Abstract
In the heart, mitochondria form a regular lattice and function as a coordinated, nonlinear network to continuously produce ATP to meet the high-energy demand of the cardiomyocytes. Cardiac mitochondria also exhibit properties of an excitable system: electrical or chemical signals can spread within or among cells in the syncytium. The detailed mechanisms by which signals pass among individual elements (mitochondria) across the network are still not completely understood, although emerging studies suggest that network excitability might be mediated by the local diffusion and autocatalytic release of messenger molecules such as reactive oxygen species and/or Ca2+. In this short review, we have attempted to described recent advances in the field of cardiac mitochondrial network excitability. Specifically, we have focused on how mitochondria communicate with each other through the diffusion and regeneration of messenger molecules to initiate and propagate waves or oscillations, as revealed by computational models of mitochondrial network.
Keywords: mitochondrial energetics, reactive oxygen species-induced reactive oxygen species release, oxidative stress, cardiovascular disease, network modeling
this article is part of a collection on Systems and Computational Approaches to Cardiovascular Physiology. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Introduction
Many biological systems have properties resembling an excitable medium, in which a wave of regenerative signal or activity propagates at a constant velocity without damping. In such a system, individual elements are connected to each other either physically, electrically, or chemically, so that signals can pass among neighboring regions in an ordered pattern (47). One characteristic of an excitable medium is that an impulse or stimulus beyond a certain threshold can trigger a response in a state variable much larger than the stimulus itself (i.e., regeneration). Another one is that the propagation of a wave is dependent on a diffusion-like local transport instead of simple diffusion over the whole network length. Cardiac muscle is a typical example of an excitable medium, in which cells are coupled electrotonically and the regenerative signals (currents) are supported by voltage gradients induced by the active movements of ions through sarcolemmal ion channels. Loss of excitability in an individual cardiac cell, or group of cells, is a harbinger of arrhythmias and sudden cardiac death.
Mitochondria are the hubs of cellular metabolic and signaling pathways, which form dynamic networks with morphologies that vary by tissue (52), for example, a highly ordered lattice in cardiac cells (8, 11, 88), versus a more irregular tubular arrangement in neurons. Functionally, the mitochondrial network displays highly nonlinear, multiplicative, and collective dynamics that span wide temporal scales (i.e., from milliseconds to hours) (10). Interestingly, the mitochondrial network also exhibits excitability, mediated by intracellular secondary messengers such as Ca2+ and reactive oxygen species (ROS). Ichas et al. (45) reported that Ca2+ can induce transitory opening of the mitochondrial permeability transition pore (mPTP), evoking Ca2+-induced Ca2+ release. Opening of the mPTP can cause release of cytochrome c, leading to initiation and propagation of apoptotic signals throughout the cell (71).
Work from our group showed that oscillations of mitochondrial inner membrane potential (ΔΨm) can be observed in either small clusters of mitochondria, synchronized throughout the whole cell (7), or as propagating waves between cells during metabolic stress (79). These events were shown to involve a mechanism that could be inhibited by ligands of the mitochondrial benzodiazepine receptor, but not by cyclosporin A (CsA), an inhibitor of mPTP (65), suggesting the involvement of mitochondrial ROS-induced ROS release (RIRR) and the activation of the inner membrane anion channel (IMAC) (5, 7, 9). Alternatively, others have found that direct laser-induced mitochondrial ΔΨm fluctuations (104), as well as traveling ΔΨm depolarization waves (23), are delayed or partially suppressed by CsA treatment, suggesting that the mPTP may also underlie RIRR-triggered responses.
Interestingly, a detailed frequency domain analysis of ΔΨm provided evidence for correlated oscillations of ΔΨm spanning a wide frequency range even under physiological conditions, indicating that the mitochondrial network can display oscillatory dynamics with a varying degree of ROS-dependent coupling under many different conditions (5). The strength of coupling is likely to determine both the spatial organization of the response (i.e., how locally restricted it is) as well its amplitude and frequency. Recent studies employing multiple wavelet analysis demonstrated that the frequency of ΔΨm oscillation depends inversely on cluster size (49), supporting the notion that the mechanism scales from high-frequency single mitochondrion fluctuations to whole cell periodic oscillations (64).
Taken together, these findings provide strong evidence that mitochondria form an excitable network that is able to convey signals across the cell. Various mechanistic hypotheses have been proposed to explain mitochondrial network excitability and depolarization wave propagation (11, 24, 91); however, it remains unclear how discrete subcellular loci could initiate the excitation waves and how subsets of mitochondria communicate to achieve wave propagation throughout the cell.
As an alternative tool, mathematical modeling has been increasingly used over the past few decades, alone, or in combination with experimental measurements to explore the complex behavior of biological systems. However, the application of this powerful tool to the study of mitochondrial network excitability has only recently begun. In this short review, we describe several recently published models of mitochondrial network excitability and oscillations that are based on observations of ROS- or Ca2+-induced ΔΨm depolarization (70, 72, 96, 98). These computational studies provide additional evidence to support the concept of mitochondrial network excitability.
Mitochondria: Important Intracellular Signaling Organelles
Mitochondria, since their discovery in the late 19th century (3, 80), have been recognized as the cell's powerhouse, producing energy to maintain various cellular functions. In addition, mitochondria function as critical signaling organelles in the delivery of signals crucial for cell survival or death (12). Mitochondria are not only a major source of ROS production but also a main target of oxidative damage. Elevated ROS accumulation and resulting mitochondrial dysfunction have been implicated in various human diseases, such as cancer, cardiovascular disease, diabetes, neurodegenerative diseases, and age-associated disease (41, 86, 89, 92, 97).
ROS source and target.
Under physiological conditions, cardiac mitochondria produce over 95% of the cell's ATP requirement, which is hydrolyzed in the cytosol to support cellular mechanical work, molecular synthesis, and ion homeostasis (62). In addition to functioning as the cellular powerhouse, mitochondria are also a major site of ROS production. Even under physiological conditions, up to 0.5–2% of electrons flowing through the electron transport chain are partially reduced to form superoxide anion (O2·−) (28). The possible electron leakage sites include complex I and complex III (60), but the precise mechanisms are still unclear. Superoxide is an unstable radical and has a short half-life; it is rapidly dismutated by superoxide dismutase (e.g., MnSOD in the mitochondrial matrix and Cu/ZnSOD in the intermembrane space and cytoplasm) to form the more stable molecule, hydrogen peroxide (H2O2). H2O2 can be further reduced to H2O by various antioxidant enzymes such as catalase, glutathione peroxidase, and peroxiredoxin (38, 68, 69). As natural by-products of oxygen metabolism, ROS are important signaling molecules that regulate gene expression and other oxygen-sensing machineries (15, 85, 87). However, excessive ROS, caused by uncontrolled production or impaired scavenging, are toxic components due to their high reactivity with large molecules such as proteins, DNA, and lipids, leading to oxidative damage (60, 69, 89). Elevated ROS can increase the permeability of mitochondrial outer membrane, facilitating the release of cytochrome c that activates the cellular apoptotic process (33, 48). Moreover, oxidative stress promotes the opening of the mPTP, causing mitochondrial swelling, loss of molecules from the matrix, and membrane rupture. It has been shown that mitochondrial ROS accumulation in cells suffering from ischemia-reperfusion can cause rapid and spatiotemporally heterogeneous discharge of ΔΨm (35), resulting in cell death, arrhythmias, and ischemia-perfusion injury (2). ROS overload could also cause a depletion of the intracellular redox pool (6) and decrease of ATP production (90). This would affect cellular Ca2+ handling, ion channel activity, and ROS-mediated signaling, which are very sensitive to intracellular redox and phosphorylation states (27, 34, 93).
Ca2+ handling and signaling.
As a ubiquitous intracellular second messenger, Ca2+ is involved in the modulation of diverse cellular functions including energy metabolism, electrical signaling, fertilization, gene transcription, and programmed cell death (21). The role of Ca2+ as a signaling molecular is highly dependent on its “local” concentration; therefore, a precise regulation of Ca2+ handling is vital. Abnormal Ca2+ handling can contribute to the pathogenesis of various diseases such as diabetes, neurodegeneration, and cardiac disease (74, 78, 81).
In cardiomyocytes, Ca2+ dynamics are controlled by a complex system composed of ion channels and exchangers located at the sarcolemma and subcellular membranes, including L-type Ca2+ channel, Na+/Ca2+ exchanger, ryanodine receptor, and sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA). Emerging evidence indicate that intracellular Ca2+ dynamics are also shaped by mitochondria (13, 14, 43). Mitochondria can influence Ca2+ cycling either directly, via inner membrane Ca2+ transport, or indirectly, via ROS-mediated signaling pathways. The ruthenium red-sensitive Ca2+ uniporter is the primary route for Ca2+ entry into the mitochondrial matrix (61), and there is strong evidence that an increase in matrix Ca2+ is important for stimulating oxidative phosphorylation at several sites, including the Ca2+-sensitive dehydrogenases of the Krebs cycle (32) and one or more sites in the electron transport chain (16). Physiological mitochondrial Ca2+ uptake may cause a measurable transient mitochondrial depolarization (53), whereas mitochondrial Ca2+ overload can trigger mPTP opening and persistent mitochondrial depolarization. The extrusion of Ca2+ from the mitochondria is mediated by a mitochondrial Na+/Ca2+ exchanger (61). A H+/Ca2+ exchanger may also be present in noncardiac tissues, and it has also been postulated that transient mPTP opening may be a third Ca2+ release mechanism (40). Under pathological conditions, mitochondria can affect Ca2+ handling indirectly by producing and releasing ROS close to the ion transporters involved in cytoplasmic Ca2+ handling. Both the L-type Ca2+ channel (44) and the ryanodine receptor (93) are sensitive to oxidative stress, as is the SERCA (51). We have recently found that in quiescent cardiomyocytes, mitochondrially derived ROS, released during self-sustained oscillations in ΔΨm, can dynamically modulate the spontaneous Ca2+ release from the ryanodine receptors (Ca2+ sparks) (99). Finally, mitochondria can function as a Ca2+ sink by taking up a large amount of Ca2+, allowing matrix free Ca2+ to be kept in the micromolar per liter range, which minimizes the osmotic effects of Ca2+ uptake (66).
