Abstract
The signaling complex consisting of the growth factor neuregulin-1 (NRG1) and its tyrosine kinase receptors ErbB2 and ErbB4 has a critical role in cardiac development and homeostasis of the structure and function of the adult heart. Recent research results suggest that targeting this signaling complex may provide a viable strategy for treating heart failure. Clinical trials are currently evaluating the effectiveness and safety of intravenous administration of recombinant NRG1 formulations in heart failure patients. Endogenous as well as administered NRG1 has multiple possible activities in the adult heart, but how these are related is unknown. It has recently been demonstrated that NRG1 administration can stimulate proliferation of cardiomyocytes, which may contribute to repair failing hearts. This review summarizes the current knowledge of how NRG1 and its receptors control cardiac physiology and biology, with special emphasis on its role in cardiomyocyte proliferation during myocardial growth and regeneration.
Keywords: cardiac repair, cardiac regeneration, cardiomyocyte differentiation
this article is part of a collection on Physiological Basis of Cardiovascular Cell and Gene Therapies. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Introduction
Heart failure is a significant and growing public health problem worldwide (74). Although new therapies have improved patient outcomes, symptomatic heart failure is still a chronically progressive disease. Exciting advances have been made with cell (32, 43, 53, 83, 94) and gene therapy (48) approaches to treat heart failure. However, few drugs targeting novel pathways for addressing heart failure are in the development pipeline (49).
One new strategy for treating heart failure, which has shown promising results in animals, targets the signaling complex of the growth factor neuregulin-1 (NRG1) and its tyrosine kinase receptors, ErbB2 and ErbB4 (39, 72, 76, 91, 92). Clinical trials have been performed (26, 42) and are proceeding to determine the effectiveness and safety of intravenous administration of recombinant NRG1 formulations for treating congestive heart failure (ClinicalTrials.gov identifiers NCT01131637, NCT01258387). However, the translation of NRG1 administration into a successful clinical therapy requires that the underlying molecular and cellular mechanisms be identified and compared and their relation to one another be characterized.
Overexpression of the NRG1 receptor subunit ErbB2 promotes uncontrolled cancer growth, and ErbB2 inhibition by Herceptin (trastuzumab) is used to treat some types of cancer. One of the side effects of trastuzumab is cardiac dysfunction (85), suggesting a possible role of endogenous NRG1 and its receptors in maintaining cardiac structure and function in adults, which was the subject of excellent recent reviews (18, 25, 34, 76). In this review, we will examine the activities and roles of NRG1 and its receptor, the tyrosine kinase heterodimer ErbB2/ErbB4, in the myocardium, focusing on their role in cardiomyocyte proliferation. Despite the long-standing controversy over to what extent adult cardiomyocytes can progress through the cell cycle (2, 66, 70), recent studies have shown that a small subpopulation can be induced to proliferate in animals (6, 10, 23, 51, 71, 73, 96) and possibly in humans (5). NRG1 is one of a small group of extracellular factors known to control this process in animals (6, 22, 23, 51, 73). The induction of cardiomyocyte proliferation for stimulating myocardial repair provides a potential new mechanism of treatment for heart failure.
A potential protein therapy approach to stimulate myocardial repair may be complementary to cell transplantation approaches, and some features may offer unique advantages. For example, since resident cells would be stimulated to proliferate, a cell source or invasive procedures for procuring and/or implanting them would not be necessary. Furthermore, daughter cells of cardiomyocytes would inherit electromechanical connections from their mother cells, guaranteeing electrical and mechanical connections to the surrounding myocardium. The duration of the effect of recombinant factors can be controlled by dose and timing of administration. In addition, since cardiomyocytes are differentiated cells and primary myocardial tumors are exceedingly rare, malignant transformation would be unlikely. Thus, if it can be translated to the clinic, administration of recombinant proteins for the stimulation of cardiac repair has several promising features (36, 47).
NRG1/ErbB Signaling Is Critical in the Nervous System, Breast, and Heart
NRG1, a member of the epidermal growth factor (EGF) family, is known to control a plethora of mechanisms in epithelial, glial, neuronal, and heart muscle cells during development. NRG1 performs different functions in different types of cells: differentiation of neuronal cells (neuronal differentiation factor), accumulation of acetylcholine receptors in skeletal muscle (acetylcholine receptor-inducing activity), and proliferation of glial cells (glial growth factor). One possible way of summarizing these diverse cellular effects is that NRG1/ErbB signaling affects developmental cell fate decisions (9).
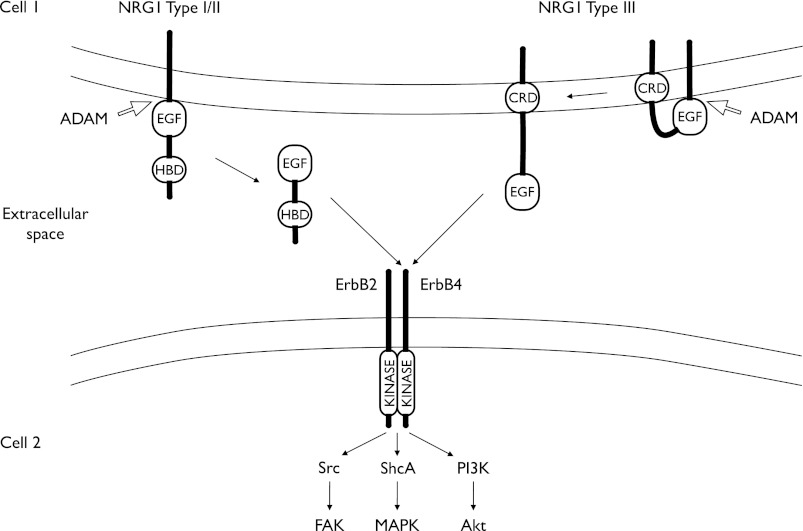
The NRG1 mRNA is transcribed from one gene, located on chromosome 8 in both mice and humans. The gene product is classified into three subgroups (I–III), which are processed as transmembranous proteins containing EGF-like domains [Fig. 1, and Olayioye et al. (68)]. Type I and II NRG1 are cleaved by metalloproteinases into soluble forms and thus act as a paracrine factors. Type III is cleaved in such a way that the extracellular EGF domain remains attached to the transmembrane domain through the cysteine-rich domain to signal in the immediate environment (Fig. 1).
Fig. 1.
Structure and function of neuregulin-1 (NRG1) and ErbB4, diagrammatically depicted in two neighboring cells. Type I–III NRG1s have epidermal growth factor-like domains (EGF). Type I/II NRG1s have a heparin-binding domain (HBD) whereas type III have a cysteine-rich domain (CRD). Both types may be subject to cleavage by a member of proteins containing a disintegrin and a metalloprotease domain (ADAM). Type I/II is released and functions as a paracrine factor. Type III remains a transmembranous protein through the CRD. NRG1/ErbB4 signaling is initiated by binding of NRG1 to ErbB4, which increases kinase activity and downstream signaling via multiple pathways. In the heart, the following pathways have been shown to be activated by NRG1: 1) Src and focal adhesion kinase (FAK); 2) Src homology domain-containing protein (ShcA) and mitogen-activated protein kinases (MAPK), and 3) phosphatidylinositol 3-kinase (PI3K) and Akt.
