Abstract
In the ischemic myocardium, extracellular potassium ([K+]o) increases to ≥20 mmol/l. To determine how lethal arrhythmias occur during ischemia, we investigated whether the increased spatial pattern of [K+]o, i.e., a regional or a global increase, affects the incidence of arrhythmias. Force, sarcomere length, membrane potential, and nonuniform intracellular Ca2+ ([Ca2+]i) were measured in rat ventricular trabeculae. A “regional” or “global” increase in [K+]o was produced by exposing a restricted region of muscle to a jet of 30 mmol/l KCl or by superfusing trabeculae with a solution containing 30 mmol/l KCl, respectively. The increase in [Ca2+]i (CaCW) during Ca2+ waves was measured (24°C, 3.0 mmol/l [Ca2+]o). A regional increase in [K+]o caused nonuniform [Ca2+]i and contraction. In the presence of isoproterenol, the regional increase in [K+]o induced sustained arrhythmias in 10 of 14 trabeculae, whereas the global increase did not induce such arrhythmias. During sustained arrhythmias, Ca2+ surged within the jet-exposed region. In the absence of isoproterenol, the regional increase in [K+]o increased CaCW, whereas the global increase decreased it. This increase in CaCW with the regional increase in [K+]o was not suppressed by 100 μmol/l streptomycin, whereas it was suppressed by 1) a combination of 10 μmol/l cilnidipine and 3 μmol/l SEA0400; 2) 20 mmol/l 2,3-butanedione monoxime; and 3) 10 μmol/l blebbistatin. A regional but not a global increase in [K+]o induces sustained arrhythmias, probably due to nonuniform excitation-contraction coupling. The same mechanism may underlie arrhythmias during ischemia.
Keywords: high extracellular potassium, calcium, ischemia, calcium waves
the majority of sudden cardiac deaths are caused by acute ventricular tachyarrhythmias (24), often triggered by acute coronary events (42). Arrhythmias associated with ischemia are generally classified as phase 1 (during the first 30 min), phase 2 (between 5 and 72 h), or phase 3 (chronic stage after infarct) (25). Among them, phase 1 arrhythmias are particularly important because they usually occur before the patient's arrival at the hospital and result in sudden death (34). Although redistribution of a number of ions, including H+, Na+, Ca2+, and K+, across the cell membrane occurs during acute ischemia and causes profound electrophysiological consequences (10, 14, 42), a rise in extracellular K+ ([K+]o) up to more than 20 mmol/l (7, 51) has been reported to play an important role in the occurrence of the phase 1 arrhythmias (9, 11, 21). However, it has not yet been established what role a rise in [K+]o plays in the initiation of these arrhythmias (7).
We have reported previously that in the myocardium with nonuniform muscle contraction, a surge of Ca2+ occurs within the border zone between the contracting and stretched regions during the relaxation phase (47). This surge induces arrhythmogenic Ca2+ waves and arrhythmias (38, 50). By determining their sustainability and frequency, we showed that the surges of Ca2+ are related to the likelihood of life-threatening arrhythmias (37). During ischemia, nonuniform muscle contraction also occurs (33), particularly at the interface between the normal and ischemic regions due to a decrease in the contractile strength within the ischemic region (1). In addition, spatially nonuniform depolarization of the membrane potential occurs during ischemia due to the rise of [K+]o within the ischemic region (7, 51). Thus, in the early stage of ischemia, the roles of nonuniform muscle contraction and/or nonuniform membrane depolarization in the occurrence of phase 1 arrhythmias have not been fully established.
Therefore, in the present study, we investigated the effects of a regional increase in [K+]o on arrhythmogenesis using a rat trabecular model. Our results indicate that a regional increase in [K+]o leads to nonuniform intracellular Ca2+([Ca2+]i), thereby increasing arrhythmogenesis due to nonuniform muscle contraction but not the depolarization of the membrane potential. A similar mechanism could play a role in the initiation of phase 1 arrhythmias during ischemia.
MATERIALS AND METHODS
Dissection and mounting of rat ventricular trabeculae.
All animal procedures were performed according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, Revised 1996) and were approved by the Ethics Review Board of Tohoku University (approval reference number: 21–98). Sprague-Dawley rats weighing 200 to 250 g were anesthetized with intraperitoneal injection of pentobarbital sodium (50 mg/kg). A total of 64 hearts were excised, and the coronary arteries were immediately perfused via the aorta with a HEPES buffered solution with 15 mmol/l KCl. Trabeculae (n = 64; length, 2.4 ± 0.2 mm; width, 241 ± 60 μm; thickness, 82 ± 4 μm in a slack condition) were dissected from the right ventricle of rats and mounted horizontally between a force transducer and a micromanipulator with a direct current torque motor, which was controlled by a personal computer, in a bath on an inverted microscope (Nikon, Japan) as described previously (37–39, 50). Preparations were equilibrated at 0.5 Hz stimulation and superfused with the HEPES buffered solution at room temperature (24°C). The composition of the HEPES solution for both dissection and perfusion was (in mmol/l) 130.8 NaCl, 5 KCl, 1.2 MgCl2, 2.8 acetate, 10 HEPES, 10 glucose, and 0.7 CaCl2; pH was adjusted to 7.4 by the addition of NaOH. The solutions were equilibrated with 100% O2.
Measurements of force, membrane potential, and sarcomere length.
Force was measured using a silicon strain gauge (model AE-801; SenSoNor, Horten, Norway). Sarcomere length (SL) was measured using laser diffraction techniques (37–39, 50), and membrane potential was measured using ultracompliant glass microelectrodes as described previously (37, 38). Briefly, for the measurement of SL, the preparations were illuminated by a He-Ne laser beam (05-LHP-925; Melles Griot), and the SL was calculated from the median of the intensity distribution of the first order diffraction pattern. For the measurement of membrane potential, flexible stepped electrodes drawn with a glass microelectrode puller (Narishige, Japan) were filled with 3 mol/l KCl.
Fura-2 loading and measurement of fluorescence.
[Ca2+]i in the trabeculae was assessed as described previously (39). Briefly, fura-2 pentapotassium salt (Molecular Probe, Eugene, OR) was microinjected electrophoretically into one cell and allowed to spread throughout the trabeculae via gap junctions. After the injection, the trabeculae were stimulated at 1 Hz for 30–60 min, i.e., until fura-2 had diffused uniformly throughout the preparation. Fluorescence of fura-2 from the muscle at excitation wavelengths of 340, 360, and 380 nm was filtered by a 490–530 nm band-pass filter (Nikon, Japan) and acquired using a photomultiplier tube (PMT; E1341 with a C1556 socket; Hamamatsu, Japan), which recorded average regional fluorescence, or an image intensified charge-coupled device camera (IIC; Hamamatsu C2400-8; Hamamatsu, Japan) at 30 frames/s to assess local [Ca2+]i.
Imaging of fura-2 fluorescence.
