Abstract
An exaggerated exercise pressor reflex (EPR) contributes to exercise intolerance and excessive sympathoexcitation in the chronic heart failure (CHF) state, which is prevented by exercise training (ExT) at an early stage in the development of CHF. We hypothesized that ExT has a beneficial effect on the exaggerated EPR by improving the dysfunction of muscle afferents in CHF. We recorded the discharge of mechanically sensitive (group III) and metabolically sensitive (group IV) afferents in response to static contraction, passive stretch, and hindlimb intra-arterial injection of capsaicin in sham+sedentary (Sed), sham+ExT, CHF+Sed, and CHF+ExT rats. Compared with sham+Sed rats, CHF+Sed rats exhibited greater responses of group III afferents to contraction and stretch, whereas the responses of group IV afferents to contraction and capsaicin were blunted. ExT prevented the sensitization of group III responses to contraction or stretch and partially prevented the blunted group IV responses to contraction or capsaicin in CHF rats. Furthermore, we investigated whether purinergic 2X (P2X) and transient receptor potential vanilloid 1 (TRPV1) receptors mediate the altered sensitivity of muscle afferents by ExT in CHF. We found that the upregulated P2X and downregulated TRPV1 receptors in L4/5 dorsal root ganglia of CHF rats were normalized by ExT. Hindlimb intra-arterial infusion of a P2X antagonist attenuated the group III response to contraction or stretch in CHF rats to a greater extent than in sham rats, which was normalized by ExT. These findings suggest that ExT improves the abnormal sensitization of muscle afferents in CHF at least, in part, via restoring the dysfunction of P2X and TRPV1 receptors.
Keywords: electrophysiology, C fiber, decerebration
a hallmark of patients suffering from chronic heart failure (CHF) is sympathoexcitation and exercise intolerance (3, 7, 42, 48). Even during moderate exercise, extreme activation of the sympathetic nervous system causes an exaggerated pressor response and hyperventilation. Although the mechanisms responsible for the increase in sympathetic outflow and exercise intolerance in CHF are not known, it has recently been suggested that this may be due, in part, to an exaggerated exercise pressor reflex (EPR) (8, 32, 39).
The EPR is a peripheral neural reflex originating in skeletal muscle that contributes significantly to the regulation of the cardiovascular system during exercise. The afferent arm of this reflex is composed of metabolically sensitive (predominantly group IV, C-fibers) and mechanically sensitive (predominately group III, A-δ fibers) afferent fibers (4, 15, 16, 24). Evidence from animal studies has demonstrated that increases in heart rate (HR), arterial pressure (AP), and sympathetic nerve activity in response to activation of this reflex are enhanced in rats with CHF induced by myocardial infarction (MI) (20, 36, 38, 39). In addition, these studies suggest that an enhanced mechanical component of this reflex (i.e., mechanoreflex) contributes to the exaggerated EPR in CHF, whereas the metaboreflex is blunted (21, 40, 41). Furthermore, our recent study (46) showed that the an enhanced mechanoreflex and blunted metaboreflex are, at least in part, due to altered sensitivities of skeletal muscle afferents in the CHF state. This includes an increased sensitivity of mechanically sensitive (group III) afferents and a blunted sensitivity of metabolically sensitive (group IV) afferents. An exaggerated EPR-induced sympathoexcitation can potentially increase cardiovascular risk and contribute to exercise intolerance during physical activity in CHF patients (8, 32, 39). A therapeutic strategy for preventing or slowing the progression of the exaggerated EPR may be beneficial in CHF patients.
Accumulating evidence suggests that long-term exercise training (ExT) as a nonpharmacological treatment for CHF increases exercise capacity, reduces sympathoexcitation, and improves cardiovascular function in CHF animals and patients (2, 18, 29). Furthermore, our recent study (46) showed that the exaggerated EPR in the CHF state can be prevented by ExT at an early stage of CHF (2 wk after coronary ligation). However, the underlying central and peripheral mechanisms by which ExT prevents the exaggerated EPR in the CHF state remain to be identified. ExT improves peripheral skeletal myopathy (e.g., muscle atrophy, decreased peripheral blood flow, fiber-type transformation, and reduced oxidative capacity) in CHF (9, 14). Therefore, it is likely that ExT affects the sensitivity of muscle afferent endings in the CHF state. To test this hypothesis, we took advantage of the technique of single fiber recording to investigate the effect of ExT at an early stage of CHF on the sensitivity of group III and IV afferents in sham-operated and CHF rats. Furthermore, based on our previous study (44) indicating that purinergic 2X receptor (P2X) and the transient receptor potential vanilloid 1 (TRPV1) receptors mediate the altered sensitivity of muscle afferents in the CHF state, we also investigated whether ExT prevented the dysfunction of P2X and TRPV1 receptors in the CHF state.
METHODS
Experiments were performed on male Sprague-Dawley rats weighing 360 to 540 g. These experiments were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center and carried out under the guidelines of the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” [DHEW Publication No. (NIH) 85-23, Revised 1996, Office of Science and Health Reports, DRR/NIH, Bethesda, MD 20205].
Model of CHF.
