Abstract
In mouse arteries, Alox15 [leukocyte-type 12/15-lipoxygenase (LO)] is assumed to regulate vascular function by metabolizing arachidonic acid (AA) to dilator eicosanoids that mediate the endothelium-dependent relaxations to AA and acetylcholine (ACh). We used Alox15−/− mice, made by targeted disruption of the Alox15 gene, to characterize its role in the regulation of blood pressure and vascular tone. Systolic blood pressures did not differ between wild-type (WT) and Alox15−/− mice between 8–12 wk of age, but Alox15−/− mice exhibited resistance toward both NG-nitro-l-arginine-methyl ester (l-NAME)- and deoxycorticosterone acetate (DOCA)/high-salt-induced hypertension. ACh relaxed mesenteric arteries and abdominal aortas of WT and Alox15−/− mice to an identical extent. The LO inhibitor nordihydroguaiaretic acid attenuated the ACh relaxations by 35% in arteries from both WT and Alox15−/− mice. Reverse-phase HPLC analysis of [14C]AA metabolites in aorta and peritoneal macrophages (PM) revealed differences. Unlike PM, aorta tissue did not produce detectable amounts of 15-hydroxyeicosatetraenoic acid. Although Alox15 mRNA was detected in aorta, high-resolution gel electrophoresis with immunodetection revealed no Alox15 protein expression. Unlike aorta, Alox15 protein was detected in PM, intestine, fat, lung, spleen, and skin from WT, but not Alox15−/−, mice. Injection of WT PM, a primary source of Alox15 protein, into Alox15−/− mice abolished their resistance toward l-NAME-induced hypertension. On the other hand, WT mice acquired resistance to l-NAME-induced hypertension after depletion of macrophages by clodronate injection. These studies indicate that Alox15 is involved in development of experimental hypertension by altering macrophage functions but not via synthesis of the vasoactive LO metabolites in mouse arteries.
Keywords: arachidonic acid, eicosanoids
lipoxygenases (LOs) are a family of nonheme iron-containing dioxygenases that incorporate molecular oxygen into unsaturated fatty acids such as linoleic acid or arachidonic acid (AA; Refs. 3, 55). Specifically, AA is metabolized by LOs to monohydroperoxyeicosatetraenoic acids (HPETEs) or further metabolized to leukotrienes, hepoxilins, or lipoxins. In rabbit arteries, endothelial 15-LO-1 converts AA to 15-HPETE that is further metabolized by a hydroperoxide isomerase and soluble epoxide hydrolase (sEH) to vasoactive hydroxyl-epoxyeicosatrienoic acids (HEETAs) and 11,12,15-trihydroxyeicosatrienoic acid (11,12,15-THETA), respectively (42, 50). The HEETAs and THETA collectively function as endothelium-derived hyperpolarizing factors (EDHFs) and mediate a portion of the endothelium-dependent relaxations to acetylcholine (ACh) and AA (6, 9, 22). Potassium channels localized to the smooth muscle cell membrane are activated by the HEETAs and THETAs causing membrane hyperpolarization and vascular relaxation (5, 20, 22). Formation of the EDHFs and subsequently the vascular hyperpolarization and relaxation are blocked by endothelium removal or LO inhibitors (5, 15, 19), indicating the involvement of endothelial LOs in the regulation of vascular tone.
In the mouse, several LOs produce 12- and 15-HPETE (3, 4, 17, 44). These include 8-LO (Alox8), platelet-type 12-LO (Alox12), epidermal-type 12-LO (Alox12e), and leukocyte-type 12/15-LO (Alox15). It is widely accepted that Alox15 is the mouse homologue of rabbit and human 15-LO-1 and so is a likely source of endothelial LO metabolites (13, 17, 45). Although the two enzymes are homologues, they produce different H(P)ETEs or HETEs. Rabbit 15-LO-1 yields a 95:5 mixture of 15(S)-HETE/12(S)-HETE, whereas Alox15 results in a ratio of 1:3. Both LOs have been implicated in atherogenesis (18, 29, 33). While 15-LO-1 plays an important role in endothelium-dependent relaxation in rabbit arteries (50), the LO that contributes to vascular relaxations in the mouse has not been fully identified.
LO inhibitors are not selective for a specific LO isozyme and most are antioxidants (46). Specific antisense oligonucleotides or small interfering RNAs selectively inhibit the expression of a single LO isozyme and determine its role in cell function (37, 50). This approach has been used in vitro but not in vivo (56). Transgenic or knockout animals also provide specific information about a particular LO isozyme (17). For example, Alox15 transgenic mice develop systolic dysfunction (31). This dysfunction is due to Alox15 upregulating monocyte chemoattractant protein 1 (MCP-1). An Alox15 knockout (Alox15−/−) mouse was developed by targeted disruption of the Alox15 gene (48). This model has been widely used to define the biological role of Alox15 in a number of pathological conditions including diabetes (2), wound healing (26), ischemia/reperfusion (52), atherosclerosis (12, 18, 23), and hypertension (1).
In the current study, we used the Alox15−/− mouse as a model to investigate the role of Alox15 in maintaining vascular tone under normotensive and hypertensive conditions. Our initial hypothesis was that Alox15 regulates vascular tone through endothelial synthesis of vasoactive AA metabolites and genetic deletion of Alox15 impairs arterial vasodilation leading to elevated blood pressure in experimental models of hypertension. In contrast to the hypothesis, there was no difference in basal blood pressure between WT and Alox15−/− mice, and the Alox15−/− mice exhibited resistance toward experimental hypertension models. Although RT-PCR analysis clearly showed transcription of Alox15 mRNA by aortas of WT mice, we could not find expression of Alox15 protein. As a consequence, there was no difference in relaxation to AA or ACh in mesenteric arteries or [14C]AA metabolism by aortas of WT and Alox15−/− mice. Since Alox15 was expressed in macrophages and absent in Alox15−/− mice, a revised hypothesis was formulated that macrophage Alox15 is necessary for the blood pressure elevation in experimental hypertension. These findings indicate that Alox15 is involved in blood pressure regulation through regulation of macrophage function, but not via endothelial synthesis of vasoactive AA metabolites, and suggest that physiological and pathological activities that are attributed to Alox15 in vasculature are caused by resident or infiltrating macrophages.
EXPERIMENTAL PROCEDURES
Chemicals.
Acetylcholine (ACh), nitro-l-arginine (l-NA), NG-nitro-l-arginine-methyl ester (l-NAME), indomethacin (Indo), nordihydroguaiaretic acid (NDGA), Triton X-100, KCl, and calcium ionophore A23187 were purchased from Sigma (St. Louis, MO). AA was purchased from Nu-Chek Prep (Elysian, MN) and [14C]AA was from PerkinElmer Life Sciences (Boston, MA). Both AA and [14C]AA were further purified by HPLC before use. Thromboxane mimetic U46619 was obtained from Cayman Chemical (Ann Arbor, MI). The thioglycollate modified Brewers medium was purchased from Becton-Dickinson (Sparks, MD). All solvents were HPLC grade and purchased from Burdick and Jackson or Sigma.
Animals.
Male WT and Alox15−/− C57BL6 mice (20–25 g) were obtained from The Jackson Laboratory (Bar Harbor, ME), and male and female WT and Alox15−/− C57BL6 mice (20–25 g) were provided by Dr. J. L. Nadler of East Virginia Medical School. These mice were produced by back-crossing Alox15−/− mice with C57BL6 WT mice to eliminate certain unwanted phenotypic characteristic of the original strain. All animals were genotyped using PCR method with the following primers: common forward 5′- GGC TGC CTG AAG AGG TAC AG-3′; knockout reverse 5′-GGG AGG ATT GGG AAG ACA AT-3′; and WT reverse 5′-CCA TAG ACG AGA CCA GCA CA-3′. Products from WT and Alox15−/− animals were 417 and 200 bp, respectively. Animal protocols were approved by the Animal Use and Care Committee of the Medical College of Wisconsin and procedures were performed in accordance with the National Institute of Health's Guide for the Use of Laboratory Animals (1996 and 2011).
Blood pressure measurement.
Systolic blood pressure of male and female WT and Alox15−/− C57BL6 mice was determined weekly between the ages of 8 and 12 wk using tail-cuff method with IITC Life Sciences Blood Pressure System (Woodland Hills, CA). The same method was used to monitor the systolic blood pressure of male mice during induced hypertension experiments. Mice were acclimated to the blood pressure chamber twice a week starting from age of 6 wk. Recordings were started after 5–10 min of acclimation. Three successful measurements were averaged as a single data point.
Experimental hypertension models.
In l-NAME-induced hypertension model, male mice received 100 mg·kg−1·day−1 of NG-nitro-l-arginine-methyl ester in regular drinking water for 5 days. The water intake was monitored during this time period to exclude any possible differences between experimental groups.
For deoxycorticosterone acetate (DOCA)/high-salt hypertension, 21-day release 50 mg DOCA pellets or vehicle pellets from Innovative Research of America (Sarasota, FL) were implanted subcutaneously while animals were under isofluorane anesthesia in male mice. During this period, mice were drinking water containing 1% sodium chloride and 0.2% potassium chloride. Blood pressure was monitored with the tail cuff method (see above).
Culturing mouse aortic endothelial cells.
Mouse aortic endothelial cells (MAECs) were isolated using the method of Kobayashi et al. (32) with slight modification. Aortas from three C57BL6 male mice were dissected, cleaned of connective tissues, and cut into sections. Aortic pieces were incubated in serum-free RPMI medium containing 0.2% collagenase H for 90 min at 37°C. MAECs were removed by gentle agitation with a Pasteur pipet. MAECs were collected by centrifugation at 1,200 rpm for 5 min and cultured in 35 mm collagen type-1-coated dishes using RPMI medium containing 20% FBS. Following the 2 h of initial attachment period, cells were washed with PBS to remove non-endothelial cells and grown in RPMI medium until confluent. MAECs were characterized by LDL uptake. Cells were washed and overlaid with medium containing 10 mM Dil-Ac-LDL for 4 h at 37°C. After three washes, cell fluorescence was determined using an inverted fluorescence microscope. Excitation and emission wavelengths were 554 and 571 nm, respectively.
Tissue preparation.
Arteries were dissected and cleaned of connective tissue in HEPES buffer (10 mM HEPES, 150 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, and 6 mM glucose pH 7.4). Fat surrounding aortas was collected during the cleaning process. Elicited macrophages were harvested from euthanized mice by peritoneal lavage using sterile PBS (100 mM NaCl, 3 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4 pH 7.4). To obtain elicited macrophages, mice were injected intraperitoneally with 2 ml of sterile 3.85% thioglycollate medium Brewer modified medium (Becton-Dickinson) 3 days before isolation. All tissues were used immediately or snap frozen in liquid nitrogen immediately after dissection and stored at −80°C until further use.
