Abstract
Consummatory responses to taste stimuli are modulated by visceral signals processed in the caudal nucleus of the solitary tract (cNST) and ventrolateral medulla. On the basis of decerebrate preparations, this modulation can occur through local brain stem pathways. Among the large number of neuropeptides and neuromodulators implicated in these visceral pathways is neuropeptide Y (NPY), which is oftentimes colocalized in catecholaminergic neurons themselves implicated in glucoprivic-induced feeding and satiety. In addition to the cNST and ventrolateral medulla, noradrenergic and NPY receptors are found in circumscribed regions of the medullary reticular formation rich in preoromotor neurons. To test the hypothesis that NPY may act as a neuromodulator on preoromotor neurons, we recorded the effects of bath application of NPY and specific Y1 and Y2 agonists on currents elicited from electrical stimulation of the rostral (taste) NST in prehypoglossal neurons in a brain stem slice preparation. A high proportion of NST-driven responses were suppressed by NPY, as well as Y1 and Y2 agonists. On the basis of paired pulse ratios and changes in membrane resistance, we concluded that Y1 receptors influence these neurons both presynaptically and postsynaptically and that Y2 receptors have a presynaptic locus. To test the hypothesis that NPY may act in concert with norepinephrine (NE), we examined neurons showing suppressed responses in the presence of a Y2 agonist and demonstrated a greater degree of suppression to a Y2 agonist/NE cocktail. These suppressive effects on preoromotoneurons may reflect a satiety pathway originating from A2 neurons in the caudal brain stem.
Keywords: oromotor, ingestion, reticular formation, norepinephrine
circuits controlling the consummatory behaviors of ingestion are located in the lower brain stem (7, 38, 40, 56). These circuits extend from the pons to the spinal medullary junction to encompass both sensory and motor nuclei, as well as specific regions of the reticular formation (RF). One region, immediately subjacent to the nucleus of the solitary tract (NST), which includes both the intermediate subdivision of the medullary RF (IRt) and the more lateral parvocellular RF (PCRt), contains a dense constellation of preoromotor neurons (29, 37, 58) that provide a potent source of excitatory drive to the oromotor nuclei (59, 60). Functional inactivation of the IRt/PCRt with either the GABA agonist muscimol or glutamatergic antagonists suppresses consummatory behavior in the awake freely moving rat (10, 11, 57).
An additional, integrative role for the IRt/PCRt is suggested by input from forebrain sites involved in homeostatic, regulatory, and motor function (9, 43, 50, 61), as well as overlapping input from brain stem orosensory and viscerosensory nuclei, including the rostral (gustatory) nucleus of the solitary tract (rNST), caudal (visceral) NST (cNST), the parabrachial nucleus, and ventrolateral medulla (8, 28, 31, 54, 64). Results from decerebrate preparations in which visceral signals, such as gastric load (49) and glucoprivation (15, 21), modify the amount of a palatable (sweet) stimulus that is consumed, suggest that these local pathways exert a potent influence over this consummatory circuitry (reviewed in Refs. 24, 45, 46). Although some amino acid-mediated excitatory and inhibitory inputs to IRt/PCRt preoromotor neurons have been identified from the rostral (gustatory) nucleus of the solitary tract (41), little is known of the neurochemical identity of other local modulatory influences on these neurons. Indirect influences on IRt/PCRt premotor neurons, however, can be inferred from brain stem/fourth ventricle infusions of neuromodulators that impact feeding behavior. Brain stem infusions of opioids (32, 34), ghrelin (20), catecholamines (53), and neuropeptide Y (12, 13, 53), all modulate ingestive behavior, and some or all of these responses may involve circuits complete within the brain stem.
The present study was undertaken to begin to identify neuromodulators that modify the influence of rNST (gustatory) stimulation on prehypoglossal neurons in the IRt/PCRt. We focused on agonists for receptors for the neuropeptide Y (NPY) family of peptides, in particular, Y1 and Y2 receptors, because they are well represented in the IRt/PCRt (33, 51) and because the brain stem contains sources for their endogenous ligands; i.e., NPY in the cNST and ventrolateral medulla, and PYY in the medial RF (19, 23, 26, 39, 48). Our results, indeed, demonstrate that activation of these receptors has a marked influence on rNST-driven responses in IRt/PCRt prehypoglossal neurons. Both Y1 and Y2 agonists cause presynaptic inhibition, and Y1 has an additional postsynaptic inhibitory effect on these responses. Because a large proportion of NPY-positive neurons in the lower brain stem colocalize with catecholamines, we also examined whether norepinephrine (NE) also modulated neurons affected by NPY. In neurons inhibited by a Y2 agonist, NE had a further suppressive effect. These inhibitory results on presumably excitatory preoromotor neurons are not easily related to the orexigenic effects of fourth ventricle NPY infusions but could be explanatory for a satiety pathway originating from A2 neurons that colocalize NPY and NE.
METHODS
Retrograde tracing.
To record from identified prehypoglossal neurons, a retrograde tracer was injected into the hypoglossal nucleus (mXII) (41). Briefly, under deep anesthesia with a combination of ketamine and xylazine (90/30 mg/kg ip), Sprague-Dawley rat pups (P7–P10) were placed in a stereotaxic frame and held in place with mouse ear cups. After opening the caudal medulla at the level of the area postrema, injections of rhodamine-labeled fluorescent microspheres (Invitrogen: 0.04 μm diameter, 50 to 100 nl) were made into the mXII 0.2 mm rostral to obex and 0.2 mm lateral to the midline. The depth was predetermined by observing microstimulation-induced lingual movements through a micropipette (tip diameter 20–40 μm) filled with 0.9% saline. The surgical wound was gently packed with gelfoam, and the skin was joined with wound clips. Pups were returned to their home cage and survived for 12–48 h to allow retrograde transport of the fluorescent tracer. All experimental protocols were approved by the Ohio State University Institutional Animal Care and Use Committee in accordance with guidelines from the National Institutes of Health.
Slice preparation.
Slices containing prehypoglossal neurons were obtained as reported previously (41, 62). Rat pups were decapitated under deep anesthesia (33% urethane, 10 ml/kg). The brain stem was quickly extracted and placed in an ice-cold, oxygenated (95% O2–5% CO2) modified Kreb's solution containing (in mM): 110 choline, 25 NaHCO3, 3 KCl, 7 MgSO4, 1.5 NaH2PO4, 10 d-glucose, and 0.5 CaCl2. The brain was blocked ∼1 mm caudal to obex and rostrally at the level of the incoming VIIth nerve and glued to a ceramic block with cyanoacrylate glue for sectioning in the coronal plane on a Vibratome 1000 (Vibratome, St. Louis, MO). Coronal slices of the brain stem were cut with a sapphire knife at 350 μM and transferred to an incubation chamber containing an oxygenated normal Kreb's solution (in mM): 124 NaCl, 25 NaHCO3, 3 KCl, 1 MgSO4, 1.5 NaH2PO4, 10 d-glucose, and 1.5 CaCl2. Sections used for recording included those from the level where NST starts to separate from the 4th ventricle to the rostral pole of the NST. Typically, four 350-μm slices were cut from this area.
Electrophysiology.
