Abstract
In the United States alone, the National Heart, Lung, and Blood Institute (NHLBI) has invested several hundred million dollars in pursuit of myocardial infarct-sparing therapies. However, due largely to methodological limitations, this investment has not produced any notable clinical application or cardioprotective therapy. Among the major methodological limitations is the reliance on animal models that do not mimic the clinical situation. In this context, the limited use of conscious animal models is of major concern. In fact, whenever possible, studies of cardiovascular physiology and pathophysiology should be conducted in conscious, complex models to avoid the complications associated with the use of anesthesia and surgical trauma. The mouse has significant advantages over other experimental models for the investigation of infarct-sparing therapies. The mouse is inexpensive, has a high throughput, and presents the ability of one to create genetically modified models. However, successful infarct-sparing therapies in anesthetized mice or isolated mouse hearts may not be successful in more complex models, including conscious mice. Accordingly, a conscious mouse model of myocardial ischemia and reperfusion has the potential to be of major importance for advancing the concepts and methods that drive the development of infarct-sparing therapies. Therefore, we describe, for the first time, the use of an intact, conscious, and unrestrained mouse model of myocardial ischemia-reperfusion and infarction. The conscious mouse model permits occlusion and reperfusion of the left anterior descending coronary artery in an intact, complex model free of the confounding influences of anesthetics and surgical trauma. This methodology may be adopted for advancing the concepts and ideas that drive cardiovascular research.
Keywords: photomicrographs, histological sections, electrocardiogram
coronary heart disease, including ischemic heart disease, angina pectoris, sudden cardiac death, and myocardial infarction, is the leading cause of death for men and women in the United States (88). Specifically, an estimated 935,000 Americans suffer a myocardial infarction each year with 610,000 new cases and 325,000 recurrent attacks (87). Prognosis after myocardial infarction is primarily dependent on the size of the infarction (25, 72, 79). Accordingly, worldwide efforts are currently underway to develop strategies to reduce infarct size. In the United States alone, the National Heart, Lung, and Blood Institute (NHLBI) has invested several hundred million dollars in pursuit of infarct-sparing therapies (54, 94). However, due largely to methodological limitations, this investment has not produced any notable clinical application or cardioprotective therapy (54, 94). In fact, to date, early coronary artery reperfusion is the only established treatment capable of consistently reducing infarct size in humans (94).
Among the major methodological limitations is the reliance on animal models that do not mimic the clinical or physiological situation. In this context, the NHLBI sponsored Consortium for preclinicAl assESsment of cARdioprotective therapies (CAESAR) has developed a strategy to focus on the use of relevant conscious animal models and models of comorbidities (54, 94) in the search for strategies to reduce infarct size. CAESAR's efforts are an attempt to assess molecular and other perturbations under physiological and functionally relevant conditions (42).
The mouse has significant advantages over other experimental models for the investigation of infarct-sparing therapies. The mouse is readily available, inexpensive, has a high throughput, and gives the investigator the ability to create genetically modified models. Thus mice can be studied for the initial screening of therapies that are promising. However, successful infarct-sparing therapies in isolated mouse hearts or anesthetized mice may not be successful in more complex models, including conscious mice. Accordingly, a conscious mouse model of myocardial ischemia and reperfusion has the potential to be of major importance for advancing the concepts and methods that drive the development of infarct-sparing therapies (105).
Therefore, we describe, for the first time, the use of an intact, conscious, and unrestrained mouse model of myocardial ischemia-reperfusion and infarction. The conscious mouse model permits occlusion and reperfusion of the left anterior descending coronary artery (LAD) in an intact, complex model free of the confounding influences of anesthetics, surgical trauma, and restraint stress (91). The myocardial ischemia and reperfusion protocol can be initiated after the resolution of the inflammation that occurs during the initial surgical preparation. This is an important consideration, because Nossuli and colleagues (73) demonstrated that the inflammation due to surgical trauma increases the background of cytokine induction and accentuates the response during myocardial ischemia and reperfusion causing significantly greater variability. The use of a chronic model eliminates these confounding effects.
MATERIALS AND METHODS
Animals.
Experimental procedures and protocols were reviewed and approved by the Animal Care and Use Committee of Wayne State University and complied with The American Physiological Society's Guiding Principles in the Care and Use of Animals. Five adult, female C57BL/6 mice (20.2 ± 0.3 g before instrumentation and 21.9 ± 0.6 g on the day the hearts were harvested) and 4 adult, male C57BL/6 mice (26.0 ± 0.6 g before instrumentation and 28.9 ± 0.3 g on the day the hearts were harvested) were studied to determine infarct size following occlusion and reperfusion of the LAD coronary artery in intact conscious mice. Three additional sham-operated (occluder inserted but not used) male mice were studied to obtain histological sections from noninfarcted hearts for the determination of chamber size and comparison with the infarcted hearts. All mice were housed on a 12 h-12 h light-dark cycle with ad libitum access to food and water.
All surgical procedures were performed using aseptic surgical techniques. Twenty minutes before the surgical procedures, the mice received the nonsteroidal anti-inflammatory agent ketoprofen (5 mg/kg sq), the antibiotic cefazolin (10 mg/kg sq), and the muscarinic cholinergic receptor antagonist methyl atropine (0.05–0.1 mg/kg sq). The ketoprofen, cefazolin, and atropine were delivered in 1–3% (body weight) of physiological saline. The saline was absorbed and offset the hypotonic effects of the anesthetic.
Subsequently, the mice were anesthetized with pentobarbital sodium (50 mg/kg ip). The intraperitoneal bolus was injected into the lower lateral abdominal quadrant to reduce risk of intraluminal intestinal injection by using a standard headdown restraint. The injection site with needle was “pinched” when the needle was withdrawn. This prevents “back tracking” of the anesthetic out of the needle track. Back tracking of the anesthetic can result in delayed induction and/or failure to reach the surgical plane. The mice were immediately placed in a standard mouse cage that was free of bedding so that particulate bedding would not obscure airways or damage corneas as anesthesia was induced. Once the mice lost the righting reflex, it became possible to begin the initial preparation of the surgical site.
To initially prepare the surgical site, the fur was removed with clean, well-lubricated fine blade clippers. The clippers were periodically checked as the heat generated can cause thermal burns that delay the healing process. The loose fur was removed with a damp cloth. After the fur was removed, the animal was ready for tracheal intubation.
To intubate the mice, a custom-made intratracheal tube was required. Specifically, a 20-mm segment of polyethylene 50 (PE 50) tubing was inserted into a 5-mm segment of PE 190 tubing that was subsequently inserted into a 7-mm segment of PE 240 tubing. Subsequently, the three segments were heat sealed together making one mouse tracheal tube. The PE 50 tubing entered the trachea and the attached PE 240 tubing was connected to a Y type connector (cat. no. 6152-0125; Nalgene Labware). The Y connector was connected to the tubing of the ventilator (SAR 830 ventilator, CWE, Ardmore, PA). The mice were pressure-controlled ventilated at a respiratory rate of 140 breaths/min and an inspiratory pressure of ∼10 cmH2O resulting in a tidal volume of ∼7.0 ml/kg. The mice were placed on a feedback-based temperature control system (model no. 40-90-8; FHC, Bowdoin, ME) for monitoring and maintaining body temperature within the physiological range. Once ventilated, the final preparation of the surgical site was conducted.
To complete the preparation of the surgical site the mice were positioned in a right lateral decubitus position and the skin was disinfected. Specifically, the surgical site was wiped in a single pass outward motion from incision site toward the fur with an iodine solution several times assuring 3–5 min of contact time. New gauze was used for each circular sweep. The same procedure was repeated using sterile alcohol-treated pads to remove the iodine solution. A sterile drape was positioned over the site and sterile petrolatum ophthalmic ointment (Puralube, E. Fougera, Melvile, NY) was placed in the eyes to prevent drying.
Thoracotomy procedures.
The hearts were approached via a left thoracotomy through the third intercostal space. In brief, a 1.5-cm incision was made in the skin over the third rib space. The muscles covering the rib were blunt dissected to expose the intercostal muscle. A 5- to 7-mm opening was made through the intercostal muscle and pleura of the third rib space using blunt dissection. The blunt tines of a Guthrie retractor (No. 17021-13, Fine Science Tools) were placed under the third and fourth rib, producing a separation of 7 mm. The pericardial sac was opened, and a coronary artery occluder made with a BV-1 tapered needle attached to 7-0 prolene suture (Ethicon 8703) was passed around the proximal segment of the LAD ∼1.0 mm from the origin by inserting the needle into the left ventricular wall and bringing it out on the other side of the coronary artery. The needle was cut from the suture, and the two ends of the suture were passed through guide tubing fashioned from a “modified” mouse thoracic carotid artery catheter (cat. no. M-CAC; Braintree Scientific). Specifically, the modification consisted of cutting the tapered end and then heat flaring the cut end. This modification prevents the guide tubing from causing tissue damage during the occlusion.
After implantation of the coronary artery occluder, the retractors were removed. The third and fourth ribs were approximated with 6-0 silk sutures. The superficial muscle covering the ribs was then apposed with 5-0 Vicryl sutures. The guide tubing with the two ends of the 7-0 suture were exteriorized at the back of the neck, filled with a sterile petrolatum ophthalmic ointment (Puralube), and sealed with a stainless steel pin to prevent a pneumothorax. Subsequently, the skin was closed with 5-0 nylon sutures. The local anesthetic bupivicaine (0.25%, 1.5 mg/kg) was injected subcutaneously at the incision site.
Subsequently, two ECG electrodes (DataSciences International, Standard Lead Coupler Kit: 276-0031-001) were sutured subcutaneously in a modified lead II configuration by placing the negative electrode slightly to the right of the manubrium and the positive electrode at the anterior axillary line along the fifth intercostal space. In addition, a third electrode was place subcutaneously, which served as the ground electrode.
All animals remained on the feedback-based temperature control system and ventilator until fully recovered from the anesthesia. Once the animal regained consciousness and was able to lift its head and maintain itself in sternal recumbency, the animal was placed in a “rodent recovery cage” (Thermocare Intensive Care Unit, Braintree Scientific, Braintree, MA). The animals were kept warm, quiet, clean, and dry and monitored every 15–30 min. Animals were returned to the housing room when fully recovered from the anesthesia and gained the ability to maintain body temperature. The nonsteroidal anti-inflammatory agent ketoprofen (5 mg/kg sq) and the antibiotic cefazolin (10 mg/kg sq) were continued for 2 days. At least 10 days were allowed for recovery. During the recovery period, the mice were handled, weighed, and acclimatized to the laboratory and investigators.
Induction of myocardial infarction.
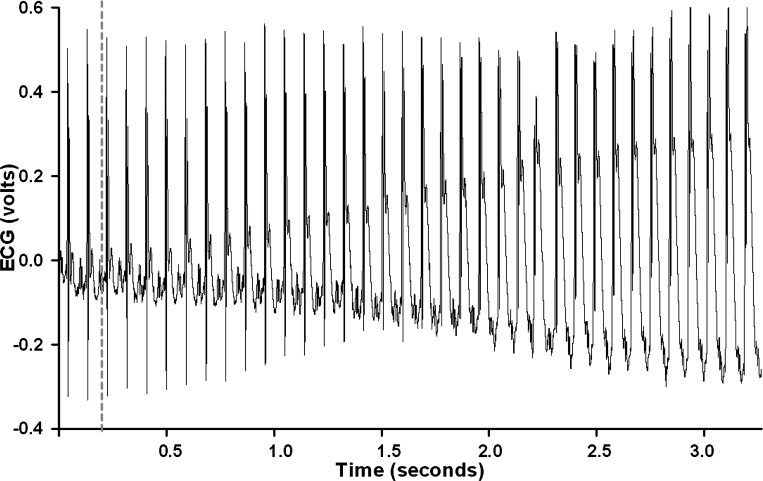
Conscious, unrestrained mice were studied in their home cages for all experiments. The temperature within the cage was monitored and maintained near the thermoneutral zone for mice of ∼27–28°C (102) by use of a circulating water pad under the cage and a Presto HeatDish Plus Parabolic Heater. The ECG was directly recorded by taping the ECG electrodes to multistranded stainless steel Teflon-coated wires [Medwire part number 316SS7/44T, OD less than 0.25 mm, the same wire used to record lumbar sympathetic nerve activity in previous studies (16, 17, 24, 95)]. ECG signals were initially amplified (1,000 times) with a Grass P511 differential preamplifier and high-impedance probe (HIP 511GA). The low- and high-pass filters were set at 0.3 Hz and 10 kHz (37). Mice were allowed to adapt to the laboratory environment for ∼1 h to ensure stable hemodynamic conditions. During this time and throughout the entire experiment, the mouse was unrestrained and able to move about the cage albeit tethered to recording wires. After the stabilization period, beat by beat, steady-state heart rate and ECG parameters were recorded over 10–15 s. Subsequently, the LAD coronary artery was temporarily occluded by use of the prolene suture. Specifically, acute coronary artery occlusion was performed by pulling up on the suture that was around the LAD and securing it to the modified catheter with a vascular clip (Schwartz straight smooth clip). Rapid changes in the ECG (peaked T wave followed by S-T segment elevation) occur within seconds of pulling on the suture, documenting coronary artery occlusion (see Fig. 1). The tension on the suture was sufficient to record the maximum elevation in the ST segment. The coronary artery occlusion was maintained for 90 min; followed by 2 h of reperfusion. It is important to note that the mice did not display any discomfort or stress during the occlusion and did not develop sustained ventricular tachycardia either during the occlusion or reperfusion periods. After the 2 h of reperfusion, the mice were returned to the animal facility.
