Abstract
Sustained hyperglycemia is associated with increased oxidative stress resulting in decreased intrarenal oxygen tension (Po2) due to increased oxygen consumption (Qo2). Chronic blockade of the main superoxide radicals producing system, the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, normalizes Qo2 by isolated proximal tubular cells (PTC) and reduces proteinuria in diabetes. The aim was to investigate the effects of acute NADPH oxidase inhibition on tubular Na+ transport and kidney Po2 in vivo. Glomerular filtration rate (GFR), renal blood flow (RBF), filtration fraction (FF), Na+ excretion, fractional Li+ excretion, and intrarenal Po2 was measured in control and streptozotocin-diabetic rats during baseline and after acute NADPH oxidase inhibition using apocynin. The effects on tubular transporters were investigated using freshly isolated PTC. GFR was increased in diabetics compared with controls (2.2 ± 0.3 vs. 1.4 ± 0.1 ml·min−1·kidney−1). RBF was similar in both groups, resulting in increased FF in diabetics. Po2 was reduced in cortex and medulla in diabetic kidneys compared with controls (34.4 ± 0.7 vs. 42.5 ± 1.2 mmHg and 15.7 ± 1.2 vs. 25.5 ± 2.3 mmHg, respectively). Na+ excretion was increased in diabetics compared with controls (24.0 ± 4.7 vs. 9.0 ± 2.0 μm·min−1·kidney−1). In controls, all parameters were unaffected. However, apocynin increased Na+ excretion (+112%) and decreased fractional lithium reabsorption (−10%) in diabetics, resulting in improved cortical (+14%) and medullary (+28%) Po2. Qo2 was higher in PTC isolated from diabetic rats compared with control. Apocynin, dimethylamiloride, and ouabain reduced Qo2, but the effects of combining apocynin with either dimethylamiloride or ouabain were not additive. In conclusion, NADPH oxidase inhibition reduces tubular Na+ transport and improves intrarenal Po2 in diabetes.
Keywords: apocynin, dimethylamiloride, ouabain, oxygen tension, oxidative stress, proximal tubular cells, rat, streptozotocin
hyperglycemia induces increased formation of reactive oxygen species (ROS) from several sources, including the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (38). The NADPH oxidase is expressed mainly in phagocytes but also in the vasculature. In smooth muscle cells it participates in the regulation of vessel tonus and blood pressure (20, 22). The diabetic kidney has an altered oxygen metabolism leading to reduced oxygen tension (Po2) throughout the kidney (29). It is also reported that isolated proximal tubular cells (PTC) from diabetic rats have increased transport-dependent oxygen consumption (Qo2) and increased phosphorylation of the regulatory NADPH oxidase subunits p47phox and p22phox (1, 5, 7). Superoxide radicals (O2·−) increase intracellular Na+ by stimulating the sodium/potassium/2-chloride (NKCC2) transporter in medullary thick ascending limbs (mTAL) (15). It was proposed that O2·− scavenge nitric oxide (NO) resulting in increased tubular Na+ transport. l-Arginine supplementation and/or addition of the superoxide dismutase mimetic tempol improves NO availability in isolated mTAL, which concomitantly reduces tubular Na+ transport (26). However, it is not known whether diabetes-induced oxidative stress will affect tubular Na+ handling in vivo. Chronic treatment of diabetic rats with apocynin prevents the increase of the regulatory cytosolic subunit p47phox as well as the increased Qo2 of isolated PTC (5). Taken together, several studies indicate that an overactivated NADPH oxidase is an important regulator of renal Qo2 in the diabetic kidney. Therefore, we hypothesize that increased NADPH oxidase activation stimulates tubular Na+ transport and reduces intrarenal Po2 in the diabetic rat kidney.
MATERIALS AND METHODS
Animals and induction of diabetes.
Age-matched male Sprague-Dawley rats (250 g body wt) were purchased from Charles River (Charles River, Sulzfeldt, Germany). Animals had free access to tap water and pelleted standard rat chow. All experiments were conducted in accordance with national guidelines and approved by the Animal Care and Use Committee of Uppsala University. Diabetes was induced by a single injection of streptozotocin (55 mg/kg body wt; Sigma-Aldrich, St. Louis, MO) in the tail vein. Animals were considered diabetic if blood glucose concentration increased to >18 mM within 24 h after injection. Blood glucose was determined with test reagent strips (Medisense, Bedford, MA) on samples obtained from the cut tip of the tail in all animals.
Experimental groups.
The experimental groups consisted of untreated control and diabetic rats with diabetes duration of 14 ± 4 days. Additional control and diabetic rats were allocated to isolation of PTC used for in vitro Qo2 measurements.
Surgical procedures.
On the day for acute experiments, the rats were anesthetized with thiobutabarbital (Inactin, 120 mg/kg body wt ip for controls and 80 mg/kg body wt ip for diabetics) and placed on a table with a servo-controlled heating pad to maintain body temperature at 37.5°C. Animals were tracheotomized and catheters placed in femoral artery and vein for withdrawal of blood samples, blood pressure monitoring, and infusion of Ringer solution (5 ml·kg−1·h−1 for controls and 10 ml·kg−1·h−1 for diabetics). The left ureter was catheterized for collection of urine for subsequent analysis, and the urinary bladder was catheterized for drainage. Left kidney was exposed through a subcostal flank incision and immobilized in a plastic cup.
Measurement of GFR, RBF, Li+ clearance, Po2, nitrate/nitrite, and TBARS.
