Abstract
The endothelial nitric oxide synthase (eNOS) requires tetrahydrobiopterin (H4B) as a cofactor and, in its absence, produces superoxide (O2·−) rather than nitric oxide (NO·), a condition referred to as eNOS uncoupling. DOCA-salt-induced hypertension is associated with H4B oxidation and uncoupling of eNOS. The present study investigated whether administration of sepiapterin or H4B recouples eNOS in DOCA-salt hypertension. Bioavailable NO· detected by electron spin resonance was markedly reduced in aortas of DOCA-salt hypertensive mice. Preincubation with sepiapterin (10 μmol/l for 30 min) failed to improve NO· bioavailability in hypertensive aortas while it augmented NO· production from control vessels, implicating a hypertension-associated deficiency in sepiapterin reductase (SPR), the rate-limiting enzyme for sepiapterin conversion to H4B. Indeed, a decreased SPR expression was observed in aortic endothelial cells, but not in endothelium-denuded aortic remains, implicating an endothelium-specific SPR deficiency. Administration of hypertensive aortas with H4B (10 μmol/l, 30 min) partially restored vascular NO· production. Combined administration of H4B and the NADPH oxidase inhibitor apocynin (100 μmol/l, 30 min) fully restored NO· bioavailability while reducing O2·− production. In angiotensin II-induced hypertension, however, aortic endothelial SPR expression was not affected. In summary, administration of sepiapterin is not effective in recoupling eNOS in DOCA-salt hypertension, due to an endothelium-specific loss in SPR, whereas coadministration of H4B and apocynin is highly efficient in recoupling eNOS. This is consistent with our previous observations that in angiotensin II hypertension, endothelial deficiency in dihydrofolate reductase is alternatively responsible for uncoupling of eNOS. Taken together, these data indicate that strategies specifically targeting at different H4B metabolic enzymes might be necessary in restoring eNOS function in different types of hypertension.
Keywords: deoxycorticosterone acetate, endothelial nitric oxide synthase uncoupling, tetrahydrobiopterin, sepiapterin reductase, superoxide, nitric oxide, vascular reduced nicotinamide adenine dinucleotide phosphatase
it has become clear during the past decade that oxidative stress plays an important role in the pathogenesis of hypertension and atherosclerosis (5, 6, 14). Vascular production of reactive oxygen species (ROS) has been predominantly attributed to vascular NADPH oxidases (NOXs) and its downstream effectors such as xanthine oxidase and uncoupled endothelial nitric oxide synthase (eNOS). A large body of evidence has demonstrated a clear intermediate role of oxidation-induced deficiency in eNOS cofactor tetrahydrobiopterin (H4B) in eNOS uncoupling (2, 3, 7, 11, 15–18, 20, 22, 27, 30, 31).
It was shown previously that eNOS uncoupling occurs in DOCA-salt-induced hypertension, consequent to vascular NOX-dependent loss of H4B (16). In this earlier study, vascular production of ROS was found to be increased in hypertensive aortas (16). The content of oxidized biopterin was markedly increased in hypertensive vessels, whereas H4B levels were significantly reduced (16). Oral H4B partially improved endothelium-dependent relaxation and nitrosyl hemoglobin levels in blood (16). These data suggest that H4B administration may be beneficial in the treatment of hypertension by recoupling of eNOS. We recently showed that supplementation of the stable H4B precursor sepiapterin to endothelial cells improved H4B and NO· bioavailabilities, which were specifically attenuated by sepiapterin reductase (SPR) RNA interference (12). Existing literature have implicated a controversial role of sepiapterin in restoring eNOS function, likely consequent to lack of analyses of SPR in various in vitro and in vivo systems. In the present study we aimed to examine whether sepiapterin can serve as a good agent to use to recouple eNOS in vivo in DOCA-salt-induced hypertension.
It turned out sepiapterin was not effective in recoupling eNOS in DOCA-salt hypertension, consequent to an endothelial SPR deficiency. Together with the previously documented contradictory effects of sepiapterin in modulating endothelium-dependent vasorelaxation in different models of vascular diseases (19, 25, 28, 32), our data seem to suggest that it is essential to determine the functionality of SPR before sepiapterin is used as a H4B precursor. In contrast, H4B supplementation on its own was partially effective in recoupling of eNOS. Coadministration of H4B and apocynin, the NOX inhibitor, normalized NO· production to control level, whereas it completely attenuated O2·− production in hypertensive aortas. This is consistent with our previous observations that in hypertension associated with elevated levels of ANG II, an endothelial deficiency dihydrofolate reductase (DHFR) alternatively mediates eNOS uncoupling (7, 11, 13, 20). Indeed, in ANG II-infused mice, aortic endothelial expression of SPR was unaffected. Taken together, these data indicate that strategies specifically targeting at different H4B metabolic enzymes might be necessary in restoring eNOS function in different types of hypertension.
MATERIALS AND METHODS
Materials and DOCA-salt hypertension.
