Abstract
Tone regulation in coronary microvessels has largely been studied in isolated vessels in the absence of myocardial tethering. Here, the potential effect of radial tethering and interstitial space connective tissue (ISCT) between coronary microvessels and the surrounding myocardium was studied. We hypothesized that rigid tethering between microvessels and the myocardium would constrain the active contraction of arterioles and is not compatible with the observed tone regulation. The ISCT between coronary microvessels and myocardium in five swine was found to increase exponentially from 0.22 ± 0.02 μm in capillaries (modified Strahler order 0) of the endocardium to 34.9 ± 7.1 μm in epicardial vessels (order 10). Microvessels with both soft tethering and ISCT gap were capable of significant changes in vessel resistance (up to an ∼1,600% increase), consistent with experimental measurements of high coronary flow reserve. Additionally, the mechanical energy required for myogenic contraction was estimated. The results indicate that rigid tethering requires up to four times more mechanical energy than soft tethering in the absence of a gap. Hence, the experimental measurements and model predictions suggest that effectiveness and efficiency in tone regulation can be achieved only if the vessel is both softly tethered to and separated from the myocardium in accordance with the experimental findings of ISCT gap. These results have fundamental implications on future simulations of coronary circulation.
Keywords: coronary vessels, tone regulation, flow regulation, vessel tethering, mechanical analysis
the coronary circulation is highly autoregulated. Network flow regulation depends on the local vasoactivity of each vessel of the network. Vascular smooth muscle (VSM) cells regulate wall tension (tone regulation) to respond to changes in intravascular pressure (myogenic), blood flow-induced wall shear stress (flow), and metabolic stimuli, with the goal to maintain a metabolic supply-demand balance under a wide range of cardiovascular activity (23). The passive and active pressure-diameter relationships (PDRs) of coronary microvessels thus play an important role in coronary flow regulation.
Liao and Kuo (38) characterized the passive PDR and studied the effects of flow attributes in isolated porcine coronary micovessels in vitro. Under a myogenic (spontaneous) stimulus, they found the greatest decrease in diameter for intermediate arterioles of the porcine left anterior descending and left circumflex vascular trees to be ∼58% (108 μm down to 45 μm). They also observed that flow and metabolic stimuli independently reduce the effect of the myogenic contraction. Cornelissen et al. (18) developed a comprehensive, empirical model of flow control for isolated vessels of a highly simplified coronary network and demonstrated good correspondence between their predictions and the in vitro experimental data of Liao and Kuo (38). Carlson and Secomb (11) and Carlson et al. (10) later developed a mechanistic approach for tone regulation and found good agreement between their predictions and the experimental data from a variety of microvessels of various species.
Although these in vitro studies have provided significant insights, little work has been done to show how active coronary microvessels interact with the myocardium. The effect of the myocardium on the passive PDR has been previously studied (1, 2), albeit the effect of the myocardium on the behavior of active vessels has yet to be determined. The latter must be included for realistic simulations of coronary flow regulation.
Although the histology of blood vessels has been well documented (17, 44, 49), a literature search revealed little data on the details of radial tethering and cross talk between the myocardium and microvessels (7, 50). Moreover, there seems to be no evidence on whether the coronary vessels are closely attached to the myocardium and, alternatively, if they are separated, on the nature of the connective tissue in the space between the vessel and myocardium [the interstitial space connective tissue (ISCT)]. Although there have been a number of studies that have hypothesized on the nature of the effect of axial and circumferential tethering on pulsatile flow in passive microvessels (16, 31, 34, 41, 45), the radial component of tethering and the size of the ISCT gap between coronary vessels and myocardium, which are essential for understanding flow regulation in the intramyocardial microvessels, have not yet been determined.
Since the myogenic control mechanism may decrease the diameter of a microvessel by up to 60% (38), the ability of that vessel to “pull” the myocardium (if rigidly tethered) is unknown. Rachev and Hayashi (43) and Zulliger et al. (51) developed separate continuum mechanical approaches for evaluating the change in the stiffness and stress-free configuration of an active vessel. Such a model, in conjunction with a detailed vessel-in-myocardium model similar to the model used by Algranati et al. (1), can be used to evaluate the constraint imposed by the myocardium on myogenic contraction. However, the requisite data on passive vessel mechanical properties, stress-free configurations, and kinematic mappings from the passive to active states of microvessels are currently unavailable.
The objectives of this study were as follows: 1) to experimentally measure the ISCT gap size between coronary vessels and the myocardium and 2) use data-based analysis to study the effects of the ISCT gap and vessel-myocardium tethering rigidity on tone regulation. We hypothesized that for effective (ability to reduce flow) and efficient (low energetic demand) tone regulation, vasoreactivity should occur unconstrained by the myocardium. Such isolation can be achieved both by a vessel-myocardium ISCT gap and by soft tethering between the vessel and myocardium.
METHODS
All animal experiments were performed in accordance with national and local ethical guidelines, including the Institute of Laboratory Animal Research guidelines, Public Health Service policy, the Animal Welfare Act, and an approved Indiana University Purdue University Indianapolis protocol regarding the use of animals in research.
Vessel-myocardium gap size.
Five hearts from normal Yorkshire swine of either sex (29.6 ± 2.1 kg body wt) were used for measurements from a recently published study (15). Briefly, myocardial tissue samples were obtained from the left ventricular free wall at three transmural locations (subepicardium, midmyocardium, and endocardium). The regions were defined by dividing the wall thickness into three equal portions. Under microscopy, each region was sliced, using ordinary razor blades, into 2 × 2 × 2-mm uniform pieces along the long axis of the myocardial fibers. The samples were then processed for histological preparation. Each section was completely surveyed for arterial and capillary vessels. Photographs of histological sections of vessels were taken, and the following measurements were made using NIS-Elements imaging software (Nikon, Melville, NY): inner circumference and the space between the media layer and the myocardium (gap) at four different locations around the vessel (north, south, east, and west). A circular diameter of the vessels was calculated by dividing the inner circumference by π.
Modeling overview.
The primary assumption of this work is that the myogenic component of activation is sensitive to the pressure difference across the wall of the microvessel (ΔP; transvascular pressure). This is consistent with Carlson and Secomb (11), who proposed that the VSM regulates the circumferential tension (T) within the wall of the vessel, since, by Laplace's law, T = (ΔP × D)/2, where D is vessel diameter. Therefore, the passive and active PDR data for isolated microvessels are sufficient to model the in vivo behavior of microvessels. According to Algranati et al. (1) and Vis et al. (48), the myocardium can be represented by a thick-walled tube surrounding each vessel, which changes ΔP and thus the vessel wall tension (48).
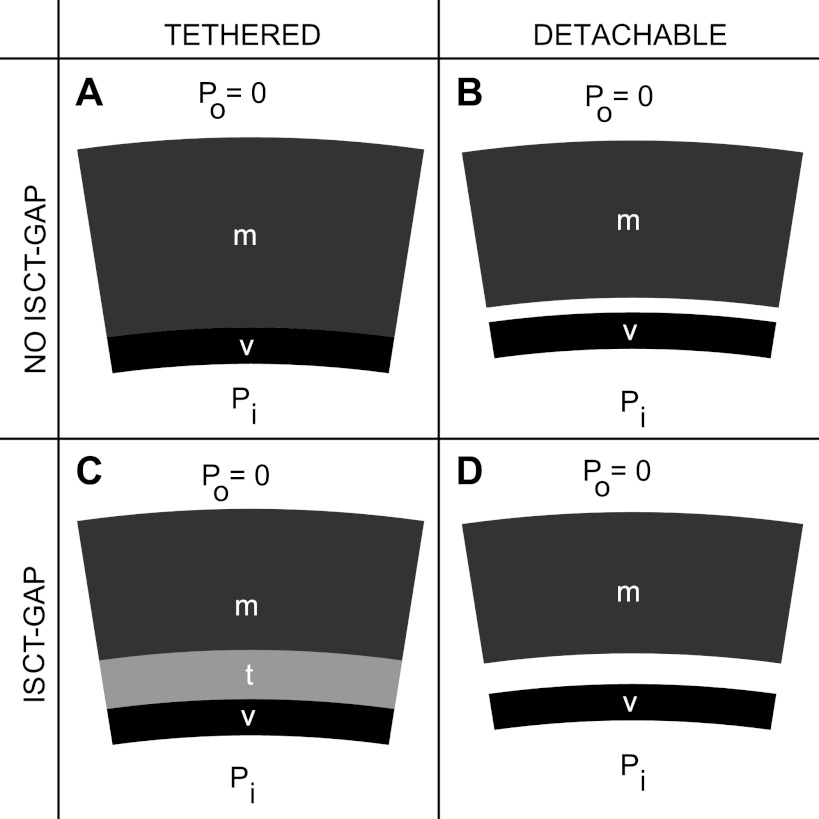
Based on the experimental evidence of the presence of an ISCT gap, and in view of absent data on the rigidity of vessel-myocardium tethering, the effects of gap and tethering rigidity were studied by considering four extreme cases: 1) no gap and rigidly tethered (NGT), 2) no gap and detachable (NGD), 3) gap and rigidly tethered (GT), and 4) gap and detachable (GD) (Fig. 1).
Fig. 1.
