Abstract
The aim of this study was to identify genes that influence iron regulation under varying dietary iron availability. Male and female mice from 20+ BXD recombinant inbred strains were fed iron-poor or iron-adequate diets from weaning until 4 mo of age. At death, the spleen, liver, and blood were harvested for the measurement of hemoglobin, hematocrit, total iron binding capacity, transferrin saturation, and liver, spleen and plasma iron concentration. For each measure and diet, we found large, strain-related variability. A principal-components analysis (PCA) was performed on the strain means for the seven parameters under each dietary condition for each sex, followed by quantitative trait loci (QTL) analysis on the factors. Compared with the iron-adequate diet, iron deficiency altered the factor structure of the principal components. QTL analysis, combined with PosMed (a candidate gene searching system) published gene expression data and literature citations, identified seven candidate genes, Ptprd, Mdm1, Picalm, lip1, Tcerg1, Skp2, and Frzb based on PCA factor, diet, and sex. Expression of each of these is cis-regulated, significantly correlated with the corresponding PCA factor, and previously reported to regulate iron, directly or indirectly. We propose that polymorphisms in multiple genes underlie individual differences in iron regulation, especially in response to dietary iron challenge. This research shows that iron management is a highly complex trait, influenced by multiple genes. Systems genetics analysis of iron homeostasis holds promise for developing new methods for prevention and treatment of iron deficiency anemia and related diseases.
Keywords: complex traits, BXD mice, quantitative trait loci analysis
iron is a critical trace element involved in nearly all biological functions. While iron is necessary for normal physiological function, too much iron can be quite toxic and lead to cell death (32). Alternatively, too little iron can lead to anemia and many other subsequent biological and behavioral complications (43). Consequently, iron must be highly regulated in all organ systems. Systemic iron homeostasis is maintained by multiple mechanisms, and malfunction in any of these may lead to disease.
Iron deficiency anemia (IDA) is one of the most common, single nutrient-deficiency diseases worldwide. According to a 2001 report from the World Health Organization, IDA affects about 2.5% of the population worldwide. Moreover, an estimated 52% of pregnant women from undeveloped or developing countries, and 20–25% of the world's infants are afflicted (37, 52). In infancy, iron deficiency has pronounced effects on multiple organ systems and can impair functions ranging from immunity (11, 48) to neurobehavioral development (44, 58). In adulthood, systemic iron deficiency causes fatigue and headache and is linked to heart failure; brain iron deficiency may lead to restless legs syndrome, and neuroleptic malignant syndrome (13, 12, 53, 40).
Dietary iron is absorbed from the intestine into blood circulation, where it is bound to transferrin and transported to different organs. Iron is then transported into target cells by the transferrin receptor (TfR) and divalent metal transporter 1 (6, 10, 19). In cells, iron is sequestered, and redox state is controlled by ferritin; iron from senescent erythrocytes is recycled by macrophages and stored in bone marrow, liver, and spleen, and in time of need, released from macrophages in the storage sites (6, 25, 49). One of the key proteins of systemic iron homeostasis is hepcidin, a peptide hormone produced by liver, which regulates both intestinal iron absorption and macrophage iron release (21, 53, 51, 68, 31). Iron-deficient diets and inflammation, which result in abnormal macrophage iron release, can give rise to anemia (37). In this study, we focused on the genetics of individual differences in susceptibility to the effects of an iron-poor diet.
The present study is a follow-up to our earlier report (29), where seven iron-related parameters were measured in a panel of recombinant inbred (RI) mouse strains that were fed a standard laboratory diet containing adequate iron. The focus of the present study was to elucidate the genetic basis of individual differences in susceptibility to being fed a low-iron diet. The animals used were 22 BXD recombinant inbred (RI) strains of mice plus the parental strains, C57BL/6J (B6) and DBA/2J (D2). In this paper, we report the analysis of seven parameters of systemic iron status: hematocrit, hemoglobin, serum iron concentration, transferrin saturation, total iron-binding capacity (TIBC), liver iron, and spleen iron. These seven measures were then subjected to principal components analysis to reveal overt and latent combinations of factors important for iron homeostasis for each dietary condition and sex. The factors identified were then subjected to quantitative trait loci (QTL) analysis to reveal chromosomal regions harboring genes that influence these factors. Recombinant inbred strains, when densely mapped for genetic polymorphisms, are especially well suited for discovery of multiple genes that influence biological and behavioral phenotypes (69). The BXD panel is densely mapped with more than 13,000 markers genome-wide.
MATERIALS AND METHODS
Animals, Treatment, and Housing
Male and female mice from 22 strains plus the parental strains, C57BL/6J and DBA/2J, of the BXD/Ty RI panel were the experimental subjects. All mice came from the Penn State University vivarium. At postnatal day 21, mice were weaned and housed in unisex groups of 2–4/cage. The mice were then randomly assigned to one of two diets, an iron-adequate diet containing 240 μg/g iron (AD, Harlan Teklad, Indianapolis, IN), or an iron-deficient diet containing ∼3 μg/g iron (ID; Harlan Teklad). The breeding stock was fed an AD diet. All mice had ad libitum access to distilled water and food. Ambient housing conditions were controlled for temperature (23 ± 2°C) and humidity (40%), and the animals were maintained on a 12:12-h light-dark cycle (lights on at 0600). At 120 days of age, all mice were killed for tissue harvest. The average number per strain × sex × diet group ranged from 10 and 12. Three groups had two animals and three groups had three animals. The total number of mice used was 1,067 (536 males, 531 females). All experimental protocols were conducted in accordance with The National Institutes of Health Animal Care guidelines and were approved by The Pennsylvania State University Institutional Animal Care and Use Committee.
Measures
All mice were weighed and killed by CO2 suffocation between the hours of 0900 and 1200. Plasma iron, hemoglobin, hematocrit, TIBC, transferrin saturation (TfS), and liver iron and spleen iron concentrations were measured using standard methods (17). Hemoglobin values were determined photometrically by using cyanmethemoglobin standard solution (Sigma Aldrich, St. Louis, MO), and hematocrit values were calculated after centrifugation (13,000 g, 5 min, room temperature) of blood samples in heparinized microcapillary tubes. Serum was stored at −20°C until assay. Plasma iron and TIBC were determined colorimetrically, and TfS was calculated by plasma iron/TIBC. Livers and spleens were rapidly removed and frozen at −80°C for photometric assessment of iron content, modified from Erikson et al. (16) and Cook et al. (14). A portion of each spleen and the same lobe of each liver were weighed and transferred into an acid-washed glass vial into which 1 ml of protein precipitant was added. The vials were covered with a marble and placed in an 80–90°C sand bath for 2 days. Samples were then cooled to room temperature and added in 50-μl increments to 3 ml chromagen for color development. Absorbance was read on a spectrophotometer at a wavelength of 535 nm. Iron concentration in each tube was determined against a standard curve and converted to iron concentration per gram of tissue.
Data Analysis
All data were evaluated by ANOVA for three between-subjects variables: strain, sex, and diet. Narrow-sense heritability estimates for each measure in each diet were calculated as SSstrain/SStotal (5). Principal component analysis (PCA) was performed on strain means for each diet group for each sex using varimax rotation of the seven parameters to examine our data for latent associations among the parameters. QTL analysis is a means to query the entire genome for DNA variants (markers) that show significant associations with the phenotype (quantitative trait) under investigation. This is the first step to identify candidate genes whose variants (alleles) affect the value of the phenotype. QTL analysis was performed using WebQTL (www.genenetwork.org) for each PCA factor. WebQTL performs 2,000 or more permutations of the strain data, and significant QTL are defined by the likelihood ratio statistic (LRS) score of correctly ordered data exceeding all other permutations 95% of the time, i.e., the 0.05 alpha level (69, 70). For each QTL, genes within ±5 Mb of the marker were evaluated using the PosMed system (http://omicspace.riken.jp/PosMed/) to identify iron-related genes. The cis-regulated, iron-related genes in this area were then placed under consideration when their expression was significantly correlated with the PCA factors and with an absolute value of r greater than 0.5 (26). We then searched the literature for articles concerning the genes and those that had been reported as related to iron-regulation were nominated as candidate genes for that factor. The gene expression profiles (GEPs) used to identify cis regulation and correlation with the PCA factors (Table 6) were provided by others and published on GeneNetwork.org. The databases include hematopoietic stem cells, liver, and spleen and are described in Table 6 (for example, the link to spleen gene expression data is www.genenetwork.org/dbdoc/IoP_SPL_RMA0509.html). Genetic correlations between the factors and published phenotypes (using BXD strain means) were performed using the GeneNetwork database.
Table 6.