Sepiapterin and apocynin were purchased from Sigma-Aldrich (St. Louis, MO). H4B was obtained from Schricks Laboratories, Switzerland. The monoclonal eNOS antibody was purchased from BD Transduction Laboratories (San Diego, CA). Male C57BL/6J mice (25–35 g; Jackson Laboratories, Bar Harbor, MN) were anesthetized with intraperitoneal ketamine (80 mg/kg; Abbott Laboratories, Chicago, IL) and xylazine (10 mg/kg; Bayer, Shawnee Mission, KS). With the use of sterile techniques, the left kidney was removed through a left-flank incision. A slow-release DOCA pellet (50 mg) was inserted subcutaneously through a midscapular incision. Drinking water was replaced by 1% saline. Control animals underwent a sham operation, and a placebo pellet was implanted subcutaneously. Water was given ad libitum. Analyses were performed 21 days after operation in mice. Blood pressure was measured using the tail-cuff method (16). The use of animals and experimental procedure were approved by the Institutional Animal Care and Usage Committee at the University of California Los Angeles and Vanderbilt University.
Acute administration of sepiapterin, H4B, and apocynin.
Acute effects of sepiapterin, H4B, apocynin, or combination of H4B and apocynin were examined by preincubating freshly isolated aortic sections with sepiapterin (10 μmol/l), H4B (10 μmol/l), or apocynin (100 μmol/l) for 30 min before analyses of NO· and O2·− productions by electron spin resonance (ESR) and HPLC, respectively. In the case of cotreatment with H4B and apocynin, the blood vessels were pretreated with apocynin (100 μmol/l) for 30 min before addition of H4B (10 μmol/l, 30 min) and consequent analyses of NO· and O2·− productions.
Chronic administration of sepiapterin.
Chronically, sepiapterin (0.5 mg·kg−1·day−1, 10 days) was used to inject DOCA-hypertensive mice via tail vein or delivered subcutaneously using an osmotic mini-pump, starting day 10 postsurgery. Aortas were harvested on day 21 for analysis of SPR protein expression by Western blotting.
Electron spin resonance determination of aortic nitric oxide bioavailability.
Animals were euthanized using CO2 inhalation. The aortas were rapidly removed and placed into chilled modified Krebs/HEPES buffer (composition in mmol/l) containing 99.01 NaCl, 4.69 KCl, 2.50 CaCl2, 1.20 MgSO4, 1.03 KH2PO4, 25.0 NaHCO3, 20.0 Na-HEPES, and 5.6 glucose (pH 7.4) and cleaned of excessive adventitial tissue, with care taken not to injure the endothelium. Aortic segments (2 mm) were incubated with freshly made NO·-specific spin trap Fe2+(DETC)2 colloid (0.5 mmol/l) for 60 min and then subjected to ESR detection of NO· production (11, 13, 20). The settings of the ESR used in these experiments were as follows: Bio-field, 3,267; field sweep, 100 G; microwave frequency, 9.78 GHz; microwave power, 40 mW; modulation amplitude, 10 G; 4,096 points resolution and receiver gain, 900.
HPLC-based dihydroethidium assay of superoxide.
It has been shown that the reaction between O2·− and dihydroethidium leads to formation of a specific product, oxy-ethidium, which can be detected using HPLC (33). We used this method to estimate intracellular O2·− production. Five 2-mm aortic segments were incubated for 20 min at 37°C with Krebs-HEPES buffer containing dihydroethidium (50 μmol/l). The vessels were then washed of dihydroethidium and incubated in Krebs-HEPES buffer for an additional hour. The segments were then placed in 300 μl cold methanol, homogenized, and filtered (0.22 μm). Separation of ethidium, oxy-ethidium, and dihydroethidium was performed using a HPLC System with a C-18 reverse phase column (Nucleosil 250-4.5 mm; Sigma-Aldrich), equipped with both UV and fluorescence detectors. Fluorescence detection at 580 nm (emission) and 480 nm (excitation) was used to monitor oxy-ethidium production. UV absorption at 355 nm was used for analysis of dihydroethidium concentration. The mobile phase was composed of a gradient containing 60% acetonitrile and 0.1% trifluoroacetic acid. Dihydroethidium, ethidium, and oxy-ethidium were separated by a linear increase in acetonitrile concentration from 37% to 47% in 23 min at a flow rate of 0.5 ml/min. Extensive validation studies showed that formation of oxy-ethidium from dihydroethidium is linearly related to the amount of O2·− generated by xanthine and xanthine oxidase and that oxy-ethidium is very stable when formed intracellularly. Further oxy-ethidium formation from dihydroethidium is not mediated by other common oxidants such as peroxynitrite, hypochlorous acid, or H2O2. This protocol has been validated previously for application to intact aortic segments (10).
Western analyses of eNOS and SPR.
Freshly isolated aortas were homogenized in buffer containing protease inhibitors and 1% Triton. Forty micrograms of proteins were separated in 10% SDS-PAGE and transferred to nitrocellulous membranes (Amersham Biosciences, Piscataway, NJ). The membranes were then probed with primary antibodies against eNOS (1:1,000) and SPR (1:1,000; mouse-specific rabbit antiserum was a generous gift of Dr. Y. S. Park from Inje University, Republic of Korea) (23), respectively, and then goat anti-mouse or rabbit secondary antibodies (Bio-Rad Laboratories, Hercules, CA). The specific targets were detected by chemiluminescence using ECL Plus reagent (Amersham Biosciences). The intensity of the target bands was analyzed with National Institutes of Health image software.