Mitochondrial Network Excitability Induced by IMAC-Mediated RIRR
Mitochondrial oscillations and RIRR: experimental observations.
Under physiological conditions, intracellular ROS are maintained at very low levels by the efficient antioxidant system. However, under certain circumstances such as ischemia, hypoxia, or substrate starvation, O2·− and H2O2 concentrations can increase dramatically as the ROS scavenging capacity is overwhelmed and/or excessive oxygen intermediates are produced. Mitochondrial ROS generation is a self-amplifying process (RIRR) that can be triggered by intracellular or exogenous ROS sources (103). One mechanism of RIRR involves the activation of IMAC (a benzodiazepine-sensitive energy-dissipating channel in the inner membrane) and depolarization of ΔΨm. Aon et al. (7, 29) showed that a local laser flash which triggers a burst of ROS production in a small number of mitochondria is followed by synchronized, cell-wide oscillations of ROS, ΔΨm, NADH, and GSH/GSSG. An interesting question to ask is how the perturbation of a few mitochondria triggers the global self-organized and synchronized spatiotemporal behavior. Early studies suggested that mitochondria are organized as clusters and may be electrically coupled through cable properties (4). On the contrary, other investigators have provided ample evidence that individual mitochondria can depolarize independent of their neighbors (36, 46, 79), arguing against electrical continuity. The precise mechanisms under which individual mitochondria communicate with each other to spread the ROS signals over the cellular distance remain unclear.
Based on previous studies (7, 11), a hypothesis based on RIRR and ROS diffusion was proposed. According to this hypothesis, a few initially depolarized mitochondria (e.g., triggered by local photooxidation) recruit their neighbors within a cluster through ROS diffusion and RIRR, amplifying the initial mitochondrial event. After a critical mass of mitochondria reach a threshold of oxidative stress, termed “mitochondrial criticality” (11), synchronized cell-wide oscillations occur. The basis of coupling in this model is ROS-mediated mitochondrial excitability, i.e., the local diffusion and regeneration of ROS within the mitochondrial network, along with a ROS-activated mitochondrial ion channel.
Mitochondrial oscillations: model simulation.
To test the hypothesis, we recently developed a computational model of the cardiac mitochondrial network (98). The model was based on the reaction-diffusion properties of O2·− and the RIRR mechanism (29), and it incorporated detailed mitochondrial energetics and ROS metabolism pathways, including O2·− production and dismutation to H2O2, O2·− release through IMAC, activation of IMAC, and scavenging and diffusion of O2·− in the cytoplasm. In the model, the mitochondrial network was divided up into individual nodes (mitochondria), with each mitochondrion chemically coupled with its four nearest neighbors (Fig. 1A) through local ROS diffusion. The dynamics of state variables of each mitochondrion are derived from the principle of mass/ion balance. At each node (i,j) in the network, the dynamics are given by:
| (1) |
where V(k) is the effective volume of species k in the mitochondria, C(k) is the concentration, R(k) is the net reaction rate and J(k) is the net transport rate. The extramitochondrial O2·− dynamics were taken to be a combination of reaction and diffusion according to the partial differential equation:
| (2) |
with the following boundary and initial conditions: and . In Eq. 2, is the diffusion coefficient of O2·− in the cytosol, and f( , t) is the net extramitochondrial O2·− production rate (release from mitochondria minus scavenging by SOD).
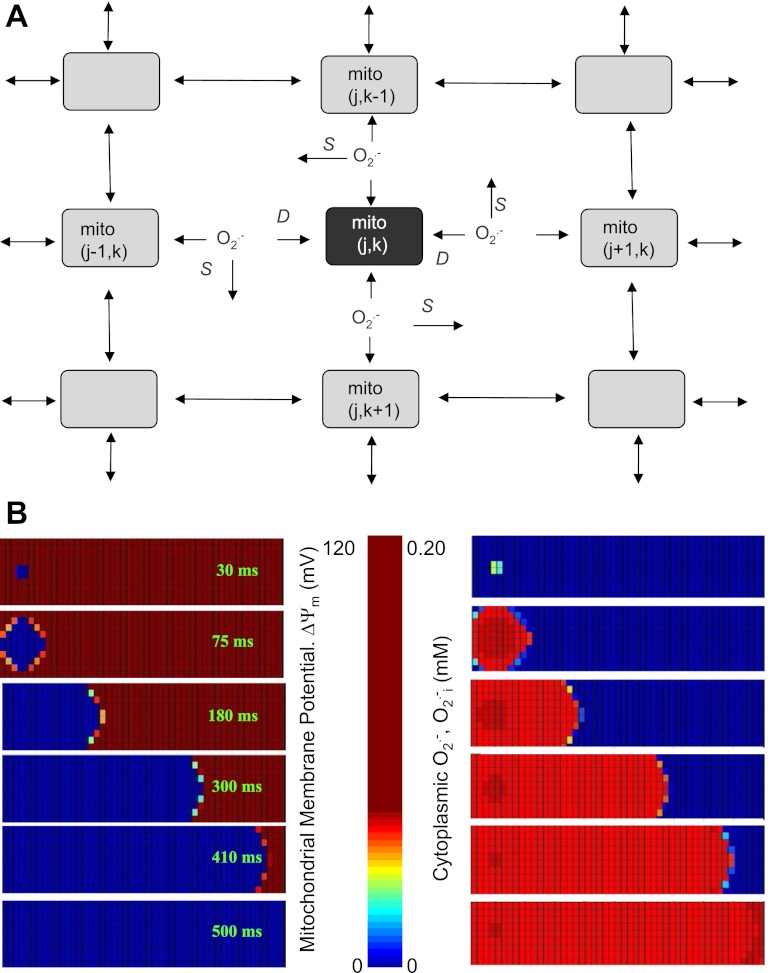
Fig. 1.
A: scheme of the two-dimensional (2-D) reaction-diffusion reactive oxygen species (ROS)-induced ROS release (RD-RIRR) mitochondrial network model. In the RD-RIRR model, neighboring mitochondria are chemically coupled with each other through superoxide anion (O2·−) diffusion. Light and dark gray indicate polarized and depolarized mitochondria, respectively. Arrows indicate release of superoxide anion (O2·−) and its effect on mitochondrial neighbors. D stands for extramitochondrial O2·− diffusion, and S for O2·− scavenging by Cu/ZnSOD and catalase. B: simulation of spatial propagations of mitochondrial inner membrane potential (ΔΨm) depolarization wave (left) and ROS wave (right) by the 2-D RD-RIRR model. This network consisted of 500 mitochondria (10 × 50), in which 6 were initially depolarized, whereas the others were polarized. Figure was obtained from (98) with permission.
This network model successfully simulated ΔΨm depolarization wave propagation observed in experiments in response to oxidative stress. Specifically, the results showed that a few oscillating mitochondria (∼1% of the total volume) could entrain the whole network into an oscillatory mode (Fig. 1B). The observations that 1) local diffusion (1 to 2 μm distances between neighboring mitochondria) of ROS can give long range (∼100 μm cellular length) global behavior and 2) the velocity of the wave and the rate of depolarization do not decline with distance from the site of wave origin demonstrate that the cardiac mitochondrial network is an excitable medium. Another insight is that the observed dynamics of the mitochondrial network do not necessarily need to invoke physical connections between mitochondria (although some may exist), as chemical communication among mitochondrial neighbors can convey information across long distances.
In addition to reproducing the experimentally observed mitochondrial oscillations and propagation of O2·− waves, the model also revealed several interesting findings that may help to elucidate the mechanisms underlying the initiation and maintenance of ΔΨm depolarization and O2·− waves.
RIRR, ROS diffusion, and wave propagation.
As shown in Fig. 1B, the network model successfully simulated the initiation and propagation of waves of O2·− and ΔΨm depolarization, the latter reproducing data obtained in cardiomyocytes exposed to oxidative stress. The key question that the model answers is how the local events scale to the network level. In the single mitochondrion model (29), RIRR occurs when O2·− produced from the electron transport chain builds up in the matrix and subsequently leaks out to a level that triggers IMAC opening via cytoplasmic activation site. The increasing ion flow across the inner membrane results in the rapid collapse of mitochondrial ΔΨm and a transient increase ROS generation, followed by a termination of the release due to both ROS scavenging at the activator site and depolarization of ΔΨm (which decreases the O2·− efflux rate). This RIRR mechanism explains very well the behavior of a single mitochondrion in response to stress. However, to elicit the whole network response, this local ROS burst must reach the neighboring mitochondria at concentration above the threshold to active their IMACs. In other words, a suprathreshold level of cytoplasmic O2·− must be delivered from the originally perturbed mitochondrion to its neighbors for the response to be regenerative.