Fig. 1.
An analog recording of the electrocardiogram (ECG) at the onset of occlusion of the left anterior descending coronary artery (LAD) in an intact, conscious, and unrestrained male mouse is shown. The onset of occlusion is indicated by the dotted line. Rapid changes in the ECG (peaked T wave and S-T segment elevation) occurred within a second of pulling on the suture, documenting coronary artery occlusion.
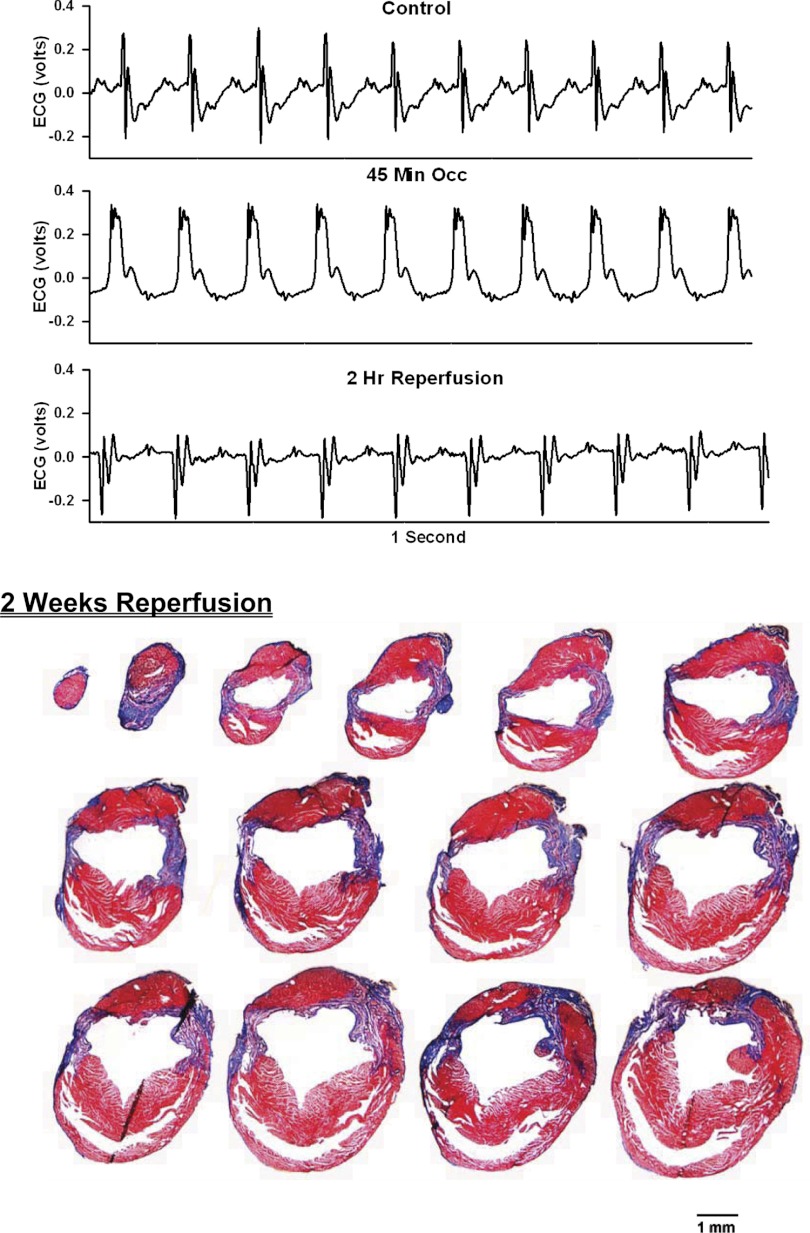
Two weeks later for the 4 male mice and 4 wk later for the 5 female mice, the mice were brought to the laboratory and the ECG was recorded for 90 min. Subsequently, the mice were deeply anesthetized and the hearts were harvested, sectioned, and processed with Masson Trichrome Stain kit (Sigma: HT15) to visualize the infarct scar. Masson Trichrome stain is useful to examine connective tissue and muscle characterized by fibrotic changes. Specifically, the cytoplasm and muscle fibers stain red, whereas the collagen displays blue (see Figs. 3–11). Using this staining protocol, infarct size scores were calculated using the length measurement approaches for infarct size described below.
Fig. 3.
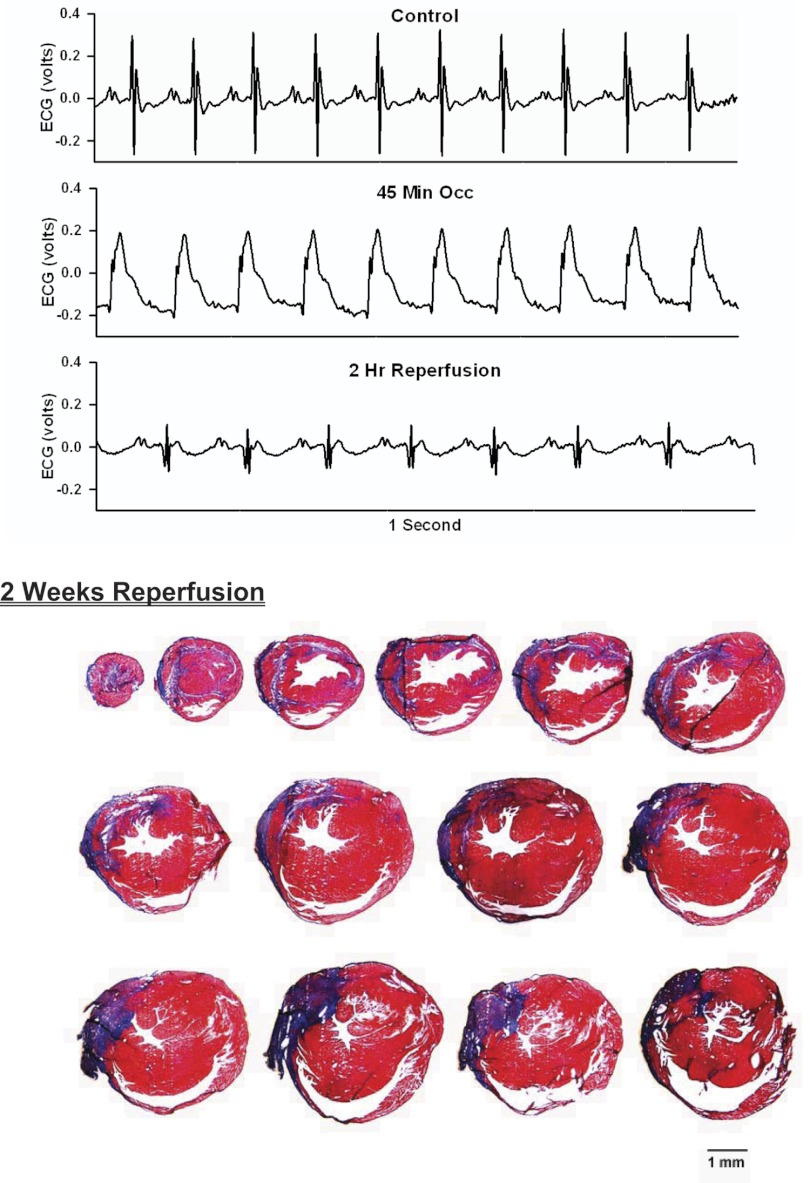
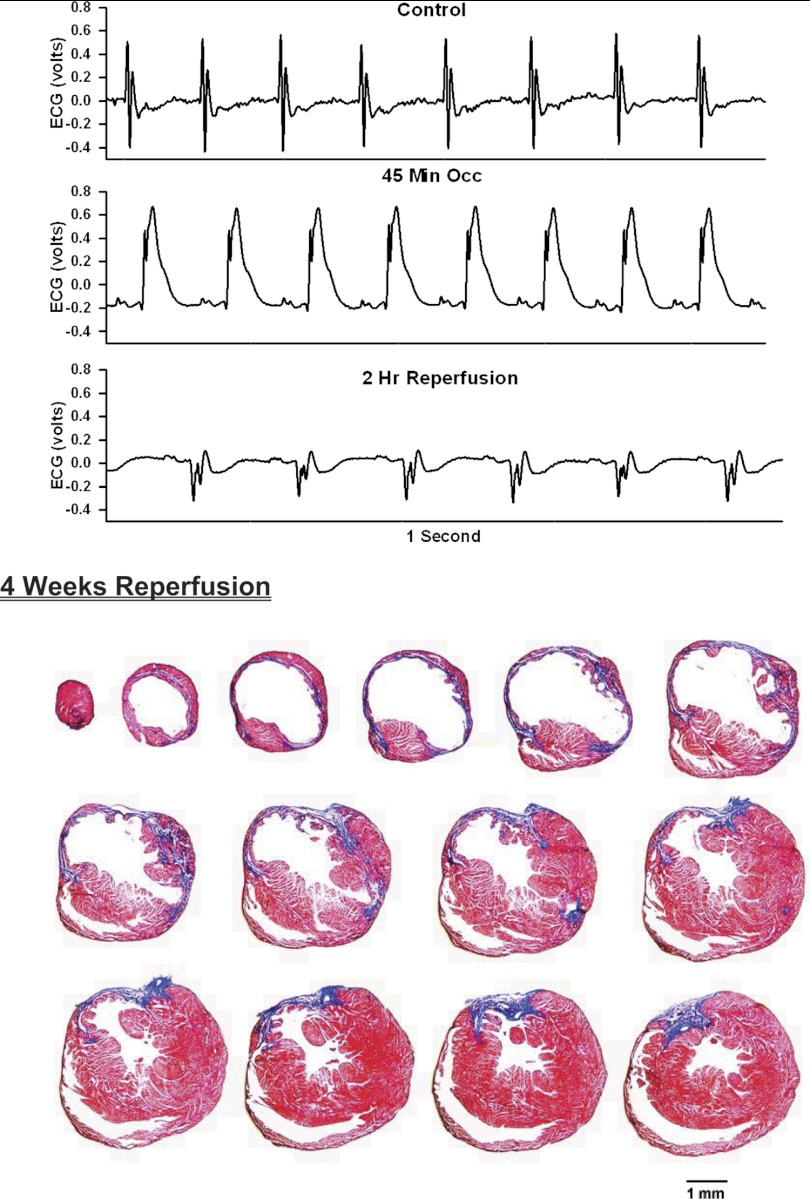
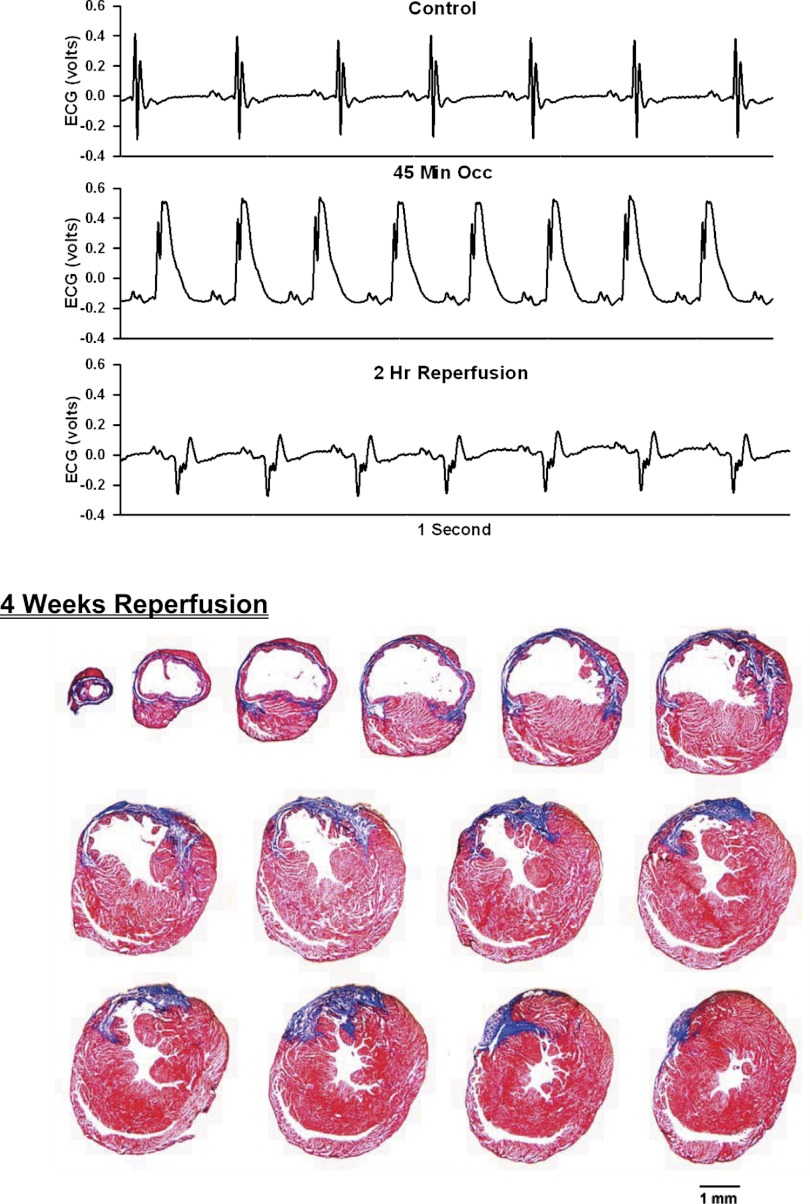
Top: 1-s analog recordings of the ECG before occlusion of the LAD (control), following 45 min of occlusion of the LAD and following 2 h of reperfusion of the LAD in a chronically instrumented, intact, conscious, and unrestrained mouse. Note the ST segment elevation at 45 min of occlusion, which remained elevated for the entire 90-min occlusion period. After 2 h of reperfusion, note the prominent Q wave, which was absent in the control condition. Bottom: photomicrographs of tissue sections taken from the infarcted heart after 2 wk of reperfusion. The sections were taken from the apex through the base of the left ventricle at 300-μm intervals. Note the intense blue staining indicating the abundance of collagen, the large left ventricular chamber area, and the thin left ventricular wall.