Glomerular filtration rate (GFR) was estimated by measurements of clearance of [3H]inulin. [3H]inulin was initially given as a bolus dose of 185 kBq (American Radiolabeled Chemicals, St. Louis, MO) and then continuously infused in Ringer solution (185 kBq·kg body wt−1·h−1). The 3H-labeled activities in urine and plasma were measured using standard liquid scintillation technique, and GFR was calculated according to GFR = U·V/P, where U and P denote the activity of [3H]inulin in urine and plasma, respectively, and V denotes the urine flow (in ml/min). After a 45-min recovery period baseline data were obtained for 30 min followed by administration of apocynin (10 mg/kg bw bolus iv) or vehicle. Thereafter, all parameters were followed for an additional two 30-min periods. Total renal blood flow (RBF) was measured using an ultrasound probe (Transonic Systems, Ithaca, NY) placed around the left renal artery. Li+ was administered as a 4-mg ip bolus of LiCl after completion of surgery followed by a continuous intravenous infusion (2.1 mg·h−1·rat−1) resulting in plasma concentration of 0.5–1.0 mM Li+. Kidneys were weighed at the end of each experiment. Urine flows were measured gravimetrically, and urinary and plasma Na+ and Li+ concentrations were determined by flame spectrophotometry (model IL543, Instrumentation Lab, Milan, Italy). Regional renal Po2 was measured using modified Clark-type microelectrodes (∼10 μm OD, Unisense, Aarhus, Denmark). Electrodes were two-point calibrated in water saturated with either Na2S2O5 (zero) or air (147 mmHg). Nitrate/nitrite was analyzed using a commercially available kit according to manufacturers' instruction (Cayman Chemicals, Ann Arbor, MI). Thiobarbituric acids reactive substances (TBARS) were measured fluorometrically (5). Briefly, 125 μl thiobarbituric acid (Merck, Darmstadt, Germany) were added to 100 μl of diluted urine sample and heated to 97°C for 60 min. Thereafter, samples were cooled on ice, and a 150-μl mixture of methanol and 1 mol/l NaOH (91:9) were added followed by centrifugation (3,000 rpm, 5 min). Fluorescence was measured in the supernatant (ex. 532 nm, em. 553 nm; Safire2, Tecan, Männedorf, Switzerland). Standards were prepared from malondialdehyd-bis-(diethylacetate) (Merck-Schuchart, Schuchart, Germany).
Measurement of Qo2 in vitro.
PTC were isolated from normoglycemic controls and diabetic rats as previously described (5). Briefly, kidney cortex was minced through a metallic mesh strainer and incubated with ice-cooled buffer solution containing collagenase (0.05% wt/vol) at 37°C for 90 min while the suspension was bubbled with a mixture of 95% O2 and 5% CO2. Thereafter, the suspension was cooled to 4°C and filtered through graded filters with pore sizes of 180, 75, 53, and 38 μm. The cells were pelleted by slow centrifugation (200 g, 2 min) and resuspended in collagenase-free buffer, a procedure that was repeated three times. The suspension was kept on ice until Qo2 was measured in an Oxygraph-2k (OROBOROS Instruments, Innsbrück, Austria). Qo2 measurements were performed using a buffer solution containing (in mM) 113.0 NaCl, 4.0 KCl, 27.2 NaHCO3, 1.0 KH2PO4, 1.2 MgCl2, 1.0 CaCl2, 10.0 HEPES, 0.5 Ca lactate, 2.0 glutamine, 50 U/ml streptomycin, and adjusted to 300 mosmol/kgH2O and pH 7.40. Glucose concentration in the medium was 5.8 mM for cells from normoglycemic controls and 23.2 mM for cells from diabetic animals (29, 30). A 50-μl cell suspension was injected into the chamber, and the rate of O2 disappearance was recorded. At the end of each experiment, 1 ml was removed from the chamber to determine protein concentration after centrifugation (15,000 g for 10 min) and resuspension in 200 μl dH2O, using DC Protein Assay (Bio-Rad Laboratories, Hercules, CA). Qo2 was calculated as the rate of O2 disappearance adjusted for protein concentration. Measurements were performed during baseline and after incubation with dimethylamiloride (DMA; 1 mmol/l) to inhibit the Na+/H+ exchanger (NHE) (4), apocynin (1 mmol/l) to inhibit the NADPH oxidase, and ouabain (2 mmol/l) to inhibit the Na+-K+-ATPase or apocynin in combination with DMA and ouabain. In all experiments samples were preincubated at 37°C for 10 min before being added to the Oxygraph-2k. The same concentration of each inhibitor as in the preincubation of the cells was present in the Oxygraph-2k before cells were added. It was also confirmed that none of the inhibitors interfered directly with technique used to measure Qo2.
Statistical analysis.
All statistical analyses were performed using GraphPad Prism software (GraphPad Software San Diego, CA). All data were analyzed by two-way repeated measure analysis of variance (ANOVA) followed by Bonferroni post hoc test. In addition, the data presented in Fig. 6 were also analyzed by one-way ANOVA followed by Bonferroni post hoc test for selected comparisons to determine between-group differences. Descriptive statistics are presented as means ± SE. For all comparisons P < 0.05 was considered significant.
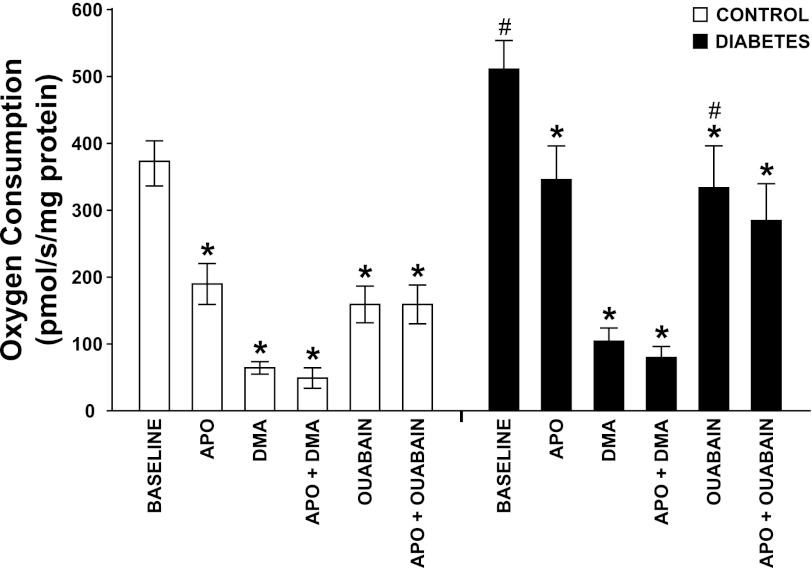
Fig. 6.