To confirm specific detection of SPR by Western blotting, an antibody clearance procedure was used. In brief, endothelial cell lysates were incubated with either rabbit IgG or rabbit-derived SPR polyclonal antibody at 4°C with rotation for 2 h. Protein A/G agarose beads were then added to the mixture for an additional 1-h incubation. After agarose beads were spinned, which pulls down SPR from cell lysates by antibody specific conjugation, the supernatant was loaded into SDS-PAGE for Western blotting.
Endothelium-specific western analysis of SPR expression.
To differentially analyze SPR expression profile in endothelial cells and endothelium-null remains of aorta, freshly isolated aortas were cut into 2-mm rings and the lumen was gently rubbed using pipette tip to remove endothelial cells into an Eppendorf tube, or whole aortas were opened longitudinally and digested with collagenase (0.6 mg/ml) at 37°C for 20 minutes before gentle removal of endothelial cells with a cotton applicator. Proteins were extracted from both collected endothelial cells and endothelium-denuded aortas, and subjected to Western blotting of eNOS or SPR as described above. Absence of eNOS was used as an index of successful removal of endothelium from aortic samples.
ANG II infusion of mice.
Wild-type C56BL6 mice were infused with ANG II (0.7 mg·kg−1·day−1) using osmotic minipumps for 14 days. The aortas were then freshly isolated for immediate analysis of endothelium-specific regulation of SPR expression.
Statistical analysis.
Differences in blood pressure and eNOS protein expression between sham-operated and DOCA-salt hypertensive mice were analyzed with Student's t-test. NO· or O2·− production from control, hypertensive, and drug-administrated control or hypertensive blood vessels was compared with one-way ANOVA. When differences were indicated, the Dunnet's post hoc test was employed. Statistical significance was set for P < 0.05. All grouped data shown in the figures were presented as means ± SE.
RESULTS
Blood pressure response and eNOS expression in DOCA-salt hypertension.
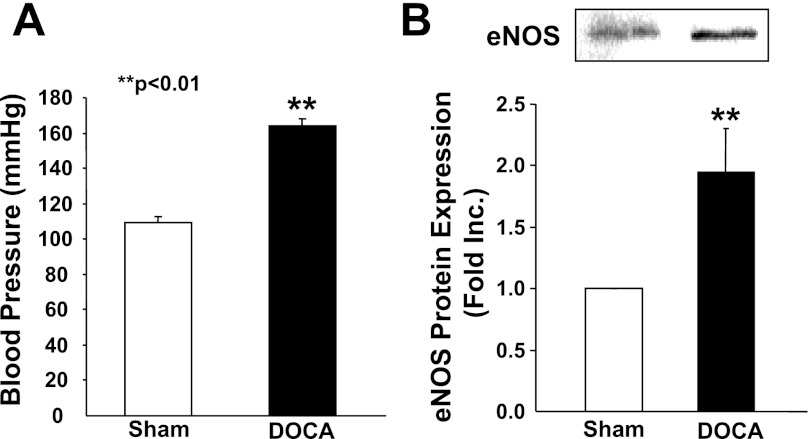
Blood pressure was increased to 163.8 ± 4.1 mmHg in hypertensive mice from 109.4 ± 3.2 mmHg in the controls (Fig. 1A). Interestingly, eNOS protein expression was significantly increased in hypertensive aortas (1.95 ± 0.35-fold) compared with that of controls (n = 6; Fig. 1B). DOCA-salt hypertension is known to have increased vascular H2O2 production. The observation that eNOS is upregulated seems consistent with previous findings that ROS, in particular H2O2, upregulate eNOS expression (4, 8). Any reduction in NO· bioavailability in these hypertensive blood vessels, therefore, is not attributed to reduced eNOS expression. Instead, because eNOS is uncoupled in DOCA-salt hypertension, this upregulation in eNOS could be detrimental.
Fig. 1.
Blood pressure response and endothelial nitric oxide synthase (eNOS) expression in DOCA-salt hypertensive mice. A: blood pressure of sham-operated control or DOCA-salt hypertensive mice was measured using a tail-cuff method. B: upregulation of eNOS protein expression in DOCA-salt hypertensive mice. Proteins were extracted from sham-operated control of DOCA-salt hypertensive aortas using a Tris-based homogenization buffer containing 1% Triton and protease inhibitors. Cellular proteins (20 μg) were separated with SDS-PAGE. eNOS protein expression was determined using a specific monoclonal antibody raised against human eNOS (BD Transduction Laboratory). Top: representative Western blot. Bottom: grouped densitometric data from 6 separate experiments. **P < 0.01 vs. sham.
Effects of sepiapterin on eNOS recoupling in DOCA-salt hypertension.