NRG1 acts through the receptor tyrosine kinases ErbB2, ErbB3, and ErbB4, which belong to the EGF-receptor family (14). Cardiomyocytes in the adult myocardium have been shown to express ErbB4 and ErbB2 (97). NRG1 binds to ErbB4 with high affinity, which in cardiomyocytes is believed to dimerize preferentially with ErbB2. In ErbB2/ErbB4 heterodimers, ErbB2 functions as a “nonautonomous amplifier” (14) by providing a very active kinase domain.
Ligand-binding to ErbB4 and dimerization with ErbB2 leads to signal transduction through the transmembrane helixes and activation of the intracellular kinase domain, leading to tyrosine phosphorylation. The phosphorylated intracellular domains then recruit signaling proteins containing phosphotyrosine-binding and Src homology-2 domains. In adult cardiomyocytes, phosphatidylinositol 3-kinase (19, 24), Src (52), and ShcA (86) are important effector molecules of ErbB2/ErbB4 signaling (Fig. 1). These proteins relay the signal to downstream signaling pathways, which regulate a variety of cell-specific functions, including differentiation, proliferation, and cell migration (14).
The ErbB4 gene is located on chromosome 1 in mice and 2 in humans. Post-transcriptional processing of the ErbB4 gene product is complex, including alternative splicing and regulated cleavage by proteases (14, 87). Alternative splicing gives rise to multiple isoforms. One splice variant of ErbB4 cannot be cleaved by matrix metalloproteinases in the juxtamembrane region and is referred to as juxtamembrane domain variant b (21). Only this variant is expressed in the adult mouse myocardium (21). Another splice variant has differential intracellular signaling to phosphatidylinositol 3-kinase and is referred to as CYT-2 (20). The functional relevance of the different splice variants in the heart is currently unknown.
Although NRG1 is a high-affinity ligand for ErbB4, it should be noted that NRG2, NRG3, NRG4, HB-EGF, and epiregulin can also activate ErbB4 (14, 93). HB-EGF has been connected to cardiac hypertrophy (41, 79), but the extent to which the other alternative ligands contribute to ErbB signaling in the heart is largely unknown.
Effects of NRG1 Administration on Cardiac Structure and Function
Multiple studies that have evaluated the NRG1/ErbB axis in cardiomyocytes, animal models, and two clinical trials are discussed throughout this review and summarized in Table 1. A preclinical study showed that intravenous administration of recombinant NRG1 improved cardiac function and prolonged survival in animal models of ischemic, dilated, and viral cardiomyopathy (60). In this study, NRG1 was administered in rats after experimental myocardial infarction (MI) daily for 10 days beginning 1 or 8 wk after MI. NRG1-injected animals showed improved ejection fraction (EF) as well as a smaller end-diastolic dimension. In an additional experiment, recombinant NRG1 was administered in rats beginning 1 wk after MI for 10 days in conjunction with angiotensin-converting enzyme (ACE) inhibitor therapy (60). The functional effects in this study, determined by hemodynamic catheterization 33 wk later, appeared to be additive, and in the survival analysis, NRG1 and ACE inhibitors showed an additive effect as well. This study also used a rat doxorubicin cardiomyopathy model and a mouse model of Coxsackie-virus myocarditis, both of which showed that NRG1 administration improved function and reduced tissue damage determined by semiquantitative histopathological examination. Furthermore, this study used a dog-pacing model, in which administration of NRG1 for 10 consecutive days showed functional improvements. The NRG1 effect was still detectable 3 wk later, suggesting that NRG1 administration may induce not only functional but also structural improvements.
Table 1.
Summary of studies of NRG1 administration in in vitro systems, animal models, and humans with relation to cardiomyocyte proliferation and differentiation and cardiac physiology and repair
| System | Formulation | Amino Acids | Administration | Measured Effects | Reference |
|---|---|---|---|---|---|
| E12.5 mouse embryo culture | NRG1-β1 | 177–244 | Injection of 50 ng per embryo, incubation in 50% rat serum | Stimulates formation of trabeculae and cardiomyocyte DNA synthesis | (35) |
| Cultured embryonic rat ventricular cardiomyocytes | GGF2 | Not provided, probably 2–246 | 30 ng/ml | Cardiomyocyte DNA synthesis, embryonic cardiomyocyte proliferation | (97) |
| Primary adult rat ventricular cardiomyocytes | NRG1-β1 | Not provided | 50 ng/ml | Cardiomyocyte DNA synthesis, karyokinesis, and cytokinesis | (23) |
| Primary adult rat ventricular cardiomyocytes | NRG1-β1 | 176–246 | 0.001–0.1 μg/ml | Cardiomyocyte DNA synthesis, karyokinesis, cytokinesis, and division | (6) |
| Primary adult rat ventricular cardiomyocytes | GGF2 | 10 ng/ml | Sarcomer integrity, protein phosphorylation | (52) | |
| Mouse, post-MI | NRG1-β1 | 176–246 | 100 μg/kg ip, for 12 wk | 10% EF improvement, reduction in EDD, reduction in infarct size, cardiomyocyte proliferation | (6) |
| Multiple animal models | NRG1-β2a | 177–237 | 3–30 μg/kg iv for 5 days | Improvement of function compared with angiotensin converting enzyme-blocker | (60) |
| Controlled human trial | NRG1-β2a | 177–237 | 0.3–1.2 μg·kg−1·day−1 iv for 10 days | 4% EF improvement (nonsignificant), reduction in EDV (CMR, P = 0.05) | (26) |
| Uncontrolled human trial | NRG1-β2a | 177–237 | 0.6–1.2 μg·kg−1·day−1 iv for 10 days | 4% EF improvement 12 wk after therapy compared with baseline (P < 0.001) | (42) |
This table may serve as a tool to compare different studies that are discussed throughout this review. Examples are ordered from development to adult and from in vitro to in vivo studies. The third column lists the amino acids corresponding to the human sequence. E12.5, embryonic day 12.5; MI, myocardial infarction; NRG1, neuregulin-1; GGF2, glial growth factor 2; EF, ejection fraction, values are absolute values, not percent changes; EDD, end-diastolic dimension; EDV, end-diastolic volume; CMR, cardiac magnetic resonance imaging.
