Abstract
Secondary lymphedema in humans is a common consequence of axillary lymph node dissection (ALND) to treat breast cancer. It is commonly hypothesized that lymphatic growth is required to increase fluid drainage and ameliorate lymphedema. Although there is a pronounced alteration in the balance of interstitial forces regulating fluid transport that sustains the chronic form of lymphedema, it is presently unknown whether changes occur to the balance of interstitial forces during acute lymphedema that may play a role in the recovery of fluid drainage. Here, we compared the relative importance of lymphangiogenesis of lymphatic vessels and interstitial flows for restoring fluid drainage and resolving acute lymphedema in the mouse foreleg after ALND. We found that removal of the axillary lymph nodes reduced lymph drainage in the foreleg at days 0 and 5 postsurgery, with fluid tracer spreading interstitially through subcutaneous tissues. Interstitial fluid drainage returned to normal by day 10, whereas functional regrowth of lymphatic vessels was first detected by indocyanine green fluorescence lymphography at day 15, demonstrating that the recovery of interstitial fluid drainage preceded the regrowth of lymphatic vessels. This was confirmed by the administration of VEGF receptor-3-neutralizing antibodies, which completely blocks lymphatic regrowth. It was found that the recovery of interstitial fluid drainage and the natural resolution of acute lymphedema produced by ALND were not hindered by VEGF receptor-3 neutralization, demonstrating that interstitial fluid drainage recovery and the resolution of acute lymphedema are lymphangiogenesis independent. The data highlight the central role of the interstitial environment in adapting to lymphatic injury to increase fluid drainage.
Keywords: vascular endothelial growth factor receptor 3, axillary lymph node dissection, interstitial flow, lymphedema
secondary lymphedema in humans is a common consequence of axillary lymph node dissection (ALND) to treat breast cancer, which disconnects lymphatic vessels and excises extracellular matrix from the axilla (9). The intervening surgical space forms into scar tissue, a matrix material that has been demonstrated to hinder lymphatic regeneration and interstitial flows (1, 21, 28, 33, 34). It has been hypothesized that an inability of the lymphatic system to adequately regenerate during normal wound repair may predispose the tissue to swell during secondary lymphedema (21, 33). For this reason, attention has recently shifted to clarifying the regulation of lymphangiogenesis by molecules expressed during wound repair (2, 10, 11, 21, 33, 35, 37).
An important characteristic of secondary lymphedema is that the chronic, lifelong swelling appears in ∼25% of patients several years after the initial lymphatic-related injury has healed, although there may be a period of acute, transient swelling that appears immediately after the surgery (9, 17, 18). The question of why the chronic form of lymphedema does not appear immediately after surgery in the human subject, when the lymphatic system has just been disrupted, remains unanswered. There is evidence that accumulating lymph may experience compensatory drainage at the expense of higher outflow resistance through a reduced number of surviving local lymphatics, rerouted lymphatics that bypass the obstructive tissue, or lymphovenous communications (17, 26). Each of these may help resolve the acute lymphedema yet fail to prevent lymphatic pump failure and chronic lymphedema (5, 26, 29).
Capillary filtration is governed by the four Starling forces, fluid pressure in the blood capillaries, fluid pressure in the interstitium, osmotic pressure in the blood capillaries, and osmotic pressure in the interstitium (27). When lymphatic drainage decreases during lymphedema, the interstitial fluid pressure increases (6, 7). This leads to tissue swelling, increased hydraulic conductivity, and interstitial matrix remodeling (13, 23). These interstitial adaptations may, in turn, increase interstitial fluid flow and reduce tissue swelling. Although there is a pronounced alteration in the balance of interstitial forces regulating fluid transport that sustains the chronic form of lymphedema, it is presently unknown whether changes occur to the balance of interstitial forces during acute lymphedema that may play a role in the recovery of fluid drainage. It is also unknown how the importance of lymphangiogenesis of lymph vessels during the acute lymphedema compares to the capacity of the microcirculation to compensate for lymph flow deficiencies through changes in the balance of interstitial forces.
Here, we recapitulated the human lymphatic injury by conducting axillary lymph node dissections in the mouse foreleg. New functional lymphatic growth was idenified with indocyanine green (ICG) fluorescent lymphography. The temporal recovery of lymphatic drainage was quantified by measuring the coverage of fluorescent fluid tracer in histological cross sections along the foreleg. Finally, the interrelationship between lymph drainage and lymph vessel regrowth were decoupled by administering VEGF receptor (VEGFR)-3-neutralizing antibodies, which has been shown in numerous studies (12, 21, 22) to prevent new lymphatic growth. These approaches allowed us to temporally correlate new lymphatic vessel growth with the recovery of interstitial fluid drainage, demonstrate conclusively that changes to the balance of interstitial forces increases fluid drainage in acute lymphedema, and compare the relative importance of lymphangiogenesis and interstitial flows for restoring drainage and resolving the acute lymphedema that follows lymphatic injury.
METHODS
Foreleg murine lymphedema model.
Female wild-type C57BL/6 or Balb/c mice (Harlan, age: 6–8 wk) were used for all experiments. Mice were anesthetized with 2.5% isoflurane mixed with O2 gas for surgical procedures and were killed at experimental end points by CO2 asphyxiation. All protocols were approved by the Animal Care and Use Committee of Michigan Technological University. Axillary lymph nodes were removed from female C57BL/6 or wild-type Balb/c mice as previously described (31). Briefly, a 5-mm-long surgical incision through the dermis was placed across the axilla on the right side, and the axillary lymph nodes were identified and excised along with visible portions of prenodal and postnodal collecting vessels under an Olympus SZX7 stereo microscope. The dermis was sutured with 6-0 suture thread (Harvard Apparatus, Holliston, MA).
Quantifying lymph drainage.
