Abstract
Oxidative stress is a molecular dysregulation in reactive oxygen species (ROS) metabolism, which plays a key role in the pathogenesis of atherosclerosis, vascular inflammation and endothelial dysfunction. It is characterized by a loss of nitric oxide (NO) bioavailability. Large clinical trials such as HOPE and HPS have not shown a clinical benefit of antioxidant vitamin C or vitamin E treatment, putting into question the role of oxidative stress in cardiovascular disease. A change in the understanding of the molecular nature of oxidative stress has been driven by the results of these trials. Oxidative stress is no longer perceived as a simple imbalance between the production and scavenging of ROS, but as a dysfunction of enzymes involved in ROS production. NADPH oxidases are at the center of these events, underlying the dysfunction of other oxidases including eNOS uncoupling, xanthine oxidase and mitochondrial dysfunction. Thus NADPH oxidases are important therapeutic targets. Indeed, HMG-CoA reductase inhibitors (statins) as well as drugs interfering with the renin-angiotensin-aldosterone system inhibit NADPH oxidase activation and expression. Angiotensin-converting enzyme (ACE) inhibitors, AT1 receptor antagonists (sartans) and aliskiren, as well as spironolactone or eplerenone, have been discussed. Molecular aspects of NADPH oxidase regulation must be considered, while thinking about novel pharmacological targeting of this family of enzymes consisting of several homologs Nox1, Nox2, Nox3, Nox4 and Nox5 in humans.
In order to properly design trials of antioxidant therapies, we must develop reliable techniques for the assessment of local and systemic oxidative stress. Classical antioxidants could be combined with novel oxidase inhibitors. In this review, we discuss NADPH oxidase inhibitors such as VAS2870, VAS3947, GK-136901, S17834 or plumbagin.
Therefore, our efforts must focus on generating small molecular weight inhibitors of NADPH oxidases, allowing the selective inhibition of dysfunctional NADPH oxidase homologs. This appears to be the most reasonable approach, potentially much more efficient than non-selective scavenging of all ROS by the administration of antioxidants.
1. Introduction
Reactive oxygen species (ROS) are important molecules regulating numerous physiological and pathological processes in the cell. As with every mechanism involved in both normal cell function and the development of disease, strategies to counteract ROS must take into account their critical importance in the normal functioning of the organism. However, we now have clear evidence that overproduction of ROS is involved in the development of a number of diseases, which range from neurological such as Parkinson’s (Mythri et al., 2011) and Alzheimer’s disease (Shaerzadeh et al., 2011), to psychiatric disorders such as schizophrenia (Powell et al., 2011) and bipolar disorder (Steckert et al., 2010), and to a majority of cardiovascular diseases (Guzik and Griendling, 2009; Szuldrzynski et al., 2010);. Many studies in experimental models and clinical reports show a link between overproduction of ROS in the vessel wall and the development of atherosclerosis, heart failure, hypertension and plaque instability (Bauersachs and Widder, 2008; Drummond et al., 2011; Guzik and Harrison, 2006). This was initially demonstrated in animal models and more recently confirmed in clinical studies of cardiovascular disorders (Berry et al., 2000; Guzik et al., 2011; Guzik et al., 2000b). Therefore, numerous attempts have been made to overcome oxidative stress in the vascular wall and to use this as a therapeutic strategy. These studies will be discussed in the present review.
In general, two ways of eliminating free radicals are possible. The first method is by scavenging, either through the administration of antioxidants or the stimulation of endogenous antioxidant systems. The second approach is more complex, but interferes with the cause of oxidative stress by inhibiting enzymes that generate ROS. While the former has been most widely used so far in both basic and clinical studies, it has not fulfilled the predicted promise of cardiovascular protection. The latter, in turn, appears to bring new possibilities in the improvement of vascular function but requires a clear understanding of the mechanisms and true nature of oxidative stress.
2. Why is oxidative stress harmful and so difficult to treat?
The pathological effects of ROS in the cardiovascular system result simultaneously from their direct actions modifying vascular cell functions and from their ability to scavenge and remove several beneficial vasoprotective compounds such as nitric oxide. The interaction between endothelium-derived relaxing factor (EDRF) and superoxide anion (O2•-) was described for the first time by the polish scientist Professor Richard Gryglewski in 1986 (Gryglewski et al., 1986). It occurs so rapidly that it makes it impossible for NO to have any biological effects. This interaction is now thought to represent the most prevalent mechanism of endothelial dysfunction, where endothelial cells are unable to provide vasoprotective agents for the vessel wall. Numerous studies in cell culture, animal models and human vessels have shown that oxidative stress, in particular superoxide anion production, is the single most important mechanism for endothelial dysfunction (Guzik and Harrison, 2006; Montezano and Touyz, 2012). While this is the case, we must not overlook the direct effects that ROS exert on vascular smooth muscle cells and fibroblasts, which are also important in the early stages of cardiovascular disease (Griendling et al., 2000). These include the direct vasoregulatory effects of individual ROS species, while others are regulated through toxic oxidation of proteins, lipids and DNA (Cai and Harrison, 2000). Overproduction of ROS activates pro-oxidative genes such as hypoxia inducible factor-1α, leading to angiogenesis and cell proliferation (Gao and Mann, 2009) as well as number of pro-inflammatory genes discussed below.
Recent studies suggest that excess production of ROS is harmful. On the other hand, they also have a role in key signaling pathways, which are important for proper cell functioning (Guzik and Harrison, 2006). At physiological concentrations they activate receptor tyrosine kinases and transcriptional factors such as NF-E2-related factor-2 (Nrf-2). This leads to antioxidant gene expression, along with many other redox sensitive transcription factors that affect the function of numerous cells and tissues (Gao and Mann, 2009). This may partially explain why the direct scavenging of ROS by antioxidant vitamins do not demonstrate a clinical benefit in the majority of large trials (Widder and Harrison, 2005).
Thus, antioxidant strategies must be carefully designed, with a strong understanding of how ROS is involved in physiologic and pathologic processes. This would help to avoid some of the adverse outcomes of these treatments such as the recently reported increase in mortality associated with high-dose vitamin E trials (Miller et al., 2005).
3. Antioxidant vitamins – a failed promise
Vitamins E, C and A have been shown to have antioxidant properties both in in vitro and in animal research studies (Anderson and Phillips, 1999; Traber and Stevens, 2011). Therefore, considering the important role that ROS play in cardiovascular disease, it was reasonable to introduce them into vascular pharmacology studies. While in most observational studies antioxidant vitamins have beneficial effects, the data from randomized clinical trials are conflicting. Observational studies must be interpreted with great caution, because individuals who use vitamin supplements usually lead healthier lifestyles than those who do not.
Flow mediated dilatation (FMD), a clinical measure of endothelial dysfunction, can be improved by administration of antioxidants in patients with diabetes mellitus, hypercholesterolemia, hyperhomocysteinemia and in smokers (Celermajer et al., 1994; Heitzer et al., 2001). One of the initial trials, the CHAOS (Cambridge Heart AntiOxidant Study) trial revealed a significant decrease in the incidence of cardiovascular events in patients treated with vitamin E (400-800 IU/day). In contrast, further studies have shown that the simple scavenging of ROS is not effective in preventing the development of cardiovascular diseases (Harrison et al., 2007). Multicenter clinical studies (HOPE, SECURE, GISSI and HPS) have not confirmed the protective effects of vitamin E on atherosclerosis progression or major cardiovascular events. In fact, a meta-analysis revealed that administration of vitamin E in doses over 400 IU/day may increase the risk of death from any cause, and as such, is currently not recommended (Miller et al., 2005).
Major antioxidant vitamin clinical trials and their outcomes are shown in Table 1 (Katsiki and Manes, 2009). These studies mainly report no effect of antioxidative vitamins on cardiovascular diseases and events, and are supported by recent systematic reviews and meta-analyses (Bjelakovic et al., 2007).
Table 1.
Summary of major antioxidant studies in cardiovascular health.
| Clinical trials | Effects | Ref. |
|---|---|---|
| Positive results | ||
| ATBC | Marginal effect on the incidence of fatal coronary heart disease in male smokers with no history of myocardial infarction of supplementation with 50mg/day of alpha-tocopherol for median follow-up of 6.1 years. |
(Virtamo et al., 1998) |
| ASAP | Significant retard of progression of common carotid atherosclerosis in men when taken twice daily 91 mg (136 IU) of d-alpha-tocopherol and 250 mg of slow-release vitamin C for three years. No effect in women and lower atherosclerosis inhibition by individual vitamins use. |
(Salonen et al., 2000) |
| CLAS | Less carotid atherosclerosis progression among nonsmoking men after coronary artery bypass graft surgery was found for high supplementary vitamin E (≥100 IU per day) use when compared with low vitamin E use. No effect of vitamin E with colestipol/niacin, as well as no effect of vitamin C (≥250 mg per day) with colestipol/niacin or placebo was found. |
(Azen et al., 1996) |
| CHAOS | Reduction of non-fatal myocardial infarction in patients with angiographically proven coronary atherosclerosis with alpha- tocopherol (capsules containing 800 IU daily for first 546 patients; 400 IU daily for remainder). Non-significant excess of cardiovascular deaths. |
(Stephens et al., 1996) |
| Fang et al. | Retardation of the early progression of transplant-associated coronary arteriosclerosis after cardiac transplantation with supplementation of vitamin C (500 mg) plus vitamin E (400 IU), each twice daily. |
(Fang et al., 2002) |
| IEISS | Lower infarct size and oxidative stress in patients with suspected acute myocardial infarction on combined treatment with the antioxidant vitamins A (50,000 IU/day), vitamin C (1,000 mg/day), vitamin E (400 mg/day), and beta-carotene (25 mg/day). |
(Singh et al., 1996) |
| SPACE | Reduction of composite cardiovascular disease endpoints and myocardial infarction in haemodialysis patients with prevalent cardiovascular disease with 800 IU/day of vitamin E supplementation. |
(Boaz et al., 2000) |
| WACS | Marginally significant reduction in the combined outcome of myocardial infarction, stroke, coronary revascularization, or CVD death among women with prior CVD with active vitamin E (600 IU every other day) treatment was observed. Fewer strokes were experienced in female with a history of CVD or 3 or more CVD risk factors with supplementation of ascorbic acid (500 mg/d) and vitamin E (600 IU every other day) for ~9.4 years. |
(Cook et al., 2007) |
| No effect | ||
| ATBC | No primary preventive effect in male smokers with no history of myocardial infarction of 20mg/day of beta-carotene on major coronary events and no influence of 50mg/day of alpha-tocopherol on nonfatal myocardial infarction for median follow-up of 6.1 years. No significant differences in the number of major coronary events in male smokers with previous myocardial infarction receiving 50mg/day of alpha-tocopherol and/or 20mg/day of beta-carotene for median follow-up of 5.3 years. |
(Rapola et al., 1996; Virtamo et al., 1998) |
| CARET | No benefits in smokers, former smokers and workers exposed to asbestos from combination of 30 mg of beta-carotene per day and 25,000 IU of retinol (vitamin A) in the form of retinyl palmitate per day for ~4.0 years |
(Omenn et al., 1996) |
| GISSI | No benefit in patients surviving recent (≤3 months) myocardial infarction from 300 mg daily of vitamin E (alpha-tocopherol) supplementation |
(1999) |
| HATS | No significantly lower atherosclerosis progression in patients with coronary disease and low plasma levels of HDL from vitamins E (d- alpha-tocopherol, 400 IU twice daily), C (500 mg twice daily) and b- carotene (12,5 mg of natural beta-carotene) and 50 microg of selenium. Atenuation of benefits from simvastatin-niacin treatment. |
(Brown et al., 2001) |
| HOPE | No apparent effects in patients at high risk for cardiovascular events from 400 IU of vitamin E for ~4.5 years treatment. |
(Yusuf et al., 2000) |
| HPS | No significant reductions in mortality from or incidence of any type of vascular disease, cancer or other major outcome among adults with coronary disease, other occlusive arterial disease or diabetes receiving 600 mg of vitamin E, 250 mg of vitamin C, and 20 mg of beta-carotene daily for 5 years. |
(2002) |
| MICRO- HOPE |
No effect on cardiovascular outcomes or nephropathy in middle-aged and elderly people with diabetes and CV disease and/or additional coronary risk factor(s) from daily administration of 400 IU of vitamin E for ~4.5 years. |
(Lonn et al., 2002) |
| MVP | No significant reduction in luminal diameter reduction and restenosis rates per segment in patients after angioplasty, receiving (30,000 IU of beta carotene, 500 mg of vitamin C, and 700 IU of vitamin E, all given twice daily for four weeks before and six months after angioplasty with extra 2000 IU of vitamin E 12 hours before angioplasty). Attenuation of beneficial probucol effects. |
(Tardif et al., 1997) |
| PHS | No early or late differences in the overall incidence of cardiovascular disease, malignant neoplasms or death from all causes among healthy men with supplementation of 50 mg of beta-carotene on alternate days for 12 years. |
(Hennekens et al., 1996) |
| PPP | No beneficial effects in primary prevention of cardiovascular events in people with at least one major risk factor of 300 mg/day of vitamin E. |
(de Gaetano, 2001) |
| SCPS | No support for a strong effect in reducing mortality from cardiovascular disease or other causes of 50 mg per day of beta- carotene supplementation during median follow-up of 8.2 years. |
(Greenberg et al., 1996) |
| SU.VI.MAX | No beneficial effects on carotid atherosclerosis and arterial stiffness in free of symptomatic chronic diseases and apparently healthy people of combination of daily antioxidants (120 mg vitamin C, 30 mg vitamin E, 6 mg beta-carotene, 100 microg selenium, and 20 mg zinc) supplementation for over ~7.2 years. |
(Zureik et al., 2004) |
| VEAPS | Null results to the IMT progression in people at low risk for CVD of 400 IU daily of vitamin E (DL-alpha-tocopherol) supplementation. |
(Hodis et al., 2002) |
| WACS | No overall effect on the myocardial infarction, stroke, coronary revascularization, or CVD death considered single or combined in female with a history of CVD or 3 or more CVD risk factors of ascorbic acid (500 mg/d), vitamin E (600 IU every other day) or beta carotene (50 mg every other day) for ~9.4 years. |
(Cook et al., 2007) |
| WAVE | No cardiovascular benefit in postmenopausal women with coronary stenosis from 400 IU of vitamin E twice daily plus 500 mg of vitamin C twice daily. Potential for harm. |
(Waters et al., 2002) |
|
Negative
results |
||
| ATBC | Increased risk of fatal coronary heart disease in male smokers with previous myocardial infarction that received either 20mg/d of beta- carotene or the combination of 50mg/d of alpha-tocopherol and 20mg/d of beta-carotene for median follow-up of 5.3 years. |
(Rapola et al., 1996) |
| CARET | May have had an adverse effect on the incidence of lung cancer and on the risk of death from lung cancer, cardiovascular disease and any cause in smokers, former smokers and workers exposed to asbestos from combination of 30 mg of beta-carotene per day and 25,000 IU of retinol (vitamin A) in the form of retinyl palmitate per day for ~4.0 years. |
(Omenn et al., 1996) |
| WAVE | Increased risk of death in postmenopausal women with coronary stenosis receiving 400 IU of vitamin E twice daily plus 500 mg of vitamin C twice daily. |
(Waters et al., 2002) |
ATBC - Alpha-Tocopherol, Beta-Carotene Trial; ASAP - Antioxidant Supplementation in Atherosclerosis Prevention Study; CLAS - Cholesterol Lowering Atherosclerosis Study; CHAOS - Cambridge Heart Antioxidant Study; IEISS - Indian Experiment of Infarct Survival Study; SPACE - Secondary Prevention with Antioxidants of Cardiovascular disease in Endstage renal disease; WACS - Women’s Antioxidant and Cardiovascular Study; CARET - β -Carotene and Retinol Efficacy Trial; GISSI - Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico; HATS - HDL-Atherosclerosis Treatment Study; HOPE - Heart Outcomes Prevention Evaluation Study; HPS - Heart Protection Study; MICRO-HOPE - Microalbuminuria, Cardiovascular, and Renal Outcomes in the HOPE study; MVP - MultiVitamins and Probucol Trial; PHS - Physicians’ Health Study; PPP - Primary Prevention Project; SCPS - Skin Cancer Prevention Study; SU.VI.MAX - Supplementation in Vitamins and Mineral Antioxidants trial; VEAPS - Vitamin E Atherosclerosis Prevention Study; WAVE - Women’s Angiographic Vitamin and Estrogen Trial.