Schematics of the four models considered, where v is vessel, m is myocardium, t is the tethering domain, Pi is the internal pressure, and Po = 0 is the external pressure. As an example, the load Pi > 0, but the vessel is active, such that it pulls the myocardium with it when tethered (A and C) but detaches from the myocardium when freely detachable (B and D). A: the no-interstitial space connective tissue (ISCT) gap and tethered case (NGT), where the tethering domain is infinitesimally small. B: the no-ISCT gap and detachable case (NGD). The vessel can freely separate from the myocardium. Under no load (Pi = 0), the vessel and myocardium are assumed to be in contact. C: the gap and tethered case (GT), with finite tethering. Here, the gap size (g) is constant for all pressure loads Pi and activation levels, but the vessel is not in direct contact with the myocardium. D: the gap and detachable case (GD). The vessel can freely separate from the myocardium, and, under no load, the vessel and myocardium are separated.
PDR of the composite.
In view of the concentric configuration of the vessel and myocardium (see Fig. A1), the radial equilibrium equation for the composite pressure (ΔPC) is the sum of the radial equilibrium for the microvessel (subscrip v) and the myocardium (subscript m) (appendix a), as follows:
| (1) |
where ΔPv(Dvo) is the pressure across the vessel as a function of the outer diameter of the microvessel (Dvo) and ΔPm(Dmi) is the pressure across the myocardium as a function of the inner diameter of the myocardium (Dmi). For the composite, ΔPC = Pi − Po = Pi, and, by Eq. A2, ΔPv = Pi − ΔPm (where Po and Pi are the pressures internal and external to the cylinder). Dvo is then solved as follows:
| (2) |
In Eq. 2, Dmi = Dvo when the vessel and myocardium are in contact. This is always true for the NGT case and only true for GD and NGD cases when the load Pi is high enough to press the vessel into the myocardium. For the GT case, Dmi = Dvo + 2g, where g is the ISCT gap thickness. For the detachable vessel, when the pressure load Pi is insufficient to push the vessel into the myocardium, then ΔPm = 0. Thus, Dmi can always be related to Dvo, and Eq. 2 was solved for ΔPv(Dvo).
Equation 2 gives the PDR of the composite vessel in myocardium for the four cases. Since D is uniquely linked to ΔP in each cylinder, the functions ΔPv(D) and ΔPm(D) are the inverse functions of the PDR for the vessel and myocardium, respectfully.
Passive and active microvessel PDR.
For tone-regulating microvessels, vessel diameter D was assumed to be a function of the pressure ΔPv and activation level (A) of 0 ≤ A ≤ 1. Microvessel diameter was composed of a passive diameter (Dp), a diameter decrease due to myogenic activation (ΔDa), and an activation level of 0 ≤ A ≤ 1 (see Fig. 3), such that
| (3) |
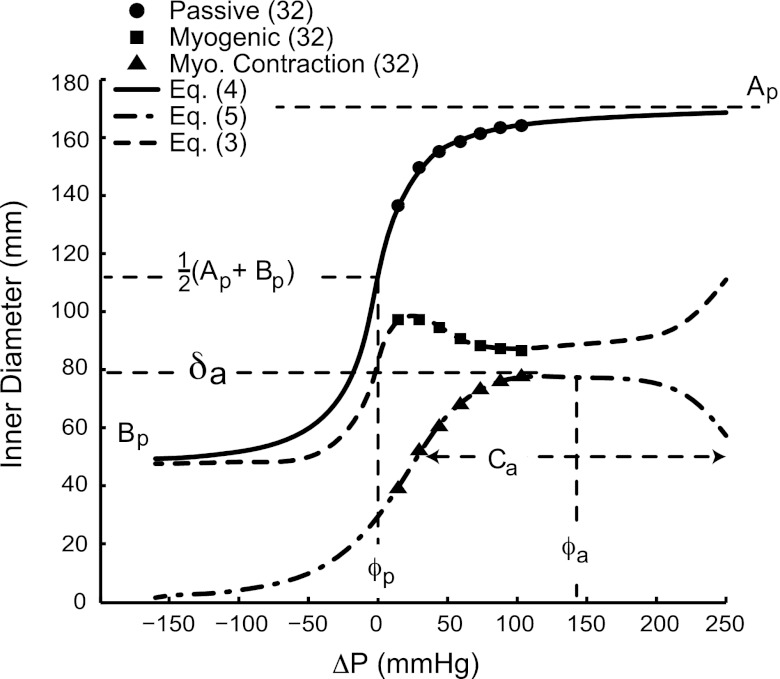
Fig. 3.
Passive, myogenic, and diameter change due to myogenic contraction data (38) and model (Eqs. 3–5) curves for an order 6 microvessel. The parameters used for the model equations were taken from Table 1.
Detailed expressions for the terms on the righthand side of Eq. 3 are now presented.
Based on empirical evidence (11, 30, 38), the passive PDR is sigmoidal in shape. Here, the sigmoidal curve was defined by a four-parameter arctan function so that it closely matched the data of Liao and Kuo (38) (see Fig. 3) with a better fit to these data than previously used sigmoidal models (see Eq. B5 of Ref. 1), namely,
| (4) |
where Ap is the asymptotic maximum passive diameter, Bp is the asymptotic minimum passive diameter, ϕp is the pressure at which the vessel is halfway between Ap and Bp, and Cp is the bandwidth of the transition between Ap and Bp (see Fig. 3).
Liao and Kuo (38) noted that the myogenic response of microvessels consisted of three phases. First, the cells contracted with increasing transmural pressure. Second, the level of contraction of the cells reached a peak, likely a property of the VSM contractile apparatus (11), and the VSM was unable to generate further tension. Third, the tension due to increasing transvascular pressure overcame the contractile force of the VSM, and the VSM passively elongated. Based on these observations and the reasons used for the choice of the form of Eq. 4, the decrease in diameter due to fully active myogenic contraction ΔDa was also governed by a four-parameter arctan function as follows:
| (5) |
where δa is the amplitude of the change in diameter, ϕa is the pressure across the vessel at which the maximum diameter change occurred, Ca is the bandwidth of myogenic activation, and a is an integer that dictates the shape of the myogenic response (see Fig. 3). The constants Ap, Bp, ϕp, Cp, δa, ϕa, Ca, and a were estimated from the passive and myogenic data (38) using fsolve in Matlab (Mathworks, Waltham, MA).
Since Liao and Kuo (38) conducted their experiments in isolated vessels, Po = 0 for the purpose of parameter estimation. Also, the constants were reevaluated for the outer diameter by using incompressibility and the unloaded wall thickness data of Frøbert et al. (24) since the data of Liao and Kuo represented the inner diameter dependence on pressure.
Finally, the pressure ΔPv as a function of D for active and passive vessels was determined numerically by solving Eq. 3 for ΔPv at a given D using fsolve in Matlab.
Passive myocardium PDR.
The PDR for the myocardium Dmi(ΔPm) was obtained in the no-ISCT gap and ISCT gap cases using continuum mechanics analysis (appendix b). The mechanics of the kinematic mapping from stress-free to loaded states of a representative cylinder of passive myocardium are a well-posed, well-studied problem, especially since most of the configurational parameters and material constants have been published (1, 25, 37, 40, 42, 48).
The remaining unknown kinematic parameters were the stress-free inner and outer radii (Rmi,SF and Rmo,SF, respectively), closed inner and outer radii (Rmi,CL and Rmo,CL, respectively), closing axial stretch ratio (λCL), and outer radius in the loaded state (Rmo,P) (Fig. B1). These parameters were estimated based on radial and axial equilibrium and from the available average data of the closed inner and outer vessel radii (Rvi,CL and Rvo,CL, respectively) (24), the in situ axial stretch ratio of the vessel (λU) (27), and the in situ stretched inner and outer vessel radii (Rvi,U and Rvo,U, respectively) (38). The details of the analysis are presented in appendix b.
Similarly to what was previously found (1), the numerical solutions from the mechanical model showed that relationship Dmi(ΔPm) is sigmoidal. Therefore, to simplify the solution of Eq. 2, Dmi(ΔPm) was approximated by the following:
| (6) |
where Am and Cm are the asymptotic maximum and minimum radii, Bm is the unloaded radius, and ϕm is the pressure at which Dmi = Am + Cm. From the mechanical analysis, Bm = g + Rvo,0, where g is the ISCT gap size and Rvo,0 is the unloaded outer radius of the vessel.
The factor of 2 was included in Eq. 6 to transform between diameter and radius, which is used in the calculation of the work of contraction below and in appendix c. Moreover, the passive sigmoid of the myocardium in Eq. 6 was chosen over the form of Eq. 4 because using an arctan function in the calculation of the work of contraction produces complex (imaginary) results in Eq. 12 and in Eq. C4 in appendix c.
Finally, by inversion of Eq. 6, ΔPm(D) takes the following form:
| (7) |
Effectiveness of flow regulation.
The ability of the myogenic response to locally regulate flow is an important aspect of tethering and gap. To measure the effect of tethering rigidity and gap on flow, the percent increase in vessel resistance due to myogenic contraction was calculated for all four cases. First, the in vivo PDR for the outer vessel diameter was mapped down to the inner diameter of the vessel using incompressibility between the unloaded and loaded case, which yielded the following:
| (8) |
where Dvi,0 and Dvo,0 are the unloaded inner and outer vessel diameters, respectively. By Poiseuille's Law, vessel resistance (ℜ) is proportional to Dvi−4. The percent increase in ℜ (%ℜ) from a passive state (subscript P) to an active state (subscript A) is given by the following:
| (9) |
where Dvi,P and Dvi,A are the lumen diameters in the passive and active states, respectively. The effectiveness (E) is defined as the relative capacity to reduce flow under myogenic activation; namely,
| (10) |
where %ℜmax is the maximum %ℜ among all four cases for each vessel order. Equations 8–10 were calculated for A = 1 and at Pi = 100 mmHg to measure the effect of uninhibited myogenic contraction on flow at a pressure within the middle of the second phase of myogenic contraction, i.e., where the myogenic effect is strongest.