The dynamics in [Ca2+]i were analyzed from a sequence of fura-2 fluorescence images with methods described previously (37–39, 50). The fluorescence images were converted to a sequence of 8-bit bitmap (BMP) images of 512 × 512 pixels. The pixel size in our optical system was 1.80 × 1.80 μm, and the temporal resolution was 33 ms. A fluorescence image at 360 nm in resting condition (reference image, ImRef) was sampled first, and images at 380 nm (Im380) were then recorded continuously at video rate. The overall kinetics of changes in [Ca2+]i throughout the preparation was obtained through the ratio images ImRef/Im380.
A custom made IDL program (Research Systems) was used to construct spatiotemporal representations of Ca2+ variations from Ca2+ time courses and to process the resulting digital images as follows. First, arrays of about 512 × 100–150 pixels including an image of the preparation were selected from all original 512 × 512 BMP frames. After subtraction of autofluorescence, ratio images (ImRef to Im380) were obtained from the reference array (selected from the reference image sampled at 360 nm) divided pixel to pixel by the corresponding 380-nm arrays (selected from every image sampled at 380 nm). The ratio images (ImRef to Im380) reflect the spatial distribution of [Ca2+]i in the muscle.
To assess [Ca2+]i dynamics during triggered arrhythmias, we analyzed the longitudinal distribution in [Ca2+]i along the muscle as described previously (37–39, 50). Briefly, longitudinal profiles of pixel ratios were computed across each individual ratio image (ImRef to Im380) as follows: 1) a region of interest (ROI) was chosen over the long axis of the preparation in the first ratio image of the sequence; 2) the profile of ratios along the long axis of the trabecula was calculated by averaging the values of pixels within the ROI vertically across the muscle; and 3) ratio profiles were computed for every ratio image of the sequence by using an identical ROI and pixel averaging procedure. Finally, individual ratio profiles were plotted as a function of time. After the ratio profiles were converted to [Ca2+]i profiles as described below, the final two-dimensional representations reflecting the spatio-temporal distribution of [Ca2+]i along the ROI were plotted.
Calculation of [Ca2+]i.
To calculate [Ca2+]i from the ratio profiles, we used the time course of the ratio (R) obtained by the standard spectro-fluorimetric method using a PMT, where R was calculated by dividing the fluorescent intensity (after subtraction of the autofluorescence of the muscle) at 340 nm excitation by that at 380 nm excitation. Consistent with results of our previous study (37–39, 50), the simultaneous use of IIC together with PMT during electrically stimulated twitch of the trabeculae confirmed that ratios calculated from digital images (ImRef to Im380) at each sampling point were closely and linearly correlated (r > 0.95) with the R obtained using the PMT. The R was converted to [Ca2+]i using the following equation: [Ca2+]i = Kd × β × (R − Rmin)/(Rmax − R), where Kd is the effective dissociation constant, Rmin is R at zero [Ca2+]i, Rmax is R at a saturating [Ca2+]i, and β is the ratio of fluorescence value for [Ca2+]i-free dye to the fluorescence value for [Ca2+]i-bound dye at 380 nm excitation. Regional [Ca2+]i in the ratio profile taken from the fluorescent images was calculated using the [Ca2+]i calibration of the PMT and the linear relation between the ratios observed with IIC and R with PMT.
To calculate the velocity of Ca2+ waves, we identified the peak of a Ca2+ transient during the Ca2+ wave at each pixel along trabeculae and plotted the time of the maximum against the position of the peak. The velocity was calculated from the slope of the fitted line to the plot, when regression analysis showed a linear relationship (r ≥ 0.9), as described previously (39).
Local superfusion of trabeculae.
Local superfusion of trabeculae was performed as described previously (37, 38, 50). In brief, a restricted region of the trabecula was exposed to a narrow “jet” of solution (∼0.06 ml/min) using a glass pipette with a right-angled tip (50∼100 μm diameter). The glass pipette was positioned in the perfusion bath perpendicular to the long axis of the muscle. The jet solution was usually in contact with the muscle in an area 200–300 μm, whereas the diameter of the jet before hitting the muscle was 100–150 μm. When the preparation was stimulated, the jet at the other side of the muscle moved, indicating the physical contact between the jet solution and the contracting muscle. The jet was still coherent behind the muscle without affecting other segments of the muscle, and its solution was discarded with the “bulk” solution perfusing the bath.
Increase in [K+]o.
To increase [K+]o “regionally,” a restricted region of a trabecula was exposed to a small jet of standard HEPES solution containing 30 mmol/l KCl. The Ca2+ in the jet solution was the same concentration as that of the HEPES solution used for superfusion. To increase [K+]o “globally,” the K+ concentration in the HEPES bath solution was increased from 5 to 30 mmol/l so that the entire trabecula was exposed to the high K+ concentration. Under these conditions, a spatially nonuniform distribution of [K+]o was improbable because trabeculae in the present study were thin and had less than five layers of myocytes (20).
Trabeculae superfused with standard HEPES solution containing 5 mmol/l KCl, without a regional or a global increase in [K+]o, were defined as the “control” throughout the measurements in the present study.
Experimental protocol.
Twitch contractions lasting more than 10 s after the cessation of electrical stimulation were defined as “sustained arrhythmias.” We used the velocity of Ca2+ waves (45) and changes in force (FCW) and [Ca2+]i (CaCW) within almost the entire trabecula during Ca2+ waves induced with electrical stimulation as predictors of arrhythmias (31). The intervals of the FCW and CaCW were defined as the time intervals between the last stimulus pulse of the electrical train and the peaks of FCW and CaCW, respectively.
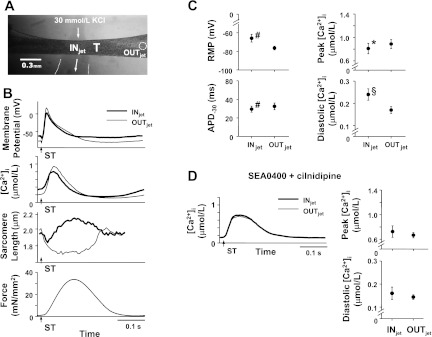
First of all, to investigate whether the 30 mmol/l KCl jet can cause spatial nonuniformity of the membrane potential, [Ca2+]i, and muscle contraction, we measured membrane potential, [Ca2+]i, and sarcomere length during electrical stimulation at 400-ms intervals within the region exposed to the 30 mmol/l KCl jet (INjet) and the region 1 mm apart from the INjet (OUTjet) (n = 14), as shown in Fig. 1A. In addition, in the presence of both 3 μmol/l SEA0400 (Taisho Pharmaceutical, Tokyo, Japan), an Na+-Ca2+ exchange blocker (36), and 10 μmol/l cilnidipine (Ajinomoto Pharmaceuticals, Tokyo, Japan), an L/N-type Ca2+ channel blocker (13), [Ca2+]i was measured during electrical stimulation within the INjet and OUTjet (n = 6).