Following 1–2 h incubation, a single slice was transferred to a custom recording chamber mounted on the stage (Siskiyou Instruments, Grants Pass, OR) of an upright microscope (Nikon, E600FN). Brain slices were held in place by a custom-made gold harp fitted with nylon mesh. The chamber was continuously perfused with warmed (32°C) oxygenated normal Kreb's solution (2 ml/min). Patch pipettes (4–6 MΩ) were formed from 1.5 mm thin-wall borosilicate glass (A-M systems, Sequim, WA) pulled on a Narishige PP-83 vertical Pipette puller. The pipette solution consisted of (in mM): 140 potassium gluconate, 10 EGTA, 10 HEPES, 1 MgCl2, 1 CaCl2, 2 ATP, and 5 NaCl. Lucifer Yellow (0.1%) was added to the pipette solution to label recorded neurons to verify double-labeled neurons and their locations.
To evoke postsynaptic currents in IRt/PCRt neurons, a bipolar stimulating electrode (67 μm, NiCr) was placed in the rNST under bright-field optics. Single (0.20 ms, 0.1 Hz, 100–150 μA) or paired pulses (0.20 ms, 30 Hz, 100–150 μA, train rate 0.10 Hz) were applied. Under IR-DIC and epifluorescence optics, patch pipettes were guided to retrogradely labeled neurons within the RF. Patch-clamp recordings were performed in whole cell configuration (model no. 2400, AM Systems) by rupturing the neuronal membrane with an intact gigaohm seal (resistance ≥1 GΩ). Neurons were excluded from the study if they did not meet the following criteria: ≥1-GΩ seal, ≥100-MΩ membrane resistance, ≥40 mV action potential amplitude, and stable resting membrane potential more negative than −40 mV. Action potential properties and membrane resistance were determined under current clamp with a series of current steps: −0.2 to 0.2 nA in 0.05 nA steps. Resting membrane potential and membrane resistance were monitored periodically throughout the recordings. Responses to electrical stimulation of the rNST were typically recorded under voltage clamp near the resting membrane potential using Clampfit 9.2 software (Molecular Devices, Union City, CA). Reported values were not corrected for a junction potential of ∼12 mV. In a subset of experiments, changes in spontaneous firing frequency were monitored in current clamp.
Drug application.
All drugs were kept as a concentrated stock solution stored at −20°C and diluted to their final concentration immediately before application. The following drugs were used: NPY (0.1–5 μM), NPY 3–36 (0.01–0.5 μM; Sigma, St. Louis, MO), a potent Y2 agonist (5, 6); d-arg-25-NPY (0.01–0.5 μM; American Peptide, Sunnyvale, CA) a Y1 agonist (22); BIIE 0246 (1 μM ), a Y2 antagonist (17) and BIBO 3304 (1 μM; Tocris, Ellisville, MO), a Y1 antagonist, (17, 63), and NE (2 - 100 μM; Sigma-Aldrich). To test the pharmacological effects on the evoked postsynaptic currents, membrane resistance, and spontaneous firing frequency, drugs were applied for 1 to 10 min following a minimum of 5 min of baseline recording. Following drug application, the normal Krebs solution was reapplied. Neurons that did not show at least a partial recovery were excluded from statistical analyses.
Data analysis.
All data were expressed as means ± SE. The amplitudes of the rNST-evoked excitatory postsynaptic currents (EPSCs) were measured following the first response to a paired pulse stimulation or following a single pulse before and after drug application. The paired pulse ratio was computed as the amplitude of the 2nd EPSC divided by the 1st EPSC. Membrane resistance was calculated as the slope of the membrane voltage against the amount of current injected. Mean spontaneous firing frequency (SFF) was measured in current clamp before and after drug application. Mean control SFF was calculated from the number of spikes occurring during a 5-min interval prior to drug perfusion; mean drug SFF was calculated as the number of spikes occurring during a 1–3-min period following drug application. Paired t-tests or repeated-measures ANOVA were used to assess drug effects (P < 0.05 criteria).
RESULTS
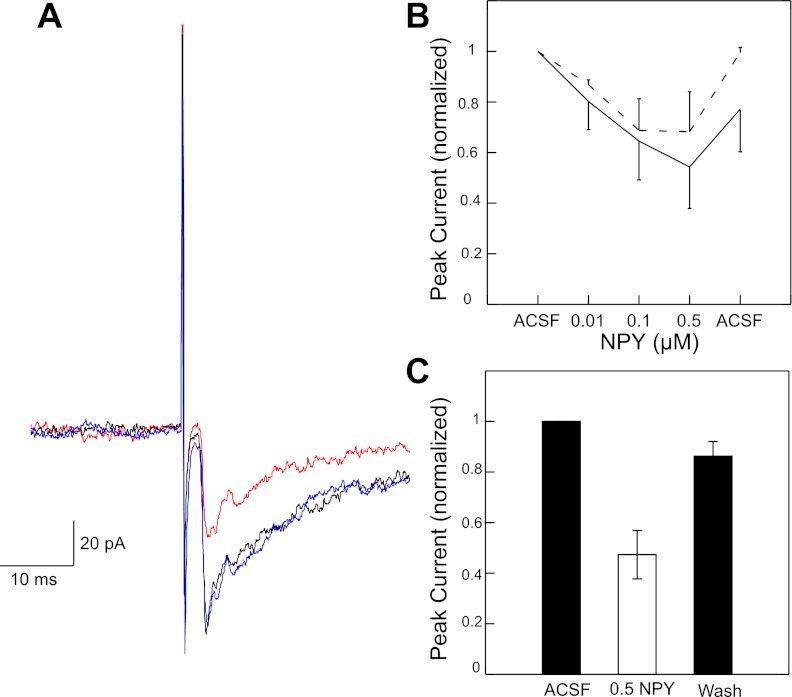
The effects of bath application of NPY, d-arg-25-NPY (Y1 agonist), and NPY3–36 (Y2 agonist) on NST stimulation-induced EPSCs were studied in a total of 125 mXII-projecting IRt/PCRt neurons. NPY suppressed rNST-evoked excitatory and inhibitory responses in a dose-dependent fashion in the subset of neurons tested with multiple concentrations (Fig. 1B). Separate repeated-measures ANOVA were performed for excitatory (P < 0.013, n = 3) and inhibitory (P < 0.033, n = 3) responses. In a larger population of neurons tested at a concentration of 0.5 μM (n = 7), the mean peak excitatory response was suppressed by nearly 60% (paired t-test: P < 0.008). To more fully determine the type and location of the receptors mediating this suppression, we examined paired pulse ratios and changes in membrane resistance to bath application of specific Y1 and Y2 agonists.
Fig. 1.
A: Stimulation of the rostral (gustatory) nucleus of the solitary tract (rNST) produces inward current during baseline condition (black). Suppression of rNST-evoked inward current following bath application of NPY (red: 0.5 μM) followed by return to baseline during washout (blue). B: dose-dependent effect of NPY on both excitatory (solid) and inhibitory (dashed) responses. C: in a larger population of neurons, 0.5 μM NPY reduces excitatory current by nearly 60%.