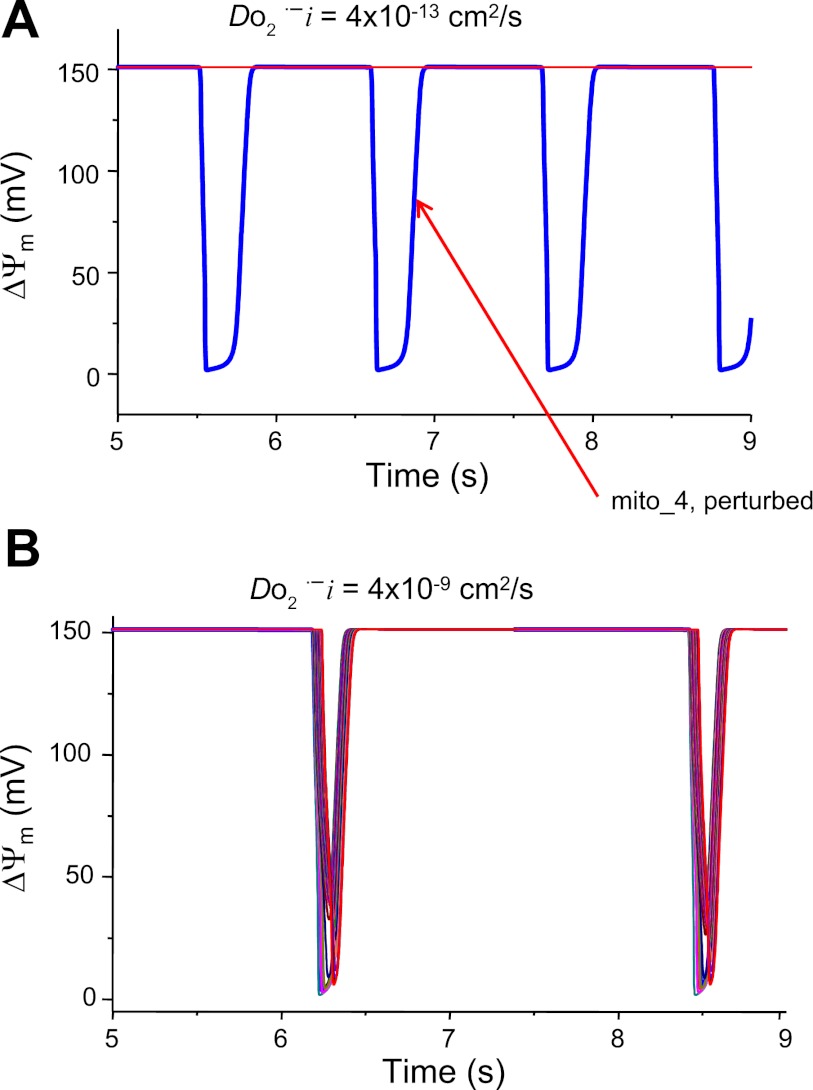
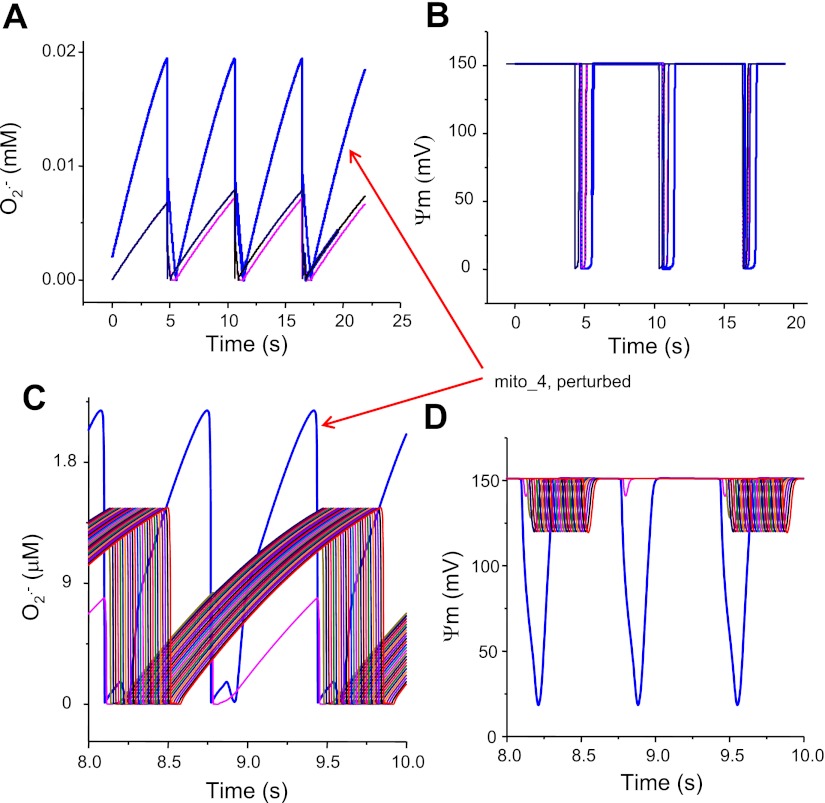
In the model, cytoplasmic O2·− surrounding a mitochondrion is governed by the following terms: O2·− release from mitochondrial matrix, the diffusion rate to its immediate neighbors, and the rate of scavenging by superoxide dismutase (Eq. 2). Increasing the diffusion rate or reducing the scavenging capacity, therefore, increases the surrounding O2·− concentration (up to suprathreshold levels), resulting in the activation of IMAC and the initiation of RIRR. The dependence on scavenger rate explains why spontaneous cell-wide oscillations can be triggered in saponin-permeabilized cardiomyocytes by decreasing the medium GSH-to-GSSG ratio (6). The role of ROS diffusion in supporting the propagation of ROS and ΔΨm depolarization waves was suggested by simulations showing that an oscillating mitochondrion (mito_4) can entrain the oscillation of other mitochondria in the network (total 30) only when the ROS diffusion coefficient is large enough (Fig. 2). ROS diffusion-mediated mitochondrial network excitability can be further demonstrated by simulations shown in Fig. 3, which show the effect of O2·− produced by the perturbed mitochondrion (i.e., high ROS production) on the entrainment of ΔΨm depolarization in other mitochondria that were not initially oxidatively stressed (i.e., they were initialized with low rates of ROS production). When O2·− produced by the perturbed mitochondrion is relatively low (0.0018 mM), the O2·− surrounding its neighbors is low as well and does not reach the threshold for activation of IMAC; therefore, propagation and entrainment of the network (30 mitochondria in total) does not occur. When the perturbed mitochondrion produces more O2·− (0.02 mM), the O2·− reaching its neighbor is sufficiently high (0.008 mM) to activate the IMAC, consequently eliciting whole network oscillations. Finally, as cytoplasmic O2·− is also governed by scavenging capability, network oscillation and propagation only occur when etSOD (concentration of cellular superoxide dismutase) is in the appropriate range. Therefore, it is the interplay between the production, scavenging, and diffusion of O2·− that determines whether a local perturbation can trigger a network response, i.e., the network excitability. When all parameters are in the right ranges such that the network is close to its criticality, perturbing even a single mitochondrion can induce cell-wide mitochondrial depolarization (98).
Fig. 2.
Effect of ROS coupling on the entrainment of mitochondrial network (30 mitochondria in a row) oscillations. Model simulations show that the diffusion (or coupling) of O2·− among neighboring mitochondria must be big enough to ensure a fast diffusion rate that can deliver enough amount of O2·− from the perturbed mitochondrion (mito_4) to its immediate neighbors to activate their inner membrane anion channels (IMACs). A: when the O2·− diffusion coefficient is low (4 × 10−13 cm2/s), the network depolarization and oscillations cannot be entrained. DO2·−i is the diffusion coefficient of O2·− in the cytosol. B: when the diffusion coefficient is 104 times higher (4 × 10−9 cm2/s), mito_4 can induce whole network depolarization and oscillations. In these 2 simulations, all model parameters are the same except the O2·− diffusion coefficient.
Fig. 3.
Effect of O2·− produced by the mito_4 on the network (30 mitochondria in total) entrainment. A: cytoplasmic O2·− level surrounding (and produced by) the perturbed mitochondrion is high (0.02 mM); as a result, the O2·− arriving the adjacent mitochondria is above the threshold (0.008 mM) to activate their IMACs and entrain the whole network oscillations (B). C: cytoplasmic O2·− level surrounding (and produced by) the mito_4 is relatively low (0.0022 mM), and the O2·− reaching its neighbors is below the threshold level (0.0015 mM); thus it cannot entrain the full depolarization of the network (D).
Mitochondrial excitability: network versus single mitochondrion.