Fig. 1.
Membrane potential, intracellular Ca2+ ([Ca2+]i), and sarcomeres within a 30 mmol/l KCl jet-exposed region (INjet) and a region at least 0.5 mm apart from the exposed region (OUTjet) at 400-ms stimulus interval ([Ca2+]o = 3 mmol/l). A: photomicrograph showing the jet of solution projected to a trabecula (T). Arrows indicate direction of jet, and a dotted circle within T indicates the OUTjet. B: representative recordings of membrane potential (temperature = 23.9°C, Experiment Number 091014), [Ca2+]i (temperature = 24.2°C, Experiment Number 090610), sarcomere length, and force (temperature = 23.5°C, Experiment Number 110404) are shown from top to bottom. Thick and thin lines indicate the recordings within the INjet and OUTjet, respectively. Arrows with ST indicate the moments of electrical stimulation. C: summary data of membrane potential (left, n = 6) and [Ca2+]i (right, n = 8) within the INjet and OUTjet. C, top left: resting membrane potential (RMP). C, bottom left: action potential duration at −30 mV (APD−30). C, top right: peak [Ca2+]i. C, bottom right: diastolic [Ca2+]i. #P < 0.01 vs. OUTjet (paired t-test); *P < 0.05 vs. OUTjet (paired t-test); §P < 0.05 vs. OUTjet (Wilcoxon signed-ranks test). D: representative recordings (left, temperature = 24.0°C, Experiment Number 100705) and summary data of [Ca2+]i (right, n = 6) within the INjet and OUTjet in the presence of both 3 μmol/l SEA0400 and 10 μmol/l cilnidipine.
Second, in the presence of 200 nmol/l isoproterenol, arrhythmias were induced by electrical stimuli at intervals of 400 ms for 30 s during a regional or global increase in [K+]o with (n = 9) and without 10 μmol/l blebbistatin (Sigma-Aldrich, St. Louis, MO), a myosin ΙΙ ATPase inhibitor (n = 22) (16, 44). Spatial changes in [Ca2+]i were then recorded during sustained arrhythmias (n = 6).
Third, trains of electrical stimuli of 7.5-s duration at 400-ms intervals were repeated every 15 s in the absence of isoproterenol since the induction of arrhythmias with isoproterenol made the analysis of their triggering mechanism difficult (n = 13). The amplitude of FCW (ΔFCW) and that of CaCW (ΔCaCW) were measured under the condition of a regional increase or a global increase in [K+]o. To further investigate the triggering mechanism of arrhythmias with the regional [K+]o, ΔFCW and ΔCaCW were measured during the regional increase in the presence of 1) both 3 μmol/l SEA0400 and 10 μmol/l cilnidipine (n = 6), 2) 100 μmol/l streptomycin, a stretch-activated channel blocker (n = 7) (19), 3) 20 mmol/l 2,3-butanedione monoxime (BDM; n = 6) (3), and 4) 10 μmol/l blebbistatin (n = 5). Furthermore, spatial changes in [Ca2+]i were recorded during the regional increase in [K+]o (n = 10).
All the measurements were performed within 10 min after the application of the 30 mmol/l KCl jet or the superfusion of solution containing 30 mmol/l KCl ([Ca2+]o, 3.0 mmol/l; SL, 2.0 μm; temperature, 24°C).
Statistics.
All measurements were expressed as means ± SE. Statistical analysis was performed using a paired t-test when the data were normally distributed. Otherwise, the Wilcoxon signed-ranks test was used. These analyses were performed using software for statistical analysis (Ekuseru-Toukei 2010; Social Survey Research Information, Tokyo, Japan). Values of P < 0.05 were considered to be significant.
All the authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
RESULTS
Nonuniformity during a regional increase in [K+]o.
As a result of a regional increase in [K+]o, the resting membrane potential was more depolarized and the action potential duration at -30 mV was shorter within the INjet versus these same values within the OUTjet (Fig. 1, B and C). Thus there was spatial nonuniformity of the membrane potential along the trabecula. Concomitantly, peak [Ca2+]i was smaller and diastolic [Ca2+]i at the moment of electrical stimuli was larger within the INjet (Fig. 1, B and C). Thus there was nonuniformity of [Ca2+]i along the trabecula. Furthermore, as shown in Fig. 1B, sarcomeres within the INjet shortened early during twitch and then were stretched passively by the contraction of cells within OUTjet. Sarcomeres within the OUTjet exhibited only shortening during twitch, exhibiting spatially nonuniform muscle contraction within the trabecula. To investigate whether membrane potential plays a role in the formation of nonuniform [Ca2+]i observed, the main pathways for the Ca2+ efflux and influx via cell membrane, that is, Na+-Ca2+ exchanger and Ca2+ channels, were blocked. In the presence of both 3 μmol/l SEA0400 and 10 μmol/l cilnidipine, peak and diastolic [Ca2+]i within the INjet did not differ from those within the OUTjet (Fig. 1D). Thus the nonuniform [Ca2+]i during the regional increase in [K+]o was secondary to the nonuniform membrane potential.
Differences in inducibility of arrhythmias due to the increased spatial pattern of [K+]o.
To investigate the effects of different spatial patterns of [K+]o on the initiation of arrhythmias, trabeculae were stimulated electrically during the regional and global increase in [K+]o in the presence of 200 nmol/l isoproterenol. Here, the regional and global increase in [K+]o decreased the developed force to 71.7 ± 5.5% (n = 10) and 26.4 ± 1.8% (n = 7) of the values before their [K+]o increase, respectively.
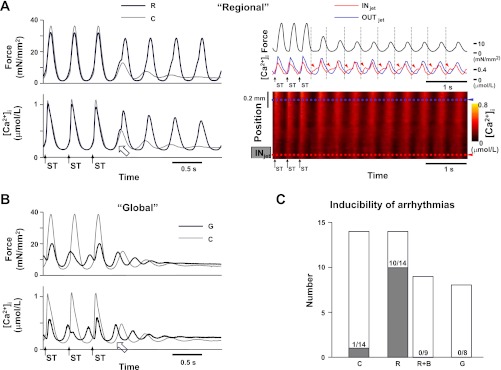
During the regional increase in [K+]o, sustained arrhythmias were induced in 10 of 14 trabeculae (Fig. 2, A and C). The amplitude of CaCW during the regional increase in [K+]o could not be determined due to interference of arrhythmias, although its ascending slope was steeper than that without the regional increase (Fig. 2A, left). The spatial changes in [Ca2+]i observed during the regional increase in [K+]o represented Ca2+ surges around the INjet during the relaxation phase of muscle contraction and always preceded the synchronous increases in [Ca2+]i of twitch contractions (Fig. 2A, right). In contrast, no arrhythmias were observed in eight trabeculae subjected to the global increase in [K+]o (Fig. 2, B and C). These results suggest that the regional but not the global increase in [K+]o causes arrhythmias. Furthermore, the surges of Ca2+ within the region showing nonuniform [Ca2+]i and contraction could play a role in arrhythmia occurrence.
