findings for an old problem. Curr Opin Pharmacol 9: 287–296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res 88: 1259–1266, 2001 [DOI] [PubMed] [Google Scholar]
- 181. Waypa GB, Guzy R, Mungai PT, Mack MM, Marks JD, Roe MW, Schumacker PT. Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ Res 99: 970–978, 2006 [DOI] [PubMed] [Google Scholar]
- 182. Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res 106: 526–535, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Waypa GB, Schumacker PT. O2 sensing in hypoxic pulmonary vasoconstriction: the mitochondrial door re-opens. Respir Physiol Neurobiol 132: 81–91, 2002 [DOI] [PubMed] [Google Scholar]
- 184. Weir EK, Wyatt CN, Reeve HL, Huang J, Archer SL, Peers C. Diphenyleneiodonium inhibits both potassium and calcium currents in isolated pulmonary artery smooth muscle cells. J Appl Physiol 76: 2611–2615, 1994 [DOI] [PubMed] [Google Scholar]
- 185. Weissmann N, Schermuly RT, Ghofrani HA, Hanze J, Goyal P, Grimminger F, Seeger W. Hypoxic pulmonary vasoconstriction—triggered by an increase in reactive oxygen species? Novartis Found Symp 272: 196–208, 2006 [PubMed] [Google Scholar]
- 186. Weissmann N, Tadic A, Hanze J, Rose F, Winterhalder S, Nollen M, Schermuly RT, Ghofrani HA, Seeger W, Grimminger F. Hypoxic vasoconstriction in intact lungs: a role for NADPH oxidase-derived H2O2? Am J Physiol Lung Cell Mol Physiol 279: L683–L690, 2000 [DOI] [PubMed] [Google Scholar]
- 188. Wesley UV, Bove PF, Hristova M, McCarthy S, vanderVliet A. Airway epithelial cell migration and wound repair by ATP-mediated activation of dual oxidase 1. J Biol Chem 282: 3213–3220, 2007 [DOI] [PubMed] [Google Scholar]
- 189. Wilhelm J, Herget J. Role of ion fluxes in hydrogen peroxide pulmonary vasoconstriction. Physiol Res 44: 31–37, 1995 [PubMed] [Google Scholar]
- 190. Wojciak-Stothard B, Tsang LY, Haworth SG. Rac and Rho play opposing roles in the regulation of hypoxia/reoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 288: L749–L760, 2005 [DOI] [PubMed] [Google Scholar]
- 191. Wolin MS, Ahmad M, Gao Q, Gupte SA. Cytosolic NAD(P)H regulation of redox signaling and vascular oxygen sensing. Antioxid Redox Signal 9: 671–678, 2007 [DOI] [PubMed] [Google Scholar]
- 192. Wolin MS, Gupte SA, Neo BH, Gao Q, Ahmad M. Oxidant-redox regulation of pulmonary vascular responses to hypoxia and nitric oxide-cGMP signaling. Cardiol Rev 18: 89–93, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Wunderlich C, Schmeisser A, Heerwagen C, Ebner B, Schober K, Braun-Dullaeus RC, Schwencke C, Kasper M, Morawietz H, Strasser RH. Chronic NOS inhibition prevents adverse lung remodeling and pulmonary arterial hypertension in caveolin-1 knockout mice. Pulm Pharmacol Ther 21: 507–515, 2008 [DOI] [PubMed] [Google Scholar]
- 194. Yamaguchi K, Asano K, Takasugi T, Kawai A, Mori M, Umeda A, Kawashiro T, Yokoyama T. Roles of antioxidant enzymes in erythrocytes on hypoxic pulmonary vasoconstriction. Adv Exp Med Biol 345: 59–66, 1994 [DOI] [PubMed] [Google Scholar]
- 195. Yang Z, Zhang A, Altura BT, Altura BM. Hydrogen peroxide-induced endothelium-dependent relaxation of rat aorta. Involvement of Ca2+ and other cellular metabolites. Gen Pharmacol 33: 325–336, 1999 [DOI] [PubMed] [Google Scholar]
- 196. Zamproni I, Grasberger H, Cortinovis F, Vigone MC, Chiumello G, Mora S, Onigata K, Fugazzola L, Refetoff S, Persani L, Weber G. Biallelic inactivation of the dual oxidase maturation factor 2 (DUOXA2) gene as a novel cause of congenital hypothyroidism. J Clin Endocrinol Metab 93: 605–610, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Zhang L, Sheppard OR, Shah AM, Brewer AC. Positive regulation of the NADPH oxidase NOX4 promoter in vascular smooth muscle cells by E2F. Free Radic Biol Med 45: 679–685, 2008 [DOI] [PubMed] [Google Scholar]
- 198. Zhang Q, Matsuzaki I, Chatterjee S, Fisher AB. Activation of endothelial NADPH oxidase during normoxic lung ischemia is KATP channel dependent. Am J Physiol Lung Cell Mol Physiol 289: L954–L961, 2005 [DOI] [PubMed] [Google Scholar]
- 199. Zhang X, Shan P, Sasidhar M, Chupp GL, Flavell RA, Choi AM, Lee PJ. Reactive oxygen species and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase mediate hyperoxia-induced cell death in lung epithelium. Am J Respir Cell Mol Biol 28: 305–315, 2003 [DOI] [PubMed] [Google Scholar]
- 200. Zhao Y, Davis HW. Hydrogen peroxide-induced cytoskeletal rearrangement in cultured pulmonary endothelial cells. J Cell Physiol 174: 370–379, 1998 [DOI] [PubMed] [Google Scholar]