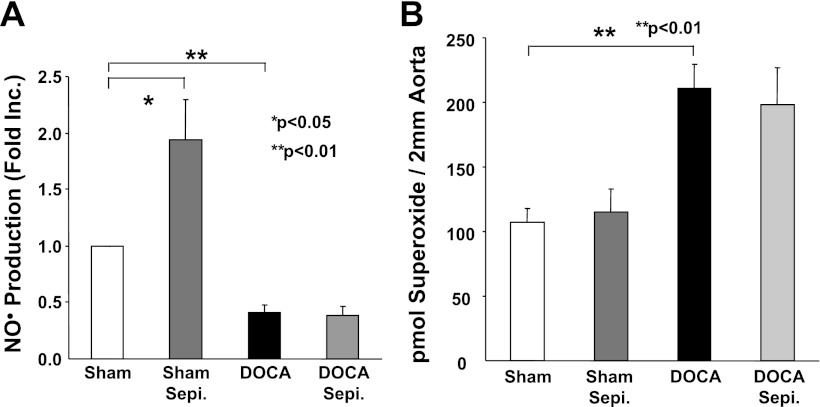
Sepiapterin is a synthetic precursor for H4B. It has been commonly used for cell and animal studies because of its stability and cell permeability (19, 25, 28, 32). To examine whether sepiapterin supplementation recouples eNOS in hypertensive aortas, freshly isolated aortic segments were incubated with sepiapterin (10 μmol/l) for 30 min before ESR measurement of NO·. Surprisingly, sepiapterin had no effect on NO· production in hypertensive vessels, whereas it doubled NO· production in the controls (Fig. 2A). Correspondingly, sepiapterin had no effect on hypertension-induced increase in vascular O2·− production (Fig. 2B). Taken together, these data indicate that sepiapterin is not effective in recoupling eNOS in DOCA-salt induced hypertension.
Fig. 2.
Sepiapterin (Sepi) is ineffective in recoupling eNOS in DOCA-salt hypertension. A: nitric oxide (NO·) production from sham-operated control and DOCA-salt hypertensive aortas in the presence of absence of sepiapterin treatment. Freshly isolated aortas (in 2-mm rings) were incubated with sepiapterin (10 μmol/l) for 30 min before NO· spin trapping with electron spin resonance (ESR). Grouped data from 5 experiments are presented (means ± SE). B: effects on vascular superoxide (O2·−) production of sepiapterin. Control and hypertensive vessels were treated with sepiapterin as described above and then subjected to a HPLC-based dihydroethidium assay of O2·−. Grouped data in picomoles O2·− per 2 mm aorta piece from 5 experiments are presented (means ± SE).
Endothelial regulation of SPR in DOCA-salt hypertension.
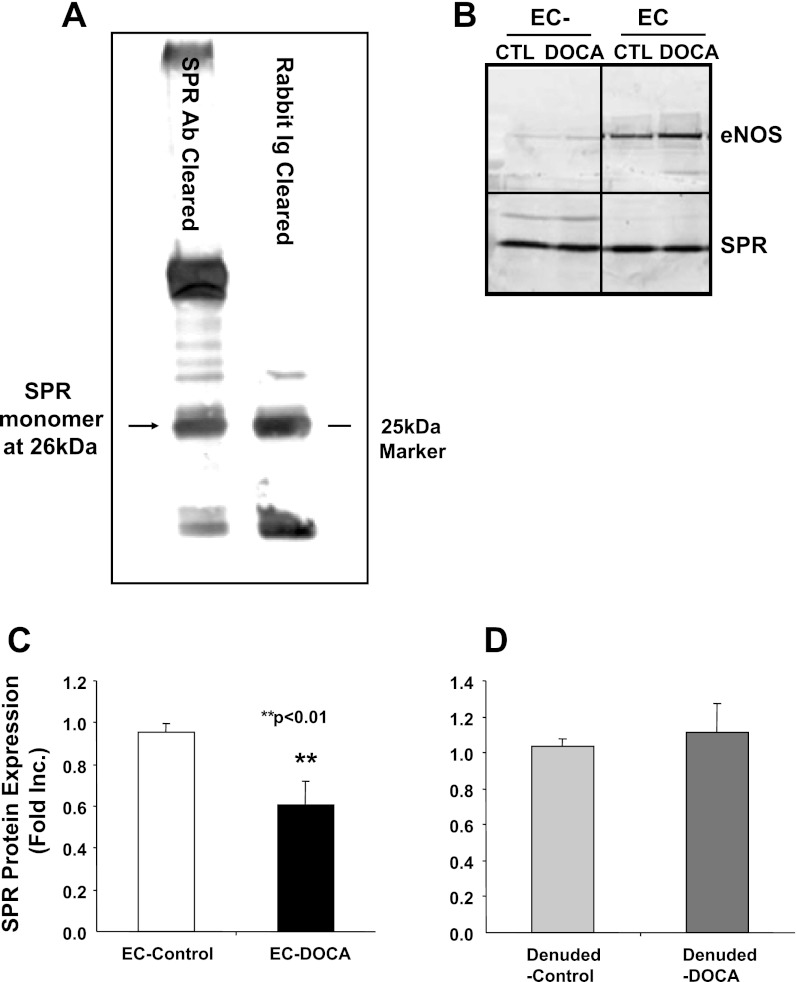
Sepiapterin is converted to dihydrobiopterin (H2B) by SPR, and the latter is then converted to H4B (24, 29). The failure of sepiapterin in restoring NO· production from hypertensive vessels seems to suggest a deficiency in SPR. We therefore examined protein expression of SPR in the control and hypertensive blood vessels using Western blotting and a polyclonal rabbit anti-mouse SPR antiserum (23). The specificity of the antiserum was examined using an antibody clearance procedure. As shown in Fig. 3A, SPR antibody clearance of endothelial cell extracts led to a reduction in a specific SPR band at around 26 kDa. We subsequently used this antibody for analysis of SPR expression from aortic endothelial cells and endothelial cell-denuded aortic remains. Interestingly, the protein abundance of SPR was apparently decreased in endothelial cells washed off from hypertensive aortas, whereas in the endothelium-denuded aortas, SPR expression was similar between control and hypertensive blood vessels (Fig. 3B for representative Western blot and Fig. 3, C and D, for grouped densitometric data). These data indicate that an endothelium-specific deficiency in SPR likely underlies the ineffectiveness of sepiapterin in recoupling eNOS.
