Abstract
Within the paraventricular nucleus (PVN), there is a balance between the excitatory and inhibitory neurotransmitters that regulate blood pressure; in hypertension, the balance shifts to enhanced excitation. Nitric oxide (NO) is an atypical neurotransmitter that elicits inhibitory effects on cardiovascular function. We hypothesized that reduced PVN NO led to elevations in blood pressure during both the onset and sustained phases of hypertension due to decreased NO synthase (NOS) and increased asymmetrical dimethylarginine (ADMA; an endogenous NOS inhibitor) and symmetric dimethylarginine (SDMA). Elevated blood pressure, in response to PVN bilateral microinjections of a NO inhibitor, nitro-l-arginine methyl ester, was blunted in renal wrapped rats during the onset of hypertension (day 7) and sustained renal wrap hypertension (day 28) compared with sham-operated rats. Adenoviruses (Ad) encoding endothelial NOS (eNOS) or LacZ microinjected into the PVN [1 × 109 plaque-forming units, bilateral (200 nl/site)] reduced mean arterial pressure compared with control (Day 7, Ad LacZ wrap: 144 ± 7 mmHg and Ad eNOS wrap: 117 ± 5 mmHg, P ≤ 0.05) throughout the study (Day 28, Ad LacZ wrap: 123 ± 1 mmHg and Ad eNOS wrap: 108 ± 4 mmHg, P ≤ 0.05). Western blot analyses of PVN NOS revealed significantly lower PVN neuronal NOS during the onset of hypertension but not in sustained hypertension. Reduced SDMA was found in the PVN during the onset of hypertension; however, no change in ADMA was observed. In conclusion, functional indexes of NO activity indicated an overall downregulation of NO in renal wrap hypertension, but the mechanism by which this occurs likely differs throughout the development of hypertension.
Keywords: asymmetrical dimethylarginine, symmetrical dimethylarginine, neurotransmission, gene transfer
the paraventricular nucleus (PVN) of the hypothalamus contains a heterogeneous cell population that participates in a multitude of physiological functions, including the regulation of blood pressure. Pathways descending from the PVN project to the rostral ventrolateral medulla and/or preganglionic sympathetic neurons in the spinal cord. These pathways control sympathetic nerve activity and blood pressure. Both excitatory and inhibitory neurotransmitters are released within the PVN, with a summation of their actions influencing neuronal activity in the descending pathways and, ultimately, blood pressure. During hypertension, the balance between excitatory and inhibitory neurotransmitters is shifted to increased excitation, resulting in increases in blood pressure and hypertension. Thus far, the temporal neurochemical changes that occur in the hypertensive process have received limited attention. By further understanding the development of hypertension, we may better understand how to anticipate and treat the outcomes.
Nitric oxide (NO) is an atypical neurotransmitter within the PVN. NO is synthesized from l-arginine by one of three NO synthase (NOS) isoforms: Ca2+-dependent endothelial NOS (eNOS), Ca2+-dependent neuronal NOS (nNOS), and Ca2+-independent inducible NOS (iNOS). Considerable evidence supports dysfunction of NOS with reduced peripheral NO availability in cardiovascular disease; there is also accumulating evidence identifying a central defect in NO availability (9, 16, 25, 26, 32, 33, 46–49, 51). Within the PVN, NO elicits inhibitory effects on cardiovascular function through its actions on sympathetic outflow (26, 29, 47). nNOS gene transfer into the PVN, PVN microinjections of sodium nitroprusside (an NO donor), and perfusions with NO-containing cerebrospinal fluid led to reductions in arterial blood pressure by increasing NO and, in turn, reducing renal sympathetic nerve activity (16, 25, 47, 48). In contrast, blockade of NOS using inhibitors of the NOS pathway (46) within the PVN or interference of the NOS pathway using gene expression techniques (33) increased arterial pressure, further indicating the important link between NO and cardiovascular end points.
One mechanism that regulates NO levels within the body is the endogenous NOS inhibitor asymmetrical dimethylarginine (ADMA). ADMA competitively displaces l-arginine from the substrate-binding site of NOS and therefore interferes with physiological NO end points. Therefore, alterations in ADMA may lead to altered bioavailable NO within the PVN and ultimately may be the cause of the increases in blood pressure. To date, there has been limited information concerning the role of ADMA and its structural isomer, symmetric dimethylarginine (SDMA), in the central nervous system. Within the periphery, ADMA has been associated with cardiovascular risk factors and correlated with established clinical markers of cardiovascular burden, including intima-media thickness in the carotid artery or left ventricular mass (6, 22, 52). In vitro SDMA has been shown not to alter NO (41). However, it has recently been suggested that SDMA may have an indirect effect on NO synthesis via inhibiting the transporter that mediates the intracellular uptake and absorption of l-arginine (7, 40). SDMA has been correlated with reductions in renal function (10). ADMA and SDMA have been localized to the brain; however, whether ADMA and SDMA are within the PVN and play a role in neuronal modulations in blood pressure are unknown.
The goal of the present series of experiments was to determine the contribution of NO in the PVN during the development of hypertension in figure 8 one-kidney renal wrap hypertensive rats. The renal wrap model of hypertension is a salt-sensitive, nongenetically mediated experimental model of hypertension that has been long used to evaluate neurohormonal changes in hypertension (8, 12, 13, 15, 27, 37, 42, 44). A multipronged approach was used to fully comprehend the physiological impact and potential mechanisms involved regarding PVN-derived NO and hypertension development and maintenance. Studies were designed to examine both the development and sustained phases of hypertension to give a complete evaluation of the role of NO in the PVN within the etiology of hypertension. Using NOS inhibitors, measures of NOS, ADMA, and SDMA in the PVN, and finally replenishing NO by increasing PVN-specific eNOS, we provide an expanded appraisal of NO in the PVN during hypertension.
METHODS
Animals
All animal procedures were followed in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the University of Texas Health Science Center and Michigan State University. Upon arrival, male Sprague-Dawley rats (275–300 g, Charles River Laboratories) were housed in clear plastic cages with wood chip bedding and allowed ad libitum access to rat chow (Teklad) and water. For all surgical procedures, anesthesia was administered by either isoflurane gas (2% in oxygen) or an intraperitoneal injection of ketamine (75 mg/kg) and xylazine (7.5 mg/kg). All surgeries were performed aseptically, and animals were treated with postsurgery analgesics.
Renal Wrap Hypertension
The kidney of the rat was exposed through a flank incision, and a figure 8 wrap was placed around the kidney with 0 suture as previously described (13). In both sham-operated (sham) and wrap animals, the contralateral kidney was removed through a separate flank incision.
Vascular Catheterization
Two to three days before the conclusion of the sham or wrap experimental protocol, animals were prepared with femoral arterial and venous catheters for direct measurements of blood pressure and blood sampling. The catheter was tunneled subcutaneously to the nape of the neck and secured with sutures for direct conscious blood pressure measurements 5–7 or 28 days after the initial surgical procedure. Catheters were flushed daily with a heparinized saline solution (20 U/ml) to maintain patency.
Telemetry
Radiotelemetry catheters (Data Sciences, St. Paul, MN) were surgically implanted into the femoral artery for continuous heart rate (HR) and blood pressure measures. The body of the telemetry transmitter was placed subcutaneously. After a 1-wk surgical recovery period, baseline arterial pressure and HR recordings were obtained (measurements taken 10 s every 10 min, 24 h/day). Telemetric recordings were only done on animals used in the gene transfer experiments to allow for monitoring of blood pressure and HR throughout the entire experimental protocol.