To determine the time course of lymph drainage recovery after lymphatic injury, axillary lymph nodes were removed from mice and an assessment was made of lymph drainage function at days 0, 5, 10, or 15 postsurgery (n = 10 mice/group). Tetramethylrhodamine-conjugated dextran (2,000,000 molecular weight, Invitrogen, Carlsbad, CA) at 1 mg/ml in PBS was used as a fluorescent lymph tracer to quantify fluid drainage in the mouse foreleg. At the specified days postsurgery, 10 μl of fluorescent tracer solution were injected intradermally into the posterior of both foreleg paws. Because the presence and distribution of the tracer across the foreleg depend on interstitial fluid drainage, the coverage of fluorescent tracer that is measured later in foreleg cross sections can serve to quantify drainage across the foreleg. Collected forelegs were cryosectioned to produce 100-μm cross sections at the elbow joint (designated as the upper location), midway between the elbow and wrist (middle location), and near the wrist (lower location). Sections were counterstained for cell nuclei with 4′,6-diamino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) and imaged under an Olympus BX51 fluorescent microscope. The fluorescent tracer area of coverage was quantified using Metamorph Offline 6.3r7 software and expressed as a percentage of the total cross-sectional area of the foreleg tissue section. To optimize conditions for fluid tracer accumulation after ALND, mice were allowed to regain activity for 30 min, 2 h, or 6 h before euthanization (n = 10) to provide time for the tracer to drain through the foreleg lymphatics. We found the greatest coverage of fluorescent dye in the foreleg of mice that were allowed to regain activity for 6 h after injection of the fluorescent dye post-ALND (data not shown). Thus, we allowed all mice to recover for 6 h after dextran injections to quantify lymph drainage postsurgery.
Neutralizing antibodies.
It has been shown that the regrowth of lymphatic collecting vessels after injury is VEGFR-3 signaling dependent (14). To clarify the importance of VEGFR-3 signaling and lymphangiogenesis of lymph vessels for lymphedema resolution, we used the ALND murine model in conjunction with VEGFR-3-blocking antibodies (n = 10 mice/group). Antagonist antibodies against mouse VEGFR-3 (mF4-31C1) were provided by ImClone Systems (New York, NY). Continuous inhibition of VEGFR-3 with 150-μl ip injections of mF4-31C1 at 0.625 mg/dose (1 injection/mouse every 5 days) has been shown to completely inhibit lymphangiogenesis in vivo (12, 22). The control group received 150-μl injections of saline. Treatment was initiated 1 day before surgery and proceeded every 5 days thereafter. An injection was not administered the day before euthanization.
Immunofluorescence and immunohistochemistry.
Immunostaining was conducted on foreleg specimens cut into 50-μm cross sections. Podoplanin was immunolabeled to detect lymphatic endothelial cells. A hamster monoclonal antibody against podoplanin (AngioBio) was used with an Alexa fluor 647 goat anti-hamster secondary antibody (Invitrogen). Cell nuclei were counterstained with DAPI (Vector Laboratories). The path taken by lymph through the foreleg after the injection of 2,000,000 molecular weight tetramethylrhodamine-conjugated dextran was identified in cross sections by immobilizing the lysine-fixable fluid tracer. Fluorescence images were captured with a Zeiss MRm camera on a Zeiss Axiovert 200M fluorescence microscope with the Apotome system. This system collects a stack of two-dimensional images that are then compressed into a single image.
Physiological measurements.
Foreleg wrist thickness was measured using Metamorph software from digital images of the mouse foreleg, and right wrist thickness was normalized to the unoperated left wrist thickness for each mouse. Arm area was measured using Metamorph software from digital images of the mouse foreleg by outlining the paw, wrist, and arm on the right side relative to the unoperated left side for each mouse. Skin thickness of the swollen and nonswollen contralateral arm of each mouse was measured with MetaMorph imaging software (Molecular Devices) from sections obtained 4 mm distal to the elbow of each arm. Thickness of the edematous skin was normalized to the contralateral (nonswollen) skin for each mouse.
Imaging of functional lymphatic vessels via ICG fluorescence lymphography.
We used ICG fluorescence lymphography to identify lymphatic vessel regeneration in the ALND model and to compare the timing of lymphatic vessel regrowth with the recovery of lymphatic drainage (n = 5 mice/group). An imaging system recently developed by Drs. N. Unno, F. Ogata, and E. M. Sevick-Muraca (19, 20, 24, 25, 32) was used to detect functional lymphatic vessels and lymph nodes in the mouse foreleg. The fluorescent near-infrared dye ICG (2.5 μl of a 5 mg/ml solution, Akorn) was injected into the mouse paw. Detection of ICG was performed with an electron multiplying charge-coupled device (C9100-13, Hamamatsu) using Hamamatsu recommendations as previously described (32). ICG was illuminated with an array of 760 nm LEDs (Epitex) placed before a 760-nm band-pass filter (model 760FS10-50, Andover) to provide the excitation light to activate ICG. A 785- and 763-nm custom-made holographic notch band rejection filter (model HNPF-785.0-2.0 and HNPF-763.0-2.0, Kaiser Optical Systems) and a 830-nm image quality band-pass filter (model 830FS20-25, Andover) were placed before the camera lens to selectively reject the excitation light and pass the emitted 830-nm wavelength. After microdose paw injections of ICG dye, images were collected over a period of 30 min to visualize the path of ICG drainage through the mouse foreleg. Nonoperated mice and the left forelegs of operated mice were imaged for control comparisons.
Statistical methods.
Between 5 and 10 animals were used for each experimental group. Data are presented as means ± SE. P values were calculated using ANOVA or a Tukey-Kramer highly significant difference test as indicated.
RESULTS
Rapid recovery of fluid drainage after ALND.
