Abstract
The sympathetic nervous system is critical for the beat-to-beat regulation of arterial blood pressure (BP). Although studies have examined age- and sex-related effects on BP control, findings are inconsistent and limited data are available in postmenopausal women. In addition, the majority of studies have focused on time-averaged responses without consideration for potential beat-to-beat alterations. Thus we examined whether the ability of muscle sympathetic nerve activity (MSNA) to modulate BP on a beat-to-beat basis is affected by age or sex. BP and MSNA were measured during supine rest in 40 young (20 men) and 40 older (20 men) healthy subjects. Beat-to-beat fluctuations in mean arterial pressure (MAP) were characterized for 15 cardiac cycles after each MSNA burst using signal averaging. The rise in MAP following an MSNA burst was similar between young men and women (+2.64 ± 0.3 vs. +2.57 ± 0.3 mmHg, respectively). However, the magnitude of the increase in MAP after an MSNA burst was reduced in older compared with young subjects (P < 0.05). Moreover, the attenuation of the pressor response was greater in older women (+1.20 ± 0.1 mmHg) compared with older men (+1.72 ± 0.2 mmHg; P < 0.05). Interestingly, in all groups, MAP consistently decreased after cardiac cycles without MSNA bursts (nonbursts) with the magnitude of fall greatest in older men. In summary, healthy aging is associated with an attenuated beat-to-beat increase in BP after a spontaneous MSNA burst, and this attenuation is more pronounced in postmenopausal women. Furthermore, our nonburst findings highlight the importance of sympathetic vasoconstrictor activity to maintain beat-to-beat BP, particularly in older men.
Keywords: aging, autonomic nervous system, hypertension, menopause, blood pressure
the prevalence of hypertension increases with age in both sexes; however, postmenopausal women are at the greatest risk (23, 26, 28, 47, 49). The underlying mechanisms for this increased risk and differential age effect in men and women remain incompletely understood. Although structural (25, 36) and hormonal (43, 44, 49) factors likely contribute, an age-related increase in sympathetic nerve activity (SNA) has also been implicated as a key factor (20, 28, 29).
The sympathetic nervous system and its ability to modulate vascular tone are paramount for the regulation of arterial blood pressure (BP). Studies using systemic pharmacological blockade (3, 20, 35) or correlational analyses (15, 28, 29) have indicated both age and sex differences in the regulation of resting muscle SNA (MSNA) and BP. However, findings are inconsistent and limited data are available in older postmenopausal women. An important caveat in these studies is the degree to which spontaneous bursts of MSNA actually cause a rise in BP. In this regard, Wallin and Nerhed (46) reported a peak rise in BP of 2 to 3 mmHg occurring ∼5.5 s following a spontaneous burst of MSNA. However, these initial analyses were performed on a heterogeneous group of subjects without consideration for the potential influence of aging or sex on the transduction of MSNA into a change in arterial BP. Surprisingly, to date, no studies have investigated how age and sex affect the ability of the sympathetic nervous system to regulate BP on a beat-to-beat basis. Importantly, an augmentation in the beat-to-beat oscillations in BP has been demonstrated with age (39), and this increased variability is suggested to be associated with end organ damage (27, 32–34). However, the cause of these fluctuations is not completely understood.
With this background in mind, the purpose of the current study was to provide a comprehensive examination of beat-to-beat changes in BP evoked by individual bursts of MSNA in healthy young and older men and women. This was accomplished by directly recording MSNA from the peroneal nerve along with beat-to-beat BP and applying signal averaging to compare the magnitude of the rise in BP following a spontaneous burst of MSNA. Given previous studies demonstrating greater sympathetic support of BP and increased BP variability with age (20, 39), we hypothesized that the magnitude of the increase in BP following a burst of MSNA is augmented in older compared with younger subjects. Furthermore, considering the hormonal changes associated with menopause and also epidemiological data showing a higher prevalence of hypertension in women with age (26, 47), we further hypothesized that the augmentation of the pressor response is more pronounced in older women compared with older men.
METHODS
Subjects.
Forty young (20 men) and 40 older (20 men) healthy subjects participated in this study. Studies were performed in a clinical research laboratory at the Mayo Clinic [n = 56; 30 young (16 men) and 26 older (13 men) subjects] and at the University of Missouri [n = 24; 10 young (4 men) and 14 older (7 men) subjects]. The records used were retrospectively analyzed from previous (13–15, 50) and ongoing studies in the respective laboratories. Of note, all data were taken during an initial baseline period before any type of intervention. The subjects were nonsmokers with no history of cardiovascular or other chronic diseases and none were using prescribed or over-the-counter medications. To minimize the effects of reproductive hormones on autonomic control of cardiovascular function, all the young women were studied in the early follicular phase of the menstrual cycle or in the low hormone phase of oral contraceptive use. In addition, all older postmenopausal women were not using hormone replacement therapy. Participants were instructed to abstain from caffeinated beverages and food for at least 12 hours and alcohol for 24 hours before the study. After receiving a detailed verbal and written explanation of the intended experimental protocol and measurements, each subject provided written informed consent. All experimental procedures and protocols conformed to the Declaration of Helsinki and were approved by the Mayo Clinic Foundation and the University of Missouri Health Sciences Institutional Review Board.
Experimental measurements.