In the single mitochondrion model of RIRR developed by Cortassa et al. (29), oscillation occurs when there is a bifurcation in the dynamics of the model, creating upper (polarized) and lower (depolarized) branches of the steady state. Stability analysis revealed that the mitochondrion could exhibit oscillatory behavior with stable limit cycles only when key parameters are within the oscillatory domain (e.g., small ROS scavenging, etSOD, or large ROS production, shunt); outside these domains, the mitochondrion displays either a stable depolarized or polarized state. For the network model, however, entrainment of mitochondria that would not normally oscillate is possible (98). This situation more closely resembles evidence from intact cardiomyocytes where we observe that the network behaves as a system of oscillators with a variable degree of ROS-dependent coupling as one moves from the physiological to the pathophysiological state (8). In other words, individual mitochondria might be oscillating at high frequencies and low amplitudes at all times but become entrained under stress to produce network-wide large amplitude, slow oscillations. The transition to synchronized oscillations has significant consequences because it induces large changes in the cellular ATP-to-ADP ratio and much larger bursts of ROS, which could impact multiple targets in the cell. As shown in Fig. 3, a high ROS-producing mitochondrion located at the center of a row of 30 mitochondria can entrain the oscillations of other low ROS-producing mitochondria, as long as ROS diffusion is rapid enough between neighboring mitochondria (determined by the diffusion coefficient). The summation of the responses of the source mitochondrion and the target mitochondria result in the O2·− concentration surrounding the low ROS-producing mitochondria to increase dramatically compared with those without ROS-dependent coupling. The elevated ROS, upon reaching the critical threshold, activates RIRR in the follower mitochondria and entrains ΔΨm oscillations. Therefore, a mitochondrion does not necessarily need to be in an “oscillatory parametric domain” to oscillate; the determining factor is the surrounding ROS concentration. The source of this ROS could be either endogenous (produced from the electron transport chain and exported through the IMAC) or exogenous (e.g., diffusing from neighboring mitochondria or extramitochondrial sources of ROS). For example, an addition of 20 μM KO2 (98) or depletion of cytoplasmic-reduced glutathione (6) can cause mitochondrial depolarization and/or ΔΨm oscillation in partially permeabilized cardiomyocytes.
Taking the question of how ΔΨm loss in individual mitochondria (flickering) scales to produce synchronization at the whole cell level one step further, Nivala et al. (64) recently developed a Markov gating model of IMAC incorporated into individual mitochondria, which were then organized into a network of mitochondria. Stochastic gating of the channel resulted in flickering of ΔΨm, and when the mitochondria were initialized with high O2·− production rates, coupling occurred in the network, resulting in propagating waves and slower periodic oscillations at the whole cell level. This study illustrates how a seemingly random high-frequency process at the nanoscale level can lead to self-organized criticality through coupling in the network.
Other potential mechanisms participating in IMAC-mediated mitochondrial oscillations.
The model simulations provide supporting evidence that local O2·− regeneration and diffusion are central to the propagation of ROS and ΔΨm depolarization waves in the mitochondrial network. Our model of RIRR is centered on the ROS-dependent activation of IMAC, based on our experimental observations that RIRR induced by substrate deprivation (65), local laser excitation (7), or GSH depletion (6) is inhibited by benzodiazepine receptor ligands, which block IMAC in isolated mitochondria (17), but does not involve mPTP activation (7). IMAC is permeable to a variety of inorganic (e.g., Cl− and Pi) and organic (e.g., citrate3−, malate2− and ATP4−) anions (17) and has properties similar to the 108pS conductance anion channel found in mitoplast patch-clamp studies (22). Other potential IMAC modulatory factors might also be suggested by these early studies. For example, Beavis and Powers (20) showed that in isolated mitochondria, IMAC is inhibited by Mg2+ from both the inside and the outside of the membrane, and this regulation is dependent on the matrix pH. However, the assay procedure they used to activate IMAC-mediated mitochondrial swelling was to totally deplete free Mg2+ from the mitochondrial matrix (18), which is unlikely to occur in the living cells. In addition, the inhibitory effect of protons and Mg2+ is highly sensitive to the temperature, suggesting that IMAC is poised to respond to small changes in pH or Mg2+ under more physiological conditions (19).
With respect to the observed RIRR-mediated mitochondrial oscillations, there is no direct experimental evidence to implicate free Mg2+ depletion and pH changes as a trigger of the RIRR, at least under our experimental conditions. Although they are not essential players in the fundamental oscillator mechanism, changes in both ions and energetics do occur during ΔΨm oscillations and they are likely to modulate either the amplitude or frequency of oscillations, as suggested by a recent study (98). It is essential to incorporate the modulation of IMAC by these factors in future modeling studies, pending additional experimental support.
Mitochondrial Network Excitability Induced by mPTP-Mediated RIRR
In addition to the IMAC-mediated pathway, RIRR might also be triggered by the ROS-activated opening of the mPTP, as originally described by Zorov et al. (103) for direct laser excitation of individual mitochondria. A slow depolarization wave (<0.1 μm/s) associated with mPTP activation was also described by Brady et al. (23), that is, slow compared with the propagation rate of RIRR measured in studies from our group (∼25 μm/s) (7, 11). The mPTP-mediated RIRR has been implicated in both the preconditioning and the apoptotic pathways in cardiac cells (46, 83). mPTP activation has been invoked to explain these phenomena, as well as the more recent observations of O2·− transients in single mitochondria (94). However, the effects of CsA on these responses are to delay (103) or decrease the frequency of the ΔΨm depolarizations (94), and other investigators have observed no CsA sensitivity (76). Moreover, these groups have found that Ca2+ is not required to activate the mPTP via RIRR, leaving the nature of the ΔΨm depolarization somewhat unclear.
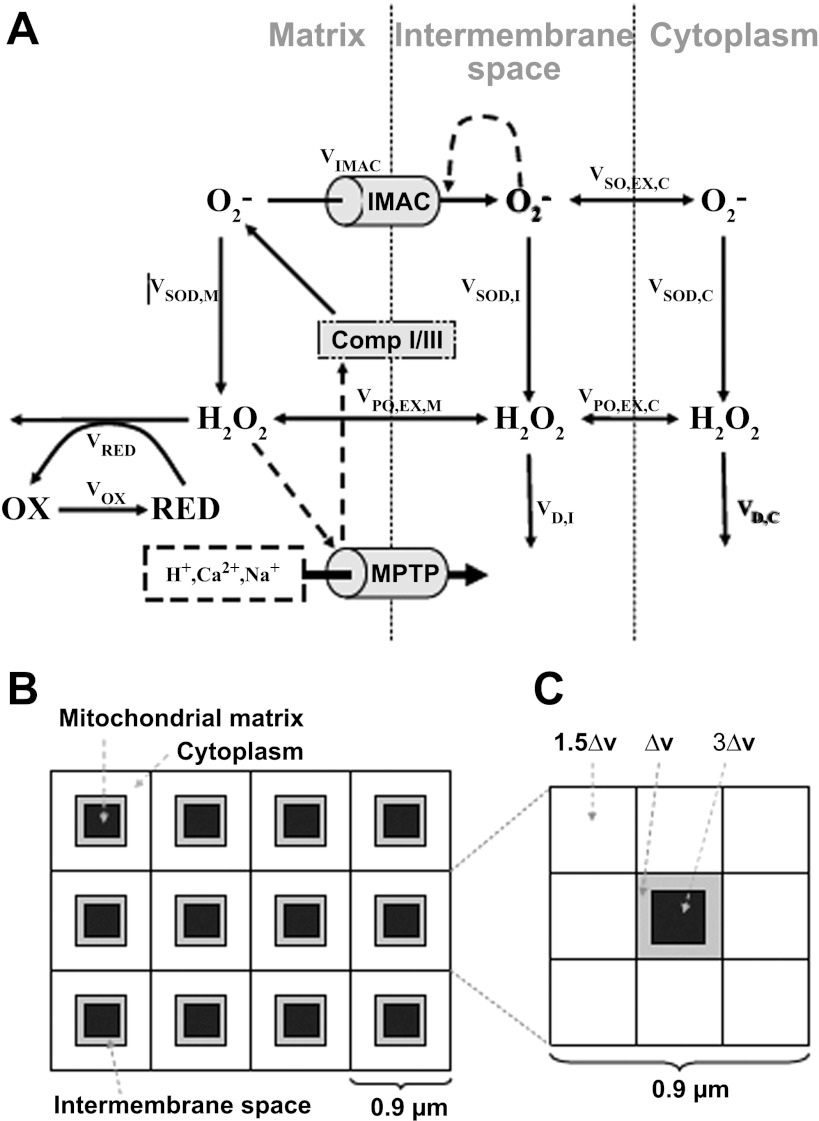
These differences in mechanistic interpretation need to be resolved in future studies, but it is likely that more than one energy-dissipating ion channel can mediate transient ΔΨm depolarization. In addition, there may be synergistic interactions among different channels. For instance, IMAC and mPTP can be activated in a sequential manner by oxidation of the GSH pool in cardiac cells (6), so it is important to consider the cooperative effects of both channels in the context of RIRR mitochondrial network excitability. Yang et al. (96) developed a mitochondrial model that included both IMAC-mediated RIRR and mPTP-mediated RIRR. In their model, mitochondria are coupled via ROS diffusion in a spatially extended two-dimensional array of voxels, each containing the single mitochondrion model, the intermembrane space, and cytoplasmic volumes (Fig. 4). The IMAC-mediated RIRR was modeled using the same strategy as that developed by Cortassa et al. (29). To simulate the mPTP-mediated RIRR, the authors assumed that the accumulation of downstream product of antioxidant pathways, such as hydroxyl radicals (modeled as the linear function of H2O2), activates the mPTP opening, leading to ΔΨm depolarization and RIRR. In this model, both O2·− and H2O2 were allowed to diffuse freely between the intermembrane space and the adjacent cytoplasmic space.