CHF was produced by left coronary artery ligation; sham-operated rats were prepared in the same manner but did not undergo coronary artery ligation. The procedure was described in our previous studies (44, 46). Briefly, the rat was ventilated at a rate of 60 breaths/min with 3% isoflurane during the surgical procedure. A left thoracotomy was performed through the fifth intercostal space, the pericardium was opened, the heart was exteriorized, and the left anterior descending coronary artery was ligated. Sham-operated rats were prepared in the same manner but did not undergo coronary artery ligation. All the rats survived the sham surgery. However, ∼70% of rats survived the coronary artery ligation surgery.
In this study, the cardiac function in all experimental animals was measured by echocardiography (VEVO 770, Visual Sonics) as previously described. In addition, at the end of each acute experiment, a Millar catheter (SPR 524; size, 3.5-Fr; Millar Instruments, Houston, TX) was advanced through the carotid artery into the left ventricle (LV) to determine LV end-diastolic pressure (LVEDP) and LV systolic pressure (LVP). The rats were then euthanized with an overdose of pentobarbital sodium. The hearts and lungs were removed, and the ratio of the infarct area to whole LV minus septum was measured.
Exercise training protocol.
All animals were divided into four groups: sham+sedentary (Sed) (n = 42), sham+ExT (n = 42), CHF+Sed (n = 42), and CHF+ExT (n = 46). Rats (CHF, n = 3; Sham, n = 2) that were unwilling to run steadily on the treadmill were excluded from the analysis. Rats were treadmill trained as described previously by us and others (27, 30). Generally, ExT was started 2 wk after coronary ligation or sham operation. Rats ran at an initial speed of 10 m/min, 0% grade; 10–15 min/day during the first week. The speed and grade of treadmill were gradually increased to 25 m/min and 10% grade, and the exercise duration was increased to 60 min/day during the second and third week. Since the fourth week, Sham and CHF rats had the same average period of time and total workload (25 m/min, 10% grade, 60 min/day, 5 days/wk, 5–7 wk). Sedentary rats were used as the control. Echocardiography was performed on all rats at the completion of ExT. To assess the effectiveness of ExT, exercise performance in all four groups was evaluated by measuring the intolerance time during treadmill running (50 m/min, 10% grade) at the completion of the ExT period. Briefly, the exercise tolerance test consisted of walking at 25 m/min (10% grade) for 2 min, followed by a quick increase in speed to 50 m/min. The intolerance time was calculated by determining the time from the 50 m/min speed was started until the rat reached exhaustion. Time to exhaustion was determined when the rat sat at the lower end of the treadmill near a shock bar for more than 10 s. Furthermore, citrate synthase activity from soleus muscle homogenate was measured spectrophotometrically as described previously (30, 46).
Acute surgical preparation.
Rats were initially anesthestized with 5% isoflourane and maintained on 2–3% isoflourane in oxygen. A jugular vein and the trachea were cannulated. As indicated above, the right carotid artery was catheterized for measurement of mean arterial pressure (MAP) and HR. Body temperature was maintained between 37°C and 38°C by a heating pad. A catheter was placed in the right iliac artery with its tip advanced to the abdominal aortic bifurcation, ensuring that the drugs were delivered to the left hindlimb through the left iliac artery without interrupting flow.
Decerebration.
The decerebration procedure was performed as described previously (44, 45, 47). Briefly, under isoflurane anesthesia, rats were placed in a stereotaxic apparatus (Stoelting, Chicago, IL) and customized spinal frame. Dexamethasone (0.2 mg iv) was given to reduce brain edema and inflammatory responses from the decerebration. The remaining intact carotid artery was isolated and ligated to reduce bleeding during decerebration. Subsequently, a portion of bone superior to the central sagittal sinus was removed. The cerebral cortex was gently aspirated to visualize the superior and inferior colliculi. With the use of a blunt instrument, the brain was perpendicularly sectioned precollicularly and the transected forebrain aspirated. A minimum recovery period of 1.25 h was employed postdecerebration before data collection began.
Recording of group III and group IV afferent impulse activity.
To directly address the issue whether ExT improves the abnormal sensitivity of group III and IV afferents in the CHF state, we recorded the impulse activity of thinly myelinated group III (Aδ) and group IV (C) fibers in sham+Sed, sham+ExT, CHF+Sed, and CHF+ExT. Group III afferents were activated by either passive stretch of the triceps surae muscles using a calibrated rack and pinion system (Harvard Apparatus) or static contraction induced by electrical stimulation of the peripheral end of L4 or L5 ventral roots, whereas group IV were activated by either static contraction or hindlimb intra-arterial (IA) injection of capsaicin. For electrical stimulation of ventral roots, a laminectomy exposing the lower lumbar portions of the spinal cord (L2-L6) was performed. The dura of the cord was cut and reflected, allowing visual identification of the L4-L6 spinal roots. The dorsal and ventral roots of L4 and L5 were carefully separated. The ventral roots were sectioned and the cut peripheral ends were positioned on insulated bipolar platinum electrodes. The exposed neural tissue was covered in a pool of warm mineral oil (37°C). The animals were secured within the spinal adaptor (Stoelting, Wood Dale, IL) by clamps placed on rostral lumbar vertebrae. Furthermore, the pelvis was stabilized with steel posts within the frame, and the hindlimb containing the triceps surae muscles under study was fixed in one position with clamps. The angle of the hip and knee was 120° and 80°, respectively. The calcaneal bone was sectioned and the Achilles' tendon connected to a force transducer (model FT-03, Grass Instruments, West Warwick, RI) for the measurement of muscle tension. Electrical stimulation was performed using a Grass Instruments S88 stimulator. Electrically induced static muscle contraction of the triceps surae was performed by stimulating the L4 or L5 ventral roots for about 30–35 s. Constant-current stimulation was used at 2.5 times motor threshold (defined as the minimum current required to produce a muscle twitch) with a pulse duration of 0.1 ms at 30–40 Hz (44). To minimize or eliminate the stimulus artifact, we recorded the group III afferent impulse from L4 dorsal root in response to static contraction induced by electrical stimulation of the L5 ventral root.