Western immunoblotting.
Tissues frozen in liquid nitrogen were pulverized and homogenized in lysis buffer (10 mM HEPES, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, and 1 mM sodium bisulfite pH 7.5) containing Complete Mini cocktail protease inhibitor (Roche Diagnostics) and 0.5% Triton X-100. Homogenates were further incubated on ice for 30 min with occasional vortexing. Macrophage and MAEC cell pellets were resuspended and homogenized directly in lysis buffer. The homogenates were centrifuged at 12,000 g for 20 min, and the supernatants were used for analysis. Usually 75 μg of total protein were applied to each line of a 7.5 or 10% SDS-PAGE gel (Criterion Precast Gel; Bio-Rad), separated, and transblotted onto nitrocellulose membrane. After blocking with 5% nonfat dry milk (Bio-Rad) in TBS-T buffer (20 mM Tris base, 150 mM NaCl, and 0.1% Tween-20), the membranes were probed with three different rabbit 12-LO primary antibodies: one generated in our laboratory (14, 50), a commercial antibody (Cayman Chemical), or an antibody provided by Dr. Colin Funk (48). The antibodies were raised against different leukocyte-type 12-LO peptides (all in 1:1,000 dilution) (14, 50). Horseradish peroxidase-conjugated goat anti-rabbit IgG (1:10,000 dilution, GE Healthcare) was used as secondary antibody. β-Actin was used as loading control detected by a mouse monoclonal anti-β-actin primary antibody (1:5,000 dilution; Santa Cruz Biotechnology) and an horseradish peroxidase-conjugated goat anti-mouse secondary antibody (1:10,000 dilution; Invitrogen). When comparing aorta and MAEC lysates, GAPDH (rabbit anti-GAPDH, 1:10,000 dilution; Thermo Scientific) was used as a loading control. Immunoreactive bands were visualized using SuperSignal West Pico or West Femto chemiluminescence reagents (Thermo Scientific).
Isometric tension in mesenteric arteries and abdominal aortas.
Arterial/aortic sections (∼1.5 mm in length) were mounted in a four-chamber wire myograph (model 610M; Danish Myo Technology) and maintained at 37°C in physiological saline solution: 119 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.17 mM MgSO4, 24 mM NaHCO3, 1.18 mM KH2PO4, 0.026 mM EDTA, and 5.5 mM glucose, gassed with O2 containing 5% CO2 as previously described (21, 57). Primary and major secondary branches of the mesenteric artery with relative diameters of 150 to 300 μm were stretched to a resting tension of 0.25 millinewtons (mN). Abdominal aortic rings were isolated and stretched to a resting tension of 1.5 mN. The vessels were stimulated three times with 60 mM KCl plus 100 nM U46619 for 8–10 min at 10-min intervals. Arteries were contracted with submaximal concentrations of U46619 (50–300 nM) to 50–90% of their maximum KCl/U46619 challenge. Cumulative concentrations of ACh (0.1 nM to 10 μM) were added. All experiments were performed on arteries pretreated with the nitric oxide (NO) synthase inhibitor NG-nitro-l-arginine (30 μM) and the cyclooxygenase inhibitor Indo (10 μM). Some experiments were performed in the presence of a LO inhibitor, NDGA (50 μM). Results are expressed as percent relaxation of the U46619-preconstricted arteries with 100% representing basal tension.
[14C]AA metabolism and RP-HPLC analysis.
Cleaned aortas were cut into 3- to 5-mm pieces and incubated for 10 min at 37°C in 5 ml HEPES buffer containing Indo (10 μM). Aortic segments were then incubated with AA (0.1 μM) plus [14C]AA (0.05 μCi). After 10 min, calcium ionophore A23187 (20 μM; Sigma) was added and the incubation continued for another 15 min. In some instances, NDGA (10 μM) was added to the first incubation. Reactions were stopped by adding ice-cold ethanol to a final concentration of 15%. The buffer was acidified (pH < 3.5) with glacial acetic acid and extracted on Bond Elute ODS solid-phase extraction columns (Agilent Technology) as previously described (42). Extracted metabolites were dried under a stream of nitrogen and stored at −40°C until analysis.
[14C]AA metabolites were resolved by reverse phase HPLC using a Nucleosil-C18 column (5 μ, 4.6 × 250 mm). The following solvent systems were used: solvent system I: solvent A was water and solvent B was acetonitrile (MeCN) containing 0.1% glacial acetic acid. The program consisted of a 40 min linear gradient from 50% solvent B in A to 100% solvent B. Flow rate was 1 ml/min. Column eluate was collected in 0.2-ml fractions (42). Solvent system II consisted of MeCN/MeOH/H2O/trifluoroacetic acid (37.5:25:37.5:0.05) with a flow rate of 0.75 ml/min (48). Column eluate was collected in 0.5-ml fractions. For both solvent systems, absorbance was monitored at 235 nm, and column fraction radioactivity was determined by liquid scintillation spectrometry. For quantitative analysis of metabolites, areas under the HPLC peaks were integrated using Beckman 32 Karat software.
RT-PCR.
Total RNA was isolated from tissues using RNeasy mini kit (Qiagen) and subjected to reverse transcription with SuperScript III First-Strand cDNA Synthesis System (Invitrogen). Samples without reverse transcriptase or RNA were prepared as controls. cDNA samples were analyzed by qPCR (iCycler; Bio-Rad) using iQ SYBR Green Supermix (BioRad) reagent. Alox15-specific primers synthesized by Eurofins MWG Operon (Huntsville, AL) were as follows: exon 3 forward 5′-CTC TCA AGG CCT GTT CAG GA-3′, reverse 5′-GTC CAT TGT CCC CAG AAC CT-3′; and exon 14 forward 5′-GCG ACG CTG CCC AAT CCT AAT C -3′, reverse 5′-CAT ATG GCC ACG CTG TTT TCT ACC-3′. Alox15 expression was normalized to the corresponding 18S levels. Primers for mouse 18S were as follows: forward 5′-AAA TCA GTT ATG GTT CCT TTG GTC-3′, reverse 5′-GCT CTA GAA TTA CCA CAG TTA TCC AA-3′. The PCR program was 94°C for 30 s, 60°C for 1 min, 72°C for 1 min, and 81°C for 15 s, repeated 40 times. Taqman qPCR gene expression assay (Applied Biosystems) was used to detect Alox15 exon 1 region. Relative abundance of each product was analyzed with the 2[−ΔΔC(T)] method using 18S gene as a control. Transcription in tissues from Alox15−/− mice was verified by PCR using primer sets of short-neo (forward 5′-GAT GGG TTC AGG GCA CCA GCA TC-3′) or long-neo (forward 5′-CGG AGG CAG AAT TCA AGG TGG ATG T-3′), both with a common reverse 5′-GGG AGG ATT GGG AAG ACA AT-3′. Amplified PCR products were separated by 1.5% agarose gel electrophoresis and visualized with ethidium bromide staining.
Flow cytometry.
The distribution of cell types in the elicited peritoneal lavage was determined by flow cytometry using monoclonal antibodies to monocytes/macrophages (anti-CD11b-APC-Cy7 and anti-F4/80-PE), T-cells (anti-CD3-PE-Cy7), B-cells (anti-B220-APC), and granulocytes (anti-Ly-6G-FITC). The cells were stained simultaneously with each antibody for 20 min on ice. They were then washed twice in PBS + 0.5% BSA (FACS buffer) and resuspended in FACS Buffer for analysis. The cells were analyzed using a BD FACS Aria II. Fluorescence minus one controls were used to ensure an accurate measurement of surface marker expression. The data were analyzed using CellQuest Pro.
Macrophage depletion with clodronate liposomes.
Clodronate liposomes were purchased from clodronateliposomes.org (Vrije Universiteit, Amsterdam, The Netherlands) at the concentration of 5 mg/ml and prepared as previously described (53). Clodronate was a gift of Roche Diagnostics (Mannheim, Germany). Mice were injected using the tail vein with either clodronate liposomes or PBS liposomes (100 μl/10 g of body wt) while they were under isofluorane anesthesia. The injection was repeated 48 h later. On the day following the second injection, mice were treated with l-NAME to induce hypertension.
Peritoneal macrophage transfer.
Elicited peritoneal macrophages were harvested from female C57BL6 mice injected with sterile thioglycollate solution 3 days earlier. Cells were washed and resuspended in PBS to have ∼8 million cells/ml. Male Alox15−/− mice were injected intraperitoneally with either 1 ml of PBS or 1 ml of macrophage suspension. Immediately after injection mice were treated with l-NAME to induce hypertension.
Statistics.
Data are presented as means ± SE. The two-tailed Student's t-test or ANOVA was used for determining the significance of observed differences between experimental values, with P < 0.05 considered statistically significant.
RESULTS
Systolic blood pressure of WT vs. Alox15−/− mice under normal or experimental hypertension conditions.