Efficiency of flow regulation.
The energetic cost of the vessel contraction may be an important aspect of flow regulation. Therefore, the energy cost of myogenic contraction at a specific load Pi between NGT and NGD vessels as well as between GT and GD vessels were compared to determine which vessel types are more energetically efficient in adjusting the vessel diameter to meet the metabolic demands of the myocardium. To compare the work of myogenic contraction, both detachable and tethered vessel types were constrained to reach the same diameter D. Based on preliminary simulations, the rigidly tethered case was found to be limited by the rigid tethering to the myocardium. Therefore, the diameter of the tethered vessel was chosen as the diameter to which both freely detachable and tethered vessels contract.
Using Laplace's law and assuming the myocardium acts on the vessel wall as an external load, the mechanical work of contraction (wC) is the line integral of wall tension along a path from the passive radius Rp = (1/2)Dp to the active radius R = (1/2)D, such that
| (11) |
where Dp is given by Eq. 6 and D is given by Eq. 5. Using Eq. 2, we then obtained the desired expression as follows:
| (12) |
R in Eq. 11 was chosen to be the active radius of the rigidly tethered vessel, and, therefore, all variables for the tethered vessel were designated with subscript T. Likewise, the variables for the detachable vessel were designated with subscript D.
The ratio of the mechanical work of contraction between tethered and detachable vessel types (wR,i) was defined as follows:
| (13) |
where wC,iT and wC,iD are the mechanical works of contraction in tethered and detachable vessel types, respectively, and i = [NG, G] is a metric of the relative cost in mechanical energy necessary for the detachable vessel to reach the same diameter as the rigidly tethered vessel. Thus, wR is a measure of the efficiency in flow regulation. The work terms in Eq. 12 were calculated and their ratio evaluated for a range of Pi and for different activation levels of the tethered vessel (AT).
RESULTS
Vessel-myocardium ISCT gap.
Figure 2A shows a representative cross section of the myocardium with an embedded arteriole (order 4), an ISCT gap surrounding the arteriole, and myocytes of the myocardium. The gap is a thin layer of collagen fibers, glycoproteins, and glycosaminoglycans (8). Figure 2B shows the measured vessel-myocardium ISCT gap size of the porcine coronary arterial tree. There was an increasing log-linear trend in ISCT gap size with Strahler order, whereas no statistical difference was found between the three myocardium layers.
Fig. 2.
A: representative image of the lumen (white), wall (black), and gap (gray) of an arteriole (order 4). B: measured gap size between the porcine coronary vessels and myocardium from order 0 to 10 vessels of the endocardial (Endo), midmyocardial (Mid), and subepicardial (Subepi) regions. Lines represent a log-linear fit to the mean gap size. Error bars are ±SE. There were no statistical differences in gap size between the layers.
Passive and active microvessel properties.
The estimated parameters of Eqs. 4 and 5 for passive and active vessels of orders 5, 6, and 7 are shown in Table 1. They were estimated from the data of passive and active vessels (38). The models fit the data of Liao and Kuo very well, with R2 values of 0.999, 0.998, and 0.999 for the passive data of orders 5, 6, and 7 and 0.992, 0.998, and 0.994 for the myogenic data of orders 5, 6, and 7, respectively (Fig. 3).
Table 1.
Vessel model parameters
|
Order |
|||
|---|---|---|---|
| Parameter | 5 | 6 | 7 |
| Ap | 173 | 259 | 440 |
| Bp | 160 | 200 | 360 |
| ϕp | 6.20 | 4.44 | 4.96 |
| Cp | 21.1 | 18.0 | 21.2 |
| δa | 17.3 | 81.8 | 81.0 |
| ϕa | 75.9 | 113 | 85.7 |
| Ca | 57.4 | 98.9 | 53.5 |
| a | 2 | 2 | 2 |
Estimated parameters for the passive pressure-diameter relationship (PDR; Eq. 4) and active PDR (Eq. 5) of the outer vessel diameter based on the in vitro data of Liao and Kuo (38). These parameters were constant for all four cases [no gap and detachable (NGD), no gap and rigidly tethered (NGT), gap and detachable (GD), and gap and rigidly tethered (GT)]. The parameters for an order 6 microvessel were used to generate the model curves in Fig. 3. Ap, asymptotic maximum passive diameter; Bp, asymptotic minimum passive diameter; ϕp, pressure at which the vessel is halfway between Ap and Bp; Cp, bandwidth of the transition between Ap and Bp; δa, amplitude of the change in diameter; ϕa, pressure across the vessel at which the maximum diameter change occurred; Ca, bandwidth of myogenic activation; a, an integer that dictates the shape of the myogenic response.
Passive myocardium PDR.
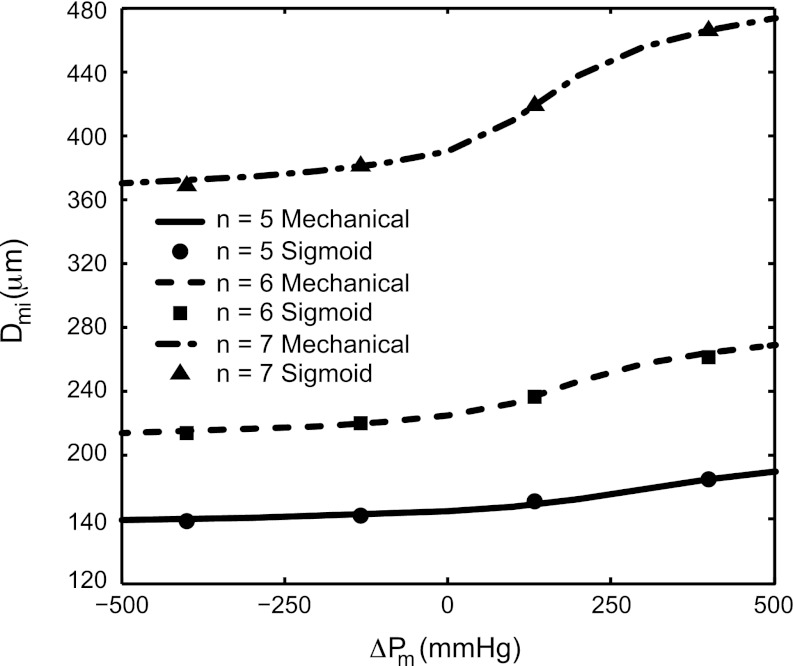
The estimated configurational parameters of the mechanical model of the myocardium are summarized in Table 2. The constants for the four-term sigmoidal representation are summarized in Table 3. Figure 4 shows a comparison of the myocardium PDR solution for the myocardium for order 5–7 microvessels in the ISCT gap case.
Table 2.
Configurational parameters
| Rvi,CL* | Rvo,CL* | λU† | Rvi,U | Rvo,U | Rmi,SF | Rmo,SF | λCL | |
|---|---|---|---|---|---|---|---|---|
| No gap | ||||||||
| Order | ||||||||
| 5 | 74.4 | 112 | 1.10 | 22.5 | 82.7 | 1091 | 1255 | 1.08 |
| 6 | 96.0 | 146 | 1.18 | 49.0 | 112 | 1434 | 1635 | 1.08 |
| 7 | 149 | 243 | 1.26 | 95.0 | 195 | 2458 | 2791 | 1.07 |
| Gap | ||||||||
| Order | ||||||||
| 5 | 74.4 | 112 | 1.10 | 22.5 | 82.7 | 1105 | 1265 | 1.07 |
| 6 | 96.0 | 146 | 1.18 | 49.0 | 112 | 1457 | 1652 | 1.06 |
| 7 | 149 | 243 | 1.26 | 95.0 | 195 | 2498 | 2821 | 1.05 |
Configuration parameters for the mechanical model of the myocardium cylinder. All radii (R) are in micrometers, and stretches (λ) are dimensionless. Data from Frøbert et al. (24) (*) of closed, unloaded inner and outer radii of the vessel (Rvi,CL and Rvo,CL) and from Guo et al. (27) (†) of the in situ axial stretch ratio (λCL) were used to determine the in situ inner and outer radii of the vessel (Rvi,U and Rvo,U). Additionally, the stress-free inner and outer radii of the myocardium (Rmi,SF and Rmo,SF) and the closing stretch ratio (λCL) for the myocardium were determined and used to calculate the continuum-based PDR of the passive myocardium. Note that the vessel configurational parameters are equal in the no-interstitial space connective tissue (ISCT) gap and ISCT gap cases, but the constants for the myocardium differ due to the gap between the vessel and myocardium under no internal load.
Table 3.
Sigmoid parameters
| Am | Bm | Cm | ϕm | |
|---|---|---|---|---|
| No-ISCT gap | ||||
| Order | ||||
| 5 | 102 | 83.0 | 78.8 | 328 |
| 6 | 142 | 113 | 106 | 254 |
| 7 | 246 | 198 | 183 | 185 |
| ISCT gap | ||||
| Order | ||||
| 5 | 108 | 85.6 | 81.3 | 285 |
| 6 | 150 | 117 | 110 | 198 |
| 7 | 260 | 205 | 190 | 132 |
Fig. 4.