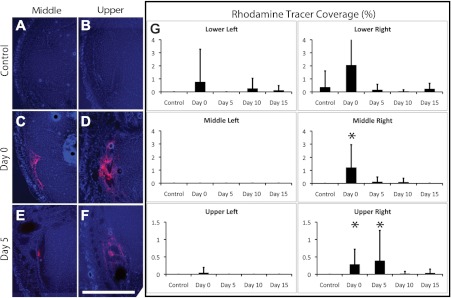
We found that the dextran fluid tracer was scarcely detectable in foreleg cross sections of nonoperated control mice (Fig. 1, A and B), consistent with the functional lymphatic system that was present in control mice, which efficiently clears the dye across the foreleg. In contrast, the fluorescent signal was most prominent in sections of operated forelegs at the middle (midway up the foreleg) and upper (near the elbow) locations (Fig. 1, C–F) at days 0 and 5, indicative of deficient lymph drainage through the foreleg at these locations and times. A statistical analysis of the measurements revealed that tracer coverage was significantly increased at the middle location of the operated right forelegs of mice at day 0 relative to the control condition (P < 0.01 by ANOVA; Fig. 1G), returning to control values at day 5. There was significantly increased tracer coverage at days 0 and 5 in the upper foreleg location relative to the control condition (P < 0.05 and P = 0.05, respectively, by ANOVA; Fig. 1G), which returned to normal levels at day 10. There were no significant differences in tracer coverage over time in the nonoperated left forelegs. Cross sections taken from the lower location did not show significance, possibly because there was a high variability in coverage area due to the close proximity to the injection site. The data demonstrate a reduced lymph drainage from the operated foreleg at days 0 and 5 and a return to normal drainage by day 10.
Fig. 1.
Rapid recovery of lymph drainage after axillary lymph node dissection (ALND) in the foreleg. A–F: cross sections from the normal (A and B) and operated mouse foreleg (C–F) at day 0 (C and D) and day 5 (E and F) postsurgery from the middle (A, C, and E) and upper (B, D, and F) regions of the foreleg of Balb/c mice. The red color in each image is the fluorescent dextran lymph fluid tracer. The blue color in each image shows 4′,6-diamidino-2-phenylindole (DAPI)-labeled cell nuclei. Scale bar in F = 0.5 mm. G: coverage of fluorescent dextran was measured for both nonoperated left and operated right forelegs at the lower, middle, and upper locations of the foreleg. The y-axis in all graphs shown in G is the percent coverage of the rhodamine fluorescent tracer present in the cross section. n = 10 mice/group. *Statistical significance relative to the control condition within the respective graph.
Fluid tracer spreads interstitially through the foreleg after ALND.
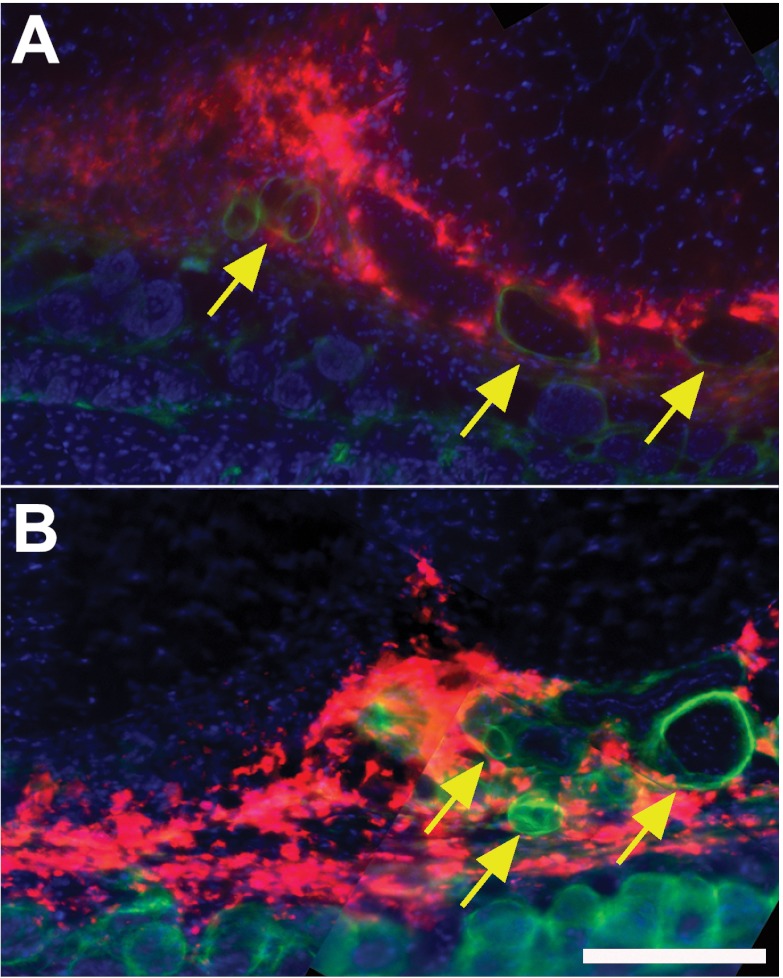
Because we found that lymph drainage was impaired at days 0 and 5 in the foreleg, we labeled cross sections for a podoplanin lymphatic vessel marker to determine the spatial distribution of the fluorescent fluid tracer with respect to lymphatic vessels. We found that the fluid tracer at days 0 and 5 was present in the interstitial space of the subcutaneous region, with the most fluid tracer located outside of podoplanin-positive structures (Fig. 2). Therefore, most of the fluid marker was seen spreading interstitially through the subcutaneous tissues of the mouse foreleg.
Fig. 2.
Lymph drains interstitially through subcutaneous tissue after ALND. Cryosections (100 μm thick) were immunostained against the lymphatic endothelial cell-specific marker podoplanin (green) after the injection of the fluorescence fluid tracer to visualize the spatial distribution of lymph within the foreleg (red) of Balb/c mice. Cell nuclei were stained with DAPI (blue). A and B: fluorescence images of the mouse foreleg at 0 (A) and 5 (B) days after ALND. Yellow arrows identify several podoplanin-positive lymph vessels. n = 10 mice/group. Scale bar in B = 50 μm.
Lymphangiogenesis of lymphatic vessels follows the recovery of fluid drainage.