Oxygen consumption by isolated proximal tubular cells from normoglycemic controls (n = 7) and hyperglycemic diabetic rats (n = 8) after incubation with the NADPH oxidase inhibitor apocynin (APO), the Na+/H+ exchanger (NHE3) inhibitor dimethylamiloride (DMA), the Na+-K+-ATPase inhibitor ouabain, and apocynin in combination with DMA or ouabain. *P < 0.05 compared with baseline within the same group. #P < 0.05 compared with corresponding control.
RESULTS
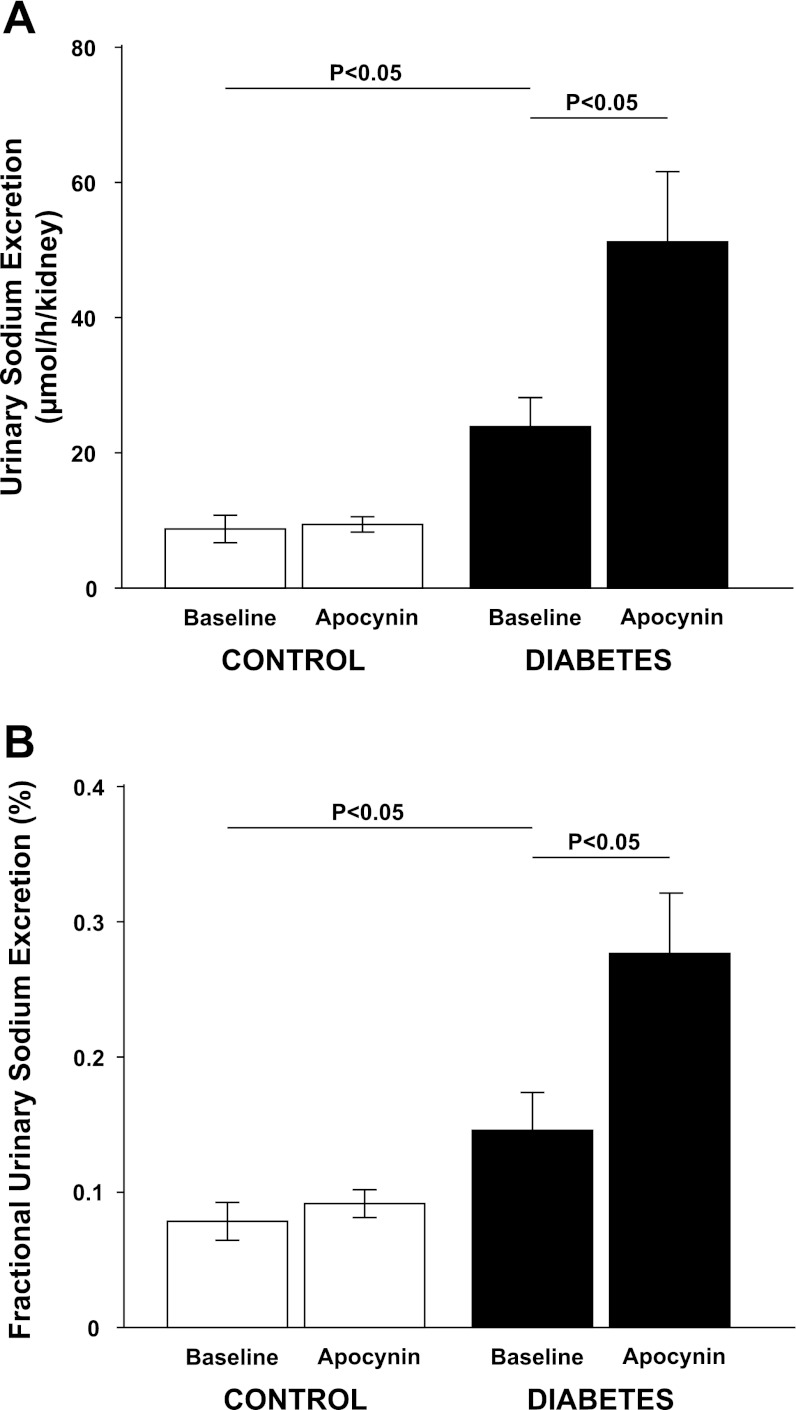
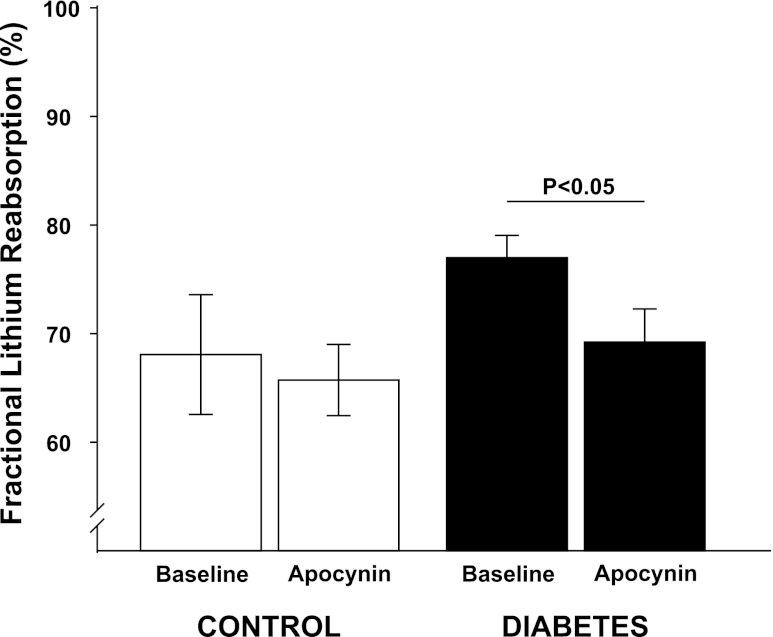
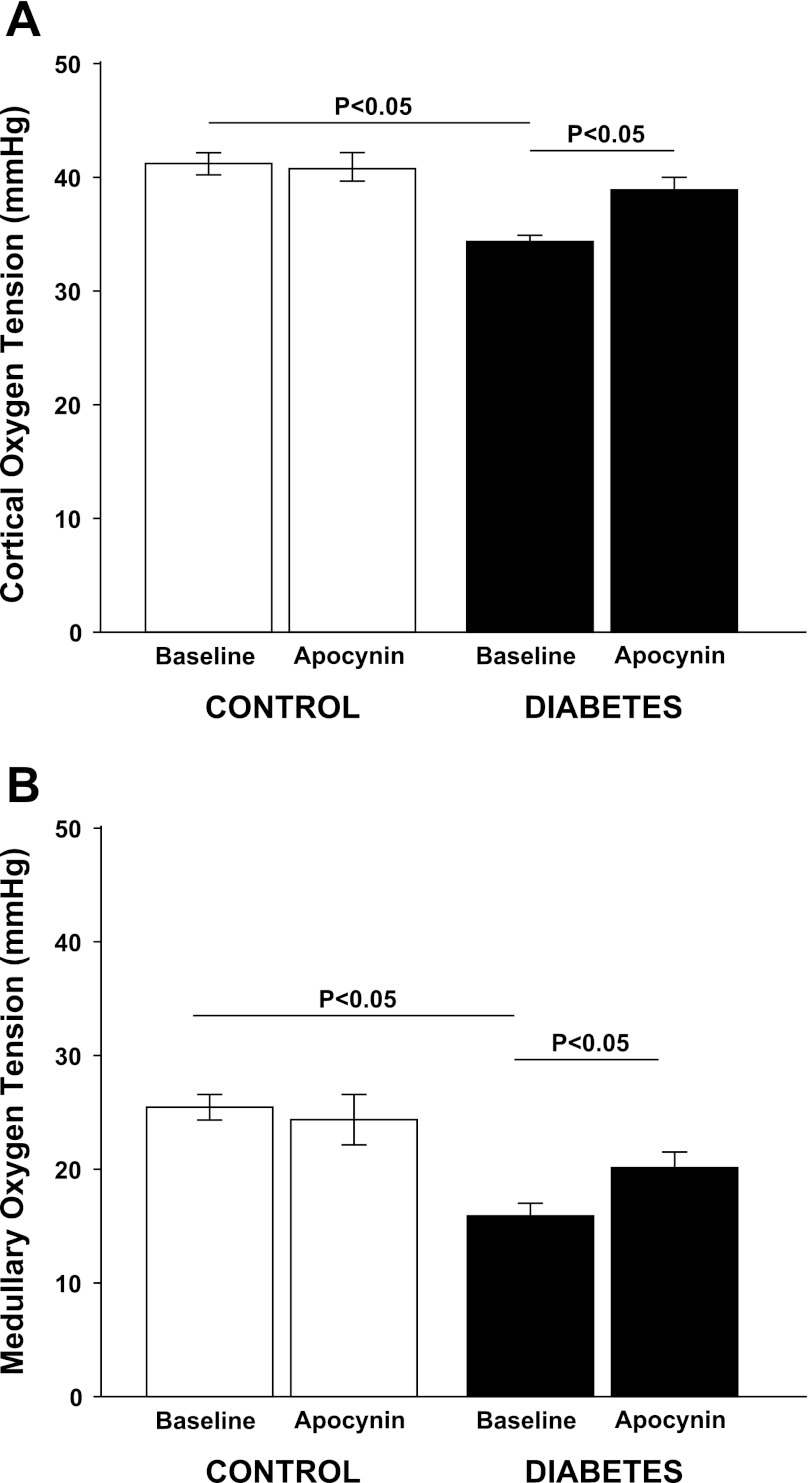
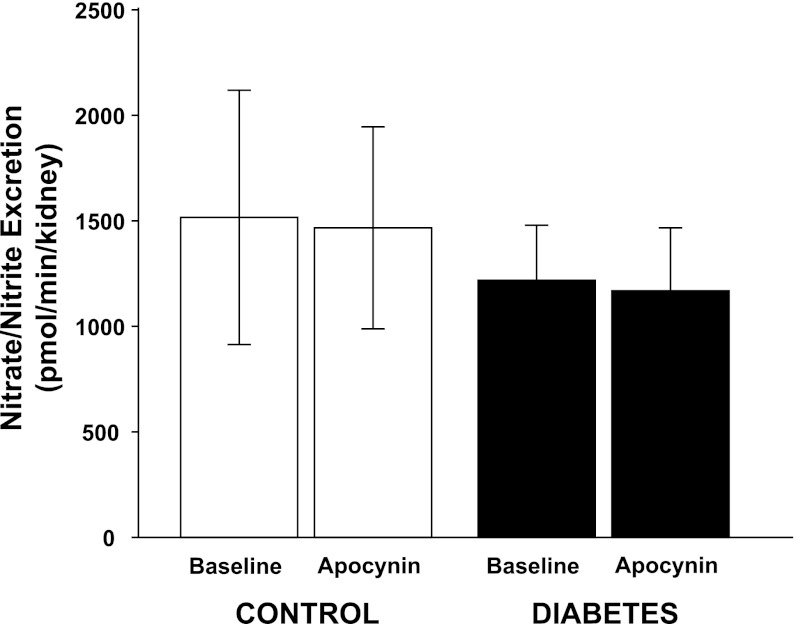
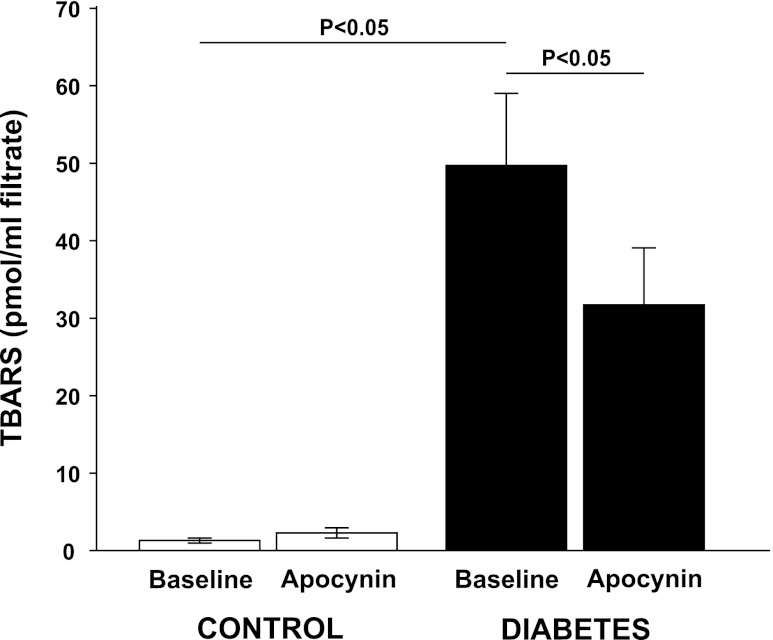
All diabetic animals developed hyperglycemia compared with controls (22.9 ± 0.7 vs. 5.6 ± 0.2 mM) and gained less weight compared with control animals (326 ± 7 vs. 357 ± 3 g). Food intake was higher in diabetic animals compared with controls (54 ± 3 vs. 26 ± 1 g/24 h). Left kidney weight was increased in diabetic animals compared with normoglycemic controls (1.85 ± 0.05 vs. 1.28 ± 0.03 g). Diabetic rats displayed 57% higher GFR than control, which was not affected by NADPH oxidase inhibition (Table 1). There was no difference in total RBF or MAP between the two groups, and NADPH oxidase inhibition did not alter any of these parameters (Table 1). Filtration fraction was elevated in diabetic animals and unaffected by NADPH oxidase inhibition (Table 1). Total and fractional urinary Na+ excretion was higher in diabetics compared with controls (+167% and +86%, respectively), and NADPH oxidase inhibition further increased absolute and fractional Na+ excretion selectively in the diabetic animals (+112% and +90%, respectively) (Fig. 1, A and