Pressure-diameter relationship (PDR) for the myocardium enclosing order 5–7 vessels, in the gap case, with mechanical model curves (mechanical in inset, Appendix B) and sigmoidal models (sigmoid in inset) from Eq. 6. The R2 values for the sigmoidal fit to the mechanical model were 0.997, 0.996, and 0.996 for the gap case and 0.996, 0.997, and 0.997 for the no-gap case (not shown) for orders 5, 6, and 7, respectively. An excessively wide pressure range of −500 to +500 mmHg was used simply to demonstrate the sigmoidal shape of the myocardial PDR.
Composite PDR.
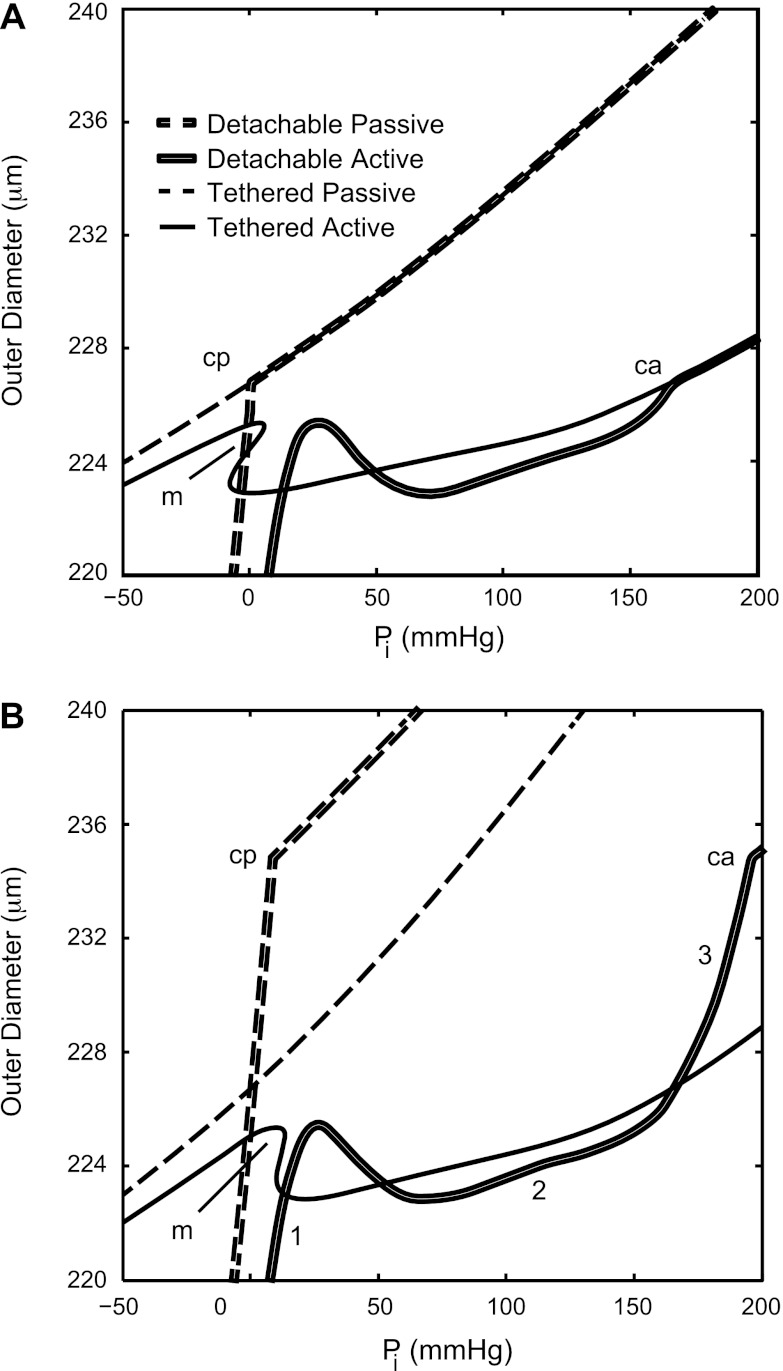
The composite model results for an order 6 vessel in the no-ISCT gap and ISCT gap cases with AT equal to the activation level of the detachable vessel (AD) equal to 0.8 are shown in Fig. 5, A and B, respectively. This combination of vessel order and activation level was chosen because order 6 is in the middle of the range of vessels considered here, and the composite results demonstrated that a moderately high AD is sufficient for vessel detachment in the NGD case. For high negative internal pressures, the detachable vessel separated from the myocardium, while the tethered vessel was restricted from contraction and constriction and the detachable diameter was always less than the tethered diameter. For high positive internal pressures, the detachable and tethered passive diameters were equal because the myocardium limits the expansion of both vessel types.
Fig. 5.
Composite PDRs for an order 6 vessel, with activation level in the tethered vessel AT = AD = 0.8, in the no-gap case (A) and the gap case (B) for detachable passive, detachable active, tethered passive, and tethered active vessels. A jump increase in composite stiffness relative to Pi can be seen at the point at which the passive detachable vessel first comes into contact with the myocardium (cp), and similarly for the active vessel (ca), where the slope of the composite curve abruptly changes. Both rigidly tethered curves showed a multivalued domain (m), whereas the detachable curves remained single valued with respect to Pi. B: the three phases of myogenic contraction are indicated with numerals (1–3) for the active PDR of the GD vessel.
For most moderate negative pressures, the active diameter of the detachable vessel was less than that of the rigidly tethered vessel. These results are not directly shown in Fig. 5 because the focus of this study was on the effect of gap and tethering on activation, which occurs solely under positive transvascular pressures.
In all orders studied, a multivalued domain developed between 60% and 100% (A = 0.6–1.0) activation levels of the rigidly tethered vessel, whereas the detachable vessel was single valued, with the exception of the order 7 NGD case.
Effectiveness of flow regulation.
Table 4 shows the relative change in vessel resistance by Eq. 9 and effectiveness in flow regulation by Eq. 10. Rvi,U and Rvo,U from Table 2 were used in Eq. 8 to calculate the diameters of the vessel lumen. The greatest increase in lumen resistance occurred for the GD case (E = 1) for all orders, where the resistance of the vessel increased by nearly 1,600% relative to the passive vessel.
Table 4.
Flow effectiveness
| NGD |
NGT |
GD |
GT |
|||||
|---|---|---|---|---|---|---|---|---|
| Passive | Active | Passive | Active | Passive | Active | Passive | Active | |
| Outer diameter | ||||||||
| Order | ||||||||
| 5 | 168 | 164 | 168 | 165 | 172 | 164 | 169 | 165 |
| 6 | 232 | 213 | 232 | 221 | 244 | 213 | 236 | 220 |
| 7 | 406 | 389 | 406 | 390 | 424 | 389 | 412 | 390 |
| Inner diameter | ||||||||
| Order | ||||||||
| 5 | 53.0 | 38.7 | 53.0 | 43.7 | 65.5 | 38.6 | 56.4 | 43.2 |
| 6 | 113 | 67.6 | 113 | 89.7 | 137 | 67.6 | 122 | 86.2 |
| 7 | 220 | 187 | 220 | 189 | 251 | 187 | 232 | 188 |
| NGD | NGT | GD | GT | |
|---|---|---|---|---|
| Percent resistance | ||||
| Order | ||||
| 5 | 252 | 118 | 728 | 189 |
| 6 | 691 | 155 | 1,584 | 298 |
| 7 | 91 | 84 | 226 | 130 |
| Effectiveness | ||||
| Order | ||||
| 5 | 0.35 | 0.16 | 1.00 | 0.26 |
| 6 | 0.44 | 0.10 | 1.00 | 0.19 |
| 7 | 0.40 | 0.37 | 1.00 | 0.58 |
Shown are outer diameters (in micrometers) of the NGD, NGT, GD, and GT cases for passive and active vessels, where internal pressure = 100 mmHg and activation level in the detached vessel = activation level in the tethered vessel = 1. Rvi,U and Rvo,U from Table 2 were used in Eq. 8 to calculate the inner diameters of the vessel (in micrometers). The percent change in resistance, calculated by Eq. 9, shows the magnitude of resistance increase, and the flow effectiveness, calculated by Eq. 10, indicated that the GD case was most effective in changing vessel resistance for all vessel orders.
Efficiency of flow regulation.
Figure 6, A and B, shows AD (appendix c, Eq. C1) as a function of Pi and AT. The NGD vessel activation was nearly identical to the NGT vessel activation for low activation levels and higher Pi (Fig. 6A). Furthermore, the activation was insufficient to separate the vessel from the myocardium under such pressure loads. As AT increased, the radius of the NGT vessel could not decrease further due to the rigid tethering to the myocardium. However, the NGD vessel at AD = AT could separate from the myocardium, and, therefore, AD decreased to match the radius of the NGT vessel.
Fig. 6.
AD in the no-ISCT gap case (A) and ISCT gap case (B) and the ratio of the work of contraction (wR in Eq. 13; wc,T/wc,D) in the no-ISCT gap case (C) and ISCT gap case (D) for an order 6 vessel as a function of Pi and AT (numeric labels). AD was calculated as in appendix c (Eq. C1) to estimate the relative work of contraction of tethered to detachable vessel types so that the detachable and tethered vessels were made to reduce the diameter to same outer diameter for Pi. The small multivalued region (*) in B is a result of the instability in the rigidly tethered composite solution. wR ≥ 1 in the no-gap case (C), but wR > 1 only under high levels of activation in the gap case (D). The multivalued region (*) in D is a result of the instability in the rigidly tethered composite solution and is a manifestation of the same point in B.