In a human cohort trial of 15 patients with ischemic heart disease and idiopathic dilative cardiomyopathy (mean age, 60 yr; New York Heart Association classes II and III; mean EF, 32%; and standard medical therapy), NRG1 was administered by daily intravenous infusions for 10 consecutive days (42). The EF, measured by cardiac magnetic resonance imaging, improved by 4%, which is in the range of what has been reported after stem cell transplantation (4, 78). The patients showed sustained improvement of the EF for a follow-up period of 80 days.
A double-blinded, randomized controlled trial of 44 patients with congestive heart failure (New York Heart Association classes II and III; EF < 40% by echocardiography; and acute MI was excluded) tested three different doses of intravenous NRG1 (0.3, 0.6, 1.2 μg/kg) given for 10 consecutive days versus standard medical therapy (26). The effects were determined by cardiac magnetic resonance imaging, which showed significant improvements of the EF and left ventricular volumes only in the 0.6 μg/kg dose group. These improvements were sustained for 90 days, suggesting lasting effects of the NRG1 administration beyond the immediate period following the infusions.
To become an acceptable therapeutic approach, the design of the animal and human studies of NRG1 administration needs to be optimized to advance the promising results observed to date. Achieving this goal may require developing greater understanding of the underlying cellular and molecular effects and mechanisms. This knowledge could answer questions about which target cells are directly and indirectly affected, what cellular responses occur after administration, and what signaling mechanisms are involved. Advanced knowledge of these mechanisms would provide essential information for conducting future basic, translational, and clinical research studies.
NRG1 and Its Receptors Control Prenatal Myocardial Development
The germline knockouts (KOs) of the NRG1, ErbB2, and ErbB4 genes all showed very similar cardiac phenotypes, i.e., the ventricular myocardial wall was thin at embryonic day (E) 9.5–10.5, leading to fetal demise (28, 55, 64). More specifically, the inner, trabecular layer of myocardium, which arises from the outer, compact layer, was thin. While NRG1 is required for myocardial trabeculation, bone morphogenic protein 10 was identified as an important factor stimulating cardiomyocyte proliferation at E9.5 in mice (30). A zebrafish ErbB2 mutant (59) also lacked myocardial trabeculations. The accessibility of zebrafish larvae for microscopic imaging allowed the investigators to determine that altered cardiomyocyte delamination was the likely underlying cellular mechanism. Complex gene regulatory mechanisms, which connect NRG1/ErbB signaling with myocardial trabeculation, have been identified in mice (54). It is important to note that proliferation and differentiation, which are mutually exclusive in skeletal muscle cells, occur simultaneously in cardiomyocytes. Thus, collectively, these findings suggest that NRG1, ErbB2, and ErbB4 are involved in controlling cardiomyocyte differentiation and migration during cardiac morphogenesis.
In cultured embryonic rat cardiomyocytes, the addition of NRG1 stimulated DNA synthesis and proliferation (97). In an embryonic heart ex vivo culturing system, the addition of NRG1 stimulated formation of trabeculae (35), which is in agreement with the phenotype of the KOs. These results support the notion that the complex of NRG1, ErbB2, and ErbB4 controls cardiomyocyte proliferation and differentiation during development. In addition, NRG1 stimulates the generation of working-type cardiomyocytes in embryonic stem cell cultures (84, 89, 98), indicating that NRG1 may also stimulate formation of cardiomyocytes from undifferentiated stem and progenitor cells.
In summary, during prenatal development, the signaling complex of NRG1, ErbB2, and ErbB4 controls growth of the ventricular myocardium. Additionally, this signaling complex may impact the generation of cardiomyocytes from stem and progenitor cells (63). In humans, germline mutations in the coding regions of the NRG1, ErbB2, and ErbB4 genes have not been identified. However, a recent study found a haplotype in intron 3 of ErbB4 to be associated with congenital heart disease consisting of left-sided obstructive lesions (62).
NRG1 and Its Receptors Control Postnatal Myocardial Development
In mice and rats, most cardiomyocytes withdraw permanently from the cell cycle and 80–90% become binucleated in the first 1 to 2 wk after birth (58, 82). The first indication that NRG1 may be relevant for cardiomyocyte cycling during this stage of development came from the observation that NRG1 stimulated DNA synthesis and survival in isolated neonatal rat cardiomyocytes in vitro (97). Cardiomyocyte-specific inactivation of a floxed ErbB4 gene using a β-myosin light chain-Cre (β-MLC2v) knock-in (12) led to recombination and deletion of exon 2 beginning around E8.5. This strategy yielded viable mice, which developed dilative cardiomyopathy at 3 mo of age (27). The proposed pathogenic mechanism involved abnormal intercellular electrical connectivity and compensatory cardiomyocyte hypertrophy (27). However, this cardiomyocyte-specific ErbB4 KO also showed higher cardiomyocyte nuclear ploidy detected at age 21 days, i.e., before the documented onset of the cardiomyopathy phenotype, raising the possibility that postnatal cardiomyocyte differentiation may also have been affected (27).
ErbB2, the preferred partner for ErbB4 in formation of the active heterodimer in cardiomyocytes (14), was inactivated using the β-MLC2v-Cre and muscle creatine kinase-Cre strategies (17). This study reported the development of dilative cardiomyopathy and proposed as a possible underlying mechanism an increased susceptibility to apoptosis (17). Another study, using a combination of a heterozygous germline KO and the β-MLC2v-Cre strategies, reported severe and partially lethal dilative cardiomyopathy with an onset at 2 mo of age (69). An effect on cardiomyocyte apoptosis and cycling was excluded. It should be noted that the aforementioned genetic deletion strategies, although cardiomyocyte-specific, were not inducible and do not allow definitive assessment of possible cellular effects in the second and third trimesters or early postnatal period.
The effect of inactivating ErbB4 at specific times during postnatal development on cardiac structure and function was determined in another study (6), which used a conditional strategy for inactivating the ErbB4 gene with the α-myosin heavy chain-MerCreMer (α-MHC-MerCreMer) approach (80). With the use of this strategy, ErbB4 was inactivated on the first day of life, which resulted in decreased cardiomyocyte cycling and division detected at 2.5 wk of age (6). Conversely, overexpressing the ErbB4 cDNA under control of the α-MHC promoter increased cardiomyocyte cycling and division, detected at 2.5 wk of age, which resulted in more cardiomyocytes with a smaller mean cellular volume. Because the heart weight was not affected, this suggests that the increased number of cardiomyocytes was offset by decreased cell size (6). In the absence of genetic modifications, injecting NRG1 into 3-wk-old healthy C57Bl6 mice increased cycling and divisions of cardiomyocytes (6).