ICG dye in nonoperated mice and the left foreleg of operated mice was seen to drain from the injection site by a large vessel (Fig. 3A; the red arrow identifies a functional lymphatic vessel) leading to the axillary lymph nodes (Fig. 3A, identified by the yellow arrow). Operated forelegs imaged directly after surgery (day 0) demonstrated dramatic lymph drainage impairment as the small ICG dye was seen to spread interstitially throughout the entire foreleg, as apparent in the image by the total limb fluorescence (Fig. 3B). Although ICG dye was distributed along the entire foreleg, the dye often appeared to pool prominently at the wound site (Fig. 3B, yellow arrow). A continued absence of functional lymphatic vessels was apparent at day 10 (Fig. 3C), with the ICG dye often seen spreading interstitially through the limb in some locations or being generally indistinguishable from background fluorescence through the foreleg. In either case, the distribution of ICG dye at day 10 was substantially less diffuse than the distribution of the dye at day 0, suggesting that adaptations had taken place over this time to concentrate interstitial flow, possibly through fluid channels. The diffuse appearance of fluid drainage at day 0 correlates temporally with the deficient lymph drainage that was quantified in tissue cross sections (Fig. 1). The less diffuse interstitial fluid drainage that was apparent from the day 10 ICG image correlates with fluid drainage recovery (Fig. 1). A new complex network of functional lymphatic vessels was detected by ICG imaging at day 15 (Fig. 3D). A prominent interstitial pooling of the dye was often seen near the wound site at day 15 (identified by the yellow arrow in Fig. 3D). It is noteworthy that the new lymphatic vessels appeared to largely bypass the region of surgical injury. Thus, it was seen that the temporal distribution of interstitial fluid drainage in the ICG images of the mouse foreleg correlated with the functional quantification that was made from the fluorescent dextran images of the foreleg cross sections. Together, the data demonstrate that the recovery of interstitial fluid drainage preceded the regrowth of lymphatic vessels in the mouse foreleg.
Fig. 3.
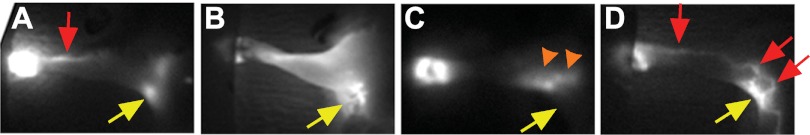
Lymph drainage recovers before lymphangiogenesis. A–D: indocyanine green (ICG) fluorescence lymphography images captured from normal (A) and operated mouse forelegs at 0 (B), 10 (C), and 15 (D) days postsurgery in Balb/c mice. Yellow arrows in A–D identify the axillary region (lymph nodes are apparent in A). Red arrows in A and D identify lymphatic vessels. Orange arrowheads in C identify the interstitial transport of fluid.
Lymphangiogenesis is not necessary for the resolution of acute lymphedema or recovery of interstitial fluid drainage.
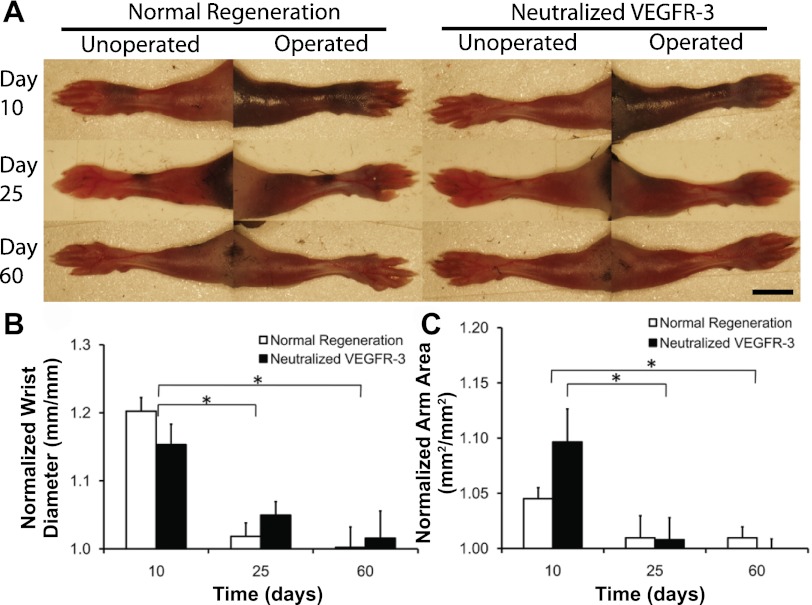
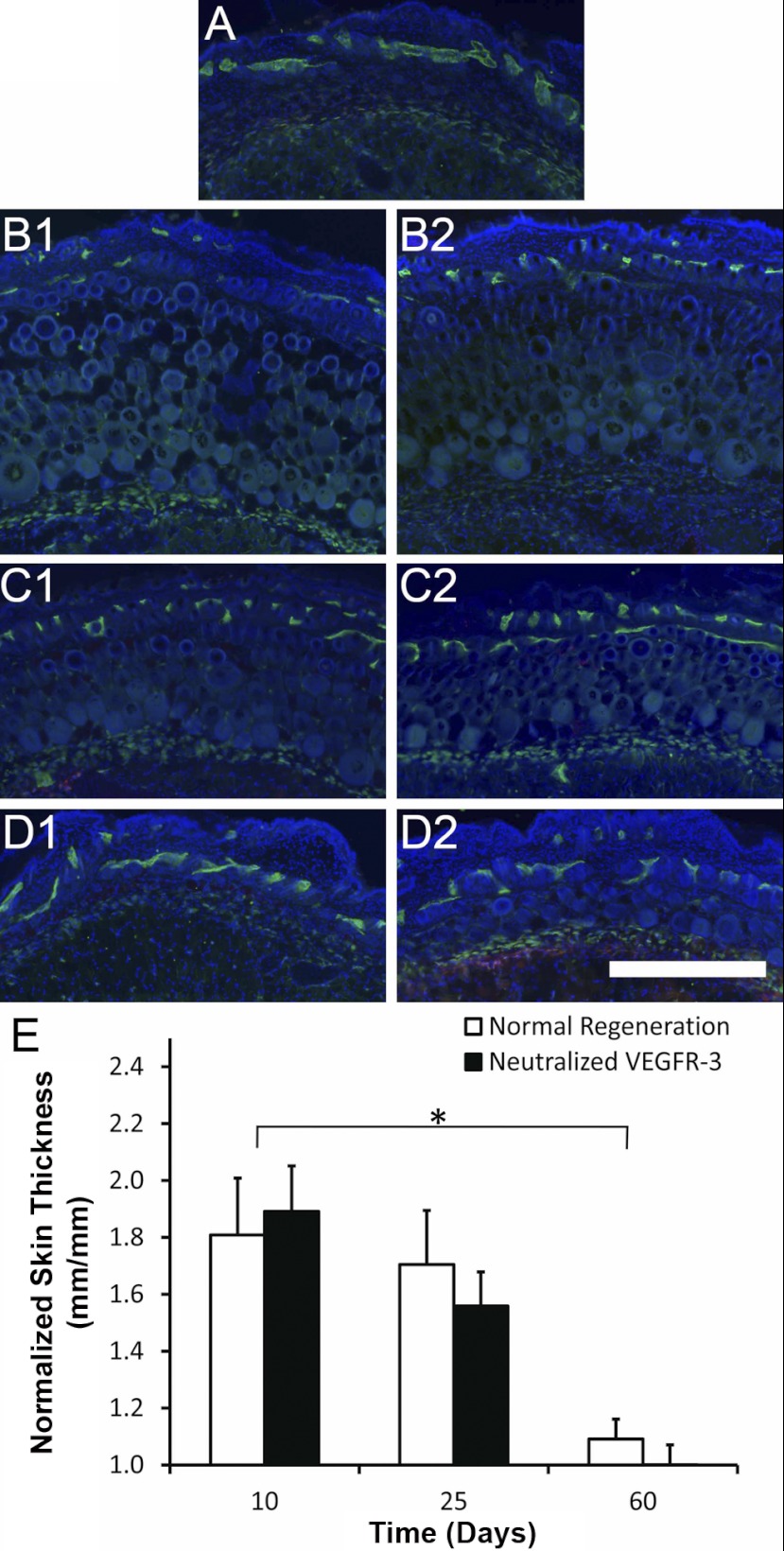