PVN Cannulation/Microinjections
Male Sprague-Dawley rats were anesthetized (75 mg/kg ketamine and 7.5 mg/kg xylazine) and placed in a stereotaxic apparatus for microinjections or prepared with bilateral guide cannulae directed at the PVN (target site: 2.0 mm caudal to the bregma, 1.2 mm lateral to the midline, 7.4 mm ventral from the skull, 10° angle). Cannulae were anchored into the skull using stainless steel screws and dental acrylic.
Histology for Determining Cannulae Placement
Rats were anesthetized using 50 mg/kg ip pentobarbital and perfused with 4% paraformaldehyde (Sigma, St. Louis, MO) in 0.1 mol/l phosphate buffer. Brains were postfixed in 4% paraformaldehyde for 24 h at 4°C, transferred to 20% sucrose in 0.1 mol/l phosphate buffer, and then stored (4°C) until stained. Brains were cut into serial sections (60 μm), placed onto slides, and dried overnight. On the second day, the slides were rinsed in 95% alcohol, 70% alcohol, distilled H2O, cresyl violet stain, distilled H2O, 70% alcohol, 95% alcohol, 100% alcohol, and xylene (3 min each). Slides were then mounted and viewed with a microscope for staining and microinjection site evaluation.
PVN Protein Isolation
Rats were anesthetized using 50 mg/kg ip pentobarbital. Brains were removed, frozen on dry ice, and cryostat sectioned (600 μm). Using a stereoscope, the PVN was localized and isolated using a tissue punch (17-gauge needle). The PVN punch was solubilized in lysis buffer [0.5 mmol/l Tris·HCl (pH 6.8), 10% SDS, and 10% glycerol] with protease inhibitors (0.5 mmol/l PMSF, 10 μg/μl aprotinin, and 10 μg/μl leupeptin, Sigma). Using an ultrasonic processor (Misonex, Farmingdale, NY), punches were homogenized (2- to 3-s pulses) and centrifuged (10 min, 5,000 rpm, at 4°C). The supernatant was collected, and protein concentration was determined using a BCA protein assay (Sigma).
Western Blot Analysis
Tissue (4:1 in denaturing sample buffer, boiled for 5 min) was separated on SDS-polyacrylamide gels and transferred to Immobilon-P membranes. Membranes were blocked for 3 h [Tris-buffered saline (TBS)-Tween 20 (TBS-T), 4% chick egg ovalbumin, and 2.5% sodium azide]. Blots were probed overnight at 4°C with primary antibody, rinsed in TBS-T with a final rinse in TBS, and incubated with the appropriate secondary antibody for 1 h at 4°C. Blots were then incubated with ECL reagents to visualize the bands.
ADMA and SDMA Analyses
Acid precipitation was performed on the entire sample volume for ADMA and SDMA analyses. PVN brain samples were prepared for HPLC analyses of ADMA and SDMA using a modification of the method of Heresztyn et al. (14) and quantified by reverse-phase liquid chromatography (Breeze Systems, Waters, Milford, MA). The average method detection limit was calculated from eight replicates of 0.75 μM DMA standard and was 0.19 μM for SDMA and 0.02 μM for ADMA. The interassay coefficient of variation was 1.7% and the intra-assay coefficient was 2.8%, as determined by a comparison of quality controls (4 replicates) included within three separate sample sets.
Data Analysis and Statistics
Data are presented as means ± SE. Blood pressure and HR during bilateral microinjections were averaged for the control period and in 15-s bins after the N-nitro-l-arginine methyl ester (l-NAME) injections using MacLab software. Band density from Western blot analyses was quantified using NIH Imaging software. When two groups were compared, the appropriate Student's t-test was used. For multiple comparisons, two-way ANOVA followed by least-significant-difference and Student-Newman-Keul's post hoc tests were used. For telemetry experiments, proc mixed and repeated-measures statistical analyses were performed using SAS statistical software (version 9.1) due to multiple measures coming from one animal. In all cases, P values of <0.05 were considered statistically significant.
Experimental Protocols
l-NAME responses.
To evaluate the physiological aspect of NO in renal wrap hypertension, acute blood pressure changes in response to bilateral PVN microinjections of l-NAME (NOS inhibitor) were examined. At the conclusion of the renal wrap or sham surgical protocol [7 days (n = 7–8) or 28 days (n = 7–9) postsurgery], the arterial catheter was attached to a pressure transducer (Cobe model 41-500, AD Instruments, Colorado Springs, CO) coupled to a data-acquisition system (MacLab, AD Instruments). Mean arterial pressure (MAP) and HR were continuously monitored at a rate of 0.1 Hz. Approximately 1 h before central injections, 30-gauge stainless steel injectors were inserted into the region, extending 1 mm past the end of the guide cannulae. After baseline blood pressure/HR measurements had been taken, l-NAME (200 nmol/site, 50 nl, microinjection pump) was injected bilaterally into the PVN in conscious animals. Blood pressure and HR were followed for an additional 30 min after the injection. At the conclusion of the microinjection experiment, the animals were euthanized (50 mg/kg ip pentobarbital), and histological analysis was performed to confirm PVN cannulae placement.
NOS.
In a separate series of experiments, PVN NOS protein was isolated from 7- and 28-day renal wrap rats and corresponding sham animals (n = 6 animals/group). Western blot analysis was then performed [probed with the following antibodies: nNOS (1:1,000), eNOS (1:500), iNOS (1:500, BD Transduction Laboratories, Rockville, MD), and tubulin (control antibody; 1:5,000, Millipore, Billerica, MA)]. Before protein isolation, the blood pressures of the animals were determined using direct arterial catheterization.
ADMA/SDMA.
Alterations in endogenous inhibitors of NOS may account for variations in NO activity. Therefore, in another experiment, PVN samples were isolated for ADMA and SDMA measurements during both the onset (n = 10) and sustained (n = 4–6) phases of renal wrap hypertension. Before PVN isolation, the blood pressures of the animals were determined using direct arterial catheterization methodologies.
eNOS gene transfer.
To evaluate whether replenishment of NOS within the PVN could reduce the elevations in blood pressure that are observed, we took advantage of the fact that eNOS protein was not detectable in the PVN (Fig. 1) and microinjected adenovirus (Ad) specific to eNOS (Ad eNOS) into the PVN. The Ad allowed us to evaluate if increased expression of eNOS occurred and chronically replenishing NOS and availability of NO within the PVN. After 1 wk of baseline telemetry blood pressure measures, the rats were anesthetized and placed in a stereotaxic apparatus (as stated above). Bilateral microinjections of an Ad vector encoding eNOS or LacZ (marker gene; used as a control) was injected into the PVN (1 × 109 plaque-forming units/ml, 200 nl/site over 2 min, n = 5–6). Ad preparation of the eNOS viral vector has been previously described (4, 5). Microinjectors were removed 15 min postinjection, the skin was sutured over the skull, and the animals were returned to their home cages. Seven days after viral injection, renal wrap or sham surgery was performed (see protocol above). At the conclusion of the experimental protocol (day 30), the animal was euthanized, the brain was removed, and PVN protein was isolated. Subsequent Western blot analyses were performed to evaluate eNOS expression.