The procedures used for single-fiber recording was similar to that described by Kaufman et al. and Mense et al. (13, 15, 25). Group III and IV fibers with endings in the triceps surae muscle were recorded from the cut peripheral ends of L4 or L5 dorsal roots. First, we determined the fibers whose receptive fields were in the triceps surae muscle. Only those group III and IV afferents whose receptive fields had been located in the triceps surae muscle were studied. The receptive fields of group III and IV muscle afferents were located by several methods as described previously (25, 44). These methods are briefly desribed as the following. The first is by slightly touching or stroking the surface of the tissue with a blunt forceps; this stimulus was repeated about once every second. This stimulus is most likely to activate mechanoreceptors in the receptive field. The second is moderate pressure. Innocuous steady deformation of the tissue for 15 s is applied using a forceps with broadened tips. Finally, noxious pressure is performed by squeezing the tissue with the same forceps for 15 s; this stimulus is perceived as painful, which is likely to activate a metaboreceptor.
Group III and IV fibers were then defined by conduction velocity. Group III fibers conduct at 1.5–15 m/s and group IV fibers conduct at less than 1.5 m/s (13, 15, 22, 44). To measure the conduction velocities of fibers with endings in the triceps surae muscle, we stimulated the tibial nerves and recorded the conduction time from the stimulating electrode to the recording electrode in the dorsal root by using Scope software (AD Instruments). The conduction distance between the two points was measured, and conduction velocity was calculated by dividing the conduction distance by the conduction time. Finally, we examined 10 sweeps from one spike to identify whether it came from a single muscle afferent or not. In some cases, if the spikes came from two separate group III afferents, we used a window discriminator (model 121, WPI, Sarasota, FL) to isolate and record the impulse of the individual fiber.
Drugs and injected solutions.
To determine whether P2X receptors mediate the potential beneficial effect of ExT on the sensitization of group III afferents in the CHF state, pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), an antagonist of the P2X receptor, was administrated via hindlimb IA infusion (10 mg/kg, 0.2 ml, 10 min) using a syringe pump (model 310; Stoelting). The responses of group III afferents to either static contraction or passive stretch were compared before and after IA treatment with PPADS in each group of rats.
Hindlimb IA bolus injections of capsaicin (0.2 or 2.0 μg/0.15 ml) were used to activate the group IV afferents in each group of rats. Briefly, a catheter was placed in the right iliac artery with its tip advanced to the abdominal aortic bifurcation, ensuring that capsaicin was delivered to the left hindlimb through the left iliac artery. It should be pointed out that we did not reversibly ligate the common iliac vein by vascular occlusion to trap capsaicin in the hindlimb as previously described (46). In that study, we limited capsaicin delivery to the left hindlimb to eliminate any concern of the cardiovascular response to capsaicin being due to a systemic rather than local effect (i.e., stimulation of afferent endings). However, because we carried out direct afferent recordings in the current study this concern is moot.
PPADS was purchased from Fisher Scientific and was dissolved in saline before use. Capsaicin was purchased from Sigma and was dissolved in alcohol and then diluted with saline.
Western blot analysis.
For Western blot analysis, rats (n = 5/each group) were anesthetized with pentobarbital sodium (40 mg/kg ip). Cardiac function such as LVEDP was first determined. Then the rats were euthanized with an overdose of pentobarbital sodium (150 mg/kg iv). The L4/L5 dorsal root ganglions (DRG) were rapidly removed and lysed with 20 mM Tris·HCl buffer, pH 8.0, containing 1% Nonidet-40, 150 mM NaCl, 1 mM EDTA, 10% glycerol, 0.1% β-mercaptoethanol, 0.5 mM dithiothreitol, and a mixture of proteinase and phosphatase inhibitors (Sigma). The hearts and lungs were also removed, and infarct size was measured as indicated above. Protein concentration was measured by the BCA protein assay method using bovine serum albumin as standard. The proteins were loaded onto a 10% SDS-PAGE gel along with protein standards (Bio-Rad Laboratories) in a separate lane for electrophoresis and then transferred to polyvinylidene fluoride membrane. The membrane was probed with rabbit polyclonal antibody against P2X3 (1:1,000 dilutions, Santa Cruz Biotechnology) or TRPV1 receptors (1:1,000 dilutions, Santa Cruz Biotechnology) and secondary antibody of goat anti-rabbit IgG (1:5,000 dilutions, Pierce Chemical). The protein signals were detected by enhanced chemiluminescence reagent (Pierce Chemical) and analyzed using UVP BioImaging Systems. GAPDH (1:1,000, Santa Cruz Biotechnology) was used to verify equal protein loading, and the densitometric results of P2X3 and TRPV1 receptors were reported as the ratio to GAPDH.