We used the ALND model in conjunction with VEGFR-3-neutralizing antibodies to confirm our finding that the recovery of interstitial fluid drainage precedes the regrowth of lymphatic vessels. We were able to determine the importance of VEGFR-3 signaling and lymphangiogenesis of lymph vessels during mouse foreleg lymphedema resolution by administering anti-VEGFR-3-neutralizing antibodies, which inhibit VEGFR-3 signaling. The ALND surgery was found to produce mild swelling of the mouse foreleg (Fig. 4, A–C) and skin (Fig. 5, A–D). It was found that the natural resolution of acute mouse foreleg lymphedema was not significantly different between saline-treated and VEGFR-3-neutralized conditions, based on measurements of wrist thickness (Fig. 4B), arm area (Fig. 4C), and skin thickness (Fig. 5E) (P > 0.05 by ANOVA). It was also found that wrist, arm, and skin swelling resolved from day 10 to day 60 for each condition (P < 0.05 by Tukey-Kramer highly significant difference test for the wrist and skin). Thus, the recovery of fluid drainage that we found to be present at day 10 led to a progressive loss of the fluid and new cellular structures that had accumulated in the foreleg up to that time. These results demonstrate that acute lymphedema resolution of the mouse foreleg does not depend on VEGFR-3 signaling or lymphatic vessel regrowth, confirming that interstitial fluid drainage recovers before the regrowth of lymph vessels.
Fig. 4.
Resolution of acute mouse foreleg lymphedema in the absence of VEGF receptor (VEGFR)-3 signaling. A: operated right and unoperated left arms of the same mouse at 10 (top), 25 (middle), and 60 (bottom) days (with different mice at each time point) under both normal [left arm (left) and right arm (right)] and VEGFR-3-neutralized [left arm (left) and right arm (right)] conditions in C57BL/6 mice. Scale bar in the bottom right image = 5 mm. B: wrist thickness was measured for both forelegs, and the swollen side was normalized to the unoperated left side to determine the percent swelling for each mouse at the wrist. C: normalized arm area. n = 10 mice/group. *Statistical significance.
Fig. 5.
Resolution of acute mouse foreleg skin swelling in the absence of VEGFR-3 signaling. A–D: lymphatic vessel endothelial hyaluronan receptor (green)-labeled images of foreleg skin in the normal mouse (A) and at 10 (B), 25 (C), and 60 (D) days after surgery to produce lymphedema with injections of saline (1) or neutralizing antibodies against VEGFR-3 (2) in C57BL/6 mice. The blue color in each image shows DAPI-labeled cell nuclei. Scale bar in the bottom right images = 0.5 mm. E: skin thickness was measured from arm sections, and the swollen side was normalized to the unoperated left side to determine the percent skin swelling for each mouse midway between the elbow and wrist. n = 10 mice/group. *Statistical significance.