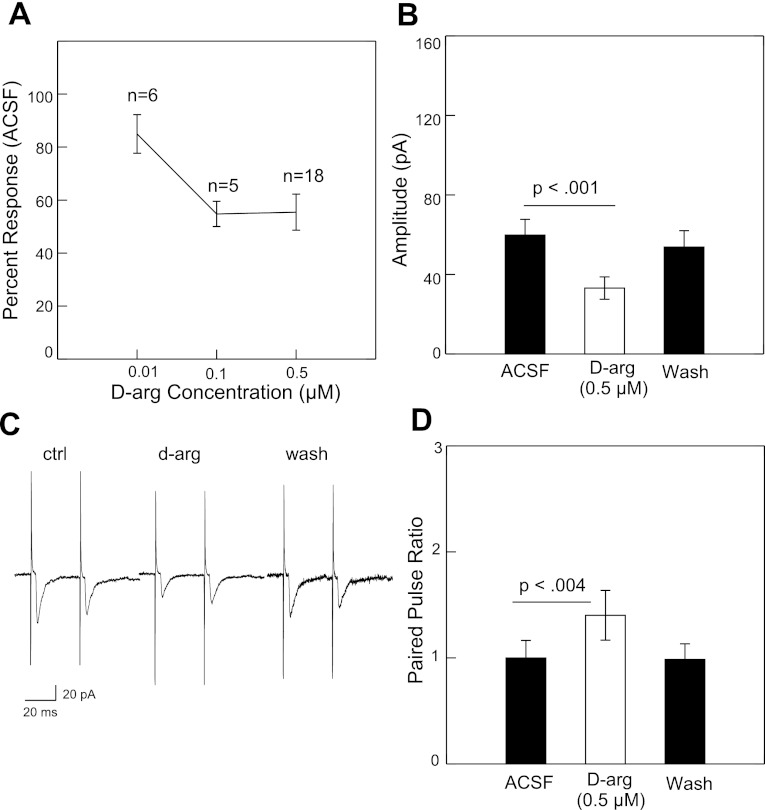
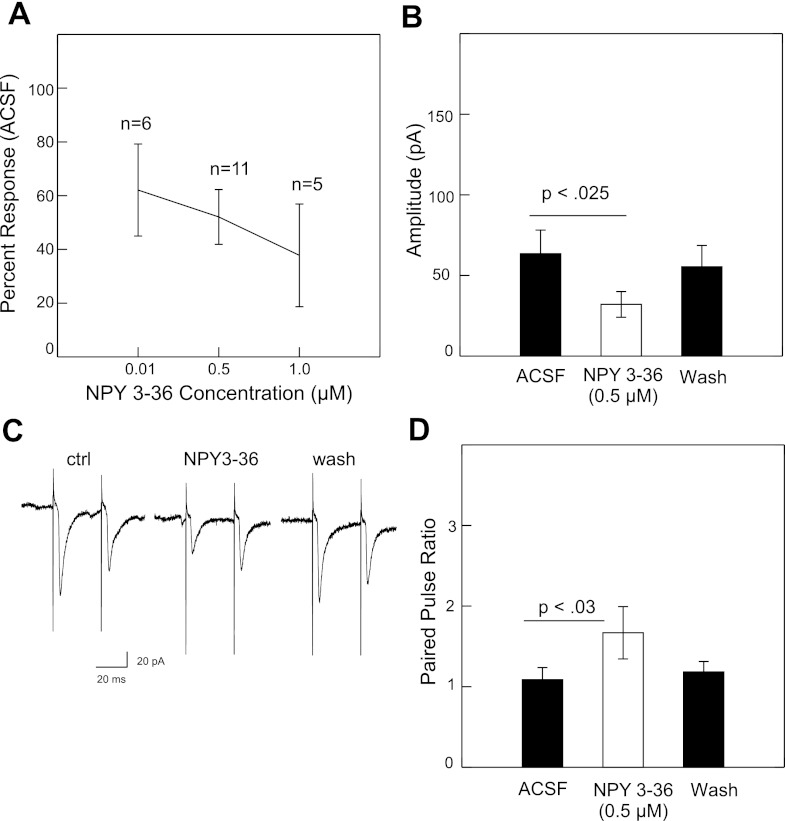
Following bath application of the Y1 receptor agonist d-arg-25-NPY (0.01–0.5 μM) or the Y2 agonist NPY3–36 (0.01–1.0 μM), there was a dose-dependent reduction in the peak amplitude of the NST-evoked EPSCs (Figs. 2A and Fig. 3A). At 0.5 μM, d-arg-25-NPY significantly suppressed the peak excitatory current from 59.8 pA to 33.1 pA (−44.7%) followed by recovery during washout to 53.8 pA (Fig. 2B). The difference between the control and drug conditions was statistically significant (P < 0.001, paired t-test, n = 18). At 0.5 μM, NPY3–36 significantly suppressed the peak excitatory current from 63.5 pA to 32 pA (−49.6%) followed by recovery during washout to 55.4 pA (Fig. 3B). The difference between the control and drug condition was also significant (P < 0.025, n = 11). Both Y1 (n = 1) and Y2 (n = 2) agonists also suppressed rNST-evoked inhibitory currents, but the small number of inhibitory responses precluded any statistical treatment.
Fig. 2.
A: mean suppression of rNST-evoked inward current by Y1 receptor agonist d-arg-25-NPY at 3 concentrations. B: suppression at 0.5 μM is statistically significant (paired t-test). The suppression recovered almost completely with washout. C: example of the increase in the paired-pulse ratio. D: average increase in paired-pulse ratio was statistically significant for the 12 out of 18 neurons that showed suppression with d-arg-25-NPY.
Fig. 3.
A: mean suppression of rNST-evoked inward current by Y2 receptor agonist NPY3–36 at 3 concentrations. B: suppression at 0.5 μM is significant (paired t-test) and almost completely recovers with washout. C: example of the increase in paired-pulse ratio for one neuron: ACSF, NPY3–36, washout. D: mean increase in paired-pulse ratio was statistically significant for the 9/11 neurons that showed suppression with d-arg-25-NPY.
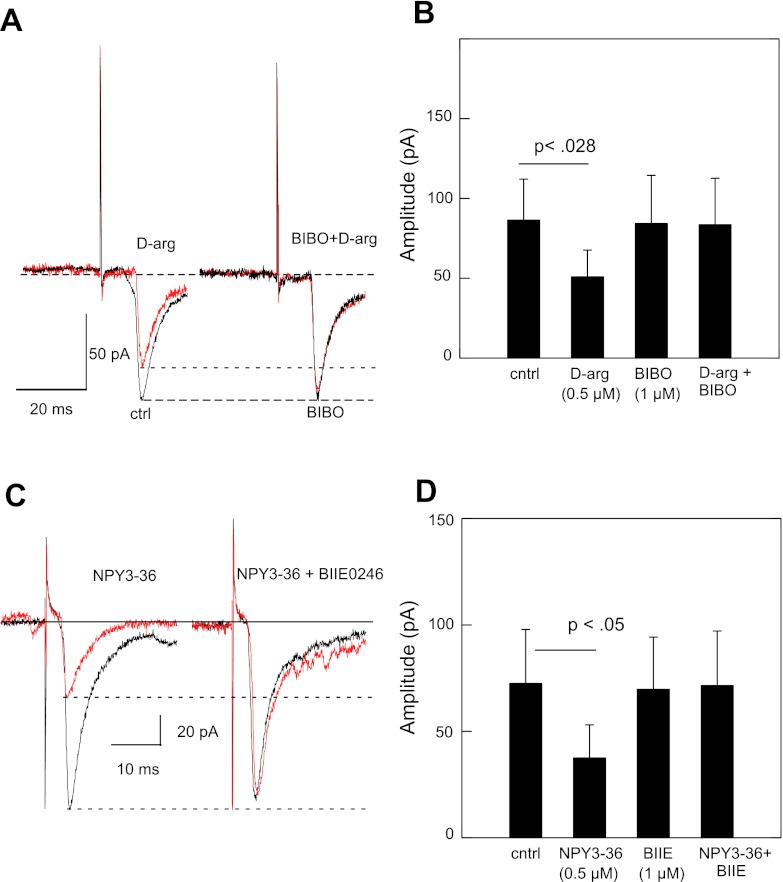
For both NPY agonists, there was a significant increase in the paired pulse ratio. For the 12 (out of 18) neurons showing some degree of suppression to d-arg-25-NPY, the mean paired-pulse ratio increased from 0.99 during control to 1.4 following drug application (P < 0.004, paired t-test) (Fig. 2D). NPY3–36 increased the paired pulse ratio from 1.1 to 1.7 for the 9 neurons (out of 11) showing response suppression (P < 0.029) (Fig. 3D). These results imply a presynaptic inhibition of the excitatory synaptic transmission by Y1 and Y2 receptors. Furthermore, the inhibitory effects of d-arg-25-NPY or NPY3–36 on the NTS-evoked EPSCs were abolished by the NPY Y1-selective antagonist BIBO3304 (1 μM, n = 4, Fig. 4, A and B) or Y2-selective antagonist BIIE0246 (1 μM, n = 4, Fig. 4, C and D).