Fig. 1.
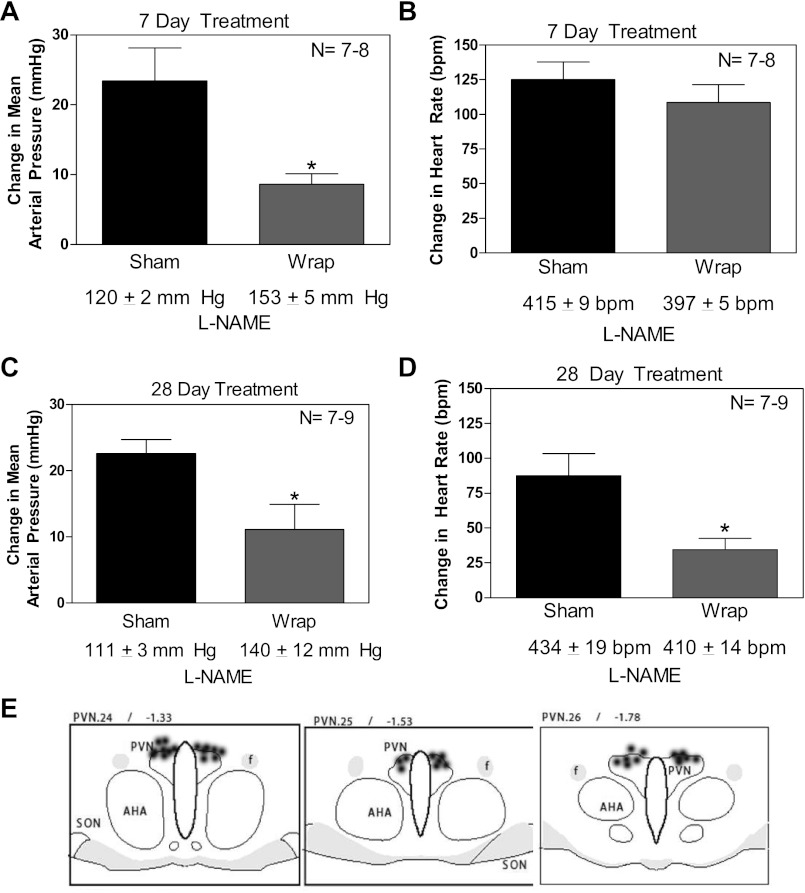
Paraventricular nucleus (PVN) microinjections of a nitric oxide synthase (NOS) inhibitor, nitro-l-arginine methyl ester (l-NAME), led to a blunted increase in mean arterial blood pressure in wrap rats compared with sham-operated (sham) rats both 7 and 28 days postsurgery (A and C). No changes in heart rate [in beats/min (bpm) were detected 7 days postsurgery (B); however, there was a significant reduction in heart rate in animals that had 28 days of renal wrap hypertension (D). Microinjection sites in the area of the paraventricular nucleus (PVN) are shown (E). AHA, anterior hyperthalmic area; SON, supraoptic nucleus; f, fornix. Values are means ± SE; n = 7–9. *P ≤ 0.05.
RESULTS
Baseline Blood Pressure and HR
Resting MAP from animals with indwelling catheters (l-NAME and ADMA/SMDA experiments) was significantly greater in the renal wrap animals compared with sham animals [7-day treatment: sham, 108 ± 5 mmHg vs. wrap, 142 ± 6 mmHg (n = 16); 28-day treatment: sham, 91 ± 3 mmHg vs. Wrap, 140 ± 8 mmHg (n = 10–12), P ≤ 0.05]. There were no significant differences in HR between sham and renal wrap animals at either time point examined.
Short-Term Blockade of NOS in the PVN
During the onset of hypertension, the MAP response to l-NAME was significantly attenuated in the renal wrap hypertensive animals; there was a 23 ± 5-mmHg increase in sham animals in contrast to a 9 ± 2-mmHg increase in renal wrap animals (P ≤ 0.05; Fig. 1A). There were no significant differences observed regarding HR between the groups (change in HR: sham, 125 ± 13 beats/min vs. renal wrap, 109 ± 13 beats/min; Fig. 1B). During sustained hypertension, (28 days postsurgery), there was also an attenuation of MAP observed in renal wrap rats compared with sham rats (23 ± 2-mmHg increase in sham rats compared with a 11 ± 4-mmHg increase in wrap rats, P ≤ 0.05; Fig. 1C). In contrast to the onset of hypertension, during sustained hypertension, there was a blunted change in HR observed in renal wrap rats (88 ± 16 beats/min in sham rats vs. 34 ± 8 beats/min in renal wrap rats, P ≤ 0.05; Fig. 1D). Histological analyses confirmed PVN cannulae placement (Fig. 1E). All cannulae placed outside of the region of the PVN were excluded from the study; no differences in responses were observed in those animals (data not shown).
NOS
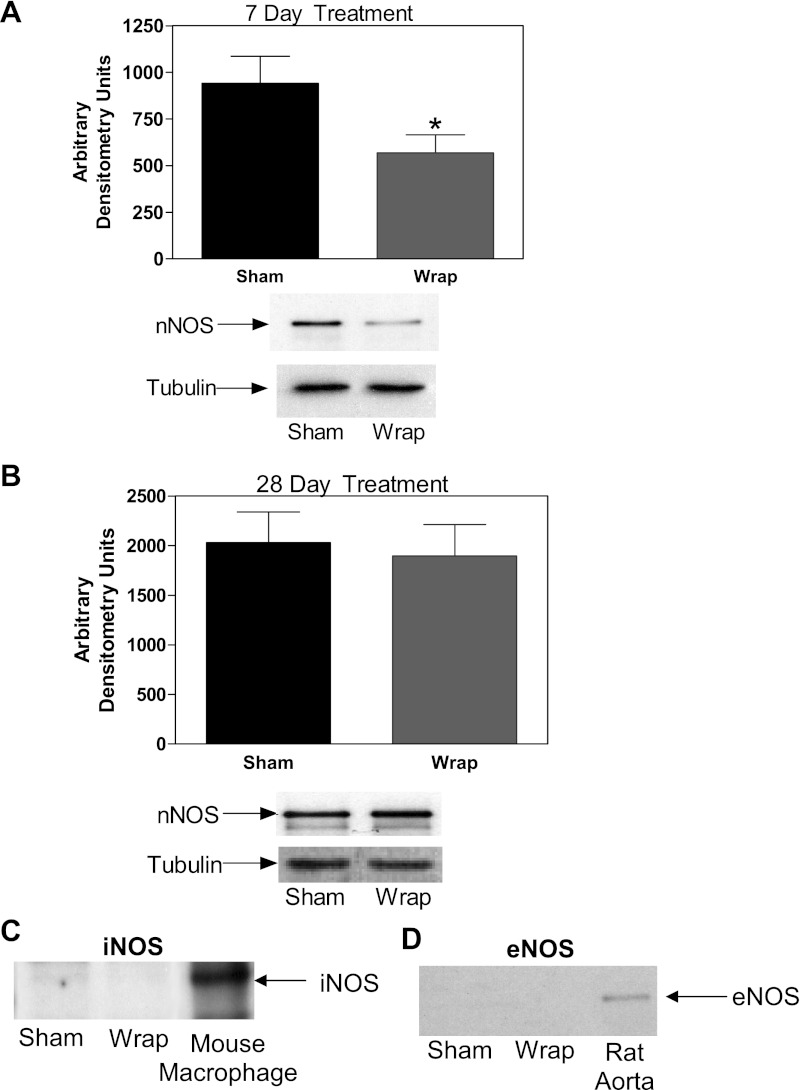
There was significantly lower nNOS protein detected in the PVN from renal wrap animals compared with sham animals during the onset of hypertension (7-day treatment: 567 ± 97 vs. 941 ± 145 arbitrary densitometry units, P ≤ 0.05; Fig. 2A). In contrast, during sustained hypertension (28 days postsurgery), this difference was no longer present (Fig. 2B). Neither eNOS nor iNOS were found at detectable levels in the PVN from either sham or wrap animals (Fig. 2, C and D; data shown from 7-day PVN samples). To ensure equal loading of protein for the Western blot analyses, tubulin was used as a comparative measure. No differences in tubulin expression were found among the experimental groups (Fig. 2, A and B).