Candidate genes by principal component
| Diet, Sex, PCA Factor | Chr; Location (Mb) | LRS Score | Candidate Gene | Gene Expression Profile Showing cis Regulation | Gene Expression Profile of Correlation with the Factor | Correlation (r) |
|---|---|---|---|---|---|---|
| Adequate Fe, Males, PCA factor 1 | 4 (77.85) | 7.56 | Ptprda | UNC Agilent G4121A Liver LOWESS Stanford (Jan06) Both Sexes, IoP Affy MOE 430v2 Spleen (May09) RMA | Iop Affy MOE 430v2 Spleen (May09) RMA | −0.626 |
| Adequate Fe, Males, PCA factor 2 | 10 (117.58) | 11.94 | Mdmlb | IoP Affy MOE 430v2 Spleen (May09) RMA | UMCG Progenitor Cells ILM6v1.1 (Apr09) transformed | −0.613 |
| Adequate Fe, Females, PCA factor 3 | 7 (97.28) | 15.07 | Picalma | UNC Agilent G4121A Liver LOWESS Stanford (Jan06) Both Sexes, IoP Affy MOE 430v2 Spleen (May09) RMA | IoP Affy MOE 430v2 Spleen (May09) RMA | −0.66 |
| 18 (42.67) | 11.80 | Tcerglb | IoP Affy MOE 430v2 Spleen (May09) RMA | IoP Affy MOE 430v2 Spleen (May09) RMA | 0.694 | |
| Deficient Fe, Females, PCA factor 2 | 15 (9.04) | 13.46 | Skp2b | IoP Affy MOE 430v2 Spleen (May09) RMA | UMCG Stem Cells ILM6v1.1 (Apr09) original | −0.584 |
| 19 (34.56) | 12.22 | Liplb | IoP Affy MOE 430v2 Spleen (May09) RMA | UNC Agilent G4121A Liver LOWESS Stanford (Jan06) Females | 0.587 | |
| Deficient Fe, Females, PCA factor 3 | 2 (80.25) | 10.33 | Frzbb | IoP Affy MOE 430v2 Spleen (May09) RMA | UTK Spleen ILM6.1 (Jan10) VST | 0.642 |
Initial gene mapping for each iron-related principal component was performed using WebQTL (www.genenetwork.org). Suggestive or significant QTL were examined for the genes contained within or near the genomic area. Those genes that were self (or cis) regulated and whose expression was correlated with the PCA were considered candidates.
Significant cis regulation.
Suggestive cis regulation.
RESULTS
Body Weight
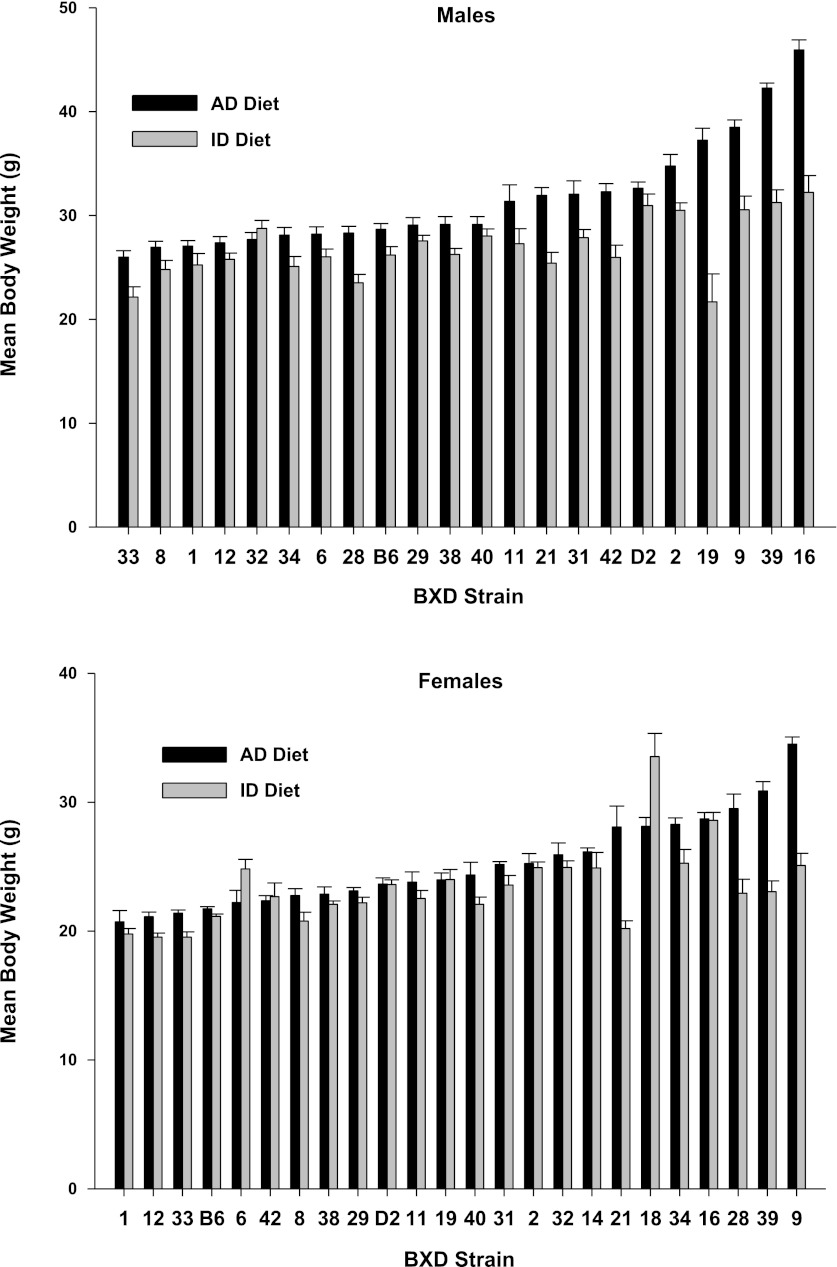
Figure 1 illustrates the effect of an ID on body weight in the animals at 4 mo of age. ANOVA showed that the main effects for strain, sex, and diet were all significant beyond P < 0.001 (F20,928 = 41.98; F1,928 = 673.32; F1,928 = 295.30, respectively), as were all of the interactions, owing to the large error degrees of freedom. Heritability estimates for body weight under the AD diet and ID diets were 0.40 and 0.25, respectively. Overall, animals fed the ID diet showed lower body weights compared with those treated with the iron-adequate (AD) diet, except for males (strain 32) and for females (strains 6, 42, and 18), which showed weight gain. There were also large differences among the strains in response to the iron-deficient diet. We performed a step-wise multiple-regression analysis of each of the seven iron-related parameters on body weight. The only parameter to have a significant impact on body weight was hematocrit with a regression coefficient of 0.218 (P < 0.05).
Fig. 1.
Body weights (g) by strain, sex, and diet for mice fed an iron-poor (ID Diet; 3 ppm [Fe]) or an iron-adequate (AD Diet; 240 ppm [Fe]) diet. The animals were fed their respective diets between weaning at postnatal day 21 until 4 mo of age, or 100 days total. Data are expressed as means ± SE.
Iron Measures: Summary Statistics
Descriptive statistics for the seven measures, by diet or sex, are presented in Table 1. The ANOVA summaries for the iron parameters are listed in Table 2. The main effect of strain was significant for all seven measures. The main effect of sex was significant for all seven measures, except for TfS. For hematocrit and hemoglobin, overall, the values for males were equal to females on the AD diet, but ID females showed higher values than ID males. For TIBC, males showed higher values than females under both diets. For plasma Fe, AD females showed higher values than AD males, while ID females showed lower values than ID males. For both liver Fe and spleen Fe, females showed higher values than males on both diets. The main effect of diet was also significant for all seven measures. The ID diet increased TIBC and reduced all other measures in both sexes compared with AD diet, except for plasma Fe in males (no change). The strain × diet interaction was significant for all seven measures. The strain × sex interaction was significant for all seven measures except for TfS and plasma Fe. The diet × sex interaction was significant for all seven measures except for TIBC. The strain × diet × sex interaction was significant for all seven measures. Narrow-sense heritability estimates (from ANOVA SSstrain/SStotal) for each measure by diet are listed in Table 3.
Table 1.