The apparent lack of clinical usefulness of ROS-scavenging approaches is not entirely explained. It appears that ROS-scavengers partially remove the selected harmful end-products, but they do not eliminate the overproduction of ROS, and the whole pathogenic process is allowed to continue. Furthermore, antioxidants, including vitamins, react with superoxide anions one billion times slower than NO. As a result, a reaction of superoxide radical with NO is thermodynamically preferred, leading to a loss of NO bioavailability, a major hallmark of vascular dysfunction. There are also studies indicating that vitamins do not penetrate into the vascular wall sufficiently, hence they may reach therapeutic levels in the plasma but are unable to do so in tissues (Micheletta et al., 2004). Finally, a tocopheroxyl radical, a strong oxidant, is formed as a product of the reaction between vitamin E and O2•-. This radical can be reconverted into vitamin E in the presence of vitamin C or coenzyme Q (Gazdikova et al., 2000). However, studies such as HPS (Heart Protection Study, Table 1), which used both vitamin E and C, were unable to demonstrate any enhanced clinical usefulness of this approach.
There are also a number of other considerations which could help us understand the lack of effectiveness of antioxidant vitamins. It could partially result from patients being in the late stages of atherosclerosis (less sensitive to antioxidant influence), non-optimal vitamin dosage, and isomer-specific properties of investigated vitamins. Additionally, some clinical trials did not report participants’ dietary habits and physical fitness, which can also be important. Although studies have failed to demonstrate the effectiveness of antioxidant vitamins in the treatment of cardiovascular disease, we should not omit them when thinking about cardiovascular pharmacology. In addition, we should explore different administration strategies and/or target patient groups.
4. Revised concept of oxidative stress – implications for antioxidant strategies
The absence of clinically effective classical antioxidant strategies led to the notion that our current understanding of oxidative stress may not be entirely accurate (Guzik and Harrison, 2006; Jones, 2006). Classically, oxidative stress has been defined as an imbalance between the endogenous production of reactive oxygen compounds (such as superoxide radical, hydrogen peroxide, hydroxyl radical) and the antioxidative potential of cells, which is related to the overall activity of enzymes such as superoxide dismutase, glutathione peroxidase and thioredoxins (Gongora et al., 2008; Halliwell, 1993). Here, the imbalance is considered on the level of the whole cell or even the whole organism. However, this may be a critical oversimplification, as it does not take into account complex molecular events that are involved in the development of oxidative stress. Moreover, it fails to acknowledge that cellular events leading to disease primarily occur in individual cellular compartments (Guzik and Harrison, 2006). Thus, measuring oxidant status in plasma or even whole cell lysates may not accurately reflect the pathogenic events, which should be inhibited and treated. In order to design successful antioxidant strategies to correct the molecular abnormalities underlying oxidative stress, we must first understand these mechanisms in detail.
5. Molecular complexity of oxidative stress generation – the central role of NADPH oxidases
The main sources of ROS include redox enzymes such as NADPH (Nicotinamide Adenine Dinucleotide Phosphate) oxidase, xanthine oxidase, lipooxygenase and cyclooxygenase. It is also important to note that these sources are continuously interacting with each other. NADPH oxidases and flavin oxidases that convert atmospheric oxygen (O2) to superoxide radical (O2•-), are at the center of these events. ROS produced by these enzymes have been shown to modify virtually all other possible sources of ROS. Changes in the structure of several oxidases caused by the overproduction of ROS from NADPH oxidases led to their dysfunction and to a further increase in ROS generation, forming a vicious cycle of oxidative stress (Figure 1). For example, reversible oxidation of sulfhydryl residues with subsequent irreversible proteolytic modification, transforms xanthine dehydrogenase (involved in purine metabolism) into ROS-generating xanthine oxidase. This conversion leads to a visible change in molecular weight (Figure 1). This phenomenon has been observed in numerous vascular disease states, diabetes, atherosclerosis (Cai and Harrison, 2000), and has also been demonstrated by us in human vasculature.
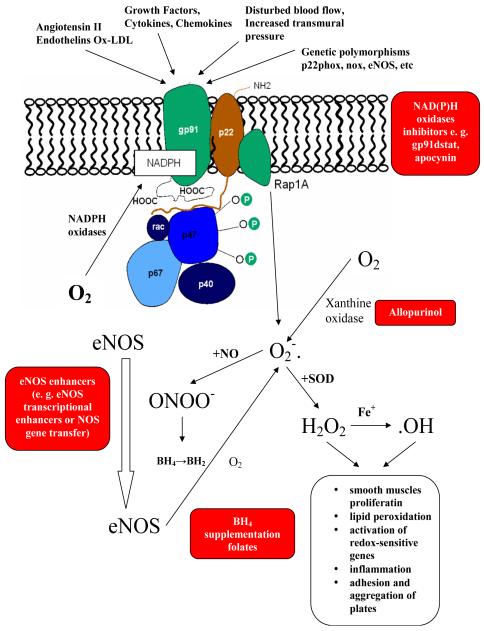
Figure 1. Central role of NADPH oxidase in the pathogenesis of vascular oxidative stress and endothelial dysfunction.
Numerous factors such as angiotensin II, endothelins, oxidized LDL (Ox-LDL), growth factors, cytokines, chemokines and disturbed blood flow increase NADPH oxidase activity, resulting in superoxide radical generation. Increased levels of O2•- cause changes in vessel walls and the development of endothelial dysfunction. ROS production also depends on the activity of other cellular oxidases such as xanthine oxidase or eNOS (Guzik and Harrison, 2006).
Another example of local modification of individual enzymes involved in ROS metabolism is endothelial nitric oxide synthase (eNOS). Primarily involved in nitric oxide (NO) production, eNOS might be harmful in pathological conditions, where it generates ROS rather than NO (Guzik et al., 2006; Pacher et al., 2007; Vasquez-Vivar et al., 2003). Oxidation of tetrahydrobiopterin (BH4), a cofactor for eNOS, or lack of its substrate L-arginine, lead to eNOS uncoupling, in which eNOS monomers generate superoxide anion rather than NO. Oxidation of BH4 to BH2 is dependent on peroxynitrite production, which results in an overproduction of superoxides by NADPH oxidases (Landmesser et al., 2003). Reaction of superoxide radicals with NO causes the production of peroxynitrite (ONOO-), resulting in a further decrease of NO concentration and oxidation of the zinc-thiolate center of NOS. Additionally, this prevents BH4 from binding to its binding site. Also, oxidation of calmodulin, which is a NOS regulator, further decreases NOS activity, contributing to oxidative stress and the molecular dysfunction of NOS (Gryglewski et al., 1986; Guzik and Harrison, 2006).
Tetrahydrobiopterin, in part through its ability to correct eNOS uncoupling, and in part through direct ROS scavenging, may be an important antioxidative compound, which has been found to be effective in some recent clinical trials (Antoniades et al., 2011b).
Folates are known to improve endothelial function and have been linked to the maintenance of BH4 bioavailability. The circulating form of folic acid is 5-methyltetrahydrofolate and has been shown to have beneficial effects on endothelial function and vascular superoxide production in human atherosclerosis. This is accomplished by preventing peroxynitrite-mediated BH4 oxidation and improving eNOS coupling (Antoniades et al., 2006).
Similar to xanthine oxidase and NOS, increased O2•- generation by NADPH oxidase induces mitochondrial oxidative damage via structural changes to the inner mitochondrial membrane and disturbs flow in the electron transport chain which enhances ROS production (Doughan et al., 2008; Ray and Shah, 2005).
Mice or cells lacking the p47phox molecule are deficient in functional NADPH oxidases and have been valuable in demonstrating NADPH oxidase involvement in the dysfunction of the previously described enzyme pathways (Landmesser et al., 2003). NADPH oxidases are found in virtually every tissue and are the major source of superoxide anions observed in the vasculature (Guzik et al., 2000b). Thus, in parallel to the two-way actions of ROS, Nox proteins have both beneficial and harmful effects. They are important signaling molecules which regulate vascular tone, expression, proliferation, migration and differentiation (Takac et al., 2012). On the other hand, cardiovascular risk factors and vascular diseases increase ROS and contribute to atherosclerosis, vascular dysfunction, hypertension, vascular hypertrophy and thrombosis (Guzik et al., 2000b; Takac et al., 2012). To successfully inhibit NADPH oxidase, it is important to understand its structure, activation and function.
6. NADPH oxidase structure and activation
The NADPH oxidase family includes several homologs, which differ in their expression, structure and function. Detailed molecular aspects of NADPH oxidases have been extensively reviewed elsewhere (Amanso and Griendling, 2012; Bedard and Krause, 2007; Morawietz, 2011; Muller and Morawietz, 2009; Takac et al., 2012). In brief, these homologs include: Nox1, Nox2, Nox3, Nox4, Nox5, Duox1 and Duox 2. The Nox isoforms contain an FAD and NADPH binding site, two heme molecules and six transmembrane domains (Table 2) (Bedard and Krause, 2007). The Duox isoforms also contain the same domains; however, a seventh transmembrane domain and peroxidase homology are present. The NADPH oxidase system contains additional key components, which include modulatory subunits (such as p22phox), two cytosolic adaptor proteins, organizer subunits (p47phox and its homolog NoxO1 or p40phox) and two activator subunits that bind GTP-Rac (p67phox and its homolog NoxA1). Two maturation factors specific for Duox (DuoxA1 and DuoxA2) have been described (Bedard and Krause, 2007). NoxO1 and NoxA1 regulate Nox1 and Nox3, while activation of Nox2 requires p40phox, p47phox and p67phox. Moreover, Nox1-4 activity is not directly dependent on intracellular calcium (Guzik et al., 2002a; Leto et al., 2009). In contrast, Nox5, Duox1 and 2 are activated by intracellular calcium (Banfi et al., 2001; Guzik et al., 2008). There are also vital differences in the nature of interaction between Nox homologues and the p22phox subunit, which may be useful in designing specific inhibitors of individual Nox enzymes (Ambasta et al., 2004; von Lohneysen et al., 2008).
Table 2. Known Nox NADPH oxidase homologues similarities and differences.
Duox oxidases have been reviewed elsewhere and are not discussed here (Al Ghouleh et al., 2011; Leto et al., 2009; Madamanchi and Runge, 2010; Selemidis et al., 2008; Streeter et al., 2012; Wingler et al., 2011).
| Characteristic | Nox1 | Nox2 | Nox3 | Nox4 | Nox5 |
|---|---|---|---|---|---|
| Electron source / transporter / acceptor / product |
Cytosolic NADPH / FAD and hemes / molecular oxygen / superoxide anion |
||||
| Structure | Reductase domains (C-terminal) with NADPH and FAD-binding sequences + six transmembrane alpha-helical segments + two heme- binding sites + three extra- and two intracellular loops |
||||
| EF hands (Ca2+ binding motifs, N-terminal) |
− | − | − | − | + |
| Active Nox complexes | |||||
| p22phox | + | + | + | + | − |
| Nox activators (GTP-Rac binding) |
NoxA1 | p67phox (NoxA2) |
NoxA1* / 2* |
− | − |
| Nox organizers (cytosolic regulatory subunits) |
NoxO1 | p40phox, p47phox (NoxO2) |
NoxO1 / 2 | − | − |
| Rho family GTP binding small proteins required for catalytic activity |
Rac1 | Rac2, Rac1† |
Rac1† | − | − |
| Main transcriptional regulation and constitutive activity |
− | − | − | + | − |
| Activated by Ca2+ intracellular increase |
− | − | − | − | + |
| Major ROS currently detected from activity |
O2•- | O2•- | O2•- | O2•-/H2O2 | O2•- |
| NADPH oxidases vascular distribution |
VSMC, EC |
EC, fibro- blasts, VSMC? macro- phages |
- inner ear - ? |
EC, VSMC, fibroblasts |
EC ?VSMC? spleen |
not required for Nox3 activity in human;
minor role in some cells; EC – endothelial cells; VSMC – vascular smooth muscle cells;
– disputable, no firm data available at present
7. NADPH oxidase localizations and functions
NADPH oxidases differ in their expression in various vascular cells. Nox1 and Nox5 are expressed primarily in smooth muscle and endothelial cells respectively. Nox2 is found in endothelial cells and adventitial fibroblasts as well as in vascular smooth muscle cells. Similarly, Nox4 has been detected in all the above mentioned cell types (Takac et al., 2012). Importantly it seems that in certain conditions more than one Nox isoform contributes to oxidative stress in individual cell types, and in some cases different Nox isoforms may physically interact (Briones et al., 2011). Nox3 is an NADPH oxidase present mainly in the inner ear, including spiral ganglion and vestibular and cochlear epithelia (Banfi et al., 2004). Duox1 and Duox2 are highly expressed, particularly in the thyroid where they participate in oxidation of thyroid hormones (Bedard and Krause, 2007; De Deken et al., 2000). Different predominant locations of individual homologs may in part be related to differences in their primary functions in health and disease. NADPH oxidase present in leukocytes is needed for the respiratory burst, producing oxygen free radicals, which kill bacteria. Loss of leukocyte NADPH oxidase Nox2 leads to chronic granulomatous disease, emphasizing the importance of this enzyme for immune defense (Gallin et al., 1992). However, as recently indicated, NADPH oxidase (Nox2 and Nox5 predominantly) presence in T cells or dendritic cells may also be important for adaptive immunity (Jackson et al., 2004). NADPH oxidases have also been described in the gastrointestinal tract and airways. Nox1 is present in colon epithelium, which produces ROS and is activated by lipopolysaccharide (LPS). Overexpression of this homologue has been reported in colon cancer (Laurent et al., 2008). In bronchial epithelial cells, Duox1 and Duox2 isoforms are involved in the generation of antimicrobial hypothiocyanite ions (Rada and Leto, 2008), although other homologs are also expressed and may be important in airway obstructive disorders (T. Guzik unpublished data). Vascular NADPH oxidases, especially Nox1, 2 and 5, produce ROS through single electron transfer and thus yield predominantly superoxide anion. An exception is Nox4, which because of a specific alteration in its E-loop, appears to be able to produce hydrogen peroxide, although this aspect of Nox4 biology is still under discussion (Dikalov et al., 2008; Takac et al., 2011). Hydrogen peroxide is an important vasodilator, which promotes endothelium-dependent relaxation and reduces blood pressure in mice (Ray et al., 2011). H2O2 increases endothelial NOS expression and activity and directly oxidizes protein kinase GIα, promoting vasodilatation (Takac et al., 2012). This may partially be responsible for the differential expression of Nox homologues during the development of atherosclerosis. Nox4 expression appears to increase stability of atherosclerotic plaques, while at earlier stages of plaque development Nox2 seems to predominate contributing to atherosclerosis progression. (Sorescu et al., 2002). This led to the conclusion that Nox4 might be involved in the maintenance of the differentiated vascular smooth muscle cell phenotype (Clempus et al., 2007). Nox5 in turn seems to be co-expressed at sites of human plaque instability (Guzik et al., 2008). The aspects discussed above point to the complex nature of NADPH oxidase regulation and their role in disease. It is also clear, that we must be cautious while thinking about pharmacological targeting of these enzymes in humans. This picture is further complicated by the fact that genetic variation of NADPH oxidase components, which is related to differential ROS production, is involved in atherosclerosis and vascular dysfunction. Genetic variation of the p22phox subunit encoding gene CYBA has been discussed in this context, however, it is warranted that all other subunits should be carefully examined, also in the context of pharmacokinetics and immunogenetics. (Guzik et al., 2008; Guzik et al., 2007; Guzik et al., 2000a; Madhur et al., 2010; Sorescu et al., 2002).