For the gapped GT and GD vessels, the situation was reversed (Fig. 6B). Due to the open gap in the GD case, the passive GD vessel radius was much greater than the passive GT vessel radius, where the gap was closed by the rigid tether. Therefore, the GD vessel radius must decrease a greater amount than the GT vessel radius, and, consequently, AD > AT for AT < 0.6. At about AT = AD = 0.8, the GD vessel radius was less than the GT vessel radius, and, therefore, AD decreased accordingly.
The predictions for the work of contraction ratio wR (Eq. 13) are shown for an order 6 vessel in the no-ISCT gap and ISCT gap cases in Fig. 6, C and D, respectively. The mechanical work of contraction for NGT vessels was always greater than the work of contraction for NGD vessels, as wR was always >1. However, the work of contraction of the GT vessel was usually less than the work of contraction for the GD vessel for similar reasons in the results seen for the level of activation of the GD case.
The apparent vertical asymptote in wR for small internal pressure loads Pi is also related to AD. As the internal pressure increased from 0 to some small value (<10 mmHg), AD increased from 0 to AT. Thus, the detachable vessel was entirely or nearly passive, and the work of contraction for the detachable vessel vanished at low pressures. Since wR is inversely proportional to wC,D, wR → ∞ for vanishing wC,D.
DISCUSSION
Overview.
The present study examined the effects of passive vessel-myocardium tethering on coronary flow regulation. The analysis was based on measured data of ISCT gap size and of passive and active properties of order 5–7 microvessels (38). The major findings of this analysis were that 1) rigidly tethered vessels cannot undergo significant diameter changes and constriction and, therefore, cannot affect coronary blood flow; 2) rigid tethering is mechanically more energetically demanding; and 3) rigid myocardial tethering may cause instability in the active PDR. These results suggest that the most effective and efficient vasoactive environment is the GD case: a system where the vessel is isolated mechanically (soft tethering or detachable) and geometrically (ISCT gap) from the myocardium. The measured ISCT gap sizes presented in this work are consistent with vessel-myocardium independence and with this conclusion.
ISCT gap size.
The presence and arrangement of connective tissue in skeletal and cardiac muscles was first described by Holmgren in 1907, who elaborated on an extracellular system of fibrils that interconnected capillaries to myocytes and myocytes to each other (33). This system has been divided into three major components: 1) a collagen network that surrounds groups of myocytes, 2) a network of collagen struts (120–150 nm in diameter) that extends from the basal laminae of a myocyte to the basal laminae of all contiguous myocytes, and 3) a network of similar size collagen struts that extends from the basal laminae of the capillaries to the basal laminae of the myocytes. Myocyte-to-myocyte struts are thought to prevent slippage of adjacent cells during the cardiac cycle, ensuring an equal stretch of adjacent myocytes during diastole (6, 8, 12). Myocyte-to-capillary struts may be important in maintaining capillary patency during the early phases of systole (6, 8, 12). Unfortunately, there is no similar description of the structural relation between myocytes and arterioles or larger coronary arteries. The present measurements are the first systematic quantification of the ISCT gap that generally consists of collagen fibers, glycoproteins, and glycosaminoglycans. These fibers are likely slack to allow free contraction of the microvessels from the myocytes. Further studies of gap thickness in vasoconstricted versus vasodilated states are needed to experimentally verify the slack hypothesis.
The increase in gap with order number (nominal diameter) of vessels accounts for the presence of adventitia (loose and dense) in the larger vessels. For the smaller arteries that lack adventitia (intima and media only), the increase in ISCT gap size with respect to vessel order likely accommodates for the greater degree of vasoconstriction of the intermediate arterioles (order 6 vessels) (38).
Microvessel properties.
The proposed models of Eqs. 3–5 fit the data of Liao and Kuo (38) well (Fig. 3) and extend the data over a much wider range of transvascular pressure. This extension introduces some uncertainty in the active PDR. For the function ΔDa(ΔPv) (Eq. 5), ϕa, Ca, and a may be subject to high variance because data for ΔPv > 102 mmHg were not available (Fig. 3). From the results of Carlson and Secomb (11), the active diameter, however, always returns to the passive diameter for high positive transvascular pressures (the third phase of myogenic contraction). To check the effects of the uncertainty in ϕa, Ca, and a, the analysis was repeated by artificially extending the second phase and delaying the third phase of myogenic contraction, which is associated with increased values of these three parameters (Fig. 3). The analysis of the extended contraction vessels suggests that the study conclusions still hold.
Myocardium properties.
As shown in Fig. 4, as vessel order (nominal vessel size) decreases, the extent of expansion under positive pressure relative to the unloaded state also greatly decreases, i.e., the myocardium is stiffer against radial expansion. This phenomenon is known as the tunnel-in-gel concept described by Fung (25).
Composite model.
The data-based modeling approach developed here was preferred over previously developed mechanical models (43, 51) since it circumvented the unavailable data of the change in stress-free configuration and the material properties of VSM under various levels of activation. Using the PDR data of Liao and Kuo (38) is advantageous because it includes all kinematic and material changes of the VSM under activation.
Although only rigidly tethered and freely detachable vessel types were considered in this work, they represent the upper and lower bound of the unknown radial tethering forces that may exist between coronary vessels and the myocardium in vivo. The real situation is likely somewhere between these bounds and, based on the results presented here, more likely closer to the detachable case.
Effectiveness of flow regulation.
The major conclusion is that tethering between the vessel and the myocardium, with or without an ISCT gap between the two tissues, would limit the constriction of the vessel lumen. Thus, the tethered vessel would likely be incapable of significant flow reduction, similar to the level that has been shown in vivo (22).
Under intensive physical activity, the coronary reserve is 2–22 times that of the resting coronary network in dogs (4) and 3.5–5 times that of the resting heart in humans (21). Since >55% of flow resistance occurs in microvessels with diameters < 50 μm (14), changes in total flow resistance are mostly due to microvessels of order 5 and less and are likely the major determinants of the high level of coronary reserve. Therefore, the constraint imposed by the myocardium on active vessels is expected to have a greater effect for vessels smaller than order 5. As shown in Fig. 4, the stiffness of the myocardium significantly increased with the decrease in vessel size. Therefore, any tone regulation in vessels smaller than order 5 would likely be negligible with rigid tethering. Consequently, the majority of flow resistance vessels (diameter < 50 μm) would be inert conduits. In vessels larger than order 7, flow control was observed to be significantly smaller compared with smaller active arterioles (Table 3 in Ref. 19).
As shown in Table 4 (%ℜ and effectiveness), the GD case allows for up to a factor of 16 increase in vessel resistance, which is nearly 5 times that of the GT case (E = 0.19). The next most effective configuration is the NGD case (E = 0.44), which is more than four times more effective in increasing vessel resistance compared with the NGT case (E = 0.10). These results suggest that the observed high level of coronary reserve is most readily achieved with detachable vessels and that the greatest increase in vessel resistance (and change in flow) is possible in the GD case.
It is important to note that high myogenic activation rarely exists because wall shear stress and metabolic demand reduce the overall activation level (10, 11, 18, 38). Since the results shown in Table 4 are given with respect to full myogenic activation (A = 1) and the GD case provides the greatest change to microvessel resistance, the GD case is the most compatible with the high coronary reserve.
Efficiency of flow regulation.
The NGT vessel always required higher mechanical energy to contract than the NGD vessel under the same internal load Pi. Although the GT vessel was more efficient with contraction than the GD vessel at low levels of activation (Fig. 6B), the GD vessel became more energy efficient than the GT vessel at high levels of activation. Therefore, rigidly tethered vessels require greater mechanical energy to contract compared with detachable vessels.
Instability in composite PDR.
For every case except GD, a multivalued PDR was predicted. Carlson and Secomb measured the multivalued diameter-tension relationship of active vessels (Figs. 1b, 2, and 4 in Ref. 11) and discussed instabilities in regulated vessel diameter (e.g. Ref. 20). In reality, the multivalued curve implies instability in the PDR in vivo. It is likely that the diameter of the active vessel in the multivalued (unstable) domain would attain the diameter where the mechanical energy is at a minimum. For the tone regulation observed in the literature for isolated vessels, it is unlikely that tone regulation of microvessels in vivo would be unstable. Moreover, due to the highly complex, asymmetric, and heterogeneous nature of the coronary network, such instabilities would cause portions of the network to lose flow regulation capability under specific levels of activation and loading.
Interestingly, rigid tethering is not essential to produce instability of the PDR in vivo, as shown for order 7. Here, order 7 NGD and NGT vessels both developed instability for moderately high levels of activation (AD = AT = 0.8). Thus, the instability is generally caused by the interaction between the myocardium and the active vessel, whether or not these structures were tethered.
Critique of the model.
