Abstract
A strict balance between self-renewal and differentiation of hematopoietic stem cells (HSCs) is required in order to maintain homeostasis, as well as to efficiently respond to injury and infections. Numbers and fate decisions made by progenitors derived from HSC must also be carefully regulated to sustain large scale production of blood cells. The complex Wnt family of molecules generally is thought to be important to these processes, delivering critical signals to HSC and progenitors as they reside in specialized niches. Wnt proteins have also been extensively studied in connection with malignancies and are causatively involved in development of several types of leukemias. However, studies with experimental animal models have produced contradictory findings regarding the importance of Wnt signals for normal hematopoiesis and lymphopoiesis. Here we will argue that dose dependency of signaling via particular Wnt pathways accounts for much, if not all of this controversy. We conclude that there seems little doubt that Wnt proteins are required to sustain normal hematopoiesis, but are likely to be presented in carefully controlled gradients in a tissue specific fashion.
Keywords: stem cell, self renewal, signaling, Wnt, Notch
Introduction
Wnt proteins are secreted lipid-modified molecules that bind to multiple-component receptor complexes on cell membranes. Appropriate ligation of those receptors is likely to be essential for normal blood cell formation, but is very complicated (reviewed in (1) (2)). For example, there are at least 19 Wnt proteins, 10 receptors, 2 co-receptors and multiple modifying molecules. The corresponding intracellular signaling pathways are no less complex, with as many as 10 proposed to mediate Wnt responses (Reviewed in(1) (2)). For simplicity, this review will focus mainly on the canonical Wnt pathway, which involves β-catenin (also known as cadherin-associated protein-β) and members of the T-cell factor (Tcf)/lymphocyte-enhancer binding factor (Lef) family. Non-canonical mechanisms utilize the planar cell polarity (PCP) pathway and the Wnt-Ca2+ pathway.
In the absence of Wnt ligands bound to the Wnt receptor complex, cytoplasmic levels of β-catenin, a key player in this pathway, are kept very low through the action of a protein complex (the so-called destruction complex) that actively targets β-catenin for degradation (Figure 1). This complex is composed of two negative regulatory kinases, including Glycogen Synthase Kinase 3β (GSK-3β) and at least two anchor proteins that also function as tumor suppressor proteins, namely Axin1 or Axin2 and APC (adenomatous polyposis coli). APC and Axin function as negative regulators of the pathway by sequestering β-catenin in the cytoplasm. Activation of this pathway by certain Wnt proteins leads to inactivation of the β-catenin destruction complex allowing its accumulation in the cytoplasm and consequently migration to the nucleus. In the nucleus β-catenin binds to members of the TCF/LEF transcription factor family, thereby converting them from transcriptional repressors into transcriptional activators (reviewed in 1). Recent evidence from stem cells in the intestine indicates that the membrane proximal events are even more complex and involve another co-receptor, namely the adult stem cell markers LGR5 (or related members LGR4 and 6) (3). These molecules are receptors for R-spondin and significantly enhance Wnt signaling in the stem cell niche(4) . It will be intriguing to figure out whether these receptors function in a similar fashion in the hematopoietic system.
Figure 1. Simplified scheme of canonical Wnt signal transduction.

In the absence of Wnt signaling free cytoplasmic β-catenin is kept at very low levels through proteosomal degradation. β-catenin degradation is accomplished through active phosphorylation at conserved regions by the ser/thr kinases glycogen synthase kinase 3β (Gsk-3β) and Casein Kinase 1 (Ck1). These proteins belong to the so called destruction complex, that also includes the scaffolding proteins axis inhibition protein 1 and 2 (Axin1 and Axin2), and the tumor suppressor protein adenomatous polyposis coli (Apc). β-catenin is phosphorylated first on Ser45 by Ck1, and then on Ser33, Ser37 and Thr41 by Gsk-3β to create recognition sites for the ubiquitin ligase β-transducin repeat-containing protein (β-Trcp), leading to its ubiquitylation and subsequent proteasomal breakdown. At the cell membrane, upon binding of a Wnt protein to the Frizzled receptor and the co-receptor low density lipoprotein receptor-related protein 5 (Lrp5) or Lrp6, the signaling cascade is initiated. Formation of the Frizzled–Lrp5/Lrp6 complex results in ser/thr kinases inhibition. This inhibition, mediated via Dishevelled (Dlv), disrupts the destruction complex and allows the stabilization of β-catenin in the cytoplasm. Accumulation of β-catenin, most probably in its amino-terminally dephosphorylated form, is followed by translocation to the nucleus where it binds to Tcf/Lef transcription factors. Tcf normally assembles a transcriptional repressor complex. Formation of the active β-catenin/Tcf transcription-factor complex culminates in the activation of Wnt target genes.
Genetic studies involving HSC in mice: gain-of-function approaches
The majority of reported studies on Wnt signaling in hematopoiesis provide evidence for a crucial role of canonical Wnt signaling in hematopoiesis(5). However, a number of studies have reported data that were interpreted to have the opposite effect, namely that there is no role for canonical Wnt signaling in hematopoiesis. As we discus here, most of these controversies can be explained by taking Wnt dosage into account.