B). Furthermore, fractional Li+ reabsorption only decreased (−10%) in diabetic animals after NADPH oxidase inhibition (Fig. 2). Po2 was reduced in both kidney cortex (−19%) and medulla (−39%) of the diabetic rats compared with controls, but NADPH oxidase inhibition normalized Po2 in cortex (+14%) and medulla (+28%) in diabetic kidneys (Fig. 3, A and B). NADPH oxidase inhibition did not affect Po2 in control kidneys. There was no difference in urinary excretion of nitrate and nitrite between the two groups, and NADPH oxidase inhibition did not have any effect (Fig. 4). Vehicle treatment did not have any effects in any of the groups (n = 6/group, data not shown). Baseline urinary excretion of TBARS was higher in diabetics compared with controls (Fig. 5). NADPH oxidase inhibition did not affect TBARS excretion in controls but reduced it by −36% diabetics. PTC isolated from diabetic rats had increased Qo2 (+36%) compared with PTC from normoglycemic controls (Fig. 6). Apocynin, DMA, ouabain, and apocynin in combination with either DMA or ouabain reduced Qo2 in PTC from both the control and diabetic rat. DMA or ouabain in combination with apocynin did not result in significantly different inhibition of Qo2 as either DMA or ouabain alone.
Table 1.
Mean arterial pressure, glomerular filtration rate, total renal blood flow, filtration fraction, urine flow, and hematocrit in normoglycemic control and hyperglycemic diabetic rats during baseline and after acute NADPH-oxidase inhibition by apocynin
| MAP, mmHg | GFR, ml•min−1•kidney−1 | RBF, ml•min−1•kidney−1 | FF | VU, μl•min−1•kidney−1 | Hct, % | |
|---|---|---|---|---|---|---|
| Normoglycemic (n = 11) | ||||||