Descriptive statistics for iron measures, by diet
| Hct, % |
Hb, g/dl |
TIBC, μg/dl |
TfS, % |
Plasma Fe, μg/dl |
Liver Fe, μg/g tissue |
Spleen Fe, μg/g tissue |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AD | ID | AD | ID | AD | ID | AD | ID | AD | ID | AD | ID | AD | ID | |
| Males | ||||||||||||||
| mean | 45.22 | 29.65 | 12.42 | 6.79 | 627.83 | 838.01 | 28.27 | 19.72 | 166.5 | 167.67 | 105.52 | 28.56 | 749.73 | 74.76 |
| SD | 3.99 | 9.94 | 1.69 | 3.03 | 212.49 | 264.45 | 10.11 | 15.68 | 46.71 | 150.24 | 48.64 | 11.56 | 481.92 | 64.83 |
| minimum | 36 | 6 | 6.96 | 0.67 | 154.3 | 253.9 | 8.3 | −0.6 | 64.2 | 9.7 | 20.3 | 13.5 | 30.9 | 12.7 |
| maximum | 57 | 528.6 | 17.47 | 528.6 | 1406.7 | 1977.5 | 62.4 | 528.6 | 304.2 | 1977.5 | 255.2 | 528.6 | 3891.7 | 528.6 |
| n | 256 | 246 | 256 | 245 | 252 | 239 | 253 | 233 | 253 | 236 | 260 | 244 | 253 | 238 |
| Females | ||||||||||||||
| mean | 45.2 | 34.21 | 12.43 | 8.14 | 595.12 | 754.2 | 32.86 | 16.42 | 182.97 | 115.71 | 153.78 | 32.92 | 1180.57 | 87.09 |
| SD | 3.89 | 8.26 | 1.85 | 2.64 | 224.21 | 209.93 | 13.02 | 13.16 | 54.31 | 100.27 | 63.34 | 28.54 | 1055.32 | 65.58 |
| minimum | 32 | 5 | 7.76 | 1 | 159.7 | 98.26 | 5.9 | 0.2 | 73.3 | 1.2 | 34.8 | 14.9 | 84.8 | 8.2 |
| maximum | 55 | 52 | 21.96 | 18.11 | 1478.9 | 1451.7 | 80.6 | 70.4 | 377.6 | 668 | 357.8 | 270.3 | 10415.3 | 576.3 |
| n | 242 | 239 | 244 | 235 | 241 | 229 | 243 | 227 | 247 | 222 | 249 | 227 | 241 | 230 |
| Sexes Combined | ||||||||||||||
| mean | 45.21 | 31.89 | 12.42 | 7.45 | 611.84 | 797 | 30.52 | 18.09 | 174.64 | 142.48 | 129.13 | 30.66 | 959.92 | 80.82 |
| SD | 3.94 | 9.42 | 1.76 | 2.92 | 218.69 | 242.72 | 11.84 | 14.57 | 51.23 | 130.94 | 61.22 | 21.57 | 841.05 | 65.42 |
| minimum | 32 | 5 | 6.96 | 0.67 | 154.3 | 98.26 | 5.9 | −0.6 | 64.2 | 1.2 | 20.3 | 13.5 | 30.9 | 8.2 |
| maximum | 57 | 52 | 21.96 | 18.11 | 1478.9 | 1977.5 | 80.6 | 87.2 | 377.6 | 741.2 | 357.8 | 270.3 | 10415.3 | 576.3 |
| n | 498 | 485 | 500 | 480 | 493 | 468 | 496 | 460 | 500 | 458 | 509 | 471 | 494 | 468 |
Table 2.
ANOVA summary
| Hct |
Hb |
TIBC |
TfS |
Plasma Fe |
Liver Fe |
Spleen Fe |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measures | df | F | P | F | P | F | P | F | P | F | P | F | P | F | P |
| Strain | 20 | 10.5 | <0.001 | 14.37 | <0.001 | 19.06 | <0.001 | 5.04 | <0.001 | 5.52 | <0.001 | 39.12 | <0.001 | 27.08 | <0.001 |
| Sex | 1 | 33.51 | <0.001 | 33.39 | <0.001 | 16.07 | <0.001 | <1 | n.s. | 12.39 | <0.001 | 216.08 | <0.001 | 79.65 | <0.001 |
| Diet | 1 | 1257.2 | <0.001 | 1573.9 | <0.001 | 231.84 | <0.001 | 193.34 | <0.001 | 13.7 | <0.001 | 3242.3 | <0.001 | 1109 | <0.001 |
| Strain×Sex | 20 | 6.07 | <0.001 | 6.96 | <0.001 | 1.64 | <0.05 | 1.4 | n.s. | 1.44 | n.s. | 6.08 | <0.001 | 7.35 | <0.001 |
| Strain×Diet | 20 | 13.36 | <0.001 | 8.44 | <0.001 | 3.59 | <0.001 | 4.41 | <0.001 | 6.21 | <0.001 | 30.13 | <0.001 | 22.44 | <0.001 |
| Sex×Diet | 1 | 35.01 | <0.001 | 25.75 | <0.001 | 1.88 | n.s. | 19.35 | <0.001 | 30.2 | <0.001 | 144.69 | <0.001 | 73.06 | <0.001 |
| Strain×Sex×Diet | 20 | 3.11 | <0.001 | 3.36 | <0.001 | 1.66 | <0.05 | 1.74 | <0.05 | 20221 | <0.005 | 10.11 | <0.001 | 6.84 | <0.001 |