Fig. 3.
Endothelim-specific regulation of sepiapterin reductase (SPR) expression in DOCA-salt hypertension. A: SPR antibody clearance of endothelial cell (EC) extracts leads to a reduction in a specific SPR band detected by Western blotting at around 26 kDa. B: representative Western blot. Freshly isolated aortas were homogenized in a Tris-based buffer containing 1% Triton and protease inhibitors. Equal amount of proteins from endothelium specific (right) or endothelium denuded (left) were separated by SDS-PAGE and SPR protein expression analyzed by Western blotting with a polyclonal antibody. Samples were run on the same gel, but lanes were removed for the final presentation. C: grouped data of endothelium-specific aortic expression of SPR (n = 5). D: grouped data of endothelium-denuded aortic expressions of SPR (n = 5). CTL, control.
Effect of chronic administration of sepiapterin on SPR expression in DOCA-salt hypertension.
To examine whether chronic administration of sepiapterin induces substrate mediated restoration of SPR expression to enable more efficient utilization of sepiapterin, wild-type C57BL6 mice were treated with sepiapterin (0.5 mg·kg−1·day−1, 10 days) after establishment of hypertension (10 days after kidney removal and implantation of DOCA tablet). Although sepiapterin increased SPR expression in sham-operated control aortic endothelial cells, it had no effects on endothelium-specific expression of SPR in hypertensive aortas (Fig. 4). These data imply that both acute and chronic sepiapterin administration have limited beneficial effects in recoupling eNOS in DOCA-salt hypertension.
Fig. 4.
The effect of chronic administration of sepiapterin on restoration of SPR expression. Mice were treated with sepiapterin at dose of 0.5 mg·kg−1·day−1 for 10 days after establishment of hypertension. Endothelium-specific SPR expression was determined by Western blot, and grouped data from 5 independent experiments are presented (means ± SE). NS, not significant.
Effects of H4B on eNOS recoupling in DOCA-salt hypertension.
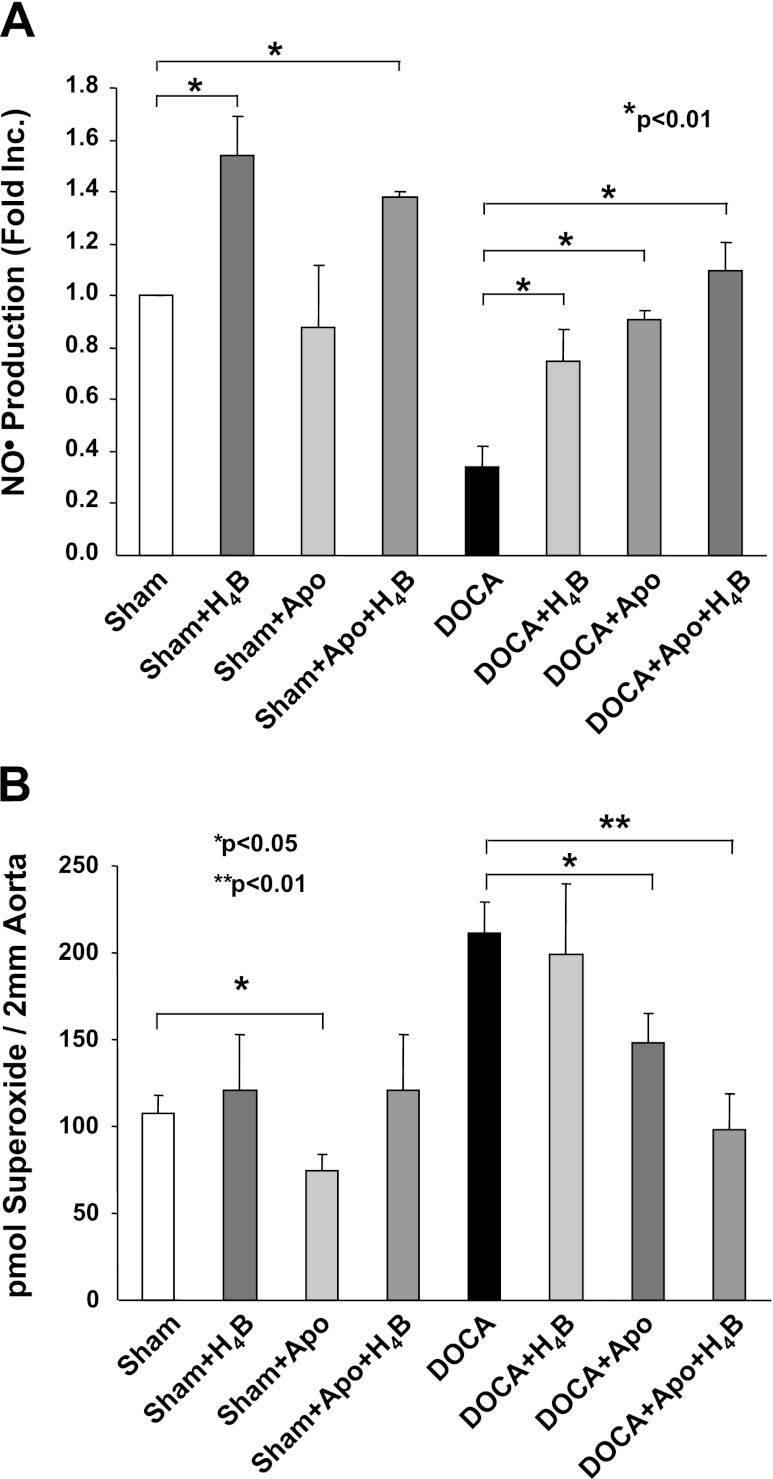
Oral administration of H4B increased nitrosyl hemoglobin levels in the blood of DOCA-salt hypertensive mice. Nitosyl hemoglobin is not a direct reflect of local vascular NO· bioavailability. It remains unclear whether H4B is effective in restoring NO· production in blood vessels. To address this question, control and hypertensive aortas were incubated with H4B (10 μmol/l, stock solution maintained in constantly nitrogen-bubbled Krebs-HEPES buffer containing 50 μmol/l deferoximine, used fresh) for 30 min before ESR analysis of NO· production. In control vessels, H4B significantly enhanced NO· production (Fig. 5A). This is consistent with earlier findings where supplementation of H4B to cultured endothelial cells increased NO· levels. Importantly, H4B partially yet significantly enhanced NO· production in hypertensive aortas (Fig. 5A).
Fig. 5.