On arrival to the laboratory, the subjects rested in the supine position during instrumentation. Arterial BP was measured either by direct arterial catheterization (n = 54) or finger photoplethysmography (Finometer; Finapres Medical Systems BV, Arnhem, The Netherlands; n = 26). Previous studies have reported similar beat-to-beat changes in BP measured by Finometer and arterial line (11, 38). For arterial catheterization, local anesthesia with 2% lidocaine was applied and a 5-cm, 20-gauge catheter was inserted into the brachial artery of the nondominant arm, using aseptic techniques. The catheter was connected to a pressure transducer and interfaced with a personal computer to monitor beat-to-beat arterial BP. For finger photoplethysmography absolute Finometer values were validated with an automated sphygmomanometer. Heart rate (HR) was continuously monitored using a lead II electrocardiogram. Respiratory movements were monitored using a strain gauge pneumobelt placed around the subject's abdomen. Multiunit recordings of postganglionic MSNA were obtained by inserting a unipolar tungsten microelectrode percutaneously through the intact skin and positioned into muscle nerve fascicles of the peroneal nerve near the fibular head. The nerve signal was processed by a pre-amplifier and an amplifier (Dept. of Bioengineering, University of Iowa, Iowa City, IA), band-pass filtered (bandwidth 700–2,000 Hz), rectified, and integrated (time constant, 0.1 s) to obtain a mean voltage neurogram. Muscle SNA recordings were identified by their characteristic pulse-synchronous burst pattern and increased neural activity in response to an end-expiratory apnea or Valsalva maneuver, without any response to arousal stimuli or stroking of the skin (31, 42, 50).
Experimental protocol: comparing beat-to-beat changes in BP following a spontaneous burst of MSNA in young and older men and women.
While the subject rested quietly in the supine position in a dimly lit room, a minimum of 5 min (9.3 ± 0.7 min) of baseline data were continuously recorded. Sympathetic bursts in the integrated neurogram were identified using a custom-built automated analysis program; burst identification was then corrected via visual inspection by a single observer (L. C. Vianna). Sympathetic activity was quantified using standard measures, including burst frequency (in bursts/min) and burst incidence (in bursts/100 heart beats). The relationship between each individual spontaneous burst of MSNA and the ensuing changes in mean arterial pressure (MAP) were characterized by using a signal-averaging technique, as described in detail elsewhere (46). Briefly, MAP measured during the cardiac cycle in which a sympathetic burst occurred was utilized to calculate changes of MAP from that value in the succeeding 15 cardiac cycles. This procedure was repeated for all cardiac cycles associated with a sympathetic burst, and the resulting mean changes of MAP were then calculated for each subject. MAP was used as the primary endpoint for this analysis; however, analyses were also performed for systolic and diastolic BP. We also repeated this procedure for those cardiac cycles that were not associated with a sympathetic burst (i.e., nonbursts). In addition, to understand the potential influence of variations in burst size on the pressor responses following a sympathetic burst, each subject had their sympathetic bursts divided into quartiles according to the height of the burst. To do this, the average height of the three highest bursts in the baseline segment was assigned a value of 100 (arbitrary units), and all other bursts within a trial were normalized with respect to this value. Beat-to-beat changes in BP following a MSNA burst were then put into quartiles based on the relative height of the burst.
Beat-to-beat changes in cardiac output (CO) and total vascular conductance (TVC) were also calculated and characterized for the 15 cardiac cycles following a burst of MSNA. Briefly, stroke volume was estimated from the arterial BP waveform (resampled at 100 Hz) using the Modelflow method through Beatscope (TNO-TPD; Biomedical Instrumentation, Amsterdam, The Netherlands), which incorporates age, sex, weight, and height adjustments. Modelflow is a nonlinear three-element model that uses the arterial input impedance, the compliance of the aorta, and total peripheral resistance to describe the relationship between aortic flow and pressure and thus compute stroke volume, as described in detail previously (18, 19, 48). This method has been shown to provide accurate beat-to-beat changes in CO (22, 48). TVC was calculated from the ratio of CO and MAP. MAP was calculated from the integral of the arterial pressure waveform.
Statistical analysis.
Statistical analyses were conducted using the SPSS statistical software package for Microsoft Windows (version 19.0; SPSS, Chicago, IL). For resting cardiovascular variables, a univariate ANOVA was performed with two independent factors (age × sex). Beat-to-beat data were compared using a three-way repeated measures ANOVA followed by Bonferroni post hoc tests. Reproducibility of the MAP changes following MSNA bursts in each group was calculated by comparing the first and the second half of each subject's baseline record using Intraclass Correlation Coefficients (ICC). Coefficient of variation for BP was calculated as the standard deviation divided by the mean. All tests were two-sided, and a P value <0.05 was considered statistically significant. Group data are presented as means ± SE. For presentation purposes, all figures are displayed using 10 rather than all 15 cardiac cycles following an MSNA burst.
RESULTS
Resting neural and cardiovascular variables.
Subject characteristics and the average resting neural and hemodynamic variables in young and older men and women are presented in Table 1. Body mass index was slightly but significantly greater in older subjects. MAP and systolic BP were significantly higher in older compared with young subjects (P = 0.002 and P = 0.003, respectively), whereas diastolic BP was similar between groups. No sex differences were observed in resting BP. HR was significantly higher in young and older women compared with the young and older men (P = 0.026). These differences in HR between groups were not dependent on age. MSNA was higher in older compared with young subjects expressed as burst frequency (P < 0.001) and burst incidence (P < 0.001). No sex differences were observed in resting MSNA. Respiratory rate (in breaths/min) was not different among the groups (13.4 ± 0.6 young men, 13.3 ± 0.5 young women, 12.4 ± 0.7 older men, and 12.6 ± 0.8 older women; age, P = 0.263; sex, P = 0.952; interaction, P = 0.732). BP variability measured using coefficient of variation (in percentage) for MAP was also not affected by age or sex (3.5 ± 0.3 young men, 3.6 ± 0.2 young women, 4.2 ± 0.4 older men, and 3.3 ± 0.2 older women; age, P = 0.423; sex, P = 0.152; interaction, P = 0.052).
Table 1.