8. Complexity of regulation and signaling of NADPH oxidases
It is not the goal of this paper to provide a comprehensive review of NADPH oxidase regulation and its role in signaling. This has been described at length in respect to both physiology and pathology elsewhere (Morawietz, 2011; Muller and Morawietz, 2009). We will however, briefly outline the major regulatory mechanisms, which may be important when considering pharmacological targeting of this family of enzymes.
NADPH oxidase activity is regulated by factors such as cytokines (TNF-α, BNF-β, IL-1), growth factors (EGF, FGF-β, IGF-1, PDGF, TGF-β1, VEGF) and hormones (e.g. insulin) (Guzik and Harrison, 2006). In addition, shear stress, flow cessation, thrombin, serotonin, endothelin-1, angiotensin-II, histamine, bradykinin, lysophosphatidic acid, phorbol 12-myristate 13-acetate (PMA), prostaglandin F2α (PGF2α) and sphingosine 1-phosphate are known to have an influence on NADPH oxidase activity (Rhee et al., 2003). Recent studies emphasize the stimulating effect of glucose, advanced glycation end-products (AGE), non-esterified fatty acids (NEFAs) and oxidized low density lipoproteins (ox-LDL) on NADPH oxidase activity as well (Ray and Shah, 2005).
Activation of NADPH oxidases may result from the stimulation of a number of cell surface receptors, such as the angiotensin II receptor, which is particularly important in hypertension and heart failure (Morawietz et al., 2001). AT1 receptor stimulation by angiotensin II may lead to phospholipase C and D (PLC and PLD) activation, both of which produce diacylglycerol (DAG). PLC also produces inositol triphosphate (IP3). DAG and IP3 mediated calcium release activate protein kinase C (PKC) (Lyle and Griendling, 2006). Phosphorylation of the p47phox subunit by PKC is required to enable binding of this subunit with others and activate NADPH oxidase (Massenet et al., 2005). NADPH oxidase may also be activated via the production of lipid second messengers, Rac (Ras superfamily of small GTPases) and guanine nucleotide exchange factors (GEF) such as Sos (son-of-sevenless). Sos may be stimulated by c-Abl (tyrosine kinase) and hypothetically by phosphatidylinositol 3,4,5-triphosphate (PIP3) (Lyle and Griendling, 2006). The last process is thought to bind Rac1 to the p67phox subunit and activate the oxidase (Ray and Shah, 2005).
There are also several feedback mechanisms which make the regulation of NADPH oxidases even more complex. For example, hydrogen peroxide activates c-Src (tyrosine kinase, cellular counterpart of v-Src), which may amplify NADPH oxidase activity through activation of c-Abl or leading to epidermal growth factor receptor (EGFR) transactivation (activates PI3-kinase which produces PIP3), thereby creating positive feedback in ROS generation. It is also worth emphasizing that while NADPH oxidases affect ROS production from other cellular sources, their own activity may be influenced by other ROS sources including those in the mitochondrial electron transport chain, cytochrome P450 monooxygenase, cyclooxygenases, lipooxygenases, peroxidases and heme oxygenases.
9. Molecular consequences of NADPH oxidase activation
Superoxide anion, a major product of NADPH oxidases, reacts with NO causing peroxynitrite generation, which is the major and best characterized mechanism of endothelial dysfunction (Guzik et al., 2002b) (Figure 1). Superoxide anion also interacts with other enzymes and is spontaneously or enzymatically dismutated to a more stable H2O2 (Rhee et al., 2003). Hydrogen peroxide may then be converted into reactive nitrogen and reactive chlorine, which attack both LDL and HDL, contributing to plaque formation (Lyle and Griendling, 2006; Nicholls and Hazen, 2004). H2O2 influences key intracellular signaling components, affecting both protein kinases and phosphatases. H2O2 modulates protein phosphorylation by inactivating phosphatases and activating some protein kinases through active site cysteine oxidation (Rhee et al., 2000). An accepted paradigm is that an increase in intracellular ROS production may inhibit tyrosine phosphatases and tip the balance toward tyrosine kinase activation. This would lead to the phosphorylation of mitogen-activated protein kinases (MAPKs), also known as extracellular signal-regulated kinases (ERKs), serine-threonine kinases and stress-activated protein kinases (SAPKs) (Irani, 2000). Akt kinase (downstream of IP 3-kinase) and caspase-3 activity are both involved in the apoptosis process, and are regulated by ROS (Irani, 2000).
ROS regulates transcription factors primarily via the formation of disulfide bonds at DNA binding domains in critical amino acid residues or indirectly by modulating redox signaling pathways (phosphorylation/dephosphorylation) (Kregel and Zhang, 2007). Both activator protein-1 (AP-1, via MAPKs pathway, especially ERK1/2 and c-Jun N-terminal protein kinases (JNKs), (Abe and Berk, 1998; Kunsch and Medford, 1999)) and NF-κB (Kunsch and Medford, 1999) have been found to be activated under oxidative stress conditions and lead to the expression of pro-inflammatory genes along with cytokine and chemokine production (Kregel and Zhang, 2007; Piette et al., 1997; Yao et al., 1994). Moreover NF-κB is involved in E-selectin, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) expression causing monocyte adhesion to endothelial cells and their activation (Abe and Berk, 1998).
Aside from modulating intracellular signaling pathways, ROS contributes to DNA, lipid and protein damage. ROS-induced DNA damage activates poly(ADP-ribose) polymerase (PARP), leading to PKC activation and a further increase in NF-κB activity (Brownlee, 2005).
In the signaling pathways described above, it is very likely that other intermediate proteins such as intracellular reducing agents (ie. glutathione, catalase, peroxidases and SOD) are involved (Guzik et al., 2005).
Through these molecular mechanisms, ROS influences platelet aggregation, monocyte migration, lipid peroxidation and redox-sensitive gene expression. ROS is also involved in the mediation of endothelial cell dysfunction, vascular smooth muscle cell (VSMC) growth, proliferation, differentiation, apoptosis and migration, as well as immune responses (Griendling and FitzGerald, 2003; Lyle and Griendling, 2006). Elucidation of these underlying mechanisms is an important first step in the development of novel treatments against atherosclerosis and vascular dysfunction (Griendling et al., 1994; Harrison et al., 2003; Pagano et al., 1998) (Figure 1, 2).
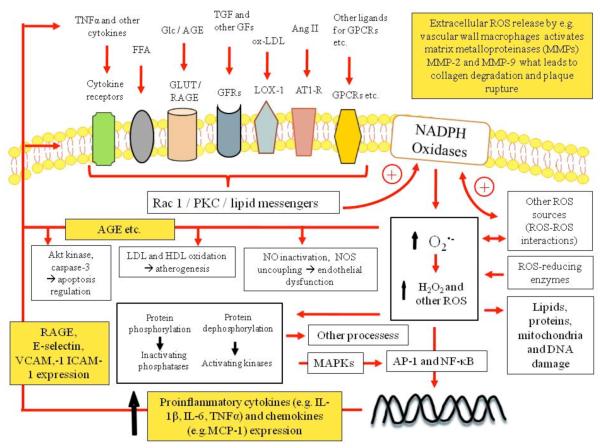
Figure 2. NADPH oxidase regulation and intracellular ROS signaling pathways.
Activation of growth factor receptors (GFR), lectin-like ox-LDL receptor-1 (LOX-1), angiotensin type 1-receptor (AT1-R) and other G protein-coupled receptors (GPCR), various cytokines and other receptors lead to Rac1 and/or protein kinase C (PKC) activation and/or lipid messenger production, which induces NADPH oxidase. Superoxide anion and hydrogen peroxide change intracellular protein phosphorylation, regulate redox sensitive gene expression, induce damage, mediate cell survival, growth, proliferation, migration and numerous other processes. MAPKs – mitogen-activated protein kinases ; AP-1 - activator protein-1; NF-κB - Nuclear factor-κB; (R)AGE – (receptor of) advanced glycation end-products; VCAM-1 - vascular cell adhesion molecule – 1; ICAM-1 - intercellular adhesion molecule – 1; MCP-1 – monocyte chemoattractant protein –1.
10. Points of targeting
Due to the complex mechanisms involved in the activation of NADPH oxidases, these enzymes can be targeted on several different levels of their activity. Firstly, decreasing NADPH oxidase expression can inhibit them. Also, the activation of NADPH oxidase can be decreased by blocking the translocation of its cytosolic subunits to the membrane. Another possibility is inhibition of the p47phox subunit, either by preventing its phosphorylation using PKC inhibitors, or by blocking its binding to other subunits. A decrease of signal transduction and inhibition of Rac 1 translocation have also been demonstrated to decrease ROS generation (Guzik and Harrison, 2006). Finally, it is important to note that strategies should not aim to inhibit all known oxidases at the same time, but rather to inhibit specific NADPH oxidase isoforms (Figure 3). These “points of targeting” can help guide the search for novel inhibitors of NADPH oxidases. However, several drugs currently used clinical in practice have been shown to decrease NADPH oxidase function.
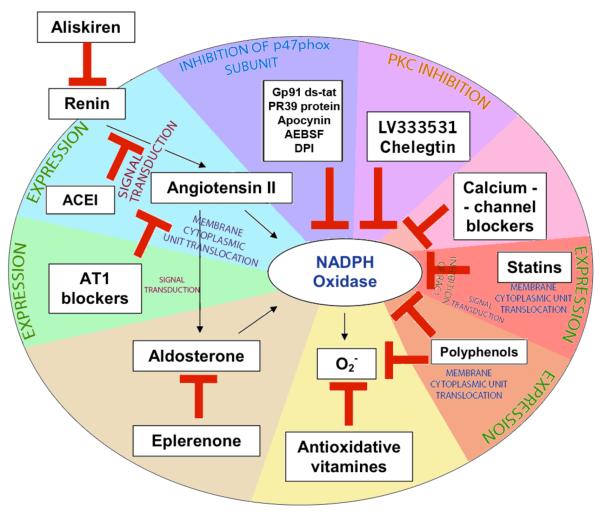
Figure 3. Points of targeting of NADPH oxidase activity.
The influence of the renin-angiotensin-aldosterone system on NADPH oxidase activity can be stopped by inhibiting renin, inhibiting angiotensin converting enzyme or by blocking angiotensin receptors. NADPH can be also blocked directly by gp91ds-tat, PR39 protein, apocynin, PKC inhibitors, calcium-channel blockers, statins and polyphenols. Antioxidative vitamins and polyphenols can deactivate superoxide radicals generated by NADPH oxidase.
10.1 Renin-angiotensin-aldosterone system blockade
The well characterized renin-angiotensin-aldosterone system (RAAS) is critical in the regulation of NADPH oxidase function. Angiotensin II increases the activity of NADPH oxidase and production of superoxide radical (Griendling et al., 1994). Moreover, in angiotensin II-dependent hypertension, an increase of Nox enzyme oxidase activity, especially Nox1, is observed. Activation of these pathways can be interrupted by inhibiting renin, inhibiting angiotensin-converting enzyme or by blocking angiotensin II receptors.
10.1.1 Angiotensin-converting enzyme inhibitors
The significant role of angiotensin and angiotensin-converting enzyme (ACE) in the pathogenesis of atherosclerosis and the beneficial clinical effects of their inhibitors have been demonstrated in many investigations (Ennezat et al., 2011). ACE inhibitors improve the function of endothelium by several mechanisms including: antioxidative properties, beneficial effects on fibrinolysis, lowering of angiotensin II and an increase in bradykinin. The protective effects of ACE inhibitors are not limited to states classically related to increased Ang II, like hypertension or heart failure. A recent study suggests that advanced glycation end products (AGE) play a role in NADPH oxidase signaling involved in diabetic vascular changes. Addition of AGE to cultured rabbit aortic smooth muscle cells increased ACE mRNA levels, expression of the receptor for advanced glycation end products (RAGE), Rac1 and p47phox membrane translocation and NF-κB activation, which led to the production of ROS. (Kamioka et al., 2010) Levels of matrix metalloproteinase (MT-MMP1 and MMP9), monocyte chemoattractant protein-1 (MCP-1) and plasminogen activator inhibitor-1 (PAI-1) were also raised. All of these effects were inhibited in the presence of temocaprilat (ACE inhibitor) or olmesartan (angiotensin II receptor antagonist) (Kamioka et al., 2010).
There is also evidence that ACE inhibition affects several mechanisms of NADPH oxidase activity, including p22phox expression, enzyme activation and even AT1 receptor levels (Miguel-Carrasco et al., 2010).