Fig. 4.
A: suppression of rNST-evoked inward current by Y1 agonist d-arg-25-NPY is blocked by inclusion of Y1 antagonist BIBO3304. B: significant reduction of mean inward current by Y1 agonist (paired t-test: P < 0.028, n = 4) compared with Y1 antagonist alone or Y1 agonist together with Y1 antagonist. C: suppression of rNST-evoked inward current by Y2 agonist NPY3–36 is blocked by inclusion of Y2 antagonist BIIE0246. B: significant reduction of mean inward current by Y2 agonist (paired t-test: P < 0.05, n = 4) compared with Y2 antagonist alone or Y2 agonist together with Y2 antagonist.
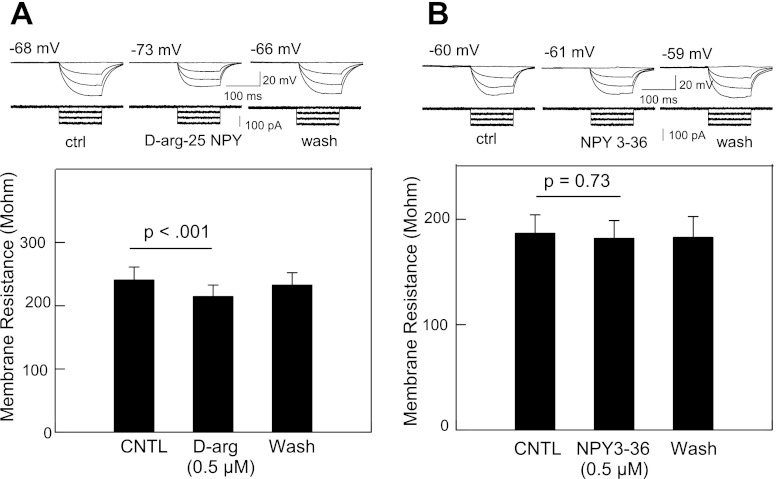
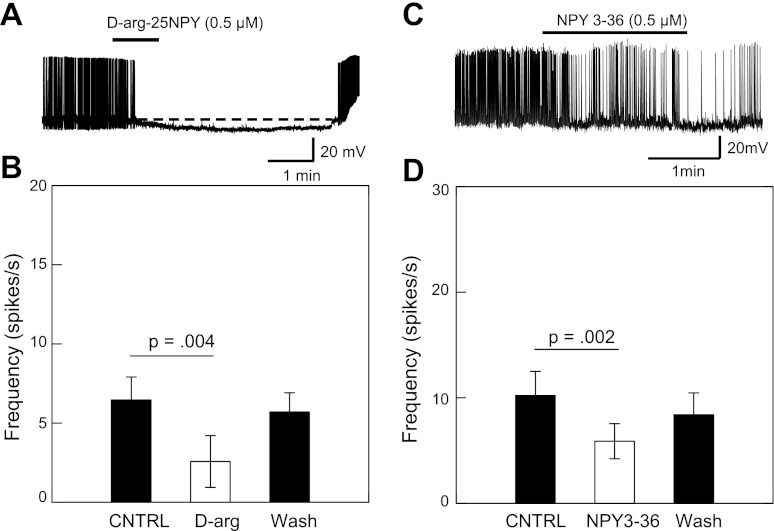
We further studied the site of inhibition by measuring membrane resistance. Perfusion of d-arg-25-NPY (0.5 μM) reduced the membrane resistance by 10.9% (P < 0.001, n = 4) (Fig. 5A); bath application of NPY3–36 (0.5 μM) had no significant effect (P = 0.38) (Fig. 5B). These results indicate that d-arg-25-NPY, but not NPY3–36, also exerts a postsynaptic effect in the inhibition of IRt/PCRt neurons to rNST stimulation. The reduction in membrane resistance following d-arg-25-NPY (0.5 μM) was blocked by the Y1-selective antagonist BIBO3304 (1 μM, n = 4; data not shown). These findings suggest that Y1 but not Y2 receptors exist in IRt/PCRt neurons. The inhibitory effect of both NPY agonists was also apparent from a reduction in mean spontaneous firing under current clamp. Application of d-arg-25-NPY (0.5 μM) reduced the spontaneous firing frequency by 60% (P < 0.004, n = 10), NPY 3–36 reduced activity by 42% (P < 0.002, n = 11) (Fig. 6).
Fig. 5.
A: d-arg-25-NPY reduces the net change of the membrane potential induced by a series of depolarizing pulses. B: significant reduction in membrane resistance following application of Y1 agonist (n = 28). C: NPY3–36 has no discernible effect on the potential change induced by depolarizing pulses. D: no significant change in membrane resistance following application of NPY3–36.
Fig. 6.
A: reduction in spontaneous activity following infusion with Y1 agonist d-arg-25NPY is accompanied by lowered resting membrane potential. B: significant reduction in spontaneous activity (paired t-test) following infusion with Y1 agonist returns to near normal following washout. C: significant reduction in spontaneous activity (paired t-test) following infusion with Y2 agonist returns to near normal following washout. Decrease in spontaneous activity was not associated with a change in resting membrane potential (not shown).