Subject characteristics and resting neural cardiovascular variables
| Blood Pressure, mmHg |
||||||||
|---|---|---|---|---|---|---|---|---|
| Age, years | Body Mass Index, kg/m2 | Heart Rate, beats/min | Burst Frequency, bursts/min | Burst Incidence, bursts/100 beats | Systolic | Diastolic | Mean Arterial | |
| Young | ||||||||
| Men | 26 ± 1 | 25 ± 1 | 131 ± 3 | 73 ± 2 | 92 ± 3 | 59 ± 2 | 14 ± 1 | 25 ± 2 |
| Women | 25 ± 1 | 23 ± 1 | 127 ± 2 | 72 ± 1 | 92 ± 1 | 65 ± 2 | 15 ± 3 | 24 ± 3 |
| Older | ||||||||
| Men | 61 ± 1 | 27 ± 1 | 138 ± 3 | 72 ± 4 | 98 ± 2 | 60 ± 2 | 29 ± 2 | 51 ± 3 |
| Women | 60 ± 2 | 25 ± 1 | 139 ± 3 | 71 ± 2 | 98 ± 2 | 63 ± 2 | 28 ± 2 | 45 ± 3 |
| P value | ||||||||
| Age | <0.001 | 0.002 | 0.003 | 0.799 | 0.002 | 0.754 | <0.001 | <0.001 |
| Sex | 0.136 | 0.026 | 0.424 | 0.372 | 0.978 | 0.026 | 0.890 | 0.289 |
| Interaction | 0.891 | 0.781 | 0.357 | 0.979 | 0.848 | 0.484 | 0.604 | 0.528 |
Values are means ± SE.
Beat-to-beat changes in BP following a spontaneous burst of MSNA in young and older men and women.
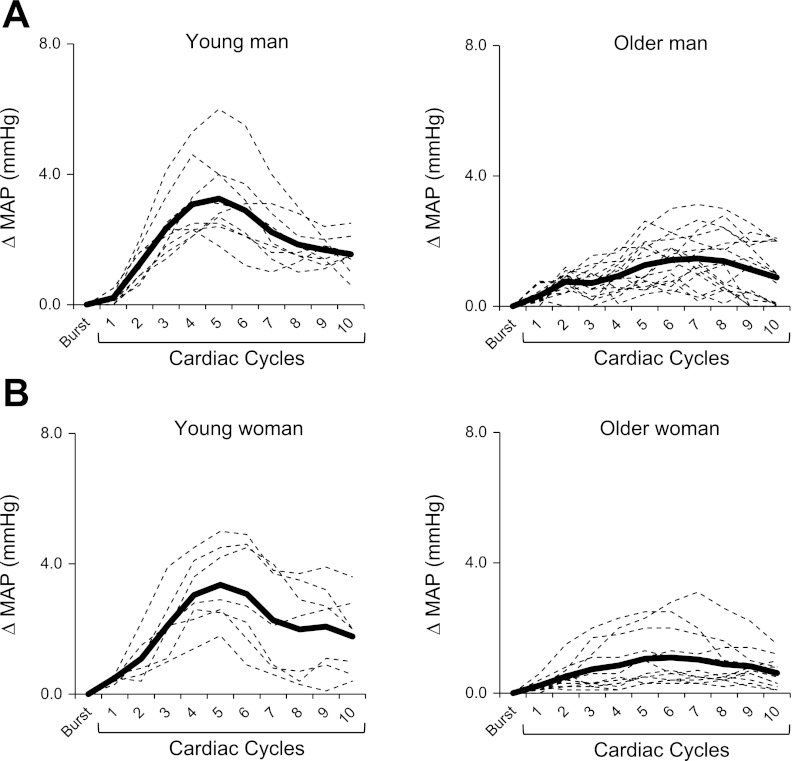
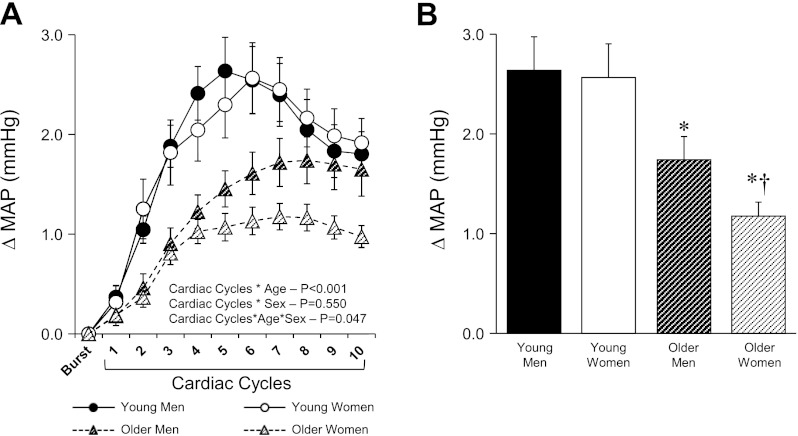
Examples of signal averaging for a young and older man (Fig. 1A) and a young and older woman (Fig. 1B) are shown in Fig. 1. Figure 2A shows the beat-to-beat increases in MAP for young and older men and women following a burst of MSNA. There was no difference in the magnitude of the peak rise in MAP between young men and women (Fig. 2B). However, the magnitude of the increase in MAP following a MSNA burst was reduced in older compared with young subjects (+1.46 ± 0.1 mmHg older vs. +2.60 ± 0.2 mmHg young; P < 0.001). In addition, the attenuation of the pressor response was more pronounced in older women compared with older men (+1.20 ± 0.1 mmHg older women vs. +1.72 ± 0.2 mmHg older men; P = 0.04). Similar age and sex differences were observed when analyses were performed with systolic and diastolic BP (data not shown). The ICC for changes in MAP following MSNA bursts between the first and second half of each subject's baseline record were high and significant (P < 0.001) in all groups, indicating reproducible and reliable measurements: young men (ICC = 0.86, 95% CI of 0.65 to 0.95), young women (ICC = 0.82, 95% CI of 0.51 to 0.93), older men (ICC = 0.88, 95% CI of 0.69 to 0.95), and older women (ICC = 0.92, 95% CI of 0.78 to 0.97). After a spontaneous MSNA burst the latency to the peak increase in MAP was significantly longer (P < 0.001) in the older (7.2 ± 0.5 s men vs. 7.1 ± 0.6 s women) compared with young group (5.6 ± 0.4 s men vs. 5.4 ± 0.3 s women), with no significant sex effect in either group.