Combined treatment with enalapril, an ACE inhibitor (30 mg/l in water solution) and paricalcitol, a vitamin D analog (200 ng 3 times a week) has been demonstrated to reduce the development of atherosclerotic plaques in ApoE-deficient (ApoE-/-) mice better than monotherapy alone (Husain et al., 2010). Studies discussed above serve the purpose of brief exemplification of various beneficial effects of ACE inhibitors on oxidative stress. There are many more studies available, which are not reviewed here.
10.1.2 Angiotensin II receptor antagonists (ARBs)
Angiotensin II receptor antagonists appear to be more effective than ACE inhibitors in vasoprotection on the molecular level. However, this does not seem to be accompanied by enhanced benefits in clinical trials. Blockade of AT1-R causes inhibition of NADPH oxidase and a decrease in O2•- production with noticeable improvement of endothelial function (Berry et al., 2000; Warnholtz et al., 1999; Zhang et al., 1999). Olmesartan (1 mg/kg) caused an increase in acetylcholine-induced vascular relaxation and was more potent in inhibiting expression of the p22phox NADPH oxidase subunit when compared to the low-affinity ARB valsartan (3 mg/kg) in stroke-prone spontaneously hypertensive rats (SHR-SP) (Takai et al., 2009a).
The effects of ARBs on NADPH oxidases are superior to those exerted by ACE inhibition. Morawietz et al compared the impact of preoperative ACE inhibition with that of AT1 receptor blocker therapy before CABG surgery on vascular gp91phox expression in internal mammary arteries in patients with coronary artery disease. Long-term treatment with ACE inhibitors had, at the prescribed dosages, no effect on vascular gp91phox expression. In contrast, pharmacological treatment with AT1 receptor antagonists resulted in significant reduction of gp91phox expression. These data suggest an antioxidative and vasoprotective effect of AT1 receptor blocker therapy by the downregulation of endothelial NADPH oxidase expression (Rueckschloss et al., 2002).
Another investigation, performed on mesenteric artery rings from male Wistar rats, suggests a role of connexins Cx37, Cx40, Cx43 in decreasing vasodilatation under the influence of endothelium-derived hyperpolarizing factor (EDHF), which was followed by higher NADPH oxidase subunit and eNOS expression. This effect was inhibited by apocynin, an NADPH oxidase inhibitor, or losartan, an ARB (Dal-Ros et al., 2010).
A recent study emphasized the role of cathepsin-S activity suppression and macrophage migration to the atherosclerotic plaque by the treatment of ApoE-deficient mice with olmesartan (3 mg/kg per day) and apocynin (Sasaki et al., 2010).
The beneficial effect of combined treatment with candesartan (1 mg/kg/day) and pioglitazone (10 mg/kg/day) has been recently described in diabetic db/db mice. In this investigation, activity of NADPH oxidase (expression of gp91phox and p22phox subunits) and vascular dysfunction were further inhibited by combined treatment than by monotherapy alone. This treatment increased Cu/Zn-SOD and EC-SOD activity and caused eNOS phosphorylation (Fukuda et al., 2010).
Hence, AT1 receptor blockers can be considered as one of the most potent drugs currently clinically available for inhibiting the activation of NADPH oxidase. However, since they act on the mechanisms of activation rather than on the oxidase themselves, their effects may not be adequately focused.
10.1.3 Renin inhibitors
Numerous studies have confirmed the antihypertensive properties of renin inhibitors such as aliskiren. A recent study suggests that this renin blocker upregulates pro-angiogenic cells and reduces atherogenesis in mice. Aliskiren (25 mg/kg/day s.c.) reduced vascular NADPH oxidase activity to 41.6% and atherosclerotic plaque area in the aortic sinus by 58% in ApoE-deficient mice (Poss et al., 2010).
In addition, studies of combined treatment with aliskiren and valsartan on mice were performed. The combination of a half-dose of both drugs (aliskiren-12.5 mg/kg/day and valsartan-4 mg/kg/day) was more successful in the attenuation of tissue oxidative stress when compared with monotherapy using either aliskiren (25 mg/kg/day) or valsartan (8 mg/kg/day) in NOS-deficient mice (Yamamoto et al., 2009). This may be particularly important in the ongoing discussion regarding the use of aliskiren in clinical practice in light of the early termination of the ALTITUDE study due to side effects related to double Ang-II signaling blockade in diabetic subjects (Harel et al., 2012).
10.1.4 Mineralocorticoid receptor blockers
Aldosterone antagonism has been used clinically for many years to treat conditions often related to oxidative stress. It has been established that spironolactone exhibits significant effects on the inhibition of oxidative stress, predominantly through the inhibition of NADPH oxidases (Virdis et al., 2002).
Mineralocorticoid receptor antagonism using eplerenone improved endothelial dysfunction and inhibited expression of the NADPH oxidase subunit p22phox in the early post-MI phase (Sartorio et al., 2007). Moreover, it has recently been suggested that eplerenone may specifically affect Nox4 expression in the kidney (Bayorh et al., 2011).
These findings were corroborated by numerous studies, which also included conditions of possible paracrine production of aldosterone independently of the RAAS. The examination of rat aortic smooth muscle cells, which were subjected to uniaxial cyclic stretching, revealed increased activity of NADPH oxidase, level of cytochrome P450 aldosterone synthase (CYP11B2) and local production of aldosterone (Ohmine et al., 2009). Eplerenone (10 μmol/l), a selective mineralocorticoid receptor antagonist, inhibited this process more effectively than olmesartan, an angiotensin II receptor blocker (Ohmine et al., 2009) (Figure 3).
Thus, the potential for mineralocorticoid receptor inhibition in the regulation of NADPH oxidases in cardiovascular diseases is promising and should be further investigated in clinical studies (Figure 3).
10.2 Statins
Numerous studies on HMG-CoA reductase inhibitors (statins) have been performed and their beneficial influence on the prevention of endothelial dysfunction has been determined (Biasucci et al., 2010). This is due to lowering of LDL-cholesterol level which enhances NO-dependent vasodilatation and inhibits oxidative stress in the vessel wall (Anderson et al., 1995). At the same time, the pleiotropic effects of statins (Takemoto and Liao, 2001) involve their ability to directly limit oxidative stress in blood vessels (Wassmann et al., 2002) (Rueckschloss et al., 2001). Multiple studies indicate the role of NADPH oxidase inhibition as a major mechanism of the pleiotropic effects of statins. HMG-CoA reductase inhibitors reduce both the activity and expression of Nox1 (Wassmann et al., 2002) and Nox2 (Rueckschloss et al., 2001). Other Nox homologs may also be affected. The effects on activation are related to the fact that membrane association of rac1-GTPase, required for NADPH oxidase activation, is dependent on geranylgeranylation (Wassmann et al., 2002). A sine qua non condition of rac1-GTPase (NADPH oxidase component) activity is its geranylation. Statins inhibit geranyl phosphate production, which has influence on Rho and Ras protein-dependent processes and thus NADPH oxidase activity (Wassmann et al., 2002). Martin et al. also showed that inhibition of Rho protein geranylation causes stimulation of peroxisome proliferator-activated receptors alpha (PPRA alpha). Activation of these receptors causes an increase in apolipoprotein A gene transcription and improves HDL synthesis in the liver (Martin et al., 2001).
At the same time statins have been shown to decrease expression of numerous NADPH oxidase subunits and homologues (Nox1, Nox2, p22phox). Thus statin effects on NADPH oxidases are multi-focal. This has been reflected by observation of their effects in numerous models of vascular disease associated with oxidative stress.
Spontaneously hypertensive rats treated with atorvastatin have reduced aortic ROS production, p22phox and Nox1 expression, as well as Rac1 translocation (Wassmann et al., 2002). Similar observations have been described in mammary arteries from atherosclerotic patients (Rueckschloss et al., 2001). To date, atorvastatin, simvastatin, rosuvastatin and fluvastatin have all been shown to inhibit NADPH oxidase activity (Guzik and Harrison, 2006).
The effects of atorvastatin have recently been compared in ApoE-deficient, atherosclerotic, mice and angiotensin II-infused, hypertensive, mice. ROS generation and NADPH oxidase activity in both groups receiving atorvastatin was reduced (Cui et al., 2009). In ApoE-deficient mice, treatment with atorvastatin reduced levels of Nox2 and Nox4 subunit mRNA, while in angiotensin II-induced hypertensive mice, only expression of Nox4 was reduced. In the latter group, membrane translocation of Rac1 was also diminished. Antihypertensive effects of atorvastatin were observed in both animal models (Cui et al., 2009).
In another investigation also focused on atorvastatin, inhibition of homocysteine-induced ROS generation diminished NADPH oxidase activation. Additionally, a decrease in Nox4 mRNA and p-p38MAPK protein expression was seen. Apoptosis of endothelial progenitor cells (EPCs) also decreased after treatment (Bao et al., 2010).
Inhibitory effects of pitavastatin on NADPH oxidase activity and rac1-GTPase activity has been shown (Yagi et al., 2010) in endothelial NOS (eNOS-/-) mice infused with angiotensin II. Perivascular fibrosis and thrombogenic abnormalities were decreased along with inhibition of ROS generation by NADPH oxidases independently from eNOS-dependent statin effects (Yagi et al., 2010). Perivascular ROS production, in part modulated by sympathetic nervous system, may be responsible for the regulation of perivascular inflammation (Lob et al., 2010) and therefore direct “anti-NADPH oxidase” effects of statins may in part contribute to their anti-inflammatory properties.
Studies in human vessels are in agreement with observations in animal models. After administration of either atorvastatin, simvastatin, rosuvastatin or fluvastatin, a decrease in ROS production and expression of p22phox and Nox1 subunits were observed in the internal mammary arteries of patients who underwent coronary artery bypass grafting (CABG) (Rueckschloss et al., 2001). Lowering of the ROS level improves endothelial function, increases NO bioavailability and limits atherogenic effects of LDL-cholesterol, the last of which is more susceptible to phagocytosis by macrophages when oxidized. In a group of patients suffering from diabetes with or without hyperlipidemia, administration of cerivastatin for 3 days caused an improvement of endothelial function. This was represented by flow-dependent dilation of the brachial artery accompanied by a noticeable increase of cGMP and NO level (Tsunekawa et al., 2001). In hypercholesterolemia, an independent mechanism of endothelial dysfunction is related to a disturbance in eNOS activation. This is attributed to changes in lipid composition of the cell membrane (Blair et al., 1999) and a deficiency of BH4 leading to eNOS uncoupling (Cosentino et al., 2001). While the majority of statins’ effects have been characterized in either model systems or clinical trials, only recently have the direct effects of statin treatment on the vascular wall been shown. These were related primarily to the reduction of vascular NADPH oxidases and a decrease in vascular superoxide production. Interestingly, part of the vasoprotective effect was dependent on tetrahydrobiopterin-mediated endothelial NO synthase coupling (Antoniades et al., 2011a; Antoniades et al., 2010).
Moreover, a strong independent association between myocardial superoxide production and in-hospital complications after cardiac surgery has been found by Antoniades et al. Importantly, oxidative stress was reduced by pre-operative statin treatment, through a Rac1-mediated suppression of NADPH oxidase activity. Taken together, these findings suggest that the inhibition of myocardial NADPH oxidases may contribute to the beneficial effects of statins (Antoniades et al., 2012).
10.3 Calcium channel blockers
An investigation comparing the anti-atherosclerotic effect of two calcium channel blockers (CCB), benidipine and cilnidipine, has recently been performed on stroke-prone spontaneously hypertensive rats (SHR-SP) (Yamamoto et al., 2010). Cardiac and renal NADPH oxidase activity was more inhibited by benidipine than by cilnidipine, followed by a lower level of superoxide production, decreasing oxidative stress. Additionally, only benidipine lowered the serum level of aldosterone (Yamamoto et al., 2010).
Azelnidipine, a dihydropyridine CCB, also has an antagonistic effect on mineralocorticoid receptors. The effects of azelnidipine were examined on uninephrectomized rats, which had been administered with aldosterone. In this model, treatment with azelnidipine (3 mg/kg/day) decreased NADPH oxidase activity and levels of p22phox and gp91phox (Nox2) mRNA expression (Fan et al., 2009).
The effects of another calcium channel blocker, nifedipine, were investigated in type 2 diabetic KK-A(y) mice. Nifedipine (1.5 mg/kg/day) decreased the activity of NADPH oxidase and increased NOS expression in white adipose tissue (Iwai et al., 2011).
The effects of calcium channel blockers have been discussed in the context of Nox5, which is a calcium dependent NADPH oxidase, and its activity has recently been demonstrated by our group in human atherosclerosis, in particular at shoulder regions of atherosclerotic plaques. However, as NOX5 is expressed predominantly, although not exclusively, in endothelial cells (BelAiba et al., 2007), concerns have been raised that endothelium lacks the channels where Ca-antagonists may work. This aspect of action of Ca-channel antagonists should be addressed by additional studies.
10.4 Cholinergic agonists/antagonists, hydralazine
The cholinergic system has been implicated in the development of atherosclerosis and endothelial dysfunction. It has been demonstrated that stimulation of the cholinergic system is associated with the inhibition of NF-κB activation. Moreover, anti-atherosclerotic effects have been observed in ApoE-knockout mice treated with donepezil, a selective reversible acetylcholinesterase inhibitor, possibly through antioxidative and anti-inflammatory effects (Inanaga et al., 2010). On the other hand, nicotine, a nicotinic acetylcholine receptor agonist, was reported in the same animal model to induce endothelial dysfunction and accelerate atherosclerosis (Heeschen et al., 2001). Hydralazine, in spite of initial suggestions to the contrary, also inhibits O2•- production independently of blood pressure reduction (Rajagopalan et al., 1996) by antioxidative effects (Munzel et al., 1996). It is difficult however, to clearly separate inhibitory effects toward NADPH oxidases and the ROS scavenging effects in this case.
10.5 Cannabinoid receptor antagonism
Recent studies have focused on the role of the cannabinoid receptor (CB1-R) in cardiovascular diseases including hypertension and atherosclerosis. Importantly, CB1-R activation is associated with increased NADPH oxidase activity, in part, through the regulation of the AT1 receptor (AT1-R) (Tiyerili et al., 2010). In ApoE-deficient mice that rimonabant, a selective CB1-R antagonist, caused improved endothelium-dependent vasodilatation, reduced NADPH oxidase activity and ROS generation in the aorta. Administration of rimonabant to cultured vascular smooth muscle cells (VSMCs) caused a decrease in AT1-R expression, NADPH oxidase activity and angiotensin II-mediated ROS generation. Treatment with the CB1-R agonist, CP55940, increased the expression of AT1 receptor confirming the link between cannabinoid and angiotensin II signaling (Tiyerili et al., 2010). These studies suggest that CB1 receptor antagonism could be a new method to affect NADPH oxidases. Indeed, CB1 blockade has been shown to reduce body weight, blood pressure and insulin resistance, improve lipid levels (Despres et al., 2005; Van Gaal et al., 2008b), limit hepatic steatosis (Tam et al., 2010) and to increase daily physical activity in mice (Zhang et al., 2011). Due to these beneficial effects, rimonabant seemed to be a perfect drug for the treatment of metabolic syndrome. Unfortunately, adverse psychiatric effects such as depressed mood disorders and anxiety (Christensen et al., 2007), irritability and aggression (Rucker et al., 2007), nausea, dizziness, diarrhea and insomnia (Van Gaal et al., 2008a) diminished hopes for the clinical use of CB1-R antagonism. Furthermore, with the recent withdrawal of rimonabant from the market, this therapeutic option is currently questionable.