In studies reporting an important role for Wnt signaling in blood and immune cell, Wnt signaling seemed to be required for normal HSC self renewal and therefore for efficient reconstitution after transplantation. The role of Wnt signaling pathways in HSCs has been studied using both gain and loss of function approaches.1,(5) The first studies on a role of Wnt signaling in the hematopoietic system focused on loss-of function models during T lymphocyte development in the thymus (6, 7) and have been extensively reviewed before (8)Studies on HSCs followed a few years later and initially used gain of function approaches. Initial attempts to retrovirally overexpress a constitutively active form of β-catenin in Bcl2-transgenic hematopoietic stem/progenitor cells led to an increase in proliferation of HSCs and repopulation capacity upon transplantation into lethally irradiated mice.(9) However, conditional overexpression of a stabilized form of β-catenin using a transgenic approach led to a block in multilineage differentiation, and a transient expansion of the HSC pool which was followed by the exhaustion of long-term HSCs(10, 11). These and other studies have created confusion concerning the importance of Wnts in maintaining numbers and integrity of HSCs. Recent findings suggest that this results from differences in levels of Wnt signaling achieved in experimental circumstances. Wnts are normally present in carefully controlled gradients within tissues, and responses to them can be concentration dependent. For example, studies with reporter strains of mice suggest levels of canonical Wnt signaling are particularly high in gut and skin, while lower levels are present in breast and central nervous system (CNS) and even more modest levels are found in hematopoietic organs (Reviewed in (12, 13), and references therein). We recently showed that an optimal activation level for Wnt signaling exists in vivo. That is, when Wnt signaling is slightly enhanced over normal levels, HSC reconstitute better, however when HSC are forced to undergo high levels of Wnt signaling, they completely fail to reconstitute irradiated recipient mice (14). For these studies, a series of transgenic mouse lines carrying different combinations of targeted mutations of the negative Wnt signaling regulator Apc were used. By combining different targeted hypomorphic alleles and a conditional deletion allele of Apc, a gradient of five different Wnt signaling levels was obtained in vivo(15) . Strict limiting-dilution competitive transplantation assays demonstrated enhanced HSC activity with activation of this pathway. However, HSCs only tolerated mild levels of Wnt signaling, corresponding to approximately 2-3 fold higher than the normal physiological levels. Both intermediate and high levels of Wnt signaling activity resulted in inability of HSC to repopulate recipient mice. Thus, different levels of activation of the pathway can account for discrepancies in previous studies. Furthermore, recent studies with Apc haploinsufficiency (16), ApcMin/+ (17)mice or a shRNA approach to knockdown Gsk-3β activity (18) showed enhanced HSC repopulation activity. These treatments probably resulted in relatively low levels of Wnt activity. In some cases, reduced long-term reconstitution capacity was observed, despite initial increased repopulation efficiency in primary recipients (19).
Genetic studies involving HSC in mice: loss-of-function approaches
Experimental approaches involving conditional deletion of β-catenin should be complementary to the over-expression studies, but again the results were confusing. For example. the Mx-Cre system has been used to drive deletion of β-catenin(20) , or both β-catenin and its homologue γ-catenin,(21, 22). However, no defects were observed in HSC function or cells within lymphopoietic tissues such as the thymus. Surprisingly, in vivo reporter assays revealed that the canonical Wnt signaling pathway was still active in HSCs despite the absence of both β- and γ-catenin. This could imply the existence of an alternative factor with the ability to transduce Wnt signals in the hematopoietic system. Alternatively, a hypomorphic allele of β-catenin may have been generated by the targeting approach. That could theoretically permit low levels of Wnt signaling that would negate hematopoietic defects. Support for this notion comes from recent studies on the role of β-catenin in mature CD8+ T cells (23). The Eomes gene is a known Wnt target gene in CD8 T cells and can be induced by Wnt3a stimulation. In T cells with the same conditionally deleted β-catenin as used in HSC (but now targeted with a mature T cell specific Cre promoter), the Wnt-induced activation of Eomes was significantly reduced, but not zero (from ∼6 fold to∼ 2 fold) Extrapolating this to the known remaining Wnt signaling in the two studies using conditionally deleted β-catenin (in a γ-catenin negative background) would suggest that the remaining low amount of Wnt activity could have been sufficient to sustain HSC and progenitor cells. Indeed, deletion of β-catenin resulted in 70% (ref) or 25% (dr. F. Radtke, personal communication) residual Wnt activity in HSCs. Together with studies in which Wnt activity in HSC was reported to be close to zero (24, 25) these findings suggest that complete absence of Wnt signaling is detrimental to HSC function, but that up to a quarter of normal activity is sufficient for normal function, slightly (2-3 fold) enhanced Wnt activity is beneficial for HSC function, whereas higher and much higher levels abrogate HSC function. It will be of importance to verify the various explanations for lack of effect of targeting β-catenin in blood cells experimentally.
In contrast to these results targeting β-catenin, three other loss-of-function studies indicated that some level of Wnt signaling is necessary for normal HSC function. These employed Wnt3a deficient mice(26), overexpression of the Wnt negative regulator DKK1 in osteoblastic stem cell niches (25), or Vav-Cre-mediated (rather than Mx-Cre) conditional deletion of β-catenin (19) . Because of their importance, each of these approaches will be reviewed separately.
Striking morphological similarities between Tcf1/Lef1 double deficient and Wnt3a deficient embryos (27, 28) suggested that Wnt3a plays non-redundant roles in several developmental processes (29, 30). Using mice with a specific germline mutation in the Wnt3a gene, we showed that Wnt signaling and more specifically Wnt3a is essential for self-renewal of fetal liver HSCs(26). Importantly, Wnt3a deficiency could not be compensated by the other Wnt proteins expressed in fetal liver and resulted in the complete inhibition of the canonical Wnt signaling pathway(24). This represents evidence that Wnt3a plays a non-redundant role in the formation or maintenance of fetal HSC.