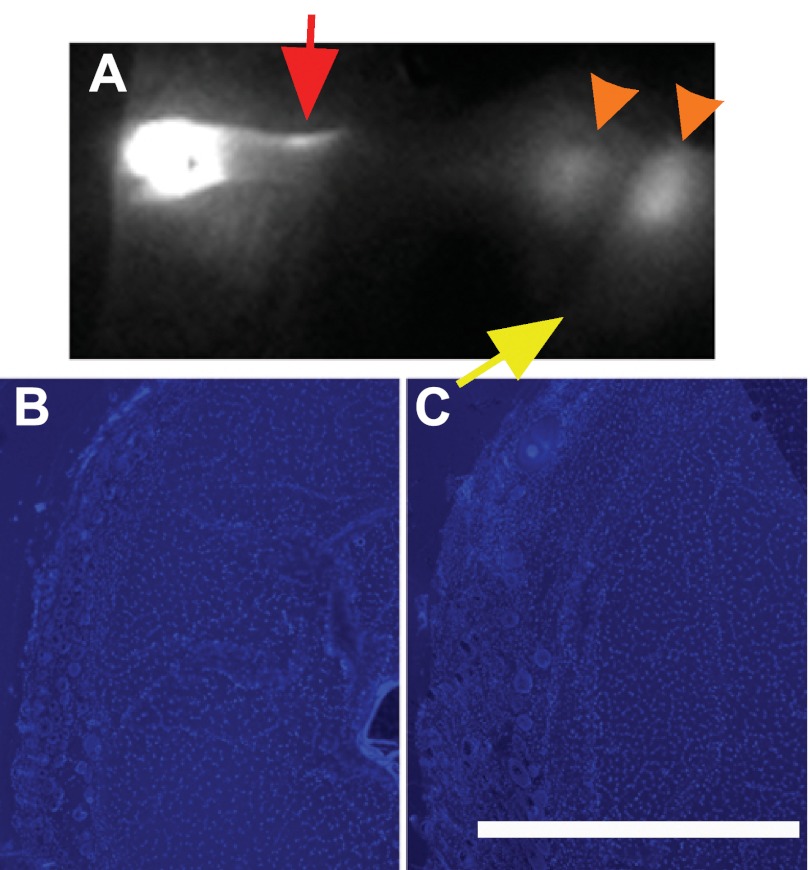
To directly confirm the recovery of fluid drainage after inhibition of lymphangiogenesis, we generated ICG images and measured the coverage of the fluid marker in foreleg cross sections in mice that had received anti-VEGFR-3-neutralizing antibodies up to day 15 post-ALND surgery. A lack of regenerated functional lymphatic vessels was apparent in the day 15 ICG image of anti-VEGFR-3-treated mice (Fig. 6A) relative to forelegs without administration of the blocking antibody (Fig. 3D), where functional lymphangiogenesis was apparent, and relative to saline-treated controls (data not shown). It was apparent that the main foreleg lymphatic vessel (Fig. 6A, red arrow), which was not directly damaged by the surgery, had regained activity despite the VEGFR-3 signaling neutralization. The ICG dye was often seen spreading interstitially across the axilla (Fig. 6A, orange arrowheads), with a possible increased involvement of the brachial lymph node. We also found that the dextran fluid tracer was scarcely detectable in the foreleg cross sections of anti-VEGFR-3-treated mice (Fig. 6, B and C), consistent with recovery of a functional drainage system in these mice, which efficiently cleared the dye across the foreleg. Coverage of the fluid marker in cross sections of the foreleg was not statistically different from the normally regenerating control mice (by ANOVA), demonstrating the functional recovery of fluid drainage in the absence of VEGFR-3 signaling. No differences in ICG imaging or fluid marker coverage were seen between Balb/c and C57BL/6 mice (data not shown). The data demonstrate that adaptations had taken place over the 15 days postsurgery to recover lymphatic function and fluid transport across the axilla in a manner that was independent of VEGFR-3 signaling and lymphangiogenesis of lymphatic vessels. These results directly demonstrate that interstitial fluid drainage recovers before the regrowth of lymph vessels after ALND in the mouse.
Fig. 6.
Lymph drainage recovers in the absence of VEGFR-3 signaling and lymphangiogenesis. A: ICG fluorescence lymphography image captured from an operated mouse foreleg at 15 days postsurgery after anti-VEGFR-3-neutralizating antibody administration in C57BL/6 mice. The yellow arrow identifies the location of the excised axillary lymph node. The red arrow identifies lymphatic vessels. Orange arrowheads identify the interstitial transport of fluid. B and C: dextran fluid marker was imaged in cross sections from VEGFR-3-neutralizing antibody-treated mice at the middle (B) and upper (C) locations of the foreleg in C57BL/6 mice. The red color in each image is the fluorescent dextran lymph fluid tracer. The blue color in each image shows DAPI-labeled cell nuclei. n = 10 mice/group. Scale bar in C = 0.5 mm.
DISCUSSION
In this study, we are the first to demonstrate that the functional recovery of fluid drainage during acute lymphedema is independent of lymphangiogenesis of lymphatic vessels in the mouse foreleg. Our functional analysis showed that fluid drainage was dramatically impaired by ALND and recovered before the regrowth of lymphatic vessels and even in the complete absence of VEGFR-3 signaling and lymphangiogenesis. The impaired lymph drainage was associated with a diffuse interstitial spreading of fluid through the foreleg subcutaneous region. Before the detection of any lymphatic vessel regrowth, the diffuse appearance had disappeared and lymph had become focused into channels. These interstitial changes were associated with fluid drainage recovery, suggesting an intrinsic adaptive capacity of the interstitium that helps to restore lymphatic drainage during acute lymphedema. We confirmed the lymphangiogenesis-independent nature of this adaptive response by showing that neutralization of VEGFR-3 and lymphangiogenesis with antagonistic antibodies did not hinder the recovery of lymph drainage. Indeed, in the absence of VEGFR-3 signaling, foreleg lymphatics were able to regain drainage function, and fluid was seen spreading interstitially near the axillary region. The data, when taken together, demonstrate the central role of the interstitial environment in adapting to lymphatic injury to increase lymph drainage.
Because the vast majority of studies assessing the properties of lymph drainage after ALND have been conducted on patients who have developed chronic lymphedema, it is not known how the interstitial environment adapts after the surgery in the human. It is often hypothesized that the lymphatic obstruction at the axilla transmits an increased pressure to the interstitium (5). Our results suggest that changes to the balance of interstitial forces after ALND may initially be helpful to restore fluid drainage and resolve acute swelling before lymphatic regrowth or in the absence of adequate lymphatic regrowth, although they may later contribute to the sustained swelling experienced during chronic lymphedema. Therefore, the present findings may help explain why the chronic form of lymphedema does not appear immediately in the human after ALND or sentinel lymph node dissection.