Fig. 4.
Scheme of mitochondrial model that includes both IMAC-mediated RIRR and mitochondrial permeability transition pore (mPTP)-mediated RIRR. In this model, mitochondria are coupled via ROS diffusion in a spatially extended 2-D array of voxels, each containing the single mitochondrion model, the intermembrane space, and cytoplasmic volumes. Reproduced from (96).
The authors showed that their model was able to simulate both IMAC-mediated and mPTP-mediated mitochondrial oscillations and depolarization wave propagation, depending on how much O2·− production (Kshunt) was induced by external factors. With relatively low Kshunt (e.g., <0.105 mM/s), mitochondria exhibited limit cycle oscillations that were regulated by IMAC-mediated RIRR, whereas when Kshunt was higher (e.g., 0.25 mM/s), the accumulated H2O2 triggered the complete opening of mPTP, forming a positive feedback loop that leads to bistability. The lower excitability of mPTP-mediated RIRR was perhaps due to the higher H2O2 scavenging rate (compared with O2·−), as increasing H2O2 degradation rate significantly reduced the velocity of wave propagation. When scaled up to the mitochondrial network, these two dynamics resulted in different spatiotemporal behaviors in the cell. Specifically, the IMAC-mediated RIRR, induced by increasing ROS production in a small area in the center of the network, triggered synchronized cell-wide ΔΨm oscillations. The resulting depolarization wave propagated at a speed of ∼25 μm/s that was comparable with that observed by Aon et al. (11). The mPTP-mediated RIRR, induced by increasing ROS production (Kshunt) in all mitochondria in the network, led to a ΔΨm depolarization wave that propagated much more slowly (∼2.1 μm/s). Because of the self-amplifying nature of the RIRR process, opening of mPTP eventually led to irreversible ΔΨm depolarization. The authors also simulated the effect of increasing Kshunt (as a linear function of time) on the network dynamics to mimic a pathological condition of progressive depletion of antioxidant capability or damage to the distal electron transport chain. They showed that under this condition, a series of mitochondrial oscillations evoked by IMAC-mediated RIRR were observed at the first, which then developed into a slow depolarization wave as a result of mPTP-mediated RIRR (Fig. 5). These results indicate that the mitochondrial network can exhibit two different types of excitabilities, fast waves induced by the IMAC-mediated RIRR (triggered by O2·−) and slower waves caused by mPTP-mediated RIRR (triggered by H2O2) processes, respectively. These two processes could occur alone or together, depending on the oxidative status of the cell. This model of differential sensitivity of IMAC and mPTP to different ROS remains hypothetical at present, and other factors may contribute to coupling between IMAC and mPTP. In this regard, it is very likely that disturbances of the Ca2+ handling subsystem associated with mitochondrial ΔΨm depolarization and the concomitant oxidative stress (99) would predispose the mPTP to open during sustained RIRR, as suggested by experimental observations (6). It should be emphasized that the details of the mechanism of activation of energy-dissipating ion channels in the mitochondrial inner membrane are not well understood and the molecular structures of IMAC and PTP have not been determined. For example, PTP activation in isolated mitochondria is strictly dependent on Ca2+ as the trigger, although its sensitivity is altered by the redox state. Limitations of available ROS probes also make it impossible to quantitatively measure local ROS concentrations near the mitochondrial inner membrane owing to the robust scavenger systems present in the matrix and the intermembrane space. Hence, certain aspects of the proposed models will require additional investigation to refine them in the future.
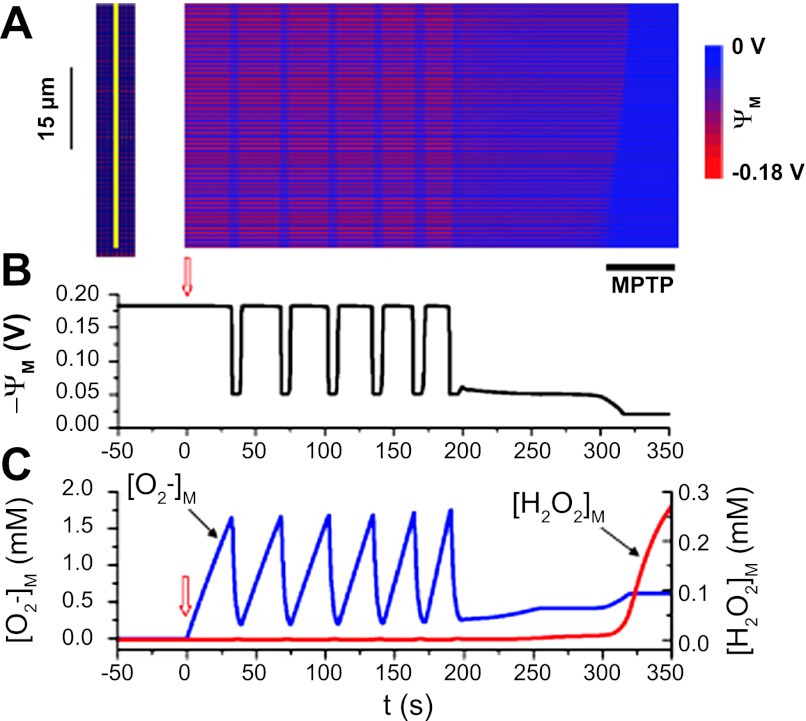
Fig. 5.
IMAC-mediated ΔΨm oscillations triggering an mPTP-mediated final depolarization wave. A: space-time plot of ΔΨm recorded along the yellow line (left). O2·− production rate increased progressively over time, most rapidly in the lower 4 rows of mitochondria {where kshunt = 0.35[0.3 + 0.7 min (1,e0.02t/150)]} and more slowly elsewhere {kshunt = 0.2[0.3 + 0.7 min (1,e0.02t/150)]}. B: average ΔΨm vs. time. C: mitochondrial O2·− (blue) and H2O2 (red) concentrations vs. time. kshunt was initially zero and started to increase at the upward arrows. Black bar below the snapshot indicates the final mPTP-mediated slow wave. Reproduced from (96).
Agent-Based Model of Mitochondrial Network Excitability
More recently, to determine the role of ROS (i.e., O2·− and H2O2) in the mediation of mitochondrial network excitability, Park et al. (72) developed a mathematical model of the mitochondrial network using an agent-based modeling (ABM) approach. The ABM technique has a rule-based modeling format that determines the probabilities of a transition to the next state at every step; therefore, it can be used to simulate interactions between agents in a complex system. Their simulations showed that mitochondrial network organization (i.e., the mitochondrial spatial distribution in the cytosol or the number of mitochondria in the cell) could significantly affect intracellular ROS propagation profiles and cellular sensitivity to oxidative stress. This was achieved by incorporating differential effects of specific free radicals (i.e., O2·− and H2O2) that have an impact on inter-mitochondrial communication. Remarkably, they showed that increasing the superoxide dismutase activity, either in the mitochondria or in the cytosol, significantly reduced the reactivity of mitochondria in a regular lattice and blocked ROS propagation, but this protective effect was largely abolished when the mitochondrial network was irregularly distributed. In contrast, raising the rate of cytosolic H2O2 decomposition capability had a protective effect on the irregular mitochondrial network but did not protect the lattice-like network. These results indicate that there is a correlation between mitochondrial organization and ROS signaling and suggest that in a regular lattice-like mitochondrial network, such as in cardiomyocytes, O2·− is the major species the regulates mitochondrial excitability, whereas in irregularly distributed mitochondria, such as in neurons, H2O2 may play a larger role in mediating RIRR and mitochondrial signal propagation. The switch between O2·−-triggered RIRR and H2O2-triggered RIRR, depending on mitochondrial network organization, is probably determined by differences in the kinetics, diffusability, and lifetime of these two messenger molecules. H2O2 is more stable compared with O2·− and can also diffuse through membranes; therefore, it can travel much farther in the cytosol and between cells.