Effect on eNOS recoupling of combined treatment of H4B and apocynin (Apo). A: vascular production of NO· was detected using ESR after the vessels being incubated with H4B (10 μmol/l) or apocynin (100 μmol/l) alone or combined. Data are presented as means ± SE from 8 independent experiments. B: control and hypertensive vessels were treated with H4B (10 μmol/l) or apocynin (100 μmol/l) alone or both and then subjected to a HPLC-based dihydroethidium assay of O2·− as described in materials and methods. Grouped data in picomoles O2·− per 2 mm aorta piece from 5 independent experiments are presented (means ± SE). ANOVA *P < 0.05, **P < 0.01.
Effects on eNOS recoupling of cotreatment with H4B and apocynin in DOCA-salt hypertension.
NOX has been found to lie upstream of uncoupled eNOS in vitro and in vivo (7, 16). When NOX remain active in endothelial cells, or in nonendothelial cells such as vascular smooth muscle, constant production of ROS could oxidize exogenously supplemented H4B to its inactive form. This may explain why H4B was only partially effective in restoring NO· production when administered alone. We next incubated aortas with apocynin (100 μmol/l, a inhibitor for NOX) for 30 min before a 30-min incubation with H4B. The hypertensive aortas were then subjected to ESR spin trapping of NO·. Apocynin alone partially improved NO· production in hypertensive blood vessels, similar to H4B alone (Fig. 5A). Intriguingly, cotreatment with both agents completely restored NO· production to control levels (Fig. 5A). In additional experiments, aortic O2·− production was quantitated using a HPLC-based dihydroethidium assay. It was shown earlier that the increase in O2·− is predominantly caused by uncoupled eNOS in hypertensive endothelium (16). As is obvious in Fig. 5B, whereas apocynin alone only partially reduced O2·− production in hypertensive aortas, combination of apocynin and H4B abolished O2·− production (Fig. 5B). Taken together; these data strongly suggest that a coadministration of H4B and apocynin is highly effective in recoupling of eNOS in DOCA-salt hypertension.
Endothelial regulation of SPR in ANG II-induced hypertension.
To determine whether an endothelial deficiency in SPR also occurs in other vascular diseases or other types of hypertension, we infused C57BL6 mice with ANG II for 14 days before analysis of aortic endothelial SPR expression as described earlier. As is obvious in Fig. 6, SPR expression in either endothelium or endothelium-denuded aortas was regulated in ANG II-dependent hypertension. This is consistent with our previous notion that a deficiency in another H4B salvage enzyme, DHFR, is responsible for ANG II uncoupling of eNOS in both ANG II-dependent hypertension and type 1 diabetes (11, 13, 20).
Fig. 6.
Regulation of SPR in ANG II-induced hypertension. A: representative Western blot for SPR. Osmotic pumps containing ANG II (0.7 mg·kg−1·day−1) were implanted subcutaneously. After 14 days, mice aortas were harvested and freshly isolated aortas were subjected to isolation of endothelial cells by using collagenase digestion as described previously (Refs. 11 and 13). Endothelial cells and denuded vessels were homogenized in a Tris-based buffer containing 1% Triton and protease inhibitors. Equal amount of proteins from endothelial cell or denuded vessels were separated by SDS-PAGE, and SPR protein expression was analyzed by Western blotting with a polyclonal antibody. B: grouped data of endothelium-specific aortic expression of SPR (n = 6). C: grouped data of endothelium-denuded aortic expressions of SPR (n = 6).