10.6 Protein kinase C inhibition
Numerous studies have confirmed that PKC is a strong NADPH oxidase activator and has significant influence on endothelial dysfunction. Chelerythrine, a PCK inhibitor, decreases the activity of this enzyme by phosphorylation of NADPH oxidase cytoplasmic subunits (Hamilton et al., 2002). It was observed that some of vascular complications of hyperglycemia are related to increased PKC activity (Tesfamariam et al., 1990).
Moreover, clinical studies have revealed that inhibition of PKC by its selective inhibitor, LY333531, causes a decrease in O2•- generation and decreased hyperglycemia-induced endothelial dysfunction (Gutterman, 2002; Guzik et al., 2002a). This pathway could have a significant role in the development of endothelial dysfunction caused by hyperlipidemia and diabetes (Gutterman, 2002; Guzik et al., 2002a). Even though a clinical application of this knowledge will be difficult due to the divergent roles of PKCs in the regulation of various cellular functions, further studies are unquestionably warranted, narrowin down isoforms involved in this process.
10.7 Naturally occurring antioxidants
While the search for novel and specific Nox inhibitors continues, the use of natural antioxidants represents a promising new approach. Polyphenols, representing more than 10000 compounds occurring naturally in foods, reduce oxidative stress and LDL oxidation (Badimon et al., 2010). As a result, the recommendation for a polyphenol rich (fruits/vegetables/whole grain foods) diet in the prevention of cardiovascular disease is still valid (Munzel et al., 2010). It was found that polyphenols, apart from their well-known superoxide radical scavenging abilities, decrease NADPH oxidase activity (Al-Awwadi et al., 2005). The ability to inhibit NADPH oxidases has been confirmed in various polyphenols in a number of tissues including vessels and platelets (Ryszawa et al., 2006). Moreover, novel polyphenolic compounds are being investigated which lack typical superoxide scavenging properties and directly inhibit NADPH oxidase, which will be discussed in the next section. The beneficial effects on cardiovascular disease reported in several studies, although not all, may partly explain the protective role of polyphenol-containing foods and beverages such as fruits, vegetables, green tea and red wine (Badimon et al., 2010). Recent studies revealed an inhibition of NADPH oxidase activity and reduction of gp91phox mRNA expression in macrophages treated with berberine (10-50 μmol/L), a plant alkaloid (Sarna et al., 2010). Also, emodin, an active component extracted from rhubarb and rhein, reduced ROS generation (Heo et al., 2010). Similar effects were observed by treatment with 3-(4′-hydroxyl-3′,5′-dimethoxyphenyl)propionic acid (HDMPPA), the active ingredient in kimchi, ellagic acid, a polyphenol present in fruits and nuts (Lee et al., 2010), apocynin (Guzik and Harrison, 2006; Liu et al., 2010) and dihomo-γ-linolenic (ω–6 ) acid (Takai et al., 2009b).
Two things should be kept in mind when designing new antioxidant strategies based on polyphenols. First, additional randomized studies with dietary interventions are needed. The second is that there exists the possibility that certain NADPH oxidase-inhibiting compounds may be extracted from plants, rather than synthesized de novo. However, we should always remember that the fact of an herbal origin of a pharmacological compound by no means indicates its safety, which should always be studied with great caution.
10.8 Direct NADPH oxidase inhibitors
Several compounds have the ability to directly inhibit NADPH oxidases. While their effects have been confirmed in vivo, major problems with currently availablt inhibitors include low specificity, associated toxicity, or their peptide character. The search for successful inhibitors continues as it would enable us to introduce our molecular knowledge of oxidative stress into clinical practice (Guzik and Griendling, 2009).
One of the first inhibitors used in model studies was diphenyliodonium, which is very potent (although in micromoolar range) but lacks specificity. It inhibits flavin oxidases, thus affects the activation of numerous enzymes including mitochondrial oxidases (Wind et al., 2010). Its greatest drawback is however toxicity. Later studies involved apocynin, a naturally occurrning NADPH oxidase inhibitor originally isolated from the roots of Picrorhiza kurroa.
Apocynin is an orally active agent that can block NADPH oxidase assembly but requires a reaction with peroxidase for its activation, and therefore does not work immediately (Stolk et al., 1994). Apocynin reduces ROS production when tested in animal models of arthritis and asthma. Oral treatment with apocynin blunted the development of hypertension, abolished the increase in vascular O2•- and prevented endothelial dysfunction in DOCA-salt hypertensive rats (Ghosh et al., 2004). However, effects of apocynin are not specific, as it has been reported to affect arachidonic acid metabolism (Engels et al., 1992), increase glutathione synthesis and to activate the AP-1 transcription factor (Lapperre et al., 1999). In addition, it has recently been shown to be a direct ROS scavenger in certain experimental conditions (Heumuller et al., 2008).
Another chemical inhibitor of NADPH oxidase, which has been used for a while is aminoethyl benzenesulphonyl-fluoride (AEBSF) (Diatchuk et al., 1997), although its effects, and in particular, specificity towards NADPH oxidases have not been satisfactory.
Several other molecules are currently being studied and have been reviewed elsewhere recently (Kim et al., 2011). These include Nox inhibitors such as VAS 2870, VAS 3947, GK-136901, plumbagin, as well as polyphenolic derivative S17834. VAS 2870 is a cell-permeable thiotriazolopyrimidine compound that is reported to act as a rapid and reversible inhibitor of NADPH oxidase activity. VAS 2870 has also been shown to efficiently abolish agonist-induced ROS generation and to offer protection against oxidative stress, with no effect on basal ROS production. VAS 2870 does not seem to have direct ROS scavenging properties, nor does it affect xanthine oxidase-mediated superoxide production. In recent studies it has been demonstrated to attenuate PDGF-dependent smooth muscle cell chemotaxis, but not proliferation (ten Freyhaus et al., 2006). Furthermore, it modifies T regulatory cell function (Efimova et al., 2011) and induces apoptosis of tumor cells (Sancho and Fabregat, 2011). A recently introduced triazolopyrimidine, VAS 3947, has also been shown to be relatively specific for NADPH oxidases. However, its usage has been limited (Wind et al., 2010). GK-136901 is an interesting candidate, as it has been suggested to be a selective Nox1/4 inhibitor. A recent study showed that it may prevent high glucose-induced ROS-dependent changes in the kidney (Sedeek et al., 2010). Plumbagin, in turn inhibits NADPH-dependent superoxide production in cell lines that express NOX4 oxidase. Although its exact mechanism of action remains unclear, the inclusion of a napthoquinone structure within the molecule may be responsible for its ROS scavenging abilities (Drummond et al., 2011).
Finally, a very promising molecule is the benzo(b)pyran-4-one derivative, S17834. It was demonstrated that S17834 inhibited NADPH oxidase activity in endothelial cell membranes, displayed anti-atherosclerotic properties, and exhibited potent anti-inflammatory properties including the ability to reduce expression of redox sensitive genes such as VCAM-1 (Cayatte et al., 2001). Its major mechanism of action involves prevention of cytosolic (mainly p47phox) binding to membrane complex of the enzyme. Also, S17834 had no effect on superoxide produced by xanthine oxidase, indicating that it does not have significant superoxide scavenger properties (Cayatte et al., 2001). Recent studies show that S17834, decreases acetylation of the sirtuin-1-dependent lysine-382 on p53 and thus, apoptotic signaling (Xu et al., 2011).
Use of these antagonists has been limited to model studies. Although we were unable to discuss all studies using various small molecule inhibitors of NADPH oxidases, it is evident that they have little to no efficacy in human vasculature or disease. In fact, the majority of these compounds may possess unspecific off-target effects, which need to be carefully reviewed and dissected. Therefore, focus has been shifted to the development of peptide inhibitors, which have been shown to truly and successfully inhibit NADPH oxidase activation, but their low stability and lack of oral availability makes them clinically unattractive.
10.9 Selective peptide NADPH oxidase inhibitors
The selective peptide NADPH oxidase inhibitor, gp91ds-tat, binds to the p47phox subunit and prevents its interaction with other subunits. Inhibition of Nox1 and Nox2, but not Nox4, was observed since Nox4 activity is p47phox-independent. So far, a decrease in O2•- production and inhibition of angiotensin II-induced blood pressure increase have been demonstrated only in animal models (Rey et al., 2001). The fact that this novel inhibitor is a peptide might limit its use to parenteral administration.
Similarly, PR39 protein, an NADPH oxidase inhibitor isolated from swine digestive tract and present in macrophages and neutrophils, binds to the SH3 domain of p47phox subunits, limiting NADPH oxidase activity (Diatchuk et al., 1997; Shi et al., 1996). However, PR39 is not completely NADPH oxidase selective and can influence other proteins possessing the SH3 domain. Finally, similar to other peptide inhibitors, PR39 cannot be administered orally.
10.10 Nuclear factor-κB inhibition
Nuclear factor kappa B (NF-κB) is a protein complex localized in the cell nucleus, involved in regulating DNA transcription. Recent studies emphasize its key role in inflammatory responses including infections, autoimmune diseases, atherosclerosis and cancer development. Moreover, NF-κB is involved in synaptic plasticity. NF-κB function depends on regulating factors such as cytokines, ROS, UV and oxidized LDL (Guzik and Harrison, 2007).
The role of NF-κB in inflammation accompanying hypertension in blood vessels was demonstrated in several investigations on animals. Miguela-Carrasco showed recently that administration of captopril (ACE inhibitor, 80mg/kg/day) normalized the expression of IL-1 beta, IL-6 and ACE mRNA levels, AT1-R and p22phox in heart ventricle muscle cells of spontaneously hypertensive rats (SHR). Additionally, increased expression and activity of NF-κB was noticed. This effect was neutralized by administration of ACE inhibitors (Miguel-Carrasco et al., 2010). Thus NF-κB can constitute a possible target for oxidative stress regulation in the context of NADPH oxidase inhibition although this requires much more data.
11. Conclusions
Oxidative stress is a molecular dysregulation in ROS production, which plays a key role in the pathogenesis of atherosclerosis and vascular dysfunction. Endothelial dysfunction is caused by overproduction of ROS, leading to a decrease in NO bioavailability. Numerous studies have shown improvement of endothelial dysfunction and oxidative stress using different treatment strategies, which include the use of statins, ACE inhibitors and angiotensin II receptor blockers.
Enthusiasm was raised by the great efficacy of antioxidant vitamins in animal models. Unfortunately, this did not translate into clinical usefulness. As a result, skeptics have been critical of the significance that oxidative stress may have in the development of certain vascular disease states. However, the failures of classical antioxidant therapies demonstrate that they are insufficient to inhibit oxidative stress. Simply ignoring the importance of ROS will never lead to the achievement of clinical benefits. Indeed, further research into selective molecular inhibitors of vascular ROS generation, particularly those that interfere with NADPH oxidase activation, are warranted.
Another important question which must be answered is whether or not antioxidant vitamins are in fact clinically beneficial. Increased dietary consumption of vitamins and polyphenols appear to convey some protective effects. Thus, is it justifiable to completely discard a simple and relatively safe antioxidant strategy? Further studies could concentrate on treating only patients with very high levels of oxidative stress and the use of more aggressive interventions. In order to do so, it is imperative that we develop reliable techniques to evaluate local and systemic oxidative stress. Moreover studies must take into account dietary vitamin consumption of studied patients. Classical antioxidants could also be combined with novel pro-oxidant enzyme inhibitors. Finally, new formulations could be designed which would allow antioxidant vitamins, along with enzyme inhibitors, to effectively reach sites of high oxidative stress.
In summary, our efforts must focus on generating small molecular weight inhibitors of NADPH oxidases. The selective inhibition of dysfunctional NADPH oxidase homologs appears to be the most reasonable approach at present, with the potential to be far more efficient than non-selective scavenging of ROS by the administration of antioxidants. However we must be aware that this is a very difficult task.