Mitochondrial Excitability Induced by mPTP-Mediated Mitochondrial Ca2+-Induced Ca2+ Release
Mitochondrial excitability through Ca2+-induced Ca2+ release (mCICR), propagated by mPTP opening, has been proposed by Ichas et al. (45). In their scheme, transitory opening of mPTP in a reversible low-conductance mode can dissipate the transmembrane potential, thus releasing any stored Ca2+ in the matrix. This Ca2+ would then be taken up by neighboring mitochondria, triggering mPTP and propagating the signal (31, 45). To simulate the experimentally observed mCICR, Selivanov et al. (82) developed a kinetic model of mitochondria that composes of Ca2+ uniporter, pH-gated mPTP, and respiratory chain. This simplified model successfully simulates all mCICR profiles observed experimentally, as well as the notable triggering characteristics of mCICR (82). To better understand the role of mPTP in the propagation of waves of depolarization and Ca2+ release, Oster et al. (70) modified the Magnus-Keizer model (55–57) by incorporating the PTP (75, 82) and mitochondrial pH buffering systems. In their model, the low conductance mPTP opening (PTPl) is assumed to be initialized by pH changes (Hm).
| (3) |
where τl is the time constant and PTPl∞ is the opening rate. To simulate the traveling of depolarization and Ca2+ waved observed by Ichas et al. (45), mitochondria were assumed to be spatially coupled through the cytosolic Ca2+ diffusion described by Fick's law. The cytoplasmic (extramitochondrial) Ca2+ dynamics (Cac) were modeled as
| (4) |
where DCa is the cytosolic Ca2+ diffusion coefficient and J represents individual Ca2+ fluxes (via Na+/Ca2+ exchanger, uniporter, PTP, and stimulus, respectively). Their model, with the inclusion of mPTP, successfully simulated the excitable behavior of mitochondria demonstrated by Ichas et al. (45), i.e., the traveling of Ca2+ and depolarization waves across the length of the domain. Interestingly, they showed that mCICR-mediated mitochondrial excitability does not require Ca2+ efflux through the permeability transition pore, since Ca2+ can flow out of mitochondria via the uniporter (operating in reverse) (39, 58, 61) and the slower mitochondrial Na+/Ca2+ exchanger. In addition, model simulations demonstrated that the pH buffering capability in the mitochondrial matrix is important in shaping the resulting dynamics, and the cyclical variations in the mitochondrial pH can lead to PTP oscillations. This is not surprising since in their model PTP is explicitly gated by proton gradient. Moreover, they proposed a heuristic model for how prolonged Ca2+ overload can induce nonreversible mPTP openings.
It is worth pointing out that the experiments of Ichas et al. (45) were done on isolated mitochondria suspended in gels that lack extramitochondrial Ca2+ buffering or nearby rapid Ca2+ sequestering pathways such as sarco(endo)plasmic reticulum uptake or plasmalemmal Ca2+ transporters and involve Ca2+ pulses well above the physiological range (>60 μM). Thus there is still little experimental evidence to indicate that mCICR-mediated mitochondrial excitability occurs in intact cells or in vivo. In fact, most studies of RIRR-mediated propagation have shown that Ca2+ is not required for ΔΨm depolarization to be propagated (7, 103), and there is evidence that enhanced Ca2+ release from neighboring SR stores (Ca2+ sparks) is a consequence, rather than a cause, of ΔΨm depolarization in the resting, nonbeating cardiomyocytes. Nevertheless, since oxidative stress sensitizes the mPTP to Ca2+ activation as mentioned above, it is possible that there is an interaction between RIRR and mCICR such that the propagation of ΔΨm depolarization has a mixed mechanism when intracellular Ca2+ overload occurs. Although cross talk between Ca2+ and ROS signaling pathways has been discussed intensively (30, 37, 95), more work is required to gain a complete understanding of the interactions between intracellular Ca2+ handling and mitochondria.
Mitochondrial Network Excitability and Cardiac Pathology
These network model simulations, in combination with experimental data, suggest that the mitochondrial network can serve as an intracellular signal amplifier by scaling up local processes to produce emergent macroscopic behaviors such as sustained oscillations, damped oscillations, bistability, or waves. Signaling molecules such as ROS, nitric oxide, Ca2+, and ADP play a prominent role in modulating the network properties, and these factors are known to change significantly in pathological situations. Ischemia-reperfusion injury, for example, is known to involve increased ROS accumulation, especially during the restoration of coronary flow and the concomitant increase in mitochondrial respiration. The increase in oxidative stress during and after ischemia can lead to rapid loss of ΔΨm in the whole heart (54, 84), along with changes in the cytoplasmic phosphorylation potential (100). Changes in the intracellular redox environment will trigger redox cascades that alter the signal transduction pathways, ion transport processes, and Ca2+ handling systems of the cell (1, 93). A well-known effect of a decrease in ATP-to-ADP ratio is the activation of sarcolemmal ATP-sensitive K+ (KATP) channels, causing shortening of action potential duration (Fig. 6) (2, 63, 67, 100), and this will profoundly affect the electrical properties of the myocardial syncytium. Oxidative stress also influences cytosolic Ca2+ transients, as many enzymes involved in Ca2+ handling pathways are sensitive to intracellular redox state and energy state, such as the ryanodine receptors and the SERCA pump (102). During whole cell metabolic oscillations, our group has previously shown that Ca2+ transients are suppressed during the phase of NADH oxidation (67). On the other hand, inhibition of sarcoplasmic reticulum Ca2+ uptake by oxidative stress has been shown to induce larger cytoplasmic Ca2+ transients in neurons (73). The implication is that the collapse of ΔΨm in a small number of depolarized mitochondria can scale to the level of the mitochondrial network and then to the whole heart because of the multiplicative interdependency of the bioenergetic, electrophysiological, and Ca2+ handling subsystems. This has been demonstrated in several experimental studies in which ventricular arrhythmias were induced by ischemia-reperfusion (2, 25), ROS exposure (42, 50, 59), or depletion of intracellular reduced glutathione (26). Treatment with ROS scavengers or mitochondrially targeted antioxidant peptides, or blocking IMAC during the metabolic stress (2, 25, 26), can significantly decrease the incidence of cardiac arrhythmias.
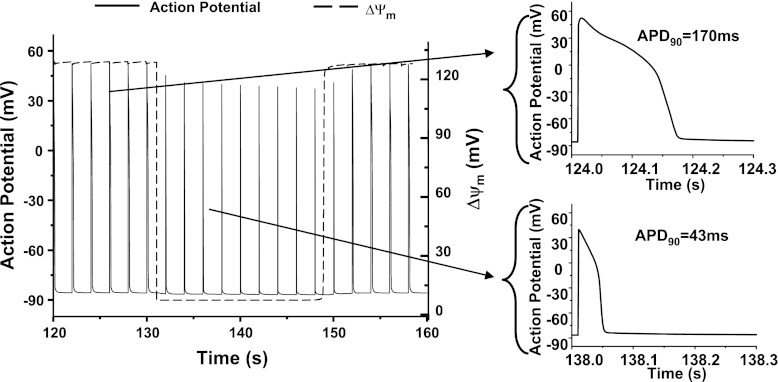
Fig. 6.
Model simulated shortening of action potential during mitochondrial depolarization. When mitochondria are polarized, the action potential duration (APD) is 170 ms, whereas when mitochondria depolarize, the APD decreased to 43 ms, representing about 75% shortening. Reproduced from (100).
Understanding the role of the cardiac mitochondrial network in intracellular signaling under both physiological and pathological conditions requires a multiscale (77) and ultimately a multicellular modeling approach. This will be extremely important in developing a detailed mechanistic knowledge of how the failure of the energy generation, combined with redox-dependent modulation of ion transporters and channels, contributes to arrhythmias. To that end, expanding on our integrated cardiac cell models of RIRR (100), we have recently developed two-dimensional models of the cardiac syncytium to study the impact of ΔΨm loss on electrical propagation (101). Preliminary results show that mitochondrial depolarization in a region of a cardiac cell monolayer through the activation of sarcolemmal KATP currents, results in shortening of the action potential, dispersion of repolarization, and increased vulnerability to reentry, providing further motivation to explore novel therapeutic strategies based on manipulation of mitochondrial inner membrane ion fluxes.
Summary
Mitochondria form dynamic networks, both morphologically and functionally, to generate energy, as well as to transmit critical intracellular signals. To investigate how mitochondria communicate with each other to initiate and propagate these signals, an increasing number of experimental and computational studies have been carried out over the past decades. Whereas more studies need to be done to understand the precise mechanism(s) behind propagated mitochondrial ΔΨm and redox waves, these studies clearly demonstrate that mitochondrial network excitability can be mediated by various messenger molecules. Our studies have focused on a mechanism of RIRR mediated by IMAC, which, in the regular lattice-like arrangement of mitochondria in the cardiomyocyte can support wave propagation, KATP channel current activation and oscillation through local O2·−-mediated coupling. Alternatively, H2O2 may transmit mitochondrial ROS effects over longer distances. In addition, mitochondrial excitability can also be evoked by mCICR and the mPTP under certain circumstances. Despite differences in the type of messenger molecule and targets implicated in different studies, the common finding is that the local diffusion of messenger molecules, in the range of few micrometers (mitochondrion size) can elicit a much longer range (∼100 μm, cellular size) synchronized behavior. In addition, the velocity of the wave and the rate of depolarization do not decline with distance from the site of wave origin, supporting a regenerative model that involves diffusive signaling between neighboring mitochondria. The results of model simulations also reinforce the concept that intermitochondrial communication represents an effective means to ensure progressive spreading of signals throughout the network.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.Z. and B.O. conception and design of research; L.Z. prepared figures; L.Z. drafted manuscript; L.Z. and B.O. edited and revised manuscript; L.Z. and B.O. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants R01-HL-105216 and R37-HL-54598 (to B. O'Rourke) and R00-HL-95648 (to L. Zhou).