DISCUSSION
The most significant new findings of this study include 1) first identification of SPR deficiency in hypertension and 2) identification of novel approaches effective in recoupling eNOS in hypertension. Consequent to the loss of SPR in the endothelium, sepiapterin is not effective in recoupling eNOS in DOCA-salt hypertensive aortas. Acute administration of sepiapterin had no effects on both NO· and O2·− productions in DOCA-salt hypertension. Chronic sepiapterin supplementation also failed to restore SPR expression. These data seem to suggest that in DOCA-salt hypertension, the salt-sensitive, low renin model of hypertension, sepiapterin is a low efficacy agent for eNOS recoupling. H4B or apocynin alone partially improved NO· bioavailability in hypertensive blood vessels. The combination of H4B and apocynin completely restored NO· production in hypertensive aortas, suggesting that ceasing O2·− production from vascular NOX while supplementing H4B is likely the most efficient in treating hypertensive uncoupling of eNOS.
As a nonenzymatically produced synthetic precursor for H4B, sepiapterin does not exist under physiological conditions. When administrated exogenously, however, sepiapterin can be converted to H4B by SPR and has been shown to increase NO· production from cultured, normal endothelial cells (1, 12). It has been shown to improve endothelium-dependent vasorelaxation in small arteries of diabetic rodents (21). Whereas sepiapterin nearly doubled NO· production in aortas isolated from control mice due to increased H4B bioavailability, it by contrast had no effects on NO· production in DOCA-salt hypertensive aortas (Fig. 2). These data suggest that there is an inability of hypertensive vessels to convert sepiapterin to H4B. Interestingly, these observations are consistent with earlier studies demonstrating that although H4B improved endothelium-dependent vasodilatation in spontaneous hypertensive rats, sepiapterin was ineffective (32). Vasquez-Vivar et al. (26) also reported that sepiapterin had no effects on aortic relaxation in hypercholesterolemic rabbit. The molecular mechanisms underlying the inconsistent effects of sepiapterin in different models, however, have not been addressed previously. Our hypothesis was that SPR, the enzyme critical for sepiapterin conversion to H4B, is deficient in the large vessels of hypertensive mice, after observing an inefficiency of sepiapterin in restoring eNOS function. Indeed, protein expression of SPR was found downregulated in aortic endothelial cells, but not in endothelial cell-denuded aortas (Fig. 3). This is the first evidence for endothelium-specific regulation of SPR in hypertension. Consequent to this regulation, sepiapterin fails to serve as a substrate for regeneration of H4B. It would be interesting to investigate whether this is specific for DOCA-salt hypertension. Of note, when we looked into ANG II-induced hypertension, aortic endothelial SPR expression was unaffected (Fig. 6).
We showed that acute treatment of DOCA-hypertensive aortas with H4B partially restored bioavailable NO· as detected by ESR and that coadministration with H4B and NOX inhibitor apocynin completely restored NO· production. It is interesting to speculate that oral administration of H4B together with apocynin would be the most effective in restoring NO· production from entire animals and likely be the most efficient in preventing atherogenesis in hypertension. H4B alone, however, only partially restored NO·. This seems consistent with earlier observations that overexpression of the H4B synthetic enzyme GTPCH I was partially effective in improving endothelial function in DOCA-salt hypertensive rats (9). The GTPCH I activity was found downregulated in DOCA animals (9).
Taken together, these data suggest that H4B itself is at least partially effective in recoupling eNOS in hypertension. However, due to its instability, it is challenging to deliver H4B successfully to enable full recovery of eNOS function. In addition, whether chronic in vivo coadministration of H4B and apocynin is equally effective as in vitro in improving endothelial function remains unclear. Thus biopterin precursors that are stable and feasible for chronic delivery are still being considered as alternative treatments for eNOS uncoupling. Although our data demonstrated that sepiapterin is not effective due to hypertensive downregulation of SPR, it also implies that gene therapy overexpressing SPR may be useful in conjunction with sepiapterin administration. In addition, it also implies that the efficacy of biopterin precursors in recoupling eNOS could largely dependent on the functions of endothelial H4B metabolic enzymes. Finally, these data are consistent with our previous observations that in hypertension associated with elevated levels of ANG II, endothelial deficiency in the H4B salvage enzyme DHFR is responsible for eNOS uncoupling. Therefore, strategies specifically targeting at different H4B metabolic enzymes might be necessary in restoring eNOS function in different types of hypertension.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.Y.Y. and H.C. interpreted results of experiments; J.Y.Y., T.W., and H.C. prepared figures; J.Y.Y. and H.C. drafted and revised manuscripts; J.Y.Y., T.W., J.B., K.M.L., J.H.O., L.M., and H.C. performed experiments; J.Y.Y., T.W., and H.C. analyzed data; D.G.H. and H.C. contributed to the conception and design of research.
ACKNOWLEDGMENTS
This work was supported by National Heart, Lung and Blood Institute (NHLBI) Grants HL-077440 and HL-088975 (to H. Cai), HL-101228 (to H. Cai), and HL-39006 (to D. G. Harrison).