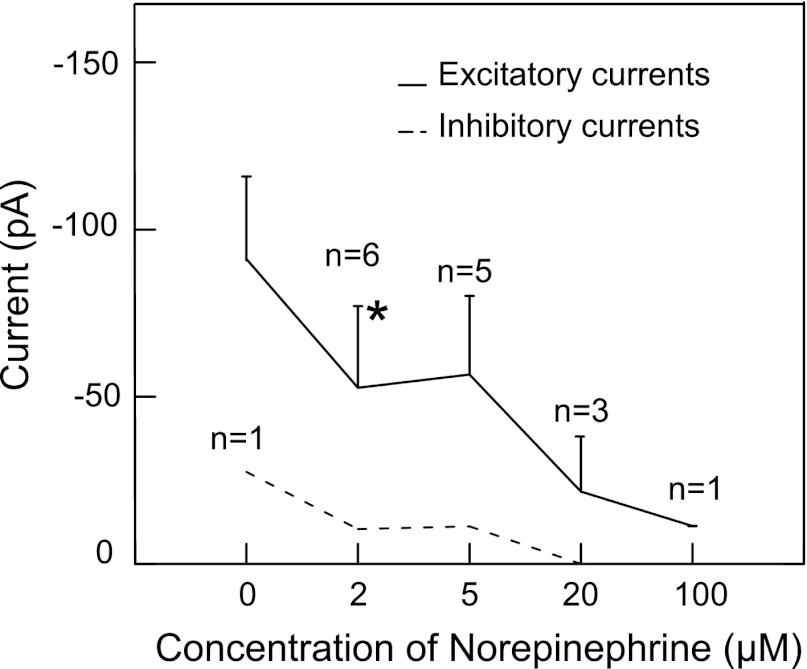
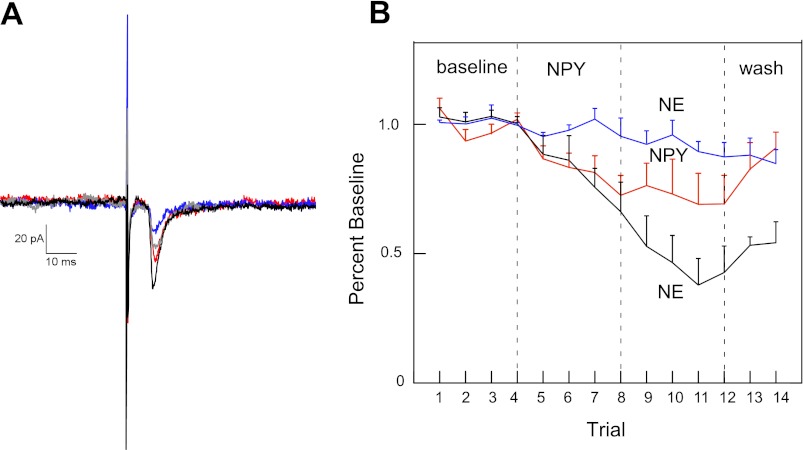
To determine whether NE was effective in RF neurons responsive to an NPY agonist, we first determined that bath application of NE produced a dose-dependent decrease in rNST-induced excitatory responses from RF neurons (Fig. 7). A concentration of 2 μM NE significantly reduced rNST-induced excitatory responses by 42% from 91.2 pA to 52.67 pA (P = 0.012). This dose was also effective in suppressing the one inhibitory response that we recorded. We then tested the effects of 2 μM NE on rNST-evoked responses in prehypoglossal RF neurons that showed at least a 10% reduction to NPY3–36 (Fig. 8). These neurons showed a further significant decrease in the rNST-evoked response compared with neurons that only received NPY3–36 (ANOVA: trial × group interaction: P = 0.035). The additional suppression in the evoked response to NE preceded by NPY3–36 was comparable to the reduction observed by NE alone. Specifically, the reduction seen between trial 8 (66% of baseline) and trial 10 (38% of baseline) represents a 42% reduction comparable to the mean reduction observed to 2 μM NE given by itself (Fig. 7).
Fig. 7.
Response of prehypoglossal neurons to stimulation of the rostral nucleus of the solitary tract in the presence of norepinephrine (0 to 100 μM). The absolute value of the inhibitory current from one neuron is plotted.
Fig. 8.
A: prehypoglossal neuron that showed suppression of the amplitude of the rostral solitary nucleus stimulation-induced excitatory postsynaptic currents (EPSC) in the presence of NPY3–36, showed a further reduction following application of norepinephrine (black: baseline; red: 0.5 μM NPY3–36; blue: 0.5 μM NPY3–36 + 0.2 μM norepinephrine; green: washout). B: this reduction was significant across a population of NPY3–36 cells showing at least a mean 10% reduction following NPY3–36 (black line: n = 6) compared with similar neurons continuing to receive only NPY3–36 (red line: n = 5). Interestingly, in four neurons not showing the 10% reduction following NPY3–36 (blue line), there was no NE effect; these neurons were excluded from the ANOVA.
Histology.
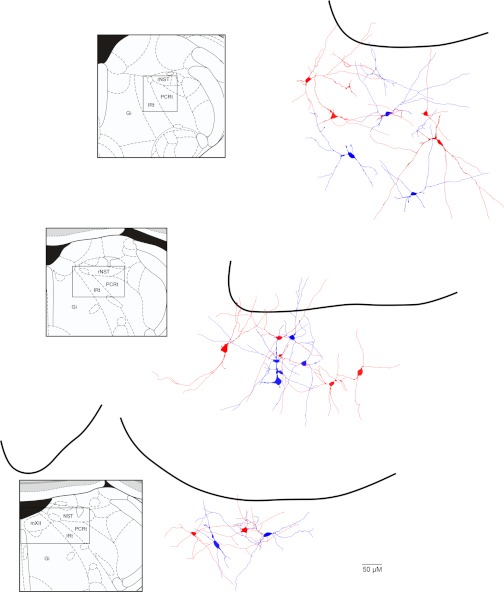
Of 125 neurons identified as prehypoglossal and located in the reticular formation subjacent to the nucleus of the solitary tract, 26 were reconstructed (Fig. 9). We targeted neurons to be subjacent to the rostral NST; however, a small proportion of them were located at an intermediate level, where the NST abuts the fourth ventricle (Fig. 9). Most of the neurons were large multipolar neurons, oftentimes, with processes extending dorsal toward or into the rNST. Neurons suppressed by Y1 and Y2 receptor agonists were intermingled with nonresponsive neurons.
Fig. 9.
Location of 26 neurons. Neurons with significant reductions (range: 29–100% suppression) following Y1 agonist (solid red), significant reductions (range: 22–100%) following Y2 agonist (solid blue), nonsignificant changes (range: 0–3%) to Y1 agonist (open red), nonsignificant changes (range: (0–3%) to Y2 agonist (open blue). Gi, nucleus gigantocellularis; IRt, intermediate subdivision of the reticular formation; mXII, hypoglossal nucleus; PCRt, parvocellular reticular formation; rNST, rostral nucleus of the solitary tract.
DISCUSSION
We can conclude from our study that Y1 receptors influence prehypoglossal neurons both presynaptically and postsynaptically and that Y2 receptors also have a presynaptic locus. Although the source of the endogenous ligand for these receptors remains unknown, the brain stem is rich in neurons expressing NPY, as well as PYY, albeit to a much lesser extent. Both of these neuromodulators are associated with homeostatic energy regulation, suggesting that IRt/PCRt preoromotor neurons are themselves a site of integration.
Location of cells.
Neurons in this study were located immediately ventral to the rNST. Compared with the location of prehypoglossal neurons described in anatomical studies (e.g., 14, 58), it is clear that our study primarily sampled the dorsal-most extent of this population, in a region similar to those reported in two previous studies (41, 62). The region immediately ventral to the rNST has the densest population of prehypoglossal neurons, as well as the densest terminal field of afferent fibers emanating from the rNST (1, 54, 55, 59). Hence, it offers the greatest likelihood for sampling rNST/prehypoglossal interactions. Save for a modest propensity for prehypoglossal neurons innervating lingual retractor motoneurons to be somewhat dorsal compared with lingual protrudor premotoneurons (16), a spatial specialization of IRt/PCRt premotoneurons has not been reported. Thus, it seems reasonable to hypothesize that the results of the present study could be extended to more ventral populations.