Wnt signaling has also been implicated in the regulation of constituent cells of the stem cell niche (31) and more specifically in maintaining osteogenic development (32-35). Furthermore, Wnt regulates expression of the VCAM-1 adhesion molecule by hematopoietic supporting stromal cells(31). Although an indirect influence of Wnt3a on fetal HSC niches is possible, Wnt reporter analysis demonstrated that HSCs are directly affected by Wnt3a deficiency(24). Moreover, given that Wnt3a is not expressed by the HSCs themselves, this environmentally determined deficiency turned into a cell-autonomous defect since these cells lost long-term reconstitution capacity of wild-type recipient mice, where Wnt3a is available (36). This indicates that Wnt signaling deficiency permanently and irreversibly impairs the self-renewal capacity of HSCs.
Evidence for an essential requirement of canonical Wnt signaling for adult HSCs was also obtained with another experimental strategy by Fleming and colleagues(25). Transgenic mice over-expressed the Wnt inhibitor Dkk1 specifically in osteoblast-like cells within bone marrow, sites thought to be part of hematopoietic HSC niches. While effects of this manipulation on HSC were subtle, serial transplantation experiments demonstrated that their self-renewal potential was compromised.
This notion was confirmed in another study using the Vav-Cre system to achieve deletion of β-catenin(19) in HSCs. This study also showed reduced self-renewal capacity suggesting the requirement of Wnt signaling for the long-term growth and maintenance of HSCs.
Together these studies show that transient inhibition of the Wnt pathway during fetal development or in the adult bone marrow niche irreversibly impairs HSC self-renewal. A possible explanation for this irreversibility may involve epigenetic modifications as a result of the absence of Wnt activation.
Other Wnt ligands and signaling pathways
Besides Wnt3a, other Wnt proteins such as Wnt5a and Wnt4 were recently implicated in regulation of hematopoietic stem/progenitor cells. Wnt4 expanded early hematopoietic progenitors through activation of non-canonical Wnt signaling (37). Intraperitoneal administration of Wnt5a into NOD/SCID mice(38) and in vitro Wnt5a exposure prior to transplantation(39) enhanced HSCs repopulation capacity. Of interest, the non-canonical Wnt5a signals may antagonize canonical Wnt signaling in HSCs. However the results do not discriminate whether this antagonistic effect is due to interaction of the intracellular pathways or just to competition between Wnt ligands for the same Frizzled receptor. Clearly, there are many possibilities for Wnt-dependent interactions between HSC and their niche microenvironment, leading to autocrine and paracrine effects in the HSC niche
Wnt signaling in the HSC niche
While many studies have addressed Wnt signaling from the perspective of HSC or developing blood cells, only recently attention has been devoted to the influence of Wnt signaling on niche component cells (mesenchymal stromal cells, osteoblasts, CXCL12-abundant reticular cells, certain peripheral nerve cells, endothelial cells and others) (40). As discussed above, with transgenic expression of DKK1 specifically in osteoblasts, HSC self renewal was severely diminished(25). However, also the niche itself was severely altered, with a reduction in trabecular bone volume. Similarly, in mice deficient for a natural inhibitor of both canonical and non-canonical Wnt signaling, the Sfrp1 gene, a self renewal defect has been reported that was dependent on the mirco environment(41). Recently, Moore and Lemischka using another natural Wnt inhibitor, namely Wif1, also showed alteration in both the niche cells and HSCs(42). Wif transgenic mice showed normal numbers of HSC, but under stress conditions self renewal was impaired and HSC quiescence was lost. Of interest, not only was Wnt signaling disturbed in the niche cells, but several other key developmental signals implicated in HSC biology were altered. These include sonic hedgehog signaling to some extend Notch signaling. The Notch and HH pathways have extensively been described in other reviews in this spotlight series(43, 44). Thus, alterations in one important self-renewal pathway not only affect that specific pathway, but also other pathways implicated in the biology of HSC (either self renewal, integrity, quiescence or apoptosis).
Wnt signaling regulates other aspects of hematopoiesis in a dosage dependent fashion
Besides a role in the regulation of HSC function, Wnt signaling has also been implicated in differentiation through the different hematopoietic lineages. One excellent example is in T-cell development, where its role was first described (7). In line with the idea that fetal and leukemic stem cells may require higher Wnt activity than normal adult HSCs, the impaired T-cell development found with conditional deletion of β-catenin in the T-cell lineage (45) also suggests that thymocyte development requires higher levels of Wnt signaling than HSC or myeloid cells. Supporting this idea, measurement of Wnt signaling activity with in vivo reporter assays, showed a remarkable difference between bone marrow stem/progenitor cells and thymocytes (approximately 5 fold higher in thymocytes, (14)) and also significant changes in Wnt reporter positive cells in various hematopoietic subpopulations. Within HSC, the LT-HSC have the highest percentage of reporter activity, with MPPs the lowest, but also CMP and GMP show significant levels and percentage of Wnt positive cells (Figure 2). Both early myeloid and B-cell progenitors display detectable levels of Wnt signaling activity, which is down-regulated as these cells differentiate to more mature stages. The thymus is of all hematopoietic organs most rich in expression of Wnt components(46) and also has the highest levels of Wnt signaling. Of interest, all mature blood cells show no detectable Wnt reporter activity, with the exception of T lymphocytes. The functional importance of this finding is subject of intense research by various laboratories around the world.
Figure 2. Differential Wnt signaling in hematopoiesis and lymphopoiesis.