| Error df | 899 | 896 | 877 | 872 | 874 | 896 | 878 |
Table 3.
Narrow sense heritability estimates for the parameters
| Hematocrit | Hemoglobin |
Plasma [Fe] |
Total Iron Binding Capacity |
Transferrin Saturation |
Liver [Fe] |
Spleen [Fe] |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AD | ID | AD | ID | AD | ID | AD | ID | AD | ID | AD | ID | AD | ID |
| 0.22 | 0.29 | 0.11 | 0.34 | 0.32 | 0.16 | 0.36 | 0.27 | 0.23 | 0.12 | 0.51 | 0.18 | 0.43 | 0.37 |
Strain Distributions for Iron Measures
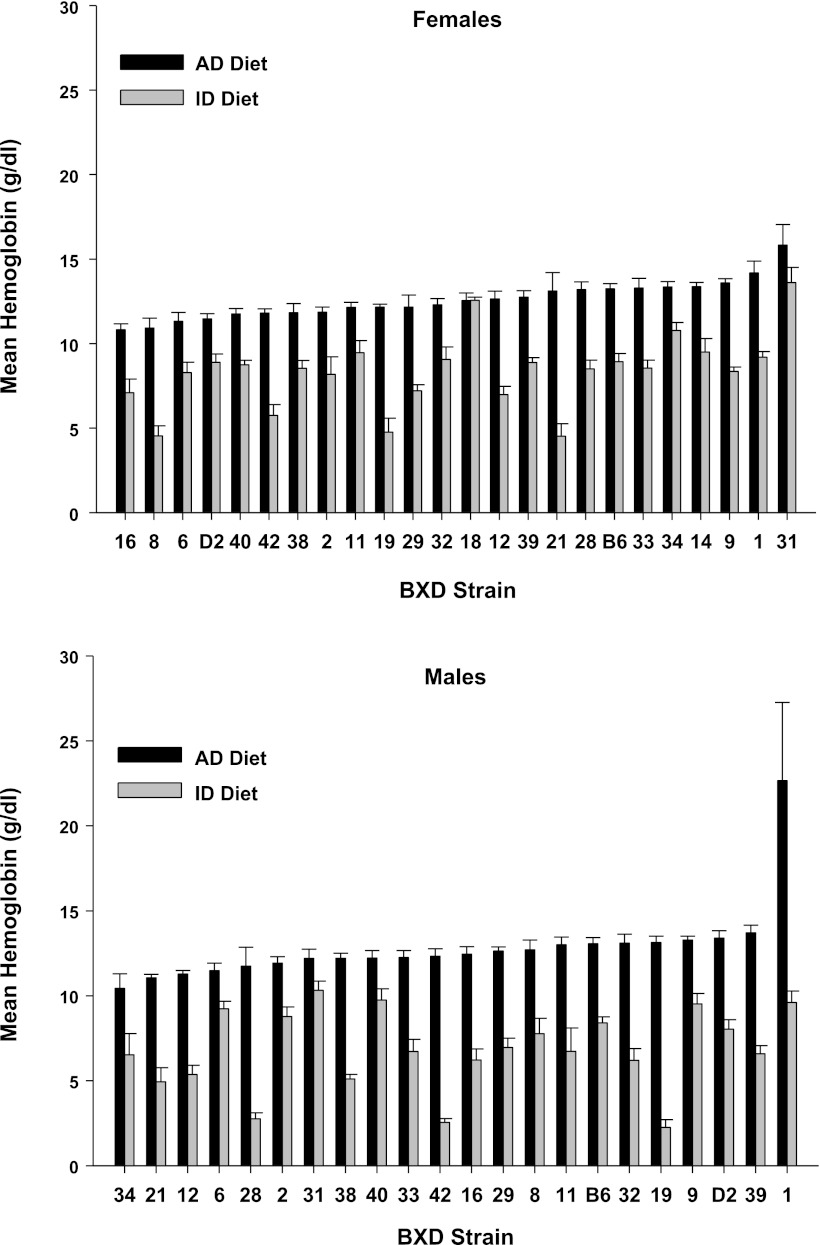
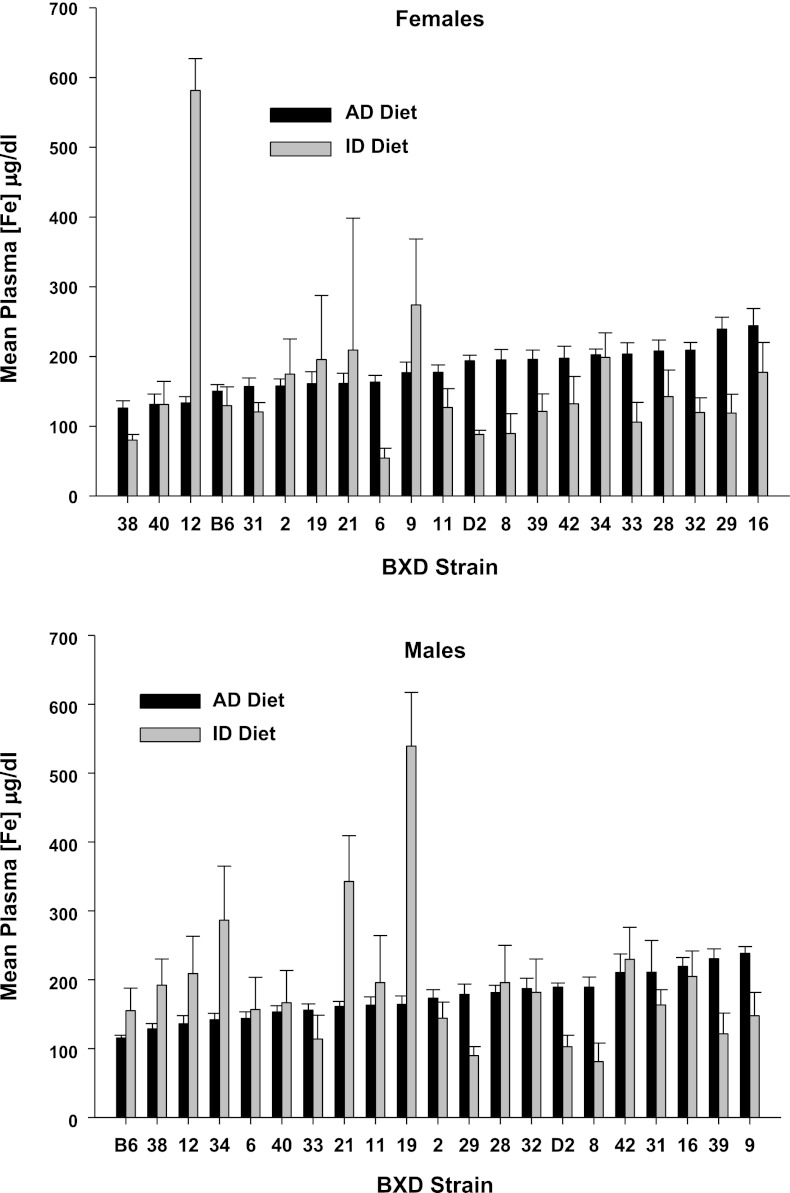
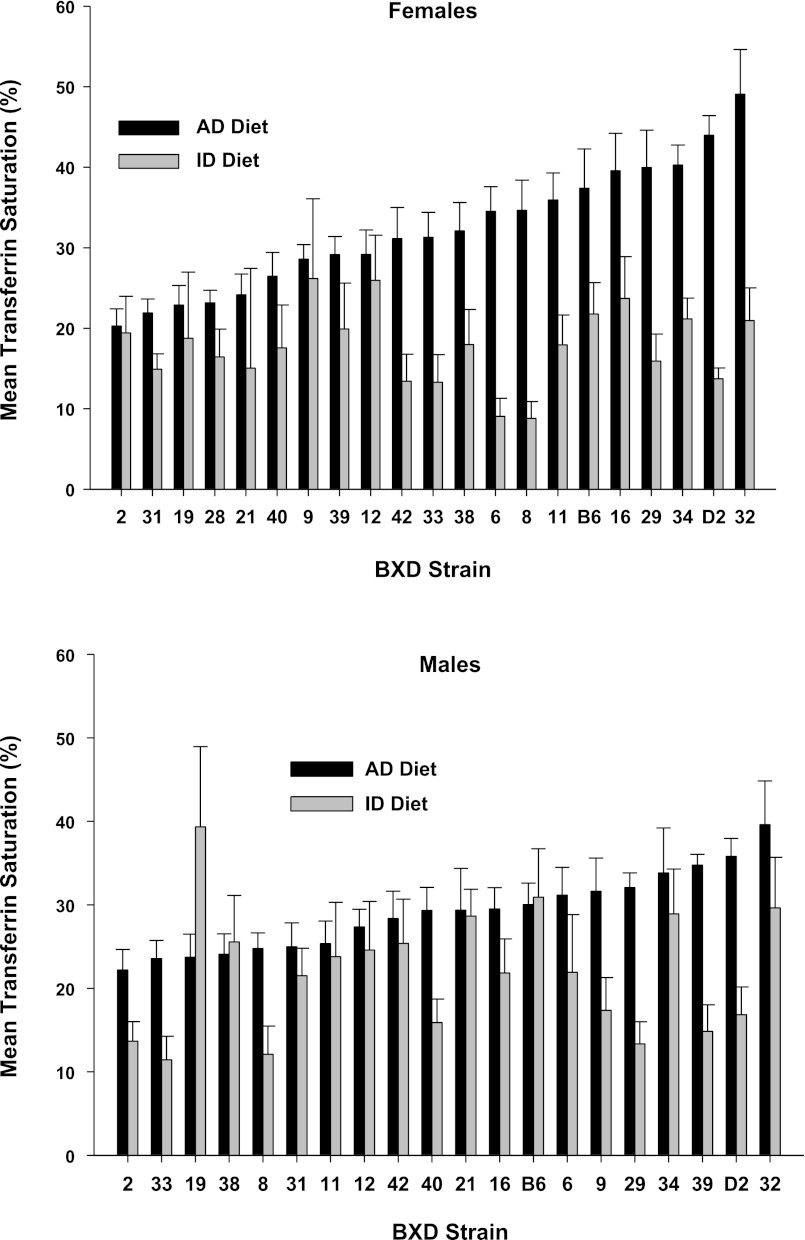
We observed continuous distributions of means among strains for each measure in each diet, as well as large strain differences in the degree of change in each measure due to ID diet, shown in Fig. 2 (hemoglobin), Fig. 3 (hematocrit), Fig. 4 (plasma iron), Fig. 5 (transferrin saturation), Fig. 6 (TIBC), Fig. 7 (liver iron), and Fig. 8 (spleen iron).
Fig. 2.
Hemoglobin values (g/dl) by strain, sex, and diet for mice fed an iron-poor (ID Diet; 3-ppm [Fe]) or an iron-adequate (AD Diet; 240-ppm [Fe]) diet. The animals were fed their respective diets between weaning at postnatal day 21 until 4 mo of age, or 100 days total. Data are expressed as means ± SE.
Fig. 3.
Hematocrit (% packed red blood cells) by strain, sex, and diet for mice fed an iron-poor (ID Diet; 3 ppm [Fe]) or iron-adequate (AD Diet; 240 ppm [Fe]) diet. The animals were fed their respective diets between weaning at postnatal day 21 until 4 mo of age, or 100 days total. Data are expressed as means ± SE.
Fig. 4.
Plasma [Fe] (μg/dl) by strain, sex, and diet for mice fed an iron-poor (ID Diet; 3 ppm [Fe]) or an iron-adequate (AD Diet; 240 ppm [Fe]) diet. The animals were fed their respective diets between weaning at postnatal day 21 until 4 mo of age, or 100 days total. Data are expressed as means ± SE.
Fig. 5.
Transferrin saturation (%) by strain, sex, and diet for mice fed an iron-poor (ID Diet; 3 ppm [Fe]) or an iron-adequate (AD Diet; 240 ppm [Fe]) diet. The animals were fed their respective diets between weaning at postnatal day 21 until 4 mo of age, or 100 days total. Data are expressed as means ± SE.
Fig. 6.
Total iron binding capacity (μg/dl) by strain, sex, and diet for mice fed an iron-poor (ID Diet; 3 ppm [Fe]) or an iron-adequate (AD Diet; 240 ppm [Fe]) diet. The animals were fed their respective diets between weaning at postnatal day 21 until 4 mo of age, or 100 days total. Data are expressed as means ± SE.
Fig. 7.
Liver [Fe] (μg/g wet tissue weight) by strain, sex, and diet for mice fed an iron-poor (ID Diet; 3 ppm [Fe]) or an iron-adequate (AD Diet; 240 ppm [Fe]) diet. The animals were fed their respective diets between weaning at postnatal day 21 until 4 mo of age, or 100 days total. Data are expressed as means ± SE.
Fig. 8.
Spleen [Fe] (μg/g wet tissue weight) by strain, sex, and diet for mice fed an iron-poor (ID Diet; 3 ppm [Fe]) or an iron-adequate (AD Diet; 240 ppm [Fe]) diet. The animals were fed their respective diets between weaning at postnatal day 21 until 4 mo of age, or 100 days total. Data are expressed as means ± SE.