Fig. 2.
Western blot analyses of neuronal NOS (nNOS) in the PVN from sham and renal wrap rats revealed lower nNOS protein in 7-day hypertensive renal wrap rats compared with normotensive sham rats (A). Neither inducible NOS (iNOS) nor endothelial NOS (eNOS) were found in quantifiable levels (mouse macrophage and rat aorta served as positive controls; C and D). There were no significant differences found in nNOS in the PVN from 28-day renal wrap and sham rats (B). Tubulin was used as a control (no differences were found regarding tubulin throughout the Western blot experiments). Values are means ± SE; n = 6. *P ≤ 0.05.
ADMA and SDMA
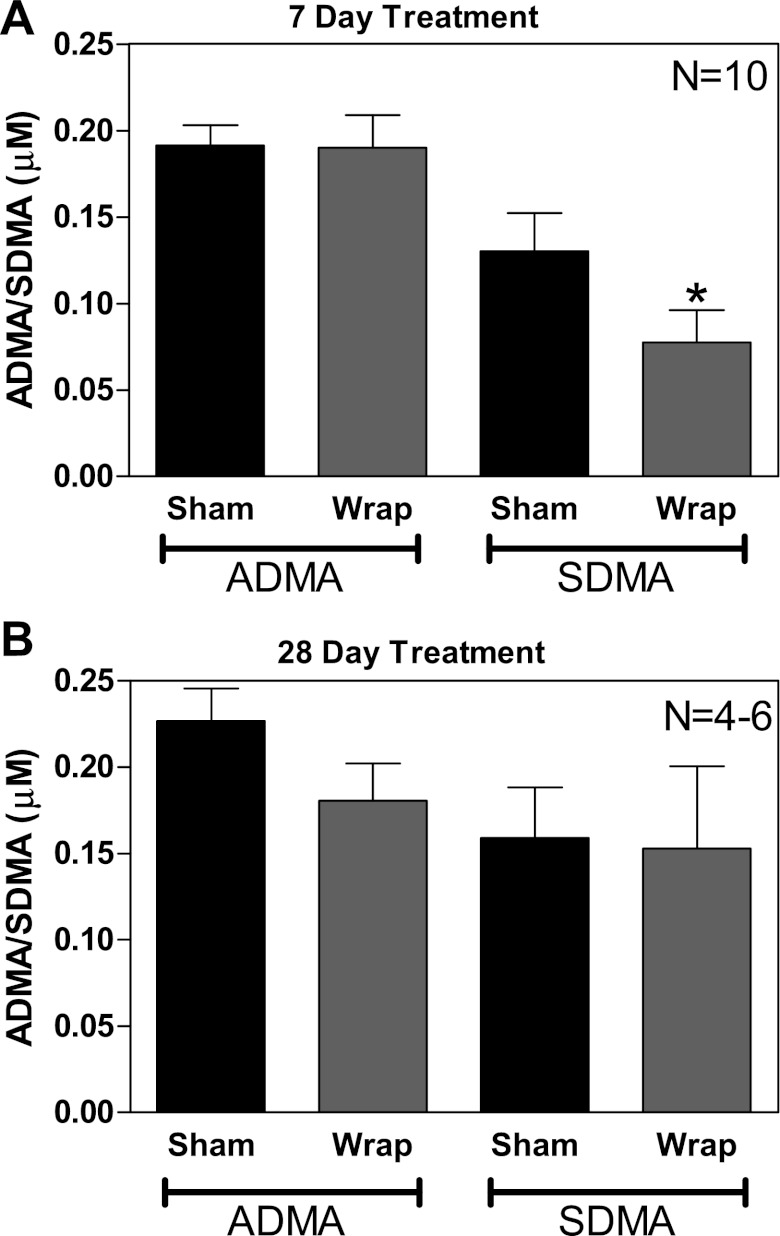
During the onset of renal wrap hypertension, we did not detect differences in ADMA in the PVN (Fig. 3A); however, there were significantly lower SDMA levels in the PVN from hypertensive animals (Fig. 3A). During sustained hypertension (28 days), there were no significant differences in PVN ADMA or SDMA (Fig. 3C).
Fig. 3.
During the onset of hypertension (7 day), renal wrap rats had detectable levels of asymmetrical dimethylarginine (ADMA) and symmetrical dimethylarginine (SDMA) in the PVN (A; n = 10). There were no significant differences in ADMA levels detected in PVN brain samples. There was, however, a significant decrease in SDMA, the noninhibitory isomer, within the PVN (A). After 28 days of renal wrap, there were detectable levels of ADMA and SDMA within the PVN (B; n = 4–6); however, no differences were detected. *P ≤ 0.05.