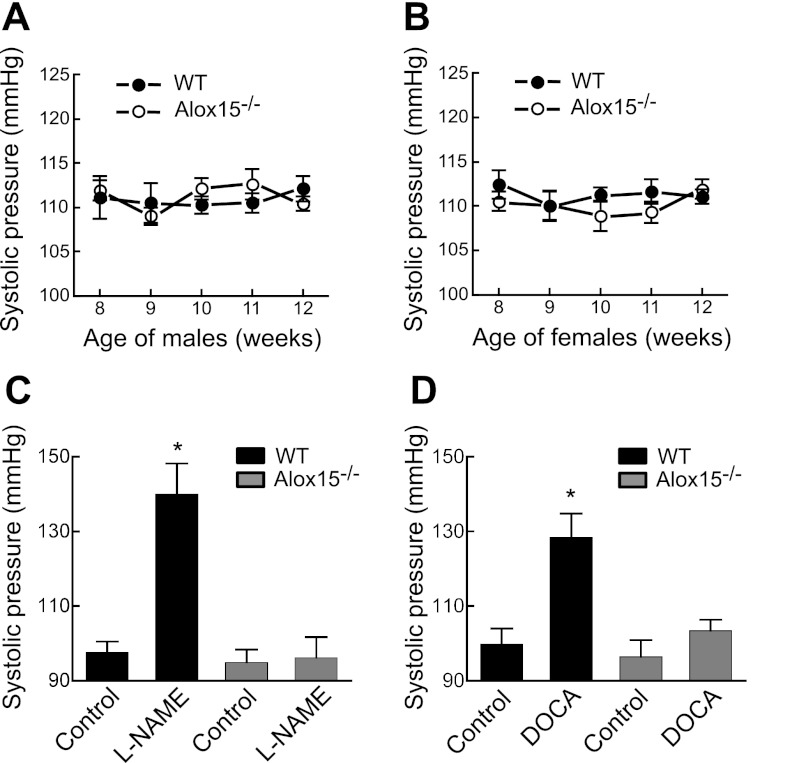
Systolic blood pressure was monitored in male and female C57BL6 WT and Alox15−/− mice between the age of 8 and 12 wk using the tail-cuff method. Throughout the 4 wk, the blood pressure of the male and female groups remained in the range of 109–112 mmHg (Fig. 1, A and B). There was no significant difference in the blood pressures at any age or when WT and Alox15−/− mice were compared. These data indicate that Alox15 gene disruption had no effect on blood pressure. However, when experimental hypertension was induced in male mice by l-NAME or DOCA/high-salt treatment, differences were observed between the groups. On a fifth day of l-NAME ingestion, the blood pressure of male WT animals was significantly increased, while the blood pressure remained unchanged in Alox15−/− mice (Fig. 1C). In female WT mice, l-NAME also increased blood pressure (105 ± 6 mmHg for control vs. 132 ± 8 mmHg with l-NAME). As with the Alox15−/− male mice, blood pressure did not increase with l-NAME treatment in Alox15−/− mice (100 ± 4 mmHg for control and 101 ± 3 mmHg for l-NAME). Since there was no difference in basal blood pressure between male and female animals, all subsequent experiments on induced hypertension were performed on male mice. Similar results were obtained when the hypertension was induced by DOCA/high-salt-diet treatment (Fig. 1D). These data indicate that the Alox15−/− mice are protected against experimentally induced hypertension.
Fig. 1.
Systolic blood pressures of wild-type (WT) and Alox15−/− mice under normal or experimental hypertension conditions. Basal blood pressure of male (A) and female (B) mice. Blood pressure of WT vs. Alox15−/− male mice under NG-nitro-l-arginine-methyl ester (l-NAME; C)- or deoxycorticosterone acetate (DOCA)/high-salt (D)-induced hypertension. Blood pressure was monitored using tail-cuff method. Each value represents the mean ± SE; n = 8–11 animals per group. ANOVA test was performed to find any significant differences. *P < 0.01 compared with control.
Vasorelaxation to ACh in WT vs. Alox15−/− mouse mesenteric arteries and abdominal aorta.
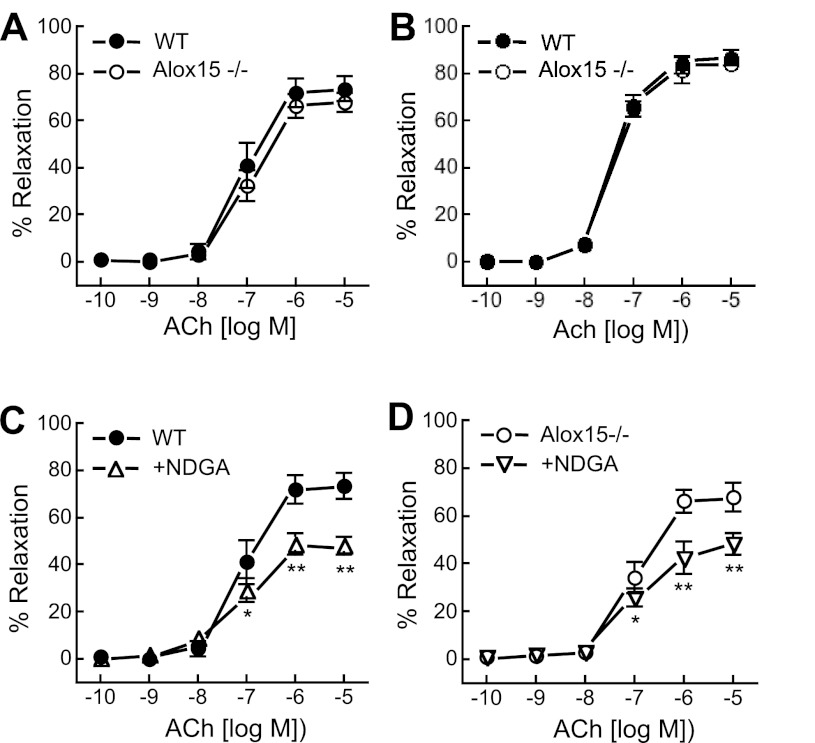
In preconstricted mesenteric arterial and abdominal aorta rings that were pretreated with Indo and l-NA, ACh (10−10 to 10−5 M) caused concentration-dependent relaxations. There was no significant difference in ACh-induced relaxations between arteries (Fig. 2A) and aortas (Fig. 2B) from WT and Alox15−/− mice. The LO inhibitor NDGA (50 μM) inhibited the relaxation of both WT and Alox15−/− arteries to ACh (10−5 M) by ∼35% (Fig. 2, C and D). Similar patterns were observed with AA-induced relaxations (data not shown). These data indicate that LO metabolites of AA contribute to ACh- and AA-mediated relaxations of mouse mesenteric arteries; however, Alox15 is not the LO mediating these relaxations.
Fig. 2.
Acetylcholine (ACh) relaxations of mouse mesenteric arteries and abdominal aortas of WT and Alox15−/− mice. Contribution of Alox15 on ACh relaxations of mesenteric artery (A) and abdominal aorta (B). Effect of the lipoxygenases (LO) inhibitor nordihydroguaiaretic acid (NDGA; 50 μM) on ACh relaxation of arteries from WT (C) and Alox15−/− (D) mice. All vessels were pretreated with 30 μM NG-nitro-l-arginine and 10 μM indomethacin (Indo) to block the nitric oxide and cyclooxygenase pathways and preconstricted with U46619. Each value represents the means ± SE; n = 10–12. *P < 0.01, **P < 0.001 compared with control.
AA metabolism by mouse aorta and peritoneal macrophages.
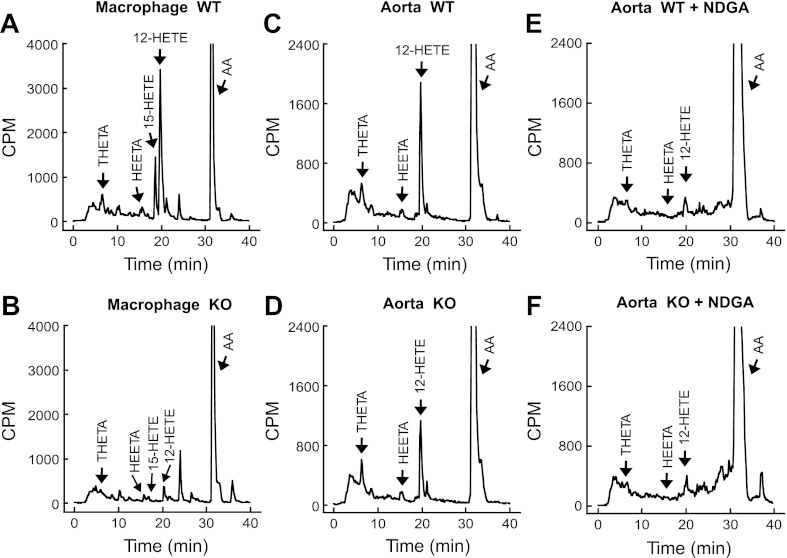
Macrophages and aortas from WT and Alox15−/− C57BL6 mice were incubated with [14C]AA in the presence of Indo and A23187. With the use of reverse-phase HPLC, solvent system I, the major [14C]AA metabolites were identified by comparing the retention times of radioactive metabolites with known standards for THETAs, HEETAs, and HETEs. The WT macrophages and aortic segments produced similar profiles of metabolites (Fig. 3, A and C). The predominant metabolite was 12-HETE in both cases. The synthesis of 15-HETE was not observed in aorta. Incubations under the same conditions with macrophages and aortic segments from Alox15−/− mice resulted in different profiles (Fig. 3, B and D). In macrophages, all four of the major metabolites were absent. In aorta, the radioactive peak comigrating with 12-HETE was decrease by ∼40%; however, the other metabolites remained unchanged. The LO inhibitor, NDGA completely blocked metabolite formation in aortas from both WT and Alox15−/− mice (Fig. 3, E and F).
Fig. 3.
Metabolism of [14C]arachidonic acid (AA) by mouse peritoneal macrophages and aortic rings. Macrophages (8 × 106) from WT (A) or Alox15−/− (B) mice and aortic rings from WT (C) or Alox15−/− (D) mice were incubated with [14C]AA in the presence of 10 μM Indo and 20 μM A23187. Effect of the LO inhibitor NDGA (10 μM) on the metabolism of [14C]AA by aortic rings from WT (E) and Alox15−/− (F) mice. Metabolites were extracted and resolved by RP-HPLC using solvent system I. Migration times of known standards are indicated in A-F. Traces represent ≥3 experiments. HEETA, hydroxyl-epoxyeicosatrienoic acids; 12-HETE, 15-hydroxyeicosatetraenoic acid; THETA, trihydroxyeicosatrienoic acid; CPM, counts/min.