Genetic Correlations Between Measures
Genetic correlations between strain means are presented in Table 4. For the AD diet, we observed significant correlations (all P < 0.05) between hemoglobin and hematocrit (in males and combined sexes), between transferrin saturation and TIBC (in both sexes), and between TIBC and plasma iron (in males and combined sexes) (Table 4). For ID diet, we observed significant correlations (all P < 0.05) between hemoglobin and hematocrit, between plasma Fe concentration and transferrin saturation, between spleen Fe concentration and hemoglobin, and between spleen Fe concentration and hematocrit, all in both sexes; also a significant correlation was found (P < 0.05) between TIBC and plasma Fe concentration in male and sex-pooled mice (Table 4).
Table 4.
Genetic correlations between measures for AD and ID diets
| Iron Measures | Males | Females | Males + Females |
|---|---|---|---|
| AD | |||
| Hb and Hct | r = 0.91 | n.s. | 0.772 |
| TIBC and TfS | r = −0.54 | −0.59 | −0.61 |
| TIBC and Plasma Fe | r = 0.69 | n.s. | 0.50 |
| ID | |||
| Hb and Hct | r = 0.93 | 0.85 | 0.89 |
| TIBC and Plasma Fe | r = 0.60 | n.s. | 0.52 |
| TfS and Plasma Fe | r = 0.82 | 0.67 | 0.63 |
| Hb and Spleen Fe | r = 0.64 | 0.67 | 0.66 |
| Hct and Spleen Fe | r = 0.66 | 0.54 | 0.54 |
These Pearson correlation coefficients were calculated using the strain means. These are genetic correlations because using strain means eliminates much of the environmentally based covariance. Hct, hematocrit; Hb, hemoglobin; TIBC, total iron binding capacity; TfS, transferrin saturation. Except where indicated, r values are significant at P < 0.05.
Genetic Correlations Between Sexes by Measures
For the AD diet, the correlation between males and females for hemoglobin was weak (r = 0.26, P > 0.05), but robust for all the other six measures (hematocrit: 0.59, TIBC: 0.86, TfS: 0.74, plasma Fe: 0.57, liver Fe: 0.62, and spleen Fe: 0.75, all P < 0.05). For the ID diet, the correlation between males and females was strong only for TIBC (0.71, P < 0.01) and were weak for the other six measures (all r <0.5, P > 0.05).
Principal Components Analysis
PCA is a method to show inter-relatedness among multiple measures, including latent associations (obscured by partial correlations). We performed PCA on strain means and not on the raw data for the seven measures. We first combined the data from both sexes by strain to explore for major factors involved in iron homeostasis for each diet. We then further analyzed the data of males and females separately for each diet to identify the major factors. Factor loadings (all eigenvalues >0.5) and the percentage of total phenotype variance accounted for by each factor are summarized in Table 5. Strain distribution graphs of the z scores for each factor are presented in Fig. 9.
Table 5.
Principal components analysis of the parameters by diet and sex
| Measure | AD Diet Males |
ID Diet Males |
AD Diet Females |
ID Diet Females |
AD Diet Sex Combined |
ID Diet Sex Combined |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | Factor 1 | Factor 2 | Factor 3 | Factor 1 | Factor 2 | Factor 3 | Factor 1 | Factor 2 | Factor 3 | Factor 1 | Factor 2 | Factor 3 | Factor 1 | Factor 2 | Factor 3 | |
| Tfs | 0.833 | 0.864 | 0.865 | 0.837 | 0.969 | |||||||||||||
| Plasma Fe | 0.983 | 0.958 | 0.708 | 0.931 | 0.641 | 0.787 | 0.526 | |||||||||||
| Hb | 0.864 | 0.89 | 0.913 | 0.762 | 0.5 | 0.902 | 0.891 | |||||||||||
| HCT | 0.861 | 0.893 | 0.81 | 0.746 | 0.881 | 0.855 | ||||||||||||
| TIBC | 0.628 | 0.56 | 0.727 | 0.65 | 0.922 | |||||||||||||
| Spleen Fe | 0.815 | 0.72 | 0.851 | 0.868 | 0.821 | |||||||||||||
| Liver Fe | 0.625 | 0.696 | 0.681 | 0.789 | 0.751 | 0.567 | 0.5 | |||||||||||
| % of Total Variance | 33.24 | 23.74 | 21.59 | 39.52 | 34.16 | 27.32 | 26.58 | 24.06 | 36.41 | 24.68 | 24.62 | 34.51 | 24.01 | 20.67 | 37.23 | 23.54 | 23.46 | |
This analysis was performed on the strain means for each of the measures and the correlation matrix evaluated by varimax rotation.
Fig. 9.
Strain distribution of principal component factors by dietary condition. Principal components analysis was performed on the means of the seven iron-related parameter estimates.
Quantitative Trait Loci Analysis of the Principal Components
QTL analysis was performed separately on the PCA factors identified for males and females. Table 6 summarizes this analysis and provides candidate genes identified by PosMed within each locus containing the gene symbol, gene description, chromosomal location, gene expression profiles, and correlation between expression and PCA.
AD, males.
FACTOR 1.
One weak QTL on chromosome 4 at 82.26 Mb (marker: rs6258088, LRS = 7.56) was observed. The bootstrap statistic is 27.7% with a width of about 3.8 Mb. One candidate gene, Ptprd, was nominated for this factor.
FACTOR 2.
One suggestive QTL on chromosome 10 at 118.41 Mb (marker: mcv25264026, LRS = 11.94) was observed. The bootstrap statistic is 40% with a width of about 4 Mb. One candidate gene, Mdm1, was nominated for this factor.
FACTOR 3.
One suggestive QTL on chromosome 14 at 41.43 Mb (marker: rs6314716, LRS = 12.54) was observed. The bootstrap statistic is 41.1%, and the width is about 2 Mb. No candidate gene was identified for this factor.
AD, females.
FACTOR 1.
We observed 1 weak QTL on chromosome 9 at 33.19 Mb (marker: rs6406454, LRS = 9.63). The bootstrap statistics is 22.2% with a width of about 2 Mb. No candidate gene was identified for this factor.
FACTOR 2.
No suggestive or significant QTL was observed.
FACTOR 3.
Two suggestive QTLs were observed, one on chromosome 7 at 95.16 Mb (marker: rs3672782, LRS = 15.07) with bootstrap statistics 55.4% (width around 0.5 Mb), and another one on chromosome 18 at 42.91 Mb (marker: rs13483326, LRS = 11.80) with bootstrap statistics 41.5% (width around 1.8 Mb). Two candidate genes, Picalm and Tcerg1, were identified for this factor.
ID, males.
FACTOR 1.
No suggestive or significant QTL was observed.
FACTOR 2.
No suggestive or significant QTL was observed.
ID, females.
FACTOR 1.
We observed one significant QTL, on chromosome 7 at 95.15 Mb (marker: rs3672782, LRS = 16.92) with bootstrap statistic of 56.3% (width around 0.8 Mb), and two suggestive QTL, one on chromosome 17 at 65.34 Mb (marker: rs13483071, LRS = 12.49), and another one on chromosome 18 at 42.91 Mb (marker: rs13483326, LRS = 11.53), with bootstrap statistics 46.1% (width around 0.5 Mb) and 33.25% (width around 3 Mb), respectively. Two (markers: rs3672782 and rs13483326) of the three identified QTLs are identical with the factor 3 in AD females.
FACTOR 2.
We observed two suggestive QTLs, one on chromosome 15 at 8.25 Mb (marker: rs13482420, LRS = 13.46) and the other one on chromosome 19 at 30.05 Mb (marker: rs13483589, LRS = 12.22). The bootstrap statistics are 37% (width around 2 Mb) and 36.4% (width around 2 Mb), respectively. Two candidate genes were identified for this factor: Skp2 and Lip1.
FACTOR 3.
We observed one suggestive QTL on chromosome 2 at 82.83 Mb (marker: rs13476608, LRS = 10.33). The bootstrap statistic is 45.7% with the width about 1 Mb. One candidate gene was nominated for this factor, Frzb.
DISCUSSION
This is the first study of its kind on dietary iron deficiency using a systems biology/genetics approach. By feeding animals from a genetic reference population diets from weaning until 4 mo of age, our aim was to model individual differences in susceptibility to the effects of dietary iron deficiency. In fact, some of the strains appeared to meet the criteria for iron-deficient anemia, i.e., increased TfS, and decreased hemaglobin, hematocrit, TIBC, and iron concentration in plasma, liver, and spleen, while other strains appeared to be unaffected by the iron-deficient diet. For plasma iron, females in one strain and males in at least four strains showed dramatic increases under iron deficiency. The extent to which this represented hemolysis, fragile erythrocytes, or leaching of iron from some other compartment is unknown. We do not know what the status of this increased iron is, bound, loosely bound, or unbound; however, as Kell (32) recently pointed out, loosely bound iron poses a problem and may be an etiological factor for cardiovascular and inflammation-related diseases. Moreover, it may contribute, together with environmental toxicants, to neurodegenerative disease, such as Parkinson's disease (75). One more remarkable finding was that the iron-poor diet produced varying degrees of splenomegaly, an observation previously reported by us (23). Iron-deficient diets also reduced body weight in most strains, and this was related to reduced hematocrits. This indicates that insufficient iron availability in the postnatal period affects overall bodily growth.