This study also showed that the cellular mechanisms of NRG1/ErbB4-controlled postnatal cardiomyocyte proliferation involved the population of mononucleated cardiomyocytes (6). This subpopulation in rodents is almost 100% at birth and decreases to 10–20% in the first 2 wk of life (58, 82). In adult mice, only few mononucleated cardiomyocytes proliferated, suggesting the existence of a privileged subpopulation or an unknown limiting mechanism. Inactivating ErbB4 decreased and overexpressing ErbB4, and injecting NRG1, increased the percentage of cycling mononucleated cardiomyocytes. However, the overall percentages of mono- and binucleated cardiomyocytes remained unchanged, indicating that NRG1-stimulated cell cycling of differentiated cardiomyocytes may lead to formation of binucleated cardiomyocytes as well as cell division. This is consistent with the notion that NRG1 stimulates proliferation and differentiation in mature cardiomyocytes and is consistent with its role in stimulating differentiation of immature cardiomyocytes during early myocardial morphogenesis (30). These results indicate that a subpopulation of differentiated, proliferation-competent cardiomyocytes exists in the mononucleated pool, in agreement with the results from cats (13) and rats (51). Molecular-genetic identifiers and characteristics of this putative subpopulation are currently unknown.
These studies indicate that NRG1 and ErbB4 are involved in controlling postnatal cardiomyocyte generation, myocardial growth, and homeostasis, involving mechanisms that include cardiomyocyte proliferation and differentiation. However, the discussed effects on cardiomyocyte proliferation and differentiation were observed in young adult mice, which may have some degree of endogenous cardiomyocyte cycling and proliferation (88), raising the question of what the effect of NRG1 may be on fully cell cycle-withdrawn cardiomyocytes.
NRG1/ErbB4 Signaling in Cardiomyocyte Proliferation in Healthy and Injured Adult Hearts
Most cardiomyocytes in the adult mammalian heart are in proliferative arrest (66, 70). The observation that blocking ErbB2 with the antibody Herceptin induced cardiomyopathy in some patients (50) suggested that the NRG1/ErbB4/ErbB2 complex is active in adult human hearts. The first indication that NRG1 may stimulate cycling of differentiated and fully cell-cycle-withdrawn cardiomyocytes came from observations that NRG1 stimulated DNA synthesis, karyokinesis, and cytokinesis in cultured adult rat ventricular cardiomyocytes (23).
The effect of NRG1 administration on cycling and proliferation of adult cardiomyocytes was evaluated in 3-mo-old C57BL6 mice (6). The stimulation by NRG1 was inhibited by inactivating the ErbB4 gene but not augmented by increasing ErbB4 expression in cardiomyocytes. This indicates that ErbB4 was required for NRG1-stimulated cardiomyocyte cycling in vivo, but overexpressing ErbB4 did not augment the effect, suggesting that other limiting mechanisms are likely active.
In adult animals, the underlying cellular mechanisms of NRG1-stimulated cardiomyocyte division involved a subpopulation of mononucleated cardiomyocytes, which have proliferative potential. This is consistent with video microscopy of cultured adult rat ventricular cardiomyocytes showing that 12.5% of mononucleated cardiomyocytes divided under NRG1 stimulation in a 6-day observation period. In vivo data in agreement with this finding include the presence of double-labeled mononucleated cardiomyocytes after sequential labeling with different thymidine analogs. This is analogous to proliferating mononucleated cardiomyocytes in growing cats (13) and periostin peptide-stimulated rat cardiomyocyte proliferation in vitro and in vivo, where mononucleated cardiomyocytes also cycle (51). Interestingly, myocardial regeneration in adult zebrafish is based on a cellular mechanism of proliferating cardiomyocytes, with almost all cardiomyocytes in zebrafish being mononucleated (90). In summary, adult mice and rats have a subpopulation of differentiated cardiomyocytes that responds to NRG1 with cell cycling and proliferation. Although humans have a higher percentage of mononucleated cardiomyocytes than rodents, the percentage of polyploid nuclei is also higher in humans, and it is currently unknown how these animal data translate into humans (53).
In vitro 5-bromo-2-deoxyuridine labeling followed by visualization of cytokinesis suggests that for NRG1-stimulated cardiomyocyte divisions, DNA synthesis precedes cytokinesis and video microscopy provides direct evidence that karyokinesis precedes cytokinesis (6). However, it is important to note that karyokinesis was not always followed by cytokinesis in cultured adult cardiomyocytes and in mouse hearts in vivo since ∼50% of cell cycles were not productive and resulted in formation of binucleated cardiomyocytes (6), suggesting that karyokinesis without subsequent cytokinesis may be another NRG1-stimulated mechanism. Since it is generally accepted that binucleated cardiomyocytes represent the terminally differentiated phenotype in rodents (82), this can be interpreted as evidence for a prodifferentiation effect of NRG1.
Stimulating and assaying cardiomyocyte cycling and proliferation are experimentally challenging (2, 3, 81). The experimental parameters that may potentially influence the outcome of NRG1-stimulation with respect to cardiomyocyte proliferation are unknown. NRG1 stimulation of cardiomyocyte cell cycle activity and proliferation was demonstrated so far only in cultured adult rat ventricular cardiomyocytes in vitro and in juvenile and young adult mice in vivo [see Table 1, and Bersell et al. (6)]. In addition, all of the animal studies presented in Table 1 involved animals with experimentally induced MI, i.e., without coronary artery disease and comorbidities, in contrast to the two reported human clinical trials. It remains to be tested whether NRG1 can stimulate cardiomyocyte proliferation in aged mice, in injury models other than MI, and in humans.
The effect of NRG1 injection was also evaluated in a mouse MI model (6). In this study, daily administration of NRG1 (Table 1) for 12 wk resulted in a 10% improvement of the EF, determined by echocardiography, and a significant reduction of the infarct scar size. At the cellular level, ∼0.7 × 106 new cardiomyocytes were generated, an increase of 17%, which was accounted for by summing up the number of cardiomyocytes predicted to be generated based on the measured phosphorylated histone H3 index over the 12-wk period (Table 2). NRG1-injected animals had smaller mean cardiomyocyte diameters and the same heart weight as controls, indicating that NRG1-administration may have an antihypertrophic effect.
Table 2.
Back-of-the-envelope calculation of NRG1-stimulated regeneration of cardiomyocytes in mice after MI
| 1 wk of treatment |
12 wk of treatment |
|||
|---|---|---|---|---|
| Control (BSA) | Test (NRG1) | Control (BSA) | Test (NRG1) | |
| BrdU+ cardiomyocytes (5 injections) | 0.04% | 0.18% | 0.01% | 0.15% |
| H3P+ cardiomyocytes | 0.01% | 0.055% | ND | ND |
| Aurora B kinase+ cardiomyocytes | 0.008% | 0.03% | ND | ND |
| Number of cardiomyocyte nuclei present in LV | 3.6 × 106 | 3.1 × 106 | 3.8 × 106 | 5.1 × 106 |
Numeric data are from Bersell et al. (6) and were rounded. Corresponding results from control [injected with bovine serum albumin (BSA)] and treated (NRG1-injected) are shown. BrdU, 5-bromo-2-deoxyuridine; H3P, phosphorylated histone H3; LV, left ventricle; ND, not done.