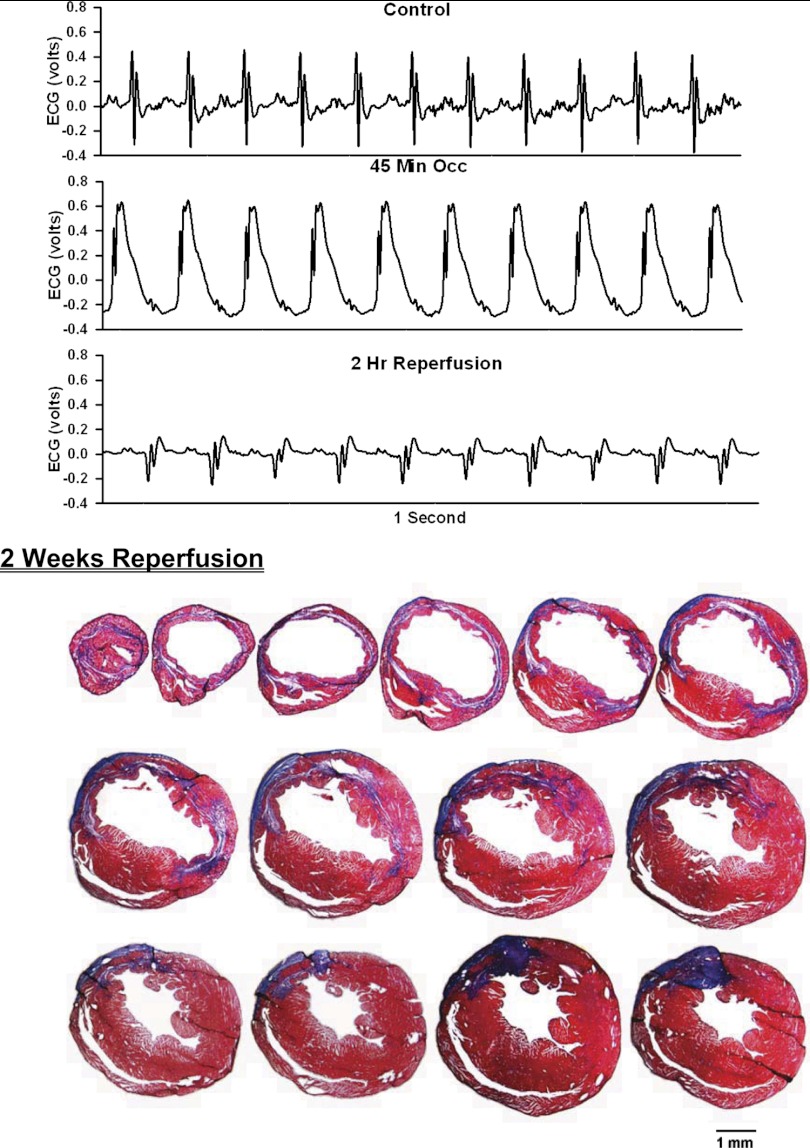
Fig. 4.
ECG before, during, and after occlusion of the LAD in a chronically instrumented, intact, conscious, unrestrained mouse (top) and photomicrographs of histological sections taken from the infarcted heart 2 wk postocclusion (bottom). See Fig. 3 legend for more details.
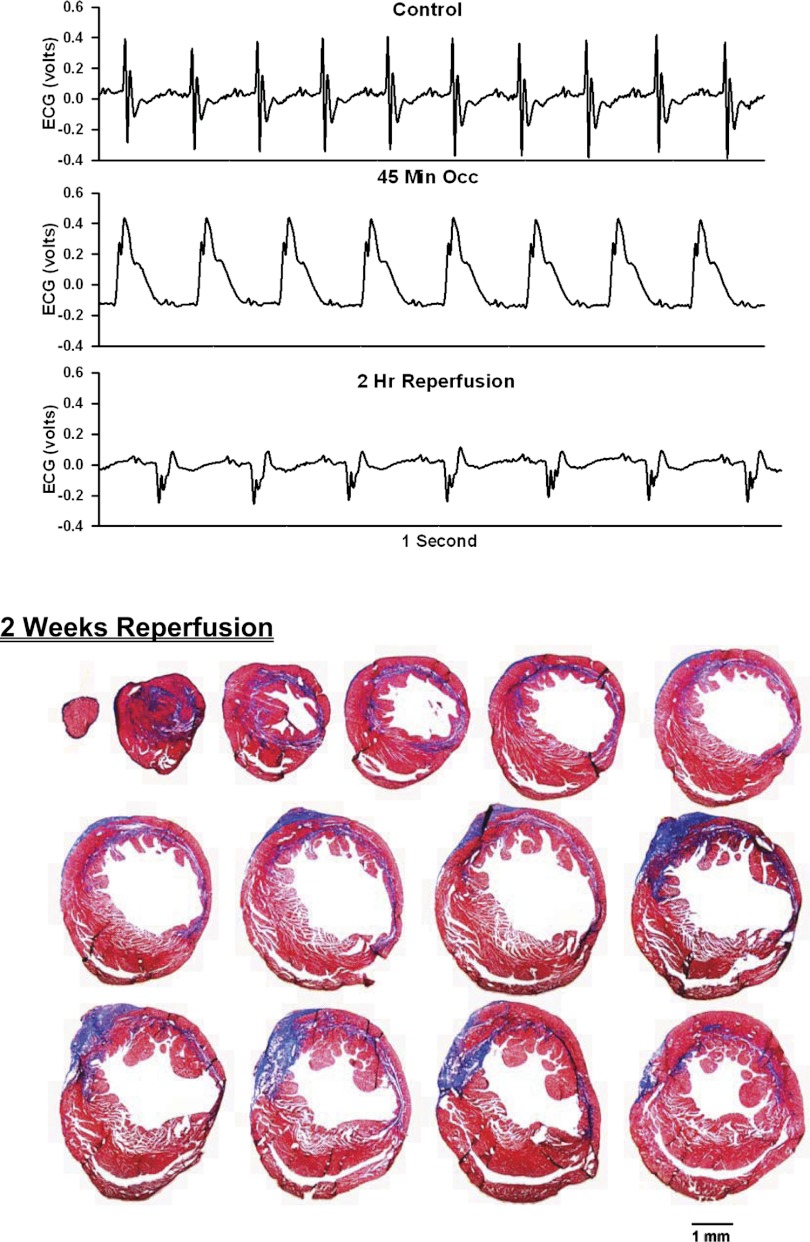
Fig. 5.
ECG before, during, and after occlusion of the LAD in a chronically instrumented, intact, conscious, unrestrained mouse (top) and photomicrographs of histological sections taken from the infarcted heart 2 wk postocclusion (bottom). See Fig. 3 legend for more details.
Fig. 6.
ECG before, during, and after occlusion of the LAD in a chronically instrumented, intact, conscious, unrestrained mouse (top) and photomicrographs of histological sections taken from the infarcted heart 2 wk postocclusion (bottom). See Fig. 3 legend for more details.
Fig. 7.
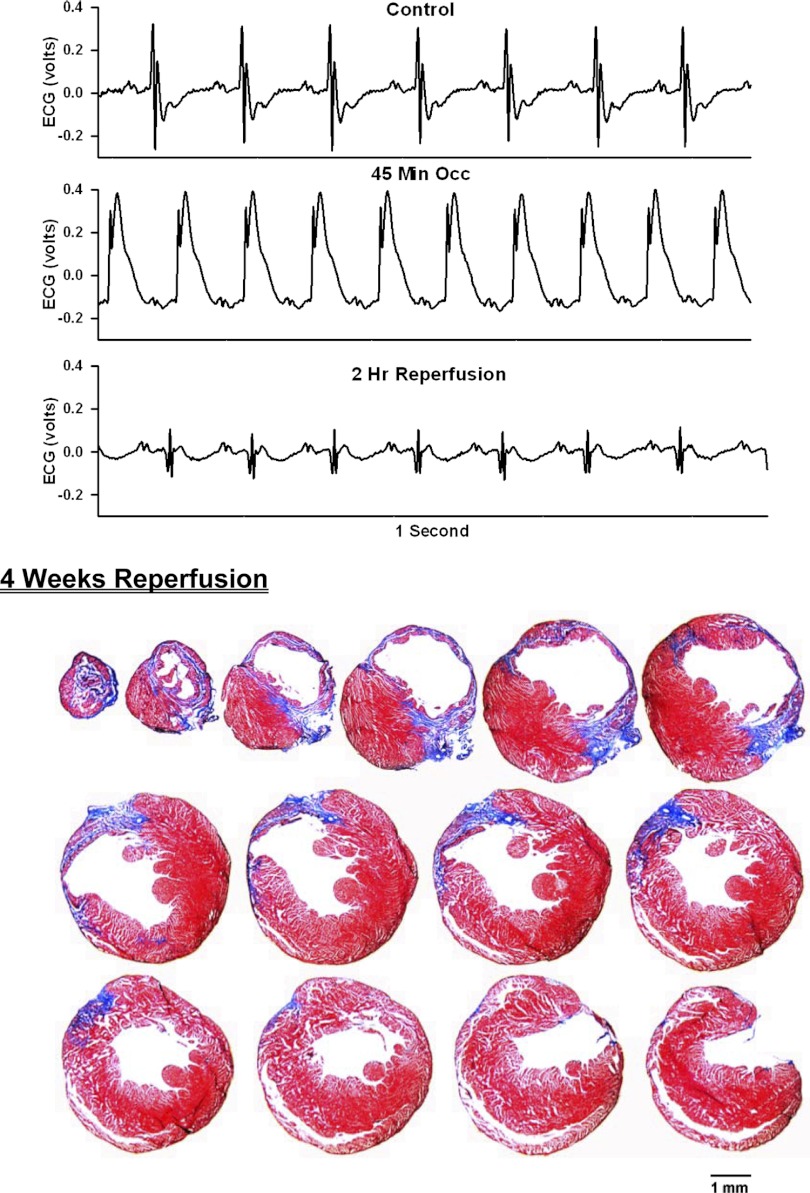
ECG before, during, and after occlusion of the LAD in a chronically instrumented, intact, conscious, unrestrained mouse (top) and photomicrographs of histological sections taken from the infarcted heart 4 wk postocclusion (bottom). See Fig. 3 legend for more details.