| Baseline | 107 ± 4 | 1.4 ± 0.1 | 8.8 ± 1.0 | 0.31 ± 0.03 | 3.4 ± 0.5 | 46 ± 0.4 |
| Apocynin | 103 ± 4 | 1.2 ± 0.1 | 9.0 ± 1.0 | 0.27 ± 0.02 | 3.5 ± 0.4 | 46 ± 0.5 |
| Diabetic (n = 13) | ||||||
| Baseline | 104 ± 2 | 2.2 ± 0.3* | 10.1 ± 0.9 | 0.45 ± 0.04* | 24.9 ± 3.5* | 48 ± 0.4* |
| Apocynin | 105 ± 3 | 2.4 ± 0.2* | 11.1 ± 1.3 | 0.42 ± 0.03* | 20.8 ± 3.2* | 49 ± 0.7* |
Values are means ± SE; n is the number of rats. MAP, mean arterial pressure; GFR, glomerular filtration rate; RBF, total renal blood flow; FF, filtration fraction; VU, urine flow; Hct, hematocrit.
P < 0.05 compared with corresponding control.
Fig. 1.
Total urinary sodium excretion (A) and fractional urinary sodium excretion (B) in normoglycemic controls (n = 11) and hyperglycemic diabetic rats (n = 13) during baseline and after a bolus dose of apocynin.
Fig. 2.
Fractional urinary reabsorption of lithium in normoglycemic controls (n = 11) and hyperglycemic diabetic rats (n = 13) during baseline and after a bolus dose of apocynin.
Fig. 3.
A: cortical oxygen tension in normoglycemic controls (n = 11) and hyperglycemic diabetic rats (n = 13) during baseline and after a bolus dose of apocynin. B: medullary oxygen tension in normoglycemic controls (n = 11) and hyperglycemic diabetic rats (n = 13) during baseline and after a bolus dose of apocynin.
Fig. 4.
Urinary excretion of nitrate and nitrite in normoglycemic controls (n = 11) and hyperglycemic diabetic rats (n = 13) during baseline and after a bolus dose of apocynin.
Fig. 5.
Urinary excretion of the lipid peroxidation marker thiobarbituric acids reactive substances (TBARS) in normoglycemic controls (n = 11) and hyperglycemic diabetic rats (n = 13) during baseline and after a bolus dose of apocynin.