Fig. 2.
Induction of sustained arrhythmias with a regional and global increase in [K+]o in the presence of 200 nmol/l isoproterenol ([Ca2+]o = 3 mmol/l). Arrows with ST indicate the moments of electrical stimulation. A, left: representative recordings of force (top) and average [Ca2+]i within the entire trabecula (bottom) during the last 3 electrical stimuli (400-ms stimulus interval) with (black lines, R) and without a regional increase in [K+]o (gray lines, C). During the regional increase in [K+]o, sustained arrhythmias were induced. The open arrow indicates an increase in [Ca2+]i during an aftercontraction with interference of an arrhythmia (temperature = 24.0°C, Experiment Number 110203). A, right: recordings of force (black lines), [Ca2+]i at the positions indicated by the dotted blue and red lines at bottom (blue and red lines), and regional changes in [Ca2+]i (bottom) during sustained arrhythmias with the regional increase in [K+]o. During the sustained arrhythmia, [Ca2+]i (red arrowheads) around the KCl jet-exposed region (INjet) surged during the relaxation phase of muscle contractions and preceded the synchronous increases in [Ca2+]i (temperature = 24.1°C, Experiment Number 101013). OUTjet indicates the region about 0.5 mm apart from the INjet. B: representative recordings of force (top) and average [Ca2+]i within the entire trabecula (bottom) during the last 3 electrical stimuli (400-ms stimulus interval) with (black lines, G) and without a global increase in [K+]o (gray lines, C). With the global increase in [K+]o, an increase in [Ca2+]i (open arrow) during an aftercontraction occurred early and seemed to be small (temperature = 23.9°C, Experiment Number 110203). C: inducibility of sustained arrhythmias with the regional (R) and global increase in [K+]o (G) and without the increase (C). “R+B” indicates inducibility of sustained arrhythmias with the regional increase in [K+]o in the presence of 10 μmol/l blebbistatin. Bars indicate the number of trabeculae in which induction of arrhythmias by electrical stimulation was attempted, and gray parts indicate the number of trabeculae in which sustained arrhythmias were actually induced. Note that the presence of blebbistatin suppressed the induction of sustained arrhythmias during the regional increase in [K+]o.
To further investigate the direct effect of nonuniform contraction on the induction of these arrhythmias, trabeculae were stimulated electrically in the presence of 10 μmol/l blebbistatin as well as 200 nmol/l isoproterenol. The presence of blebbistatin decreased developed force by electrical stimulation to 5.4 ± 1.0% (n = 9) of the predrug values and minimized the contractile differences along the trabeculae. Interestingly, despite the presence of isoproterenol, no arrhythmias were observed in nine trabeculae during the regional increase in [K+]o in the presence of blebbistatin (Fig. 2C), suggesting that by uncoupling excitation-contraction coupling, the conditions that favor arrhythmias were removed.
CaCW and velocity of Ca2+ waves during the regional increase in [K+]o.
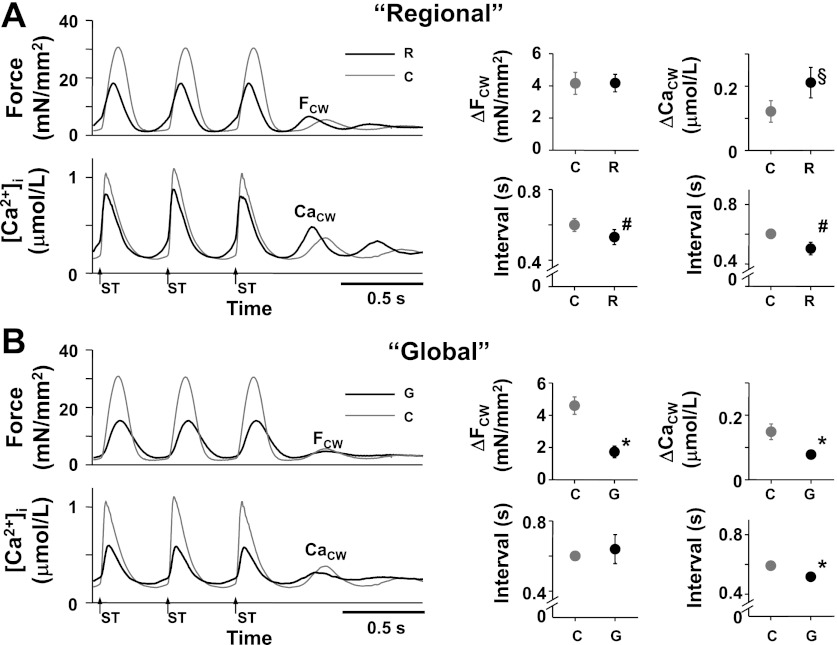
To investigate how arrhythmias were induced during the regional increase in [K+]o, FCW and CaCW were determined in the absence of isoproterenol because interference of arrhythmias with isoproterenol made their measurement difficult. The regional and global increase in [K+]o decreased developed force to 60.3 ± 3.7% (n = 11) and 30.4 ± 4.4% (n = 6) of the values before their [K+]o increase, respectively. During the regional increase in [K+]o, an early FCW and an early, large CaCW were induced by electrical stimulation at 400-ms intervals for 7.5 s (Fig. 3A). In contrast, with the global increase in [K+]o, a small FCW and an early, small CaCW were induced (Fig. 3B). Because it has been reported that an early, large CaCW is important for the initiation of triggered arrhythmias (31), we assumed that this enhancement of CaCW during the regional increase in [K+]o was involved in the initiation of arrhythmias observed in Fig. 2, A and C.
Fig. 3.
Effect of the regional (R) and global increase in [K+]o (G) on the aftercontractions (FCW) and [Ca2+]i (CaCW) during the aftercontractions in the absence of isoproterenol. ΔFCW and ΔCaCW indicate the amplitude of the FCW and that of CaCW, respectively. Arrows with ST indicate the moments of electrical stimulation. A, left: representative recordings of force (top) and average [Ca2+]i within the entire trabecula (bottom) during the last 3 electrical stimuli (400-ms stimulus interval) with (black lines, R) and without the regional increase in [K+]o (gray lines, C) ([Ca2+]o = 3 mmol/l, temperature = 23.7°C, Experiment Number 110221). Right (4 panels): summary data of the effect of the regional increase in [K+]o on the FCW and CaCW. At top (2 panels), the ΔFCW (n = 11) and ΔCaCW (n = 7). At bottom (2 panels), the intervals of the FCW and CaCW with (black symbols, R) and without the regional increase in [K+]o (gray symbols, C). #P < 0.01 vs. C (paired t-test); §P < 0.05 vs. C (Wilcoxon signed-ranks test). B, left: representative recordings of force (top) and average [Ca2+]i within the entire trabecula (bottom) during the last 3 electrical stimuli (400-ms stimulus interval) with (black lines, G) and without a global increase in [K+]o (gray lines, C) ([Ca2+]o = 3 mmol/l, temperature = 23.7°C, Experiment Number 110221). At right (4 panels): summary data of the effect of the global increase in [K+]o on the FCW and CaCW. Top right (2 panels): the ΔFCW (n = 11) and ΔCaCW (n = 6). Bottom right (2 panels): intervals of the FCW and CaCW with (black symbols, G) and without a global increase in [K+]o (gray symbols, C). *P < 0.05 vs. C (paired t-test).