Contact between HSCs and niche cells enables many autocrine and paracrine interactions not only influencing the biology of HSCs but also of niche cells. In the simplest model, niche constituent cells secrete Wnt proteins that regulate HSC functions. However, interactions between HSCs and niche cells may also alter the secretion of Wnt proteins or HSC may also secrete Wnt proteins that influence stromal cells. We argue that precise amounts of Wnt are required to maintain HSC integrity, while different levels may be needed to support processes such as lymphopoiesis. Different percentages and Wnt signaling levels on a per cell basis have been reported. Color coding indicates a combination of the levels of Wnt signaling observed in a certain cell type and the percentage of cells responsive to Wnt signals.
Using the targeted approach of the APC tumor suppressor gene, our recent study (14)also provides evidence for an essential and dosage-dependent regulation of hematopoiesis by Wnt signals. A differential optimum of Wnt signaling activation was observed in HSCs, myeloid development and early thymocytes, with HSCs having the lower requirements and early thymocytes having the highest ones. Importantly, very high Wnt signaling impaired both HSC self-renewal and differentiation through the different hematopoietic lineages (Figure 3).
Figure 3. Differential optimum of Wnt signaling strength in HSCs and early myeloid and T-cell progenitors.
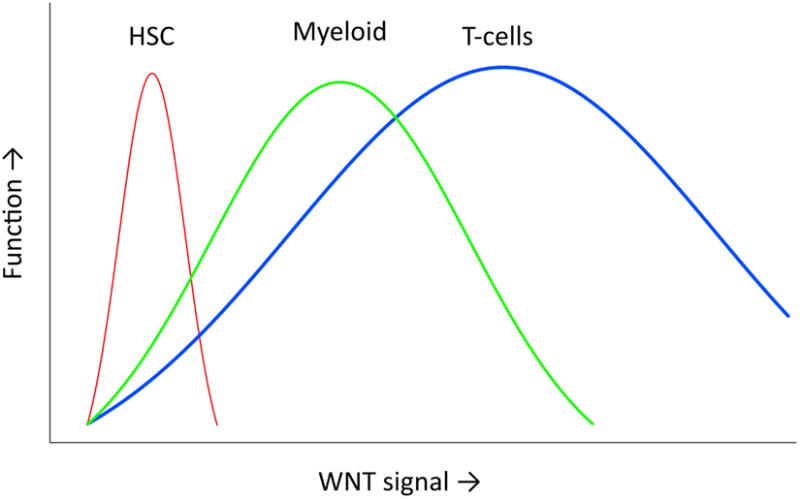
The graph displays the effects of increasing Wnt signaling above normal levels. For each cell lineage investigated (HSC, myeloid progenitors, thymocytes) a different optimal level of Wnt signaling activation was observed. It is possible that at the high end of Wnt signaling not only loss of integrity and apoptosis are induced, but also a pre leukemic stage, for instance by inducing self renewal properties on cell types normally lacking this property.
Artificial stimulation of canonical Wnt pathways confers multilineage differentiation potential on lymphoid and myeloid progenitors (47). Also, exposure to Wnt 3a producing stromal cells caused reacquisition of some stem-cell like characteristics by committed lymphoid progenitors (48). These observations raise the interesting possibility that early differentiation events may be reversible and Wnt signaling is important for maintenance of stem cell properties. This is consistent with the fact that Wnt3a helps promote establishment of induced pluripotent stem cell lines from adult tissues(49, 50). While Wnt3a blocks B lymphoid lineage progression in mice and humans, it is promoted by the non-canonical ligand Wnt5a (51, 52) . These effects have yet to be studied with respect to required levels of Wnt signaling.
Canonical Wnt signaling has also been implicated in the self-renewal of other stem cell compartments in the gut, mammary gland, skin and ES cells. Notably, Wnt signaling influences the capacity of ES cells to differentiate into the three main germ layers: ectoderm, mesoderm and definitive endoderm, in a dosage-dependent fashion(53). These Wnt dosage-dependent effects also seem to hold true for adult self-renewing tissues such as gut and skin, though the underlying cellular and molecular mechanisms still remain poorly understood (54). In addition, different levels of activation of the pathway confer varying degrees of tumor susceptibility in different tissues (55). It will be important to study whether hematopoietic malignancies where Wnt signaling is involved also have different and specific requirements of Wnt signaling strength. Finally, as particular Wnt family molecules could be implicated in some of these processes, they may represent candidates for new therapeutic approaches.
Interactions of Wnt signals with other key developmental pathways to regulate HSCs
In addition to the regulation of hematopoiesis by means of levels, timing and duration of Wnt signals, the outcome may be determined by the cellular context in which these signals are received. This may partially be explained by other signaling pathways activated at the same time. Indeed Wnt and Notch, as well as other signaling pathways such as Hedgehog, were previously shown to synergistically or antagonistically regulate each other. (56-58). The recent work on Wif1 transgenics underscores how closely linked thee pathways are (42). It has been proposed that Wnt and Notch signaling affect each other in regulating HSCs (57). However, the molecular and/or biochemical mechanisms underlying these processes are still poorly understood. Gsk-3β is a potential candidate to mediate this crosstalk since it regulates the stability/degradation of both β-catenin and NICD (59)and its inhibition affects HSC function through mechanisms involving regulation of both Wnt and Notch target genes (60). Additionally, Jagged1 is a Wnt/β-catenin target gene in other systems (61), implying that a cross-talk involving these pathways may occur between neighboring cells.
Aberrant Wnt signaling in leukemias
Over the last couple of years it has become apparent that deregulated Wnt signaling is important in development of hematological malignancies. Although the underlying mechanisms are sometimes unclear, mutations leading to overexpression of Wnt genes or mutations in key Wnt signaling molecules appear to be important. Also epigenetic changes in Wnt molecules have been reported, although the functional consequences for Wnt signaling and leukemogenesis remained unclear(62, 63). Methylation of Wnt antagonists has prognostic relevance in acute myeloid leukemia(AML) (64). Secreted Frizzled-related protein genes (sFRPs), have been found to be inactivated by promoter hypermethylation in ALL and AML(65).