DISCUSSION
The new findings from the present study are that diabetes-induced oxidative stress, originating from the NADPH oxidase, stimulates tubular Na+ transport and contributes to intrarenal hypoxia. This was evident as substantially decreased fractional reabsorption of Li+ (−10%) and reduced PTC transport-dependent Qo2 (−32%) after NADPH oxidase inhibition. This indicates an effect of NADPH oxidase inhibition in the proximal tubule in diabetes. However, concomitant effects in more distal parts of the nephron are likely since it is well known that Li+ also is handled by mTALs and collecting ducts. This is further supported by the more pronounced alterations in total and fractional urinary Na+ excretion (+112% and +90%, respectively). The reduced tubular Na+ transport resulted in a concomitant increase in kidney Po2 in both cortex and medulla. Furthermore, NADPH oxidase inhibition reduced Qo2 in control and diabetic PTC isolated from both control and diabetic rats, but the effect of NADPH oxidase inhibition was not additive to that of inhibiting either the Na+-K+-ATPase using ouabain or NHE3 using DMA. These results demonstrate that active tubular transport by the PTC is directly linked to NADPH oxidase activity. Kidney Po2 is determined by the delivery of oxygen by RBF, the extraction from hemoglobin, and Qo2 related to basal metabolism and active tubular transport (31). Diabetes-induced glomerular hyperfiltration is present early in both patients and animal models of insulinopenic diabetes. Several theories have been suggested, but the underlying cause is still under debate (33). One implication for this phenomenon is that more electrolytes are filtered across the glomerular membrane into the primary urine, which requires increased tubular transport and increased Qo2 to maintain Na+ balance. Previous studies have established that the diabetic kidney is working under reduced intrarenal Po2 (6, 29), and this study confirmed these observations. There are two possible explanations for the reduced kidney Po2 in diabetes; the first is related to increased tubular Na+ transport and the second is increased formation of ROS. However, the increased oxidative stress appears to be the most important factor since treatment with the antioxidant dl-α-tocopherol throughout the course of diabetes normalized renal Po2 without affecting GFR (29). One explanation might be that ROS formation and tubular transport are closely connected since both exogenous and endogenous O2·− stimulates NKCC and NHE isoform 3 in mTAL in isolated tubules (15, 16, 27). Furthermore, O2·− interferes with cofactors required for NO synthesis by inhibiting dihydropteridine reductase, the enzyme responsible for reduction of dihydrobiopterin (BH2) to tetrahydrobiopterin (BH4) (18), O2·− causes depletion of BH4 resulting in uncoupling of NOS, which further increases the O2·− production (40). Reduced plasma concentrations of BH4 have been demonstrated in models of type 2 diabetes (25). BH4 supplementation improves vascular function in isolated aortic rings from diabetic rat (34) as well as endothelium-dependent vasodilation in type 2 diabetic patients (10), indicating that BH4 levels are compromised in diabetes. Indeed, it has been shown that NO concentration is reduced in the cortex of diabetic rats (28) making it relevant since NO itself can directly inhibit tubular electrolyte transport mediated by NKCC2, NHE1, and NHE3 in mTAL (11). However, acute NADPH oxidase inhibition did not increase NO production, as estimated by urinary excretion of nitrate/nitrite. It is still possible that NO is involved since there is an important difference between NO production and NO bioavailability. The latter is highly influenced by oxidative stress, and acute NADPH oxidase inhibition did lower the urinary excretion of the lipid peroxidation marker TBARS in the diabetic animals. It should be noted that urinary TBARS has previously been demonstrated to correlate with the increased oxidative stress in diabetes measured by in vivo electron spin resonance (37).
Li+ clearance has been used as a marker of proximal tubular reabsorption (19), although Li+ also can be reabsorbed in the mTAL and cortical collecting duct. In this study, NADPH oxidase inhibition decreased fractional reabsorption of Li+, indicating that O2·− directly influences proximal tubular transport in vivo. A recent report demonstrates that diabetes induce increased transport-dependent QO2 in isolated mTAL via protein kinase C-α and NADPH oxidase activation (41). Thus the reduced Na+ reabsorption after NADPH oxidase inhibition is likely to be influenced by altered transport also in this tubular segment. However, the involvement of the proximal tubule is verified by the in vitro experiments demonstrating reduced transport-dependent Qo2 in the isolated PTC after NADPH oxidase inhibition. Inhibition of either NADPH oxidase or Na+-K+-ATPase reduced Qo2, but the effects were not additive suggesting a direct effect on transport-dependent Qo2 in the proximal tubule. Qo2 was higher in PTC from diabetic rats during baseline but also after Na+-K+-ATPase inhibition suggesting increased basal Qo2. This is in agreement with previous reports and may be explained by diabetes-induced mitochondrial uncoupling reducing the efficiency to produce ATP (9, 32). Proximal tubular transport is mainly achieved by the NHE3. Juncos and coworkers (16) demonstrated that O2·− in isolated perfused mTAL directly affects NHE3 transport, where 25–30% of the total filtered Na+ is reabsorbed by NKCC2 (70–80%) and NHE3 (20–30%). They demonstrated that O2·− stimulates luminal NHE3, whereas basolateral NHE2 is inhibited, which will provide more H+ for luminal transport (16). Since NHE3 is reabsorbing the vast majority of filtered Na+ in the proximal tubule, O2·− from an overactivated NADPH oxidase in diabetes has the potential to affect proximal tubular Na+ transport as evident from this study where fractional excretion of Li+ as well as total Na+ excretion was increased exclusively in diabetic rats by inhibition of the NADPH oxidase. The reason why NADPH oxidase inhibition reduces transport-dependent Qo2 in PTC from both controls and diabetics, but exclusively induces natriuresis the diabetic rats, is currently not known. It may be speculated that downstream segments may be able to compensate for any increased Na+ load caused by NADPH oxidase inhibition in controls, whereas diabetics already reach the limit due to glomerular hyperfiltration. In addition, increasing tubular flow or electrolyte delivery to mTAL activates the NADPH oxidase, coupling metabolic activity in this segment to O2·− production (13, 21). Another explanation might be that general NADPH oxidase inhibition actually inhibits any compensatory transport capacity in the downstream tubular segments only in the diabetic condition, and it is therefore possible to observe the natriuretic effects. Late proximal and early distal flow is increased in diabetes, which also can contribute to the altered electrolyte handling (35). However, it is unknown if these mechanisms apply to the proximal tubule, but the results from present study indicate that NADPH oxidase mediated O2·− affects proximal tubule NHE3 since there was no effect of NADPH oxidase inhibition when NHE3 activity was blocked.