Immunohistochemistry.
Rats (n = 5/each group) were anesthetized with pentobarbital sodium (40 mg/kg ip). Cardiac function such as LVEDP was first determined. At the end of the experiment the rats were perfused through the aorta, first with 100 ml heparinized saline followed by 500 ml 4% paraformaldehyde in 0.1 mol/l sodium phosphate buffer (PBS, pH = 7.4). The L4/L5 DRGs were immediately dissected and immersed in 4% paraformaldehyde in 0.1 mol/l PBS (pH = 7.2) overnight at 4°C. The tissues were then transferred to 30% sucrose in PBS and kept in the solution until they sank to the bottom. Thereafter, the blocks were rapidly frozen, and 14-μm sections were cut on a Leica cryostat and thawed onto gelatin-coated slides. Since the slices were 14 μm thick, and the DRG neuron soma ranged from <10 μm to over 100 μm, the same neuron could potentially be counted twice. Therefore, we counted every seventh slice.
For triple immunostaining of P2X3 or TRPV1 receptors, sections were stained with the isolectin IB4 (a C-fiber neuron marker) (43) and NF200 (an A-fiber neuron marker) (6). Ganglionic sections, after being preincubated in 10% goat serum for 60 min, were incubated with rabbit anti-P2X3 or anti-TRPV1 antibody (1:200 dilution, Santa Cruz Biotechnology) and mouse anti-NF200 antibody (1:200 dilution, Abcam, Cambridge, MA) overnight at 4°C. Sections were then washed with PBS and incubated with fluorescence-conjugated secondary antibody (1:200, Alexa 488-conjugated goat anti-rabbit IgG and pacific blue-conjugated goat anti-mouse IgG, Invitrogen) and Alexa FluorR 568 conjugated isolectin-B4 (1:200, Invitrogen) for 60 min at room temperature. After three washes with PBS, the sections were mounted on precleaned microscope slides. Slides were observed under a Leica fluorescent microscope with corresponding filters. Pictures were captured by a digital camera system. No staining was seen when a negative control was performed with PBS instead of the primary antibody (data not shown).
Counts were obtained from the total number of IB4- (C fiber) or NF200-positive (A fiber) neurons. The percentage of neurons positive for P2X3 or TRPV1 for A or C neurons was calculated. A neuron was considered to be “positive” when the measured intensity of immunostaining was more than five times greater than the background. Ten sections from each DRG were analyzed for a total of 5 rats in each group.
Data acquisition and statistical analysis.
Muscle tension and impulse activity of group III and IV afferents were acquired using PowerLab software (AD Instruments). Baseline values were determined by analyzing at least 30 s of the data before muscle contraction. The peak response was determined during the period of the greatest change from baseline. The tension-time index (TTI) was calculated by integrating the area between the tension trace and the baseline level (expressed in kg × s). Peak developed tension was calculated by subtracting the resting tension from the peak tension and is expressed in grams. All values are expressed as means ± SE. Differences between groups were determined by a two-way ANOVA followed by the Tukey post hoc test. Changes in TTI, peak developed tension, and discharges of group III and IV afferents before and after arterial administration of chemicals were determined by paired t-test. P < 0.05 was considered statistically significant.
RESULTS
Evaluation of body weight, organ weight, baseline hemodynamics, and exercise performance.
Echocardiographic and hemodynamic measurements of all groups of rats are summarized in Table 1. The heart weight and lung weight-to-body weight ratios were significantly higher in CHF+Sed rats than that in sham+Sed rats, suggesting cardiac hypertrophy and substantial pulmonary congestion in the CHF state. Moreover, in CHF+Sed rats, a gross examination revealed a dense scar in the anterior ventricular wall. The mean infarct area was 42.7 ± 1.8% of the LV area. No infarcts were identified in sham+Sed rats. Pleural fluid and ascites were also found in the CHF+Sed rats but none in the sham+Sed rats. Compared with sham+Sed rats, there was a slight but significant decrease in baseline MAP in CHF+Sed rats. However, there was no significant difference in baseline HR between sham+Sed and CHF+Sed rats. Furthermore, CHF+Sed rats exhibited elevated LVEDP and reduced ejection fraction and fractional shortening compared with sham+Sed rats, indicating decreased cardiac function. Finally, CHF+Sed rats exhibited a significant decrease in exercise performance compared with sham+Sed rats, indicating that exercise intolerance occurs in these CHF+Sed rats. After ExT, indices of cardiac function were not significantly different between CHF+Sed and CHF+ExT rats, whereas exercise intolerance was reduced in CHF+ExT rats. ExT did not significantly affect MAP and HR in sham and CHF rats. Citrate synthase (CS) activity, an important marker of skeletal muscle aerobic metabolism, increased by 52% in the soleus muscle of sham+ExT rats and by 45% in CHF+ ExT rats compared with their respective controls (Table 1).
Table 1.