Presynaptic vs. postsynaptic inhibition.
Excitatory input from rNST stimulation produces an inward current via non-NMDA glutamate receptors (41). In the present study, bath application of the Y1 agonist d-arg-25-NPY reduced the rNST-evoked inward current and suppressed spontaneous action potentials. Both of these effects were blocked by the specific Y1 receptor antagonist BIBO3304. An increase in the paired-pulse ratio and a decrease in membrane resistance following d-arg-25-NPY indicated both a presynaptic and postsynaptic location for Y1 receptors. Although Y1 receptors have not been specifically localized to prehypoglossal neurons in the IRt/PCRt, Y1 receptor-like immunoreactivity is well represented in this general region (33). A postsynaptic location for Y1 receptors with inhibitory effects is present in other central neurons, including orexin-containing neurons in the lateral hypothalamus (22), as well as brain stem neurons. For example, Y1 agonists postsynaptically suppressed excitatory currents in dorsal motor nucleus (DMN) neurons in response to cNST stimulation (6).
Y2 receptor-mediated inhibition appeared to be presynaptic to IRt/PCRt prehypoglossal neurons, and Y2R immunoreactivity appears not only well represented in the lower brain stem (18, 42) but is clearly concentrated in the IRt/PCRt compared with either the more lateral or medial RF (see Fig. 3I in Ref. 51). Similar Y2-mediated presynaptic inhibition of glutamatergic input was also observed in orexin neurons in the lateral hypothalamus (22), as well as in DMN neurons in response to cNST stimulation (4).
Possible sources of endogenous ligand.
The endogenous ligand(s) for Y1 and Y2 receptors is(are) most likely of brain stem origin, as NPY neurons in the hypothalamus do not appear to project to the brain stem reticular formation (2). Neurons expressing NPY, however, are found in both the caudal NST and ventral medulla, oftentimes colocalized with catecholamines (19, 26, 39, 48), and there is extensive overlap of Y1, Y2, and A2α adrenergic receptors in the IRt/PCRt where we did our recordings (see Fig. 4E in Ref. 47) (33, 51). The results of the present study demonstrating that NE acts in a parallel fashion to a Y2 agonist in suppressing rNST-induced responses is consistent with brain stem CA/NPY neurons as a (single) source for these ligands. In addition to NPY, PYY is also an endogenous ligand for Y1 and Y2 receptors and has been localized to neurons in the medial RF (3, 23, 44). PYY-immunoreactive fibers are evident in the medullary RF, including the IRt/PCRt.
Functional significance.
NPY is a potent orexigenic peptide (52). In addition to its role in promoting food intake via hypothalamic pathways (reviewed in Ref. 65), there is growing evidence that NPY endogenous to the lower brain stem promotes food intake as well. Thus, infusions of NPY into the 4th ventricle lead to increased food consumption, similar to that observed following third ventricular infusions (12, 13, 53) Likewise, glucoprivation-induced feeding appears to depend on intact brain stem catecholamine-containing NPY neurons (CA/NPY) (35, 36, 46) and glucoprivation-induced feeding can be elicited in decerebrate preparations (15, 21). Thus, a parsimonious argument might simply hold that brain stem CA/NPY neurons augment food intake by direct action on preoromotor neurons. However, there are important unresolved issues with this chain of reasoning.
First, the majority of CA/NPY neurons implicated in glucoprivically induced feeding are in the ventral medulla, somewhat removed from the fourth ventricle (reviewed in Ref. 46). Although C1 neurons, many of which contain NPY (19, 48), traverse the preoromotor RF substrate en route to the cNST (8), it is not known whether this projection specifically includes C1 neurons colocalizing NPY, nor is it known if this pathway actually terminates on preoromotor neurons. Indeed, even if this were the case, it is difficult to envision how the inhibitory effects that we observed would produce an orexigenic effect. Activation of Y1 receptors is typically associated with a profound increase in food intake (e.g., 30). Thus, inhibition of (primarily) excitatory inputs from the rNST to prehypoglossal neurons, which are also primarily excitatory, runs counter to the expected increase in evoked activity that one might expect if activation of Y1 receptors were orexigenic. Interestingly, however, this paradoxical effect parallels the influence of NPY on lateral hypothalamic neurons (22), where it was observed that Y1 agonists postsynaptically inhibit identified hypocretin/orexin neurons. On the face of it, this suppression would also be unexpected if effects were related to feeding, as hypothalamic NPY and oxexin/hypocretin both increase feeding. Indeed, the authors discuss NPY's inhibition of orexin/hypocretin neurons in the context of arousal as an alternative to feeding mechanisms. Similarly, we cannot rule out a potential role for NPY's effect on preoromotor neurons that is not directly related to food intake.
Nor is this paradox resolved by the recognition that Y1 receptors also bind PYY. Like NPY, PYY induces an orexigenic response when infused into the fourth ventricle (13). Although it is likely that PYY-positive neurons, located in nucleus gigantocellularis of the medullary RF project to the IRt/PCRt (3, 23, 44), these PYY-positive neurons are in close association with melanin-concentrating hormone and orexin-positive fibers, orexigenic peptides originating from the hypothalamus.
Unlike Y1 receptor activation, however, Y2 receptor activation is associated with suppressing food intake, at least in some studies (e.g., 27, 42). Because A2 neurons are strongly implicated in satiety mechanisms (reviewed in Ref. 45), including those that survive decerebration (25), an alternative hypothesis to an orexigenic (excitatory) role for NPY on prehypoglossal neurons could involve the colocalization of NPY in a subset of A2 neurons (19, 48). In that case, the inhibition of preoromotor neurons observed in the present study could represent a brain stem satiety pathway originating from A2 neurons in the cNST, in which the release of NPY and/or NE suppresses excitatory input from the rNST. The observation that NE acts in parallel with NPY agonists supports such a pathway, although it remains to be determined whether such effects actually originate from A2 neurons.
Perspectives and Significance
Considerable progress has been made in identifying neural and hormonal pathways that signal metabolic need or surfeit. Ultimately, these signals must influence neurons that control the actual behavior of feeding and a number of studies suggest that the IRt/PCRt is a critical region of the medullary reticular formation for the coordination and expression of oromotor consummatory behavior. The present paper demonstrates that activation of specific receptors for NPY or NE, neuromodulators with powerful effects on feeding behavior, also modulate the activity of preoromotor neurons that control lingual motoneurons. This study emphasizes the integrative capacity of brain stem circuits and suggests that these neuromodulators are likely to be but one of many in the hindbrain that mediates control of consummatory behavior.
GRANTS
This work was supported by National Institutes of Health Grants DC-00416 and DC-00417.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Z.C., S.P.T., and J.B.T. conception and design of research; Z.C. performed experiments; Z.C. and J.B.T. analyzed data; Z.C., S.P.T., and J.B.T. interpreted results of experiments; Z.C. and J.B.T. prepared figures; Z.C., S.P.T., and J.B.T. drafted manuscript; Z.C., S.P.T., and J.B.T. edited and revised manuscript; Z.C., S.P.T., and J.B.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Ken Herman for technical assistance.