Diabetes upregulates the local renin-angiotensin system in the kidney in mesangial cells (24) and PTC (14) due to increased oxidative stress with a subsequent increase in locally produced angiotensin II (ANG II), as well as upregulation of ANG II AT1 receptors. ANG II is normally mediating biphasic activation of NHE3-mediated Na+ transport. The biphasic regulation is lost during increased oxidative stress leading to a significantly stronger ANG II-mediated Na+ transport (2, 3). Indeed, increased cortical NHE3 activity has been observed in the diabetic rat without any increase in protein levels (17). Furthermore, ANG II acting on AT1 receptors activates the NADPH oxidase in mTAL with a further increase in O2·− production (12). This could provide an explanation to how NADPH oxidase inhibition reduces proximal tubular transport exclusively in the diabetic animals.
The mechanisms behind the early diabetic glomerular hyperfiltration are unknown and proposed theories involve an increased proximal tubular reabsorption, mainly through sodium-dependent glucose transporters (SGLTs), since phlorizin normalize early distal Na+ delivery (39). Increased proximal tubular reabsorption with a subsequent reduction in distal Na+ delivery will be interpreted by macula densa as reduced GFR. Interestingly, reducing tubular Na+ transport and increasing fractional Li+ excretion by inhibiting the NADPH oxidase did not alter GFR and RBF in the present study. This suggests that other mechanisms than altered tubular Na+ transport determine GFR in diabetes, or that acutely reduced oxidative stress activates compensatory mechanisms to maintain normal GFR and RBF in the diabetic kidney. Urine flow was unaltered despite increased natriuresis after NADPH oxidase inhibition, which may reflect differential regulation of tubular Na+ transport and water permeability. Potential mechanisms for such differential regulation are beyond the scope of this study.
Finally, this study confirms that increased tubular load of electrolytes due to the diabetes-induced glomerular hyperfiltration is not causing the reduction in kidney Po2 per se, since Po2 was improved in both the cortex and medulla by NADPH oxidase inhibition, despite unchanged GFR and RBF. Previous studies have demonstrated that chronic apocynin treatment during diabetes reduces proteinuria and prevents histological alterations, providing further evidence for oxidative stress being an important factor for the development of diabetic nephropathy (1).
In conclusion, an overactivated NADPH oxidase in diabetes results in increased transport-dependent Qo2 and increased tubular Na+ transport with a subsequent reduction in cortical and medullary Po2 that is improved by acute NADPH oxidase inhibition.
Perspectives and Significance
Diabetic patients are at increased risk for development of hypertension and already in uncomplicated early diabetes display increased Na+ retention (8, 23, 36). One contributing factor might be increased tubular electrolyte transport due to increased oxidative stress resulting in volume expansion and hypertension. Increased tubular transport contributing to Na+ retention induced by enhanced O2·− production by the NADPH oxidase is now evident in both proximal tubule and mTAL (41). More research is needed to further clarify the regulation of tubular electrolyte transport and the potential link to increased arterial blood pressure in diabetes.
GRANTS
The work from our laboratory presented in this paper was supported by the Swedish Research Council, the Swedish Society for Medical Research, the Lars Hierta Foundation, the Magnus Bergvall Foundation, the Åke Wiberg Foundation and NIH/NIDDK K99/R00 grant (DK077858).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.P. and F.P. conception and design of research; P.P. performed experiments; P.P. and F.P. analyzed data; P.P., P.H., and F.P. interpreted results of experiments; P.P. and F.P. prepared figures; P.P. drafted manuscript; P.P., P.H., and F.P. edited and revised manuscript; P.P., P.H., and F.P. approved final version of manuscript.
REFERENCES
- 1. Asaba K, Tojo A, Onozato ML, Goto A, Quinn MT, Fujita T, Wilcox CS. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int 67: 1890–1898, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Banday AA, Lokhandwala MF. Angiotensin II-mediated biphasic regulation of proximal tubular Na+/H+ exchanger 3 is impaired during oxidative stress. Am J Physiol Renal Physiol 301: F364–F370, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Banday AA, Lokhandwala MF. Oxidative stress causes renal angiotensin II type 1 receptor upregulation, Na+/H+ exchanger 3 overstimulation, and hypertension. Hypertension 57: 452–459, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Counillon L, Scholz W, Lang HJ, Pouyssegur J. Pharmacological characterization of stably transfected Na+/H+ antiporter isoforms using amiloride analogs and a new inhibitor exhibiting anti-ischemic properties. Mol Pharmacol 44: 1041–1045, 1993 [PubMed] [Google Scholar]
- 5. Edlund J, Fasching A, Liss P, Hansell P, Palm F. The roles of NADPH oxidase and nNOS for the increased oxidative stress and the oxygen consumption in the diabetic kidney. Diabetes Metab Res Rev 26: 349–356, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edlund J, Hansell P, Fasching A, Liss P, Weis J, Glickson JD, Palm F. Reduced oxygenation in diabetic rat kidneys measured by T2* weighted magnetic resonance micro-imaging. Adv Exp Med Biol 645: 199–204, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Etoh T, Inoguchi T, Kakimoto M, Sonoda N, Kobayashi K, Kuroda J, Sumimoto H, Nawata H. Increased expression of NAD(P)H oxidase subunits, NOX4 and p22phox, in the kidney of streptozotocin-induced diabetic rats and its reversibity by interventive insulin treatment. Diabetologia 46: 1428–1437, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Feldt-Rasmussen B, Mathiesen ER, Deckert T, Giese J, Christensen NJ, Bent-Hansen L, Nielsen MD. Central role for sodium in the pathogenesis of blood pressure changes independent of angiotensin, aldosterone and catecholamines in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 30: 610–617, 1987 [DOI] [PubMed] [Google Scholar]