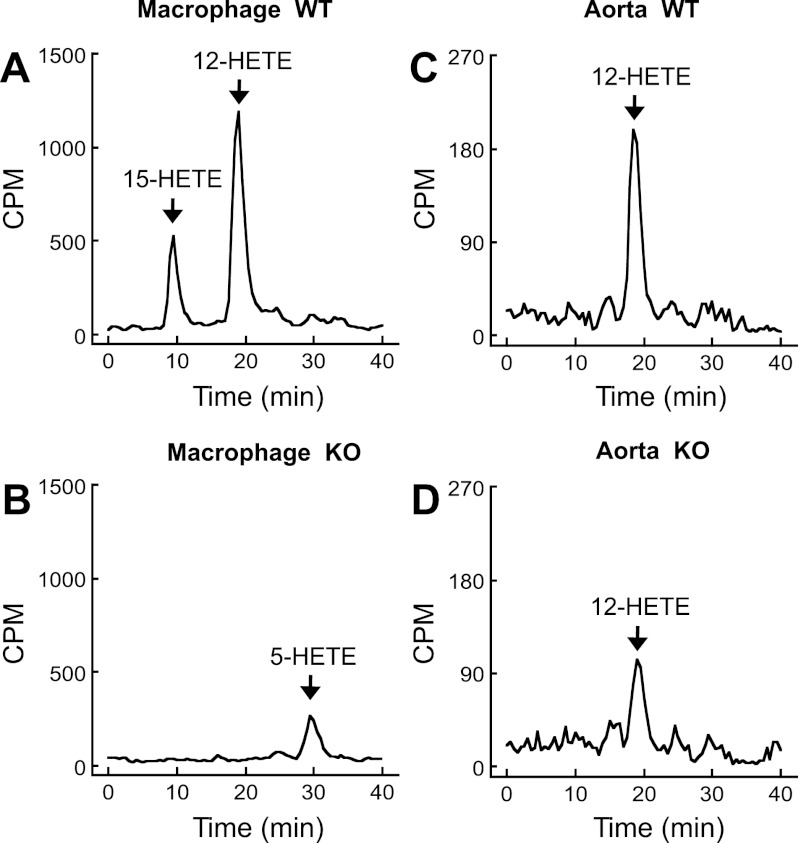
To focus on HETE synthesis, the extracted samples were also analyzed by reverse-phase HPLC, solvent system II. Macrophages from WT mice synthesized both 12- and 15-HETE (Fig. 4A). The 15-HETE and 12-HETE were synthesized in a ratio of 1:3 (Fig. 4A), the ratio described for Alox15 (34, 50). Macrophages from Alox15−/− mice synthesized 5-HETE, but neither 15-HETE nor 12-HETE was produced suggesting activation of the Alox5 pathway (48) (Fig. 4B). In contrast, only 12-HETE was synthesized by aortic rings (Fig. 4, C and D). The synthesis of 12-HETE was reduced, but not eliminated, in aortas from Alox15−/− mice compared with aortas from WT mice.
Fig. 4.
Metabolism of [14C]AA to HETEs by macrophages (8 × 106) from WT (A) and Alox15−/− (B) mice or aortic rings from WT (C) and Alox15−/− (D) mice. Macrophages or aortic rings were incubated with [14C]AA in the presence of 10 μM Indo and 20 μM A23187. Metabolites were extracted and resolved by RP-HPLC using solvent system II. Migration times of known standards are indicated in A–D. Traces represent 3 experiments.
Alox15 protein expression in mouse tissues.
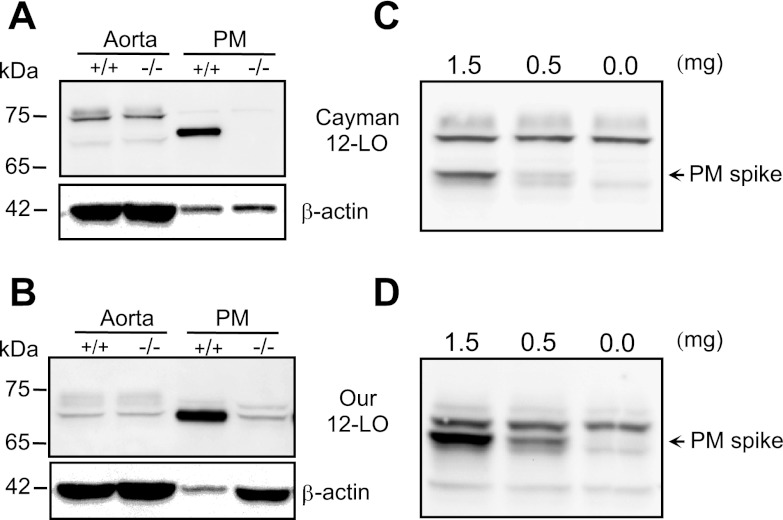
Initially, Alox15 protein content was determined by immunoblotting in aorta and peritoneal macrophages of WT and ALOX15−/− mice using two different 12-LO primary antibodies. The expression of Alox15 was high in macrophages from WT mice but absent in macrophages from Alox15−/− mice (Fig. 5, A and B). On the other hand, Alox15 expression did not differ between aortas from WT and Alox15−/− mice (Fig. 5, A and B). It is important to note the difference between the two antibodies. Both 12-LO antibodies detected a 70-kDa immunoreactive protein in macrophages but detected different proteins in aortas. The antibody generated in our laboratory detected a 70-kDa immunoreactive protein in aortas, and this protein comigrated with the macrophage LO protein (Fig. 5B). In contrast, the Cayman 12-LO antibody primarily detected an immunoreactive protein of 76 kDa (Fig. 5A). Thus using Cayman 12-LO antibody without the macrophage LO protein as a positive control can lead to an erroneous identification of Alox15. The third 12-LO antibody from Dr. Colin Funk gave as almost identical result to Cayman (data not shown). It detected a 70-kDa protein in macrophages and a 76-kDa protein in aortic homogenates. Since there was no difference between the immunoreactive band densities of aortas from WT and Alox15−/− mice, we used a high-resolution 7.5% gel and added macrophage lysate protein to confirm the identity of the immunoreactive bands. The same aortic lysates were spiked with 0.0, 0.5, or 1.5 μg of macrophage proteins. With the use of both the Cayman 12-LO antibody (Fig. 5C) and our 12-LO antibody (Fig. 5D), Fig. 5, C and D, indicates no expression of an immunoreactive protein in aorta that comigrates with the macrophage Alox15 protein after the nonspecific bands were resolved with the high-resolution gel. Thus the 76- and 70-kDa proteins in aortic lysates do not represent Alox15 but another LO or a nonspecific, cross-reacting protein.
Fig. 5.
Expression of Alox15 protein in mouse aorta and peritoneal macrophages. Western immunoblot of lysates prepared from aorta and peritoneal macrophages (PM) detected with Cayman Chemical 12-LO primary antibody (A) or 12-LO antibody raised in our laboratory (B). β-Actin was used as a loading control. Aorta lysates were spiked with 0.0, 0.5, or 1.5 μg of peritoneal macrophage total lysate protein, proteins resolved with a high resolution (7.5%) gel and detected with Cayman (C) or our (D) 12-LO antibodies. +/+, WT; −/−, Alox15 gene deletion. Representative blots of 3 experiments.
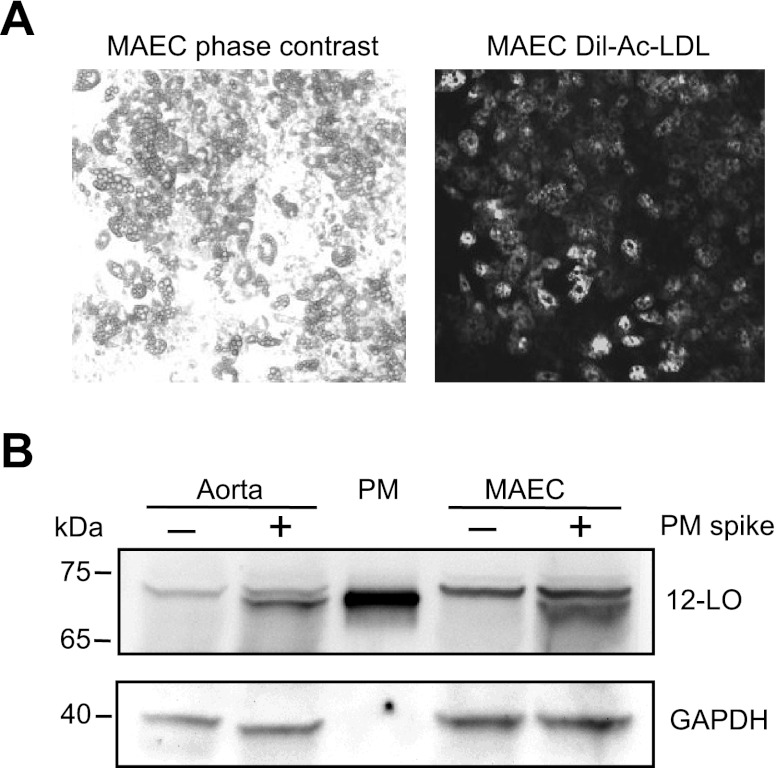
To exclude the possibility that Alox15 protein was not detected in aorta due to the low ratio of endothelium to smooth muscle, we isolated and cultured the aortic endothelium. MAEC culture was characterized by fluorescently labeled LDL uptake. As it is visible in Fig. 6A by comparing the phase contrast and the fluorescent images, all cells exhibited uptake of DiI-Ac-LDL, a characteristic only for endothelium. Similarly to aorta, the MAECs did not have an immunoreactive protein comigrating with the macrophage Alox15 protein (Fig. 6B).
Fig. 6.
Analysis of Alox15 expression in mouse aorta and cultured mouse aortic endothelial cells. A: identification of endothelial cells by Dil-Ac-LDL uptake. Isolated mouse aortic endothelial cells (MAECs) were visualized by phase-contrast microscopy (left) and by fluorescence microscopy (right) after 4 h of incubation with 10 mg/ml Dil-Ac-LDL. A and B: same representative area of the endothelial culture. B: representative blot of 3 Western immunoblot experiments on 50 μg/lane lysates prepared from the aorta and MAEC with (+) or without (−) addition of 0.5 μg of PM protein. GAPDH was used as a loading control.