The wide variation in each measure across the lines suggests that iron management is a complex trait under the influence of multiple genes and that this genetic effect interacts with dietary iron intake. The large between-strain variations in the response to the ID diet can be used to elucidate mechanisms underlying individual differences in susceptibility to iron-deficiency anemia and other iron deficiency-related disorders in humans (2, 6, 7, 50).
From a systems biology perspective, the ID diet altered PCA factor structures compared with the iron-adequate diet, recruiting at least one of the two iron storage depots (spleen, liver) to the first factor in both sexes. This is consistent with the fact that an iron-poor diet mobilizes iron availability from storage compartments. Exactly what physiological mechanisms account for the changes in the other PCA factor structures remain to be seen, but this observation may provide insight into understanding the pathophysiology of iron deficiency with or without anemia.
Principal Components Analysis and Candidate Genes
PCA has been shown to be useful for identifying apparent and latent composite variables that influence composite traits (29, 60). This is particularly valuable for genetic mapping of synthetic variables involved in biological systems, such as iron homeostasis. During the course of our QTL analysis, we identified 7 candidate genes within the loci detected. Moreover, we propose that polymorphisms in these genes at least partially underlie individual differences in the regulation of iron homeostasis and susceptibility to the effects of an iron-poor diet.
The expression of two genes, Ptprd and Mdm1, were significantly correlated with PCA factor 1 (hemoglobin and hematocrit) and PCA factor 2 (plasma Fe and TIBC), respectively in male mice fed the AD diet. Ptprd encodes for protein tyrosine phosphatase receptor type D, which is a key regulator in brain development, specifically in the process of neurogenesis (54) and migration of cortical interneurons (18). Genome-wide association studies in humans have revealed PTPRD as a risk factor for restless legs syndrome (RLS) (57, 74); however, the mechanism accounting for its role in RLS is currently unknown. One of the pathological characteristics of RLS is low iron content in the substantia nigra, and a significant percentage of patients with RLS are responsive to iron treatment (9, 13, 66). The single nucleotide polymorphism (SNP) density near Ptprd in BXD RI mice is high (3,476), thereby making it a good candidate underlying individual differences in iron regulation.
Mdm1 encodes a nuclear protein, transformed mouse 3T3 cell double minute 1, and its involvement in iron homeostasis might be mediated by another gene Ifng (interferon gamma), which regulates iron homeostasis in inflammation. Indeed, Mdm1 has been reported to be in linkage disequilibrium with Ifng on chromosome 10 among inbred mouse strains (63). During inflammation, Ifng activates macrophages (37) and regulates expression of the genes for TfRs (33), and the hormone hepcidin (20), resulting in alteration of macrophages iron recycling and iron metabolism (33, 61, 62). Depressions in plasma iron and in the ratio of plasma iron to TIBC are common signs of inflammation-related anemia (76). Notably, the expression of Mdm1 was significantly correlated with these two iron parameters (PCA factor 2 loadings in AD males) in our study. Thus, we nominate Mdm1 as a candidate gene involved in iron homeostasis and the polymorphism of Mdm1 might give us a clue to better understand the crosstalk among iron homeostasis, predisposition to iron deficiency, inflammation, and anemia.
Picalm (phosphatidylinositol-binding clathrin assembly protein), and Tcerg1 (transcription elongation regulator 1, also known as CA150) are the two candidate genes whose expression are significantly correlated with PCA factor 3 (loadings: transferrin saturation and liver Fe) in AD female mice. We hypothesize that these two genes are involved in iron homeostasis by regulating clathrin-mediated endocytosis (CME) of iron (transferrin) and the downstream cellular utilization of endocytosed iron. Erythorid cells acquire iron via CME of transferrin (71). Picalm is involved in clathrin-mediated endocytosis (CME) (64) and has recently been shown to be crucial in hematopoiesis and iron metabolism (34). Klebig et al. (34) reported that Picalmfit1 mutations resulted in truncated Picalm protein, which alters transferrin endocytosis and subsequent cellular iron uptake and iron transport from hemoglobin and the liver to other tissues. This is consistent with our PCA/QTL finding that Picalm expression variation among strains contributed to strain variation in transferrin saturation and liver Fe concentration in AD females; moreover, the SNP density of Picalm in BXD mice is 300.
Tcerg1 might be involved in CME of iron (likely by interacting with another protein, Htt). Htt, coding for huntingtin, is the causative gene in Huntington's disease, and the disruption of iron homeostasis has been well characterized in Huntington's disease (28, 48). Both in vivo and in vitro experiments show that the WW domain containing protein Tcerg1 can interact with Htt (27). Dysfunction of Tcerg1 causes binding and coaggregation with Htt in striatal and cortical neurons as Huntington's disease progresses and Tcerg1 overexpression can rescue striatal cell death when Htt mutations exist (4). Htt is ubiquitously expressed throughout the body with its exact physiological function unclear. Recent Htt knockout studies in zebrafish showed that Htt deficiency causes a variety of developmental defects, including abnormal iron homeostasis with evidence of reduced hemoglobin production, increased transferrin receptor 1 transcription, and decreased accessibility of endocytosed iron for cellular use (24, 45). Even though there is no evidence so far showing the involvement of Tcerg1 or the interaction between Tcerg1 and Htt in iron homeostasis, nevertheless, we believe that to rule out Tcerg1 at this time may be premature.
Two candidate genes were identified to account for PCA factor 2 (loadings: transferrin saturation and plasma iron concentration) in ID female mice: Skp2 (S-phase kinase-associated protein 2) and Lip1 (lysosomal acid lipase 1). Skp2 is an F-box-containing ubiquitin ligase required for p21 (cyclin-dependent kinase inhibitor 1A) proteolysis, which controls the cell cycle G1/S transition, including during hematopoiesis (3, 56). Moreover, it has recently been reported that iron depletion alters p21 expression and blocks cells at the G1/S interface, subsequently inhibiting hematopoiesis (1, 22, 38). Thus polymorphisms of Skp2 could alter p21 proteolysis, thereby altering hematopoiesis and causing abnormal iron metabolism as a consequence. Lip1 knockout mice have been shown to develop macrophage malfunction (73), considering the crucial role of macrophages in iron regulation (35, 67); we thus nominate Lip1 as the candidate gene.
The candidate gene identified for factor 3 (loading: hemoglobin) in ID female mice is Frzb, a secreted frizzled-related protein that antagonizes Wnt signaling by regulating Wnt5a activity (15, 41, 42). The coordination between Frzb and Wnt5a has been shown to play an important role in inner ear development (41), and cancer progression (15, 36, 59). No effect of Frzb or Wnt5a on iron homeostasis has yet to be reported. Wnt5a signaling has, however, been shown to be important in erythropoiesis during development (65). Considering the relationship between erythropoiesis and iron regulation (72, 8), we nominated Frzb as the candidate gene.
Perspectives and Significance
Forward genetic analysis of complex traits in genetic reference populations of animals has opened the inquiry into polygenic influence on important biological functions. QTL mapping is a valuable resource for elucidating the mechanisms underlying individual differences in iron biology. Recently, McLachlan et al. (46) identified several candidate genes related to strain differences in basal liver iron inbred mice using haplotype analysis.
Iron deficiency and its consequences constitute a major public health problem worldwide. We now report that individual differences in susceptibility to its effects are driven, in part, by genetics, and we have identified candidate genes that may mediate this susceptibility. While our bioinformatics analysis points to specific candidate genes, functional validation of these findings in animals will be needed to pinpoint their involvement in iron regulation. One major implication of this work is the promise of follow-up on human genome-wide association studies. One such study was reported by McLaren et al. (47). Considering that the mouse and human genomes are more than 90% syntenic, what we find in humans can inform the mouse genome and help us to elucidate biological mechanisms involved in individual differences in susceptibility to iron deficiency.
GRANTS
This study was supported, in part, by National Institues of Health Grants PO1 AG-021190 to C. J. Earley, ES-019103 to J. C. Fleet, a NRSA Fellowship F31NS060393 to L. C. Jellen and a grant from the Restless Legs Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: L.Y., E.L.U., L.C.J., and A.T. performed experiments; L.Y. and L.C.J. analyzed data; L.Y., E.L.U., R.P.A., J.C.F., and B.C.J. interpreted results of experiments; L.Y. prepared figures; L.Y. and B.C.J. drafted manuscript; L.Y., E.L.U., L.C.J., C.J.E., J.C.F., and B.C.J. edited and revised manuscript; B.C.J. conception and design of research; B.C.J. approved final version of manuscript.