While the data-based model analysis presented here reduces the number of overall assumptions, there are some limitations. The present analysis assumed cylindrical arterial microvessels. Vessel collapse and resulting elliptical cross-sectional geometry have not been considered. First, tethering of the microvessels to the surrounding myocardium by means of connective tissue fibers, while allowing for diameter reduction due to myogenic contraction up to a certain level, may impede and prevent complete vessel collapse. In experimental studies, vessel collapse has not been observed (32). In addition, myogenic contraction occurs solely under positive transvascular pressure, whereas vessel collapse occurs solely under negative transvascular pressure. Hence, it was assumed here that there is thus no need to include vessel collapse in analysis of myogenic response.
In Eq. 4, some terms were estimated from data that were remote from the range of interest (Table 1). For example, Bp, which dominates the response at high negative ΔPv, was estimated from the available data, which was restricted to moderate positive ΔPv. As such, the estimated Bp is subject to higher variance compared with the parameters estimated by interpolation within the available data. For the present study, this limitation is unimportant because myogenic contraction occurs only under positive transvascular pressures, where the passive and active PDR are less sensitive to Bp.
The available data of the configurational parameters (Table 2; Rvi,CL, Rvo,CL, λU, Rvi,U, and Rvo,U) (24, 27, 38) and gap size (Fig. 2b) are also subject to variability. An analysis of confidence intervals was not done since all four cases (gap, no gap, rigid, and detachable) were estimated based on the same data. Hence, the variance in the data should affect the parameters of all four cases similarly. Gap size (Fig. 2B), on the other hand, does affect the difference between the gap and no-gap cases (Rmi,SF, Rmo,SF, λCL, and the parameters from Table 3). Based on the results of our analysis, if the gap size is higher than the average level used, the benefit of gap will be even higher. If the gap size is smaller, then the results of the gap case will be closer to the no-gap case (higher energetic demand, lower flow reserve). If gap size substantially decreases, then our analysis suggests that the observed flow reserve cannot be achieved.
The present analysis relates to the passive myocardium. The conclusion regarding the necessity of detachment between intramyocardial vessels and the myocardium does not only hold in the case of stiffer contracted myocardium as well, but is actually expected to be of higher importance in that state. With stiffer, activated myocardium, VSM cells have to apply higher forces and expend more energy to contract against the resistance of the stiffer, contracted myocardium compared with the case of passive myocardium. Hence, the need for freedom of the vessel to contract (i.e., detachment from the myocardium) is even more critical in the active myocardium.
Cyclic myocardial stiffening and vascular compression strongly affect vessel collapse and flow directionality in vivo. However, the time scale of myocardial contraction (∼1 s) is between one and two orders of magnitude shorter than that of myogenic contraction (between 16 s and 2 min), as shown in Fig. 3A of the Kuo et al. study (36) and in other studies (32, 39, 47). Since these two mechanisms occur on largely different time scales, it is anticipated that tone regulation is insensitive to cyclic events, such as myocardial contraction and vessel compression, but rather depends on currently unknown representative levels of the dynamic intravascular (Pi) and extravascular (ΔPm) pressures (such as time-averaged, peak amplitude). Thus, the results presented here address only the steady-state myogenic response.
The present analysis, although based on available data (24, 26, 38, 40), has not been fully validated. To this end, one would need composite data not only on the available vessel in vitro PDR in the passive and active states (data that was used in the analysis) but, in addition, PDR data of the same sized vessels under in vivo conditions (e.g., embedded and tethered to the myocardium) in both the passive and controlled myogenic states. The currently available in vivo data on pressure and diameter of microvessels (3, 9, 13, 14, 35, 46) are insufficient since they relate either to vasodilated vessels or to vessels under an unknown in vivo spontaneous activation level determined by the composite effects of pressure, flow, and metabolism.
The mechanical energy of contraction investigated here relates solely to transient myogenic vessel contraction and not to the energy required to maintain the activated state. The latter case is a different issue since under sustained contraction of VSM, Ca2+ concentration, cross-bridge phosphorylation, and ATP consumption rate fall, a phenomenon termed the latch state (29).
Significance and impact.
This work highlights the importance of understanding the interaction between vessel tone and surrounding tissues since this has significant impact on the ability to locally regulate blood flow. These results further highlight the effects of cardiovascular disease, which may affect the environment external to the coronary vasculature. For example, hypertensive heart disease has been shown to stiffen the extracellular matrix of the myocardium (5). While this ultimately reduces the extent of myocardial contraction, it may also increase the tethering stiffness and, thus, limit the flow regulation capacity in the coronary vasculature. Additionally, the stiffness of the myocardium increases during the first 2 wk after anteroapical infarct but then becomes more compliant (28). With respect to flow regulation, a stiffer myocardium is equivalent to stiffer tethering, which would also cause reduced coronary flow regulation.
Conclusions and summary.
The effect of myocardial tethering on coronary flow regulation was studied using data-based models of the passive and active PDR of microvessels of the coronary network and measured vessel-myocardium ISCT gap. The PDRs of the microvessels and myocardium were considered under four extreme configurations of vessel-myocardium gap and tethering rigidity. The results suggest that the most effective and efficient configuration for flow regulation requires soft tethering and a gap between the microvessels and the myocardium, which was measured histologically.
GRANTS
This work was supported by United States-Israel Binational Science Foundation Grant 2009029 and by National Heart, Lung, and Blood Institute Grant HL-055554-12 (to G. S. Kassab).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.M.Y., G.S., and Y.L. conception and design of research; J.M.Y. and J.S.C. analyzed data; J.M.Y. and J.S.C. interpreted results of experiments; J.M.Y. and J.S.C. prepared figures; J.M.Y. drafted manuscript; J.M.Y., J.S.C., G.S.K., and Y.L. edited and revised manuscript; J.M.Y., J.S.C., G.S.K., and Y.L. approved final version of manuscript; J.S.C. performed experiments.
APPENDIX A: MECHANICS OF PRESSURIZED CYLINDRICAL VESSELS
The transvascular pressure across the wall of a cylindrical vessel ΔP was obtained using radial equilibrium, namely, the following derivation:
| (A1) |
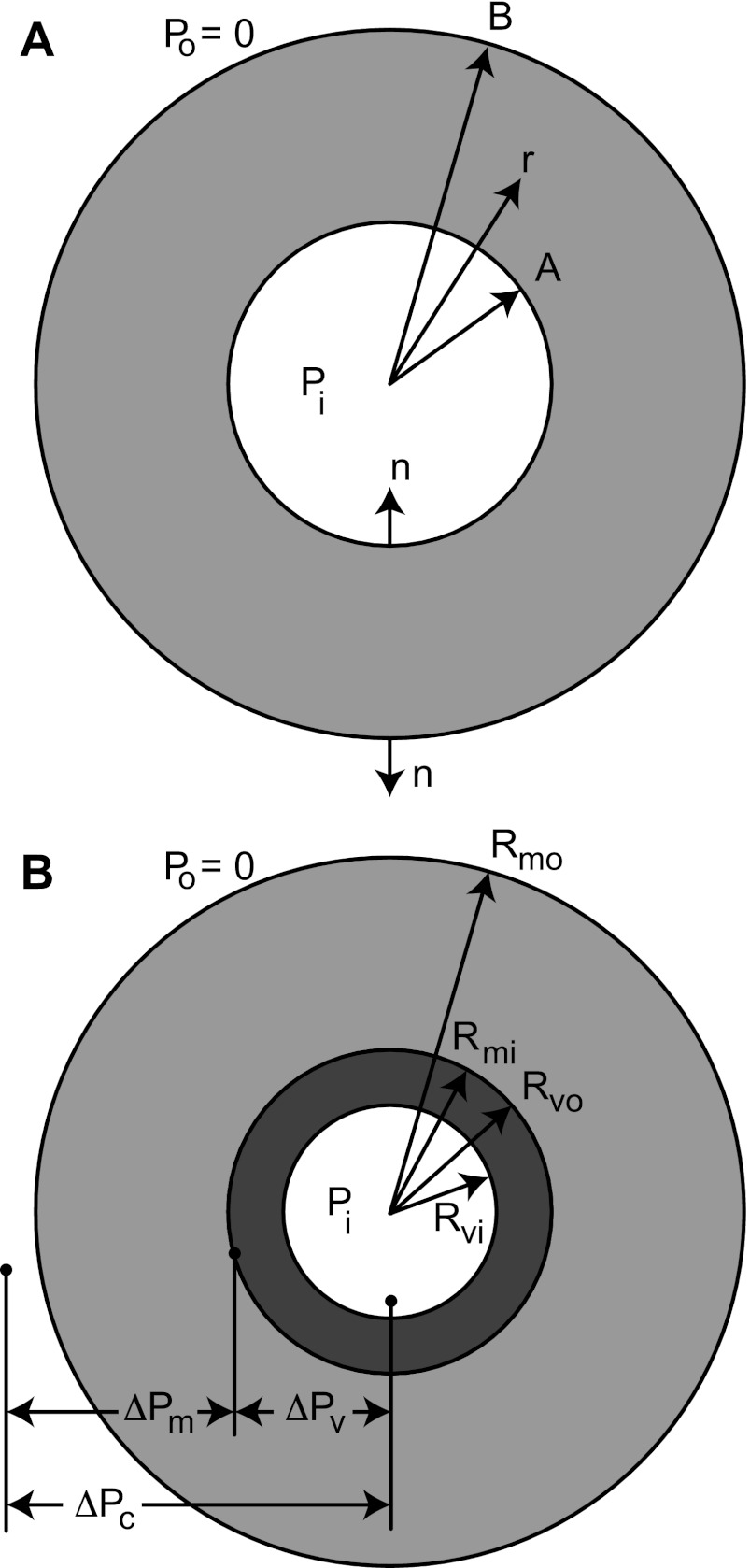
where σrr is radial stress, r is the radius, σθθ is circumferential stress, ni and no are the unit normals of the vessel in the radial direction of the inner and outer vessel wall, respectively, Pi and Po are the pressures internal and external to the cylinder (see Fig. A1A), A is the inner radius of the vessel, and B is the outer radius of the vessel. For the present analysis, Po = 0.