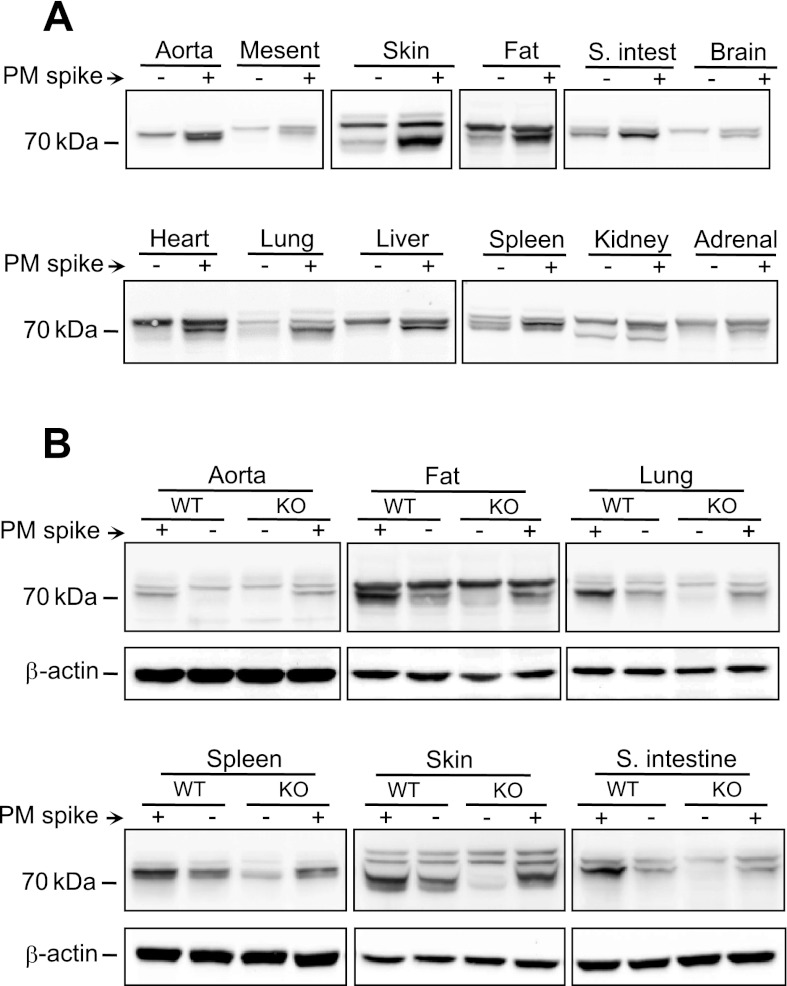
Alox15 expression was determined in mouse organs including mesenteric artery, aortic fat, heart, lung, liver, spleen, kidney, adrenal gland, skin, small intestine, and brain (Fig. 7A). Aortic fat, lung, spleen, skin, and small intestine samples showed immunoreactive bands that comigrated with the macrophage Alox15 protein. Then, tissues from WT and Alox15−/− mice were compared for the Alox15 expression. All tissues that had immunoreactive bands comigrating with the macrophage protein showed a clear reduction or elimination of Alox15 protein in samples from Alox15−/− mice compared with WT mice (Fig. 7B). Aortas were included as a negative control.
Fig. 7.
Western immunoblot analysis of Alox15 expression in tissues from WT and Alox15−/− mice. A: samples prepared from WT aorta, mesenteric artery, heart, liver, lung, spleen, kidney, adrenal gland, skin, small intestine, and brain with (+) or without (−) addition of 0.5 μg of PM protein. B: comparison of aorta, aortic fat, lung, spleen, skin, and small intestine of WT (+/+) and Alox15−/− (−/−) mice with (+) or without (−) 0.5 μg of peritoneal macrophage protein spike. β-Actin was used as a loading control. Representative blots of 3 experiments are shown.
RT-PCR analyses of mouse tissues.
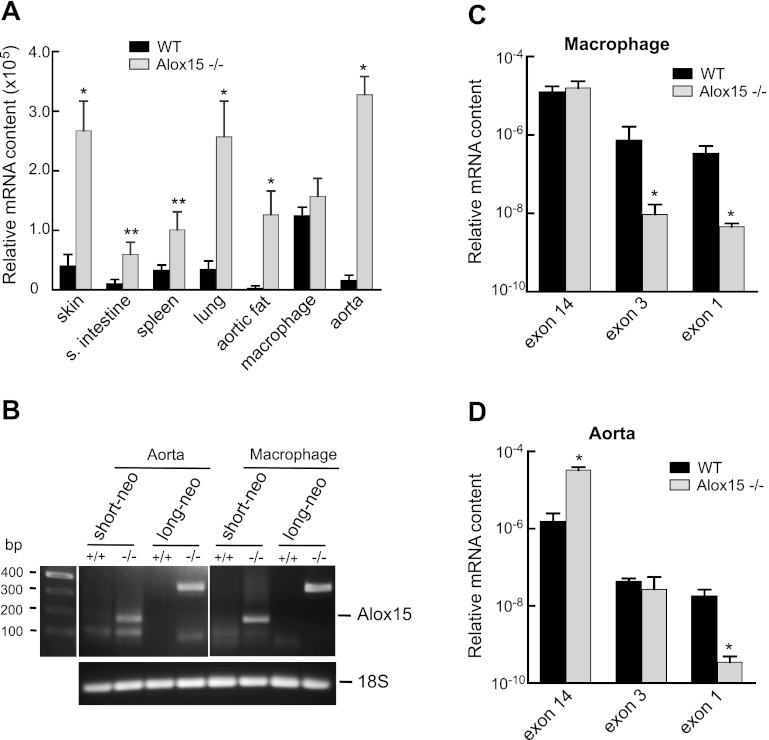
RT-PCR was performed to confirm the presence of Alox15 mRNA in tissues where the protein expression was detected in WT mice and the absence of Alox15 mRNA in tissues from Alox15−/− mice. The relative mRNA content in different tissues was initially determined using a primer set designed for exon 14 of the Alox15 gene (Fig. 8A). Alox15 mRNA was detected in every tissue examined including aorta even though it did not express the protein. It was also detected in all tissues from Alox15−/− mice. In some cases, the intensity of the signal was significantly higher in tissues from Alox15−/− mice compared with tissues from WT mice. To examine whether the disrupted Alox15 gene was being transcribed, we designed two additional primer sets (short-neo and long-neo). Forward primer of short-neo annealed to exon 2 of the Alox15 gene, while the forward primer of long-neo annealed to a region spanning the border of exons 1 and 2. The common reverse counterpart annealed to the neo cassette sequence, which made the product appearance possible only if the neo sequence was present in the mRNA. As can be seen on Fig. 8B, the 159- and 320-bp products for short-neo and long-neo primers, respectively, appeared in aortas and macrophages from Alox15−/− mice but not from WT mice. Murine 18S was used as a loading control. Two additional primer sets were designed for exon 3 and 1. These primers were used to investigate the relationship between the ratio of the mRNA signals in WT and Alox15−/− samples and the influence of the position of the primer set (Fig. 8, C and D). PCR products of the expected size were obtained with the two sets of primers in samples from aorta and macrophages. These PCR products from aortas and macrophages of WT and Alox15−/− mice were sequenced (Retrogen). The sequences of the products from the exon 3 primers were identical to each other and matched the reported sequence of Alox15 (48, 50). The same was true for the exon 14 primer products. The signal with macrophage and aortic samples from WT to Alox15−/− mice differed when primers for exon 3 were used. The abundance of mRNA was decreased in aortas from Alox15−/− mice when exon 1 primers were used (Fig. 8D). Thus the selection of primers influences the detection of the mRNA abundance from macrophages and aorta of WT and Alox15−/− mice.
Fig. 8.
RT-PCR detection of Alox15 mRNA expression in mouse tissues. A: comparison of Alox15 mRNA expression in tissues of WT and Alox15−/− mice using a primer set for exon 14. B: detection of neo-cassette sequence in aorta and macrophage of WT (+/+) and Alox15−/− (−/−) mice using short-neo (159 bp product) or long-neo (320 bp product) primer sets. Murine 18S was used as a loading control. Representative gel of 3 experiments. Relative mRNA content using primers for exon 14, exon 3, and exon 1 in macrophage (C) and aorta (D) of WT and Alox15−/− mice. In A, C, and D, values are means ± SE; n = 6–8. * P < 0.01, **P < 0.001 for Alox15−/− compared with WT.
Role of 12/15-LO in development of l-NAME-induced hypertension.
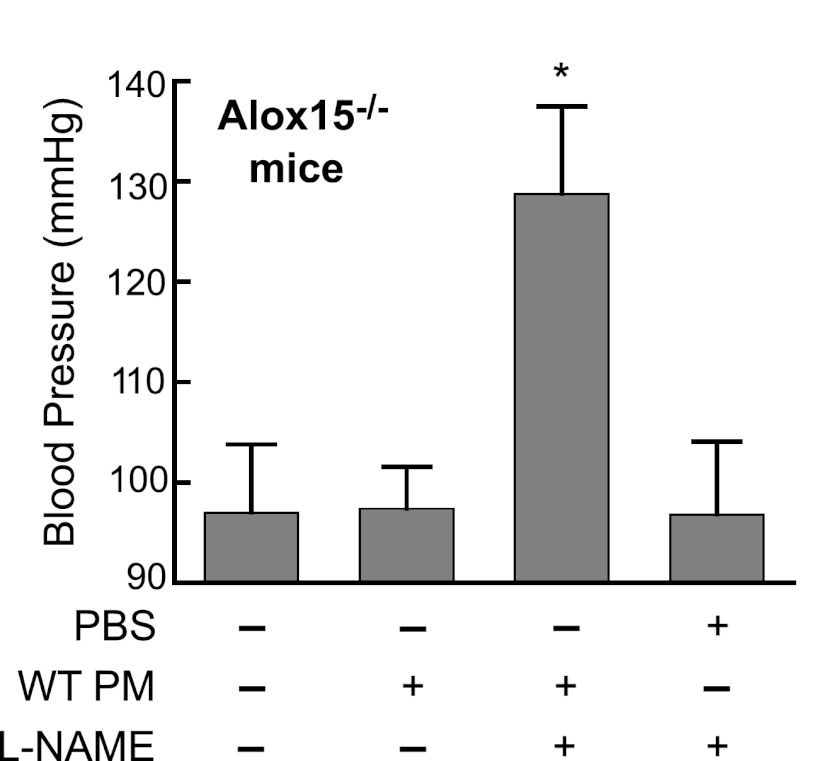
Since the only known difference between WT and Alox15−/− mice is the lack of 12/15-LO expression in macrophages of the latter, we designed a rescue experiment to restore the macrophage functions by transfusing WT peritoneal macrophages into Alox15−/− mice. Elicited peritoneal macrophages harvested from female WT mice were injected intraperitoneally into male Alox15−/− mice followed by induction of hypertension. As seen in Fig. 9, the Alox15−/− mice receiving WT macrophages showed a significant increase of blood pressure after 5 days of l-NAME treatment. The blood pressure of animals receiving vehicle plus l-NAME treatment remained at the baseline level. These data suggest the involvement of macrophage 12/15-lipoxygenase in processes leading to development of an experimental hypertension.
Fig. 9.
Systolic blood pressure of Alox15−/− mice in l-NAME-induced hypertension model. Mice were injected intraperitoneally with either 8 × 106 WT PM or vehicle (PBS) followed by induction of hypertension (l-NAME). Blood pressure was determined on a fifth day of the l-NAME treatment by tail-cuff method. Control group did not receive any treatment. Values are means ± SD; n = 5. *P < 0.01, compared with PBS + l-NAME group.
Effect of macrophage depletion on blood pressure in l-NAME-induced hypertension.