Interestingly, we often saw new lymph vessels bypassing the surgical defect (Fig. 3D). We also frequently observed fluorescent fluid tracer pooling at the wound site, even at latter time points when lymphatic vessels had regrown. When VEGFR-3 was neutralized, we saw fluid spreading interstitially near, but not across, the site of surgical obstruction (Fig. 6A). This is suggestive that wound healing products may serve as endogenous inhibitors of lymphatic regeneration and interstitial flow. Indeed, we (21) have recently shown that a simple collagen material implant that minimizes scar formation can effectively improve lymphatic regeneration across the site of injury and increase lymphatic drainage from the swollen limb. The inhibitory nature of the scar matrix is also supported by animal studies (2, 10) and clinical reports (28, 34) showing a reduced ability of lymphatics to reconnect through scar tissue. Whereas there is ample space for lymphatics to bypass the small surgical defect in the mouse, the site of surgery (and thus the space that forms into scar tissue) is substantially larger in humans. The extensive scarring that occurs in humans may limit the extent of endogenous lymphatic regeneration and rerouting as well as the ability of the extracellular matrix to remodel in ways that increase the interstitial drainage of accumulating fluid. These previous studies, when taken together with results from the present study, suggest that reduced scar formation at the site of obstruction may serve the therapeutic purpose of reducing resistance to interstitial outflow and increasing lymphatic regeneration.
It is widely believed that lymphatic regeneration is necessary to resolve the acute lymphedema that is produced in mouse models of lymphatic injury and that molecular therapies that promote lymphatic growth and hasten the natural resolution of acute lymphedema in the mouse may be useful for treating chronic lymphedema in the human (8, 15, 30, 36). Although transient swelling is often observed after surgery in the human (16), the appearance of chronic lymphedema follows a substantial delay of several years and never appears in most women. Therefore, the swelling that is produced in the mouse is more similar to acute lymphedema than to chronic lymphedema in the human. Moreover, it is unclear how conclusions regarding the therapeutic effectiveness of stimulated lymphangiogenesis drawn from acute lymphedema models may apply to chronic lymphedema patients. Similar to what we have found in the mouse, we speculate that changes to the balance of interstitial forces in the human acts to quickly restore lymph drainage after ALND or sentinel lymph node dissection and resolve acute lymphedema before the functional regrowth of lymphatic vessels via lymphangiogenesis. Whereas adaptations of interstitial forces may be sufficient to resolve acute lymphedema, the increased lymph drainage may not be adequate to prevent chronic lymphedema from appearing at a later time. Whereas we have found that lymphangiogenesis is not required for restoring lymph drainage during acute lymphedema, new lymphatic growth may be important for increasing lymphatic reserve to reduce susceptibility for developing chronic lymphedema.
GRANTS
This work was funded by National Institutes of Health Grants R21-AR-053094, R21-HL-093568, and R15-HL-093705.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: U.M., E.M.B., and E.L.O. performed experiments; U.M., E.M.B., E.L.O., J.R.S., and J.G. analyzed data; U.M. and J.G. drafted manuscript; U.M., E.M.B., E.L.O., J.R.S., and J.G. approved final version of manuscript; J.G. conception and design of research; J.G. interpreted results of experiments; J.G. prepared figures; J.G. edited and revised manuscript.
ACKNOWLEDGMENTS
The authors give special thanks to Trent Jansen for assisting with the Zeiss Apotome imaging.
REFERENCES
- 1. Avraham T, Clavin NW, Daluvoy SV, Fernandes J, Soares MS, Corderio AP, Mehrara BJ. Fibrosis is a key inhibitor of lymphatic regneration. Plast Reconstr Surg 124: 438– 450, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Avraham T, Daluvoy S, Zampell J, Yan A, Haviv YS, Rockson SG, Mehrara BJ. Blockade of transforming growth factor-β1 accelerates lymphatic regeneration during wound repair. Am J Pathol 177: 3202– 3214, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bates DO. An interstitial hypothesis for breast cancer related lymphoedema. Pathophysiology 17: 289– 294, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bates DO, Levick JR, Mortimer PS. Starling pressures in the human arm and their alteration in postmastectomy oedema. J Physiol 477: 355– 363, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bates DO, Levick JR, Mortimer PS. Subcutaneous interstitial fluid pressure and arm volume in lymphoedema. Int J Microcirc Clin Exp 11: 359– 373, 1992 [PubMed] [Google Scholar]
- 8. Cheung L, Han J, Beilhack A, Joshi S, Wilburn P, Dua A, An A, Rockson SG. An experimental model for the study of lymphedema and its response to therapeutic lymphangiogenesis. Biodrugs 20: 363– 370, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Clarke D, Martinez A, Cox RS. Analysis of cosmetic results and complications in patients with stage-I and stage-Ii breast-cancer treated by biopsy and irradiation. Int J Radiat Oncol 9: 1807– 1813, 1983 [DOI] [PubMed] [Google Scholar]
- 10. Clavin NW, Avraham T, Fernandez J, Daluvoy SV, Soares MA, Chaudhry A, Mehrara BJ. TGF-β1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol 295: H2113– H2127, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Goldman J, Conley KA, Raehl A, Bondy DM, Pytowski B, Swartz MA, Rutkowksi JM, Jaroch DB, Ongstad EL. Regulation of lymphatic capillary regeneration by interstitial flow in skin. Am J Physiol Heart Circ Physiol 292: H2176– H2183, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Goldman J, Rutkowski JM, Shields JD, Pasquier MC, Cui Y, Schmokel HG, Willey S, Hicklin DJ, Pytowski B, Swartz MA. Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis. FASEB J 21: 1003– 1012, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Guyton AC, Scheel K, Murphree D. Interstitial fluid pressure. 3. Its effect on resistance to tissue fluid mobility. Circ Res 19: 412– 419, 1966 [DOI] [PubMed] [Google Scholar]