REFERENCES
- 1. Abate C, Patel L, Rauscher FJ, 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science 249: 1157–1161, 1990 [DOI] [PubMed] [Google Scholar]
- 2. Akar FG, Aon MA, Tomaselli GF, O'Rourke B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest 115: 3527–3535, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Altmann R. Die Elementarorganismen und ihre Beziehungen zu den Zellen. Leipzig: Verlag yon Veit &Comp, 1890 [Google Scholar]
- 4. Amchenkova AA, Bakeeva LE, Chentsov YS, Skulachev VP, Zorov DB. Coupling membranes as energy-transmitting cables. I. Filamentous mitochondria in fibroblasts and mitochondrial clusters in cardiomyocytes. J Cell Biol 107: 481–495, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aon MA, Cortassa S, Akar FG, Brown DA, Zhou L, O'Rourke B. From mitochondrial dynamics to arrhythmias. Int J Biochem Cell Biol 41: 1940–1948, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aon MA, Cortassa S, Maack C, O'Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem 282: 21889–21900, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aon MA, Cortassa S, Marban E, O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem 278: 44735–44744, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Aon MA, Cortassa S, O'Rourke B. The fundamental organization of cardiac mitochondria as a network of coupled oscillators. Biophys J 91: 4317–4327, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aon MA, Cortassa S, O'Rourke B. Mitochondrial oscillations in physiology and pathophysiology. Adv Exp Med Biol 641: 98–117, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aon MA, Cortassa S, O'Rourke B. On the network properties of mitochondria. In: Molecular System Bioenergetics, edited by Saks Valdur. Darmstadt, Wiley-VCH, 2007, p. 111–135 [Google Scholar]
- 11. Aon MA, Cortassa S, O'Rourke B. Percolation and criticality in a mitochondrial network. Proc Natl Acad Sci USA 101: 4447–4452, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aon MC, S, O'Rourke B. Energy for life. In: Molecular System Bioenergetics. Darmstadt: Wiley-VCH, 2007 [Google Scholar]
- 13. Babcock DF, Herrington J, Goodwin PC, Park YB, Hille B. Mitochondrial participation in the intracellular Ca2+ network. J Cell Biol 136: 833–844, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Babcock DF, Hille B. Mitochondrial oversight of cellular Ca2+ signaling. Curr Opin Neurobiol 8: 398–404, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Baines CP, Goto M, Downey JM. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Mol Cell Cardiol 29: 207–216, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Balaban RS, Bose S, French SA, Territo PR. Role of calcium in metabolic signaling between cardiac sarcoplasmic reticulum and mitochondria in vitro. Am J Physiol Cell Physiol 284: C285–C293, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Beavis AD. Properties of the inner membrane anion channel in intact mitochondria. J Bioenerg Biomembr 24: 77–90, 1992 [DOI] [PubMed] [Google Scholar]
- 18. Beavis AD, Garlid KD. The mitochondrial inner membrane anion channel. Regulation by divalent cations and protons. J Biol Chem 262: 15085–15093, 1987 [PubMed] [Google Scholar]
- 19. Beavis AD, Powers M. Temperature dependence of the mitochondrial inner membrane anion channel: the relationship between temperature and inhibition by magnesium. J Biol Chem 279: 4045–4050, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Beavis AD, Powers MF. On the regulation of the mitochondrial inner membrane anion channel by magnesium and protons. J Biol Chem 264: 17148–17155, 1989 [PubMed] [Google Scholar]
- 21. Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70: 23–49, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Borecky J, Jezek P, Siemen D. 108-pS channel in brown fat mitochondria might be identical to the inner membrane anion channel. J Biol Chem 272: 19282–19289, 1997 [PubMed] [Google Scholar]
- 23. Brady NR, Elmore SP, van Beek JJ, Krab K, Courtoy PJ, Hue L, Westerhoff HV. Coordinated behavior of mitochondria in both space and time: a reactive oxygen species-activated wave of mitochondrial depolarization. Biophys J 87: 2022–2034, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brady NR, Hamacher-Brady A, Westerhoff HV, Gottlieb RA. A wave of reactive oxygen species (ROS)-induced ROS release in a sea of excitable mitochondria. Antioxid Redox Signal 8: 1651–1665, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Brown D, Aon MA, Akar FG, Liu T, Sorarrain N, O'Rourke B. Effect of 4'-chlorodiazepam on cellular excitation-contraction coupling and ischemia-reperfusion injury in rabbit heart. Cardiovasc Res 79: 141–149, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brown DA, Aon MA, Frasier CR, Sloan RC, Maloney AH, Anderson EJ, O'Rourke B. Cardiac arrhythmias induced by glutathione oxidation can be inhibited by preventing mitochondrial depolarization. J Mol Cell Cardiol 48: 673–679, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cadenas E. Mitochondrial free radical production and cell signaling. Mol Aspects Med 25: 17–26, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev 59: 527–605, 1979 [DOI] [PubMed] [Google Scholar]
- 29. Cortassa S, Aon MA, Winslow RL, O'Rourke B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys J 87: 2060–2073, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Csordás G, Hajnóczky G. SR/ER-mitochondrial local communication: calcium and ROS. Biochim Biophys Acta 1787: 1352–1362, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Giorgi F, Lartigue L, Ichas F. Electrical coupling and plasticity of the mitochondrial network. Cell Calcium 28: 365–370, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Denton RM, McCormack JG. Ca2+ as a 2nd messenger within mitochondria of the heart and other tissue. Annu Rev Physiol 52: 451–466, 1990 [DOI] [PubMed] [Google Scholar]
- 33. Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol 10: 369–377, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Droge W. Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Duchen MR. Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. J Physiol 516: 1–17, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duchen MR, Leyssens A, Crompton M. Transient mitochondrial depolarizations reflect focal sarcoplasmic reticular calcium release in single rat cardiomyocytes. J Cell Biol 142: 975–988, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Feissner RF, Skalska J, Gaum WE, Sheu SS. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front Biosci 14: 1197–1218, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem 64: 97–112, 1995 [DOI] [PubMed] [Google Scholar]
- 39. Gunter KK, Gunter TE. Transport of calcium by mitochondria. J Bioenerg Biomembr 26: 471–485, 1994 [DOI] [PubMed] [Google Scholar]
- 40. Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol Cell Physiol 258: C755–C786, 1990 [DOI] [PubMed] [Google Scholar]
- 41. Harman D. Role of free radicals in aging and disease. Ann NY Acad Sci 673: 126–141, 1992 [DOI] [PubMed] [Google Scholar]
- 42. Hearse DJ, Kusama Y, Bernier M. Rapid electrophysiological changes leading to arrhythmias in the aerobic rat heart. Photosensitization studies with rose bengal-derived reactive oxygen intermediates. Circ Res 65: 146–153, 1989 [DOI] [PubMed] [Google Scholar]
- 43. Herrington J, Park YB, Babcock DF, Hille B. Dominant role of mitochondria in clearance of large Ca2+ loads from rat adrenal chromaffin cells. Neuron 16: 219–228, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Hool LC, Corry B. Redox control of calcium channels: from mechanisms to therapeutic opportunities. Antioxid Redox Signal 9: 409–435, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell 89: 1145–1153, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest 113: 1535–1549, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Krinsky V, Swinney editors H. Wave and Patterns in Biological and Chemical Excitable Media. Amsterdam: North-Holland, 1991 [Google Scholar]
- 48. Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med 6: 513–519, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Kurz FT, Aon MA, O'Rourke B, Armoundas AA. Spatio-temporal oscillations of individual mitochondria in cardiac myocytes reveal modulation of synchronized mitochondrial clusters. Proc Natl Acad Sci USA 107: 14315–14320, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kusama Y, Bernier M, Hearse DJ. Singlet oxygen-induced arrhythmias. Dose- and light-response studies for photoactivation of rose bengal in the rat heart. Circulation 80: 1432–1448, 1989 [DOI] [PubMed] [Google Scholar]
- 51. Kuster GM, Lancel S, Zhang J, Communal C, Trucillo MP, Lim CC, Pfister O, Weinberg EO, Cohen RA, Liao R, Siwik DA, Colucci WS. Redox-mediated reciprocal regulation of SERCA and Na+-Ca2+ exchanger contributes to sarcoplasmic reticulum Ca2+ depletion in cardiac myocytes. Free Radic Biol Med 48: 1182–1187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kuznetsov AV, Hermann M, Saks V, Hengster P, Margreiter R. The cell-type specificity of mitochondrial dynamics. Int J Biochem Cell Biol 41: 1928–1939, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Loew LM, Carrington W, Tuft RA, Fay FS. Physiological cytosolic Ca2+ transients evoke concurrent mitochondrial depolarizations. Proc Natl Acad Sci USA 91: 12579–12583, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lyon AR, Joudrey PJ, Jin D, Nass RD, Aon MA, O'Rourke B, Akar FG. Optical imaging of mitochondrial function uncovers actively propagating waves of mitochondrial membrane potential collapse across intact heart. J Mol Cell Cardiol 49: 565–575, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Magnus G, Keizer J. Minimal model of β-cell mitochondrial Ca2+ handling. Am J Physiol Cell Physiol 273: C717–C733, 1997 [DOI] [PubMed] [Google Scholar]