Fig. A1.
A: scheme of a pressurized cylindrical tube. This system is the basis for the mechanics of a vessel-in-myocardium system. B: general cylindrical configuration for the vessel (dark)-in-myocardium (light) system. The inner and outer radii of the vessel and myocardium are designated Rvi, Rvo, Rmi, and Rmo, respectively. For the NGD case, Rmi = Rvo when the cylinders are in contact. This was always true for the NGT case, never possible for the GT case, and sometimes possible for GD cases.
For the vessel in myocardium composite (Fig. A1B), the radial equilibrium for the composite pressure (ΔPc) is then given by the following:
| (A2) |
APPENDIX B: PDR OF THE PASSIVE MYOCARDIUM
The PDR Dmi(ΔPm) was obtained using continuum mechanics analysis. The kinematic mapping from cylindrical coordinates in the stress-free (SF) open state (rSF, θSF, zSF) to the cylindrical coordinates in the closed state (CL) state (rCL, θCL, zCL), and from the SF state to the loaded (P) state (rP, θP, zP) were used to derive the Cauchy stresses in CL and P states. These stresses in the SF state were then used in the equation of radial equilibrium to determine Dmi(ΔPm). However, the stress-free inner and outer radii (Rmi,SF and Rmo,SF), closed inner and outer radii (Rmi,CL and Rmo,CL), closed axial stretch ratio (λCL), and loaded outer radius (Rmo,P) are unknown.
Using the passive PDR data of Liao and Kuo (38), the inner radius of vessel in the closed, unloaded (U), in situ axial length state (Rvi,U) was obtained. Frøbert et al. (24) measured the radii and wall thicknesses of SF and CL states of vessel orders 6–11. Using this database, measurements of opening angles of vessels from Guo et al. (26), measurements of in situ axial stretch of microvessels (λU) from Guo et al. (27), and using incompressibility, the outer radius of the vessel in the U state (Rvo,U) was obtained. By Vis et al. (48), the cross-sectional area of the myocardium was seven times that of the vessel in the U state. Thereby, Rmi,CL = Rvo,U + g and Rmo,CL = were obtained, where g is the gap size taken as the mean values of ISCT gap size from the midmyocardial sections.
To complete the set of kinematic constants, the unknowns Rmi,SF, Rmo,SF, and λCL were estimated by solving radial and axial equilibrium. The kinematic mapping of cylindrical coordinates from SF to CL states was prescribed by the following:
| (B1) |
where χm = π/(π − Φm) is the circumferential stretch due to the closing of the myocardium, Φm = 2.75 radians (37) is the opening angle measured from the inner surface of the sector subtended by two equal arcs, and
| (B2) |
is the axial stretch due to closing. Using incompressibility, we obtained the following:
| (B3) |
The deformation gradient from SF to CL states is FCL = diag(rSF/λCLχCLrCL, χCLrCL/rSF, λCL), which reflects this kinematic mapping.
The Cauchy stresses in the CL state were derived from the strain energy function of the passive myocardium [w(E)], such that
| (B4) |
where the “·” symbol represents the dot product between the tensors. Taking the convex set of material constants used by Liu et al. (40) for the myocardium, the strain energy density function w (in kPa) was defined as follows:
| (B5) |
where the Green-Lagrange strain components (Err, Eθθ, and Ezz) were provided by the following:
| (B6) |
where FCL = diag(rSF/λCLχCLrCL, χCLrCL/rSF, λCL) is the deformation gradient from SF the CL states and I is the identity tensor.
Radial equilibrium was expressed using Eq. A1 from appendix a as follows:
| (B7) |
where ΔPCL is the transmural pressure across the myocardium cylinder wall in the CL state (CL is unloaded and thus ΔPCL = 0) and σθθ,CL and σrr,CL are the circumferential and radial stresses in the CL state measured from the SF state. Axial equilibrium from SF to CL states was then written as follows:
| (B8) |
where fz,CL = 0 is the axial load, which also vanishes because the closed myocardium cylinder is unloaded.
The fsolve function in Matlab was used to implement Eqs. B1–B8 to numerically solve two unknown SF radii of the myocardium, Rmi,SF and Rmo,SF. For numerical procedures using fsolve, the function residual was set to <10−12.
With the SF radii and closing stretch known, the kinematic mapping of cylindrical coordinates from SF to P states was specified as follows:
| (B9) |
where
| (B10) |
is the axial stretch from CL to P states. Since the myocardium surrounding the vessel was locally representative of a region containing a single microvessel but globally part of a tissue containing many vessels, the outer radius of the myocardium was chosen to be fixed. By imposing this boundary condition, the outer surface of the myocardium cylinder was fixed in the radial direction, yielding Rmo,P = Rmo,CL.
Finally, the radial coordinate in the P state was related to the radial coordinate in the SF state by using incompressibility, giving the following:
| (B11) |
The Cauchy stresses in the P state were obtained using the following:
| (B12) |
where w is given in Eq. B5, EP = (1/2)(FPT ⋅ FP − I), and FP = diag(rSF/λCLλPχCLrP, χCLrP/rSF, λCLλP) is the deformation gradient from SF to P states.
Radial equilibrium from Eq. A1 was used again, yielding the following:
| (B13) |
where ΔPP is the transmural pressure across the myocardium wall. Letting ΔPP = ΔPm and Rmi,P = (1/2)Dmi, Eq. B13 represents the continuum mechanics-based PDR of the myocardium (Fig. B1).
Fig. B1.
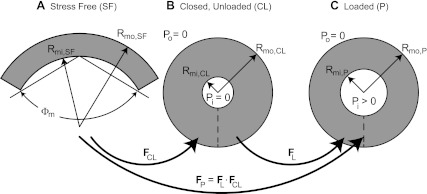
Configurational map of the stress-free (SF; A), closed and unloaded (CL; B), and loaded (P; C) myocardium for the continuum-based mechanical model. A: the SF configuration is an open sector measured by inner and outer radii Rmi,SF and Rmo,SF and opening angle Φm. B: the CL configuration is represented by inner and outer radii Rmi,CL and Rmo,CL. The dashed line represents the free edges of the SF sector that meet to close the cylinder and Pi and Po vanish. The kinematic mapping from SF to CL states is represented by FCL. C: the loaded configuration is represented by inner and outer radii Rmi,P and Rmo,P. Here, Pi > 0 and Po = 0 and the kinematic mapping from CL to P states is represented by FL. The full kinematic mapping from SF to P states is then FP = FL ⋅ FCL.
Equation B13 was solved numerically to give Rmi,P → Rmi,P(ΔPm) = (1/2)Dmi(ΔPm) using the fsolve function in Matlab. A pressure range of ΔPm = [−1,600, +1,600] mmHg was used to give an adequate pressure range for approximating the PDR of the myocardium through Eq. 6.
APPENDIX C: WORK OF CONTRACTION
As both tethered (subscript T) and detachable (subscript D) vessel types reach the same outer diameter, the activation level of the detachable vessel (AD) may not be equal to the activation level of the tethered vessel (AT). Therefore, AD was calculated using Eq. 3, such that
| (C1) |
where Rp,D is the passive diameter of the detachable vessel and ΔRa,D is the maximum myogenic diameter change due to contraction of the detachable vessel. The resulting AD was limited to the feasible range 0 ≤ AD ≤ 1, and therefore any states outside of this range were not included in the analysis.
For the rigidly tethered and detachable vessels, the work of activation (wC) was given by the following:
| (C2a) |
| (C2b) |
When the detachable vessel separates from the myocardium, ΔPm = 0, and the myocardium no longer affects the PDR of the vessel. The diameter at which this occurs is Rm,0. Therefore, the upper bound of integration for the second integral in Eq. C2 must be appropriately updated, so that
| (C3a) |
with
| (C3b) |
Additionally, if Rp,D < Bm, the vessel is already separated from the myocardium in the passive state, and the second integral on the righthand side of Eq. C3a vanishes. Using Eq. 7, the solution to Eq. C3a has an analytical solution of the following form:
| (C4) |
The integral on the righthand side of Eq. C4 takes the following form:
| (C5) |
It should be noted that for the GT and GD cases, the inner diameter of the myocardium Dmi = 2g + Dvo, where g is the measured ISCT gap size.
Using Eq. 7, the work of contraction for the four vessel types was derived to yield
| (C6) |
In Eq. C6, km,i = ln(Am,i − Bm,i) − ln(Bm,i − Cm,i), with i = [NG, G], is a combination of sigmoidal constants for the myocardium without (NG) and with (G) the gap. Additionally, = g + Rp,GT and = g + RGT account for the rigid gap in the GT and GD cases.