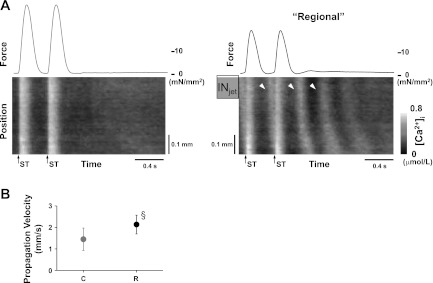
To determine whether the spatially nonuniform [Ca2+]i was related to the enlarged CaCW observed during the regional increase in [K+]o (Fig. 3A), spatial changes in [Ca2+]i were determined. As shown in Fig. 4A, the Ca2+ surged around the INjet (arrowheads) after the electrical stimulation during the regional increase in [K+]o and propagated as Ca2+ waves through the trabeculae. Without the regional increase in [K+]o, Ca2+ waves propagated slowly and sometimes could not be induced (Fig. 4, A and B). This suggests that acceleration of Ca2+ waves underlies the enlarged CaCW observed during the regional increase in [K+]o.
Fig. 4.
Effect of the regional increase in [K+]o on spatial changes in [Ca2+]i in the absence of isoproterenol. A: representative recordings of force (top) and spatial changes in [Ca2+]i (bottom) during the last 2 electrical stimuli (400-ms stimulus interval) with (right) and without the regional increase in [K+]o (left). At right, Ca2+ surged repeatedly (arrowheads) around the jet-exposed region (INjet) and propagated as waves through the trabeculae after the trains of electrical stimuli. Arrows with ST indicate the moments of electrical stimulation. Without the regional increase in [K+]o, the Ca2+ waves disappeared (left) ([Ca2+]o = 3.0 mmol/l, temperature = 24.1°C, Experiment Number 090507). B: summary data of the effect of the regional increase in [K+]o on the velocity of Ca2+ waves. The propagation velocity of Ca2+ waves increased significantly during the regional increase in [K+]o (R), compared with that without the increase (C, n = 9). §P < 0.01 vs. C (Wilcoxon signed-ranks test).
To minimize the [Ca2+]i spatial gradients produced by nonuniform membrane depolarization along the trabeculae (Fig. 1, C and D), both 3 μmol/l SEA0400 and 10 μmol/l cilnidipine were added. In the presence of both drugs, the regional increase in [K+]o resulted in both a small FCW and a small CaCW (Fig. 5A), similar to those observed during the global increase in [K+]o protocol (Fig. 3B). In contrast, the early FCW and the early, large CaCW during the regional increase in [K+]o (Fig. 3A) remained unchanged in the presence of streptomycin (Fig. 5B), suggesting that stretch-activated channels are not related to the measured changes in the FCW and CaCW.
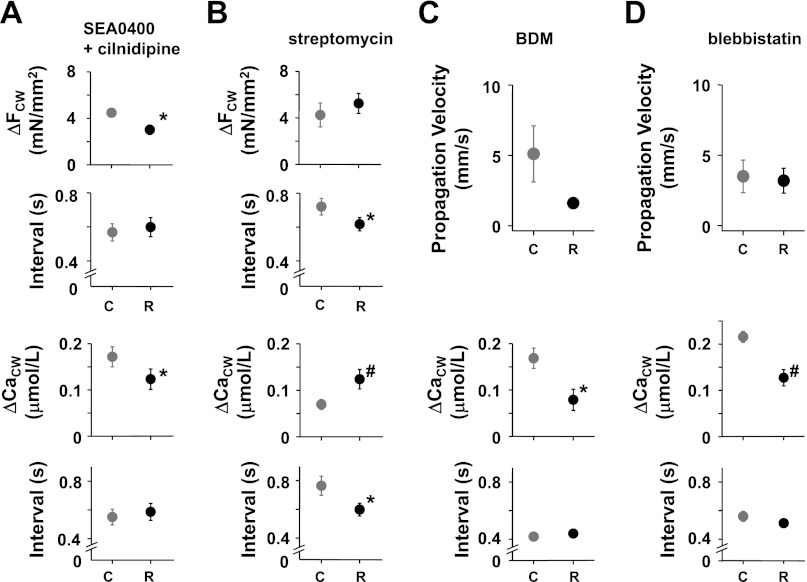
Fig. 5.
Effects of nonuniform [Ca2+]i and contraction on the aftercontractions (FCW) and [Ca2+]i (CaCW) during the aftercontractions with the regional increase in [K+]o in the absence of Isoproterenol. A and B, top 2 panels: amplitudes (ΔFCW) and intervals of the FCW. A and B, bottom 2 panels: amplitudes (ΔCaCW) and intervals of the CaCW. C and D, top 2 panels: propagation velocity of Ca2+ waves. C and D, bottom 2 panels: the ΔCaCW and the intervals of the CaCW. Black and gray symbols indicate the changes with (R) and without the regional increase in [K+]o (C), respectively. *P < 0.05 vs. C; #P < 0.01 vs. C (paired t-test). A: effect of the regional increase in [K+]o on the FCW and CaCW in the presence of both 3 μmol/l SEA0400, an Na+-Ca2+ exchange blocker, and 10 μmol/l cilnidipine, an L/N-type Ca2+ channel blocker (n = 6). B: effect of the regional increase in [K+]o on the FCW and CaCW in the presence of 100 μmol/l streptomycin, a stretch-activated channel blocker (n = 7). C: effect of the regional increase in [K+]o on the propagation velocity of Ca2+ waves and CaCW in the presence of 20 mmol/l 2,3-butanedione monoxime (BDM) (n = 5). D: effect of the regional increase in [K+]o on the propagation velocity of Ca2+ waves and CaCW in the presence of 10 μmol/l blebbistatin (n = 5).