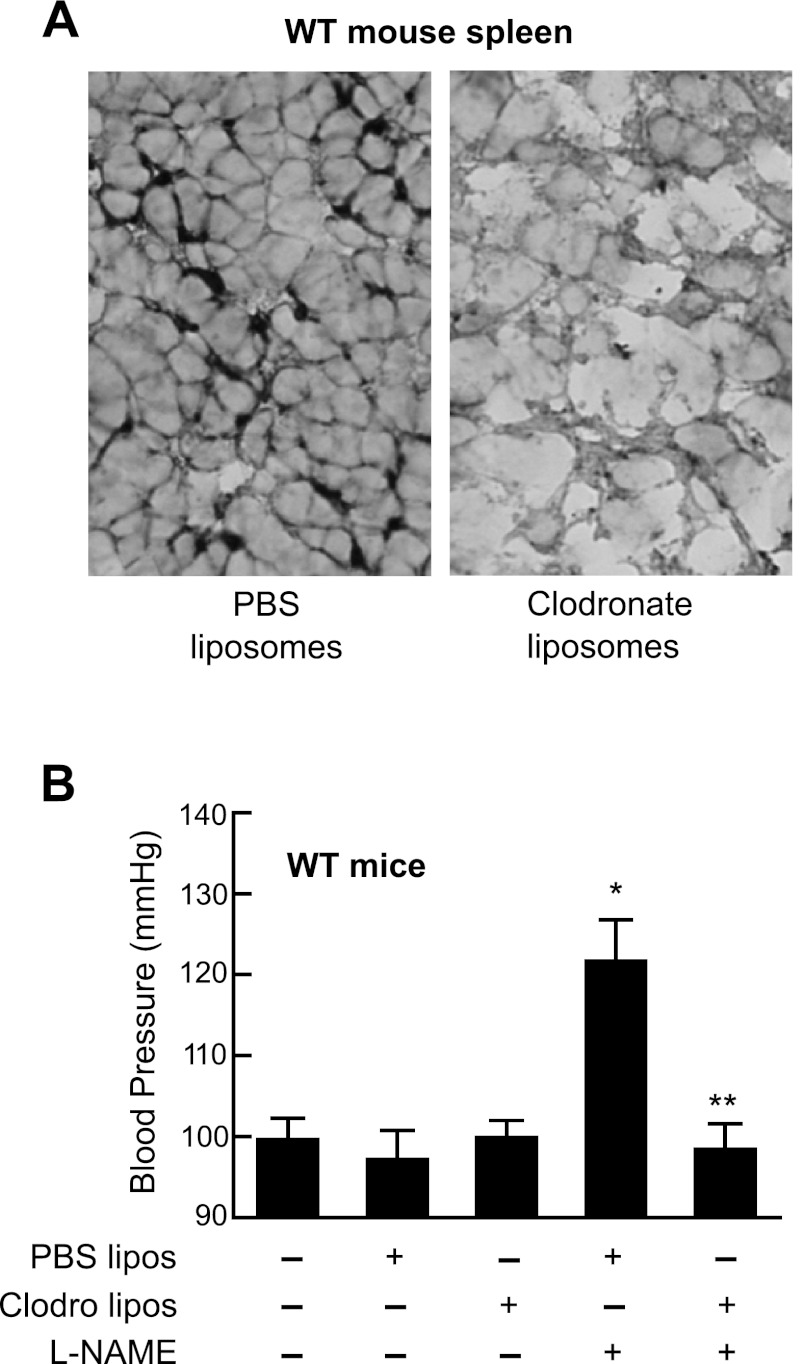
The distribution of cell types in the elicited peritoneal lavage was 85 ± 5% macrophage, 12 ± 3% lymphocytes, and 3 ± 1% granulocytes as determined by flow cytometry. T-lymphocyes were isolated by fluorescence cell sorting and lysates analyzed by immunoblotting for Alox15. No Alox15 was detected in T-lymphocytes. Thus macrophages are the source of Alox15 in peritoneal lavage cells. To prove the involvement of macrophages in l-NAME-induced hypertension, macrophages were depleted from the whole body of male WT mice using the clodronate-liposome injection method. The effectiveness of the depletion was confirmed by assessing the macrophage content of spleens from animals injected with either control PBS liposomes or clodronate liposomes (Fig. 10A). Clodronate-liposome treatment depleted the spleen macrophages. CD68-positive dark staining spots were absence from clodronate-treated (Fig. 10A, right) but present in PBS-liposome-treated spleens (Fig. 10A, left). We then determined the susceptibility of macrophage-depleted animals to l-NAME-induced hypertension. The WT mice that received PBS liposomes developed elevated blood pressures with l-NAME treatment, while the blood pressures of mice depleted of macrophages remained at basal values after 5 days of l-NAME treatment (Fig. 10B). These results suggest a pivotal role of macrophages in development of experimental hypertension.
Fig. 10.
Effect of clodronate treatment on the macrophage content of the spleen (A) and blood pressure of C57BL6 mice with l-NAME-induced hypertension (B). A: spleens of PBS-liposome (left)- or clodronate-liposome (right)-injected mice were subjected to immunohistochemical staining with anti-macrophage CD68 antibody. B: mice were injected with either PBS liposomes (PBS lipos) or clodronate liposomes (Clodro lipos) followed by induction of hypertension with l-NAME. Blood pressure was determined on a fifth day of l-NAME treatment by the tail-cuff method. Control group did not receive any treatment. Values are means ± SD; n = 6–8. *P < 0.01, compared with control or PBS liposomes. **P < 0.001, compared with PBS liposomes + l-NAME group.
DISCUSSION
The contribution of EDHF to the regulation of vascular tone is widely accepted. As an endogenous vasodilator, it mediates a portion of the endothelium-dependent relaxations to ACh, bradykinin, and shear stress (5, 9). The chemical identity of the mediator of the EDHF response varies among species and vessel types. In murine arteries, EETs, H2O2, and C-type natriuretic peptide have been shown to function as EDHFs (8, 28, 40). Recently, we (21) demonstrated that LO metabolites of AA also mediate EDHF activity in murine arteries. The non-NO, non-PG-mediated relaxations of mesenteric arteries were blocked by high extracellular potassium and inhibited by apamin that blocks potassium channel activity. Thus the relaxations were EDHF mediated. Additionally, the relaxations to ACh and AA were inhibited by LO inhibitors and an inhibitor of hydroperoxide isomerase. When mouse arteries were incubated with AA, the major metabolites were identified as LO products including 12-HETE, HEETA, 11,14,15-THETA, and 11,12,15-THETA. These studies indicate that mouse mesenteric arteries, like rabbit mesenteric arteries, synthesize LO metabolites that mediate the relaxations to ACh and AA (21, 57). In rabbit arteries, 15-LO-1 was identified as the endothelial LO, and 11(R),12(S),15(S)-THETA was the biologically active stereoisomer causing vascular relaxation through activation of smooth muscle apamin-sensitive K+ channels (6, 20, 22, 50). Similarly, 15-LO is expressed in bovine coronary endothelial cells (38). In murine arteries, the endothelial LO mediating the relaxations to ACh is not established even though Alox15 is considered a vascular LO (21, 51). LO inhibitors nonselectively block the activity of all LO isozymes so are of no value in determining the specific LO mediating a biological response. As a result, we used mice with deletion of the Alox15 gene to determine the contribution of this LO to endothelium-dependent relaxations and blood pressure (48).
There was no difference in the basal blood pressures between male or female WT and Alox15−/− mice. This is in agreement with others (1). Inhibition of endothelial NO synthase with l-NAME or deletion of the gene in male and female WT mice elevates the blood pressure indicating that NO is involved in blood pressure regulation (43). However, when l-NAME hypertension was induced in male and female Alox15−/− mice, the mice were completely resistant. This finding contradicts the results published by Anning et al. (1). This resistance of Alox15−/− mice to experimental hypertension is not unique for NO inhibition. Similar resistance was observed for angiotensin II-induced hypertension (1), and in the present study, the Alox15−/− mice were resistant to DOCA/high-salt-induced hypertension. This indicates the involvement of Alox15 in several forms of experimental hypertension.
Previous studies (21, 57) indicate that mouse and rabbit mesenteric arteries synthesize LO metabolites that mediate the relaxations to ACh and AA. However, the non-NO, non-PG mediated relaxations to ACh and the relaxations to AA were identical in mesenteric arteries from WT and Alox15−/− mice indicating that an AA metabolite of Alox15 is not involved in the relaxations. Interestingly, the ACh-induced relaxations in mesenteric arteries from WT and Alox15−/− mice are inhibited by the LO inhibitor NDGA. Thus an LO other than vascular Alox15 is involved in the synthesis of vasoactive AA metabolites that mediate the relaxations to ACh and AA.
Alox15 is the mouse homolog of rabbit 15-LO-1 and human leukocyte-type 15-LO (17, 48). Both 15-LO-1 and Alox15 are 12/15-LOs and synthesize both 15-HETE and 12-HETE (17, 34, 48). However, there are differences in the two LOs. The 12-HETE-to-15-HETE ratio changes from 1:12 with 15-LO-1 in the rabbit vasculature (35) to 3:1 with Alox15 in mouse macrophages (10). This issue is even further complicated. Unlike mouse macrophages, mouse arteries produce very little, if any, 15-HETE. In a previous study (21), we identified 15-HETE in mouse aortic incubates; however, it was a minor component. One possible explanation is that the metabolism of 15-HPETE by hydroperoxide isomerase to HEETA and THETA results in a decreased amount of 15-HETE without a change in 12-HETE. However, the comparison of profiles of AA metabolites in aorta and peritoneal macrophages in this study raises questions of the identity of the vascular LO. In contrast to macrophages, the aorta tissue produces little 15-HETE and the synthesis of HEETA and THETA is not reduced by disruption of Alox15 gene. These results are consistent with the relaxation studies and indicate that the LO responsible for AA metabolism in aorta is not Alox15 but rather another LO, which is not altered in Alox15−/− mice (48). For example, a novel form of platelet-type 12-LO in human vascular smooth muscle cells produces 12-HETE (39). It is not known if the mouse has a similar vascular 12-LO.
The lack of a role for Alox15 in arteries is further supported by our measurements of protein expression. Western immunoblots using three different antibodies against different 12-LO epitopes failed to detect Alox15 expression in aorta. Peritoneal macrophages, which express Alox15 abundantly, were used as an internal standard to identify the Alox15 band in the midst of closely migrating nonspecific ones. With the use of this approach, Alox15 was detected in lung, spleen, small intestine, fat, and skin but not aorta or mesenteric arteries. In many cases, Alox15 was detected in tissues that have resident macrophages (7, 11, 26, 31). The presence of Alox15 in these tissues was further confirmed by its absence in tissues from Alox15−/− mice. The detection of Alox15 in small intestine and its absence in intestines of Alox15−/− mice are particularly interesting, since Kawajiri et al. (30) failed to detect Alox15 expression in mouse intestinal tissue.