In Chronic Myeloid Leukemia (CML) caused by the t(9,22) translocation leading to the production of the abnormal BCR-ABL fusion protein, Wnt signaling is activated during blast crises (66). The reasons for this abnormal Wnt signaling are unknown. As discussed above, β-catenin deletion significantly reduced CML development in murine models(19) and, of MLL-AF9 or HoxA9 and Meis1a induced acute myelogenous leukemia (AML) (67) In addition, in treatment insensitive BCR-ABL+ CML, β-catenin is crucial to the survival of leukemic cells (68). As AML is characterized by aberrant Wnt activation (69) (67), this raises the interesting possibility that Wnt confers stem cell properties on the leukemic stem cells (LSC) (70). Therefore, similarly to HSCs in embryos, LSCs may have a higher requirement of Wnt activity than adult HSC in bone marrow.
This idea is further supported by recent work on AML stem cells in a MLL-AF9 leukemia model in mice. In this study by Williams, Amstrong, Scadden and coworkers, normal HSC, Leukemic Stem Cells (LSC) and a preleukemic stem cells (pre LSC) are identified (71). Pre LSC contain immortalized HSC and progenitors with self renewal properties and the ability to give rise to leukemia, depending on secondary mutational hits. In full blown AML samples (both in mouse and human) cell intrinsic Wnt signaling is well established (ref Muller Ridow leukemia reference), but Pre LSC and HSC are dependent on and under control of niche derived Wnt signals. Using the DKK1 transgenic model, the investigators show that the LSC occupy a distinct niche than HSC, with different dosages of external Wnt signals.
Also in models for T-Acute Lymphoblastic Leukemia (ALL), thymus specific expression of activated β-catenin leads to development of thymic lymphomas(72). This work in mouse models has to be extended to cohorts of patients with rigorous assessment of the status of the Wnt pathway, to indicate whether deregulated Wnt signaling is a causative factor in human leukemias as well (73).In summary, current evidence suggests that Wnt signaling is capable of conferring stem cell properties on leukemic stem cells(74). Thus, targeting aberrant Wnt signaling may be a means to directly affect leukemic stem cells, in contrast to most conventional chemotherapy that often is less affective at targeting leukemia initiating cells than the bulk of the malignant cells.
Concluding remarks
HSC self-renewal, specification, commitment and differentiation are complex processes regulated by intricate networks of signals and transcription factors that have to be tightly orchestrated. It has been a long-standing challenge to mimic the niche signals HSCs receive, in order to expand and manipulate them for clinical purposes. Such clinical applications not only refer to HSCS, but also to IPS cells differentiated into blood cells (75) The possibility of achieving these goals by stimulating HSCs with signaling ligands such as Wnt is very attractive(75). However, changes in delicate balances between these factors may lead to leukemia (76), immunodeficiencies or autoimmunity. Unraveling these mechanisms will therefore be essential in order to fully explore the potential of HSCs and translate this basic knowledge into clinical applications. The deregulation of Wnt signaling as a causative factor in leukemogenesis is becoming more and more apparent, especially in leukemic model systems in the mouse. Given that many pharmacological small-molecule inhibitors of Wnt signaling are being tested, these developments open up new therapeutic strategies that target aberrant Wnt signaling in hematological malignancies (77).
Acknowledgments
T.C.L is partially supported by “Fundação para a Ciência e a Tecnologia – Portugal”. F.J.T.S is supported in part by the Association of International Cancer Research (AICR) and a TOP-Grant from The Netherlands Organisation for Health Research and Development (ZonMW). M.H.B. is supported by a grant from the Netherlands Institute for Regenerative Medicine (NIRM). M.I and P.W.K. are supported by grant AI20069 from the National Institutes of Health.
Footnotes
Authors have no conflict of interest to disclose.