REFERENCES
- 1. Alcantara O, Boldt DH. Iron deprivation blocks multilineage haematopoietic differentiation by inhibiting induction of p21(WAF1/CIP1). Br J Haematol 137: 252–261, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Allen RP. Race, iron status and restless legs syndrome. Sleep Med 3: 467–468, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Amador V, Ge S, Santamaría PG, Guardavaccaro D, Pagano M. APC/C(Cdc20) controls the ubiquitin-mediated degradation of p21 in prometaphase. Mol Cell 27: 462–473, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arango M, Holbert S, Zala D, Brouillet E, Pearson J, Régulier E, Thakur AK, Aebischer P, Wetzel R, Déglon N, Néri C. CA150 expression delays striatal cell death in overexpression and knock-in conditions for mutant huntingtin neurotoxicity. J Neurosci 26: 4649–459, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belknap JK. Effect of within-strain sample size on QTL detection mapping using recombinant inbred mouse strains. Behav Genet 28: 29–38, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Bothwell TH, Charlton RW, Cook JD, Finch CA. Iron Metabolism in Man. Oxford, UK: Blackwell Scientific, 1979 [Google Scholar]
- 7. Brittenham G. Disorders of Iron Metabolism: Iron Deficiency and Iron Overload. In: Hematology: Basic Principles and Practice. 5th ed., edited by Hoffman R., Benz EJ., Shattil SJ., Furie B., Silberstein LE., McGlave P., Heslop H. Philadelphia, PA: Elsevier Churchill Livingstone, 2008 [Google Scholar]
- 8. Camaschella C, Pagani A. Iron and erythropoiesis: a dual relationship. Int J Hematol 93: 21–26, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Catoire H, Dion PA, Xiong L, Amari M, Gaudet R, Girard SL, Noreau A, Gaspar C, Turecki G, Montplaisir JY, Parker JA, Rouleau GA. Restless legs syndrome-associated MEIS1 risk variant influences iron homeostasis. Ann Neurol 70: 170–175, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Cheng Y, Zak O, Aisen P, Harrison SC, Walz T. Structure of the human transferrin receptor-transferrin complex. Cell 116: 565–576, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Coe CL, Lubach GR, Shirtcliff EA. Maternal stress during pregnancy predisposes for iron deficiency in infant monkeys impacting innate immunity. Pediatr Res 61: 520–524, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Connor JR, Ponnuru P, Lee BY, Podskalny GD, Alam S, Allen RP, Earley CJ, Yang QX. Postmortem and imaging based analyses reveal CNS decreased myelination in restless legs syndrome. Sleep Med 12: 614–619, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Connor JR, Ponnuru P, Wang XS, Patton SM, Allen RP, Earley CJ. Profile of altered brain iron acquisition in restless legs syndrome. Brain 134: 959–968, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cook GA, King MT, Veech RL. Changes in liver inorganic pyrophosphate content during ethanol metabolism. Adv Exp Med Biol 132: 433–440, 1980 [DOI] [PubMed] [Google Scholar]
- 15. Ekström EJ, Sherwood V, Andersson T. Methylation and loss of secreted frizzled-related protein 3 enhances melanoma cell migration and invasion. PLoS One 6: e18674, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Erikson KM, Jones BC, Hess EJ, Zhang Q, Beard JL. Iron deficiency decreases dopamine D1 and D2 receptors in rat brain. Pharmacol Biochem Behav 69: 409–418, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Erikson KM, Pinero DJ, Connor JR, Beard JL. Regional brain iron, ferritin and transferrin concentrations during iron deficiency and iron repletion in developing rats. J Nutr 127: 2030–2038, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Faux C, Rakic S, Andrews W, Yanagawa Y, Obata K, Parnavelas JG. Differential gene expression in migrating cortical interneurons during mouse forebrain development. J Comp Neurol 518: 1232–1248, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Fleming MD, Andrews NC. Mammalian iron transport: an unexpected link between metal homeostasis and host defense. J Lab Clin Med 132: 464–468, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Frazier MD, Mamo LB, Ghio AJ, Turi JL. Hepcidin expression in human airway epithelial cells is regulated by interferon-γ. Respir Res 12: 100, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gaetano C, Massimo L, Alberto M. Control of iron homeostasis as a key component of macrophage polarization. Haematologica 95: 1801–1803, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gazitt Y, Reddy SV, Alcantara O, Yang J, Boldt DH. A new molecular role for iron in regulation of cell cycling and differentiation of HL-60 human leukemia cells: iron is required for transcription of p21(WAF1/CIP1) in cells induced by phorbol myristate acetate. J Cell Physiol 187: 124–135, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Gibson JN, Jellen LC, Unger EL, Morahan G, Mehta M, Earley CJ, Allen RP, Lu L, Jones BC. Genetic analysis of iron-deficiency effects on the mouse spleen. Mamm Genome 22: 556–562, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Henshall TL, Tucker B, Lumsden AL, Nornes S, Lardelli MT, Richards RI. Selective neuronal requirement for huntingtin in the developing zebrafish. Hum Mol Genet 18: 4830–4842, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell 117: 285–297, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Hitzemann R, Reed C, Malmanger B, Maureen L, Hitzemann B, Cunningham B, McWeeney S, Belknap J, Harrington C, Buck K, Phillips T, Crabbe J. On the integration of alcohol-related quantitative trait loci and gene expression analyses. Alcohol Clin Exp Res 28: 1437–1448, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Holbert S, Denghien I, Kiechle T, Rosenblatt A, Wellington C, Hayden MR, Margolis RL, Ross CA, Dausset J, Ferrante RJ, Néri C. The Gln-Ala repeat transcriptional activator CA150 interacts with huntingtin: neuropathologic and genetic evidence for a role in Huntington's disease pathogenesis. Proc Natl Acad Sci USA 98: 1811–1816, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Imarisio S, Carmichael J, Korolchuk V, Chen CW, Saiki S, Rose C, Krishna G, Davies JE, Ttofi E, Underwood BR, Rubinsztein DC. Huntington's disease: from pathology and genetics to potential therapies. Biochem J 412: 191–209, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Jones BC, Beard JL, Gibson JN, Unger EL, Allen RP, McCarthy KA, Earley CJ. Systems genetic analysis of peripheral iron parameters in the mouse. Am J Physiol Regul Integr Comp Physiol 293: R116–R124, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Jones BC, Reed CL, Hitzemann R, Wiesinger JA, McCarthy KA, Buwen JP, Beard JL. Quantitative genetic analysis of ventral midbrain and liver iron in BXD recombinant inbred mice. Nutr Neurosci 6: 369–377, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Kaplan J, Ward DM, De Domenico I. The molecular basis of iron overload disorders and iron-linked anemias. Int J Hematol 93: 14–20, 2011 [DOI] [PubMed] [Google Scholar]
- 32. Kell DB. Towards a unifying, systems biology understanding of large-scale cellular death and destruction caused by poorly liganded iron: Parkinson's, Huntington's, Alzheimer's, prions, bactericides, chemical toxicology and others as examples. Arch Toxicol 84: 825–889, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim S, Ponka P. Effects of interferon-gamma and lipopolysaccharide on macrophage iron metabolism are mediated by nitric oxide-induced degradation of iron regulatory protein 2. J Biol Chem 275: 6220–6226, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Klebig ML, Wall MD, Potter MD, Rowe EL, Carpenter DA, Rinchik EM. Mutations in the clathrin-assembly gene Picalm are responsible for the hematopoietic and iron metabolism abnormalities in fit1 mice. Proc Natl Acad Sci USA 100: 8360–8365, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci USA 102: 1324–1328, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kongkham PN, Northcott PA, Croul SE, Smith CA, Taylor MD, Rutka JT. The SFRP family of WNT inhibitors function as novel tumor suppressor genes epigenetically silenced in medulloblastoma. Oncogene 29: 3017–3024, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Konijn AM. Iron metabolism in inflammation. Baillieres Clin Haematol 7: 829–849, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Kramer JL, Baltathakis I, Alcantara OS, Boldt DH. Differentiation of functional dendritic cells and macrophages from human peripheral blood monocyte precursors is dependent on expression of p21 (WAF1/CIP1) and requires iron. Br J Haematol 117: 727–734, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Lee AL, Okam MM. Anemia in pregnancy. Hematol Oncol Clin N Am 25: 241–259, 2011 [DOI] [PubMed] [Google Scholar]