It is important to note the significantly higher dose of NRG1 (100 μg/kg ip) and longer duration of administration (12 wk) used in this study (6) compared with those in the human trials (0.3–1.2 μg/kg iv for 10 days) and prior animal studies [3–30 μg/kg iv for 5 days; (26, 42, 60); see Table 1]. In addition, it should be noted that different versions of recombinant NRG1 have been applied (Table 1), i.e., full-length NRG1 (amino acids 2–244), comprised of the heparin-binding and the EGF-like domains and an EGF-like domain-only NRG1 (approximately, amino acids 176–246). The functional differences between the dosing and administration regimens and recombinant products are currently unknown.
The notion that NRG1 may stimulate some differentiated cardiomyocytes to divide raises the question of how they divide. In differentiated cardiomyocytes that were actively in karyokinesis, the sarcomeric Z disks and M bands were disassembled in the region of the midzone of the mitotic spindle (6). In cytokinesis, sarcomeric structures were absent from the division plane (6). Thus the observed sarcomeric disassembly was similar to the disassembly described in dividing fetal cardiomyocytes (1) and in regenerating zebrafish hearts (44). This leads to the hypothesis that the intracellular NRG1 effects must include signaling pathways that influence the integrity of the sarcomeric apparatus. These data suggest that NRG1/ErbB signaling may be directly or indirectly connected to the sarcomere structure.
A different study evaluated 10 daily injection of NRG1 in rats with MI and other models of myocardial injury (60). This aforementioned study demonstrated significant functional improvements of the combination of NRG1 with ACE inhibitor compared with a control group receiving only ACE inhibitor.
Potential Effects of NRG1 Administration that Do Not Involve Cardiomyocyte Proliferation
NRG1 administration may activate other cellular processes in those cardiomyocytes that do not proliferate. These processes may be relevant for understanding the effects of therapeutic strategies that target the NRG1/ErbB2/ErbB4 complex. NRG1 has been shown to be antiapoptotic in cultured adult rat ventricular cardiomyocytes via activating the Akt pathway (24). This mechanism is consistent with the cardioprotective function of endogenous NRG1, released from the vascular endothelium (16, 57), in the acute phase after experimental MI (33). However, NRG1 injection 1 wk after MI did not affect the percentage of cardiomyocyte apoptosis (6). NRG1 administration may also be involved with regulation of the structure of cardiomyocyte sarcomeres as it affects anthracyclin-induced myofibril disarray in cell culture (11, 24, 52, 77) and improves anthracycline-induced myocardial dysfunction in mice (7). However, the cardiomyocyte-specific KOs of ErbB2 and ErbB4 did not show myofibril disarray, despite having dilative cardiomyopathy (17, 27, 69). In addition, NRG1 administration alters the transcription of many genes in the heart; for example, it lowers levels of α- and β-MHC and increases cardiac MLC kinase (31) levels and phosphorylation in vivo, which may affect the molecular composition and regulation of the sarcomeres. Furthermore, NRG1 addition to isolated cardiomyocytes regulated the activity of the sarco(endo)plasmic reticulum Ca2+-ATPase 2, thereby improving calcium handling (8). If operative in vivo, this mechanism may improve diastolic dysfunction. In this context, it is interesting to note that the administration of NRG1 decreased inotropy in isolated papillary muscles (56). In a different report, heterozygous NRG1 KO animals displayed altered regulation of cardiomyocyte excitation and contraction by muscarinic and adrenergic agonists (67), suggesting an effect of endogenous NRG1 on autonomic control of cardiomyocyte contractile function. In summary, both endogenously produced and exogenously administered NRG1 activate many potentially beneficial processes in the heart.
Thus far, only the cardiomyocyte-autonomous effects of NRG1 administration have been considered. However, NRG1, whether endogenously produced or administered, may also affect cells other than cardiomyocytes. Perhaps NRG1 stimulates differentiation of resident stem and progenitor cells into cardiomyocytes, as suggested by its cardiomyogenic effect in embryonic stem cell cultures (84, 89, 98). However, the detection of cell cycle events in (genetically labeled) cardiomyocytes suggests this is unlikely to be the case in adult mice in vivo, although effects on immature progenitor cells and cardiomyocytes may coexist (6). An additional important consideration is the possibility that NRG1 may not only act on cells of the cardiomyocyte lineage but rather on resident noncardiomyocytes, which are often generally called cardiac fibroblasts (45, 95). This possible effect of NRG1 on cardiac fibroblasts may involve complex and currently unknown paracrine and juxtacrine pathways (18, 29, 65). In addition, it has been proposed that NRG1-administration may regulate angiogenesis, which may in turn contribute to its beneficial effects (34, 38, 46, 75).
The fact that NRG1 administration has multiple effects in the heart is not sufficient to conclude that it is the only or the physiological ligand of ErbB4 in the myocardium. In fact, HB-EGF, a high-affinity ligand for the EGF receptor (EGFR, also called ErbB1) and ErbB4, is expressed in the heart (40, 41). Conditional inactivation of HB-EGF induces cardiac dysfunction and dilative cardiomyopathy (41), but the effect of administration of HB-EGF on cardiac structure and function in myocardial disease models is unknown. Although HB-EGF stimulated cell-cycle activity in embryonic cardiomyocytes in vitro (37), its effect in adult cardiomyocytes was small compared with NRG1, fibroblast growth factor, and periostin peptide (6). Furthermore, the EGFR (ErbB1), which serves as the primary receptor for HB-EGF, has been linked to cardiomyocyte hypertrophy (79), suggesting that differences may exist between the cellular effects of NRG1 and HB-EGF on cardiomyocytes.
Effects of NRG1 Administration Outside of the Heart
In light of efforts to advance NRG1 administration into a clinical therapy, it is important to consider the potential effects outside of the heart, which may give rise to side effects. Since both the nervous system and the breast gland express NRG1 receptors in adults, these organs may show side effects. Injection of NRG1 and ErbB receptor antagonists into the rostral ventrolateral medulla, an important region involved in central cardiovascular regulation, affected heart rate and blood pressure (61). Although it is unknown whether NRG1 administered by injection into the peritoneum or blood stream is able to cross the blood-brain barrier, these findings and the association between ErbB signaling and mental disorders (15) suggest the potential for side effects on the nervous system.
Since NRG1 stimulates cellular proliferation, a theoretical risk for cancer growth exists. However, it is important to note that the mechanism of stimulation of cancer growth by ErbB overexpression is constitutive receptor activity, i.e., ligand independent (9, 14). In contrast, physiological ErbB2 or ErbB4 action is ligand dependent. This fundamental difference may explain why tumor formation after long-term NRG1 administration has not been reported.