REFERENCES
- 1. Baker TA, Milstien S, Katusic ZS. Effect of vitamin C on the availability of tetrahydrobiopterin in human endothelial cells. J Cardiovasc Pharmacol 37: 333– 338, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Cai H. Hydrogen peroxide regulation of endothelial function: mechanisms, consequences and origins. Cardiovasc Res 68: 26– 36, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Cai H. NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ Res 96: 818– 822, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Cai H, Davis ME, Drummond GR, Harrison DG. Induction of endothelial NO synthase by hydrogen peroxide via a Ca2+/calmodulin-dependent protein kinase II/janus kinase 2-dependent pathway. Arterioscler Thromb Vasc Biol 21: 1571– 1576, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci 24: 471– 478, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840– 844, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci USA 102: 9056– 9061, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drummond GR, Cai H, Davis ME, Ramasamy S, Harrison DG. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ Res 86: 347– 354, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Du YH, Guan YY, Alp NJ, Channon KM, Chen AF. Endothelium-specific GTP cyclohydrolase I overexpression attenuates blood pressure progression in salt-sensitive low-renin hypertension. Circulation 117: 1045– 1054, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol 287: C895– C902, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Gao L, Chalupsky K, Stefani E, Cai H. Mechanistic insights into folic acid-dependent vascular protection: dihydrofolate reductase (DHFR)-mediated reduction in oxidant stress in endothelial cells and angiotensin II-infused mice. A novel HPLC-based fluorescent assay for DHFR activity. J Mol Cell Cardiol 47: 752– 760, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gao L, Pung YF, Zhang J, Chen P, Wang T, Li M, Meza M, Toro L, Cai H. Sepiapterin reductase regulation of endothelial tetrahydrobiopterin and nitric oxide bioavailability. Am J Physiol Heart Circ Physiol 297: H331– H339, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gao L, Siu KL, Chalupsky K, Nguyen A, Chen P, Weintraub NL, Galis Z, Cai H. Role of uncoupled endothelial nitric oxide synthase in abdominal aortic aneurysm formation: treatment with folic acid. Hypertension 59: 158– 166, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harrison DG. Endothelial function and oxidant stress. Clin Cardiol 20: II-11– II-17, 1997 [PubMed] [Google Scholar]
- 15. Heinzel B, John M, Klatt P, Bohme E, Mayer B. Ca2+/calmodulin-dependent formation of hydrogen peroxide by brain nitric oxide synthase. Biochem J 281: 627– 630, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201– 1209, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 103: 1282– 1288, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun 263: 681– 684, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Mitchell BM, Dorrance AM, Ergul A, Webb RC. Sepiapterin decreases vasorelaxation in nitric oxide synthase inhibition-induced hypertension. J Cardiovasc Pharmacol 43: 93– 98, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Oak JH, Cai H. Attenuation of angiotensin II signaling recouples eNOS and inhibits nonendothelial NOX activity in diabetic mice. Diabetes 56: 118– 126, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Pannirselvam M, Verma S, Anderson TJ, Triggle CR. Cellular basis of endothelial dysfunction in small mesenteric arteries from spontaneously diabetic (db/db−/−) mice: role of decreased tetrahydrobiopterin bioavailability. Br J Pharmacol 136: 255– 263, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pou S, Pou WS, Bredt DS, Snyder SH, Rosen GM. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem 267: 24173– 24176, 1992 [PubMed] [Google Scholar]
- 23. Seong C, Kim YA, Chung HJ, Park D, Yim J, Baek K, Park YS, Han K, Yoon J. Isolation and characterization of the Drosophila melanogaster cDNA encoding the sepiapterin reductase. Biochim Biophys Acta 1443: 239– 244, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Thony B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J 347: 1– 16, 2000 [PMC free article] [PubMed] [Google Scholar]
- 25. Tiefenbacher CP, Bleeke T, Vahl C, Amann K, Vogt A, Kubler W. Endothelial dysfunction of coronary resistance arteries is improved by tetrahydrobiopterin in atherosclerosis. Circulation 102: 2172– 2179, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Vasquez-Vivar J, Duquaine D, Whitsett J, Kalyanaraman B, Rajagopalan S. Altered tetrahydrobiopterin metabolism in atherosclerosis: implications for use of oxidized tetrahydrobiopterin analogues and thiol antioxidants. Arterioscler Thromb Vasc Biol 22: 1655– 1661, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA 95: 9220– 9225, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vasquez-Vivar J, Martasek P, Whitsett J, Joseph J, Kalyanaraman B. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: an EPR spin trapping study. Biochem J 362: 733– 739, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Werner-Felmayer G, Golderer G, Werner ER. Tetrahydrobiopterin biosynthesis, utilization and pharmacological effects. Curr Drug Metab 3: 159– 173, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Wever RM, van Dam T, van Rijn HJ, de Groot F, Rabelink TJ. Tetrahydrobiopterin regulates superoxide and nitric oxide generation by recombinant endothelial nitric oxide synthase. Biochem Biophys Res Commun 237: 340– 344, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem 273: 25804– 25808, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Yang D, Levens N, Zhang JN, Vanhoutte PM, Feletou M. Specific potentiation of endothelium-dependent contractions in SHR by tetrahydrobiopterin. Hypertension 41: 136– 142, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med 34: 1359– 1368, 2003 [DOI] [PubMed] [Google Scholar]