Fig. 8.
ECG before, during, and after occlusion of the LAD in a chronically instrumented, intact, conscious, unrestrained mouse (top) and photomicrographs of histological sections taken from the infarcted heart 4 wk postocclusion (bottom). See Fig. 3 legend for more details.
Fig. 9.
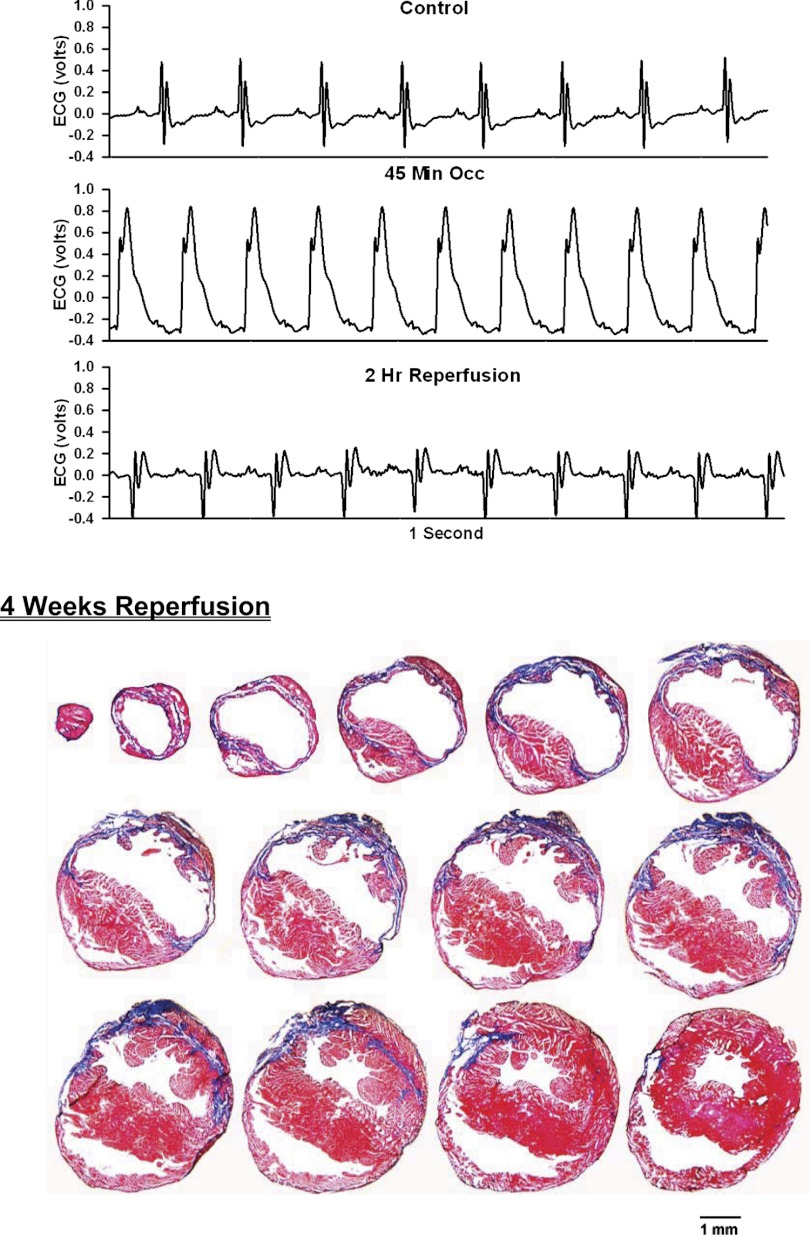
ECG before, during, and after occlusion of the LAD in a chronically instrumented, intact, conscious, unrestrained mouse (top) and photomicrographs of histological sections taken from the infarcted heart 4 wk postocclusion (bottom). See Fig. 3 legend for more details.
Fig. 10.
ECG before, during, and after occlusion of the LAD in a chronically instrumented, intact, conscious, unrestrained mouse (top) and photomicrographs of histological sections taken from the infarcted heart 4 wk postocclusion (bottom). See Fig. 3 legend for more details.
Fig. 11.
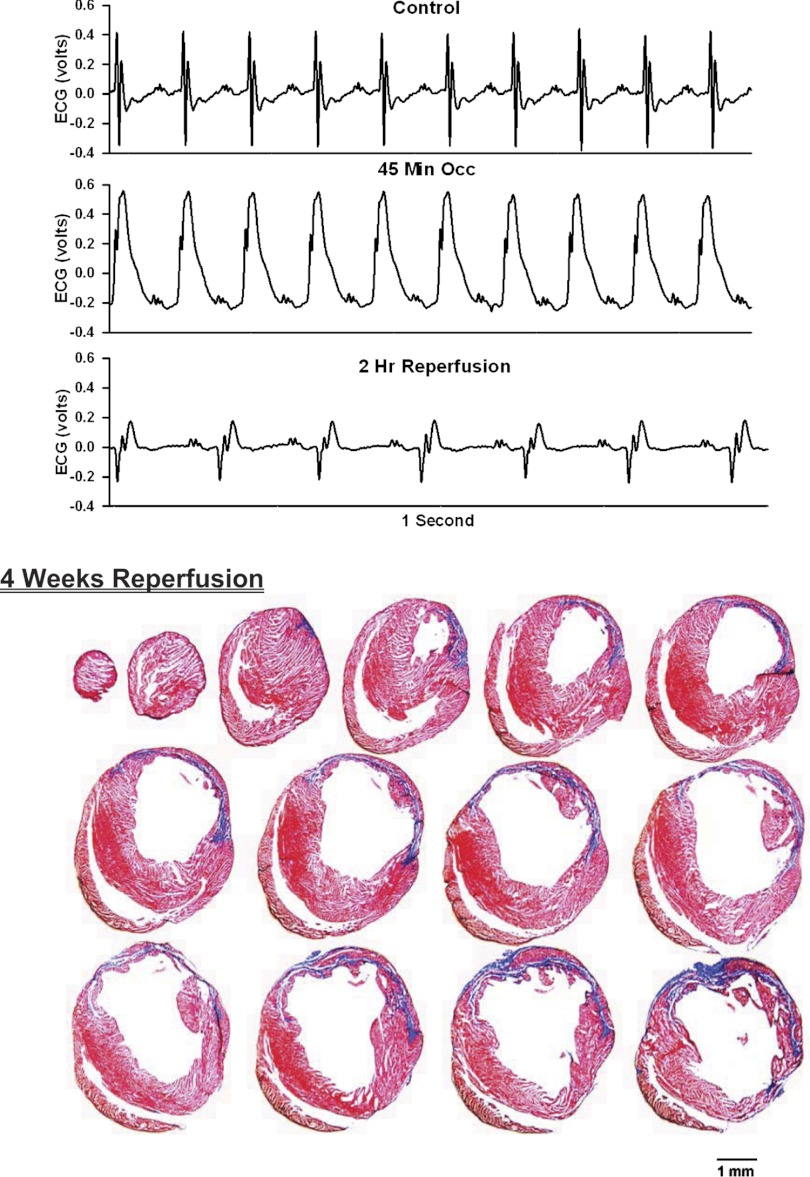
ECG before, during, and after occlusion of the LAD in a chronically instrumented, intact, conscious, unrestrained mouse (top) and photomicrographs of histological sections taken from the infarcted heart 4 wk postocclusion (bottom). See Fig. 3 legend for more details.
Preparation of heart sections.
The heart was excised under deep anesthesia. The left and right atria and large vessels were removed, and the heart was quickly rinsed in 10 mM Tris, 0.9% NaCl, 0.05% thimerosal in 10 mM phosphate buffer, pH 7.4 (TPBS) and then immersion fixed in formaldehyde-zinc fixative for 60 min, washed in TPBS (3 × 10 min) then cryoprotected overnight in 30% sucrose (prepared in half strength TPBS). Subsequently, the heart was embedded in OCT compound and sliced transversely from the apex to the base at 10-μm intervals with the use of a cryostat. An interval of 300 μm was maintained between each section. All sections were thaw mounted on Superfrost Plus slides and stained with Masson Trichrome for quantitative analysis of infarct size.
Measurement of infarct size.
After an experimental acute myocardial infarction, infarct size is typically measured as the area of the infarcted region in relation to the area at risk (70, 76, 81, 103, 115). However, after a chronic myocardial infarction, cardiac structural remodeling consisting of chamber dilatation and wall thinning in the infarcted region with hypertrophy in the viable region may confound the area-based approaches (68, 77, 78). Specifically, the opposing changes of wall thickness in the necrotic and viable myocardium may result in spurious measurements of the infarcted area. Despite these potentially confounding variables (progressive thinning of the infarcted wall with reduction in the volume of the infarcted region) area measurements are also used in the chronic infarct setting (51, 52, 104). However, to account for the cardiac structural remodeling in the chronic model of myocardial infarction, histological measurement of the arc length of the infarct scar is commonly used (46, 75, 78, 80, 103).
Length measurement.
All histological sections were examined with an Olympus BH-2 microscope using a ×1 objective. Our primary end point was infarct size. To achieve this end point, every section from apex to base (300 μm apart) totaling 14–15 sections per heart was quantified from the digital photomicrographs using image analysis software (Image J 1.45s). Four lengths, including epicardial and endocardial infarct lengths and epicardial and endocardial circumferences, were traced manually. Endocardial infarct length was taken as the length of the endocardial infarct scar surface that included greater than 50% of the whole thickness of myocardium (103). Epicardial infarct length was taken as the length of the transmural infarct region (see Figs. 3–11 and Ref. 103).Epicardial infarct ratio was obtained by dividing the sum of the epicardial infarct lengths from all sections by the sum of epicardial circumferences from all sections. Endocardial infarct ratio was calculated similarly. Infarct size derived from this approach was calculated as [(epicardial infarct ratio + endocardial infarct ratio)/2] × 100 (103).
Data analysis.
All physiological recordings were sampled at 4 kHz, and the data were expressed as means ± SE. An unpaired t-test was used to determine differences in infarct size following 2 and 4 wk of reperfusion. A one-way ANOVA was used to determine differences in left ventricular chamber size in noninfarcted hearts and infarcted hearts following 2 and 4 wk of reperfusion.
RESULTS
Figure 1 presents an analog recording of the ECG at the onset of occlusion of the LAD (gray dotted line) in an intact, conscious, and unrestrained male mouse. Rapid changes in the ECG (peaked T wave and S-T segment elevation) occurred within a second of pulling on the suture, documenting coronary artery occlusion.