Fig. 1.
Examples of the signal averaging for a young and older man (A) and a young and older woman (B) illustrating the changes in mean arterial pressure (MAP) following spontaneous bursts of muscle sympathetic nerve activity (MSNA). The dashed lines represent the average MAP response for 10 MSNA bursts, and the thick line represents the mean MAP response for all MSNA bursts in the baseline segment.
Fig. 2.
Summary data for young and older men and women showing beat-to-beat (A) and peak (B) changes in MAP following a MSNA burst. Values are means ± SE. *Significantly different from young men and women (P < 0.05); †significantly different from older men (P < 0.05).
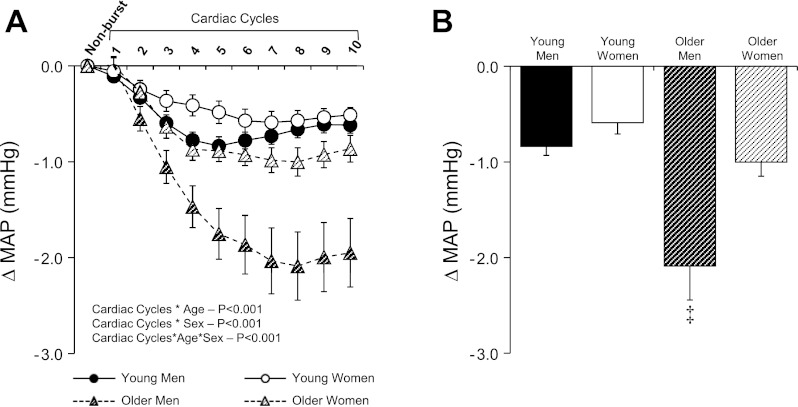
The beat-to-beat changes in MAP for those cardiac cycles that were not associated with a sympathetic burst are presented in Fig. 3A. In all groups, MAP consistently decreased following cardiac cycles without MSNA bursts (Fig. 3). However, the magnitude of fall in MAP was greatest in older men (Fig. 3B; P < 0.001). Similar age and sex differences for cardiac cycles without bursts were observed when analyses were performed with systolic and diastolic BP (data not shown).
Fig. 3.
Summary data for young and older men and women showing beat-to-beat (A) and peak (B) changes in MAP following cardiac cycles that were not associated with MSNA burst (i.e., nonburst). Values are means ± SE. ‡Significantly different from older women and young men and women (P < 0.05).
Beat-to-beat changes in TVC and CO following a spontaneous burst of MSNA in young and older men and women.
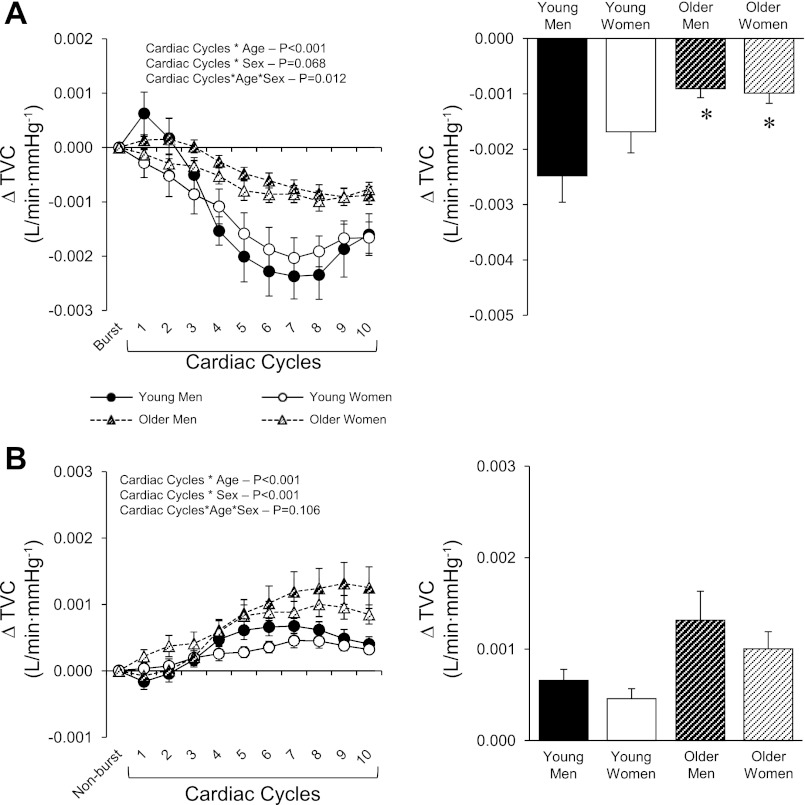
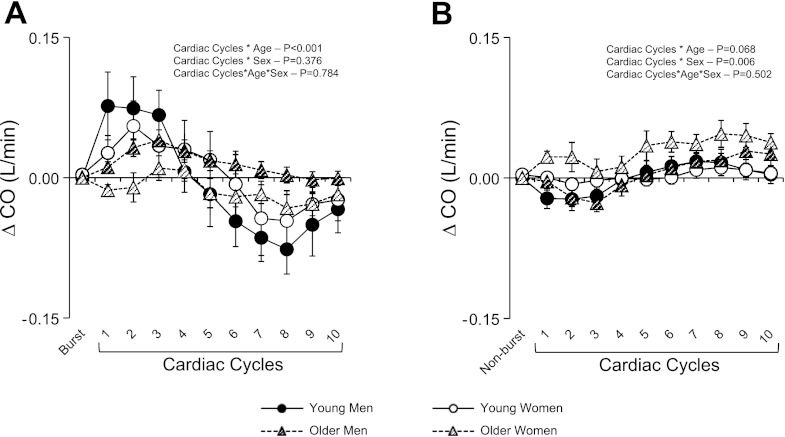
Along with increases in MAP, TVC decreased following a MSNA burst in all groups (Fig. 4A). When compared with young men and women, the magnitude of decrease in TVC was attenuated in the older men and women (P < 0.001). When cardiac cycles without an MSNA burst were taken into consideration, TVC was increased in all groups (Fig. 4B). However, increases in TVC were greater in older compared with young subjects (P < 0.001). There was also a sex effect in which men exhibited greater increases in TVC compared with women (Fig. 4B; P < 0.001). In regards to CO, beat-to-beat changes following a MSNA burst did not reveal any sex differences (P = 0.376), but there was an age effect (P < 0.001) with smaller changes in the older subjects, particularly the older women (Fig. 5A). Importantly, CO appeared to contribute mainly to the initiation of the pressor response since at the time of the peak MAP response CO was near or below baseline in all groups. The changes in CO for cardiac cycles without an MSNA burst were minimal, although a sex effect was observed (Fig. 5B; P = 0.006).