- 40. Lee JW. Serum iron in catatonia and neuroleptic malignant syndrome. Biol Psychiatry 44: 499–507, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Liu W, Li L, Li G, Garritano F, Shanske A, Frenz DA. Coordinated molecular control of otic capsule differentiation: functional role of Wnt5a signaling and opposition by sfrp3 activity. Growth Factors 26: 343–354, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Lories RJ, Peeters J, Bakker A, Tylzanowski P, Derese I, Schrooten J, Thomas JT, Luyten FP. Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis Rheum 56: 4095–4103, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Lozoff B. Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. J Nutr 141: 740S–746S, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lukowski AF, Koss M, Burden MJ, Jonides J, Nelson CA, Kaciroti N, Jimenez E, Lozoff B. Iron deficiency in infancy and neurocognitive functioning at 19 years: evidence of long-term deficits in executive function and recognition memory. Nutr Neurosci 13: 54–70, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lumsden AL, Henshall TL, Dayan S, Lardelli MT, Richards RI. Huntingtin-deficient zebrafish exhibit defects in iron utilization and development. Hum Mol Genet 16: 1905–1920, 2007 [DOI] [PubMed] [Google Scholar]
- 46. McLachlan S, Lee SM, Steele TM, Hawthorne PL, Zapala MA, Eskin E, Schork NJ, Anderson GJ, Vulpe CD. In Silico QTL mapping of basal liver iron levels in inbred mouse strains. Physiol Genomics 43: 136–147, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McLaren CE, Garner CP, Constantine CC, McLachlan S, Vulpe CD, Snively BM, Gordeuk VR, Nickerson DA, Cook JD, Leiendecker-Foster C, Beckman KB, Eckfeldt JH, Barcellos LF, Murray JA, Adams PC, Acton RT, Killeen AA, McLaren GD. Genome-wide association study identifies genetic loci associated with iron deficiency. PLoS One 6: e17390, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morrison PJ, Nevin NC. Serum iron, total iron binding capacity and ferritin in early Huntington disease patients. Ir J Med Sci 163: 236–237, 1994 [DOI] [PubMed] [Google Scholar]
- 49. Mullick S, Rusia U, Sikka M, Faridi MA. Impact of iron deficiency anaemia on T lymphocytes and their subsets in children. Indian J Med Res 124: 647–654, 2006 [PubMed] [Google Scholar]
- 50. Muñoz M, García-Erce JA, Remacha ÁF. Disorders of iron metabolism. Part II: iron deficiency and iron overload. J Clin Pathol 64: 287–296, 2011 [DOI] [PubMed] [Google Scholar]
- 51. Nicolas G, Bennoun M, Porteu A, Mativet S, Beaumont C, Grandchamp B, Sirito M, Sawadogo M, Kahn A, Vaulont S. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci USA 99: 4596–4601, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Peirano PD, Algarín CR, Chamorro RA, Reyes SC, Durán SA, Garrido M, Lozoff B. Sleep alterations and iron deficiency anemia in infancy. Sleep Med 11: 637–642 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loreal O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem 276: 7811–7819, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Reinhard J, Horvat-Bröcker A, Illes S, Zaremba A, Knyazev P, Ullrich A, Faissner A. Protein tyrosine phosphatases expression during development of mouse superior colliculus. Exp Brain Res 199: 279–297, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rosebush PI, Mazurek MF. Serum iron and neuroleptic malignant syndrome. Lancet 338: 149–151, 1991 [DOI] [PubMed] [Google Scholar]
- 56. Sarmento LM, Huang H, Limon A, Gordon W, Fernandes J, Tavares MJ, Miele L, Cardoso AA, Classon M, Carlesso N. Notch1 modulates timing of G1-S progression by inducing SKP2 transcription and p27 Kip1 degradation. J Exp Med 202: 157–168, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schormair B, Kemlink D, Roeske D, Eckstein G, Xiong L, Lichtner P, Ripke S, Trenkwalder C, Zimprich A, Stiasny-Kolster K, Oertel W, Bachmann CG, Paulus W, Högl B, Frauscher B, Gschliesser V, Poewe W, Peglau I, Vodicka P, Vávrová J, Sonka K, Nevsimalova S, Montplaisir J, Turecki G, Rouleau G, Gieger C, Illig T, Wichmann HE, Holsboer F, Müller-Myhsok B, Meitinger T, Winkelmann J. PTPRD (protein tyrosine phosphatase receptor type delta) is associated with restless legs syndrome. Nat Genet 40: 946–948, 2008 [DOI] [PubMed] [Google Scholar]
- 58. Shafir T, Angulo-Barroso R, Su J, Jacobson SW, Lozoff B. Iron deficiency anemia in infancy and reach and grasp development. Infant Behav Dev 32: 366–375, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shi Y, He B, You L, Jablons DM. Roles of secreted frizzled-related proteins in cancer. Acta Pharmacol Sin 28: 1499–1504, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Shlens J. A tutorial on principal component analysis. http://www.snl.salk.edu/∼shlens/pca.pdf, 2009
- 61. Sow FB, Alvarez GR, Gross RP, Satoskar AR, Schlesinger LS, Zwilling BS, Lafuse WP. Role of STAT1, NF-κB, and C/EBPbeta in the macrophage transcriptional regulation of hepcidin by mycobacterial infection and IFN-γ. J Leukoc Biol 86: 1247–1258, 2009 [DOI] [PubMed] [Google Scholar]
- 62. Sow FB, Florence WC, Satoskar AR, Schlesinger LS, Zwilling BS, Lafuse WP. Expression and localization of hepcidin in macrophages: a role in host defense against tuberculosis. J Leukoc Biol 82: 934–945, 2007 [DOI] [PubMed] [Google Scholar]
- 63. Taylor BA, Rowe L, Grieco D. Close linkage of Mdm-1, a gene amplified and overexpressed in a transformed 3T3 cell line, with gamma interferon (Ifg) on chromosome 10 of the mouse. Mamm Genome 3: 700–704, 1992 [DOI] [PubMed] [Google Scholar]
- 64. Tebar F, Bohlander SK, Sorkin A. Clathrin assembly lymphoid myeloid leukemia (CALM) protein: localization in endocytic-coated pits, interactions with clathrin, and the impact of overexpression on clathrin-mediated traffic. Mol Biol Cell 10: 2687–2702, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tsiftsoglou AS, Vizirianakis IS, Strouboulis J. Erythropoiesis: model systems, molecular regulators, and developmental programs. IUBMB Life 61: 800–830, 2009 [DOI] [PubMed] [Google Scholar]
- 66. Wang J, O'Reilly B, Venkataraman R, Mysliwiec V, Mysliwiec A. Efficacy of oral iron in patients with restless legs syndrome and a low-normal ferritin: A randomized, double-blind, placebo-controlled study. Sleep Med 10: 973–975, 2009 [DOI] [PubMed] [Google Scholar]
- 67. Wang J, Pantopoulos K. Regulation of cellular iron metabolism. Biochem J 434: 365–381, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Weinstein DA, Roy CN, Fleming MD, Loda MF, Wolfsdorf JI, Andrews NC. Inappropriate expression of Hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood 100: 3776–3781, 2002 [DOI] [PubMed] [Google Scholar]
- 69. Williams RW, Gu J, Qi S, Lu L. The genetic structure of recombinant inbred mice: high-resolution consensus maps for complex trait analysis. Genome Biol 2: 0046.1–0046.18, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Williams RW. Significant threshold. From the WebQTL Glossary—A GeneNetwork Resource. The data are available at http://www.genenetwork.org/glossary.html, 2004
- 71. Wingert RA, Brownlie A, Galloway JL, Dooley K, Fraenkel P, Axe JL, Davidson AJ, Barut B, Noriega L, Sheng X, Zhou Y, Zon LI. The chianti zebrafish mutant provides a model for erythroid-specific disruption of transferrin receptor 1. Development 131: 6225–6235, 2004 [DOI] [PubMed] [Google Scholar]
- 72. Wrighting DM, Andrews NC. Iron homeostasis and erythropoiesis. Curr Top Dev Biol 82: 141–167, 2008 [DOI] [PubMed] [Google Scholar]
- 73. Yan C, Lian X, Li Y, Dai Y, White A, Qin Y, Li H, Hume DA, Du H. Macrophage-specific expression of human lysosomal acid lipase corrects inflammation and pathogenic phenotypes in lal-/- mice. Am J Pathol 169: 916–926, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yang Q, Li L, Yang R, Shen GQ, Chen Q, Foldvary-Schaefer N, Ondo WG, Wang QK. Family-based and population-based association studies validate PTPRD as a risk factor for restless legs syndrome. Mov Disord 26: 516–519, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yin L, Lu L, Prasad K, Richfield EK, Unger EL, Xu J, Jones BC. Genetic-based, differential susceptibility to paraquat neurotoxicity in mice. Neurotoxicol Teratol 33: 415–421, 2011 [DOI] [PubMed] [Google Scholar]
- 76. Yip R, Dallman PR. The roles of inflammation and iron deficiency as causes of anemia. Am J Clin Nutr 48: 1295–1300, 1988 [DOI] [PubMed] [Google Scholar]