Finally, to determine whether nonuniform muscle contraction is related to the enhancement of CaCW shown in Fig. 3A, 20 mmol/l BDM or 10 μmol/l blebbistatin was added to minimize the contractile differences along the observed trabecula. In the presence of BDM or blebbistatin, developed force by electrical stimulation decreased to 2.7 ± 0.7% (n = 6) or 10.9 ± 1.0% (n = 5) of the predrug values, respectively. In the presence of BDM or blebbistatin, the regional increase in [K+]o did not accelerate Ca2+ waves anymore, resulting in a small CaCW (compare Fig. 5, C and D, with Fig. 3A). These changes are again similar to those observed during the global increase in [K+]o (Fig. 3B) and suggest that blebbistatin suppresses the induction of arrhythmias during the regional increase in [K+]o despite the presence of isoproterenol, as shown in Fig. 2C. Thus, during the regional increase in [K+]o, the nonuniformity of [Ca2+]i and contraction but not the depolarization of the resting membrane potential accelerates the potentially arrhythmogenic Ca2+ waves and enhances the CaCW, causing the arrhythmias observed in Fig. 2.
DISCUSSION
The present study characterized the effects of a regional or a global increase in [K+]o on arrhythmogenesis in intact rat trabeculae. To the best of our knowledge, it shows for the first time that a regional but not a global increase in [K+]o increases arrhythmogenesis, probably due to spatially nonuniform [Ca2+]i and nonuniform muscle contraction, which causes dissociation of Ca2+ from the myofilaments, particularly at the interface between the shortening and stretched region.
Nonuniformity of membrane potential, [Ca2+]i, and muscle contraction.
The observation that the 30 mmol/l KCl jet depolarized the resting membrane potential and shortened the action potential duration within the INjet (Fig. 1, B and C) is consistent with past reports (9, 28). This regional change in membrane potential resulted in spatial nonuniformity of membrane potential as observed during ischemia (32). It has been reported that depolarization of the membrane potential decreases the net efflux of Ca2+ through a Na+-Ca2+ exchanger (40) and that the shortened action potential reduces the Ca2+ influx through the Ca2+ channel (6). Thus disappearance of nonuniformity of [Ca2+]i after the inhibition of both the Na+-Ca2+ exchanger by SEA0400 and the Ca2+ channels by cilnidipine (Fig. 1D) suggests that this nonuniform [Ca2+]i during the regional increase in [K+]o was caused by the nonuniform membrane potential.
Nonuniformity and arrhythmias.
Regional application of a high [K+]o jet resulted in an early, large ΔCaCW (Fig. 3A), accelerated the arrhythmogenic Ca2+ waves (Fig. 4), and induced sustained arrhythmias in the presence of isoproterenol (Fig. 2, A and C). These observations suggest that arrhythmias during the regional increase in [K+]o were triggered by enhancement of delayed afterdepolarizations (DADs) since in the endocardium (35), the earlier and larger occurrence of CaCW reflects larger DADs (20) and the velocity of Ca2+ waves correlates with the amplitude of DADs (45). In addition, the early, large ΔCaCW and the accelerated Ca2+ waves during the regional increase in [K+]o are caused by nonuniform [Ca2+]i and contraction for the following three reasons. First, a global increase in [K+]o decreased the ΔCaCW in the absence of isoproterenol (Fig. 3B), and sustained arrhythmias were not inducible in the presence of isoproterenol (Fig. 2, A and C). Second, after minimization of nonuniform [Ca2+]i by the inhibition of both the Na+-Ca2+ exchanger and the Ca2+ channels, the ΔCaCW decreased during the regional increase in [K+]o (Fig. 5A). Third, after minimization of nonuniform contraction by the inhibition of muscle contraction, the ΔCaCW decreased and the velocity of Ca2+ waves did not increase anymore during the regional increase in [K+]o (Fig. 6, C and D), and sustained arrhythmias were never induced in the presence of isoproterenol (Fig. 2C). Taken together, these results suggest that the regional increase in [K+]o enhanced arrhythmogenesis through spatially nonuniform [Ca2+]i and nonuniform muscle contraction.
As for the initiation of Ca2+ waves, two mechanisms have been reported. One is SR Ca2+ leak due to Ca2+ overload (47), and the other is Ca2+ dissociation from the myofilaments in the myocardium with nonuniform contraction (37, 38, 50). In the myocardium with nonuniform contraction, regional differences in contractile strength may cause paradoxical stretching and shortening of the impaired muscle by contractions of the more viable neighboring muscle. During the relaxation of the neighboring muscle, Ca2+ is dissociated from the myofilaments due to the paradoxical shortening of the impaired muscle, induces additional Ca2+ release from the SR (38), and thus forms larger initiators of Ca2+ waves (Ca2+ surges) (50). Ca2+ waves propagate faster by this larger initiator (39) and cause triggered arrhythmias (37). Ter Keurs et al. (46) named this sequence of events reverse excitation-contraction coupling (RECC). In the present study as well, the Ca2+ surged around the INjet during sustained arrhythmias (Fig. 2A) and propagated as Ca2+ waves during the regional increase in [K+]o (Fig. 4). Thus it is probable that in the present study, arrhythmias occurred through RECC.
Clinical implications.
After the onset of ischemia, [K+]o increases to more than 20 mmol/l (7, 51) due to 1) increased K+ efflux through ATP-dependent K+ channels (4, 27, 41), 2) decreased active K+ influx through Na+/K+ pump (41, 48), and 3) shrinkage of extracellular space. This rise in [K+]o shows a triphasic pattern (7), which overlaps that of the occurrence of phase 1 arrhythmias. Some of the known electrophysiological effects of acute ischemia can be mimicked by the rise in [K+]o (22, 23), and alteration of [K+]o due to modulation of ATP-dependent K+ channels affects the occurrence of arrhythmias (17, 29). Based on these findings, it has been proposed that the rise in [K+]o is strongly related to phase 1 arrhythmias. In addition, it has been reported that an elevation of catecholamine also occurs 10 min after the onset of ischemia (43). Thus it is reasonable to infer that the mechanisms of arrhythmias during the early phase of ischemia are similar to those studied here.
As for mechanisms underlying phase 1 arrhythmias in animal models, several candidates such as re-entry (2, 30), triggered activity (49) due to gap junctional uncoupling (15), and mechanically induced membrane depolarization (26) have been reported. Here, we propose that the Ca2+ dissociated from the myofilaments within the border zone between the contracting and stretched region (37, 38, 50) is also involved in the occurrence of phase 1 arrhythmias since nonuniform muscle contraction actually occurs at the interface between normal and ischemic tissue during acute ischemia (33).
Interestingly, it has been reported that the incidence of phase 1 arrhythmias is related to left ventricular wall stress (8), which may affect the regional differences in contractile strength in the myocardium during nonuniform contraction. Additionally, Gd3+, a stretch-activated channel blocker, does not suppress phase 1 arrhythmias (5) in the same way that streptomycin did not affect arrhythmogenesis in the present study (Fig. 5B). Furthermore, it has been reported that Ca2+ channel blockers prevented the onset of arrhythmias during ischemia (12, 18) in the same way that cilnidipine played a role in the disappearance in nonuniform [Ca2+]i in the present study (Figs. 1C and 5A). Thus the reports in the past are not contradictory to the hypotheses proposed in the present study.