Hemodynamic, morphological, and exercise performance data in sham and CHF rats following exercise training
| Sham + Sed (n = 42) | Sham + ExT (n = 40) | CHF + Sed (n = 42) | CHF + ExT (n = 43) | |
|---|---|---|---|---|
| Body weight, g | 462 ± 9 | 410 ± 6* | 485 ± 9 | 429 ± 8# |
| Heart weight, mg | 1,511 ± 20 | 1,515 ± 22 | 2,540 ± 30* | 2,469 ± 31* |
| HW/BW, mg/g | 3.3 ± 0.1 | 3.7 ± 0.1 | 5.2 ± 0.1* | 5.7 ± 0.1* |
| WLW/BW, mg/g | 5.0 ± 0.3 | 5.1 ± 0.3 | 9.5 ± 0.5* | 9.0 ± 0.5* |
| MAP, mmHg | 109.6 ± 2.5 | 104.2 ± 2.5 | 90.5 ± 2.0* | 99.5 ± 2.2 |
| HR, beats/min | 363.5 ± 7.5 | 344.3 ± 6.9 | 379.1 ± 6.8 | 367.1 ± 6.9 |
| LVEDP, mmHg | 0.3 ± 0.4 | −0.2 ± 0.7 | 19.1 ± 1.6* | 17.3 ± 1.5* |
| EF, % | 76.1 ± 0.8 | 78.0 ± 0.8 | 37.9 ± 1.7* | 39.5 ± 1.6* |
| FS, % | 45.4 ± 0.7 | 46.1 ± 0.7 | 23.1 ± 1.0* | 24.2 ± 1.0* |
| Infarct size, % | 0 | 0 | 42.7 ± 1.8* | 40.2 ± 2.0* |
| CS, μmol•g−1•min−1 | 16.6 ± 1.0 | 25.1 ± 1.2* | 9.2 ± 0.8* | 17.2 ± 1.0# |
| Exercise performance, min | 3.4 ± 0.1 | 7.1 ± 0.1* | 1.2 ± 0.1* | 3.1 ± 0.1# |
Values are means ± SE. Sed, sedentary; ExT, excerise trained;, CHF, chronic heart failure; BW, body weight; HW, heart weight; WLW, wet lung weight; MAP, mean arterial pressure; LVEDP, left ventricle end-diastolic pressure; EF, ejection fraction; FS, fractional shortening; CS, citrate synthase activity.
P < 0.05 vs. sham+Sed.
P < 0.05 vs. CHF+Sed.
Characteristics of group III and group IV afferents.
We recorded the impulse activity of 122 group III afferents (31 fibers from sham+Sed rats, 26 fibers from sham+ExT rats, 32 fibers from CHF+Sed rats, and 33 fibers from CHF+ExT rats) and 86 group IV afferents (23 fibers from sham+Sed rats, 19 fibers from sham+ExT rats, 21 fibers from CHF+Sed rats, and 23 fibers from CHF+ExT rats), each of which had its receptive field in the triceps surae muscle including the calcaneal tendon and the junction and the belly of this muscle. For the most part, group III afferents responded to light mechanical probing of their receptive fields, whereas group IV afferents responded to noxious pinching of their receptive fields. Group IV afferents did not respond to gentle stroking or nonnoxious pinching of the muscles. The conduction velocities of group III fibers were not different among all four groups (sham+sed, 5.5 ± 0.6 m/s; sham+ExT, 6.1 ± 0.7 m/s; CHF+Sed, 6.5 ± 0.7 m/s; CHF+ExT, 5.9 ± 0.5 m/s). Similarly, the conduction velocities of group IV fibers were not different among four groups (sham+sed, 0.65 ± 0.13 m/s; sham+ExT, 0.82 ± 0.11 m/s; CHF+Sed, 0.72 ± 0.13 m/s; CHF+ExT, 0.58 ± 0.14 m/s).
Discharge of group III afferents in response to either static contraction or passive stretch in sham and CHF with and without ExT.
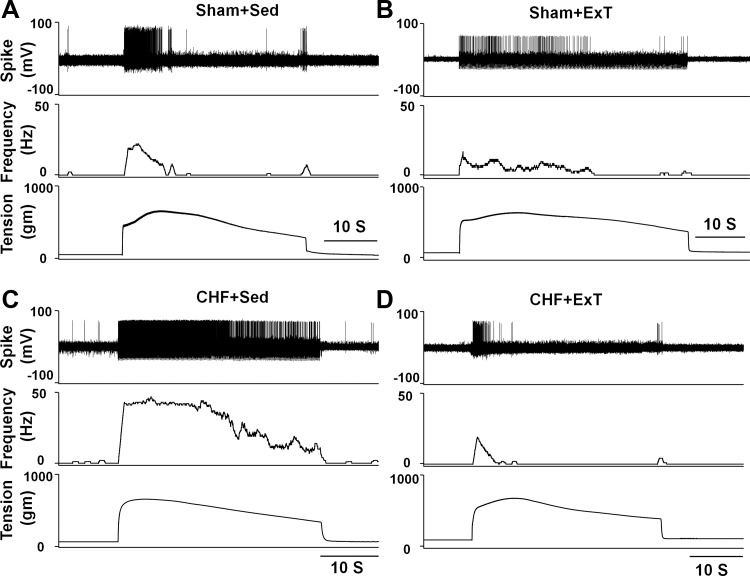
In sham+Sed rats, 20 of 31 group III fibers were activated by static contraction. Furthermore, 12 of 20 group III fibers were also responsive to passive stretch, a purely mechanical stimulus. In the remaining 11 group III fibers that were unresponsive to static contraction, 6 were still activated by stretch, consistent with the previous findings of us and others (10, 44), suggesting that there is not complete overlap between stretch-sensitive and contraction-sensitive group III fibers. A similar result was observed in the other three groups. In all four groups, those group III fibers that did not respond to either static contraction or passive stretch were consequently excluded from data analysis. A common characteristic of a group III afferent response to static contraction was a sudden explosive burst of discharge during the onset of contraction, followed by an adaptive decrease during the steady-state period of contraction (Fig. 1). A similar phenomenon was seen in stretch-induced discharge of group III afferents.