References
- 1.Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008 Aug;8(8):581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- 2.Malhotra S, Kincade PW. Wnt-related molecules and signaling pathway equilibrium in hematopoiesis. Cell Stem Cell. 2009 Jan 9;4(1):27–36. doi: 10.1016/j.stem.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker N, Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology. May;138(5):1681–1696. doi: 10.1053/j.gastro.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Carmon KS, Gong X, Lin Q, Thomas A, Liu Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A. Jul 12;108(28):11452–11457. doi: 10.1073/pnas.1106083108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staal FJ, Sen JM. The canonical Wnt signaling pathway plays an important role in lymphopoiesis and hematopoiesis. Eur J Immunol. 2008 Jul;38(7):1788–1794. doi: 10.1002/eji.200738118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulroy T, McMahon JA, Burakoff SJ, McMahon AP, Sen J. Wnt-1 and Wnt-4 regulate thymic cellularity. Eur J Immunol. 2002 Apr;32(4):967–971. doi: 10.1002/1521-4141(200204)32:4<967::AID-IMMU967>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Staal FJ, Meeldijk J, Moerer P, Jay P, van de Weerdt BC, Vainio S, et al. Wnt signaling is required for thymocyte development and activates Tcf-1 mediated transcription. Eur J Immunol. 2001 Jan;31(1):285–293. doi: 10.1002/1521-4141(200101)31:1<285::AID-IMMU285>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 8.Staal FJ, Clevers HC. Wnt signaling in the thymus. Curr Opin Immunol. 2003 Apr;15(2):204–208. doi: 10.1016/s0952-7915(03)00003-7. [DOI] [PubMed] [Google Scholar]
- 9.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003 May 22;423(6938):409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 10.Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol. 2006 Oct;7(10):1048–1056. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- 11.Scheller M, Huelsken J, Rosenbauer F, Taketo MM, Birchmeier W, Tenen DG, et al. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat Immunol. 2006 Oct;7(10):1037–1047. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- 12.Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes & development. 2008 Sep 1;22(17):2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aoki K, Taketo MM. Methods in molecular biology. Vol. 468. Clifton, NJ: 2008. Tissue-specific transgenic, conditional knockout and knock-in mice of genes in the canonical Wnt signaling pathway; pp. 307–331. [DOI] [PubMed] [Google Scholar]
- 14.Luis TC, Naber BA, Roozen PP, Brugman MH, de Haas EF, Ghazvini M, et al. Canonical Wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell. 2011;9(4):345–356. doi: 10.1016/j.stem.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Kielman MF, Rindapaa M, Gaspar C, van Poppel N, Breukel C, van Leeuwen S, et al. Apc modulates embryonic stem-cell differentiation by controlling the dosage of beta-catenin signaling. Nat Genet. 2002 Dec;32(4):594–605. doi: 10.1038/ng1045. [DOI] [PubMed] [Google Scholar]
- 16.Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009 Mar 20;136(6):1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lane SW, Sykes SM, Al-Shahrour F, Shterental S, Paktinat M, Lo Celso C, et al. The Apc(min) mouse has altered hematopoietic stem cell function and provides a model for MPD/MDS. Blood. 2010 Apr 29;115(17):3489–3497. doi: 10.1182/blood-2009-11-251728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J, Zhang Y, Bersenev A, O'Brien WT, Tong W, Emerson SG, et al. Pivotal role for glycogen synthase kinase-3 in hematopoietic stem cell homeostasis in mice. J Clin Invest. 2009 Dec;119(12):3519–3529. doi: 10.1172/JCI40572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007 Dec;12(6):528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cobas M, Wilson A, Ernst B, Mancini SJ, MacDonald HR, Kemler R, et al. Beta-catenin is dispensable for hematopoiesis and lymphopoiesis. J Exp Med. 2004 Jan 19;199(2):221–229. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeannet G, Scheller M, Scarpellino L, Duboux S, Gardiol N, Back J, et al. Long-term, multilineage hematopoiesis occurs in the combined absence of beta-catenin and gamma-catenin. Blood. 2008 Jan 1;111(1):142–149. doi: 10.1182/blood-2007-07-102558. [DOI] [PubMed] [Google Scholar]
- 22.Koch U, Wilson A, Cobas M, Kemler R, Macdonald HR, Radtke F. Simultaneous loss of beta- and gamma-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008 Jan 1;111(1):160–164. doi: 10.1182/blood-2007-07-099754. [DOI] [PubMed] [Google Scholar]
- 23.Prlic M, Bevan MJ. Cutting Edge: {beta }-Catenin Is Dispensable for T Cell Effector Differentiation, Memory Formation, and Recall Responses. J Immunol. 2011 Aug 15;187(4):1542–1546. doi: 10.4049/jimmunol.1100907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luis TC, Naber BA, Fibbe WE, van Dongen JJ, Staal FJ. Wnt3a nonredundantly controls hematopoietic stem cell function and its deficiency results in complete absence of canonical Wnt signaling. Blood. 2010 Jul 22;116(3):496–497. doi: 10.1182/blood-2010-04-282624. [DOI] [PubMed] [Google Scholar]
- 25.Fleming HE, Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008 Mar 6;2(3):274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luis TC, Weerkamp F, Naber BA, Baert MR, de Haas EF, Nikolic T, et al. Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood. 2009 Jan 15;113(3):546–554. doi: 10.1182/blood-2008-06-163774. [DOI] [PubMed] [Google Scholar]