REFERENCES
- 1. Beckman ME, Whitehead MC. Intramedullary connections of the rostral nucleus of the solitary tract in the hamster. Brain Res 557: 265–279, 1991 [DOI] [PubMed] [Google Scholar]
- 2. Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci USA 95: 15043–15048, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Broome M, Hokfelt T, Terenius L. Peptide YY (PYY)-immunoreactive neurons in the lower brain stem and spinal cord of rat. Acta Physiol Scand 125: 349–352, 1985 [DOI] [PubMed] [Google Scholar]
- 4. Browning KN, Kalyuzhny AE, Travagli RA. Mu-opioid receptor trafficking on inhibitory synapses in the rat brainstem. J Neurosci 24: 7344–7352, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Browning KN, Travagli RA. Modulation of inhibitory neurotransmission in brainstem vagal circuits by NPY and PYY is controlled by cAMP levels. Neurogastroenterol Motil 21: e1309–e1126, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Browning KN, Travagli RA. Neuropeptide Y and peptide YY inhibit excitatory synaptic transmission in the rat dorsal motor nucleus of the vagus. J Physiol 549: 775–785, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brozek G, Zhuravin IA, Megirian D, Bures J. Localization of the central rhythm generator involved in spontaneous consummatory licking in rats: functional ablation and electrical brain stimulation studies. Proc Natl Acad Sci USA 93: 3325–3329, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Card JP, Sved JC, Craig B, Raizada M, Vazquez J, Sved AF. Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: Implications for the central control of cardiovascular regulation. J Comp Neurol 499: 840–859, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Cassell MD, Gedney MT, Agassandian KA. Network architecture of central amygdala output shows strong connectional bias towards oro-lingual sensory and reflex circuits. Soc Neurosci Abstracts 594.–10., 2003 [Google Scholar]
- 10. Chen Z, Travers JB. Inactivation of amino acid receptors in medullary reticular formation modulates and suppresses ingestion and rejection responses in the awake rat. Am J Physiol Regul Integr Comp Physiol 285: R68–R83, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Chen Z, Travers SP, Travers JB. Muscimol infusions in the brain stem reticular formation reversibly block ingestion in the awake rat. Am J Physiol Regul Integr Comp Physiol 280: R1085–R1094, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Corp ES, McQuade J, Krasnicki S, Conze DB. Feeding after fourth ventricular administration of neuropeptide Y receptor agonists in rats. Peptides 22: 493–499, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Corp ES, Melville LD, Greenberg D, Gibbs J, Smith GP. Effect of fourth ventricular neuropeptide Y and peptide YY on ingestive and other behaviors. Am J Physiol Regul Integr Comp Physiol 259: R317–R323, 1990 [DOI] [PubMed] [Google Scholar]
- 14. Cunningham ET, Jr, Sawchenko PE. Dorsal medullary pathways subserving oromotor reflexes in the rat: implications for the central neural control of swallowing. J Comp Neurol 417: 448–466, 2000 [PubMed] [Google Scholar]
- 15. Darling RA, Ritter S. 2-Deoxy-d-glucose, but not mercaptoacetate, increases food intake in decerebrate rats. Am J Physiol Regul Integr Comp Physiol 297: R382–R386, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dobbins EG, Feldman JL. Differential innervation of protruder and retractor muscles of the tongue in rat. J Comp Neurol 357: 376–394, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Dumont Y, Cadieux A, Doods H, Pheng LH, Abounader R, Hamel E, Jacques D, Regoli D, Quirion R. BIIE0246, a potent and highly selective non-peptide neuropeptide Y Y(2) receptor antagonist. Br J Pharmacol 129: 1075–1088, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dumont Y, Fournier A, St-Pierre S, Quirion R. Comparative characterization and autoradiographic distribution of neuropeptide Y receptor subtypes in the rat brain. J Neurosci 13: 73–86, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Everitt BJ, Hokfelt T, Terenius L, Tatemoto K, Mutt V, Goldstein M. Differential co-existence of neuropeptide Y (NPY)-like immunoreactivity with catecholamines in the central nervous system of the rat. Neuroscience 11: 443–462, 1984 [DOI] [PubMed] [Google Scholar]
- 20. Faulconbridge LF, Grill HJ, Kaplan JM. Distinct forebrain and caudal brainstem contributions to the neuropeptide Y mediation of ghrelin hyperphagia. Diabetes 54: 1985–1993, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Flynn FW, Grill HJ. Insulin elicits ingestion in decerebrate rats. Science 221: 188–190, 1983 [DOI] [PubMed] [Google Scholar]
- 22. Fu LY, Acuna-Goycolea C, van den Pol AN. Neuropeptide Y inhibits hypocretin/orexin neurons by multiple presynaptic and postsynaptic mechanisms: tonic depression of the hypothalamic arousal system. J Neurosci 24: 8741–8751, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glavas MM, Grayson BE, Allen SE, Copp DR, Smith MS, Cowley MA, Grove KL. Characterization of brainstem peptide YY (PYY) neurons. J Comp Neurol 506: 194–210, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Grill HJ. Leptin and the systems neuroscience of meal size control. Front Neuroendocrinol 31: 61–78, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grill HJ, Smith GP. Cholecystokinin decreases sucrose intake in chronic decerebrate rats. Am J Physiol Regul Integr Comp Physiol 254: R853–R856, 1988 [DOI] [PubMed] [Google Scholar]
- 26. Harfstrand A, Fuxe K, Terenius L, Kalia M. Neuropeptide Y-immunoreactive perikarya and nerve terminals in the rat medulla oblongata: relationship to cytoarchitecture and catecholaminergic cell groups. J Comp Neurol 260: 20–35, 1987 [DOI] [PubMed] [Google Scholar]
- 27. Henry M, Ghibaudi L, Gao J, Hwa JJ. Energy metabolic profile of mice after chronic activation of central NPY Y1, Y2, or Y5 receptors. Obes Res 13: 36–47, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Hermann GE, Kohlerman NJ, Rogers RC. Hepatic-vagal and gustatory afferent interactions in the brainstem of the rat. J Auton Nerv Syst 9: 477–495, 1983 [DOI] [PubMed] [Google Scholar]
- 29. Holstege G, Kuypers HG, Dekker JJ. The organization of the bulbar fibre connections to the trigeminal, facial and hypoglossal motor nuclei. II. An autoradiographic tracing study in cat. Brain 100: 264–286, 1977 [PubMed] [Google Scholar]
- 30. Kalra SP, Kalra PS. To subjugate NPY is to improve the quality of life and live longer. Peptides 28: 413–418, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karimnamazi H, Travers JB. Differential projections from gustatory responsive regions of the parabrachial nucleus to the medulla and forebrain. Brain Res 813: 283–302, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Kinzeler NR, Travers SP. μ-Opioid modulation in the rostral solitary nucleus and reticular formation alters taste reactivity: evidence for a suppressive effect on consummatory behavior. Am J Physiol Regul Integr Comp Physiol 301: R690–R700, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kopp J, Xu ZQ, Zhang X, Pedrazzini T, Herzog H, Kresse A, Wong H, Walsh JH, Hokfelt T. Expression of the neuropeptide Y Y1 receptor in the CNS of rat and of wild-type and Y1 receptor knock-out mice. Focus on immunohistochemical localization. Neuroscience 111: 443–532, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Kotz CM, Billington CJ, Levine AS. Opioids in the nucleus of the solitary tract are involved in feeding in the rat. Am J Physiol Regul Integr Comp Physiol 272: R1028–R1032, 1997 [DOI] [PubMed] [Google Scholar]