Fig. 4.
Summary data for young and older men and women showing beat-to-beat (left) and peak (right) changes in total vascular conductance (TVC) for cardiac cycles with (A) and without (B) a MSNA burst. Values are means ± SE. *Significantly different from young men and women (P < 0.05).
Fig. 5.
Summary data for young and older men and women showing beat-to-beat changes in cardiac output (CO) for cardiac cycles with (A) and without (B) a MSNA burst. Values are means ± SE. *Significantly different from young men and women (P < 0.05).
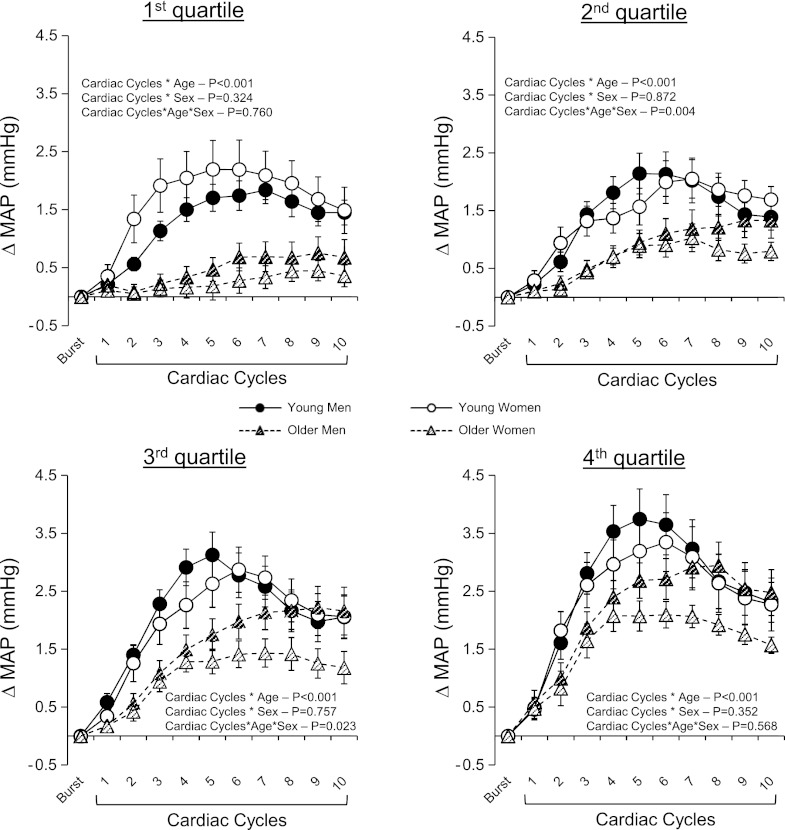
Influence of burst size on beat-to-beat changes in MAP following a spontaneous burst of MSNA.
Figure 6 shows the beat-to-beat changes in MAP following a spontaneous burst of MSNA divided into quartiles according to the relative size of the MSNA burst (i.e., burst height). There was a clear graded effect of burst size on the magnitude of change of MAP following a MSNA burst that was consistently attenuated in the older men and women compared with young men and women with no significant sex effect in either group.
Fig. 6.
Summary data showing beat-to-beat changes in MAP following a MSNA burst when taking burst size into consideration for young and older men and women. Each subject had their sympathetic bursts divided into quartiles according to the height of the burst. Values are means ± SE.
DISCUSSION
A major novel finding of this study is that the magnitude of rise in BP following a spontaneous burst of MSNA was significantly reduced in older subjects, an effect that was more marked in postmenopausal women. Moreover, when no burst of MSNA occurred, a fall in BP was observed in all groups demonstrating the importance of SNA for the beat-to-beat maintenance of BP. Notably, the older men had the largest fall in BP following cardiac cycles without a burst, whereas older women responded similarly to young men and women. These data suggest older men are highly reliant on SNA for the beat-to-beat maintenance of BP and may help explain the greater resting MSNA in this group. In contrast, the small increase in BP following a MSNA burst combined with the slight fall when no bursts were present suggests that SNA has minimal influences on resting beat-to-beat fluctuations in BP in older women. Collectively, these findings provide novel insight into underlying age and sex differences in the sympathetic control of beat-to-beat BP in healthy individuals.