Summary
The in vivo and in vitro studies discussed in this article collectively help us to appreciate the role of NRG1/ErbB4 signaling in cardiomyocyte proliferation. The mechanisms of action of endogenously produced as well as administered recombinant NRG1 in the mammalian heart are beginning to be understood, and stimulation of cardiomyocyte proliferation may be part of it. Fundamental research will be necessary to map out in detail the mechanisms of action, both for endogenous regulation and for stimulation of cardiomyocyte proliferation with recombinant NRG1. This research should help in fulfilling the promise of therapeutic approaches to treating heart failure with recombinant NRG1 preparations, a strategy that awaits validation in humans.
GRANTS
Our research was supported by the Department of Cardiology and the Translational Investigator Program (Children's Hospital Boston); National Heart, Lung, and Blood Institute Grants K08-HL-085143 and R01-HL-106302; the Children's Cardiomyopathy Foundation; and the Geneen Trust.
DISCLOSURES
Children's Hospital Boston has filed a patent application on the use of NRG1 to treat heart failure. At this point, it is not clear whether the patent will be granted nor whether there will be royalties.
AUTHOR CONTRIBUTIONS
B.W. and B.K. conception and design of research; B.W. performed experiments; B.W. and B.K. interpreted results of experiments; B.W. prepared figures; B.W. and B.K. drafted manuscript; B.W. and B.K. edited and revised manuscript; B.W. and B.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We apologize to the many scientists whose work we were not able to credit because of space restrictions. Original work has been cited at the expense of many excellent reviews. We thank our colleagues for many stimulating suggestions and helpful discussions and thank B. Polizzotti, S. Suresh, C. Edwards, and K. Bersell for comments on this text.
REFERENCES
- 1. Ahuja P, Perriard E, Perriard JC, Ehler E. Sequential myofibrillar breakdown accompanies mitotic division of mammalian cardiomyocytes. J Cell Sci 117: 3295– 3306, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Ahuja P, Sdek P, MacLellan WR. Cardiac myocyte cell cycle control in development, disease, and regeneration. Physiol Rev 87: 521– 544, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anversa P, Kajstura J. Ventricular myocytes are not terminally differentiated in the adult mammalian heart. Circ Res 83: 1– 14, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med 355: 1222– 1232, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science 324: 98– 102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 138: 257– 270, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Bian Y, Sun M, Silver M, Ho KK, Marchionni MA, Caggiano AO, Stone JR, Amende I, Hampton TG, Morgan JP, Yan X. Neuregulin-1 attenuated doxorubicin-induced decrease in cardiac troponins. Am J Physiol Heart Circ Physiol 297: H1974– H1983, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brero A, Ramella R, Fitou A, Dati C, Alloatti G, Gallo MP, Levi R. Neuregulin-1beta1 rapidly modulates nitric oxide synthesis and calcium handling in rat cardiomyocytes. Cardiovasc Res 88: 443– 452, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Bublil EM, Yarden Y. The EGF receptor family: spearheading a merger of signaling and therapeutics. Curr Opin Cell Biol 19: 124– 134, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Chaudhry HW, Dashoush NH, Tang H, Zhang L, Wang X, Wu EX, Wolgemuth DJ. Cyclin A2 mediates cardiomyocyte mitosis in the postmitotic myocardium. J Biol Chem 279: 35858– 35866, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Chen B, Peng X, Pentassuglia L, Lim CC, Sawyer DB. Molecular and cellular mechanisms of anthracycline cardiotoxicity. Cardiovasc Toxicol 7: 114– 121, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Chen J, Kubalak SW, Minamisawa S, Price RL, Becker KD, Hickey R, Ross J, Jr, Chien KR. Selective requirement of myosin light chain 2v in embryonic heart function. J Biol Chem 273: 1252– 1256, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Chen X, Wilson RM, Kubo H, Berretta RM, Harris DM, Zhang X, Jaleel N, MacDonnell SM, Bearzi C, Tillmanns J, Trofimova I, Hosoda T, Mosna F, Cribbs L, Leri A, Kajstura J, Anversa P, Houser SR. Adolescent feline heart contains a population of small, proliferative ventricular myocytes with immature physiological properties. Circ Res 100: 536– 544, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 7: 505– 516, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci 7: 575– 580, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Cote GM, Miller TA, Lebrasseur NK, Kuramochi Y, Sawyer DB. Neuregulin-1alpha and beta isoform expression in cardiac microvascular endothelial cells and function in cardiac myocytes in vitro. Exp Cell Res 311: 135– 146, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J, Jr, Chien KR, Lee KF. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med 8: 459– 465, 2002 [DOI] [PubMed] [Google Scholar]
- 18. De Keulenaer GW, Doggen K, Lemmens K. The vulnerability of the heart as a pluricellular paracrine organ: lessons from unexpected triggers of heart failure in targeted ErbB2 anticancer therapy. Circ Res 106: 35– 46, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Doggen K, Ray L, Mathieu M, Mc Entee K, Lemmens K, De Keulenaer GW. Ventricular ErbB2/ErbB4 activation and downstream signaling in pacing-induced heart failure. J Mol Cell Cardiol 46: 33– 38, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Elenius K, Choi CJ, Paul S, Santiestevan E, Nishi E, Klagsbrun M. Characterization of a naturally occurring ErbB4 isoform that does not bind or activate phosphatidyl inositol 3-kinase. Oncogene 18: 2607– 2615, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Elenius K, Corfas G, Paul S, Choi CJ, Rio C, Plowman GD, Klagsbrun M. A novel juxtamembrane domain isoform of HER4/ErbB4. Isoform-specific tissue distribution and differential processing in response to phorbol ester. J Biol Chem 272: 26761– 26768, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Engel FB, Hsieh PC, Lee RT, Keating MT. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc Natl Acad Sci USA 103: 15546– 15551, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev 19: 1175– 1187, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, Kelly RA, Sawyer DB. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol 35: 1473– 1479, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Fuller SJ, Sivarajah K, Sugden PH. ErbB receptors, their ligands, and the consequences of their activation and inhibition in the myocardium. J Mol Cell Cardiol 44: 831– 854, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Gao R, Zhang J, Cheng L, Wu X, Dong W, Yang X, Li T, Liu X, Xu Y, Li X, Zhou M. A phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J Am Coll Cardiol 55: 1907– 1914, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Garcia-Rivello H, Taranda J, Said M, Cabeza-Meckert P, Vila-Petroff M, Scaglione J, Ghio S, Chen J, Lai C, Laguens RP, Lloyd KC, Hertig CM. Dilated cardiomyopathy in Erb-b4-deficient ventricular muscle. Am J Physiol Heart Circ Physiol 289: H1153– H1160, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature 378: 390– 394, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 103: 1204– 1219, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grego-Bessa J, Luna-Zurita L, del Monte G, Bolos V, Melgar P, Arandilla A, Garratt AN, Zang H, Mukouyama YS, Chen H, Shou W, Ballestar E, Esteller M, Rojas A, Perez-Pomares JM, de la Pompa JL. Notch signaling is essential for ventricular chamber development. Dev Cell 12: 415– 429, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gu X, Liu X, Xu D, Li X, Yan M, Qi Y, Yan W, Wang W, Pan J, Xu Y, Xi B, Cheng L, Jia J, Wang K, Ge J, Zhou M. Cardiac functional improvement in rats with myocardial infarction by up-regulating cardiac myosin light chain kinase with neuregulin. Cardiovasc Res 88: 334– 343, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Hansson EM, Lindsay ME, Chien KR. Regeneration next: toward heart stem cell therapeutics. Cell Stem Cell 5: 364– 377, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Hedhli N, Huang Q, Kalinowski A, Palmeri M, Hu X, Russell RR, Russell KS. Endothelium-derived neuregulin protects the heart against ischemic injury. Circulation 123: 2254– 2262, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hedhli N, Russell KS. Cytostatic drugs, neuregulin activation of erbB receptors, and angiogenesis. Curr Hypertens Rep 12: 411– 417, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Hertig CM, Kubalak SW, Wang Y, Chien KR. Synergistic roles of neuregulin-1 and insulin-like growth factor-I in activation of the phosphatidylinositol 3-kinase pathway and cardiac chamber morphogenesis. J Biol Chem 274: 37362– 37369, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Hwang H, Kloner RA. Improving regenerating potential of the heart after myocardial infarction: factor-based approach. Life Sci 86: 461– 472, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, Srivastava D. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell 16: 233– 244, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Iivanainen E, Paatero I, Heikkinen SM, Junttila TT, Cao R, Klint P, Jaakkola PM, Cao Y, Elenius K. Intra- and extracellular signaling by endothelial neuregulin-1. Exp Cell Res 313: 2896– 2909, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Iwamoto R, Mekada E. ErbB and HB-EGF signaling in heart development and function. Cell Struct Funct 31: 1– 14, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Iwamoto R, Mine N, Kawaguchi T, Minami S, Saeki K, Mekada E. HB-EGF function in cardiac valve development requires interaction with heparan sulfate proteoglycans. Development 137: 2205– 2214, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Iwamoto R, Yamazaki S, Asakura M, Takashima S, Hasuwa H, Miyado K, Adachi S, Kitakaze M, Hashimoto K, Raab G, Nanba D, Higashiyama S, Hori M, Klagsbrun M, Mekada E. Heparin-binding EGF-like growth factor and ErbB signaling is essential for heart function. Proc Natl Acad Sci USA 100: 3221– 3226, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jabbour A, Hayward CS, Keogh AM, Kotlyar E, McCrohon JA, England JF, Amor R, Liu X, Li XY, Zhou MD, Graham RM, Macdonald PS. Parenteral administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur J Heart Fail 13: 83– 92, 2011 [DOI] [PubMed] [Google Scholar]
- 43. Janssens S. Stem cells in the treatment of heart disease. Annu Rev Med 61: 287– 300, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Jopling C, Sleep E, Raya M, Marti M, Raya A, Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 464: 606– 609, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kakkar R, Lee RT. Intramyocardial fibroblast myocyte communication. Circ Res 106: 47– 57, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kalinowski A, Plowes NJ, Huang Q, Berdejo-Izquierdo C, Russell RR, Russell KS. Metalloproteinase-dependent cleavage of neuregulin and autocrine stimulation of vascular endothelial cells. FASEB J 24: 2567– 2575, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kanashiro-Takeuchi RM, Schulman IH, Hare JM. Pharmacologic and genetic strategies to enhance cell therapy for cardiac regeneration. J Mol Cell Cardiol 51: 619– 625, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kawase Y, Ladage D, Hajjar RJ. Rescuing the failing heart by targeted gene transfer. J Am Coll Cardiol 57: 1169– 1180, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kaye DM, Krum H. Drug discovery for heart failure: a new era or the end of the pipeline? Nat Rev Drug Discov 6: 127– 139, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Keefe DL. Trastuzumab-associated cardiotoxicity. Cancer 95: 1592– 1600, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Kuhn B, Del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med 13: 962– 969, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Kuramochi Y, Guo X, Sawyer DB. Neuregulin activates erbB2-dependent src/FAK signaling and cytoskeletal remodeling in isolated adult rat cardiac myocytes. J Mol Cell Cardiol 41: 228– 235, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Laflamme MA, Murry CE. Heart regeneration. Nature 473: 326– 335, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lai D, Forrai A, Liu X, Wolstein O, Michalicek J, Ahmed I, Garratt AN, Birchmeier C, Zhou M, Hartley L, Robb L, Feneley MP, Fatkin D, Harvey RP. Neuregulin 1 sustains the gene regulatory network in both trabecular and nontrabecular myocardium. Circ Res 107: 715– 727, 2010 [DOI] [PubMed] [Google Scholar]
- 55. Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature 378: 394– 398, 1995 [DOI] [PubMed] [Google Scholar]
- 56. Lemmens K, Fransen P, Sys SU, Brutsaert DL, De Keulenaer GW. Neuregulin-1 induces a negative inotropic effect in cardiac muscle: role of nitric oxide synthase. Circulation 109: 324– 326, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Lemmens K, Segers VF, Demolder M, De Keulenaer GW. Role of neuregulin-1/ErbB2 signaling in endothelium-cardiomyocyte cross-talk. J Biol Chem 281: 19469– 19477, 2006 [DOI] [PubMed] [Google Scholar]
- 58. Li F, Wang X, Capasso JM, Gerdes AM. Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J Mol Cell Cardiol 28: 1737– 1746, 1996 [DOI] [PubMed] [Google Scholar]
- 59. Liu J, Bressan M, Hassel D, Huisken J, Staudt D, Kikuchi K, Poss KD, Mikawa T, Stainier DY. A dual role for ErbB2 signaling in cardiac trabeculation. Development 137: 3867– 3875, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu X, Gu X, Li Z, Li X, Li H, Chang J, Chen P, Jin J, Xi B, Chen D, Lai D, Graham RM, Zhou M. Neuregulin-1/erbB-activation improves cardiac function and survival in models of ischemic, dilated, and viral cardiomyopathy. J Am Coll Cardiol 48: 1438– 1447, 2006 [DOI] [PubMed] [Google Scholar]