In conclusion, results of the present study show that a regional but not a global increase in [K+]o increases arrhythmogenesis due to nonuniform [Ca2+]i and muscle contraction, which causes Ca2+ to dissociate from the myofilaments, particularly at the interface between the shortening and stretched region. The same mechanism may also be important for the occurrence of arrhythmias during the early phase of ischemia.
Study limitations.
Acute myocardial ischemia causes redistribution of a number of ions across the cell membrane, including net [K+]i loss and subsequent [K+]o accumulation (4, 41, 48). In the present study, however, only the effects of the regional increase in [K+]o were measured. Thus this model does not precisely mimic electrophysiological consequences due to the influence of acute ischemia on the activity of a variety of ion channels and transporters (10, 14, 42). Nevertheless, the results in the present study are still important because it shows for the first time that a regional increase in [K+]o causes arrhythmias by itself, probably due to spatially nonuniform [Ca2+]i and nonuniform muscle contraction.
GRANTS
This work was supported by Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research No. 2359025 (to M. Miura) and National Heart, Lung, and Blood Institute Grant HL-066140 (to P. A. Boyden).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.M. conception and design of research; M.M., T.H., N.M., T. Nagano, and T. Nishio performed experiments; M.M. and T.H. analyzed data; M.M. and P.A.B. interpreted results of experiments; M.M. prepared figures; M.M. drafted manuscript; M.M., P.A.B., and C.S. edited and revised manuscript; M.M., P.A.B., and C.S. approved final version of manuscript.
REFERENCES
- 1. Allen DG, Orchard CH. Myocardial contractile function during ischemia and hypoxia. Circ Res 60: 153– 168, 1987 [DOI] [PubMed] [Google Scholar]
- 2. Arnar DO, Xing D, Martins JB. Overdrive pacing of early ischemic ventricular tachycardia: evidence for both reentry and triggered activity. Am J Physiol Heart Circ Physiol 288: H1124– H1130, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Backx PH, Gao WD, Azan-Backx MD, Marban E. Mechanism of force inhibition by 2,3-butanedione monoxime in rat cardiac muscle: roles of [Ca2+]i and cross-bridge kinetics. J Physiol 476: 487– 500, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bai Y, Wang J, Lu Y, Shan H, Yang B, Wang Z. Phospholipid lysophosphatidylcholine as a metabolic trigger and HERG as an ionic pathway for extracellular K+ accumulation and “short QT syndrome” in acute myocardial ischemia. Cell Physiol Biochem 20: 417– 428, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Barrabés JA, Garcia-Dorado D, Agulló L, Rodríguez-Sinovas A, Padilla F, Trobo L, Soler-Soler J. Intracoronary infusion of Gd3+ into ischemic region does not suppress phase Ib ventricular arrhythmias after coronary occlusion in swine. Am J Physiol Heart Circ Physiol 290: H2344– H2350, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Cannell MB, Berlin JR, Lederer WJ. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science 238: 1419– 1423, 1987 [DOI] [PubMed] [Google Scholar]
- 7. Carmeliet E. Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol Rev 79: 917– 1017, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Coronel R, Wilms-Schopman FJG, de Groot JR. Origin of ischemia-induced phase 1b ventricular arrhythmias in pig hearts. J Am Coll Cardiol 39: 166– 176, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Coronel R. Heterogeneity in extracellular potassium concentration during early myocardial ischaemia and reperfusion: implications for arrhythmogenesis. Cardiovasc Res 28: 770– 777, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Coronel R, Fiolet JWT, Wilms-Schopman FJG, Schaapherder AFM, Johnson TA, Gettes LS, Janse MJ. Distribution of extracellular potassium and its relation to electrophysiologic changes during acute myocardial ischemia in the isolated perfused porcine heart. Circulation 77: 1125– 1138, 1988 [DOI] [PubMed] [Google Scholar]
- 11. Curtis MJ. Characterisation, utilisation and clinical relevance of isolated perfused heart models of ischaemia-induced ventricular fibrillation. Cardiovasc Res 39: 194– 215, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Curtis MJ, Macleod BA, Walker MJA. Models for the study of arrhythmias in myocardial ischaemia and infarction: the use of the rat. J Mol Cell Cardiol 19: 399– 419, 1987 [DOI] [PubMed] [Google Scholar]
- 13. Daitoku K, Seya K, Furukawa K, Motomura S. Assessment of the effects of L- and N-type Ca2+ channel blocking drugs using canine blood-perfused papillary muscle preparations. Tohoku J Exp Med 212: 415– 422, 2007 [DOI] [PubMed] [Google Scholar]
- 14. de Diego C, Pai RK, Chen F, Xie LH, De Leeuw J, Weiss JN, Valderrábano M. Electrophysiological consequences of acute regional ischemia/reperfusion in neonatal rat ventricular myocyte monolayers. Circulation 118: 2330– 2337, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Groot JR, Coronel R. Acute ischemia-induced gap junctional uncoupling and arrhythmogenesis. Cardiovasc Res 62: 323– 334, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Farman GP, Tachampa K, Mateja R, Cazorla O, Lacampagne A, de Tombe PP. Blebbistatin: use as inhibitor of muscle contraction. Pflügers Arch 455: 995– 1005, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Flagg TP, Patton B, Masia R, Mansfield C, Lopatin AN, Yamada KA, Nichols CG. Arrhythmia susceptibility and premature death in transgenic mice overexpressing both SUR1 and Kir6.2[ΔN30,K185Q] in the heart. Am J Physiol Heart Circ Physiol 293: H836– H845, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Gwilt M, Henderson CG, Orme J, Rourke JD. Effects of drugs on ventricular fibrillation and ischaemic K+ loss in a model of ischaemia in perfused guinea-pig hearts in vitro. Eur J Pharmacol 220: 231– 236, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Hamill OP, McBride DW., Jr The pharmacology of mechanogated membrane ion channels. Pharmacol Rev 48: 231– 252, 1996 [PubMed] [Google Scholar]
- 20. Hanley PJ, Young AA, LeGrice IJ, Edgar SG, Loiselle-Dimensional Configuration of perimysial collagen fibres in rat cardiac muscle at resting and extended sarcomere lengths. J Physiol 517: 831– 837, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris AS, Bisteni A, Russell RA, Brigham JC, Firestone JE. Excitatory factors in ventricular tachycardia resulting from myocardial ischemia; potassium a major excitant. Science 119: 200– 203, 1954 [DOI] [PubMed] [Google Scholar]