- 14. Ikomi F, Kawai Y, Nakayama J, Ogiwara N, Sasaki K, Mizuno R, Ohhashi T. Critical roles of VEGF-C-VEGF receptor 3 in reconnection of the collecting lymph vessels in mice. Microcirculation 15: 1– 13, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Jin da P, An A, Liu J, Nakamura K, Rockson SG. Therapeutic responses to exogenous VEGF-C administration in experimental lymphedema: immunohistochemical and molecular characterization. Lymphat Res Biol 7: 47– 57, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Kissin MW, Querci della Rovere G, Easton D, Westbury G. Risk of lymphoedema following the treatment of breast cancer. Br J Surg 73: 580– 584, 1986 [DOI] [PubMed] [Google Scholar]
- 17. Mortimer PS. The pathophysiology of lymphedema. Cancer 83: 2798– 2802, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Mortimer PS, Bates DO, Brassington HD, Stanton AWB, Strachan DP, Levick JR. The prevalence of arm oedema following treatment for breast cancer. Qjm-Mon J Assoc Phys 89: 377– 380, 1996 [Google Scholar]
- 19. Ogata F, Azuma R, Kikuchi M, Koshima I, Morimoto Y. Novel lymphography using indocyanine green dye for near-infrared fluorescence labeling. Ann Plast Surg 58: 652– 655, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Ogata F, Narushima M, Mihara M, Azuma R, Morimoto Y, Koshima I. Intraoperative lymphography using indocyanine green dye for near-infrared fluorescence labeling in lymphedema. Ann Plast Surg 59: 180– 184, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Ongstad EL, Bouta EM, Roberts JE, Uzarski JS, Gibbs SE, Sabel MS, Cimmino VM, Roberts MA, Goldman J. Lymphangiogenesis-independent resolution of experimental edema. Am J Physiol Heart Circ Physiol 299: H46– H54, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pytowski B, Goldman J, Persaud K, Wu Y, Witte L, Hicklin DJ, Skobe M, Boardman KC, Swartz MA. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst 97: 14– 21, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Rutkowski JM, Markhus CE, Gyenge CC, Alitalo K, Wiig H, Swartz MA. Dermal collagen and lipid deposition correlate with tissue swelling and hydraulic conductivity in murine primary lymphedema. Am J Pathol 176: 1122– 1129, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sevick-Muraca EM, Sharma R, Rasmussen JC, Marshall MV, Wendt JA, Pham HQ, Bonefas E, Houston JP, Sampath L, Adams KE, Blanchard DK, Fisher RE, Chiang SB, Elledge R, Mawad ME. Imaging of lymph flow in breast cancer patients after microdose administration of a near-infrared fluorophore: feasibility study. Radiology 246: 734– 741, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sharma R, Wang W, Rasmussen JC, Joshi A, Houston JP, Adams KE, Cameron A, Ke S, Kwon S, Mawad ME, Sevick-Muraca EM. Quantitative imaging of lymph function. Am J Physiol Heart Circ Physiol 292: H3109– H3118, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Stanton AW, Modi S, Mellor RH, Levick JR, Mortimer PS. Recent advances in breast cancer-related lymphedema of the arm: lymphatic pump failure and predisposing factors. Lymphat Res Biol 7: 29– 45, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Starling EH. On the absorption of fluids from the connective tissue spaces. J Physiol 19: 312– 326, 1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suami H, Pan WR, Taylor GI. The lymphatics of the skin filled by a dermal backflow: an observation in a scarred cadaver leg. Lymphology 40: 122– 126, 2007 [PubMed] [Google Scholar]
- 29. Szuba A, Pyszel A, Jedrzejuk D, Janczak D, Andrzejak R. Presence of functional axillary lymph nodes and lymph drainage within arms in women with and without breast cancer-related lymphedema. Lymphology 40: 81– 86, 2007 [PubMed] [Google Scholar]
- 30. Szuba A, Skobe M, Karkkainen MJ, Shin WS, Beynet DP, Rockson NB, Dakhil N, Spilman S, Goris ML, Strauss HW, Quertermous T, Alitalo K, Rockson SG. Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB J 16: 1985– 1987, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Tammela T, Saaristo A, Holopainen T, Lyytikka J, Kotronen A, Pitkonen M, Abo-Ramadan U, Yla-Herttuala S, Petrova TV, Alitalo K. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med 13: 1458– 1466, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Unno N, Inuzuka K, Suzuki M, Yamamoto N, Sagara D, Nishiyama M, Konno H. Preliminary experience with a novel fluorescence lymphography using indocyanine green in patients with secondary lymphedema. J Vasc Surg 45: 1016– 1021, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Uzarski J, Drelles MB, Gibbs SE, Ongstad EL, Goral JC, McKeown KK, Raehl AM, Roberts MA, Pytowski B, Smith MR, Goldman J. The resolution of lymphedema by interstitial flow in the mouse tail skin. Am J Physiol Heart Circ Physiol 294: H1326– H1334, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Warren AG, Slavin SA. Scar lymphedema–fact or fiction? Ann Plas Surg 59: 41– 45, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Yan A, Avraham T, Zampell JC, Aschen SZ, Mehrara BJ. Mechanisms of lymphatic regeneration after tissue transfer. PLos One 6: e17201, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yoon YS, Murayama T, Gravereaux E, Tkebuchava T, Silver M, Curry C, Wecker A, Kirchmair R, Hu CS, Kearney M, Ashare A, Jackson DG, Kubo H, Isner JM, Losordo DW. VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. J Clin Invest 111: 717– 725, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zampell JC, Yan A, Avraham T, Andrade V, Malliaris S, Aschen S, Rockson SG, Mehrara BJ. Temporal and spatial patterns of endogenous danger signal expression after wound healing and in response to lymphedema. Am J Physiol Cell Physiol 300: C1107– C1121, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]