Fig. 1.
Representative recordings showing the discharge of mechanically sensitive (group III) afferents in response to static contraction induced by electrical stimulation of L5 ventral root in sham+sedentary (sham+Sed) (CV, 4.1 m/s, A), sham+exercised trained (Sham+ExT) (CV, 3.0 m/s, B), chromic heart failure+sedentary (CHF+Sed) (CV, 4.7 m/s, C), and CHF+ExT rats (CV, 5.3 m/s, D).
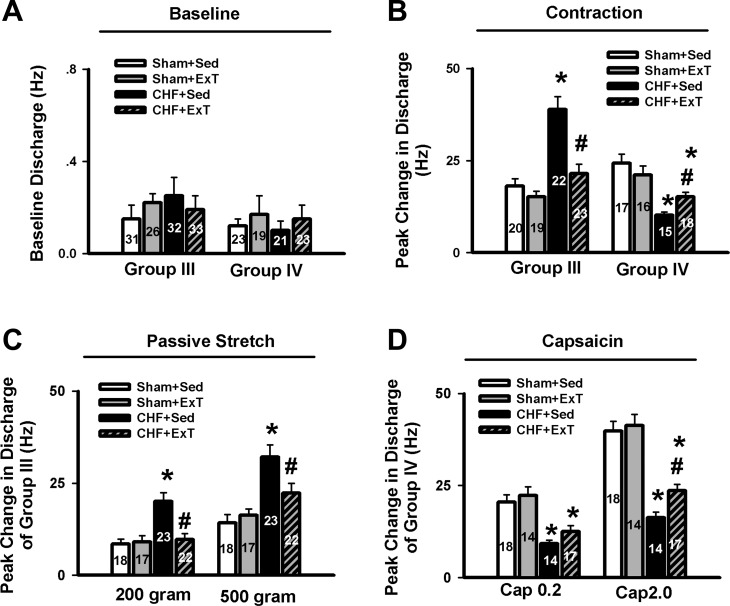
Compared with sham+Sed rats, CHF+Sed rats exhibited 1) an exaggerated peak response of group III afferents to either static contraction or passive stretch (Figs. 1 and 2) and 2) the increased duration of group III afferent discharge during either contraction or stretch (contraction, 23.0 ± 1.4 vs. 13.4 ± 0.8 s, CHF+Sed vs. sham+Sed, P < 0.05; stretch, 21.6 ± 1.2 vs. 11.5 ± 0.8 s, CHF+Sed vs. sham+Sed, P < 0.05), which was consistent with our previous findings (44) that group III afferents are sensitized in CHF rats. However, 8–10 wk of ExT prevented the increased peak response of group III afferents to both contraction and stretch in CHF rats without any effect in sham rats (Figs. 1 and 2). In addition, ExT also prevented the increased firing duration of group III afferents during either contraction or stretch in CHF rats (contraction, 23.0 ± 1.4 vs. 13.7 ± 1.0 s, CHF+Sed vs. CHF+ExT, P < 0.05; stretch, 21.6 ± 1.2 vs. 12.6 ± 0.9 s, CHF+Sed vs. CHF+ExT, P < 0.05) with no effect in sham rats (contraction, 13.4 ± 0.8 vs. 15.4 ± 1.1 s, sham+Sed vs. sham+ExT, P > 0.05; stretch, 11.5 ± 0.8 vs. 13.0 ± 1.0 s, sham+Sed vs. sham+ExT, P > 0.05).
Fig. 2.
A and B: mean data showing the baseline discharge of group III and IV afferents (A) and the responses of group III and IV afferents to static contraction induced by electrical stimulation of L5 ventral root (B) in Sham+Sed, Sham+ExT, CHF+Sed, and CHF+ExT rats. C and D: mean data showing the discharge of group III and IV afferents in response to two levels of passive stretch (C) and two doses of capsaicin (D), respectively, in Sham+Sed, Sham+ExT, CHF+Sed, and CHF+ExT rats. The digit in the bar graph indicates the number of recording fibers. Data are expressed as means ± SE. *P < 0.05 vs. sham+Sed, #P < 0.05 vs. CHF+Sed.
Discharge of group IV afferents in response to either static contraction or capsaicin in sham and CHF with and without ExT.
Seventeen of 23 group IV fibers in sham+Sed rats, 16 of 21 group IV fibers in sham+ExT rats, 15 of 19 group IV fibers in CHF+Sed rats, and 18 of 23 group IV fibers in CHF+ExT rats were activated by static contraction. In all contraction-sensitive group IV afferents, 14 fibers in sham+Sed rats, 12 fibers in sham+ExT rats, 11 fibers in CHF+Sed rats, and 14 fibers in CHF+ExT rats were also responsive to hindlimb IA injection of capsaicin. Those group IV fibers that did not respond to either static contraction or capsaicin were consequently excluded from data analysis, including 2 fibers in sham+Sed rats, 3 fibers in sham+ExT rats, 1 fiber in CHF+Sed rats, and 2 fibers in CHF+ExT rats.