- 27.Galceran J, Farinas I, Depew MJ, Clevers H, Grosschedl R. Wnt3a-/--like phenotype and limb deficiency in Lef1(-/-)Tcf1(-/-) mice. Genes Dev. 1999 Mar 15;13(6):709–717. doi: 10.1101/gad.13.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994 Jan;8(2):174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- 29.Okamura RM, Sigvardsson M, Galceran J, Verbeek S, Clevers H, Grosschedl R. Redundant regulation of T cell differentiation and TCRalpha gene expression by the transcription factors LEF-1 and TCF-1. Immunity. 1998 Jan;8(1):11–20. doi: 10.1016/s1074-7613(00)80454-9. [DOI] [PubMed] [Google Scholar]
- 30.Schilham MW, Wilson A, Moerer P, Benaissa-Trouw BJ, Cumano A, Clevers HC. Critical involvement of Tcf-1 in expansion of thymocytes. J Immunol. 1998 Oct 15;161(8):3984–3991. [PubMed] [Google Scholar]
- 31.Malhotra S, Kincade PW. Canonical Wnt pathway signaling suppresses VCAM-1 expression by marrow stromal and hematopoietic cells. Exp Hematol. 2009 Jan;37(1):19–30. doi: 10.1016/j.exphem.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005 May;8(5):739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005 May;8(5):727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Rodda SJ, McMahon AP. Development. 16. Vol. 133. Cambridge, England: 2006. Aug, Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors; pp. 3231–3244. [DOI] [PubMed] [Google Scholar]
- 35.Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002 May 16;346(20):1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 36.Reya T, O'Riordan M, Okamura R, Devaney E, Willert K, Nusse R, et al. Wnt signaling regulates B lymphocyte proliferation through a LEF-1 dependent mechanism. Immunity. 2000 Jul;13(1):15–24. doi: 10.1016/s1074-7613(00)00004-2. [DOI] [PubMed] [Google Scholar]
- 37.Louis I, Heinonen KM, Chagraoui J, Vainio S, Sauvageau G, Perreault C. The signaling protein Wnt4 enhances thymopoiesis and expands multipotent hematopoietic progenitors through beta-catenin-independent signaling. Immunity. 2008 Jul 18;29(1):57–67. doi: 10.1016/j.immuni.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 38.Murdoch B, Chadwick K, Martin M, Shojaei F, Shah KV, Gallacher L, et al. Wnt-5A augments repopulating capacity and primitive hematopoietic development of human blood stem cells in vivo. Proc Natl Acad Sci U S A. 2003 Mar 18;100(6):3422–3427. doi: 10.1073/pnas.0130233100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nemeth MJ, Topol L, Anderson SM, Yang Y, Bodine DM. Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proc Natl Acad Sci U S A. 2007 Sep 25;104(39):15436–15441. doi: 10.1073/pnas.0704747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suda T, Arai F. Wnt signaling in the niche. Cell. 2008 Mar 7;132(5):729–730. doi: 10.1016/j.cell.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 41.Renstrom J, Istvanffy R, Gauthier K, Shimono A, Mages J, Jardon-Alvarez A, et al. Secreted frizzled-related protein 1 extrinsically regulates cycling activity and maintenance of hematopoietic stem cells. Cell Stem Cell. 2009 Aug 7;5(2):157–167. doi: 10.1016/j.stem.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 42.Schaniel C, Sirabella D, Qiu J, Niu X, Lemischka IR, Moore KA. Wnt-inhibitory factor 1 dysregulation of the bone marrow niche exhausts hematopoietic stem cells. Blood. 2011 Sep 1;118(9):2420–2429. doi: 10.1182/blood-2010-09-305664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pajcini KV, Speck NA, Pear WS. Notch signaling in mammalian hematopoietic stem cells. Leukemia. Jun 7;25(10):1525–1532. doi: 10.1038/leu.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mar BG, Amakye D, Aifantis I, Buonamici S. The controversial role of the Hedgehog pathway in normal and malignant hematopoiesis. Leukemia. 2011 Jun 10;25(11):1665–1673. doi: 10.1038/leu.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Y, Banerjee D, Huelsken J, Birchmeier W, Sen JM. Deletion of beta-catenin impairs T cell development. Nat Immunol. 2003 Dec;4(12):1177–1182. doi: 10.1038/ni1008. [DOI] [PubMed] [Google Scholar]
- 46.Weerkamp F, Baert MR, Naber BA, Koster EE, de Haas EF, Atkuri KR, et al. Wnt signaling in the thymus is regulated by differential expression of intracellular signaling molecules. Proc Natl Acad Sci U S A. 2006 Feb 28;103(9):3322–3326. doi: 10.1073/pnas.0511299103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baba Y, Garrett KP, Kincade PW. Constitutively active beta-catenin confers multilineage differentiation potential on lymphoid and myeloid progenitors. Immunity. 2005 Dec;23(6):599–609. doi: 10.1016/j.immuni.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baba Y, Yokota T, Spits H, Garrett KP, Hayashi S, Kincade PW. Constitutively active beta-catenin promotes expansion of multipotent hematopoietic progenitors in culture. J Immunol. 2006 Aug 15;177(4):2294–2303. doi: 10.4049/jimmunol.177.4.2294. [DOI] [PubMed] [Google Scholar]
- 49.Hudson JE, Zimmermann WH. Tuning Wnt-signaling to enhance cardiomyogenesis in human embryonic and induced pluripotent stem cells. J Mol Cell Cardiol. Sep;51(3):277–279. doi: 10.1016/j.yjmcc.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 50.Katoh M. Network of WNT and other regulatory signaling cascades in pluripotent stem cells and cancer stem cells. Curr Pharm Biotechnol. Feb 1;12(2):160–170. doi: 10.2174/138920111794295710. [DOI] [PubMed] [Google Scholar]
- 51.Malhotra S, Baba Y, Garrett KP, Staal FJ, Gerstein R, Kincade PW. Contrasting responses of lymphoid progenitors to canonical and noncanonical Wnt signals. J Immunol. 2008 Sep 15;181(6):3955–3964. doi: 10.4049/jimmunol.181.6.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dosen G, Tenstad E, Nygren MK, Stubberud H, Funderud S, Rian E. Wnt expression and canonical Wnt signaling in human bone marrow B lymphopoiesis. BMC Immunol. 2006;7:13. doi: 10.1186/1471-2172-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaspar C, Fodde R. APC dosage effects in tumorigenesis and stem cell differentiation. Int J Dev Biol. 2004;48(5-6):377–386. doi: 10.1387/ijdb.041807cg. [DOI] [PubMed] [Google Scholar]