Chronic Increases in NOS and NO Availability in the PVN
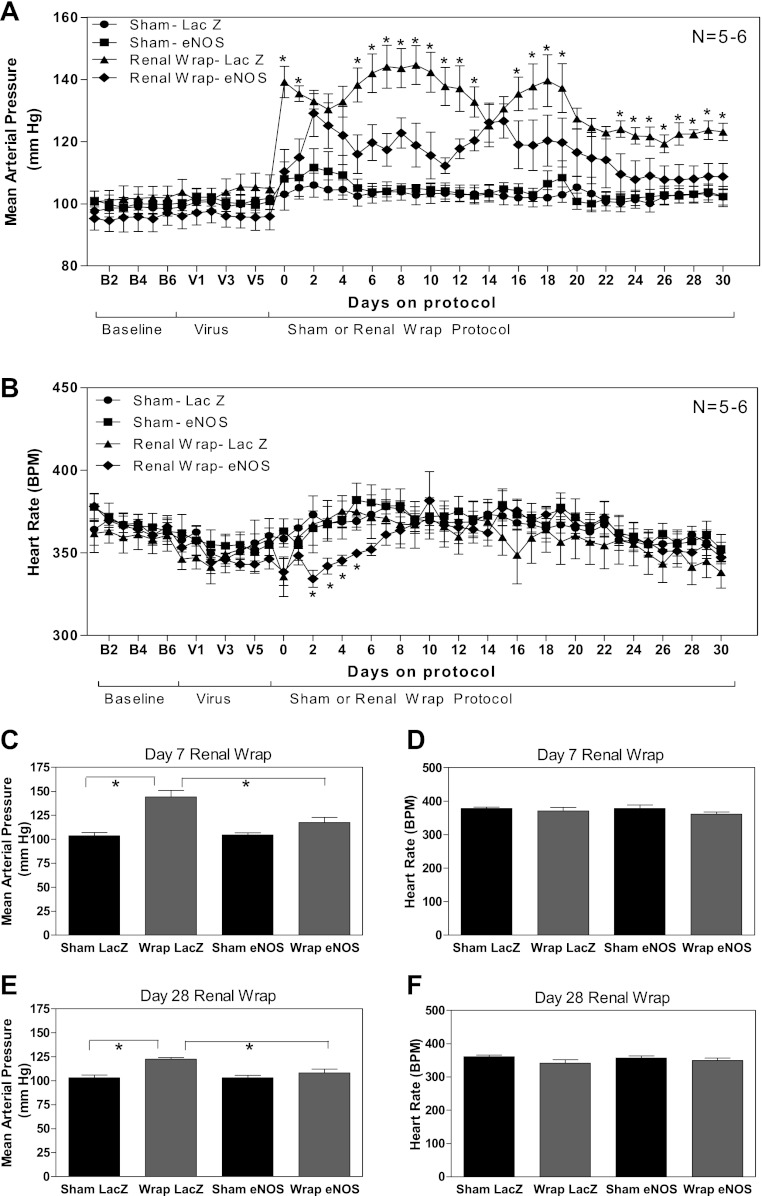
No differences in weight gain were observed between the groups (data not shown), nor were differences in baseline MAP and HR (monitored via radiotelemetry in this study) observed before or after viral injections (before renal wrap/sham surgical intervention). MAP from sham Ad eNOS-treated rats was not different compared with sham Ad LacZ-treated rats, suggesting that increasing eNOS chronically in a nonhypertensive state did not alter blood pressure. However, during hypertension, rats given Ad eNOS had significantly reduced MAP over the course of the hypertension, as determined by ANOVA (Fig. 4A). During the traditionally tested “onset” of hypertension time point of 7 days postsurgery, there were significant elevations in MAP found in Ad LacZ-treated renal wrap animals compared with sham animals. In contrast, in the PVN, Ad eNOS-treated animals had a significant reduction in MAP (P < 0.05; Fig. 4C), which continued through the remainder of the study (Fig. 4, A and E). Regarding HR, after the induction of renal wrap hypertension, there was a transient reduction in HR in Ad eNOS-treated wrap rats compared with Ad LacZ-treated wrap rats (days 2–5) postsurgery (P ≤ 0.05) that quickly returned to the same level as the other treatments for the remainder of the protocol (Fig. 4B).
Fig. 4.
A: PVN adenoviral (Ad) eNOS administration had no effect on baseline blood pressure (V1–V6); however, Ad eNOS-treated wrap rats had a significantly blunted increase in blood pressure (days 0–30). In addition, wrap Ad eNOS-treated rats were not significantly different from sham animals at the beginning of the treatment protocol (days 0–3) or at the conclusion of the experiment (days 23–30). B: PVN Ad eNOS administration had no effect on baseline blood pressure (V1–V6); however, during the beginning of the sham/renal wrap protocol, there was a significant difference in heart rate (days 2–5) in wrap Ad eNOS-treated rats compared with wrap Ad LacZ-treated rats. *P ≤ 0.05, wrap Ad LacZ-treated group vs. wrap Ad eNOS-treated group. C and D: mean arterial pressure (MAP) and heart rate (HR) are shown at 7 days after surgery. MAP was lower in sham-operated vs. renal wrap rats treated with Ad LacZ in the PVN. In renal wrap rats, administration of Ad eNOS in the PVN reduced MAP compared with Ad LacZ-treated animals. There was no difference between renal warp and sham-operated animals treated with Ad eNOS in the PVN. No differences in HR were observed among the 4 groups of animals. E and F: MAP and HR are shown at 28 days after surgery. MAP was higher in renal wrap rats treated with Ad LacZ in the PVN. Renal wrap rats treated with Ad eNOS had lower MAP compared with hypertensive animals treated with Ad LacZ. HR was not different among the 4 groups of animals.
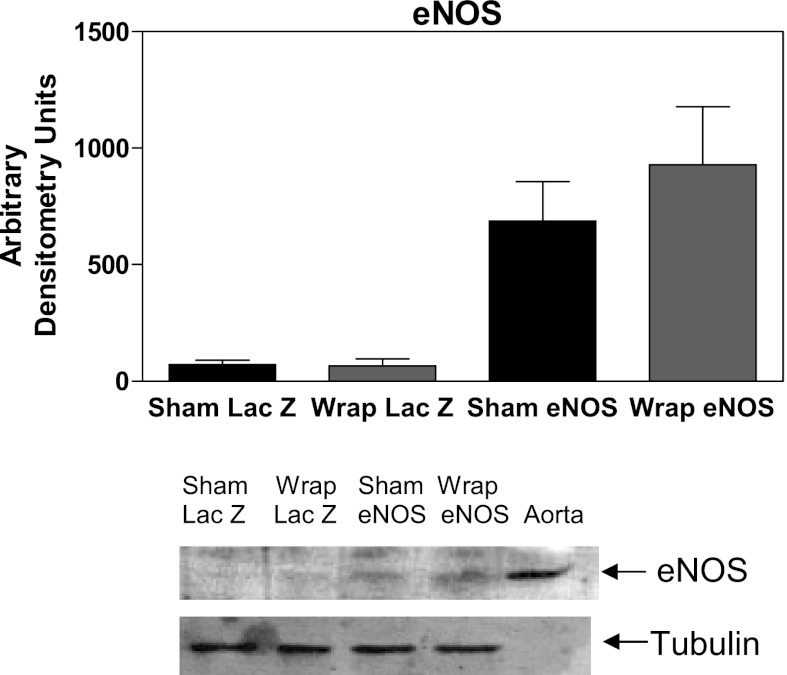
Western blot analysis of PVN lysates from the animals at the conclusion of the Ad experiments demonstrated that eNOS was present within the PVN of rats treated with Ad (n = 5–6; Fig. 5). Of note, there were also trace amounts of eNOS detected in Ad LacZ-treated animals (Fig. 5). There were no significant differences in eNOS found between sham and wrap rats (Fig. 5). These experiments confirmed that eNOS was expressed within the PVN in Ad eNOS-treated rats; however, due to the nature of limited to no eNOS being present during naive conditions, quantification of expression was not possible. A previous study (28), using an angiotensin type 1 receptor dominant negative Ad, demonstrated specific PVN targeting of Ad viruses.
Fig. 5.
Representative Western blot analysis of the PVN isolated at the conclusion of the Ad study (day 30; rat aorta was used as a positive control). No significant differences were observed in eNOS levels between sham and wrap Ad eNOS-treated groups.