With the absence of Alox15 expression in the vasculature, it is not surprising that genetic disruption of the Alox15 gene did not affect ACh- or AA-mediated relaxation. The LO inhibitor NDGA significantly attenuated the ACh-induced relaxations to a similar extent in mesenteric arteries from both WT and Alox15−/− mice, indicating that vasoactive metabolites from another LO contributes to the relaxations. Similarly, the basal systolic blood pressures of both male and female mice were not affected by the disruption of Alox15. The Alox15−/− mouse has been widely used to define the biological role of Alox15 in a number of pathological conditions including diabetes (2), wound healing (26), ischemia/reperfusion (52) atherosclerosis (12, 18, 23), and hypertension (1). While Alox15 is not expressed in the vasculature under normal conditions, it is possible that its expression is induced in the vasculature in some of these conditions. More likely, the infiltrating macrophages or leukocytes are the source of Alox15 in these pathological processes.
The vascular expression of Alox15 was further studied using three sets of RT-PCR primers: one used by Chakrabarti et al. (7) designed for exon 3, one used by Shappell et al. (45) for exon 14, and a Taqman expression assay designed for exon 1 of the Alox15 mRNA. PCR experiments with the three primers indicated the presence of Alox15 mRNA in tissue samples including arteries from WT and Alox15−/− mice. In some cases, the Alox15 signal was greater in samples from Alox15−/− than WT mice. The elevated Alox15 signal in tissues from Alox15−/− mice seemingly contradicts to findings of Huo et al. (29) that Alox15 mRNA was diminished in most organs of the mice following an Alox15−/− bone marrow replacement. The difference in results between the two studies is due to the location of the primer sets used. The relative expression of the mRNA product does not affect protein levels of Alox15 since the neo insert with the in-frame stop codon prevents translation.
We detected Alox15 mRNA in mouse aorta, which might come from the vascular cells or adherent macrophages or coagulated blood that remained after tissue preparation. The presence of Alox15 mRNA combined with the lack of immunodetectable Alox15 protein raises the possibility of posttranscriptional regulation of Alox15 in blood vessels. A similar phenomenon was reported in cultured human endothelial cells (39). IL-4 induced the transcription of the 15-LO-1 gene; however, this did not result in functional or immunodetectable 15-LO-1 protein (36). Similarly, translation of the 15-LO-1 gene is regulated by hn-RNP proteins (41). It is not known if similar regulation of translation occurs with the mouse 15-LO-1 homolog, Alox15. Endogenous expression of Alox15 mRNA is clearly without functional significance since the metabolism of AA and relaxations to ACh and AA did not differ between the WT and Alox15−/− blood vessels.
When it became clear that experimental hypertension is not regulated by AA metabolites of Alox15 formed in vascular endothelium, we turned our attention to the extravascular source of lipoxygenase. Platelet-type 12-LO was implicated in spontaneous hypertension in rats, characterized by elevated 12-HETE production (24, 47), but we could not detect significant difference of 12-HETE production between WT and Alox15−/− arteries. This finding is in agreement with the results of Dr. Colin Funk (48), showing that platelet-type 12-LO is unaffected by disruption of the Alox15 gene. WT peritoneal macrophages are the main source of the Alox15 protein while the Alox15−/− mice are lacking any expression. To test the hypothesis that macrophage Alox15 is mediating the development of experimental hypertension, we designed a rescue experiment. Injection of elicited macrophages harvested from WT mice into the Alox15−/− mice restored their sensitivity towards l-NAME-induced hypertension. In a recently published working hypothesis (27) of how the immune response to the initial stimuli causes hypertension, macrophages and T lymphocytes are proposed to play a prominent role. Activated macrophages release NO and variety of cytokines such as TNF-α, IL-6, and IL-1 (25). The Alox15 pathway specifically was implicated in the production of several cytokines. IL-12 and IFN-γ were down regulated in the Alox15 KO mice (58), while in 12/15-LO transgenic mice IL-1β, IL-6, IL-12, and MCP-1 expression were increased (54). Alox15 products, 12-HETE, and 12-HPETE upregulated the expression of proinflammatory cytokines such as TNF-α, MCP-1, IL-6, and IL-12 (7). These cytokines along with released free radicals may serve as direct hypertensive stimuli or indirect stimuli through alteration of T-cell functions (27).
Our peritoneal lavage consisted mostly of macrophages, but it also contained 12% lymphocytes. Alox15 was not detected in T lymphocytes so macrophages are the source of Alox15 in peritoneal lavage cells. Although the involvement of T lymphocytes in l-NAME-induced hypertension has not been excluded, the macrophage depletion experiment indicates the importance of macrophages. Macrophage-depleted WT mice became resistant to l-NAME-induced hypertension. Clodronate injected in liposomal vehicles depletes only phagocytic cells from the whole organism leaving the number of T and B cells unaffected (16). However, it was also shown that the absence of macrophages delayed T-cell infiltration (16), and this delay might be a crucial step in promoting hypertension. This is supported by the finding that DOCA/high-salt-induced hypertension cannot be maintained by athymic nude mice (49).
In summary, these studies indicate that Alox15 mRNA expression in the mouse vasculature is apparently not translated since the Alox15 protein and enzymatic activity are not detected. As a result, the relaxations to ACh and AA and blood pressures are identical in arteries from WT and Alox15−/− mice. We found the macrophage Alox15 expression is essential for developments of experimental hypertension. The role of macrophages was implicated by eliminating hypertension in macrophage-deprived WT mice and by restoring the hypertension in Alox15−/− mice transfused with WT macrophages.
GRANTS
These studies were supported by National Heart, Lung, and Blood Institute Grant HL-37981.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.K., C.C., D.M., L.S., and K.M.G. performed experiments; T.K., C.C., D.M., L.S., and K.M.G. analyzed data; T.K. interpreted results of experiments; T.K. prepared figures; T.K. drafted manuscript; T.K., K.M.G., and W.B.C. edited and revised manuscript; K.M.G. and W.B.C. conception and design of research; W.B.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Drs. Mark T. McNally and Vera L. Tarakanova for helpful discussions on the RT-PCR data. Dr. Jeffrey E Woodliff helped in the design and interpretation of the flow cytometry experiments. We thank Dr. David X. Zhang for advice in culturing MAECs. We thank Chuely Vang for technical assistance and Gretchen Barg for secretarial assistance.
REFERENCES
- 1.Anning PB, Coles B, Bermudez-Fajardo A, Martin PEM, Levison BS, Hazen SL, Funk CD, Kuhn H, O'Donnell VB. Elevatged endothelial nitric oxide bioactivity and resistance to angiotensin-dependent hypertension in 12/15-lipoxygenase knockout mice. Am J Pathol 166: 653–662, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bleich D, Chen S, Zipser B, Sun D, Funk CD, Nadel JA. Resistance to type 1 diabetes induction in 12-lipoxygenase knockout mice. J Clin Invest 103: 1431–1436, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brash AR. Lipoxygenases: occurrences, function, catalysis, and acquisition of substrate. J Biol Chem 274: 23679–23682, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Bryant RW, Bailey JM. Rearrangement of 15-hydroperoxy-eicosatetraenoic acid (15-HPETE) during incubations with hemoglobin: a model for platelet lipoxygenase metabolism. Prog Lipid Res 20: 279–281, 1981 [DOI] [PubMed] [Google Scholar]
- 5.Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension 49: 590–596, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Campbell WB, Spitzbarth N, Gauthier KM, Pfister SL. 11,12,15-Trihydroxyeicosatrienoic acid mediates acetylcholine-induced relaxations in the rabbit aorta. Am J Physiol Heart Circ Physiol 285: H2648–H2656, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti SK, Cole BK, Wen Y, Keller SR, Nadel JA. 12/15-Lipoxygenase products induce inflamation and impair insulin signaling in 3T3-Li adipocytes. Obesity 17: 1657–1663, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci USA 100: 1426–1431, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chawengsub Y, Gauthier KM, Campbell WB. Role of arachidonic acid lipoxygenase metabolites in the regulation of vascular tone. Am J Physiol Heart Circ Physiol 297: H495–H507, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen XS, Kurre U, Jenkins NA, Copeland NG, Funk CD. cDNA cloning, expression, mutagenesis of C-terminal isoleucine, genomic structure, and chromosomal localizations of murine 12-lipoxygenases. J Biol Chem 269: 13979–13987, 1994 [PubMed] [Google Scholar]
- 11.Coffey MJ, Natarajan R, Chumley PH, Coles B, Thimmalapura PR, Nowell M, Kuhn H, Lewis MJ, Freeman BA, O'Donnell VB. Catalytic consumption of nitric oxide by 12/15-lipoxygenase: inhibition of monocyte soluble guanylate cyclase activation. Proc Natl Acad Sci USA 98: 8006–8011, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cyrus T, Pratico D, Zhao L, Witztum JL, Rader DJ, Rokach J, FitzGerald GA, Funk CD. Absence of 12/15-lipoxygenase expression decreases lipid peroxidation and atherogenesis in apolipoprotein E-deficient mice. Circulation 103: 2277–2282, 2001 [DOI] [PubMed] [Google Scholar]
- 13.De Marzo N, Sloane DL, Dicharry S, Highland E, Sigal E. Cloning and expression of an airway epithelial 12-lipoxygenase. Am J Physiol Lung Cell Mol Physiol 262: L198–L207, 1992 [DOI] [PubMed] [Google Scholar]
- 14.Endsley MPAN, Isbell MA, Wheelock CE, Hammock BD, Falck JR, Campbell WB, Nithipatikom K. Diverse roles of 2-arachidonoylglycerol in invasion of prostate carcinoma cells: location, hydrolysis and 12-lipoxygenase metabolism. Int J Cancer 121: 984–991, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Förstermann U, Neufang B. The endothelium-dependent vasodilator effect of acetylcholine: characterization of the endothelial relaxing factor with inhibitors of arachidonic acid metabolism. Eur J Pharmacol 103: 65–70, 1984 [DOI] [PubMed] [Google Scholar]