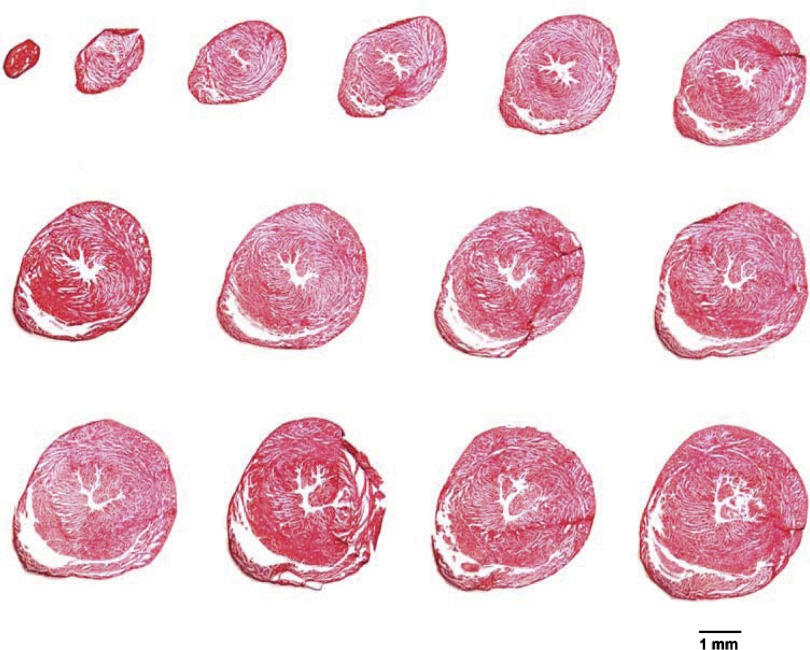
Photomicrographs of myocardial tissue sections taken from one noninfarcted male mouse heart are presented in Fig. 2. The sections were taken from the apex through the base of the left ventricle at 300-μm intervals. Note the absence of collagen (i.e., no blue stain), the small left ventricular chamber area, and the thick left ventricular wall.
Fig. 2.
Photomicrographs of myocardial sections from one noninfarcted male mouse heart are shown. The sections were taken from the apex through the base of the left ventricle at 300-μm intervals and processed with Masson Trichrome stain. Note the absence of collagen (i.e., no blue stain), small left ventricular chamber area, and thick left ventricular wall.
Analog recordings of the ECG before occlusion of the LAD (control), following 45 min of occlusion of the LAD and following 2 h of reperfusion of the LAD in each of the intact, conscious mice are presented in the top panels of Figs. 3–11. Coronary artery occlusion produced rapid changes in the ECG (peaked T wave and S-T segment elevation; Fig. 1). The ST segment was elevated at 45 min of occlusion and remained elevated during the entire 90 min occlusion. After 2 h of reperfusion, note the prominent Q wave, which was absent in the control condition. These changes in the ECG were observed in all mice.
Photomicrographs of myocardial sections taken from each of the infarcted hearts after 2 wk of reperfusion (Figs. 3–6) or 4 wk of reperfusion (Figs. 7–11) are presented in the bottom panel. The sections were taken from the apex through the base of the left ventricle at 300-μm intervals. Note the intense blue staining indicating the abundance of collagen, the large left ventricular chamber area, and the thin left ventricular wall.
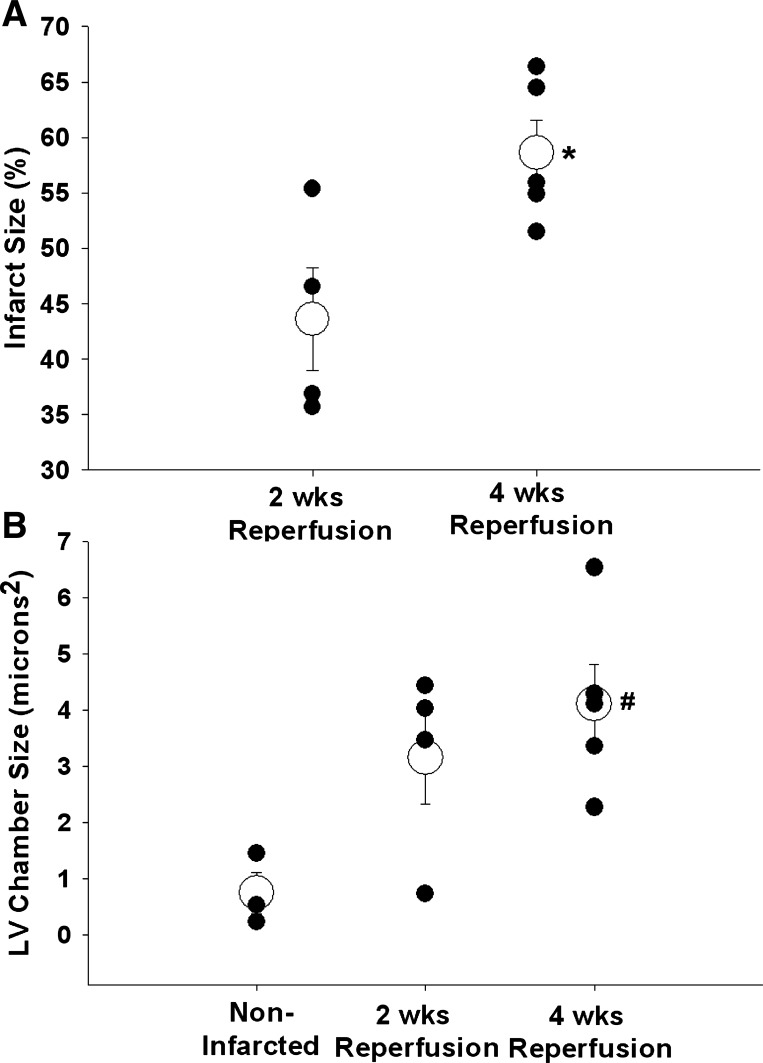
The myocardial ischemia and reperfusion protocol and a myocardial infarction was induced in 4 male and 5 female intact, conscious, and unrestrained mice. Fourteen days (4 male mice) or twenty-eight days (5 female mice) after the ischemia and reperfusion protocol, the hearts were harvested, sectioned, and stained with Masson trichrome to visualize the infarct scar. Subsequently, the infarct size scores were calculated using the length measurement approach based on epicardial and endocardial scar arc lengths. The average infarct size (open circles) and individual infarct sizes (closed circles) for males after 2 wk of reperfusion and females after 4 wk of reperfusion are presented in Fig. 12A. Although infarct size following 4 wk of reperfusion was greater than infarct size following 2 wk of reperfusion, it must be noted that sex differences may have accounted for this effect. Figure 12B presents the average (open circles) and individual (closed circles) left ventricular chamber sizes from the three noninfarcted hearts as well as the hearts from males after 2 wk of reperfusion and females after 4 wk of reperfusion. Left ventricular chamber size after 4 wk of reperfusion was significantly larger compared with the chamber size of the noninfarcted hearts but not different from the chamber size after 2 wk of reperfusion.
Fig. 12.
A: average infarct size (open circles) and individual infarct sizes (closed circles) for males after 2 wk of reperfusion and females after 4 wk of reperfusion. B: average (open circles) and individual (closed circles) left ventricular chamber sizes for the 3 noninfarcted hearts as well as the hearts from males after 2 wk of reperfusion and females after 4 wk of reperfusion. *P < 0.05, 4 wk reperfusion vs. 2 wk reperfusion; #P < 0.05, 4 wk reperfusion vs. noninfarcted.
DISCUSSION
Worldwide efforts are currently underway using mice to identify infarct sparing strategies. Often, the therapeutic effects of infarct sparing strategies of mice are not fully appreciated, due in part to the difficulty of measuring physiological responses in complex, conscious models free of the confounding influences of anesthetics, surgical trauma, and the use of crystalloid perfused hearts that are devoid of many of the vital components of blood. To address this concern, as well as provide a resource for investigators using wild-type mice or available spontaneous or engineered mouse mutants, we used existing technology, surgical techniques, and experimental protocols for the study of myocardial ischemia-reperfusion and infarction in the intact, conscious, and unrestrained mouse.
Specifically, we describe, for the first time, the use of an intact, conscious, and unrestrained mouse model of myocardial ischemia-reperfusion and infarction. The conscious mouse model permits occlusion and reperfusion of the LAD after recovery from the anesthesia and surgical trauma. This is an important consideration since it allows for the resolution of the inflammation that occurs during the initial surgical preparation (13, 39, 74). Furthermore, many anesthetic agents confer protection against ischemia-reperfusion injury across species (20, 34, 98, 105) as well as in a variety of tissues, including heart (20, 22, 58, 82, 100), brain (47, 106, 114), kidney (43), lung (56), and liver (8). Anesthesia also significantly alters the autonomic nervous system (36), and disturbances in cardiac autonomic balance play a critical role in the determination of infarct size. Specifically, reductions in parasympathetic activity or increases in sympathetic activity increase the morbidity and mortality associated with myocardial infarction (11). Accordingly, avoiding these complications associated with the use of anesthesia has the potential to improve the validity and clinical significance of the results.
Successful infarct-sparing therapies in isolated hearts may not be successful in more complex models, including conscious mice, because perfusion solutions that are devoid of many of the vital components of blood may confound the response to myocardial ischemia and reperfusion. Specifically, isolated perfused heart preparations, which are devoid of humoral influences and neuronal regulation, most often use a hemoglobin-free perfusate that requires an unphysiologically high arterial oxygen tension (28) and coronary perfusion rate (9). Furthermore, because the perfusate is devoid of many of the vital components of blood, the crystalloid-perfused hearts exhibit significantly more edema than blood-perfused hearts after ischemia and reperfusion (23, 83). In addition, the increased edema alters myocardial function and contributes to ultrastructural damage (7). Thus, although precise control of the determents of myocardial oxygen consumption, e.g., preload, afterload, heart rate, and contractility are possible with this model, results should be confirmed in complex, conscious systems.
In addition, inflammation contributes significantly to the development and progression of cardiovascular diseases. Indicators of systemic inflammation, such as C-reactive protein, a marker of low-grade inflammation (1, 2, 86), and serum levels of myeloperoxidase are indicative of the risk for adverse cardiovascular events (5, 14). Furthermore, it is well recognized that myocardial ischemia elicits an acute inflammatory response. Importantly, the inflammatory response is markedly augmented by reperfusion. Specifically, reperfusion elevates proinflammatory cytokines and infiltration of neutrophils in the tissue (27, 55, 108). Accordingly, a significant part of myocardial injury after ischemia and reperfusion is attributable to the inflammatory processes (13, 39, 74). In this context, the surgical trauma associated with open-chest preparations is also associated with inflammatory responses. Specifically, acute surgical trauma associated with open-chest preparations in sham-operated animals results in highly variable background levels of inflammation that are indistinguishable from those of ischemic-reperfused animals (15, 71). Since the background levels of inflammation associated with surgical trauma in open-chest preparations compromised the ability to assess inflammation directly due to myocardial ischemia and reperfusion, Michael and colleagues (71) developed a chronic model of myocardial ischemia and reperfusion in dogs that allowed for the resolution of the surgical trauma and inflammation that occur during the initial surgical preparations of the animals before the induction of the ischemia and reperfusion protocol. This is an important consideration because acute surgical trauma increases background levels of inflammatory markers and accentuates and primes the response causing significantly greater variability (73). Similarly, environmental stimuli that induces inflammation predisposes individuals to more severe cardiovascular injury in response to an ischemic events (21, 38, 44, 85, 109, 113). In this context, Nossuli and colleagues (73) demonstrated that the inflammation due to the surgical trauma increases the background of cytokine induction and accentuates the response causing significantly greater variability. The use of a chronic model eliminates these limitations.
Current American College of Cardiology/American Heart Association guidelines recommend reperfusion therapy (thrombolytic therapy, primary percutaneous transluminal coronary angioplasty, or coronary artery bypass grafting) be administered to all patients regardless of age, sex, or race who have symptoms suggestive of a myocardial infarction and who present to the hospital within 12 h of symptom onset and have diagnostic changes on their 12-lead ECG (ST segment elevation or bundle-branch block) (3, 89). Accordingly, most patients experiencing an acute myocardial infarction receive reperfusion therapy (6). Furthermore, the open-artery hypothesis suggests that survival after a myocardial infarction depends more on improved left ventricular remodeling and healing, electrical stability, and myocardial perfusion than on reduction in infarct size (12, 40, 49, 53, 79, 110). Thus survival may improve even when reperfusion therapy is administered late, after irreversible necrosis has occurred. Thus early or late reperfusion is recommended for individuals with myocardial infarction. However, many studies investing infarct-sparing strategies in animal models use permanent rather than temporary coronary artery occlusion. The model described in this communication permits occlusion and reperfusion of the LAD.