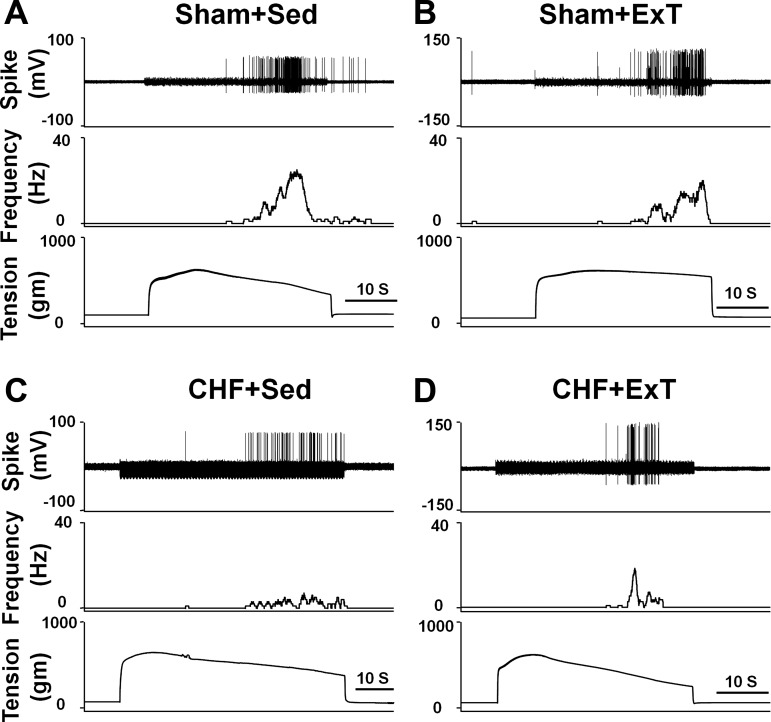
Although both respond to static contraction, group IV afferents often do not discharge at the onset of contraction as do group III afferents. Instead, the onset latencies of group IV afferents responding to static contraction were longer than those of group III afferents (Fig. 3). Compared with sham+Sed rats, CHF+Sed rats exhibited a decreased peak response of group IV afferents to either static contraction or capsaicin. Furthermore, there was an increased latency of group IV afferents in response to contraction in CHF+Sed rats (21.1 ± 1.8 s, n = 15, P < 0.05) compared with sham+Sed rats (15.8 ± 0.8, n = 17). All data mentioned above indicated that that the group IV afferents are desensitized in CHF +Sed rats, which was consistent with our previous finding in CHF rats (44). ExT only partially prevents the blunted peak response of group IV afferents to either static contraction or capsaicin in CHF rats without any effect in sham rats (Figs. 2 and 3). ExT also prevented the increased latency of group IV afferents in response to contraction in CHF rats (15.3 ± 0.9 vs. 21.1 ± 1.8 s, CHF+ExT vs. CHF+Sed, P < 0.05) without any effect in sham rats (14.3 ± 0.9 vs. 15.8 ± 0.8 s, CHF+ExT vs. CHF+Sed, P > 0.05).
Fig. 3.
Representative recordings showing the discharge of metabolically sensitive (group IV) afferents in response to static contraction induced by electrical stimulation of L5 ventral root in sham+Sed (CV, 1.2 m/s, A), Sham+ExT (CV, 0.82 m/s, B), CHF+Sed (CV, 0.73 m/s, C), and CHF+ExT rats (CV, 1.0 m/s, D).
Effect of PPADS on the discharge of group III afferents in response to either static contraction or passive stretch in sham and CHF with and without ExT.
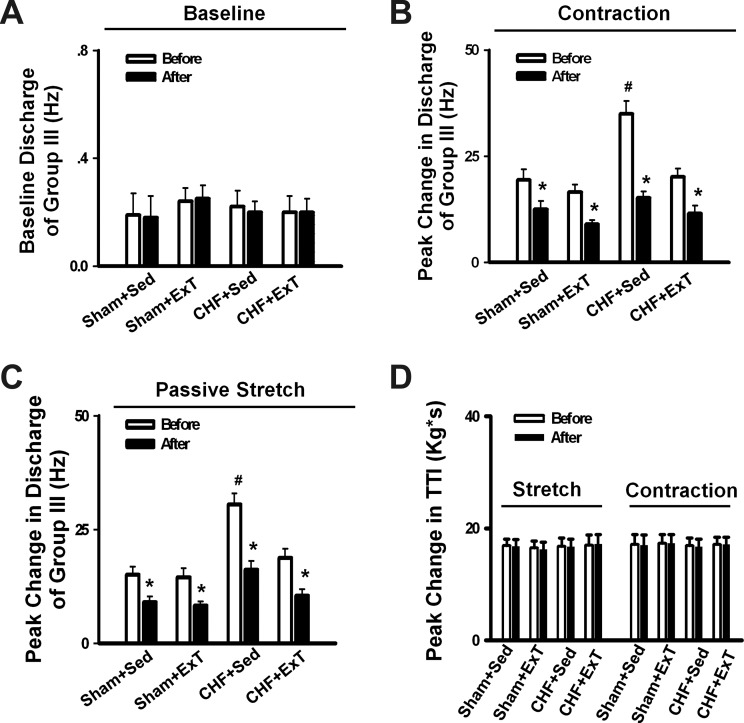
Our recent study (44) demonstrated that P2 receptors mediate the abnormal sensitization of group III afferents in CHF. To determine whether ExT prevents the abnormal sensitization of group III afferents by restoring the dysfunction of P2 receptors PPADS, an antagonist of P2 receptors, was administrated via hindlimb IA infusion in sham+Sed, sham+ExT, CHF+Sed, and CHF+ExT rats. As shown in Fig. 4, PPADS did not change the baseline activity of group III afferents (Fig. 4A), whereas it attenuated the discharge of group III afferents in response to either static contraction or passive stretch in all four groups (Fig. 4, B and C). Furthermore, PPADS attenuated the discharge of group III afferents in response to either static contraction (Fig. 4B) or passive stretch (Fig. 4C) in CHF+Sed rats to a greater extent than in sham+Sed rats, which was normalized by ExT (n = 10/each group). There was no significant difference in the effect of PPADS on the discharge of group III afferents in response to either static contraction or passive stretch between sham+Sed and sham+ExT rats. There was no significant difference in TTI generated by static contraction or passive stretch before and after PPADS administration in all four groups (Fig. 4D).