DISCUSSION
The overarching goal in our study was to examine whether a reduction in the activity of the NO system within the PVN was involved in the development and maintenance of hypertension and, if so, to begin to more closely examine how this reduction in NO activity may occur. During both the onset and sustained phases of renal wrap hypertension, reduced PVN NO activity clearly contributed to the elevations in blood pressure observed. This was demonstrated by a blunted elevation in blood pressure in response to l-NAME injections during both phases of renal wrap hypertension. Moreover, when NO was reintroduced into the PVN, via Ad eNOS targeted to the PVN, the overexpression of eNOS led to significantly blunted renal wrap-mediated increases in blood pressure throughout the progression of the disease. Ad eNOS had no effect on the blood pressure or HR in sham animals, suggesting the changes in NO to be relevant only during hypertension. Together, these observations support the importance of the NO system in keeping blood pressure at normotensive levels. However, differences observed in the expression of NOS in the PVN of hypertensive animals during the onset and established phases of hypertension suggest that different mechanisms may be responsible for the functional suppression of the NOS system at these times. To this end, levels of endogenous NOS inhibitors, ADMA/SDMA, were measured and did not provide evidence for a sustained mechanism of reduced NO activity.
The hallmark observation was that blockade of the NOS system with l-NAME resulted in reduced pressor responses in renal wrap hypertensive animals. This observation is consistent with observations in other models of hypertension (16, 29, 32, 45) and heart failure (26, 29, 46, 48). This is further supported by the work of Rossi et al. (33), who showed that further inhibition of NOS with a dominant negative nNOS virus localized to the PVN further elevates MAP in two-kidney, one-clip hypertension. Their findings indicated that chronic interference of nNOS reduced total NO and further potentiated increases in blood pressure by modulation of sympathoexcitation. The reduced functional contribution of the NOS system in all of these cardiovascular models may be the result of changes in factors that can modulate the expression of NOS or its activity, such as superoxide, changes in cofactor activity, or other undetermined mechanisms.
Immunostaining has provided biochemical evidence that NOS in the PVN is downregulated in the spontaneously hypertensive rat (SHR), a chronic heart failure rat model, a diabetic rat model, and a diet-induced obesity rat model (1, 11, 30, 34, 39, 45, 48, 49, 51). In addition, in two chronic models of hypertensive rats, central mineralocorticoid-induced hypertension and chronic renal failure, a significant decrease in the amount of nNOS mRNA was found in the hypothalamus and rostral and caudal ventrolateral medulla (39, 45). In the present study, decreases in nNOS were only observed during the onset of hypertension. This observation is in contrast with Qadri et al. (32), who found attenuated NOS in the cerebral cortex and brain stem of prehypertensive or onset hypertensive SHRs but no changes in the hypothalamus. Ferrari and Fior-Chadi (9) examined age-matched Wistar-Kyoto rats and SHR at 15 days and 1, 2, 4, 8, and 12 mo and also found no changes in nNOS mRNA or protein in the PVN at any time point. In our study, the reduction in nNOS only occurred early during the development of the condition, before when Ferrari and Fior-Chadi (9) evaluated the PVN. Consequently, decreased expression of NOS protein may contribute to reduced NO activity in the initiation of the hypertensive process. In established hypertension, other mechanisms are likely responsible for the reduced functional contribution of NO. While reduced NOS enzyme activity is a mechanistic candidate, there is no evidence that brain NOS activity is reduced in hypertension. In fact, in established SHR hypertension, NOS activity was significantly increased in the hypothalamus and brain stem, which was attributed to a compensatory mechanism to the elevation in blood pressure (32). Therefore, other possible mechanisms must be considered.
ADMA and SDMA are potent endogenous NOS inhibitors; therefore, it stands to reason that another mechanism by which NO activity may be altered within the PVN could be alterations in ADMA and SDMA within the PVN. In 1970, Kakimoto and Akazawa (17) were the first to isolate and describe ADMA and SDMA. Since then, ADMA has been found to be the most potent endogenous NOS inhibitor and has been shown to correlate with both traditional as well as nontraditional cardiovascular risk factors (17, 20). SDMA, at first, was dismissed as having no real function, but has since been found to also indirectly inhibit NOS and subsequent NO production via the y+ transporter, which is responsible for the uptake of l-arginine and has been suggested to be an endogenous marker of renal function (20, 21). ADMA has been localized in microdialysates from the rat prefrontal cortex (53); however, there is no knowledge of ADMA and SDMA within the PVN or whether ADMA and SDMA are important in regulating NO within the PVN and subsequent changes regarding pathophysiological outcomes. Our study revealed that ADMA and SDMA were localized within the PVN. In addition, there were significant reductions in SDMA during the onset of hypertension. This reduction in SDMA may serve as a compensatory mechanism in an attempt to normalize the reduction in NOS-created NO. In light of reduced nNOS, even with reduced SDMA, the PVN may be unable to maintain the NO balance required to regulate blood pressure. This hypothesis was illustrated in the experiments in which we observed a blunted response to l-NAME. Once again, however, during the sustained phase of renal wrap hypertension, no differences in PVN ADMA or SDMA were detected. Thus, the questions remain as to what is the mechanism for the reduced NO activity during sustained hypertension and does it involve mechanisms that may affect the bioavailability of NO in the synapse?
It is well established that NO actively reacts with superoxide to form peroxynitrite, a highly cytotoxic reactive nitrogen species, while also further lowering the bioavailability of NO (2, 3). ROS production is increased in human and experimental hypertension and, moreover, plays an important role in blood pressure regulation (3, 18, 23, 38). In the brain, ROS are recognized for their involvement in neurodegenerative diseases, but they have recently been implicated in modulating blood pressure and sympathetic nerve activity (4, 10, 11, 35, 47, 50). In addition, recent observations in cardiac myocytes have demonstrated a unique interaction between NOS and superoxide such that a deficiency of nNOS leads to a profound increase in xanthine oxidoreductase-mediated superoxide production (19). nNOS also has the ability to produce ROS. Under reduced levels of (6R)-tetrahydro-l-biopterin, nNOS possesses the capability of uncoupling and then producing superoxide instead of NO (31, 35, 43). Thus, an interaction between NO with ROS generated by neurotransmitter activation may be a NOS- and ADMA/SDMA-independent mechanism by which NO reduction can also occur in the later stages of hypertension.
The blunted responses to l-NAME in early and established hypertension supported the concept of reduced available NO in the PVN of hypertensive animals. During the onset of hypertension, this may be associated with a reduced nNOS enzyme presence, while the mechanism of decreased NO activity in the later stages of hypertension remains uncertain. However, there is evidence that if NO activity is increased in cardiovascular disease, measured indexes reflect an improvement of the pathological condition. PVN microinjections of sodium nitroprusside (a NO donor) and perfusions with NO-containing cerebrospinal fluid have led to reductions in arterial blood pressure by increasing bioavailable NO and inhibiting renal sympathetic nerve activity mediated through alterations in GABA (16, 25, 47, 48). In addition, overexpression of nNOS after gene transfer into the PVN potentiated the increase in renal sympathetic nerve discharge, blood pressure, and HR observed after a microinjection of the NOS inhibitor NG-monomethyl-l-arginine (l-NMMA) into the PVN compared with rats given viral vectors containing β-galactosidase (24, 25). PVN microinjections of the NOS inhibitor l-NMMA resulted in attenuated increases in sympathetic nerve activity in an experimental rat chronic heart failure model (47, 48). Our Ad eNOS experiments also suggested that if the bioavailability of NO was increased before the induction of the hypertension induced-decline of NO, the increase in blood pressure was significantly blunted throughout the progression of hypertension. Additional studies in which chronic elevations in NO occur later during the development of the hypertension will also shed further light on the impact that the early changes in NO have on overall hypertension maintenance as well as be important from a clinical perspective. These data further implicate NO in an inhibitory role in controlling sympathetic nervous system function.