An important consideration is that most myocardial infarctions in humans occur in the absence of anesthetic agents and surgical trauma and the hearts are perfused with blood. Furthermore, most myocardial ischemia and reperfusion protocols cannot be conducted in humans. Thus the study of relevant complex, conscious models are essential (54, 94) and animals must be considered. In this context, the mice did not exhibit signs of pain, distress, or agitation during or after the ischemia and reperfusion protocol. Accordingly, we do not have evidence that the mice experience pain during myocardial ischemia. This may be due, in part, to the fact that myocardial ischemic episodes are asymptomatic (silent) in as many as 80% of cases (4, 18, 69). Similarly, myocardial infarction is silent in up to 68% of cases (4, 18, 45, 69). Thus the majority of myocardial ischemic episodes are silent, indicating an inability or failure to sense ischemic damage or stress on the heart (32). In fact, chest pain has a low specificity as an indicator of myocardial ischemia and twice as many people die from a silent heart attack compared with those that experienced a myocardial infarction with chest pain (30, 50).
In support of this concept, mice produce a variety of vocalizations, including vocalizations audible to humans, as well as ultrasonic vocalizations. Ultrasonic vocalizations utilize frequencies higher than 30 kHz (90), and therefore cannot be detected directly by human ears. A number of studies have shown that mice produce ultrasonic vocalizations during stress (31, 33, 97, 101, 111). We used an ultrasonic frequency detector and have not heard vocalizations during coronary artery occlusion and reperfusion in the mouse suggesting that the mice are not stressed. Furthermore, visual inspection of the animal's appearance, posture, and spontaneous behavior suggest the absence of pain or discomfort. Specifically, there is an absence of the, well-known symptoms of pain; e.g., piloerection, unkempt coat/rough hair, hunched posture, apathy, aggression, or self-mutilation.
Limitations.
Genetically modified mice are increasingly used in cardiovascular research (92). Importantly, recent reports on the sequencing of both the human and mice genomes revealed that, to date, only 300 or so genes appear to be unique to one species or the other (107). Furthermore, human and mouse proteins show 80% homology (10). Yet, despite this conservation there are significant differences between mice and humans in cardiovascular function. Such differences are not surprising since the two species differ markedly in heart size, body mass, oxygen consumption, heart rate, and lifespan. Accordingly, a word of caution is required as it is increasingly important to understand the potential limitations of extrapolating data from mice to humans (10, 92, 107).
Specifically, caution should be used when comparing data obtained in species with high heart rates (mice, rats) to data obtained in species with low heart rates (rabbits, dogs, humans) because electrophysiology properties, autonomic regulation, and hemodynamic function are species dependent as a result of differences in heart size, body mass, oxygen consumption, and heart rate (10). Thus mice may not reflect human cardiovascular physiology as closely as larger mammals. Accordingly, extrapolation of mouse data to the human physiological condition must be made with caution; and the murine model may best be used to establish “proof of concepts” that will need to be confirmed in other models that more closely approximate human physiology.
While caution in interpreting preclinical data obtained in mice is clearly warranted, mice will continue to be an important model for human cardiovascular research and will be essential for progress in understanding cardiovascular function in health and disease. For example, numerous studies highlight the relevancy of the mouse as a model of human disease (10, 26, 29, 35, 48, 57, 84, 93, 96, 112).
An additional consideration is that even within small animals with high heart rates (rats and mice) responses to coronary artery occlusion are markedly different. Specifically, we preformed virtually identical procedures in conscious rats many times (19, 59–67). Every rat developed sustained ventricular tachycardia within 10 min of coronary artery occlusion. We expected the mouse to develop sustained ventricular arrhythmia as well, however, this never happened. These data are consistent with previous reports, documenting that it is nearly impossible to elicit ischemia- or reperfusion-induced sustained ventricular arrhythmias in the mouse (99). Understanding the differences between species may advance the concepts and ideas that drive cardiovascular research.
Perspectives and Significance
This paper describes the application of existing technology, surgical techniques, and experimental protocols for the study of myocardial ischemia-reperfusion and infarction in the chronically instrumented, intact, conscious, and unrestrained mouse. The methodology allows for the accurate documentation of infarct size following coronary artery occlusion and reperfusion in conscious mice. Investigators may be encouraged to adopt these existing procedures to their investigations of myocardial infarction since highly reliable data can be obtained in mice under physiological conditions.
It is important to acknowledge that the data gathered from experiments performed at many levels, from molecules to humans, have been and will be critical in developing infarct sparing strategies. Accordingly, a wide range of investigations, rather than a single model or experimental technique, is required to develop novel infarct sparing strategies (41). In this context, the conscious mouse provides an additional tool in the prevention, treatment, and rehabilitation from cardiovascular disease.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-88615 (S. E. DiCarlo) and HL-98945 (J-P. Jin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.L.L., H.-Z.F., J.-P.J., and S.E.D. conception and design of research; H.L.L., H.J., and S.E.D. performed experiments; H.L.L., H.J., and S.E.D. analyzed data; H.L.L. and S.E.D. interpreted results of experiments; H.L.L., H.J., and S.E.D. prepared figures; H.L.L. and S.E.D. drafted manuscript; H.L.L. and S.E.D. edited and revised manuscript; H.L.L., H.J., H.-Z.F., J.-P.J., and S.E.D. approved final version of manuscript.
REFERENCES
- 1. Albert MA. The role of C-reactive protein in cardiovascular disease risk. Curr Cardiol Rep 2: 274–279, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Albert MA, Glynn RJ, Ridker PM. Plasma concentration of C-reactive protein and the calculated Framingham Coronary Heart Disease Risk Score. Circulation 108: 161–165, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC, Jr, Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction–executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation 110: 588–636, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Aronow Silent MI WS. Prevalence and prognosis in older patients diagnosed by routine electrocardiograms. Geriatrics 58: 24–8, 40, 2003 [PubMed] [Google Scholar]
- 5. Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Munzel T, Simoons ML, Hamm CW. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation 108: 1440–1445, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Barron HV, Bowlby LJ, Breen T, Rogers WJ, Canto JG, Zhang Y, Tiefenbrunn AJ, Weaver WD. Use of reperfusion therapy for acute myocardial infarction in the United States: data from the National Registry of Myocardial Infarction 2. Circulation 97: 1150–1156, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Barsotti A, Di NP, Dini FL, Soccio M, Di IP, Gallina S, Di MM, Modesti A. Effect of acute increase of interstitial myocardial fluid on ventricular function in isolated working rat hearts. J Med 29: 137–158, 1998 [PubMed] [Google Scholar]
- 8. Beck-Schimmer B, Breitenstein S, Urech S, De CE, Wittlinger M, Puhan M, Jochum W, Spahn DR, Graf R, Clavien PA. A randomized controlled trial on pharmacological preconditioning in liver surgery using a volatile anesthetic. Ann Surg 248: 909–918, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Bergmann SR, Clark RE, Sobel BE. An improved isolated heart preparation for external assessment of myocardial metabolism. Am J Physiol Heart Circ Physiol 236: H644–H661, 1979 [DOI] [PubMed] [Google Scholar]
- 10. Berul CI. Electrophysiological phenotyping in genetically engineered mice. Physiol Genomics 13: 207–216, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Billman GE. Cardiac autonomic neural remodeling and susceptibility to sudden cardiac death: effect of endurance exercise training. Am J Physiol Heart Circ Physiol 297: H1171–H1193, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Braunwald E, Kim CB. Late establishment of patency of the infarct-related artery. In: Management of Acute Myocardial Infarction, edited by Julian D, Braunwald E. Philadelphia, PA: Saunders, 1994 [Google Scholar]
- 13. Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Invest 76: 1713–1719, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brennan ML, Penn MS, Van LF, Nambi V, Shishehbor MH, Aviles RJ, Goormastic M, Pepoy ML, McErlean ES, Topol EJ, Nissen SE, Hazen SL. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med 349: 1595–1604, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Chandrasekar B, Mitchell DH, Colston JT, Freeman GL. Regulation of CCAAT/Enhancer binding protein, interleukin-6, interleukin-6 receptor, and gp130 expression during myocardial ischemia/reperfusion. Circulation 99: 427–433, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Chen CY, DiCarlo SE. Daily exercise and gender influence arterial baroreflex regulation of heart rate and nerve activity. Am J Physiol Heart Circ Physiol 271: H1840–H1848, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Chen CY, DiCarlo SE, Scislo TJ. Daily spontaneous running attenuated the central gain of the arterial baroreflex. Am J Physiol Heart Circ Physiol 268: H662–H669, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Cohn PF, Fox KM, Daly C. Silent myocardial ischemia. Circulation 108: 1263–1277, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Collins HL, DiCarlo SE. Acute exercise increases the ventricular arrhythmia threshold via the intrinsic adenosine receptor system in conscious hypertensive rats. Am J Physiol Heart Circ Physiol 289: H1020–H1026, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Cope DK, Impastato WK, Cohen MV, Downey JM. Volatile anesthetics protect the ischemic rabbit myocardium from infarction. Anesthesiology 86: 699–709, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Cozzi E, Hazarika S, Stallings HW, III, Cascio WE, Devlin RB, Lust RM, Wingard CJ, Van Scott MR. Ultrafine particulate matter exposure augments ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol 291: H894–H903, 2006 [DOI] [PubMed] [Google Scholar]
- 22. De Hert SG, Turani F, Mathur S, Stowe DF. Cardioprotection with volatile anesthetics: mechanisms and clinical implications. Anesth Analg 100: 1584–1593, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Deng Q, Scicli AG, Lawton C, Silverman NA. Coronary flow reserve after ischemia and reperfusion of the isolated heart. Divergent results with crystalloid versus blood perfusion. J Thorac Cardiovasc Surg 109: 466–472, 1995 [DOI] [PubMed] [Google Scholar]