References
- Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- Abe J, Berk BC. Reactive oxygen species as mediators of signal transduction in cardiovascular disease. Trends in cardiovascular medicine. 1998;8:59–64. doi: 10.1016/S1050-1738(97)00133-3. [DOI] [PubMed] [Google Scholar]
- Al-Awwadi NA, Araiz C, Bornet A, Delbosc S, Cristol JP, Linck N, Azay J, Teissedre PL, Cros G. Extracts enriched in different polyphenolic families normalize increased cardiac NADPH oxidase expression while having differential effects on insulin resistance, hypertension, and cardiac hypertrophy in high-fructose-fed rats. Journal of agricultural and food chemistry. 2005;53:151–157. doi: 10.1021/jf048919f. [DOI] [PubMed] [Google Scholar]
- Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM, Kelley EE, Bauer PM, Darley-Usmar V, Shiva S, Cifuentes-Pagano E, Freeman BA, Gladwin MT, Pagano PJ. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free radical biology & medicine. 2011;51:1271–1288. doi: 10.1016/j.freeradbiomed.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanso AM, Griendling KK. Differential roles of NADPH oxidases in vascular physiology and pathophysiology. Front Biosci (Schol Ed) 2012;4:1044–1064. doi: 10.2741/s317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- Anderson D, Phillips BJ. Comparative in vitro and in vivo effects of antioxidants. Food Chem Toxicol. 1999;37:1015–1025. doi: 10.1016/s0278-6915(99)00089-7. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Meredith IT, Yeung AC, Frei B, Selwyn AP, Ganz P. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. The New England journal of medicine. 1995;332:488–493. doi: 10.1056/NEJM199502233320802. [DOI] [PubMed] [Google Scholar]
- Antoniades C, Bakogiannis C, Leeson P, Guzik TJ, Zhang MH, Tousoulis D, Antonopoulos AS, Demosthenous M, Marinou K, Hale A, Paschalis A, Psarros C, Triantafyllou C, Bendall J, Casadei B, Stefanadis C, Channon KM. Rapid, direct effects of statin treatment on arterial redox state and nitric oxide bioavailability in human atherosclerosis via tetrahydrobiopterin-mediated endothelial nitric oxide synthase coupling. Circulation. 2011a;124:335–345. doi: 10.1161/CIRCULATIONAHA.110.985150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades C, Bakogiannis C, Tousoulis D, Reilly S, Zhang MH, Paschalis A, Antonopoulos AS, Demosthenous M, Miliou A, Psarros C, Marinou K, Sfyras N, Economopoulos G, Casadei B, Channon KM, Stefanadis C. Preoperative atorvastatin treatment in CABG patients rapidly improves vein graft redox state by inhibition of Rac1 and NADPH-oxidase activity. Circulation. 2010;122:S66–73. doi: 10.1161/CIRCULATIONAHA.109.927376. [DOI] [PubMed] [Google Scholar]
- Antoniades C, Cunnington C, Antonopoulos A, Neville M, Margaritis M, Demosthenous M, Bendall J, Hale A, Cerrato R, Tousoulis D, Bakogiannis C, Marinou K, Toutouza M, Vlachopoulos C, Leeson P, Stefanadis C, Karpe F, Channon KM. Induction of vascular GTP-cyclohydrolase I and endogenous tetrahydrobiopterin synthesis protect against inflammation-induced endothelial dysfunction in human atherosclerosis. Circulation. 2011b;124:1860–1870. doi: 10.1161/CIRCULATIONAHA.111.029272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades C, Demosthenous M, Reilly S, Margaritis M, Zhang MH, Antonopoulos A, Marinou K, Nahar K, Jayaram R, Tousoulis D, Bakogiannis C, Sayeed R, Triantafyllou C, Koumallos N, Psarros C, Miliou A, Stefanadis C, Channon KM, Casadei B. Myocardial redox state predicts in-hospital clinical outcome after cardiac surgery effects of short-term pre-operative statin treatment. Journal of the American College of Cardiology. 2012;59:60–70. doi: 10.1016/j.jacc.2011.08.062. [DOI] [PubMed] [Google Scholar]
- Antoniades C, Shirodaria C, Warrick N, Cai S, de Bono J, Lee J, Leeson P, Neubauer S, Ratnatunga C, Pillai R, Refsum H, Channon KM. 5-methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation. 2006;114:1193–1201. doi: 10.1161/CIRCULATIONAHA.106.612325. [DOI] [PubMed] [Google Scholar]
- Azen SP, Qian D, Mack WJ, Sevanian A, Selzer RH, Liu CR, Liu CH, Hodis HN. Effect of supplementary antioxidant vitamin intake on carotid arterial wall intima-media thickness in a controlled clinical trial of cholesterol lowering. Circulation. 1996;94:2369–2372. doi: 10.1161/01.cir.94.10.2369. [DOI] [PubMed] [Google Scholar]
- Badimon L, Vilahur G, Padro T. Nutraceuticals and atherosclerosis: human trials. Cardiovasc Ther. 2010;28:202–215. doi: 10.1111/j.1755-5922.2010.00189.x. [DOI] [PubMed] [Google Scholar]
- Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, Krause KH. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem. 2001;276:37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- Bao XM, Wu CF, Lu GP. Atorvastatin inhibits homocysteine-induced oxidative stress and apoptosis in endothelial progenitor cells involving Nox4 and p38MAPK. Atherosclerosis. 2010;210:114–121. doi: 10.1016/j.atherosclerosis.2009.11.032. [DOI] [PubMed] [Google Scholar]
- Bauersachs J, Widder JD. Endothelial dysfunction in heart failure. Pharmacol Rep. 2008;60:119–126. [PubMed] [Google Scholar]
- Bayorh MA, Rollins-Hairston A, Adiyiah J, Lyn D, Eatman D. Eplerenone suppresses aldosterone/ salt-induced expression of NOX-4. J Renin Angiotensin Aldosterone Syst. 2011;12:195–201. doi: 10.1177/1470320310391330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological reviews. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- BelAiba RS, Djordjevic T, Petry A, Diemer K, Bonello S, Banfi B, Hess J, Pogrebniak A, Bickel C, Gorlach A. NOX5 variants are functionally active in endothelial cells. Free Radic Biol Med. 2007;42:446–459. doi: 10.1016/j.freeradbiomed.2006.10.054. [DOI] [PubMed] [Google Scholar]
- Berry C, Hamilton CA, Brosnan MJ, Magill FG, Berg GA, McMurray JJ, Dominiczak AF. Investigation into the sources of superoxide in human blood vessels: angiotensin II increases superoxide production in human internal mammary arteries. Circulation. 2000;101:2206–2212. doi: 10.1161/01.cir.101.18.2206. [DOI] [PubMed] [Google Scholar]
- Biasucci LM, Biasillo G, Stefanelli A. Inflammatory markers, cholesterol and statins: pathophysiological role and clinical importance. Clin Chem Lab Med. 2010;48:1685–1691. doi: 10.1515/CCLM.2010.277. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. Jama. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- Blair A, Shaul PW, Yuhanna IS, Conrad PA, Smart EJ. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J Biol Chem. 1999;274:32512–32519. doi: 10.1074/jbc.274.45.32512. [DOI] [PubMed] [Google Scholar]
- Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, Knecht A, Weissgarten Y, Brunner D, Fainaru M, Green MS. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): randomised placebo-controlled trial. Lancet. 2000;356:1213–1218. doi: 10.1016/s0140-6736(00)02783-5. [DOI] [PubMed] [Google Scholar]
- Briones AM, Tabet F, Callera GE, Montezano AC, Yogi A, He Y, Quinn MT, Salaices M, Touyz RM. Differential regulation of Nox1, Nox2 and Nox4 in vascular smooth muscle cells from WKY and SHR. J Am Soc Hypertens. 2011;5:137–153. doi: 10.1016/j.jash.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, Frohlich J, Albers JJ. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. The New England journal of medicine. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circulation research. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Cayatte AJ, Rupin A, Oliver-Krasinski J, Maitland K, Sansilvestri-Morel P, Boussard MF, Wierzbicki M, Verbeuren TJ, Cohen RA. S17834, a new inhibitor of cell adhesion and atherosclerosis that targets nadph oxidase. Arterioscler Thromb Vasc Biol. 2001;21:1577–1584. doi: 10.1161/hq1001.096723. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. Journal of the American College of Cardiology. 1994;24:1468–1474. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, Manson JE. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women’s Antioxidant Cardiovascular Study. Archives of internal medicine. 2007;167:1610–1618. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino F, Barker JE, Brand MP, Heales SJ, Werner ER, Tippins JR, West N, Channon KM, Volpe M, Luscher TF. Reactive oxygen species mediate endothelium-dependent relaxations in tetrahydrobiopterin-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:496–502. doi: 10.1161/01.atv.21.4.496. [DOI] [PubMed] [Google Scholar]
- Cui W, Matsuno K, Iwata K, Ibi M, Katsuyama M, Kakehi T, Sasaki M, Ikami K, Zhu K, Yabe-Nishimura C. NADPH oxidase isoforms and anti-hypertensive effects of atorvastatin demonstrated in two animal models. Journal of pharmacological sciences. 2009;111:260–268. doi: 10.1254/jphs.09148fp. [DOI] [PubMed] [Google Scholar]
- Dal-Ros S, Oswald-Mammosser M, Pestrikova T, Schott C, Boehm N, Bronner C, Chataigneau T, Geny B, Schini-Kerth VB. Losartan prevents portal hypertension-induced, redox-mediated endothelial dysfunction in the mesenteric artery in rats. Gastroenterology. 2010;138:1574–1584. doi: 10.1053/j.gastro.2009.10.040. [DOI] [PubMed] [Google Scholar]
- De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem. 2000;275:23227–23233. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- de Gaetano G. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet. 2001;357:89–95. doi: 10.1016/s0140-6736(00)03539-x. [DOI] [PubMed] [Google Scholar]
- Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. The New England journal of medicine. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- Diatchuk V, Lotan O, Koshkin V, Wikstroem P, Pick E. Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds. J Biol Chem. 1997;272:13292–13301. doi: 10.1074/jbc.272.20.13292. [DOI] [PubMed] [Google Scholar]
- Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circulation research. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimova O, Szankasi P, Kelley TW. Ncf1 (p47phox) is essential for direct regulatory T cell mediated suppression of CD4+ effector T cells. PLoS One. 2011;6:e16013. doi: 10.1371/journal.pone.0016013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels F, Renirie BF, Hart BA, Labadie RP, Nijkamp FP. Effects of apocynin, a drug isolated from the roots of Picrorhiza kurroa, on arachidonic acid metabolism. FEBS letters. 1992;305:254–256. doi: 10.1016/0014-5793(92)80680-f. [DOI] [PubMed] [Google Scholar]
- Ennezat PV, Vannesson C, Bouabdallaoui N, Marechaux S, Asseman P, LeJemtel TH. Imagine how many lives you save: angiotensin-converting enzyme inhibition for atherosclerotic vascular disease in the present era of risk reduction. Expert Opin Pharmacother. 2011;12:883–897. doi: 10.1517/14656566.2011.543675. [DOI] [PubMed] [Google Scholar]
- Fan YY, Kohno M, Nakano D, Hitomi H, Nagai Y, Fujisawa Y, Lu XM, Fu H, Du J, Ohmori K, Hosomi N, Kimura S, Kiyomoto H, Nishiyama A. Inhibitory effects of a dihydropyridine calcium channel blocker on renal injury in aldosterone-infused rats. Journal of hypertension. 2009;27:1855–1862. doi: 10.1097/HJH.0b013e32832dda6f. [DOI] [PubMed] [Google Scholar]
- Fang JC, Kinlay S, Beltrame J, Hikiti H, Wainstein M, Behrendt D, Suh J, Frei B, Mudge GH, Selwyn AP, Ganz P. Effect of vitamins C and E on progression of transplant-associated arteriosclerosis: a randomised trial. Lancet. 2002;359:1108–1113. doi: 10.1016/S0140-6736(02)08154-0. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Nakamura T, Kataoka K, Nako H, Tokutomi Y, Dong YF, Ogawa H, Kim-Mitsuyama S. Potentiation by candesartan of protective effects of pioglitazone against type 2 diabetic cardiovascular and renal complications in obese mice. Journal of hypertension. 2010;28:340–352. doi: 10.1097/HJH.0b013e32833366cd. [DOI] [PubMed] [Google Scholar]
- Gallin JI, Leto TL, Rotrosen D, Kwong CH, Malech HL. Delineation of the phagocyte NADPH oxidase through studies of chronic granulomatous diseases of childhood. Curr Opin Immunol. 1992;4:53–56. doi: 10.1016/0952-7915(92)90124-w. [DOI] [PubMed] [Google Scholar]
- Gao L, Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res. 2009;82:9–20. doi: 10.1093/cvr/cvp031. [DOI] [PubMed] [Google Scholar]
- Gazdikova K, Gvozdjakova A, Kucharska J, Spustova V, Braunova Z, Dzurik R. Malondialdehyde and selected antioxidant plasma levels in conservatively treated patients with kidney diseases. Bratislavske lekarske listy. 2000;101:490–494. [PubMed] [Google Scholar]
- Ghosh M, Wang HD, McNeill JR. Role of oxidative stress and nitric oxide in regulation of spontaneous tone in aorta of DOCA-salt hypertensive rats. Br J Pharmacol. 2004;141:562–573. doi: 10.1038/sj.bjp.0705557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gongora MC, Lob HE, Landmesser U, Guzik TJ, Martin WD, Ozumi K, Wall SM, Wilson DS, Murthy N, Gravanis M, Fukai T, Harrison DG. Loss of extracellular superoxide dismutase leads to acute lung damage in the presence of ambient air: a potential mechanism underlying adult respiratory distress syndrome. Am J Pathol. 2008;173:915–926. doi: 10.2353/ajpath.2008.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ER, Baron JA, Karagas MR, Stukel TA, Nierenberg DW, Stevens MM, Mandel JS, Haile RW. Mortality associated with low plasma concentration of beta carotene and the effect of oral supplementation. JAMA : the journal of the American Medical Association. 1996;275:699–703. doi: 10.1001/jama.1996.03530330043027. [DOI] [PubMed] [Google Scholar]
- Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circulation research. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circulation research. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Gutterman DD. Vascular dysfunction in hyperglycemia: is protein kinase C the culprit? Circulation research. 2002;90:5–7. [PubMed] [Google Scholar]
- Guzik B, Chwala M, Matusik P, Ludew D, Skiba D, Wilk G, Mrowiecki W, Batko B, Cencora A, Kapelak B, Sadowski J, Korbut R, Guzik TJ. Mechanisms of increased vascular superoxide production in human varicose veins. Polskie Archiwum Medycyny Wewnetrznej. 2011;121:279–286. [PMC free article] [PubMed] [Google Scholar]
- Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, Harrison DG. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. Journal of the American College of Cardiology. 2008;52:1803–1809. doi: 10.1016/j.jacc.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik TJ, Griendling KK. NADPH oxidases: molecular understanding finally reaching the clinical level? Antioxid Redox Signal. 2009;11:2365–2370. doi: 10.1089/ars.2009.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik TJ, Harrison DG. Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug discovery today. 2006;11:524–533. doi: 10.1016/j.drudis.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Harrison DG. Endothelial NF-kappaB as a mediator of kidney damage: the missing link between systemic vascular and renal disease? Circulation research. 2007;101:227–229. doi: 10.1161/CIRCRESAHA.107.158295. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002a;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Olszanecki R, Sadowski J, Kapelak B, Rudzinski P, Jopek A, Kawczynska A, Ryszawa N, Loster J, Jawien J, Czesnikiewicz-Guzik M, Channon KM, Korbut R. Superoxide dismutase activity and expression in human venous and arterial bypass graft vessels. J Physiol Pharmacol. 2005;56:313–323. [PubMed] [Google Scholar]
- Guzik TJ, Sadowski J, Guzik B, Jopek A, Kapelak B, Przybylowski P, Wierzbicki K, Korbut R, Harrison DG, Channon KM. Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:333–339. doi: 10.1161/01.ATV.0000196651.64776.51. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, West NE, Black E, McDonald D, Ratnatunga C, Pillai R, Channon KM. Functional effect of the C242T polymorphism in the NAD(P)H oxidase p22phox gene on vascular superoxide production in atherosclerosis. Circulation. 2000a;102:1744–1747. doi: 10.1161/01.cir.102.15.1744. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, West NE, Black E, McDonald D, Ratnatunga C, Pillai R, Channon KM. Vascular superoxide production by NAD(P)H oxidase: association with endothelial dysfunction and clinical risk factors. Circulation research. 2000b;86:E85–90. doi: 10.1161/01.res.86.9.e85. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, West NE, Pillai R, Taggart DP, Channon KM. Nitric oxide modulates superoxide release and peroxynitrite formation in human blood vessels. Hypertension. 2002b;39:1088–1094. doi: 10.1161/01.hyp.0000018041.48432.b5. [DOI] [PubMed] [Google Scholar]
- Halliwell B. The role of oxygen radicals in human disease, with particular reference to the vascular system. Haemostasis. 1993;23(Suppl 1):118–126. doi: 10.1159/000216921. [DOI] [PubMed] [Google Scholar]
- Hamilton CA, Brosnan MJ, Al-Benna S, Berg G, Dominiczak AF. NAD(P)H oxidase inhibition improves endothelial function in rat and human blood vessels. Hypertension. 2002;40:755–762. doi: 10.1161/01.hyp.0000037063.90643.0b. [DOI] [PubMed] [Google Scholar]
- Harel Z, Gilbert C, Wald R, Bell C, Perl J, Juurlink D, Beyene J, Shah PS. The effect of combination treatment with aliskiren and blockers of the renin-angiotensin system on hyperkalaemia and acute kidney injury: systematic review and meta-analysis. BMJ. 2012;344:e42. doi: 10.1136/bmj.e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- Harrison DG, Gongora MC, Guzik TJ, Widder J. Oxidative stress and hypertension. J Am Soc Hypertens. 2007;1:30–44. doi: 10.1016/j.jash.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Heeschen C, Jang JJ, Weis M, Pathak A, Kaji S, Hu RS, Tsao PS, Johnson FL, Cooke JP. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med. 2001;7:833–839. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, Willett W, Peto R. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. The New England journal of medicine. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- Heo SK, Yun HJ, Noh EK, Park SD. Emodin and rhein inhibit LIGHT-induced monocytes migration by blocking of ROS production. Vascular pharmacology. 2010;53:28–37. doi: 10.1016/j.vph.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- Hodis HN, Mack WJ, LaBree L, Mahrer PR, Sevanian A, Liu CR, Liu CH, Hwang J, Selzer RH, Azen SP, Grp VR. Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis - The Vitamin E Atherosclerosis Prevention Study (VEAPS) Circulation. 2002;106:1453–1459. doi: 10.1161/01.cir.0000029092.99946.08. [DOI] [PubMed] [Google Scholar]
- Husain K, Suarez E, Isidro A, Ferder L. Effects of Paricalcitol and Enalapril on Atherosclerotic Injury in Mouse Aortas. Am J Nephrol. 2010;32:296–304. doi: 10.1159/000319445. [DOI] [PubMed] [Google Scholar]
- Inanaga K, Ichiki T, Miyazaki R, Takeda K, Hashimoto T, Matsuura H, Sunagawa K. Acetylcholinesterase inhibitors attenuate atherogenesis in apolipoprotein E-knockout mice. Atherosclerosis. 2010;213:52–58. doi: 10.1016/j.atherosclerosis.2010.07.027. [DOI] [PubMed] [Google Scholar]
- Irani K. Oxidant signaling in vascular cell growth, death, and survival : a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circulation research. 2000;87:179–183. doi: 10.1161/01.res.87.3.179. [DOI] [PubMed] [Google Scholar]
- Iwai M, Kanno H, Inaba S, Senba I, Sone H, Nakaoka H, Horiuchi M. Nifedipine, a calcium-channel blocker, attenuated glucose intolerance and white adipose tissue dysfunction in type 2 diabetic KK-A(y) mice. American journal of hypertension. 2011;24:169–174. doi: 10.1038/ajh.2010.198. [DOI] [PubMed] [Google Scholar]
- Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004;5:818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- Kamioka M, Ishibashi T, Sugimoto K, Uekita H, Nagai R, Sakamoto N, Ando K, Ohkawara H, Teramoto T, Maruyama Y, Takeishi Y. Blockade of renin-angiotensin system attenuates advanced glycation end products-mediated signaling pathways. Journal of atherosclerosis and thrombosis. 2010;17:590–600. doi: 10.5551/jat.3624. [DOI] [PubMed] [Google Scholar]
- Katsiki N, Manes C. Is there a role for supplemented antioxidants in the prevention of atherosclerosis? Clin Nutr. 2009;28:3–9. doi: 10.1016/j.clnu.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Kim JA, Neupane GP, Lee ES, Jeong BS, Park BC, Thapa P. NADPH oxidase inhibitors: a patent review. Expert Opin Ther Pat. 2011;21:1147–1158. doi: 10.1517/13543776.2011.584870. [DOI] [PubMed] [Google Scholar]
- Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. American journal of physiology. Regulatory, integrative and comparative physiology. 2007;292:R18–36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- Kunsch C, Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circulation research. 1999;85:753–766. doi: 10.1161/01.res.85.8.753. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapperre TS, Jimenez LA, Antonicelli F, Drost EM, Hiemstra PS, Stolk J, MacNee W, Rahman I. Apocynin increases glutathione synthesis and activates AP-1 in alveolar epithelial cells. FEBS letters. 1999;443:235–239. doi: 10.1016/s0014-5793(98)01723-2. [DOI] [PubMed] [Google Scholar]
- Laurent E, McCoy JW, 3rd, Macina RA, Liu W, Cheng G, Robine S, Papkoff J, Lambeth JD. Nox1 is over-expressed in human colon cancers and correlates with activating mutations in K-Ras. International journal of cancer. 2008;123:100–107. doi: 10.1002/ijc.23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WJ, Ou HC, Hsu WC, Chou MM, Tseng JJ, Hsu SL, Tsai KL, Sheu WH. Ellagic acid inhibits oxidized LDL-mediated LOX-1 expression, ROS generation, and inflammation in human endothelial cells. J Vasc Surg. 2010;52:1290–1300. doi: 10.1016/j.jvs.2010.04.085. [DOI] [PubMed] [Google Scholar]
- Leto TL, Morand S, Hurt D, Ueyama T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal. 2009;11:2607–2619. doi: 10.1089/ars.2009.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhou J, An W, Lin Y, Yang Y, Zang W. Apocynin attenuates pressure overload-induced cardiac hypertrophy in rats by reducing levels of reactive oxygen species. Canadian journal of physiology and pharmacology. 2010;88:745–752. doi: 10.1139/y10-063. [DOI] [PubMed] [Google Scholar]
- Lob HE, Marvar PJ, Guzik TJ, Sharma S, McCann LA, Weyand C, Gordon FJ, Harrison DG. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension. 2010;55:277–283. doi: 10.1161/HYPERTENSIONAHA.109.142646. 276p following 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonn E, Yusuf S, Hoogwerf B, Pogue J, Yi Q, Zinman B, Bosch J, Dagenais G, Mann JF, Gerstein HC. Effects of vitamin E on cardiovascular and microvascular outcomes in high-risk patients with diabetes: results of the HOPE study and MICRO-HOPE substudy. Diabetes Care. 2002;25:1919–1927. doi: 10.2337/diacare.25.11.1919. [DOI] [PubMed] [Google Scholar]
- Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology (Bethesda) 2006;21:269–280. doi: 10.1152/physiol.00004.2006. [DOI] [PubMed] [Google Scholar]
- Madamanchi NR, Runge MS. NADPH oxidases and atherosclerosis: unraveling the details. American journal of physiology. Heart and circulatory physiology. 2010;298:H1–2. doi: 10.1152/ajpheart.01020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G, Duez H, Blanquart C, Berezowski V, Poulain P, Fruchart JC, Najib-Fruchart J, Glineur C, Staels B. Statin-induced inhibition of the Rho-signaling pathway activates PPARalpha and induces HDL apoA-I. J Clin Invest. 2001;107:1423–1432. doi: 10.1172/JCI10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massenet C, Chenavas S, Cohen-Addad C, Dagher MC, Brandolin G, Pebay-Peyroula E, Fieschi F. Effects of p47phox C terminus phosphorylations on binding interactions with p40phox and p67phox. Structural and functional comparison of p40phox and p67phox SH3 domains. J Biol Chem. 2005;280:13752–13761. doi: 10.1074/jbc.M412897200. [DOI] [PubMed] [Google Scholar]
- Micheletta F, Natoli S, Misuraca M, Sbarigia E, Diczfalusy U, Iuliano L. Vitamin E supplementation in patients with carotid atherosclerosis: reversal of altered oxidative stress status in plasma but not in plaque. Arterioscler Thromb Vasc Biol. 2004;24:136–140. doi: 10.1161/01.ATV.0000104028.07929.72. [DOI] [PubMed] [Google Scholar]
- Miguel-Carrasco JL, Zambrano S, Blanca AJ, Mate A, Vazquez CM. Captopril reduces cardiac inflammatory markers in spontaneously hypertensive rats by inactivation of NF-kB. Journal of inflammation (London, England) 2010;7:21. doi: 10.1186/1476-9255-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Annals of internal medicine. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- Montezano AC, Touyz RM. Reactive oxygen species and endothelial function--role of nitric oxide synthase uncoupling and Nox family nicotinamide adenine dinucleotide phosphate oxidases. Basic Clin Pharmacol Toxicol. 2012;110:87–94. doi: 10.1111/j.1742-7843.2011.00785.x. [DOI] [PubMed] [Google Scholar]
- Morawietz H. Endothelial NADPH oxidases: friends or foes? Basic research in cardiology. 2011;106:521–525. doi: 10.1007/s00395-011-0188-6. [DOI] [PubMed] [Google Scholar]
- Morawietz H, Weber M, Rueckschloss U, Lauer N, Hacker A, Kojda G. Upregulation of vascular NAD(P)H oxidase subunit gp91phox and impairment of the nitric oxide signal transduction pathway in hypertension. Biochem Biophys Res Commun. 2001;285:1130–1135. doi: 10.1006/bbrc.2001.5312. [DOI] [PubMed] [Google Scholar]
- Muller G, Morawietz H. Nitric oxide, NAD(P)H oxidase, and atherosclerosis. Antioxid Redox Signal. 2009;11:1711–1731. doi: 10.1089/ars.2008.2403. [DOI] [PubMed] [Google Scholar]
- Munzel T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J. 2010;31:2741–2748. doi: 10.1093/eurheartj/ehq396. [DOI] [PubMed] [Google Scholar]
- Munzel T, Kurz S, Rajagopalan S, Thoenes M, Berrington WR, Thompson JA, Freeman BA, Harrison DG. Hydralazine prevents nitroglycerin tolerance by inhibiting activation of a membrane-bound NADH oxidase. A new action for an old drug. J Clin Invest. 1996;98:1465–1470. doi: 10.1172/JCI118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mythri RB, Venkateshappa C, Harish G, Mahadevan A, Muthane UB, Yasha TC, Bharath M.M. Srinivas, Shankar SK. Evaluation of markers of oxidative stress, antioxidant function and astrocytic proliferation in the striatum and frontal cortex of Parkinson’s disease brains. Neurochemical research. 2011;36:1452–1463. doi: 10.1007/s11064-011-0471-9. [DOI] [PubMed] [Google Scholar]
- Nicholls SJ, Hazen SL. The role of myeloperoxidase in the pathogenesis of coronary artery disease. Japanese journal of infectious diseases. 2004;57:S21–22. [PubMed] [Google Scholar]
- Ohmine T, Miwa Y, Takahashi-Yanaga F, Morimoto S, Maehara Y, Sasaguri T. The involvement of aldosterone in cyclic stretch-mediated activation of NADPH oxidase in vascular smooth muscle cells. Hypertens Res. 2009;32:690–699. doi: 10.1038/hr.2009.76. [DOI] [PubMed] [Google Scholar]
- Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, Hammar S. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. The New England journal of medicine. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiological reviews. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano PJ, Chanock SJ, Siwik DA, Colucci WS, Clark JK. Angiotensin II induces p67phox mRNA expression and NADPH oxidase superoxide generation in rabbit aortic adventitial fibroblasts. Hypertension. 1998;32:331–337. doi: 10.1161/01.hyp.32.2.331. [DOI] [PubMed] [Google Scholar]
- Piette J, Piret B, Bonizzi G, Schoonbroodt S, Merville MP, Legrand-Poels S, Bours V. Multiple redox regulation in NF-kappaB transcription factor activation. Biological chemistry. 1997;378:1237–1245. [PubMed] [Google Scholar]
- Poss J, Werner C, Lorenz D, Gensch C, Bohm M, Laufs U. The renin inhibitor aliskiren upregulates pro-angiogenic cells and reduces atherogenesis in mice. Basic research in cardiology. 2010;105:725–735. doi: 10.1007/s00395-010-0120-5. [DOI] [PubMed] [Google Scholar]
- Powell SB, Sejnowski TJ, Behrens MM. Behavioral and neurochemical consequences of cortical oxidative stress on parvalbumin-interneuron maturation in rodent models of schizophrenia. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada B, Leto TL. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contributions to microbiology. 2008;15:164–187. doi: 10.1159/000136357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapola JM, Virtamo J, Haukka JK, Heinonen OP, Albanes D, Taylor PR, Huttunen JK. Effect of vitamin E and beta carotene on the incidence of angina pectoris. A randomized, double-blind, controlled trial. Jama. 1996;275:693–698. doi: 10.1001/jama.1996.03530330037026. [DOI] [PubMed] [Google Scholar]
- Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, Shah AM. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol. 2011;31:1368–1376. doi: 10.1161/ATVBAHA.110.219238. [DOI] [PubMed] [Google Scholar]
- Ray R, Shah AM. NADPH oxidase and endothelial cell function. Clin Sci (Lond) 2005;109:217–226. doi: 10.1042/CS20050067. [DOI] [PubMed] [Google Scholar]
- Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(−) and systolic blood pressure in mice. Circulation research. 2001;89:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Science’s STKE : signal transduction knowledge environment. 2000;2000:pe1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Chang TS, Bae YS, Lee SR, Kang SW. Cellular regulation by hydrogen peroxide. Journal of the American Society of Nephrology : JASN. 2003;14:S211–215. doi: 10.1097/01.asn.0000077404.45564.7e. [DOI] [PubMed] [Google Scholar]
- Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335:1194–1199. doi: 10.1136/bmj.39385.413113.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckschloss U, Galle J, Holtz J, Zerkowski HR, Morawietz H. Induction of NAD(P)H oxidase by oxidized low-density lipoprotein in human endothelial cells: antioxidative potential of hydroxymethylglutaryl coenzyme A reductase inhibitor therapy. Circulation. 2001;104:1767–1772. doi: 10.1161/hc4001.097056. [DOI] [PubMed] [Google Scholar]
- Rueckschloss U, Quinn MT, Holtz J, Morawietz H. Dose-dependent regulation of NAD(P)H oxidase expression by angiotensin II in human endothelial cells: protective effect of angiotensin II type 1 receptor blockade in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2002;22:1845–1851. doi: 10.1161/01.atv.0000035392.38687.65. [DOI] [PubMed] [Google Scholar]
- Ryszawa N, Kawczynska-Drozdz A, Pryjma J, Czesnikiewicz-Guzik M, Adamek-Guzik T, Naruszewicz M, Korbut R, Guzik TJ. Effects of novel plant antioxidants on platelet superoxide production and aggregation in atherosclerosis. J Physiol Pharmacol. 2006;57:611–626. [PubMed] [Google Scholar]
- Salonen JT, Nyyssonen K, Salonen R, Lakka HM, Kaikkonen J, Porkkala-Sarataho E, Voutilainen S, Lakka TA, Rissanen T, Leskinen L, Tuomainen TP, Valkonen VP, Ristonmaa U, Poulsen HE. Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) study: a randomized trial of the effect of vitamins E and C on 3-year progression of carotid atherosclerosis. Journal of internal medicine. 2000;248:377–386. doi: 10.1046/j.1365-2796.2000.00752.x. [DOI] [PubMed] [Google Scholar]
- Sancho P, Fabregat I. The NADPH oxidase inhibitor VAS2870 impairs cell growth and enhances TGF-beta-induced apoptosis of liver tumor cells. Biochem Pharmacol. 2011;81:917–924. doi: 10.1016/j.bcp.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Sarna LK, Wu N, Hwang SY, Siow YL, O K. Berberine inhibits NADPH oxidase mediated superoxide anion production in macrophages. Canadian journal of physiology and pharmacology. 2010;88:369–378. doi: 10.1139/Y09-136. [DOI] [PubMed] [Google Scholar]
- Sartorio CL, Fraccarollo D, Galuppo P, Leutke M, Ertl G, Stefanon I, Bauersachs J. Mineralocorticoid receptor blockade improves vasomotor dysfunction and vascular oxidative stress early after myocardial infarction. Hypertension. 2007;50:919–925. doi: 10.1161/HYPERTENSIONAHA.107.093450. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kuzuya M, Nakamura K, Cheng XW, Hayashi T, Song H, Hu L, Okumura K, Murohara T, Iguchi A, Sato K. AT1 blockade attenuates atherosclerotic plaque destabilization accompanied by the suppression of cathepsin S activity in apoE-deficient mice. Atherosclerosis. 2010;210:430–437. doi: 10.1016/j.atherosclerosis.2009.12.031. [DOI] [PubMed] [Google Scholar]
- Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM, Hebert RL. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol. 2010;299:F1348–1358. doi: 10.1152/ajprenal.00028.2010. [DOI] [PubMed] [Google Scholar]
- Selemidis S, Sobey CG, Wingler K, Schmidt HH, Drummond GR. NADPH oxidases in the vasculature: molecular features, roles in disease and pharmacological inhibition. Pharmacology & therapeutics. 2008;120:254–291. doi: 10.1016/j.pharmthera.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Shaerzadeh F, Ahmadiani A, Esmaeili MA, Ansari N, Asadi S, Tusi SK, Sonboli A, Ghahremanzamaneh M, Khodagholi F. Antioxidant and antiglycating activities of Salvia sahendica and its protective effect against oxidative stress in neuron-like PC12 cells. Journal of natural medicines. 2011;65:455–465. doi: 10.1007/s11418-011-0519-9. [DOI] [PubMed] [Google Scholar]
- Shi J, Ross CR, Leto TL, Blecha F. PR-39, a proline-rich antibacterial peptide that inhibits phagocyte NADPH oxidase activity by binding to Src homology 3 domains of p47 phox. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6014–6018. doi: 10.1073/pnas.93.12.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RB, Niaz MA, Rastogi SS, Rastogi S. Usefulness of antioxidant vitamins in suspected acute myocardial infarction (the Indian experiment of infarct survival-3) The American journal of cardiology. 1996;77:232–236. doi: 10.1016/s0002-9149(97)89384-8. [DOI] [PubMed] [Google Scholar]
- Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- Steckert AV, Valvassori SS, Moretti M, Dal-Pizzol F, Quevedo J. Role of oxidative stress in the pathophysiology of bipolar disorder. Neurochemical research. 2010;35:1295–1301. doi: 10.1007/s11064-010-0195-2. [DOI] [PubMed] [Google Scholar]
- Stephens NG, Parsons A, Schofield PM, Kelly F, Cheeseman K, Mitchinson MJ. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS) Lancet. 1996;347:781–786. doi: 10.1016/s0140-6736(96)90866-1. [DOI] [PubMed] [Google Scholar]
- Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. American journal of respiratory cell and molecular biology. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- Streeter J, Thiel W, Brieger K, Miller FJ., Jr Opportunity Nox: The Future of NADPH Oxidases as Therapeutic Targets in Cardiovascular Disease. Cardiovascular therapeutics. 2012 doi: 10.1111/j.1755-5922.2011.00310.x. [DOI] [PubMed] [Google Scholar]
- Szuldrzynski K, Zalewski J, Machnik A, Zmudka K. Elevated levels of 8-iso-prostaglandin F2alpha in acute coronary syndromes are associated with systemic and local platelet activation. Polskie Archiwum Medycyny Wewnetrznej. 2010;120:19–24. [PubMed] [Google Scholar]
- Takac I, Schroder K, Brandes RP. The nox family of NADPH oxidases: friend or foe of the vascular system? Curr Hypertens Rep. 2012;14:70–78. doi: 10.1007/s11906-011-0238-3. [DOI] [PubMed] [Google Scholar]
- Takac I, Schroder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai S, Jin D, Ikeda H, Sakonjo H, Miyazaki M. Significance of angiotensin II receptor blockers with high affinity to angiotensin II type 1 receptors for vascular protection in rats. Hypertens Res. 2009a;32:853–860. doi: 10.1038/hr.2009.116. [DOI] [PubMed] [Google Scholar]
- Takai S, Jin D, Kawashima H, Kimura M, Shiraishi-Tateishi A, Tanaka T, Kakutani S, Tanaka K, Kiso Y, Miyazaki M. Anti-atherosclerotic effects of dihomo-gamma-linolenic acid in ApoE-deficient mice. Journal of atherosclerosis and thrombosis. 2009b;16:480–489. doi: 10.5551/jat.no430. [DOI] [PubMed] [Google Scholar]
- Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712–1719. doi: 10.1161/hq1101.098486. [DOI] [PubMed] [Google Scholar]
- Tam J, Vemuri VK, Liu J, Batkai S, Mukhopadhyay B, Godlewski G, Osei-Hyiaman D, Ohnuma S, Ambudkar SV, Pickel J, Makriyannis A, Kunos G. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010;120:2953–2966. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif JC, Cote G, Lesperance J, Bourassa M, Lambert J, Doucet S, Bilodeau L, Nattel S, de Guise P. Probucol and multivitamins in the prevention of restenosis after coronary angioplasty. Multivitamins and Probucol Study Group. The New England journal of medicine. 1997;337:365–372. doi: 10.1056/NEJM199708073370601. [DOI] [PubMed] [Google Scholar]
- ten Freyhaus H, Huntgeburth M, Wingler K, Schnitker J, Baumer AT, Vantler M, Bekhite MM, Wartenberg M, Sauer H, Rosenkranz S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res. 2006;71:331–341. doi: 10.1016/j.cardiores.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B, Brown ML, Deykin D, Cohen RA. Elevated glucose promotes generation of endothelium-derived vasoconstrictor prostanoids in rabbit aorta. J Clin Invest. 1990;85:929–932. doi: 10.1172/JCI114521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiyerili V, Zimmer S, Jung S, Wassmann K, Naehle CP, Lutjohann D, Zimmer A, Nickenig G, Wassmann S. CB1 receptor inhibition leads to decreased vascular AT1 receptor expression, inhibition of oxidative stress and improved endothelial function. Basic research in cardiology. 2010;105:465–477. doi: 10.1007/s00395-010-0090-7. [DOI] [PubMed] [Google Scholar]
- Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011;51:1000–1013. doi: 10.1016/j.freeradbiomed.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunekawa T, Hayashi T, Kano H, Sumi D, Matsui-Hirai H, Thakur NK, Egashira K, Iguchi A. Cerivastatin, a hydroxymethylglutaryl coenzyme a reductase inhibitor, improves endothelial function in elderly diabetic patients within 3 days. Circulation. 2001;104:376–379. doi: 10.1161/hc2901.094094. [DOI] [PubMed] [Google Scholar]
- Van Gaal L, Pi-Sunyer X, Despres JP, McCarthy C, Scheen A. Efficacy and safety of rimonabant for improvement of multiple cardiometabolic risk factors in overweight/obese patients: pooled 1-year data from the Rimonabant in Obesity (RIO) program. Diabetes Care. 2008a;31(Suppl 2):S229–240. doi: 10.2337/dc08-s258. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Scheen AJ, Rissanen AM, Rossner S, Hanotin C, Ziegler O. Long-term effect of CB1 blockade with rimonabant on cardiometabolic risk factors: two year results from the RIO-Europe Study. Eur Heart J. 2008b;29:1761–1771. doi: 10.1093/eurheartj/ehn076. [DOI] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Kalyanaraman B, Martasek P. The role of tetrahydrobiopterin in superoxide generation from eNOS: enzymology and physiological implications. Free Radic Res. 2003;37:121–127. doi: 10.1080/1071576021000040655. [DOI] [PubMed] [Google Scholar]
- Virdis A, Neves MF, Amiri F, Viel E, Touyz RM, Schiffrin EL. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension. 2002;40:504–510. doi: 10.1161/01.hyp.0000034738.79310.06. [DOI] [PubMed] [Google Scholar]
- Virtamo J, Rapola JM, Ripatti S, Heinonen OP, Taylor PR, Albanes D, Huttunen JK. Effect of vitamin E and beta carotene on the incidence of primary nonfatal myocardial infarction and fatal coronary heart disease. Archives of internal medicine. 1998;158:668–675. doi: 10.1001/archinte.158.6.668. [DOI] [PubMed] [Google Scholar]
- von Lohneysen K, Noack D, Jesaitis AJ, Dinauer MC, Knaus UG. Mutational analysis reveals distinct features of the Nox4-p22 phox complex. J Biol Chem. 2008;283:35273–35282. doi: 10.1074/jbc.M804200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnholtz A, Nickenig G, Schulz E, Macharzina R, Brasen JH, Skatchkov M, Heitzer T, Stasch JP, Griendling KK, Harrison DG, Bohm M, Meinertz T, Munzel T. Increased NADH-oxidase-mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin-angiotensin system. Circulation. 1999;99:2027–2033. doi: 10.1161/01.cir.99.15.2027. [DOI] [PubMed] [Google Scholar]
- Wassmann S, Laufs U, Muller K, Konkol C, Ahlbory K, Baumer AT, Linz W, Bohm M, Nickenig G. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2002;22:300–305. doi: 10.1161/hq0202.104081. [DOI] [PubMed] [Google Scholar]
- Waters DD, Alderman EL, Hsia J, Howard BV, Cobb FR, Rogers WJ, Ouyang P, Thompson P, Tardif JC, Higginson L, Bittner V, Steffes M, Gordon DJ, Proschan M, Younes N, Verter JI. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2002;288:2432–2440. doi: 10.1001/jama.288.19.2432. [DOI] [PubMed] [Google Scholar]
- Widder JD, Harrison DG. Can vitamin E prevent cardiovascular events and cancer? Nat Clin Pract Cardiovasc Med. 2005;2:510–511. doi: 10.1038/ncpcardio0291. [DOI] [PubMed] [Google Scholar]
- Wind S, Beuerlein K, Eucker T, Muller H, Scheurer P, Armitage ME, Ho H, Schmidt HH, Wingler K. Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. British journal of pharmacology. 2010;161:885–898. doi: 10.1111/j.1476-5381.2010.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler K, Hermans JJ, Schiffers P, Moens A, Paul M, Schmidt HH. NOX1, 2, 4, 5: counting out oxidative stress. British journal of pharmacology. 2011;164:866–883. doi: 10.1111/j.1476-5381.2011.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Jiang B, Hou X, Shi C, Bachschmid MM, Zang M, Verbeuren TJ, Cohen RA. High-fat diet increases and the polyphenol, S17834, decreases acetylation of the sirtuin-1-dependent lysine-382 on p53 and apoptotic signaling in atherosclerotic lesion-prone aortic endothelium of normal mice. J Cardiovasc Pharmacol. 2011;58:263–271. doi: 10.1097/FJC.0b013e3182239eb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi S, Akaike M, Aihara K, Ishikawa K, Iwase T, Ikeda Y, Soeki T, Yoshida S, Sumitomo-Ueda Y, Matsumoto T, Sata M. Endothelial nitric oxide synthase-independent protective action of statin against angiotensin II-induced atrial remodeling via reduced oxidant injury. Hypertension. 2010;55:918–923. doi: 10.1161/HYPERTENSIONAHA.109.146076. [DOI] [PubMed] [Google Scholar]
- Yamamoto E, Kataoka K, Dong YF, Nakamura T, Fukuda M, Nako H, Ogawa H, Kim-Mitsuyama S. Benidipine, a dihydropyridine L-type/T-type calcium channel blocker, affords additive benefits for prevention of cardiorenal injury in hypertensive rats. Journal of hypertension. 2010;28:1321–1329. doi: 10.1097/HJH.0b013e3283388045. [DOI] [PubMed] [Google Scholar]
- Yamamoto E, Kataoka K, Dong YF, Nakamura T, Fukuda M, Tokutomi Y, Matsuba S, Nako H, Nakagata N, Kaneko T, Ogawa H, Kim-Mitsuyama S. Aliskiren enhances the protective effects of valsartan against cardiovascular and renal injury in endothelial nitric oxide synthase-deficient mice. Hypertension. 2009;54:633–638. doi: 10.1161/HYPERTENSIONAHA.109.133884. [DOI] [PubMed] [Google Scholar]
- Yao KS, Xanthoudakis S, Curran T, O’Dwyer PJ. Activation of AP-1 and of a nuclear redox factor, Ref-1, in the response of HT29 colon cancer cells to hypoxia. Molecular and cellular biology. 1994;14:5997–6003. doi: 10.1128/mcb.14.9.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- Zhang H, Schmeisser A, Garlichs CD, Plotze K, Damme U, Mugge A, Daniel WG. Angiotensin II-induced superoxide anion generation in human vascular endothelial cells: role of membrane-bound NADH-/NADPH-oxidases. Cardiovasc Res. 1999;44:215–222. doi: 10.1016/s0008-6363(99)00183-2. [DOI] [PubMed] [Google Scholar]
- Zhang LN, Gamo Y, Sinclair R, Mitchell SE, Morgan DG, Clapham JC, Speakman JR. Effects of Chronic Oral Rimonabant Administration on Energy Budgets of Diet-Induced Obese C57BL/6 Mice. Obesity (Silver Spring) 2011 doi: 10.1038/oby.2011.357. [DOI] [PubMed] [Google Scholar]
- Zureik M, Galan P, Bertrais S, Mennen L, Czernichow S, Blacher J, Ducimetiere P, Hercberg S. Effects of long-term daily low-dose supplementation with antioxidant vitamins and minerals on structure and function of large arteries. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:1485–1491. doi: 10.1161/01.ATV.0000136648.62973.c8. [DOI] [PubMed] [Google Scholar]