Several studies have shown that the sympathetic nervous system plays an important role in the development of hypertension (7, 9, 12); however, this is not a universal finding (21). Although it is clear that resting MSNA is elevated with age (15, 20, 28, 29), the degree to which the increase in MSNA contributes to hypertension remains unclear. Importantly, in contrast with our hypothesis, we found an attenuated rise in BP following MSNA bursts in older subjects. These data suggest that at least on a beat-to-beat basis the sympathetic nervous system is not contributing to greater increases in BP with age, particularly in older women. However, the larger fall in BP in older men when no bursts of MSNA were present suggests a greater sympathetic vasoconstrictor tone under resting conditions that appears selective to this group. Given the known attenuation of α-adrenergic receptor responsiveness in older men (5, 6), these data suggest higher resting MSNA burst frequency is somewhat compensating and providing a greater sympathetic constrictor support of BP. Interestingly, this effect is not present in older women. Although limited studies have been performed in postmenopausal women, a recent study (13) indicates that α-adrenergic sensitivity may also be attenuated in this group. Nevertheless, our findings demonstrate, for the first time, a greater sympathetic support of beat-to-beat BP in older men that is not present in older women.
In young subjects, we found no difference between men and women in the rise in BP following a spontaneous MSNA burst. These data were somewhat surprising given findings that forearm vasoconstrictor responses to α-agonists are blunted in young women compared with men (10). In general agreement, following systemic ganglionic blockade, young women have been shown to exhibit lower tonic autonomic nervous system support of arterial BP (3). Furthermore, a positive relationship between resting MSNA and total peripheral resistance has been reported in young men but not young women (14). In contrast, our findings indicate no sex differences in the beat-to-beat control of BP via MSNA. The reason for these differing results is unclear but several possibilities warrant discussion. First, there are differences in the temporal resolution of data analyses between studies. Indeed, we are assessing spontaneous beat-to-beat changes over a discrete period (i.e., 15 s), whereas previous studies have primarily used pharmacological interventions and examined time-averaged responses from longer term steady-state periods ranging from 30 s to 5 min (10, 12, 14). In addition, when pharmacological blockade is used, drugs are administrated in the luminal side of the blood vessel, whereas when MSNA is assessed, nerve fibers are releasing norepinephrine on the abluminal side. Finally, we are using BP as our main end point, whereas previous studies have relied on local or systemic vascular changes only. In this regard, we also see clear reductions in TVC following spontaneous MSNA bursts that are of similar magnitude in young men and women. These findings for beat-to-beat sympathetic transduction to TVC are consistent with the lack of a sex difference in the beat-to-beat rise in BP following a MSNA burst in the young subjects.
In a closed loop system, the control of resting MSNA and beat-to-beat BP is a dynamic process with MSNA not only driving BP responses but also being evoked by changes in BP (12, 21). Indeed, although several studies have shown that sympathetic vascular transduction is paramount for the regulation of BP (i.e., peripheral arc), the arterial baroreflex control of MSNA (i.e., neural arc) represents a critical component for the maintenance of BP (1, 8, 14, 16, 30). Interestingly, Hart et al. (17) have recently shown that MSNA responsiveness to changes in BP via the arterial baroreflex (spontaneous sympathetic baroreflex sensitivity) is associated with resting MSNA in young and older men and postmenopausal women but not in young women. Thus the sex effect present in young women is lost with age and likely due to the loss of sex hormones with menopause. On the other hand, the data from the present study highlight the importance of MSNA as a driver of beat-to-beat BP fluctuations, and this can be influenced by both age and sex. Collectively, these data suggest that in the investigation of age and sex effects on BP control it is not only important to consider the afferent side of the baroreflex (neural arc) but also the efferent transduction side (peripheral arc).
In agreement with the current findings, Sugiyama et al. (41) also suggested a diminished BP response to MSNA bursts in older men. Importantly, we have extended these results by including young and older women, demonstrating a clear age effect in that postmenopausal women exhibit an attenuated pressor response following a burst of MSNA when compared with young women. In fact, as noted above, the older women had the smallest increases in BP of any group studied including the older men. To further investigate potential age and sex effects on MSNA control of beat-to-beat BP, we examined the potential influence of variations in burst size on the BP responses following each MSNA burst. We observed a graded effect of burst size with the smallest bursts evoking the smallest increase in BP, whereas the largest MSNA bursts were associated with the greatest rises in BP. This was consistent for all groups. Importantly, in general, taking into account burst size reinforced the significant blunting of the BP response following MSNA bursts with age and sex. Although these findings cannot establish strict causality, they strongly implicate sympathetic activity in producing these transient elevations in BP.
Although the mechanisms for the age-related decrease in BP responses to a given MSNA burst are unclear, several possibilities require discussion. First, it is reasonable to suggest that the decreased effectiveness of MSNA to elicit increases in BP in older subjects may be due to reduced α-adrenergic sensitivity (5, 6, 13). Alternatively, older subjects may have a reduction in norepinephrine release (4) per burst of MSNA; however, this remains unknown. In either case, a compensatory response of increased resting MSNA with age may be required to support BP (12, 13, 15, 20, 29). Because respiration can exert potent effects on MSNA and BP (2, 24, 37), we monitored respiratory excursions in all subjects. However, we did not observe any differences in respiratory rate between groups, suggesting that the frequency of respiration likely does not account for the observed differences in BP responses to MSNA bursts. Nonetheless, future studies with more comprehensive assessments of respiratory function may provide additional insight into respiratory-sympathetic coupling and its effects on the transduction of MSNA into beat-to-beat BP changes.
Perspectives.
Little information is available regarding how the sympathetic nervous system regulates BP on a beat-to-beat basis in humans. In the early 80s, Wallin and Nerhed (46) reported a consistent transient rise in BP of ∼2.5 mmHg following a MSNA burst in a heterogeneous group of subjects. To our surprise, no studies have followed up on these initial findings. Thus we extended these findings by examining how age and sex affect the ability of the sympathetic nervous system to regulate beat-to-beat BP. We demonstrate that the increase in BP following a spontaneous burst of MSNA was remarkably attenuated in older individuals, an effect that was most marked in postmenopausal women. Moreover, when no burst of MSNA occurred, older men had the largest fall in BP, whereas older women responded similarly to young women and men. Thus age-related effects cannot be generalized, and sex must be considered. The extent to which hormone replacement therapy influences the MSNA-BP relationships in postmenopausal women remains to be determined.