REFERENCES
- 1.Algranati D, Kassab GS, Lanir Y. Mechanisms of myocardium-coronary vessel interaction. Am J Physiol Heart Circ Physiol 298: H861–H873, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Algranati D, Kassab GS, Lanir Y. Why is the subendocardium more vulnerable to ischemia? A new paradigm. Am J Physiol Heart Circ Physiol 300: H1090–H1100, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashikawa K, Kanatsuka H, Suzuki T, Takishima T. A new microscopic system for the continuous oservation of the coronary microcirculation in the beating canine left ventricle. Microvasc Res 28: 387–394, 1984 [DOI] [PubMed] [Google Scholar]
- 4.Austin RE, Jr, Aldea GS, Coggins DL, Flynn AE, Hoffman JI. Profound spatial heterogeneity of coronary reserve. Discordance between patterns of resting and maximal myocardial blood flow. Circ Res 67: 319–331, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest 117: 568–575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borg TK, Caulfield JB. The collagen matrix of the heart. Fed Proc 40: 2037–2041, 1981 [PubMed] [Google Scholar]
- 7.Borg TK, Caulfield JB. Morphology of connective tissue in skeletal muscle. Tissue Cell 12: 197–207, 1980 [DOI] [PubMed] [Google Scholar]
- 8.Borg TK, Sullivan T, Ivy J. Functional arrangement of connective tissue in striated muscle with emphasis of cardiac muscle. Scan Electron Microsc: 1775–1784, 1982 [PubMed] [Google Scholar]
- 9.Buckberg GD, Fixler DE, Archi JP, Hoffman JIE. Experimental subendocardial ischemia in dogs with normal coronary arteries. Circ Res 30: 67–81, 1972 [DOI] [PubMed] [Google Scholar]
- 10.Carlson BE, Arciero JC, Secomb TW. Theoretical model of blood flow autoregulation: roles of myogenic, shear-dependent, and metabolic responses. Am J Physiol Heart Circ Physiol 295: H1572–H1579, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson BE, Secomb TW. A theoretical model for the myogenic response based on the length-tension characteristics of smooth msucle. Microcirculation 12: 327–338, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Caulfield JB, Borg TK. The collagen network of the heart. Lab Invest 40: 364–372, 1979 [PubMed] [Google Scholar]
- 13.Chilian WM. Microvascular pressures and resistances in the left ventricular subepicardium and subendocardium. Circ Res 69: 561–570, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Chilian WM, Eastham CL, Marcus ML. Microvascular distribution of coronary vascular resistance in beating left ventricle. Am J Physiol Heart Circ Physiol 251: H779–H788, 1986 [DOI] [PubMed] [Google Scholar]
- 15.Choy JS, Kassab GS. Wall thickness of coronary vessels varies transmurally in the LV but not the RV: Implicatin on local stress distribution. Am J Physiol Heart Circ Physiol 297: H750–H758, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cinthio M, Ahlgren ÅR, Bergkvist J, Jansson T, Persson HW, Lindström K. Longitudinal movements and resulting shear strain of the arterial wall. Am J Physiol Heart Circ Physiol 291: H394–H402, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Clark JM, Glagov S. Transmural organization of the arterial media. The lamellar unit revisited. Arterioscler Thromb Vasc Biol 5: 19–34, 1985 [DOI] [PubMed] [Google Scholar]
- 18.Cornelissen AJM, Dankelman J, VanBavel E, Spaan JAE. Balance between myogenic, flow-dependent, and metabolic flow control in coronary arterial tree: a model study. Am J Physiol Heart Circ Physiol 282: H2224–H2237, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Davis MJ. Myogenic response gradient in an arteriolar network. Am J Physiol Heart Circ Physiol 264: H2168–H2179, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Davis MJ, Gore RW. Length-tension relationship of vascular smooth-muscle in sigle arterioles. Am J Physiol Heart Circ Physiol 256: H630–H640, 1989 [DOI] [PubMed] [Google Scholar]
- 21.Detry JMR. The pathophysiology of myocardial ischemia. Europ Heart J 17: 48–52, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Downey JM, Kirk ES. Inhibition of coronary blood flow by a vascular waterfall mechanism. Circ Res 36: 753–760, 1975 [DOI] [PubMed] [Google Scholar]
- 23.Dunker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev 88: 1009–1086, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Frøbert O, Gregersen H, Bjerre J, Bagger JP, Kassab GS. Relation between zero-stress state and branching order of porcine left coronary arterial tree. Am J Physiol Heart Circ Physiol 275: H2283–H2290, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Fung YC. Biomechanics: Mechanical Properties of Living Tissues. New York: Springer, 1993 [Google Scholar]
- 26.Guo X, Kassab GS. Distribution of stress and strain along the porcine aorta and coronary arterial tree. Am J Physiol Heart Circ Physiol 286: H2361–H2368, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Guo X, Liu Y, Kassab GS. Diameter-dependent axial pre-stretch of porcine coronary arteries and veins. J Appl Physiol 112: 982–989, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta KB, Ratcliffe MB, Fallert MA, Edmunds LH, Jr, Bogen DK. Changes in passive mechanical stiffness of myocardial tissue with aneurysm formation. Circulation 89: 2315–2326, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Hai C, Murphy RA. Cross-bridge phosphorylation and regulation of latch state in smooth muscle. Am J Physiol Cell Physiol 254: C99–C106, 1988 [DOI] [PubMed] [Google Scholar]
- 30.Hamza LH, Dang Q, Lu X, Mian A, Molloi S, Kassab GS. Effect of passive myocardium on the compliance of porcine coronary arteries. Am J Physiol Heart Circ Physiol 285: H653–H660, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Hodis S, Zamir M. Arterial wall tethering as a distant boundary condition. Phys Rev E 80: 051913, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Hoffman JI, Spaan JAE. Pressure-flow relations in coronary ciruclation. Physiol Rev 70: 331–390, 1990 [DOI] [PubMed] [Google Scholar]
- 33.Holmgren E. Ueber die trophospongien der querges treiften muskelfasern, nebst Bemerkungen ueber den allgemeinen bau dieser fasern. Arch Mikrosk Anat 71: 165–247, 1907 [Google Scholar]
- 34.Humphrey JD, Na S. Elastodynamics and arterial wall stress. Ann Biomed Eng 30: 509–523, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Kajiya F, Yada T, Hiramatsu O, Ogasawara Y, Inai Y, Kajiya M. Coronary microcirculation in the beating heart. Med Biol Eng Comput 46: 411–419, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Kuo L, Davis MJ, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arteries. Am J Physiol Heart Circ Physiol 259: H1063–H1070, 1990 [DOI] [PubMed] [Google Scholar]
- 37.Lanir Y, Hayam G, Abovsky M, Zlotnick AY, Uretzky G, Nevo E, Ben-Haim SA. Effect of myocardial swelling on residual strain in the left ventricle of the rat. Am J Physiol Heart Circ Physiol 270: H1736–H1743, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Liao JC, Kuo L. Interaction between adenosine and flow-induced dilation in coronary microvascular network. Am J Physiol Heart Circ Physiol 272: H1571–H1581, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Kassab GS. Vascular metabolic dissipation in Murray's law. Am J Physiol Heart Circ Physiol 292: H1336–H1339, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Zhang W, Kassab GS. Effects of myocardial constraint on the passive mechanical behaviors of the coronary vessel wall. Am J Physiol Heart Circ Physiol 294: H514–H523, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Misra JC, Choudhury KR. An analysis of the flow of blood through thick-walled vessels, considering the effect of tethering. Rheol Acta 23: 548–555, 1984 [Google Scholar]
- 42.Omens JH, Fung YC. Residual strain in rat left ventricle. Circ Res 66: 459–468, 1990 [DOI] [PubMed] [Google Scholar]
- 43.Rachev A, Hayashi K. Theoretical study of the effects of vascular smooth muscle contraction on strain and stress distributions in arteries. Ann Biomed Eng 27: 459–468, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Rhodin JA. Architecture of the vessel wall. In: Comprehensive Physiology. Ann Arbor, MI: Am. Physiol. Soc., 2011, p. 1–31. [Google Scholar]
- 45.Steelman SM, Wu Q, Wager HP, Yeh AT, Humphrey JD. Perivascular tethering modulates the geometry and biomechanics of cerebral arterioles. J Biomech 43: 2717–2721, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toyota E, Ogasawara Y, Hiramatsu O, Tachibana H, Kajiya F, Yamomori S, Chilian WM. Dynamics of flow velocities in endocardial and epicardial coronary arterioles. Am J Physiol Heart Circ Physiol 288: H1598–H1603, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Tsoukias NM, Kavdia M, Popel AS. A theoretical model of nitric oxide transport in arterioles: frequency- vs. amplitude-dependent control of cGMP formation. Am J Physiol Heart Circ Physiol 286: H1043–H1056, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Vis MA, Sipkema P, Westerhof N. Modeling pressure-area relations of coronary blood vessels embedded in cardiac muscle in diastole and systole. Am J Physiol Heart Circ Physiol 268: H2531–H2543, 1995 [DOI] [PubMed] [Google Scholar]
- 49.Wasano K, Yamamoto T. Tridimensional architecture of elastic tissue in the rat aorta and femoral artery - a scanning electron microscope study. J Electron Micros 32: 33–44, 1983 [PubMed] [Google Scholar]
- 50.Westerhof N, Boer C, Lamberts RR, Sipkema P. Cross-talk between cardiac muscle and coronary vasculature. Physiol Rev 86: 1263–1308, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Zulliger MA, Rachev A, Stergiopulos N. A constitutive formulation of arterial mechanics including vascular smooth muscle tone. Am J Physiol Heart Circ Physiol 287: H1335–H1343, 2004 [DOI] [PubMed] [Google Scholar]