- 54.Silva-Vargas V, Lo Celso C, Giangreco A, Ofstad T, Prowse DM, Braun KM, et al. Beta-catenin and Hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Dev Cell. 2005 Jul;9(1):121–131. doi: 10.1016/j.devcel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 55.Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer. 2001 Oct;1(1):55–67. doi: 10.1038/35094067. [DOI] [PubMed] [Google Scholar]
- 56.Blank U, Karlsson G, Karlsson S. Signaling pathways governing stem-cell fate. Blood. 2008 Jan 15;111(2):492–503. doi: 10.1182/blood-2007-07-075168. [DOI] [PubMed] [Google Scholar]
- 57.Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005 Mar;6(3):314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- 58.Trowbridge JJ, Moon RT, Bhatia M. Hematopoietic stem cell biology: too much of a Wnt thing. Nat Immunol. 2006 Oct;7(10):1021–1023. doi: 10.1038/ni1006-1021. [DOI] [PubMed] [Google Scholar]
- 59.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997 Jul 1;16(13):3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trowbridge JJ, Xenocostas A, Moon RT, Bhatia M. Glycogen synthase kinase-3 is an in vivo regulator of hematopoietic stem cell repopulation. Nat Med. 2006 Jan;12(1):89–98. doi: 10.1038/nm1339. [DOI] [PubMed] [Google Scholar]
- 61.Estrach S, Ambler CA, Lo Celso C, Hozumi K, Watt FM. Jagged 1 is a beta-catenin target gene required for ectopic hair follicle formation in adult epidermis. Development. 2006 Nov;133(22):4427–4438. doi: 10.1242/dev.02644. [DOI] [PubMed] [Google Scholar]
- 62.Bennett LB, Taylor KH, Arthur GL, Rahmatpanah FB, Hooshmand SI, Caldwell CW. Epigenetic regulation of WNT signaling in chronic lymphocytic leukemia. Epigenomics. Feb 1;2(1):53–70. doi: 10.2217/epi.09.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roman-Gomez J, Cordeu L, Agirre X, Jimenez-Velasco A, San Jose-Eneriz E, Garate L, et al. Epigenetic regulation of Wnt-signaling pathway in acute lymphoblastic leukemia. Blood. 2007 Apr 15;109(8):3462–3469. doi: 10.1182/blood-2006-09-047043. [DOI] [PubMed] [Google Scholar]
- 64.Valencia A, Roman-Gomez J, Cervera J, Such E, Barragan E, Bolufer P, et al. Wnt signaling pathway is epigenetically regulated by methylation of Wnt antagonists in acute myeloid leukemia. Leukemia. 2009 Sep;23(9):1658–1666. doi: 10.1038/leu.2009.86. [DOI] [PubMed] [Google Scholar]
- 65.Jost E, Schmid J, Wilop S, Schubert C, Suzuki H, Herman JG, et al. Epigenetic inactivation of secreted Frizzled-related proteins in acute myeloid leukaemia. Br J Haematol. 2008 Sep;142(5):745–753. doi: 10.1111/j.1365-2141.2008.07242.x. [DOI] [PubMed] [Google Scholar]
- 66.Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004 Aug 12;351(7):657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, Krivtsov AV, Sinha AU, North TE, Goessling W, Feng Z, et al. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. Mar 26;327(5973):1650–1653. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu Y, Chen Y, Douglas L, Li S. beta-Catenin is essential for survival of leukemic stem cells insensitive to kinase inhibition in mice with BCR-ABL-induced chronic myeloid leukemia. Leukemia. 2009 Jan;23(1):109–116. doi: 10.1038/leu.2008.262. [DOI] [PubMed] [Google Scholar]
- 69.Muller-Tidow C, Steffen B, Cauvet T, Tickenbrock L, Ji P, Diederichs S, et al. Translocation products in acute myeloid leukemia activate the Wnt signaling pathway in hematopoietic cells. Mol Cell Biol. 2004 Apr;24(7):2890–2904. doi: 10.1128/MCB.24.7.2890-2904.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eaves CJ, Humphries RK. Acute myeloid leukemia and the Wnt pathway. N Engl J Med. Jun 17;362(24):2326–2327. doi: 10.1056/NEJMcibr1003522. [DOI] [PubMed] [Google Scholar]
- 71.Lane SW, Wang YJ, Lo Celso C, Ragu C, Bullinger L, Sykes SM et al. Differential niche and Wnt requirements during acute myeloid leukemia progression. Blood. 2011 Jul 15;118(10):2849–56. doi: 10.1182/blood-2011-03-345165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guo Z, Dose M, Kovalovsky D, Chang R, O'Neil J, Look AT, et al. Beta-catenin stabilization stalls the transition from double-positive to single-positive stage and predisposes thymocytes to malignant transformation. Blood. 2007 Jun 15;109(12):5463–5472. doi: 10.1182/blood-2006-11-059071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weerkamp F, van Dongen JJ, Staal FJ. Notch and Wnt signaling in T-lymphocyte development and acute lymphoblastic leukemia. Leukemia. 2006 Jul;20(7):1197–1205. doi: 10.1038/sj.leu.2404255. [DOI] [PubMed] [Google Scholar]
- 74.Heidel FH, Mar BG, Armstrong SA. Self-renewal related signaling in myeloid leukemia stem cells. Int J Hematol. Aug;94(2):109–117. doi: 10.1007/s12185-011-0901-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Staal FJ, Baum C, Cowan C, Dzierzak E, Hacein-Bey-Abina S, Karlsson S, et al. Stem cell self-renewal: lessons from bone marrow, gut and iPS toward clinical applications. Leukemia. Jul;25(7):1095–1102. doi: 10.1038/leu.2011.52. [DOI] [PubMed] [Google Scholar]
- 76.Sengupta A, Banerjee D, Chandra S, Banerji SK, Ghosh R, Roy R, et al. Deregulation and cross talk among Sonic hedgehog, Wnt, Hox and Notch signaling in chronic myeloid leukemia progression. Leukemia. 2007 May;21(5):949–955. doi: 10.1038/sj.leu.2404657. [DOI] [PubMed] [Google Scholar]
- 77.Misaghian N, Ligresti G, Steelman LS, Bertrand FE, Basecke J, Libra M, et al. Targeting the leukemic stem cell: the Holy Grail of leukemia therapy. Leukemia. 2009 Jan;23(1):25–42. doi: 10.1038/leu.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]