- 24. DiCarlo SE, Chen CY, Collins HL. Onset of exercise increases lumbar sympathetic nerve activity in rats. Med Sci Sports Exerc 28: 677–684, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Domanski MJ, Mahaffey K, Hasselblad V, Brener SJ, Smith PK, Hillis G, Engoren M, Alexander JH, Levy JH, Chaitman BR, Broderick S, Mack MJ, Pieper KS, Farkouh ME. Association of myocardial enzyme elevation and survival following coronary artery bypass graft surgery. JAMA 305: 585–591, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Drici MD, Arrighi I, Chouabe C, Mann JR, Lazdunski M, Romey G, Barhanin J. Involvement of IsK-associated K+ channel in heart rate control of repolarization in a murine engineered model of Jervell and Lange-Nielsen syndrome. Circ Res 83: 95–102, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res 53: 31–47, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Friedman BJ, Grinberg OY, Grinberg SA, Swartz HM. Myocardial oxygen tension in isolated erythrocyte-perfused rat hearts and comparison with crystalloid media. J Mol Cell Cardiol 29: 2855–2858, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Geisterfer-Lowrance AA, Christe M, Conner DA, Ingwall JS, Schoen FJ, Seidman CE, Seidman JG. A mouse model of familial hypertrophic cardiomyopathy. Science 272: 731–734, 1996 [DOI] [PubMed] [Google Scholar]
- 30. Gottlieb SO, Gottlieb SH, Achuff SC, Baumgardner R, Mellits ED, Weisfeldt ML, Gerstenblith G. Silent ischemia on Holter monitoring predicts mortality in high-risk postinfarction patients. JAMA 259: 1030–1035, 1988 [PubMed] [Google Scholar]
- 31. Gourbal BE, Barthelemy M, Petit G, Gabrion C. Spectrographic analysis of the ultrasonic vocalisations of adult male and female BALB/c mice. Naturwissenschaften 91: 381–385, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Gutterman DD. Silent myocardial ischemia. Circ J 73: 785–797, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Haack B, Markl H, Ehret G. Sound Communication between parents and offspring. In: The Auditory Psychobiology of the Mouse, edited by Willott J. Springfield, IL: Thomas, 1983 [Google Scholar]
- 34. Haessler R, Kuzume K, Chien GL, Wolff RA, Davis RF, Van Winkle DM. Anaesthetics alter the magnitude of infarct limitation by ischaemic preconditioning. Cardiovasc Res 28: 1574–1580, 1994 [DOI] [PubMed] [Google Scholar]
- 35. Hagendorff A, Schumacher B, Kirchhoff S, Luderitz B, Willecke K. Conduction disturbances and increased atrial vulnerability in Connexin40-deficient mice analyzed by transesophageal stimulation. Circulation 99: 1508–1515, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Halliwill JR, Billman GE. Effect of general anesthesia on cardiac vagal tone. Am J Physiol Heart Circ Physiol 262: H1719–H1724, 1992 [DOI] [PubMed] [Google Scholar]
- 37. Hartley CJ, Taffet GE, Reddy AK, Entman ML, Michael LH. Noninvasive cardiovascular phenotyping in mice. ILAR J 43: 147–158, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Hazarika S, Van Scott MR, Lust RM. Severity of myocardial injury following ischemia-reperfusion is increased in a mouse model of allergic asthma. Am J Physiol Heart Circ Physiol 292: H572–H579, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Hearse DJ, Bolli R. Reperfusion induced injury: manifestations, mechanisms, and clinical relevance. Cardiovasc Res 26: 101–108, 1992 [DOI] [PubMed] [Google Scholar]
- 40. Hochman JS, Choo H. Limitation of myocardial infarct expansion by reperfusion independent of myocardial salvage. Circulation 75: 299–306, 1987 [DOI] [PubMed] [Google Scholar]
- 41. Janse MJ, Opthof T, Kleber AG. Animal models of cardiac arrhythmias. Cardiovasc Res 39: 165–77, 1998 [PubMed] [Google Scholar]
- 42. Janssen PM. Challenges in cardiac muscle physiology. Front Physiol 1: 2, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Julier K, da SR, Garcia C, Bestmann L, Frascarolo P, Zollinger A, Chassot PG, Schmid ER, Turina MI, von Segesser LK, Pasch T, Spahn DR, Zaugg M. Preconditioning by sevoflurane decreases biochemical markers for myocardial and renal dysfunction in coronary artery bypass graft surgery: a double-blinded, placebo-controlled, multicenter study. Anesthesiology 98: 1315–1327, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Kaiser Air pollution J. Evidence mounts that tiny particles can kill. Science 289: 22–23, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Kannel WB, Abbott RD. Incidence and prognosis of unrecognized myocardial infarction. An update on the Framingham study. N Engl J Med 311: 1144–1147, 1984 [DOI] [PubMed] [Google Scholar]
- 46. Kanno S, Lerner DL, Schuessler RB, Betsuyaku T, Yamada KA, Saffitz JE, Kovacs A. Echocardiographic evaluation of ventricular remodeling in a mouse model of myocardial infarction. J Am Soc Echocardiogr 15: 601–609, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Kapinya KJ, Lowl D, Futterer C, Maurer M, Waschke KF, Isaev NK, Dirnagl U. Tolerance against ischemic neuronal injury can be induced by volatile anesthetics and is inducible NO synthase dependent. Stroke 33: 1889–1898, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Kasahara H, Wakimoto H, Liu M, Maguire CT, Converso KL, Shioi T, Huang WY, Manning WJ, Paul D, Lawitts J, Berul CI, Izumo S. Progressive atrioventricular conduction defects and heart failure in mice expressing a mutant Csx/Nkx2.5 homeoprotein. J Clin Invest 108: 189–201, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kim CB, Braunwald E. Potential benefits of late reperfusion of infarcted myocardium. The open artery hypothesis. Circulation 88: 2426–2436, 1993 [DOI] [PubMed] [Google Scholar]
- 50. Krantz DS, Hedges SM, Gabbay FH, Klein J, Falconer JJ, Merz CN, Gottdiener JS, Lutz H, Rozanski A. Triggers of angina and ST-segment depression in ambulatory patients with coronary artery disease: evidence for an uncoupling of angina and ischemia. Am Heart J 128: 703–712, 1994 [DOI] [PubMed] [Google Scholar]
- 51. Kudo M, Wang Y, Wani MA, Xu M, Ayub A, Ashraf M. Implantation of bone marrow stem cells reduces the infarction and fibrosis in ischemic mouse heart. J Mol Cell Cardiol 35: 1113–1119, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Lal A, Veinot JP, Ganten D, Leenen FH. Prevention of cardiac remodeling after myocardial infarction in transgenic rats deficient in brain angiotensinogen. J Mol Cell Cardiol 39: 521–529, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Lamas GA, Flaker GC, Mitchell G, Smith SC, Jr, Gersh BJ, Wun CC, Moye L, Rouleau JL, Rutherford JD, Pfeffer MA. Effect of infarct artery patency on prognosis after acute myocardial infarction The Survival and Ventricular Enlargement Investigators. Circulation 92: 1101–1109, 1995 [DOI] [PubMed] [Google Scholar]
- 54. Lefer DJ, Bolli R. Development of an NIH consortium for preclinicAl AssESsment of CARdioprotective therapies (CAESAR): a paradigm shift in studies of infarct size limitation. J Cardiovasc Pharmacol Ther 16: 332–339, 2011 [DOI] [PubMed] [Google Scholar]
- 55. Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg 75: S715–S720, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Liu R, Ishibe Y, Ueda M. Isoflurane-sevoflurane adminstration before ischemia attenuates ischemia-reperfusion-induced injury in isolated rat lungs. Anesthesiology 92: 833–840, 2000 [DOI] [PubMed] [Google Scholar]
- 57. London B, Jeron A, Zhou J, Buckett P, Han X, Mitchell GF, Koren G. Long QT and ventricular arrhythmias in transgenic mice expressing the N terminus and first transmembrane segment of a voltage-gated potassium channel. Proc Natl Acad Sci USA 95: 2926–2931, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lucchinetti E, Jamnicki M, Fischer G, Zaugg M. Preconditioning by isoflurane retains its protection against ischemia-reperfusion injury in postinfarct remodeled rat hearts. Anesth Analg 106: 17–23, table, 2008 [DOI] [PubMed] [Google Scholar]
- 59. Lujan HL, Britton SL, Koch LG, DiCarlo SE. Reduced susceptibility to ventricular tachyarrhythmias in rats selectively bred for high aerobic capacity. Am J Physiol Heart Circ Physiol 291: H2933–H2941, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Lujan HL, Chen Y, DiCarlo SE. Paraplegia increased cardiac NGF content, sympathetic tonus and the susceptibility to ischemia-induced ventricular tachycardia in conscious rats. Am J Physiol Heart Circ Physiol 296: H1364–H1372, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lujan HL, DiCarlo SE. T5 spinal cord transection increases susceptibility to reperfusion-induced ventricular tachycardia by enhancing sympathetic activity in conscious rats. Am J Physiol Heart Circ Physiol 293: H3333–H3339, 2007 [DOI] [PubMed] [Google Scholar]
- 62. Lujan HL, DiCarlo SE. Sex differences to myocardial ischemia and β-adrenergic receptor blockade in conscious rats. Am J Physiol Heart Circ Physiol 294: H1523–H1529, 2008 [DOI] [PubMed] [Google Scholar]
- 63. Lujan HL, DiCarlo SE. Partial hindlimb occlusion reduced the susceptibility to sustained ventricular tachycardia in conscious rats. J Cardiovasc Pharmacol Ther 14: 199–206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lujan HL, Kramer VA, DiCarlo SE. Electro-acupuncture decreases the susceptibility to ventricular tachycardia in conscious rats by reducing cardiac metabolic demand. Am J Physiol Heart Circ Physiol 292: H2550–H2555, 2007 [DOI] [PubMed] [Google Scholar]
- 65. Lujan HL, Kramer VJ, DiCarlo SE. Sex influences the susceptibility to reperfusion-induced sustained ventricular tachycardia and β-adrenergic receptor blockade in conscious rats. Am J Physiol Heart Circ Physiol 293: H2799–H2808, 2007 [DOI] [PubMed] [Google Scholar]
- 66. Lujan HL, Krishnan S, DiCarlo SE. Cardiac spinal deafferentation reduces the susceptibility to sustained ventricular tachycardia in conscious rats. Am J Physiol Regul Integr Comp Physiol 301: R775–R782, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lujan HL, Palani G, Zhang L, DiCarlo SE. Targeted ablation of cardiac sympathetic neurons reduces the susceptibility to ischemia-induced sustained ventricular tachycardia in conscious rats. Am J Physiol Heart Circ Physiol 298: H1330–H1339, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lutgens E, Daemen MJ, de Muinck ED, Debets J, Leenders P, Smits JF. Chronic myocardial infarction in the mouse: cardiac structural and functional changes. Cardiovasc Res 41: 586–593, 1999 [DOI] [PubMed] [Google Scholar]
- 69. Meiltz A, Ciaroni S. [Silent myocardial ischaemia: a deafening silence]. Rev Med Suisse Romande 1: 613–616, 2005 [PubMed] [Google Scholar]
- 70. Michael LH, Ballantyne CM, Zachariah JP, Gould KE, Pocius JS, Taffet GE, Hartley CJ, Pham TT, Daniel SL, Funk E, Entman ML. Myocardial infarction and remodeling in mice: effect of reperfusion. Am J Physiol Heart Circ Physiol 277: H660–H668, 1999 [DOI] [PubMed] [Google Scholar]
- 71. Michael LH, Hunt JR, Weilbaecher D, Perryman MB, Roberts R, Lewis RM, Entman ML. Creatine kinase and phosphorylase in cardiac lymph: coronary occlusion and reperfusion. Am J Physiol Heart Circ Physiol 248: H350–H359, 1985 [DOI] [PubMed] [Google Scholar]
- 72. Miller TD, Christian TF, Hopfenspirger MR, Hodge DO, Gersh BJ, Gibbons RJ. Infarct size after acute myocardial infarction measured by quantitative tomographic 99mTc sestamibi imaging predicts subsequent mortality. Circulation 92: 334–341, 1995 [DOI] [PubMed] [Google Scholar]
- 73. Nossuli TO, Lakshminarayanan V, Baumgarten G, Taffet GE, Ballantyne CM, Michael LH, Entman ML. A chronic mouse model of myocardial ischemia-reperfusion: essential in cytokine studies. Am J Physiol Heart Circ Physiol 278: H1049–H1055, 2000 [DOI] [PubMed] [Google Scholar]