- 22. Hill JL, Gettes LS. Effect of acute coronary artery occlusion on local myocardial extracellular K+ activity in swine. Circulation 61: 768– 778, 1980 [DOI] [PubMed] [Google Scholar]
- 23. Hirche H, Franz CHR, Bös L, Bissig R, Lang R, Schramm M. Myocardial extracellular K+ and H+ increase and noradrenaline release as possible cause of early arrhythmias following acute coronary artery occlusion in pigs. J Mol Cell Cardiol 12: 579– 593, 1980 [DOI] [PubMed] [Google Scholar]
- 24. Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med 345: 1473– 1482, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Janse MJ, Wit AL. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev 69: 1049– 1169, 1989 [DOI] [PubMed] [Google Scholar]
- 26. Jie X, Gurev V, Trayanova N. Mechanisms of mechanically induced spontaneous arrhythmias in acute regional ischemia. Circ Res 106: 185– 192, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. John SA, Kondo R, Wang SY, Goldhaber JI, Weiss JN. Connexin-43 hemichannels opened by metabolic inhibition. J Biol Chem 274: 236– 240, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Kagiyama Y, Hill JL, Gettes LS. Interaction of acidosis and increased extracellular potassium on action potential characteristics and conduction in guinea pig ventricular muscle. Circ Res 51: 614– 623, 1982 [DOI] [PubMed] [Google Scholar]
- 29. Kantor PF, Coetzee WA, Carmeliet EE, Dennis SC, Opie LH. Reduction of ischemic K+ loss and arrhythmias in rat hearts. Effect of glibenclamide, a sulfonylurea. Circ Res 66: 478– 485, 1990 [DOI] [PubMed] [Google Scholar]
- 30. Kaplinsky E, Ogawa S, Balke CW, Dreifus LS. Two periods of early ventricular arrhythmia in the canine acute myocardial infarction model. Circulation 60: 397– 403, 1979 [DOI] [PubMed] [Google Scholar]
- 31. Katra RP, Laurita KR. Cellular mechanism of calcium-mediated triggered activity in the heart. Circ Res 96: 535– 542, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Lakireddy V, Baweja P, Syed A, Bub G, Boutjdir M, El-Sherif N. Contrasting effects of ischemia on the kinetics of membrane voltage and intracellular calcium transient underlie electrical alternans. Am J Physiol Heart Circ Physiol 288: H400– H407, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Lew WYW, Chen Z, Guth B, Covell JW. Mechanisms of augmented segment shortening in nonischemic areas during acute ischemia of the canine left ventricle. Circ Res 56: 351– 358, 1985 [DOI] [PubMed] [Google Scholar]
- 34. Löwel H, Dobson A, Keil U, Herman B, Hobbs MST, Stewart A, Arstila M, Miettinen H, Mustaniemi H, Tuomilehto J. Coronary heart disease case fatality in four countries. A community study. Circulation 88: 2524– 2531, 1993 [DOI] [PubMed] [Google Scholar]
- 35. Maruyama M, Joung B, Tang L, Shinohara T, On YK, Han S, Choi EK, Kim DH, Shen MJ, Weiss JN, Lin SF, Chen PS. Diastolic intracellular calcium-membrane voltage coupling gain and postshock arrhythmias. Role of Purkinje fibers and triggered activity. Circ Res 106: 399– 408, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matsuda T, Arakawa N, Takuma K, Kishida Y, Kawasaki Y, Sakaue M, Takahashi K, Takahashi T, Suzuki T, Ota T, Hamano-Takahashi A, Onishi M, Tanaka Y, Kameo K, Baba A. SEA0400, a novel and selective inhibitor of the Na+-Ca2+ exchanger, attenuates reperfusion injury in the in vitro and in vivo cerebral ischemic models. J Pharmacol Exp Ther 298: 249– 256, 2001 [PubMed] [Google Scholar]
- 37. Miura M, Nishio T, Hattori T, Murai N, Stuyvers BD, Shindoh C, Boyden PA. Effect of nonuniform muscle contraction on sustainability and frequency of triggered arrhythmias in rat cardiac muscle. Circulation 121: 2711– 2717, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miura M, Wakayama Y, Endoh H, Nakano M, Sugai Y, Hirose M, ter Keurs HEDJ, Shimokawa H. Spatial non-uniformity of excitation-contraction coupling can enhance arrhythmogenic delayed afterdepolarizations in rat cardiac muscle. Cardiovasc Res 80: 55– 61, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Miura M, Boyden PA, ter Keurs HEDJ. Ca2+ waves during triggered propagated contractions in intact trabeculae. Am J Physiol Heart Circ Physiol 274: H266– H276, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Philipson KD, Nishimoto AY. Na+-Ca2+ exchange is affected by membrane potential in cardiac sarcolemmal vesicles. J Biol Chem 255: 6880– 6882, 1980 [PubMed] [Google Scholar]
- 41. Rodríguez B, Ferrero JM, Jr, Trénor B. Mechanistic investigation of extracellular K+ accumulation during acute myocardial ischemia: a simulation study. Am J Physiol Heart Circ Physiol 283: H490– H500, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest 115: 2305– 2315, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schömig A, Fischer S, Kurz T, Richardt G, Schömig E. Nonexocytotic release of endogenous noradrenaline in the ischemic and anoxic rat heart: mechanism and metabolic requirements. Circ Res 60: 194– 205, 1987 [DOI] [PubMed] [Google Scholar]
- 44. Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin ΙΙ inhibitor. Science 299: 1743– 1747, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Sugai Y, Miura M, Hirose M, Wakayama Y, Endoh H, Nishio T, Watanabe J, ter Keurs HEDJ, Shirato K, Shimokawa H. Contribution of Na+/Ca2+ exchange current to the formation of delayed afterdepolarizations in intact rat ventricular muscle. J Cardiovasc Pharmacol 53: 517– 522, 2009 [DOI] [PubMed] [Google Scholar]
- 46. ter Keurs HE. The interaction of Ca2+ with sarcomeric proteins: role in function and dysfunction of the heart. Am J Physiol Heart Circ Physiol 302: H38– H50, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. ter Keurs HEDJ, Boyden PA. Calcium and arrhythmogenesis. Physiol Rev 87: 9457– 9506, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Terkildsen JR, Crampin EJ, Smith NP. The balance between inactivation and activation of the Na+-K+ pump underlies the triphasic accumulation of extracellular K+ during myocardial ischemia. Am J Physiol Heart Circ Physiol 293: H3036– H3045, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Verkerk AO, Veldkamp MW, Coronel R, Wilders R, van Ginneken ACG. Effects of cell-to-cell uncoupling and catecholamines on Purkinje and ventricular action potentials: implications for phase-1b arrhythmias. Cardiovasc Res 51: 30– 40, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Wakayama Y, Miura M, Stuyvers BD, Boyden PA, ter Keurs HE. Spatial nonuniformity of excitation-contraction coupling causes arrhythmogenic Ca2+ waves in rat cardiac muscle. Circ Res 96: 1266– 1273, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Wilde AAM, Aksnes G. Myocardial potassium loss and cell depolarisation in ischaemia and hypoxia. Cardiovasc Res 29: 1– 15, 1995 [PubMed] [Google Scholar]