When compared with women, as men age it appears that MSNA becomes increasingly more important for the maintenance of beat-to-beat BP. Importantly, older men exhibit a large fall in BP when no bursts of MSNA are present, suggesting an augmented reliance upon MSNA to maintain resting BP. Indeed, although a fall in BP for cardiac cycles without an MSNA burst was seen in all groups, it was ∼132% greater in older men. Thus, despite the fact that aging is associated with increased synthesis and action of vasoconstrictors (4, 40, 45), such as endothelin, and angiotensin II, our results highlight the importance and necessity of higher sympathetic outflow to the skeletal muscle vasculature in older men to regulate and maintain beat-to-beat BP. Of note, the larger falls in BP may be expected to contribute to greater BP variability in older men (39), which has been suggested to be associated with end organ damage (27, 32–34). However, we did not observe any differences in the coefficient of variation of BP between the older men and any of the other groups, suggesting that the overall distribution of BP is comparable. Nevertheless, it appears that older men may be more reliant on MSNA for maintaining BP beat-to-beat.
Conclusions
Healthy aging is associated with attenuated beat-to-beat changes in BP following a spontaneous burst of MSNA. Of note, this attenuation is more pronounced in postmenopausal women. Furthermore, our nonburst findings highlight on a beat-to-beat basis the importance of sympathetic vasoconstrictor activity to maintain BP, particularly in older men. Collectively, these findings provide novel insight into underlying age and sex differences in the sympathetic control of beat-to-beat BP in healthy individuals.
GRANTS
This research is the result of work supported with resources by the National Heart, Lung, and Blood Institute Grants HL-093167 (to P. J. Fadel) and HL-83947 (to M. J. Joyner).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.C.V., M.J.J., and P.J.F. conception and design of research; L.C.V., E.C.H., N.C., M.J.J., and P.J.F. performed experiments; L.C.V., E.C.H., and S.T.F. analyzed data; L.C.V., E.C.H., S.T.F., N.C., M.J.J., and P.J.F. interpreted results of experiments; L.C.V. and P.J.F. prepared figures; L.C.V. and P.J.F. drafted manuscript; L.C.V., E.C.H., S.T.F., N.C., M.J.J., and P.J.F. edited and revised manuscript; L.C.V., E.C.H., S.T.F., N.C., M.J.J., and P.J.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank volunteer subjects for time and effort.
REFERENCES
- 1. Akimoto T, Sugawara J, Ichikawa D, Terada N, Fadel PJ, Ogoh S. Enhanced open-loop but not closed-loop cardiac baroreflex sensitivity during orthostatic stress in humans. Am J Physiol Regul Integr Comp Physiol 301: R1591– R1598, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Badra LJ, Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KU, Eckberg DL. Respiratory modulation of human autonomic rhythms. Am J Physiol Heart Circ Physiol 280: H2674– H2688, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D, Seals DR. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation 111: 494– 498, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional alpha-adrenergic vasoconstriction in healthy men. Circulation 106: 1349– 1354, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Dinenno FA, Joyner MJ. Alpha-adrenergic control of skeletal muscle circulation at rest and during exercise in aging humans. Microcirculation 13: 329– 341, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic alpha-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol 567: 311– 321, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Esler M. Sympathetic nervous activation in essential hypertension: commonly neglected as a therapeutic target, usually ignored as a drug side effect. Hypertension 55: 1090– 1091, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Fadel PJ. Arterial baroreflex control of the peripheral vasculature in humans: rest and exercise. Med Sci Sports Exerc 40: 2055– 2062, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Fisher JP, Fadel PJ. Therapeutic strategies for targeting excessive central sympathetic activation in human hypertension. Exp Physiol 95: 572– 580, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freedman RR, Sabharwal SC, Desai N. Sex differences in peripheral vascular adrenergic receptors. Circ Res 61: 581– 585, 1987 [DOI] [PubMed] [Google Scholar]
- 11. Harms MP, Wesseling KH, Pott F, Jenstrup M, Van Goudoever J, Secher NH, Van Lieshout JJ. Continuous stroke volume monitoring by modelling flow from non-invasive measurement of arterial pressure in humans under orthostatic stress. Clin Sci (Lond) 97: 291– 301, 1999 [PubMed] [Google Scholar]
- 12. Hart EC, Charkoudian N. Sympathetic neural mechanisms in human blood pressure regulation. Curr Hypertens Rep 13: 237– 243, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the beta-adrenergic receptors. J Physiol 589: 5285– 5297, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension 53: 571– 576, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hart EC, Joyner MJ, Wallin BG, Johnson CP, Curry TB, Eisenach JH, Charkoudian N. Age-related differences in the sympathetic-hemodynamic balance in men. Hypertension 54: 127– 133, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hart EC, Joyner MJ, Wallin BG, Karlsson T, Curry TB, Charkoudian N. Baroreflex control of muscle sympathetic nerve activity: a nonpharmacological measure of baroreflex sensitivity. Am J Physiol Heart Circ Physiol 298: H816– H822, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hart EC, Wallin BG, Curry TB, Joyner MJ, Karlsson T, Charkoudian N. Hysteresis in the sympathetic baroreflex: role of baseline nerve activity. J Physiol 589: 3395– 3404, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jansen JR, Schreuder JJ, Mulier JP, Smith NT, Settels JJ, Wesseling KH. A comparison of cardiac output derived from the arterial pressure wave against thermodilution in cardiac surgery patients. Br J Anaesth 87: 212– 222, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Jansen JR, Wesseling KH, Settels JJ, Schreuder JJ. Continuous cardiac output monitoring by pulse contour during cardiac surgery. Eur Heart J 11, Suppl I: 26– 32, 1990 [DOI] [PubMed] [Google Scholar]