- 61. Matsukawa R, Hirooka Y, Nishihara M, Ito K, Sunagawa K. Neuregulin-1/ErbB signaling in rostral ventrolateral medulla is involved in blood pressure regulation as an antihypertensive system. J Hypertens 29: 1735– 1742, 2011 [DOI] [PubMed] [Google Scholar]
- 62. McBride KL, Zender GA, Fitzgerald-Butt SM, Seagraves NJ, Fernbach SD, Zapata G, Lewin M, Towbin JA, Belmont JW. Association of common variants in ERBB4 with congenital left ventricular outflow tract obstruction defects. Birth Defects Res A Clin Mol Teratol 91: 162– 168, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mercola M, Ruiz-Lozano P, Schneider MD. Cardiac muscle regeneration: lessons from development. Genes Dev 25: 299– 309, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature 378: 386– 390, 1995 [DOI] [PubMed] [Google Scholar]
- 65. Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol 50: 280– 289, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ Res 92: 139– 150, 2003 [DOI] [PubMed] [Google Scholar]
- 67. Okoshi K, Nakayama M, Yan X, Okoshi MP, Schuldt AJ, Marchionni MA, Lorell BH. Neuregulins regulate cardiac parasympathetic activity: muscarinic modulation of beta-adrenergic activity in myocytes from mice with neuregulin-1 gene deletion. Circulation 110: 713– 717, 2004 [DOI] [PubMed] [Google Scholar]
- 68. Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 19: 3159– 3167, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ozcelik C, Erdmann B, Pilz B, Wettschureck N, Britsch S, Hubner N, Chien KR, Birchmeier C, Garratt AN. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc Natl Acad Sci USA 99: 8880– 8885, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pasumarthi KB, Field LJ. Cardiomyocyte cell cycle regulation. Circ Res 90: 1044– 1054, 2002 [DOI] [PubMed] [Google Scholar]
- 71. Pasumarthi KB, Nakajima H, Nakajima HO, Soonpaa MH, Field LJ. Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ Res 96: 110– 118, 2005 [DOI] [PubMed] [Google Scholar]
- 72. Pentassuglia L, Sawyer DB. The role of neuregulin-1beta/ErbB signaling in the heart. Exp Cell Res 315: 627– 637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Reiss K, Cheng W, Ferber A, Kajstura J, Li P, Li B, Olivetti G, Homcy CJ, Baserga R, Anversa P. Overexpression of insulin-like growth factor-1 in the heart is coupled with myocyte proliferation in transgenic mice. Proc Natl Acad Sci USA 93: 8630– 8635, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart Disease and Stroke Statistics—2012 update: a report from the American Heart Association. Circulation 125: e2– e220, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Russell KS, Stern DF, Polverini PJ, Bender JR. Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. Am J Physiol Heart Circ Physiol 277: H2205– H2211, 1999 [DOI] [PubMed] [Google Scholar]
- 76. Sawyer DB, Caggiano A. Neuregulin-1beta for the treatment of systolic heart failure. J Mol Cell Cardiol 51: 501– 505, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sawyer DB, Zuppinger C, Miller TA, Eppenberger HM, Suter TM. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation 105: 1551– 1554, 2002 [DOI] [PubMed] [Google Scholar]
- 78. Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med 355: 1210– 1221, 2006 [DOI] [PubMed] [Google Scholar]
- 79. Shah BH, Catt KJ. A central role of EGF receptor transactivation in angiotensin II -induced cardiac hypertrophy. Trends Pharmacol Sci 24: 239– 244, 2003 [DOI] [PubMed] [Google Scholar]
- 80. Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res 89: 20– 25, 2001 [DOI] [PubMed] [Google Scholar]
- 81. Soonpaa MH, Field LJ. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ Res 83: 15– 26, 1998 [DOI] [PubMed] [Google Scholar]
- 82. Soonpaa MH, Kim KK, Pajak L, Franklin M, Field LJ. Cardiomyocyte DNA synthesis and binucleation during murine development. Am J Physiol Heart Circ Physiol 271: H2183– H2189, 1996 [DOI] [PubMed] [Google Scholar]
- 83. Steinhauser ML, Lee RT. Regeneration of the heart. EMBO Mol Med 3: 701– 712, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sun M, Yan X, Bian Y, Caggiano AO, Morgan JP. Improving murine embryonic stem cell differentiation into cardiomyocytes with neuregulin-1: differential expression of microRNA. Am J Physiol Cell Physiol 301: C21– C30, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Telli ML, Hunt SA, Carlson RW, Guardino AE. Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J Clin Oncol 25: 3525– 3533, 2007 [DOI] [PubMed] [Google Scholar]
- 86. Vanderlaan RD, Hardy WR, Kabir MG, Pasculescu A, Jones N, deTombe PP, Backx PH, Pawson T. The ShcA phosphotyrosine docking protein uses distinct mechanisms to regulate myocyte and global heart function. Circ Res 108: 184– 193, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Veikkolainen V, Vaparanta K, Halkilahti K, Iljin K, Sundvall M, Elenius K. Function of ERBB4 is determined by alternative splicing. Cell Cycle 10: 2647– 2657, 2011 [DOI] [PubMed] [Google Scholar]
- 88. Walsh S, Ponten A, Fleischmann BK, Jovinge S. Cardiomyocyte cell cycle control and growth estimation in vivo—an analysis based on cardiomyocyte nuclei. Cardiovasc Res 86: 365– 373, 2010 [DOI] [PubMed] [Google Scholar]
- 89. Wang Z, Xu G, Wu Y, Guan Y, Cui L, Lei X, Zhang J, Mou L, Sun B, Dai Q. Neuregulin-1 enhances differentiation of cardiomyocytes from embryonic stem cells. Med Biol Eng Comput 47: 41– 48, 2009 [DOI] [PubMed] [Google Scholar]
- 90. Wills AA, Holdway JE, Major RJ, Poss KD. Regulated addition of new myocardial and epicardial cells fosters homeostatic cardiac growth and maintenance in adult zebrafish. Development 135: 183– 192, 2008 [DOI] [PubMed] [Google Scholar]
- 91. Xu Y, Li X, Zhou M. Neuregulin-1/ErbB signaling: a druggable target for treating heart failure. Curr Opin Pharmacol 9: 214– 219, 2009 [DOI] [PubMed] [Google Scholar]
- 92. Yan X, Morgan JP. Neuregulin1 as novel therapy for heart failure. Curr Pharm Des 17: 1808– 1817, 2011 [DOI] [PubMed] [Google Scholar]
- 93. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2: 127– 137, 2001 [DOI] [PubMed] [Google Scholar]
- 94. Yi BA, Wernet O, Chien KR. Pregenerative medicine: developmental paradigms in the biology of cardiovascular regeneration. J Clin Invest 120: 20– 28, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zeisberg EM, Kalluri R. Origins of cardiac fibroblasts. Circ Res 107: 1304– 1312, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhang Y, Li TS, Lee ST, Wawrowsky KA, Cheng K, Galang G, Malliaras K, Abraham MR, Wang C, Marban E. Dedifferentiation and proliferation of mammalian cardiomyocytes. PLoS One 5: e12559, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem 273: 10261– 10269, 1998 [DOI] [PubMed] [Google Scholar]
- 98. Zhu WZ, Xie Y, Moyes KW, Gold JD, Askari B, Laflamme MA. Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ Res 107: 776– 786, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]