A recent study by Zheng et al. (50) using Ad nNOS has linked decreases in nNOS to enhanced glutamate transmission within the PVN by increasing NR1 expression in an experimental model of rat heart failure. Within the PVN, the inhibitory neurotransmitter NO can act as part of negative feedback system for the excitatory glutamatergic system (24). Regarding the downstream influence alterations in NO may have, a recent study (36) has found that within neuronal cell lines, NO serves as a negative regulator of angiotensin type 1 receptor expression. Reductions in NO may be mediating their effects on blood pressure via glutamatergic and angiotenergic-mediated mechanisms.
In conclusion, the mechanisms by which PVN NO is involved in the onset and maintenance of hypertension are not yet fully understood. However, NO dysregulation clearly plays a role throughout the hypertension development process. Reduced nNOS, while it may influence the NO-induced increases in blood pressure observed during the early stages of hypertension development, does not continue throughout the etiology of hypertension. Nevertheless, increasing available NO during the development of hypertension blunts the severe elevations in blood pressure that occur during renal wrap hypertension, thus providing a methodology by which one may be able to reduce the severity of this cardiovascular disease. Further studies will assist in understanding the cellular mechanisms affecting NOS expression, activity, and regulation in the PVN in the pathophysiological states affecting the cardiovascular system.
GRANTS
Support for this work was provided by National Institutes of Health Grants K99/R00-HL-087927 (to C. A. Northcott) and R01-GM-077352 (A. F. Chen) and by American Diabetes Association Grant Grant 7-08-RA-23 (to A. F. Chen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.A.N., C.H.-L., K.P.P., A.F.C., L.G.D., and J.R.H. conception and design of research; C.A.N., S.B., and T.C. performed experiments; C.A.N., T.C., and C.H.-L. analyzed data; C.A.N., T.C., C.H.-L., K.P.P., A.F.C., L.G.D., and J.R.H. interpreted results of experiments; C.A.N. prepared figures; C.A.N. drafted manuscript; C.A.N., S.B., T.C., C.H.-L., K.P.P., A.F.C., L.G.D., and J.R.H. approved final version of manuscript; S.B. and J.R.H. edited and revised manuscript.
REFERENCES
- 1. Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature 347: 768–770, 1990 [DOI] [PubMed] [Google Scholar]
- 2. Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci 24: 471–478, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Chen AF, Jiang SW, Crotty TB, Tsutsui M, Smith LA, O'Brien T, Katusic ZS. Effects of in vivo adventitial expression of recombinant endothelial nitric oxide synthase gene in cerebral arteries. Proc Natl Acad Sci USA 94: 12568–12573, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen AF, O'Brien T, Tsutsui M, Kinoshita H, Pompili VJ, Crotty TB, Spector DJ, Katusic ZS. Expression and function of recombinant endothelial nitric oxide synthase gene in canine basilar artery. Circ Res 80: 327–335, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Chirinos JA, David R, Bralley JA, Zea-Diaz H, Munoz-Atahualpa E, Corrales-Medina F, Cuba-Bustinza C, Chirinos-Pacheco J, Medina-Lezama J. Endogenous nitric oxide synthase inhibitors, arterial hemodynamics, and subclinical vascular disease: the PREVENCION Study. Hypertension 52: 1051–1059, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Closs EI, Basha FZ, Habermeier A, Forstermann U. Interference of l-arginine analogues with l-arginine transport mediated by the y+ carrier hCAT-2B. Nitric Oxide 1: 65–73, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Cunningham JT, Herrera-Rosales M, Martinez MA, Mifflin S. Identification of active central nervous system sites in renal wrap hypertensive rats. Hypertension 49: 653–658, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Ferrari MF, Fior-Chadi DR. Differential expression of nNOS mRNA and protein in the nucleus tractus solitarii of young and aged Wistar-Kyoto and spontaneously hypertensive rats. J Hypertens 23: 1683–1690, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Goonasekera CD, Rees DD, Woolard P, Frend A, Shah V, Dillon MJ. Nitric oxide synthase inhibitors and hypertension in children and adolescents. J Hypertens 15: 901–909, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Gordon FJ, Haywood JR, Brody MJ, Johnson AK. Effect of lesions of the anteroventral third ventricle (AV3V) on the development of hypertension in spontaneously hypertensive rats. Hypertension 4: 387–393, 1982 [DOI] [PubMed] [Google Scholar]
- 12. Haywood JR, Buggy J, Fink GD, DiBona GF, Johnson AK, Brody MJ. Alterations in cerebrospinal fluid sodium and osmolality in rats during one-kidney, one-wrap renal hypertension. Clin Exp Pharmacol Physiol 11: 545–549, 1984 [DOI] [PubMed] [Google Scholar]
- 13. Haywood JR, Williams SF, Ball NA. Contribution of sodium to the mechanism of one-kidney, renal-wrap hypertension. Am J Physiol Heart Circ Physiol 247: H797–H803, 1984 [DOI] [PubMed] [Google Scholar]
- 14. Heresztyn T, Worthley MI, Horowitz JD. Determination of l-arginine and NG, NG- and NG,NG′-dimethyl-l-arginine in plasma by liquid chromatography as AccQ-Fluor fluorescent derivatives. J Chromatogr B Analyt Technol Biomed Life Sci 805: 325–329, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Herzig TC, Buchholz RA, Haywood JR. Effects of paraventricular nucleus lesions on chronic renal hypertension. Am J Physiol Heart Circ Physiol 261: H860–H867, 1991 [DOI] [PubMed] [Google Scholar]
- 16. Horn T, Smith PM, McLaughlin BE, Bauce L, Marks GS, Pittman QJ, Ferguson AV. Nitric oxide actions in paraventricular nucleus: cardiovascular and neurochemical implications. Am J Physiol Regul Integr Comp Physiol 266: R306–R313, 1994 [DOI] [PubMed] [Google Scholar]
- 17. Kakimoto Y, Akazawa S. Isolation and identification of NG,NG- and NG,N′G-dimethyl-arginine, Nε-mono-, di-, and trimethyllysine, and glucosylgalactosyl- and galactosyl-δ-hydroxylysine from human urine. J Biol Chem 245: 5751–5758, 1970 [PubMed] [Google Scholar]
- 18. Kerr S, Brosnan MJ, McIntyre M, Reid JL, Dominiczak AF, Hamilton CA. Superoxide anion production is increased in a model of genetic hypertension: role of the endothelium. Hypertension 33: 1353–1358, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Khan SA, Lee K, Minhas KM, Gonzalez DR, Raju SV, Tejani AD, Li D, Berkowitz DE, Hare JM. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc Natl Acad Sci USA 101: 15944–15948, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kielstein JT, Fliser D, Veldink H. Asymmetric dimethylarginine and symmetric dimethylarginine: axis of evil or useful alliance? Semin Dial 22: 346–350, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Kielstein JT, Salpeter SR, Bode-Boeger SM, Cooke JP, Fliser D. Symmetric dimethylarginine (SDMA) as endogenous marker of renal function–a meta-analysis. Nephrol Dial Transplant 21: 2446–2451, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Kielstein JT, Zoccali C. Asymmetric dimethylarginine: a novel marker of risk and a potential target for therapy in chronic kidney disease. Curr Opin Nephrol Hypertens 17: 609–615, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Lerman LO, Nath KA, Rodriguez-Porcel M, Krier JD, Schwartz RS, Napoli C, Romero JC. Increased oxidative stress in experimental renovascular hypertension. Hypertension 37: 541–546, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Li YF, Mayhan WG, Patel KP. NMDA-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am J Physiol Heart Circ Physiol 281: H2328–H2336, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Li YF, Roy SK, Channon KM, Zucker IH, Patel KP. Effect of in vivo gene transfer of nNOS in the PVN on renal nerve discharge in rats. Am J Physiol Heart Circ Physiol 282: H594–H601, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Li YF, Wang W, Mayhan WG, Patel KP. Angiotensin-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am J Physiol Regul Integr Comp Physiol 290: R1035–R1043, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Martin DS, Haywood JR. Reduced GABA inhibition of sympathetic function in renal-wrapped hypertensive rats. Am J Physiol Regul Integr Comp Physiol 275: R1523–R1529, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Northcott CA, Watts S, Chen Y, Morris M, Chen A, Haywood JR. Adenoviral inhibition of AT1a receptors in the paraventricular nucleus inhibits acute increases in mean arterial blood pressure in the rat. Am J Physiol Regul Integr Comp Physiol 299: R1202–R1211, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Patel KP, Li YF, Hirooka Y. Role of nitric oxide in central sympathetic outflow. Exp Biol Med 226: 814–824, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Patel KP, Zhang K, Zucker IH, Krukoff TL. Decreased gene expression of neuronal nitric oxide synthase in hypothalamus and brainstem of rats in heart failure. Brain Res 734: 109–115, 1996 [PubMed] [Google Scholar]
- 31. Pou S, Pou WS, Bredt DS, Snyder SH, Rosen GM. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem 267: 24173–24176, 1992 [PubMed] [Google Scholar]
- 32. Qadri F, Arens T, Schwarz EC, Hauser W, Dendorfer A, Dominiak P. Brain nitric oxide synthase activity in spontaneously hypertensive rats during the development of hypertension. J Hypertens 21: 1687–1694, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Rossi NF, Maliszewska-Scislo M, Chen H, Black SM, Sharma S, Ravikov R, Augustyniak RA. Neuronal nitric oxide synthase within paraventricular nucleus: blood pressure and baroreflex in two-kidney, one-clip hypertensive rats. Exp Physiol 95: 845–857, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sadler CJ, Wilding JP. Reduced ventromedial hypothalamic neuronal nitric oxide synthase and increased sensitivity to NOS inhibition in dietary obese rats: further evidence of a role for nitric oxide in the regulation of energy balance. Brain Res 1016: 222–228, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Shang T, Kotamraju S, Kalivendi SV, Hillard CJ, Kalyanaraman B. 1-Methyl-4-phenylpyridinium-induced apoptosis in cerebellar granule neurons is mediated by transferrin receptor iron-dependent depletion of tetrahydrobiopterin and neuronal nitric-oxide synthase-derived superoxide. J Biol Chem 279: 19099–19112, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Sharma NM, Zheng H, Li YF, Patel KP. Nitric oxide inhibits the expression of AT1 receptors in neurons. Am J Physiol Cell Physiol; doi: 10.1152/ajpcell.00258.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Siragy HM, Carey RM. Protective role of the angiotensin AT2 receptor in a renal wrap hypertension model. Hypertension 33: 1237–1242, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Somers MJ, Mavromatis K, Galis ZS, Harrison DG. Vascular superoxide production and vasomotor function in hypertension induced by deoxycorticosterone acetate-salt. Circulation 101: 1722–1728, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Takeda Y, Miyamori I, Yoneda T, Furukawa K, Inaba S, Takeda R, Mabuchi H. Brain nitric oxide synthase messenger RNA in central mineralocorticoid hypertension. Hypertension 30: 953–956, 1997 [DOI] [PubMed] [Google Scholar]
- 40. Tojo A, Welch WJ, Bremer V, Kimoto M, Kimura K, Omata M, Ogawa T, Vallance P, Wilcox CS. Colocalization of demethylating enzymes and NOS and functional effects of methylarginines in rat kidney. Kidney Int 52: 1593–1601, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Vallance P, Leone A, Calver A, Collier J, Moncada S. Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis. J Cardiovasc Pharmacol 20, Suppl 12: S60–S62, 1992 [DOI] [PubMed] [Google Scholar]
- 42. VanNess JM, Hinojosa-Laborde C, Craig T, Haywood JR. Effect of sinoaortic deafferentation on renal wrap hypertension. Hypertension 33: 476–481, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Vasquez-Vivar J, Hogg N, Martasek P, Karoui H, Pritchard KA, Jr, Kalyanaraman B. Tetrahydrobiopterin-dependent inhibition of superoxide generation from neuronal nitric oxide synthase. J Biol Chem 274: 26736–26742, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Vitela M, Herrera-Rosales M, Haywood JR, Mifflin SW. Baroreflex regulation of renal sympathetic nerve activity and heart rate in renal wrap hypertensive rats. Am J Physiol Regul Integr Comp Physiol 288: R856–R862, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Ye S, Nosrati S, Campese VM. Nitric oxide (NO) modulates the neurogenic control of blood pressure in rats with chronic renal failure (CRF). J Clin Invest 99: 540–548, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang K, Li YF, Patel KP. Blunted nitric oxide-mediated inhibition of renal nerve discharge within PVN of rats with heart failure. Am J Physiol Heart Circ Physiol 281: H995–H1004, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Zhang K, Mayhan WG, Patel KP. Nitric oxide within the paraventricular nucleus mediates changes in renal sympathetic nerve activity. Am J Physiol Regul Integr Comp Physiol 273: R864–R872, 1997 [DOI] [PubMed] [Google Scholar]
- 48. Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am J Physiol Regul Integr Comp Physiol 275: R728–R734, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Zhang K, Zucker IH, Patel KP. Altered number of diaphorase (NOS) positive neurons in the hypothalamus of rats with heart failure. Brain Res 786: 219–225, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Zheng H, Liu X, Li Y, Sharma NM, Patel KP. Gene transfer of neuronal nitric oxide synthase to the paraventricular nucleus reduces the enhanced glutamatergic tone in rats with chronic heart failure. Hypertension 58: 966–973, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zheng H, Mayhan WG, Bidasee KR, Patel KP. Blunted nitric oxide-mediated inhibition of sympathetic nerve activity within the paraventricular nucleus in diabetic rats. Am J Physiol Regul Integr Comp Physiol 290: R992–R1002, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Zoccali C, Mallamaci F, Maas R, Benedetto FA, Tripepi G, Malatino LS, Cataliotti A, Bellanuova I, Boger R. Left ventricular hypertrophy, cardiac remodeling and asymmetric dimethylarginine (ADMA) in hemodialysis patients. Kidney Int 62: 339–345, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Zotti M, Schiavone S, Tricarico F, Colaianna M, D'Apolito O, Paglia G, Corso G, Trabace L. Determination of dimethylarginine levels in rats using HILIC-MS/MS: an in vivo microdialysis study. J Sep Sci 31: 2511–2515, 2008 [DOI] [PubMed] [Google Scholar]