- 20. Jones PP, Shapiro LF, Keisling GA, Jordan J, Shannon JR, Quaife RA, Seals DR. Altered autonomic support of arterial blood pressure with age in healthy men. Circulation 104: 2424– 2429, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Joyner MJ, Charkoudian N, Wallin BG. A sympathetic view of the sympathetic nervous system and human blood pressure regulation. Exp Physiol 93: 715– 724, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim A, Deo SH, Vianna LC, Balanos GM, Hartwich D, Fisher JP, Fadel PJ. Sex differences in carotid baroreflex control of arterial blood pressure in humans: relative contribution of cardiac output and total vascular conductance. Am J Physiol Heart Circ Physiol 301: H2454– H2465, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leuzzi C, Modena MG. Hypertension in postmenopausal women: pathophysiology and treatment. High Blood Press Cardiovasc Prev 18: 13– 18, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Macefield VG, Wallin BG. Modulation of muscle sympathetic activity during spontaneous and artificial ventilation and apnoea in humans. J Auton Nerv Syst 53: 137– 147, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol 105: 1652– 1660, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mosca L, Manson JE, Sutherland SE, Langer RD, Manolio T, Barrett-Connor E. . Cardiovascular disease in women: a statement for healthcare professionals from the American Heart Association Writing Group Circulation 96: 2468– 2482, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Mussalo H, Vanninen E, Ikaheimo R, Laitinen T, Hartikainen J. Short-term blood pressure variability in renovascular hypertension and in severe and mild essential hypertension. Clin Sci (Lond) 105: 609– 614, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 45: 522– 525, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension 21: 498– 503, 1993 [DOI] [PubMed] [Google Scholar]
- 30. Ogoh S, Fisher JP, Young CN, Raven PB, Fadel PJ. Transfer function characteristics of the neural and peripheral arterial baroreflex arcs at rest and during postexercise muscle ischemia in humans. Am J Physiol Heart Circ Physiol 296: H1416– H1424, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Padilla J, Young CN, Simmons GH, Deo SH, Newcomer SC, Sullivan JP, Laughlin MH, Fadel PJ. Increased muscle sympathetic nerve activity acutely alters conduit artery shear rate patterns. Am J Physiol Heart Circ Physiol 298: H1128– H1135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parati G, Bilo G, Mancia G. Blood pressure measurement in research and in clinical practice: recent evidence. Curr Opin Nephrol Hypertens 13: 343– 357, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Parati G, Faini A, Valentini M. Blood pressure variability: its measurement and significance in hypertension. Curr Hypertens Rep 8: 199– 204, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Parati G, Frattola A, Di Rienzo M, Mancia G. Blood pressure variability. Importance in research and in clinical hypertension Arq Bras Cardiol 67: 131– 133, 1996 [PubMed] [Google Scholar]
- 35. Schmitt JA, Joyner MJ, Charkoudian N, Wallin BG, Hart EC. Sex differences in alpha-adrenergic support of blood pressure. Clin Auton Res 20: 271– 275, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seals DR, Moreau KL, Gates PE, Eskurza I. Modulatory influences on ageing of the vasculature in healthy humans. Exp Gerontol 41: 501– 507, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Seals DR, Suwarno NO, Dempsey JA. Influence of lung volume on sympathetic nerve discharge in normal humans. Circ Res 67: 130– 141, 1990 [DOI] [PubMed] [Google Scholar]
- 38. Shi X, Gallagher KM, SA SM, Bryant KH, Raven PB. Diminished forearm vasomotor response to central hypervolemic loading in aerobically fit individuals. Med Sci Sports Exerc 28: 1388– 1395, 1996 [DOI] [PubMed] [Google Scholar]
- 39. Shi X, Huang G, Smith SA, Zhang R, Formes KJ. Aging and arterial blood pressure variability during orthostatic challenge. Gerontology 49: 279– 286, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Stauffer BL, Westby CM, Greiner JJ, Van Guilder GP, Desouza CA. Sex differences in endothelin-1-mediated vasoconstrictor tone in middle-aged and older adults. Am J Physiol Regul Integr Comp Physiol 298: R261– R265, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sugiyama Y, Matsukawa T, Shamsuzzaman AS, Okada H, Watanabe T, Mano T. Delayed and diminished pressor response to muscle sympathetic nerve activity in the elderly. J Appl Physiol 80: 869– 875, 1996 [DOI] [PubMed] [Google Scholar]
- 42. Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age J Physiol 274: 621– 637, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thornton SN. Angiotensin inhibition and longevity: a question of hydration. Pflügers Arch 461: 317– 324, 2011 [DOI] [PubMed] [Google Scholar]
- 44. Traish AM, Kypreos KE. Testosterone and cardiovascular disease: an old idea with modern clinical implications. Atherosclerosis 214: 244– 248, 2011 [DOI] [PubMed] [Google Scholar]
- 45. Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension 50: 403– 409, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Wallin BG, Nerhed C. Relationship between spontaneous variations of muscle sympathetic activity and succeeding changes of blood pressure in man. J Auton Nerv Syst 6: 293– 302, 1982 [DOI] [PubMed] [Google Scholar]
- 47. Wenger NK. Hypertension and other cardiovascular risk factors in women. Am J Hypertens 8: 94s– 99s, 1995 [DOI] [PubMed] [Google Scholar]
- 48. Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol 74: 2566– 2573, 1993 [DOI] [PubMed] [Google Scholar]
- 49. Yanes LL, Reckelhoff Postmenopausal hypertension JF. Am J Hypertens 24: 740– 749, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Young CN, Deo SH, Chaudhary K, Thyfault JP, Fadel PJ. Insulin enhances the gain of arterial baroreflex control of muscle sympathetic nerve activity in humans. J Physiol 588: 3593– 3603, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]