- 9. Friederich M, Fasching A, Hansell P, Nordquist L, Palm F. Diabetes-induced up-regulation of uncoupling protein-2 results in increased mitochondrial uncoupling in kidney proximal tubular cells. Biochim Biophys Acta 1777: 935–940, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia 43: 1435–1438, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Herrera M, Ortiz PA, Garvin JL. Regulation of thick ascending limb transport: role of nitric oxide. Am J Physiol Renal Physiol 290: F1279–F1284, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Herrera M, Silva GB, Garvin JL. Angiotensin II stimulates thick ascending limb superoxide production via protein kinase C(alpha)-dependent NADPH oxidase activation. J Biol Chem 285: 21323–21328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hong NJ, Garvin JL. Flow increases superoxide production by NADPH oxidase via activation of Na-K-2Cl cotransport and mechanical stress in thick ascending limbs. Am J Physiol Renal Physiol 292: F993–F998, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Hsieh TJ, Zhang SL, Filep JG, Tang SS, Ingelfinger JR, Chan JS. High glucose stimulates angiotensinogen gene expression via reactive oxygen species generation in rat kidney proximal tubular cells. Endocrinology 143: 2975–2985, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Juncos R, Garvin JL. Superoxide enhances Na-K-2Cl cotransporter activity in the thick ascending limb. Am J Physiol Renal Physiol 288: F982–F987, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Juncos R, Hong NJ, Garvin JL. Differential effects of superoxide on luminal and basolateral Na+/H+ exchange in the thick ascending limb. Am J Physiol Regul Integr Comp Physiol 290: R79–R83, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Klisic J, Nief V, Reyes L, Ambuhl PM. Acute and chronic regulation of the renal Na/H+ exchanger NHE3 in rats with STZ-induced diabetes mellitus. Nephron Physiol 102: p27–p35, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Komori Y, Hyun J, Chiang K, Fukuto JM. The role of thiols in the apparent activation of rat brain nitric oxide synthase (NOS). J Biochem 117: 923–927, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Koomans HA, Boer WH, Dorhout Mees EJ. Evaluation of lithium clearance as a marker of proximal tubule sodium handling. Kidney Int 36: 2–12, 1989 [DOI] [PubMed] [Google Scholar]
- 20. Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285: R277–R297, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Li N, Zhang G, Yi FX, Zou AP, Li PL. Activation of NAD(P)H oxidase by outward movements of H+ ions in renal medullary thick ascending limb of Henle. Am J Physiol Renal Physiol 289: F1048–F1056, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Modlinger P, Chabrashvili T, Gill PS, Mendonca M, Harrison DG, Griendling KK, Li M, Raggio J, Wellstein A, Chen Y, Welch WJ, Wilcox CS. RNA silencing in vivo reveals role of p22phox in rat angiotensin slow pressor response. Hypertension 47: 238–244, 2006 [DOI] [PubMed] [Google Scholar]
- 23. O'Hare JP, Roland JM, Walters G, Corrall RJ. Impaired sodium excretion in response to volume expansion induced by water immersion in insulin-dependent diabetes mellitus. Clin Sci (Lond) 71: 403–409, 1986 [DOI] [PubMed] [Google Scholar]
- 24. Ohashi N, Urushihara M, Satou R, Kobori H. Glomerular angiotensinogen is induced in mesangial cells in diabetic rats via reactive oxygen species–ERK/JNK pathways. Hypertens Res 33: 1174–1181, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Okumura M, Masada M, Yoshida Y, Shintaku H, Hosoi M, Okada N, Konishi Y, Morikawa T, Miura K, Imanishi M. Decrease in tetrahydrobiopterin as a possible cause of nephropathy in type II diabetic rats. Kidney Int 70: 471–476, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Ortiz PA, Garvin JL. Interaction of O(2)(-) and NO in the thick ascending limb. Hypertension 39: 591–596, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Ortiz PA, Garvin JL. Superoxide stimulates NaCl absorption by the thick ascending limb. Am J Physiol Renal Physiol 283: F957–F962, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Palm F, Buerk DG, Carlsson PO, Hansell P, Liss P. Reduced nitric oxide concentration in the renal cortex of streptozotocin-induced diabetic rats: effects on renal oxygenation and microcirculation. Diabetes 54: 3282–3287, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Palm F, Cederberg J, Hansell P, Liss P, Carlsson PO. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia 46: 1153–1160, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Palm F, Hansell P, Ronquist G, Waldenstrom A, Liss P, Carlsson PO. Polyol-pathway-dependent disturbances in renal medullary metabolism in experimental insulin-deficient diabetes mellitus in rats. Diabetologia 47: 1223–1231, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Palm F, Nordquist L. Renal oxidative stress, oxygenation, and hypertension. Am J Physiol Regul Integr Comp Physiol 301: R1229–R1241, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Persson MF, Franzen S, Catrina SB, Dallner G, Hansell P, Brismar K, Palm F. Coenzyme Q10 prevents GDP-sensitive mitochondrial uncoupling, glomerular hyperfiltration and proteinuria in kidneys from db/db mice as a model of type 2 diabetes. Diabetologia 55: 1535–1543, 2012 [DOI] [PubMed] [Google Scholar]
- 33. Persson P, Hansell P, Palm F. Tubular reabsorption and diabetes-induced glomerular hyperfiltration. Acta Physiol (Oxf) 200: 3–10, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pieper GM. Acute amelioration of diabetic endothelial dysfunction with a derivative of the nitric oxide synthase cofactor, tetrahydrobiopterin. J Cardiovasc Pharmacol 29: 8–15, 1997 [DOI] [PubMed] [Google Scholar]
- 35. Pollock CA, Lawrence JR, Field MJ. Tubular sodium handling and tubuloglomerular feedback in experimental diabetes mellitus. Am J Physiol Renal Fluid Electrolyte Physiol 260: F946–F952, 1991 [DOI] [PubMed] [Google Scholar]
- 36. Roland JM, O'Hare JP, Walters G, Corrall RJ. Sodium retention in response to saline infusion in uncomplicated diabetes mellitus. Diabetes Res 3: 213–215, 1986 [PubMed] [Google Scholar]
- 37. Sano T, Umeda F, Hashimoto T, Nawata H, Utsumi H. Oxidative stress measurement by in vivo electron spin resonance spectroscopy in rats with streptozotocin-induced diabetes. Diabetologia 41: 1355–1360, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Sonta T, Inoguchi T, Tsubouchi H, Sekiguchi N, Kobayashi K, Matsumoto S, Utsumi H, Nawata H. Evidence for contribution of vascular NAD(P)H oxidase to increased oxidative stress in animal models of diabetes and obesity. Free Radic Biol Med 37: 115–123, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol 10: 2569–2576, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA 95: 9220–9225, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang J, Pollock JS, Carmines PK. NADPH oxidase and PKC contribute to increased na transport by the thick ascending limb during Type 1 diabetes. Hypertension 59: 431–436 [DOI] [PMC free article] [PubMed] [Google Scholar]