- 56. Magnus G, Keizer J. Model of β-cell mitochondrial calcium handling and electrical activity. I. Cytoplasmic variables. Am J Physiol Cell Physiol 274: C1158–C1173, 1998 [DOI] [PubMed] [Google Scholar]
- 57. Magnus G, Keizer J. Model of β-cell mitochondrial calcium handling and electrical activity. II. Mitochondrial variables. Am J Physiol Cell Physiol 274: C1174–C1184, 1998 [DOI] [PubMed] [Google Scholar]
- 58. Montero M, Alonso MT, Albillos A, Garcia-Sancho J, Alvarez J. Mitochondrial Ca2+-induced Ca2+ release mediated by the Ca2+ uniporter. Mol Biol Cell 12: 63–71, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Morita M, Akai S, Hosomi H, Tsuneyama K, Nakajima M, Yokoi T. Drug-induced hepatotoxicity test using gamma-glutamylcysteine synthetase knockdown rat. Toxicol Lett 189: 159–165, 2009 [DOI] [PubMed] [Google Scholar]
- 60. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nicholls D, Akerman K. Mitochondrial calcium transport. Biochim Biophys Acta 683: 57–88, 1982 [DOI] [PubMed] [Google Scholar]
- 62. Nicholls D, Ferguson S. Bioenergetics 3. Biochemistry (Moscow) 818–819, 2002 [Google Scholar]
- 63. Nichols CG, Lederer WJ. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol Heart Circ Physiol 261: H1675–H1686, 1991 [DOI] [PubMed] [Google Scholar]
- 64. Nivala M, Korge P, Weiss JN, Qu Z. Linking flickering to waves and whole-cell oscillations in a mitochondrial network model. Biophys J 101: 2102–2111, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. O'Rourke B. Pathophysiological and protective roles of mitochondrial ion channels. J Physiol 529: 23–36, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. O'Rourke B, Cortassa S, Akar F, Aon M. Mitochondrial ion channels in cardiac function and dysfunction. Novartis Found Symp 287: 140–151, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. O'Rourke B, Ramza BM, Marban E. Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science 265: 962–966, 1994 [DOI] [PubMed] [Google Scholar]
- 68. Okado-Matsumoto A, Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem 276: 38388–38393, 2001 [DOI] [PubMed] [Google Scholar]
- 69. Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol 47: 143–183, 2007 [DOI] [PubMed] [Google Scholar]
- 70. Oster AM, Thomas B, Terman D, Fall CP. The low conductance mitochondrial permeability transition pore confers excitability and CICR wave propagation in a computational model. J Theor Biol 273: 216–231, 2011 [DOI] [PubMed] [Google Scholar]
- 71. Pacher P, Hajnoczky G. Propagation of the apoptotic signal by mitochondrial waves. EMBO J 20: 4107–4121, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Park J, Lee J, Choi C. Mitochondrial network determines intracellular ROS dynamics and sensitivity to oxidative stress through switching inter-mitochondrial messengers. PLoS One 6: e23211, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Paschen W, Mengesdorf T. Endoplasmic reticulum stress response and neurodegeneration. Cell Calcium 38: 409–415, 2005 [DOI] [PubMed] [Google Scholar]
- 74. Perreault CL, Meuse AJ, Bentivegna LA, Morgan JP. Abnormal intracellular calcium handling in acute and chronic heart failure: role in systolic and diastolic dysfunction. Eur Heart J 11: 8–21, 1990 [DOI] [PubMed] [Google Scholar]
- 75. Pokhilko AV, Ataullakhanov FI, Holmuhamedov EL. Mathematical model of mitochondrial ionic homeostasis: three modes of Ca2+ transport. J Theor Biol 243: 152–169, 2006 [DOI] [PubMed] [Google Scholar]
- 76. Pouvreau S. Superoxide flashes in mouse skeletal muscle are produced by discrete arrays of active mitochondria operating coherently. PLoS One 5: 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Qu Z, Garfinkel A, Weiss JN, Nivala M. Multi-scale modeling in biology: how to bridge the gaps between scales? Prog Biophys Mol Biol 107: 21–31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rasola A, Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis 12: 815–833, 2007 [DOI] [PubMed] [Google Scholar]
- 79. Romashko DN, Marban E, O'Rourke B. Subcellular metabolic transients and mitochondrial redox waves in heart cells. Proc Natl Acad Sci USA 95: 1618–1623, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Schimper AF. Untersuchungen fiber die Chlorophyllk6rper und die ihnen homologen Gebilde. Jb wiss Botanik 247, 1885 [Google Scholar]
- 81. Scoote M, Williams AJ. Myocardial calcium signalling and arrhythmia pathogenesis. Biochem Biophys Res Commun 322: 1286–1309, 2004 [DOI] [PubMed] [Google Scholar]
- 82. Selivanov VA, Ichas F, Holmuhamedov EL, Jouaville LS, Evtodienko YV, Mazat JP. A model of mitochondrial Ca2+-induced Ca2+ release simulating the Ca2+ oscillations and spikes generated by mitochondria. Biophys Chem 72: 111–121, 1998 [DOI] [PubMed] [Google Scholar]
- 83. Shanmuganathan S, Hausenloy DJ, Duchen MR, Yellon DM. Mitochondrial permeability transition pore as a target for cardioprotection in the human heart. Am J Physiol Heart Circ Physiol 289: H237–H242, 2005 [DOI] [PubMed] [Google Scholar]
- 84. Slodzinski M, Aon M, O'Rourke B. Glutathione oxidation as a trigger of mitochondrial depolarization and oscillation in intact hearts. J Mol Cell Cardiol 45: 11, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270: 296–299, 1995 [DOI] [PubMed] [Google Scholar]
- 86. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44–84, 2007 [DOI] [PubMed] [Google Scholar]
- 87. Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem 273: 18092–18098, 1998 [DOI] [PubMed] [Google Scholar]
- 88. Vendelin M, Beraud N, Guerrero K, Andrienko T, Kuznetsov AV, Olivares J, Kay L, Saks VA. Mitochondrial regular arrangement in muscle cells: a “crystal-like” pattern. Am J Physiol Cell Physiol 288: C757–C767, 2005 [DOI] [PubMed] [Google Scholar]
- 89. Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39: 359–407, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang SB, Foster DB, Rucker J, O'Rourke B, Kass DA, Van Eyk JE. Redox regulation of mitochondrial ATP synthase/novelty and significance. Circ Res 109: 750–757, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Weiss JN, Yang L, Qu Z. Systems biology approaches to metabolic and cardiovascular disorders: network perspectives of cardiovascular metabolism. J Lipid Res 47: 2355–2366, 2006 [DOI] [PubMed] [Google Scholar]
- 92. Wiesner RJ, Zsurka G, Kunz WS. Mitochondrial DNA damage and the aging process: facts and imaginations. Free Radic Res 40: 1284–1294, 2006 [DOI] [PubMed] [Google Scholar]
- 93. Xia R, Stangler T, Abramson JJ. Skeletal muscle ryanodine receptor is a redox sensor with a well defined redox potential that is sensitive to channel modulators. J Biol Chem 275: 36556–36561, 2000 [DOI] [PubMed] [Google Scholar]
- 94. Yan Y, Liu J, Wei C, Li K, Xie W, Wang Y, Cheng H. Bidirectional regulation of Ca2+ sparks by mitochondria-derived reactive oxygen species in cardiac myocytes. Cardiovasc Res 77: 432–441, 2008 [DOI] [PubMed] [Google Scholar]
- 95. Yan Y, Wei CL, Zhang WR, Cheng HP, Liu J. Cross-talk between calcium and reactive oxygen species signaling. Acta Pharmacol Sin 27: 821–826, 2006 [DOI] [PubMed] [Google Scholar]
- 96. Yang L, Korge P, Weiss JN, Qu Z. Mitochondrial oscillations and waves in cardiac myocytes: insights from computational models. Biophys J 98: 1428–1438, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yi S. Free radicals, antioxidant enzymes, carcinogenesis. Free Radic Biol Med 8: 583–599, 1990 [DOI] [PubMed] [Google Scholar]
- 98. Zhou L, Aon MA, Almas T, Cortassa S, Winslow RL, O'Rourke B. A reaction-diffusion model of ROS-induced ROS release in a mitochondrial network. PLoS Comput Biol 6: e1000657, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhou L, Aon MA, Liu T, O'Rourke B. Dynamic modulation of Ca2+ sparks by mitochondrial oscillations in isolated guinea pig cardiomyocytes under oxidative stress. J Mol Cell Cardiol 51: 632–639, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhou L, Cortassa S, Wei AC, Aon MA, Winslow RL, O'Rourke B. Modeling cardiac action potential shortening driven by oxidative stress-induced mitochondrial oscillations in guinea pig cardiomyocytes. Biophys J 97: 1843–1852, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhou L, Plank G, Cortassa S, Greenstein JL, Winslow RL, Trayanova N, O'Rourke B. Effects of mitochondrial depolarization on electrical propagation in an integrated multiscale model of the myocardium. In: 29th Annual Scientific Sessions. San Francisco, CA: Heart Rhythm Society, 2008, p. S109 [Google Scholar]
- 102. Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res 71: 310–321, 2006 [DOI] [PubMed] [Google Scholar]
- 103. Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 192: 1001–1014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta 1757: 509–517, 2006 [DOI] [PubMed] [Google Scholar]