- 16.Fox A, Koulmanda M, Mandel TE, van Rooijen N, Harrison LC. Evidence that macrophages are required for T-cell infiltration and rejection of fetal pig pancreas xenografts in nonobese diabetic mice. Transplantation 66: 1407–1416, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Funk CD. The molecular biology of mammalian lipoxygenases and the quest for eicosanoid function using lipoxygenase-deficient mice. Biochim Biophys Acta 304: 65–84, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Funk CD, Cyrus T. 12/15-Lipoxygenase, oxidative modification of LDL and atherogenesis. Trends Cardiovasc Med 11: 116–124, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Furchgott RF, Zawadzki JW. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980 [DOI] [PubMed] [Google Scholar]
- 20.Gauthier KM, Chawengsub Y, Goldman DM, Conrow RE, Anjaiah S, Falck JR, Campbell WB. 11(R),12(S),15(S)-trihydroxyeicosatrienoic acid: an endothelium-derived 15-lipoxygenase metabolite that relaxes rabbit aorta. Am J Physiol Heart Circ Physiol 294: H1467–H1472, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Gauthier KM, Goldman DH, Aggarwal NT, Chawengsub Y, Falck JR, Campbell WB. Role of arachidonic acid lipoxygenase metabolites in acetylcholine relaxations of mouse arteries. Am J Physiol Heart Circ Physiol 300: H725–H735, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gauthier KM, Spitzbarth N, Edwards EM, Campbell WB. Apamin-sensitive K+ currents mediate arachidonic acid-induced relaxations of rabbit aorta. Hypertension 43: 413–419, 2004 [DOI] [PubMed] [Google Scholar]
- 23.George J, Afek A, Shaish A, Levkovitz H, Bloom N, Cyrus T, Zhao L, Funk CD, Sigal E, Harats D. 12/15-lipoxygenase gene disruption attenuates atherogenesis in LDL receptor-deficient mice. Circulation 104: 1646–1650, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Nunez D, Claria J, Rivera F, Poch E. Increased levels of 12(S)-HETE in patients with essential hypertension. Hypertension 37: 334–338, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Gordon S. The role of the macrophage in immune regulation. Res Immunol 149: 685–688, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Gronert K, Maheshwari N, Khan MT, Hassan IR, Dunn MJ, Schwartzman ML. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem 280: 15267–15278, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Wey CM. Inflamation, immunity, and hypertension. Hypertension 57: 132–140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hercule HC, Schunck WH, Gross V, Seringer J, Leung FP, Weldon SM, Goncalves A, Huang Y, Luft FC, Gollasch M. Interaction between P450 eicosanoids and nitric oxide in the control of arterial tone in mice. Arterioscler Thromb Vasc Biol 29: 54–60, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Huo Y, Zhao L, Hyman MC, Shashkin P, Harry BL, Burcin T, Forlow SB, Stark MA, Smith DF, Clarke S, Srinivasan S, Hedrick CC, Pratico D, Witztum JL, Nadel JA, Funk CD, Ley K. Critical role of macrophage 12/15-lipoxygenase for atherosclerosis in apolipoprotein E-deficient mice. Circulation 110: 2024–2031, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Kawajiri H, Hsi LC, Kamitani H, Ikawa H, Geller M, Ward T, Eling TE, Glasgow WC. Arachidonic and linoleic acid metabolism in mouse intestinal tissue: evidence for novel lipoxygenase activity. Arch Biochem Biophys 398: 51–60, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Kayama Y, Minamino T, Toko H, Sakamoto M, Shimizu I, Takahashi H, Okada S, Tateno K, Moriya J, Yokoyama M, Nojima A, Yoshimura M, Egashira K, Aburatani H, Komuro I. Cardiac 12/15 lipoxygenase-induced inflamation is involved in heart failure. J Exp Med 206: 1565–1574, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi M, Inoue K, Warabi E, Minami T, Kodama T. A simple method for isolating mouse aortic endothelial cells. J Atheroscler Thromb 12: 138–142, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Kuhn H, Chan L. The role of 15-lipoxygenase in atherogenesis: a pro- and antiatherogenic actions. Curr Opin Lipidol 8: 111–117, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Kuhn H, Thiele BJ. Arachidonate 15-lipoxygenase. J Lipid Mediat Cell Signal 12: 157–170, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Kuhn H, Thiele BJ, Ostareck-Lederer A, Stender H, Suzuki H, Yoshimoto T, Yamamoto S. Bacterial expression, purification and partial characterization of recombinant rabbit reticulocyte 15-lipoxygenase. Biochim Biophys Acta 1168: 73–78, 1993 [DOI] [PubMed] [Google Scholar]
- 36.Lee YW, Kuhn H, Kaiser S, Kenning G, Daugherty A, Toborek M. Interleukin-4 induces transcription of the 15-lipoxygenase-1 gene in human endothelial cells. J Lipid Res 42: 783–791, 2001 [PubMed] [Google Scholar]
- 37.Li SL, Dwarakanath RS, Cai Q, Lanting L, Natarajan R. Effect of silencing leukocyte-type 12/15-lipoxygenase using short interfering RNAs. J Lipid Res 46: 220–229, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Li X, Hong S, Li PL, Zhang Y. Docosahexanoic acid-induced coronary arterial dilation: action of 17S-hydroxy docasahexanoic acid on K channel activity. J Pharmacol Exp Ther 336: 891–899, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Limor R, Weisinger G, Gilad S, Knoll E, Sharon O, Jaffe A, Kohen F, Berger E, Lifschizt-Mercer B, Stern N. A novel form of platelet-type 12-lipoxygenase mRNA in human vascular smooth muscle cells. Hypertension 38: 864–871, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kandaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest 106: 1521–1530, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ostareck DH, Ostareck-Lederer A, Wilm M, Thiele BJ, Mann M, Hentze MW. mRNA silencing in erythroid differentiation: hnRNP K and hnRNP E1 regulate 15-lipoxygenase translation from the 3' end. Cell 89: 597–606, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Pfister SL, Spitzbarth N, Nithipatikom K, Edgemond WS, Falck JR, Campbell WB. Identification of 11,14,15- and 11,12,15-trihydroxyeicosatrienoic acids as endothelium-derived relaxing factors of rabbit aorta. J Biol Chem 273: 30879–30887, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Scotland RS, Madhani M, Chauhan SD, Moncada S, Andresen J, Nilsson H, Hobbs AJ, Ahulwalia A. Investigation of vascular responses in endothelial nitric oxide synthase/cyclooxygenase-1 double knockout mice: key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation 111: 796–803, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Serhan CN, Hamberg M, Samuelson B. Lipoxins: a new series of biologically active compounds. In: Prostaglandins, Leukotriens and Lipoxins, edited by Bailey JM. New York: Plenum, 1985, p. 3–16 [Google Scholar]
- 45.Shappell SB, Olson SJ, Hannah SE, Manning S, Roberts RL, Masumori N, Jisaka M, Boeglin WE, Vader V, Dave DS, Shook MF, Thomas TZ, Funk CD, Brash AR, Matusik RJ. Elevated expression of 12/15-lipoxygenase and cyclooxygenase-2 in a transgenic mouse model of prostate carcinoma. Cancer Res 63: 2256–2267, 2003 [PubMed] [Google Scholar]
- 46.Shappell SB, Taylor AA, Hughes H, Mitchell JR, Anderson DC, Smith CW. Comparison of antioxidant and nonantioxidant lipoxygenase inhibitors on neutrophil function. Implications for pathogenesis of myocardial reperfusion injury. J Pharmacol Exp Ther 252: 531–538, 1990 [PubMed] [Google Scholar]
- 47.Stern N, Kisch ES, Knoll E. Platelet lipoxygenasein spontaneously hypertensive rats. Hypertension 27: 1149–1152, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Sun D, Funk CD. Disruption of 12/15-lipoxygenase expression in peritoneal macrophages. J Biol Chem 271: 24055–24062, 1996 [PubMed] [Google Scholar]
- 49.Svendsen UG. Evidence for an initial, thymus independent and a chronic, thymus dependent phase of DOCA and salt hypertension in mice. Acta Pathol Microbiol Scand A 84: 523–528, 1976 [DOI] [PubMed] [Google Scholar]
- 50.Tang X, Holmes BB, Nithipatikom K, Hillard CJ, Kuhn H, Campbell WB. Reticulocyte 15-lipoxygenase-1 is important in acetylcholine-induced endothelium-dependent vasorelaxation in rabbit aorta. Arterioscler Thromb Vasc Biol 26: 78–84, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Taylor AM, Hanchett R, Natarajan R, Hedrick CC, Forrest S, Nadler J, McNamara CA. The effects of leukocyte-type 12/15-lipoxygenase on Id3-mediated vascular smooth muscle cell growth. Arterioscler Thromb Vasc Biol 25: 2069–2074, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Leyen K, Kim HY, Lee SR, Jin G, Arai K, Lo EH. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke 37: 3014–3018, 2006 [DOI] [PubMed] [Google Scholar]
- 53.van Rooijen N, van Kesteren-Hendrikx E. “In vivo” depletion of macrophages by liposome-mediated “suicide”. Methods Enzymol 373: 3–16, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Wen Y, Gu J, Chakrabarti SK, Aylor K, Marshall JJ, Takahashi Y, Yoshimoto T, Nadler JL. The role of 12/15-lipoxygenase in the expression of interleukin-6 and tumor necrosis factor-a. Endocrinology 148: 1313–1322, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto S. Mammalian lipoxygenases: molecular structures and functions. Biochim Biophys Acta 1128: 117–131, 1992 [DOI] [PubMed] [Google Scholar]
- 56.Yuan H, Lanting L, Xu ZG, Li SL, Swiderski P, Putta S, Jonnalagadda M, Kato M, Natarajan R. Effect of cholesterol-tagged small interfering RNAs targeting 12/15-lipoxygenase on parameters of diabetic nephropathy in mouse model of type 1 diabetes. Am J Physiol Renal Physiol 295: F605–F617, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang DX, Gauthier KM, Chawengsub Y, Campbell WB. ACh-induced relaxations of rabbit small mesenteric arteries: role of arachidonic acid metabolites and K. Am J Physiol Heart Circ Physiol 293: H152–H159, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Zhao L, Cuff CA, Moss E, Wille U, Cyrus T, Klein EA, Pratico D, Rader DJ, Hunter CA, Pure E, Funk CD. Selective interleukine-12 synthesis defect in 12/15-lipoxygenase-deficient macrophages associated with reduced atheroschlerosis in a mouse model of familial hypercholesterolemia. J Biol Chem 277: 35350–35356, 2002 [DOI] [PubMed] [Google Scholar]