- 35. Li AJ, Wang Q, Dinh TT, Ritter S. Simultaneous silencing of Npy and Dbh expression in hindbrain A1/C1 catecholamine cells suppresses glucoprivic feeding. J Neurosci 29: 280–287, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li AJ, Wang Q, Ritter S. Participation of hindbrain AMP-activated protein kinase in glucoprivic feeding. Diabetes 60: 436–442, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li YQ, Takada M, Mizuno N. Premotor neurons projecting simultaneously to two orofacial motor nuclei by sending their branched axons. A study with a fluorescent retrograde double-labeling technique in the rat. Neurosci Lett 152: 29–32, 1993 [DOI] [PubMed] [Google Scholar]
- 38. Lund JP, Kolta A, Westberg KG, Scott G. Brainstem mechanisms underlying feeding behaviors. Curr Opin Neurobiol 8: 718–724, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Murakami S, Okamura H, Pelletier G, Ibata Y. Differential colocalization of neuropeptide Y- and methionine-enkephalin-Arg6-Gly7-Leu8-like immunoreactivity in catecholaminergic neurons in the rat brain stem. J Comp Neurol 281: 532–544, 1989 [DOI] [PubMed] [Google Scholar]
- 40. Nakamura Y, Katakura N. Generation of masticatory rhythm in the brainstem. Neurosci Res 23: 1–19, 1995 [PubMed] [Google Scholar]
- 41. Nasse J, Terman D, Venugopal S, Hermann G, Rogers R, Travers JB. Local circuit input to the medullary reticular formation from the rostral nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 295: R1391–R1408, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Naveilhan P, Hassani H, Canals JM, Ekstrand AJ, Larefalk A, Chhajlani V, Arenas E, Gedda K, Svensson L, Thoren P, Ernfors P. Normal feeding behavior, body weight and leptin response require the neuropeptide Y Y2 receptor. Nat Med 5: 1188–1193, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Notsu K, Tsumori T, Yokota S, Sekine J, Yasui Y. Posterior lateral hypothalamic axon terminals are in contact with trigeminal premotor neurons in the parvicellular reticular formation of the rat medulla oblongata. Brain Res 1244: 71–81, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Pieribone VA, Brodin L, Friberg K, Dahlstrand J, Soderberg C, Larhammar D, Hokfelt T. Differential expression of mRNAs for neuropeptide Y-related peptides in rat nervous tissues: possible evolutionary conservation. J Neurosci 12: 3361–3371, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol 300: R222–R235, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ritter S, Dinh TT, Li AJ. Hindbrain catecholamine neurons control multiple glucoregulatory responses. Physiol Behav 89: 490–500, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Rosin DL, Zeng D, Stornetta RL, Norton FR, Riley T, Okusa MD, Guyenet PG, Lynch KR. Immunohistochemical localization of alpha 2A-adrenergic receptors in catecholaminergic and other brainstem neurons in the rat. Neuroscience 56: 139–155, 1993 [DOI] [PubMed] [Google Scholar]
- 48. Sawchenko PE, Swanson LW, Grzanna R, Howe PR, Bloom SR, Polak JM. Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol 241: 138–153, 1985 [DOI] [PubMed] [Google Scholar]
- 49. Seeley RJ, Grill HJ, Kaplan JM. Neurological dissociation of gastrointestinal and metabolic contributions to meal size control. Behav Neurosci 108: 347–352, 1994 [PubMed] [Google Scholar]
- 50. Shammah-Lagnado SJ, Costa MS, Ricardo JA. Afferent connections of the parvocellular reticular formation: a horseradish peroxidase study in the rat. Neuroscience 50: 403–425, 1992 [DOI] [PubMed] [Google Scholar]
- 51. Stanic D, Brumovsky P, Fetissov S, Shuster S, Herzog H, Hokfelt T. Characterization of neuropeptide Y2 receptor protein expression in the mouse brain. I. Distribution in cell bodies and nerve terminals. J Comp Neurol 499: 357–390, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Stanley BG, Kyrkouli SE, Lampert S, Leibowitz SF. Neuropeptide Y chronically injected into the hypothalamus: a powerful neurochemical inducer of hyperphagia and obesity. Peptides 7: 1189–1192, 1986 [DOI] [PubMed] [Google Scholar]
- 53. Taylor K, Lester E, Hudson B, Ritter S. Hypothalamic and hindbrain NPY, AGRP and NE increase consummatory feeding responses. Physiol Behav 90: 744–750, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Travers JB. Efferent projections from the anterior nucleus of the solitary tract of the hamster. Brain Res 457: 1–11, 1988 [DOI] [PubMed] [Google Scholar]
- 55. Travers JB, DiNardo LA, Karimnamazi H. Medullary reticular formation activity during ingestion and rejection in the awake rat. Exp Brain Res 130: 78–92, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Travers JB, DiNardo LA, Karimnamazi H. Motor and premotor mechanisms of licking. Neurosci Biobehav Rev 21: 631–647, 1997 [DOI] [PubMed] [Google Scholar]
- 57. Travers JB, Herman K, Travers SP. Suppression of third ventricular NPY-elicited feeding following medullary reticular formation infusions of muscimol. Behav Neurosci 124: 225–233, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Travers JB, Norgren R. Afferent projections to the oral motor nuclei in the rat. J Comp Neurol 220: 280–298, 1983 [DOI] [PubMed] [Google Scholar]
- 59. Travers JB, Yoo JE, Chandran R, Herman K, Travers SP. Neurotransmitter phenotypes of intermediate zone reticular formation projections to the motor trigeminal and hypoglossal nuclei in the rat. J Comp Neurol 488: 28–47, 2005 [DOI] [PubMed] [Google Scholar]
- 60. Turman JE, Jr, Chandler SH. Immunohistochemical localization of glutamate and glutaminase in guinea pig trigeminal premotoneurons. Brain Res 634: 49–61, 1994 [DOI] [PubMed] [Google Scholar]
- 61. Van Daele DJ, Fazan VP, Agassandian K, Cassell MD. Amygdala connections with jaw, tongue and laryngo-pharyngeal premotor neurons. Neuroscience 177: 93–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Venugopal S, Boulant JA, Chen Z, Travers JB. Intrinsic membrane properties of pre-oromotor neurons in the intermediate zone of the medullary reticular formation. Neuroscience 168: 31–47, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wieland HA, Engel W, Eberlein W, Rudolf K, Doods HN. Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO 3304 and its effect on feeding in rodents. Br J Pharmacol 125: 549–555, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zerari-Mailly F, Pinganaud G, Dauvergne C, Buisseret P, Buisseret-Delmas C. Trigemino-reticulo-facial and trigemino-reticulo-hypoglossal pathways in the rat. J Comp Neurol 429: 80–93, 2001 [DOI] [PubMed] [Google Scholar]
- 65. Zheng H, Lenard NR, Shin AC, Berthoud HR. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes (Lond) 33: Suppl 2 S8–S13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]