- 74. Opie LH. Reperfusion injury and its pharmacologic modification. Circulation 80: 1049–1062, 1989 [DOI] [PubMed] [Google Scholar]
- 75. Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature 410: 701–705, 2001 [DOI] [PubMed] [Google Scholar]
- 76. Park SW, Lee SY, Park SJ, Lee SC, Gwon HC, Kim DK. Quantitative assessment of infarct size in vivo by myocardial contrast echocardiography in a murine acute myocardial infarction model. Int J Cardiol 97: 393–398, 2004 [DOI] [PubMed] [Google Scholar]
- 77. Patten RD, Aronovitz MJ, ras-Mejia L, Pandian NG, Hanak GG, Smith JJ, Mendelsohn ME, Konstam MA. Ventricular remodeling in a mouse model of myocardial infarction. Am J Physiol Heart Circ Physiol 274: H1812–H1820, 1998 [DOI] [PubMed] [Google Scholar]
- 78. Pfeffer JM, Pfeffer MA, Fletcher PJ, Braunwald E. Progressive ventricular remodeling in rat with myocardial infarction. Am J Physiol Heart Circ Physiol 260: H1406–H1414, 1991 [DOI] [PubMed] [Google Scholar]
- 79. Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 81: 1161–1172, 1990 [DOI] [PubMed] [Google Scholar]
- 80. Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA, Braunwald E. Myocardial infarct size and ventricular function in rats. Circ Res 44: 503–512, 1979 [DOI] [PubMed] [Google Scholar]
- 81. Piot CA, Padmanaban D, Ursell PC, Sievers RE, Wolfe CL. Ischemic preconditioning decreases apoptosis in rat hearts in vivo. Circulation 96: 1598–1604, 1997 [DOI] [PubMed] [Google Scholar]
- 82. Pravdic D, Sedlic F, Mio Y, Vladic N, Bienengraeber M, Bosnjak ZJ. Anesthetic-induced preconditioning delays opening of mitochondrial permeability transition pore via protein Kinase C-epsilon-mediated pathway. Anesthesiology 111: 267–274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Qiu Y, Hearse DJ. Comparison of ischemic vulnerability and responsiveness to cardioplegic protection in crystalloid-perfused versus blood-perfused hearts. J Thorac Cardiovasc Surg 103: 960–968, 1992 [PubMed] [Google Scholar]
- 84. Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science 267: 1831–1834, 1995 [DOI] [PubMed] [Google Scholar]
- 85. Reed MD, Gigliotti AP, McDonald JD, Seagrave JC, Seilkop SK, Mauderly JL. Health effects of subchronic exposure to environmental levels of diesel exhaust. Inhal Toxicol 16: 177–193, 2004 [DOI] [PubMed] [Google Scholar]
- 86. Ridker PM. High-sensitivity C-reactive protein, inflammation, and cardiovascular risk: from concept to clinical practice to clinical benefit. Am Heart J 148: S19–S26, 2004 [DOI] [PubMed] [Google Scholar]
- 87. Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, De SG, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation 123: e18–e209, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart Disease and Stroke Statistics–2012 Update: A Report From the American Heart Association. Circulation 125: e2–e220, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ryan TJ, Anderson JL, Antman EM, Braniff BA, Brooks NH, Califf RM, Hillis LD, Hiratzka LF, Rapaport E, Riegel BJ, Russell RO, Smith EE, Jr, Weaver WD. ACC/AHA guidelines for the management of patients with acute myocardial infarction A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction). J Am Coll Cardiol 28: 1328–1428, 1996 [DOI] [PubMed] [Google Scholar]
- 90. Sales GD. Ultrasound and aggressive behaviour in rats and other small mammals. Anim Behav 20: 88–100, 1972 [DOI] [PubMed] [Google Scholar]
- 91. Sanchez O, Arnau A, Pareja M, Poch E, Ramirez I, Soley M. Acute stress-induced tissue injury in mice: differences between emotional and social stress. Cell Stress Chaperones 7: 36–46, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Schaper W, Winkler B. Of mice and men–the future of cardiovascular research in the molecular era. Cardiovasc Res 39: 3–7, 1998 [DOI] [PubMed] [Google Scholar]
- 93. Schott JJ, Benson DW, Basson CT, Pease W, Silberbach GM, Moak JP, Maron BJ, Seidman CE, Seidman JG. Congenital heart disease caused by mutations in the transcription factor NKX2–5. Science 281: 108–111, 1998 [DOI] [PubMed] [Google Scholar]
- 94. Schwartz LL, Kloner RA, Arai AE, Baines CP, Bolli R, Braunwald E, Downey J, Gibbons RJ, Gottlieb RA, Heusch G, Jennings RB, Lefer DJ, Mentzer RM, Murphy E, Ovize M, Ping P, Przyklenk K, Sack MN, Vander Heide RS, Vinten-Johansen J, Yellon DM. New horizons in cardioprotection: recommendations from the 2010 National Heart, Lung, and Blood Institute Workshop. Circulation 124: 1172–1179, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Scislo TJ, DiCarlo SE. Gender difference in cardiopulmonary reflex inhibition of sympathetic nerve activity. Am J Physiol Heart Circ Physiol 267: H1537–H1543, 1994 [DOI] [PubMed] [Google Scholar]
- 96. Simon AM, Goodenough DA, Paul DL. Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Curr Biol 8: 295–298, 1998 [DOI] [PubMed] [Google Scholar]
- 97. Sipos ML, Kerchner M, Nyby JG. An ephemeral sex pheromone in the urine of female house mice (Mus domesticus). Behav Neural Biol 58: 138–143, 1992 [DOI] [PubMed] [Google Scholar]
- 98. Sloan RC, Rosenbaum M, O'Rourke D, Oppelt K, Frasier CR, Waston CA, Allan AG, Brown DA. High doses of ketamine-xylazine anesthesia reduce cardiac ischemia-reperfusion injury in guinea pigs. J Am Assoc Lab Anim Sci 50: 349–354, 2011 [PMC free article] [PubMed] [Google Scholar]
- 99. Stables CL, Curtis MJ. Development and characterization of a mouse in vitro model of ischaemia-induced ventricular fibrillation. Cardiovasc Res 83: 397–404, 2009 [DOI] [PubMed] [Google Scholar]
- 100. Stadnicka A, Marinovic J, Ljubkovic M, Bienengraeber MW, Bosnjak ZJ. Volatile anesthetic-induced cardiac preconditioning. J Anesth 21: 212–219, 2007 [DOI] [PubMed] [Google Scholar]
- 101. Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science 295: 1493–1500, 2002 [DOI] [PubMed] [Google Scholar]
- 102. Swoap SJ, Overton JM, Garber G. Effect of ambient temperature on cardiovascular parameters in rats and mice: a comparative approach. Am J Physiol Regul Integr Comp Physiol 287: R391–R396, 2004 [DOI] [PubMed] [Google Scholar]
- 103. Takagawa J, Zhang Y, Wong ML, Sievers RE, Kapasi NK, Wang Y, Yeghiazarians Y, Lee RJ, Grossman W, Springer ML. Myocardial infarct size measurement in the mouse chronic infarction model: comparison of area- and length-based approaches. J Appl Physiol 102: 2104–2111, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Virag JI, Murry CE. Myofibroblast and endothelial cell proliferation during murine myocardial infarct repair. Am J Pathol 163: 2433–2440, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Walsh RS, Tsuchida A, Daly JJ, Thornton JD, Cohen MV, Downey JM. Ketamine-xylazine anaesthesia permits a KATP channel antagonist to attenuate preconditioning in rabbit myocardium. Cardiovasc Res 28: 1337–1341, 1994 [DOI] [PubMed] [Google Scholar]
- 106. Wang C, Jin LJ, Jung HH, Zuo Z. Pretreatment with volatile anesthetics, but not with the nonimmobilizer 1,2-dichlorohexafluorocyclobutane, reduced cell injury in rat cerebellar slices after an in vitro simulated ischemia. Brain Res 1152: 201–208, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis SE, Attwood J, Baertsch R, Bailey J, Barlow K, Beck S, Berry E, Birren B, Bloom T, Bork P, Botcherby M, Bray N, Brent MR, Brown DG, Brown SD, Bult C, Burton J, Butler J, Campbell RD, Carninci P, Cawley S, Chiaromonte F, Chinwalla AT, Church DM, Clamp M, Clee C, Collins FS, Cook LL, Copley RR, Coulson A, Couronne O, Cuff J, Curwen V, Cutts T, Daly M, David R, Davies J, Delehaunty KD, Deri J, Dermitzakis ET, Dewey C, Dickens NJ, Diekhans M, Dodge S, Dubchak I, Dunn DM, Eddy SR, Elnitski L, Emes RD, Eswara P, Eyras E, Felsenfeld A, Fewell GA, Flicek P, Foley K, Frankel WN, Fulton LA, Fulton RS, Furey TS, Gage D, Gibbs RA, Glusman G, Gnerre S, Goldman N, Goodstadt L, Grafham D, Graves TA, Green ED, Gregory S, Guigo R, Guyer M, Hardison RC, Haussler D, Hayashizaki Y, Hillier LW, Hinrichs A, Hlavina W, Holzer T, Hsu F, Hua A, Hubbard T, Hunt A, Jackson I, Jaffe DB, Johnson LS, Jones M, Jones TA, Joy A, Kamal M, Karlsson EK, Karolchik D, Kasprzyk A, Kawai J, Keibler E, Kells C, Kent WJ, Kirby A, Kolbe DL, Korf I, Kucherlapati RS, Kulbokas EJ, Kulp D, Landers T, Leger JP, Leonard S, Letunic I, Levine R, Li J, Li M, Lloyd C, Lucas S, Ma B, Maglott DR, Mardis ER, Matthews L, Mauceli E, Mayer JH, McCarthy M, McCombie WR, McLaren S, McLay K, McPherson JD, Meldrim J, Meredith B, Mesirov JP, Miller W, Miner TL, Mongin E, Montgomery KT, Morgan M, Mott R, Mullikin JC, Muzny DM, Nash WE, Nelson JO, Nhan MN, Nicol R, Ning Z, Nusbaum C, O'Connor MJ, Okazaki Y, Oliver K, Overton-Larty E, Pachter L, Parra G, Pepin KH, Peterson J, Pevzner P, Plumb R, Pohl CS, Poliakov A, Ponce TC, Ponting CP, Potter S, Quail M, Reymond A, Roe BA, Roskin KM, Rubin EM, Rust AG, Santos R, Sapojnikov V, Schultz B, Schultz J, Schwartz MS, Schwartz S, Scott C, Seaman S, Searle S, Sharpe T, Sheridan A, Shownkeen R, Sims S, Singer JB, Slater G, Smit A, Smith DR, Spencer B, Stabenau A, Stange-Thomann N, Sugnet C, Suyama M, Tesler G, Thompson J, Torrents D, Trevaskis E, Tromp J, Ucla C, Ureta-Vidal A, Vinson JP, Von Niederhausern AC, Wade CM, Wall M, Weber RJ, Weiss RB, Wendl MC, West AP, Wetterstrand K, Wheeler R, Whelan S, Wierzbowski J, Willey D, Williams S, Wilson RK, Winter E, Worley KC, Wyman D, Yang S, Yang SP, Zdobnov EM, Zody MC, Lander ES. Initial sequencing and comparative analysis of the mouse genome. Nature 420: 520–562, 2002 [DOI] [PubMed] [Google Scholar]
- 108. Welbourn CR, Goldman G, Paterson IS, Valeri CR, Shepro D, Hechtman HB. Pathophysiology of ischaemia reperfusion injury: central role of the neutrophil. Br J Surg 78: 651–655, 1991 [DOI] [PubMed] [Google Scholar]
- 109. Wellenius GA, Coull BA, Godleski JJ, Koutrakis P, Okabe K, Savage ST, Lawrence JE, Murthy GG, Verrier RL. Inhalation of concentrated ambient air particles exacerbates myocardial ischemia in conscious dogs. Environ Health Perspect 111: 402–408, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. White HD, Cross DB, Elliott JM, Norris RM, Yee TW. Long-term prognostic importance of patency of the infarct-related coronary artery after thrombolytic therapy for acute myocardial infarction. Circulation 89: 61–67, 1994 [DOI] [PubMed] [Google Scholar]
- 111. Wysocki CJ, Nyby J, Whitney G, Beauchamp GK, Katz Y. The vomeronasal organ: primary role in mouse chemosensory gender recognition. Physiol Behav 29: 315–327, 1982 [DOI] [PubMed] [Google Scholar]
- 112. Yang Q, Sanbe A, Osinska H, Hewett TE, Klevitsky R, Robbins J. In vivo modeling of myosin binding protein C familial hypertrophic cardiomyopathy. Circ Res 85: 841–847, 1999 [DOI] [PubMed] [Google Scholar]
- 113. Yang Z, Day YJ, Toufektsian MC, Ramos SI, Marshall M, Wang XQ, French BA, Linden J. Infarct-sparing effect of A2A-adenosine receptor activation is due primarily to its action on lymphocytes. Circulation 111: 2190–2197, 2005 [DOI] [PubMed] [Google Scholar]
- 114. Zhao P, Peng L, Li L, Xu X, Zuo Z. Isoflurane preconditioning improves long-term neurologic outcome after hypoxic-ischemic brain injury in neonatal rats. Anesthesiology 107: 963–970, 2007 [DOI] [PubMed] [Google Scholar]
- 115. Zhu B, Sun Y, Sievers RE, Browne AE, Pulukurthy S, Sudhir K, Lee RJ, Chou TM, Chatterjee K, Parmley WW. Comparative effects of pretreatment with captopril and losartan on cardiovascular protection in a rat model of ischemia-reperfusion. J Am Coll Cardiol 35: 787–795, 2000 [DOI] [PubMed] [Google Scholar]