Fig. 4.
Mean data showing the effect of pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), a P2X antagonist, on the baseline discharge (A) and the responses of group III afferents to either static contraction (B) or passive stretch (C) in Sham+Sed, Sham+ExT, CHF+Sed, and CHF+ExT rats. D: effect of PPADS on the tension time index (TTI) produced by passive stretch or static contraction in Sham+Sed, Sham+ExT, CHF+Sed, and CHF+ExT rats. Data are expressed as means ± SE. n = 8–10/each group. *P < 0.05 vs. before, #P < 0.05 vs. Sham+Sed.
Protein expressions of P2X3 and TRPV1 receptors in the dorsal root ganglion.
Western blot analysis was used to detect P2X3 and TRPV1 protein levels in the intact dorsal root ganglions (DRGs) from sham+Sed, sham+ExT, CHF+Sed, and CHF+ExT rats. As shown in Fig. 5A (left), the P2X3 protein level was significantly increased by ∼2.1-fold in the L4/L5 DRG of CHF+Sed rats (n = 5) compared with the sham+Sed control (n = 5), which was prevented by ExT. In contrast to the P2X3 protein expression in DRG, TRPV1 protein was significantly decreased by ∼56% in the L4/L5 DRG of CHF+Sed rats compared with the sham+Sed control (Fig. 5B), which was only partially prevented by ExT.
Fig. 5.
Western blot data showing the protein expression of purigenic 2X (P2X3) (A) and transient receptor potential vanilloid 1 (TRPV1) (B) receptors in L4/L5 dorsal root ganglion (DRG) in Sham+Sed, Sham+ExT, CHF+Sed, and CHF+ExT rats. Data are expressed as means ± SE. n = 6/each group. *P < 0.05 vs. sham+Sed, #P < 0.05 vs. CHF+Sed.
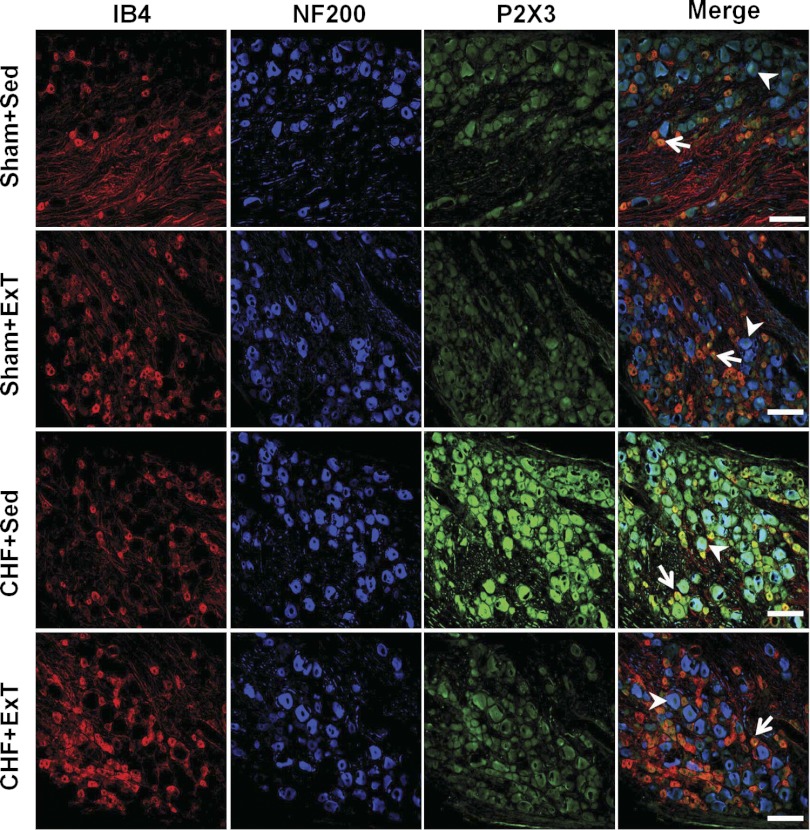
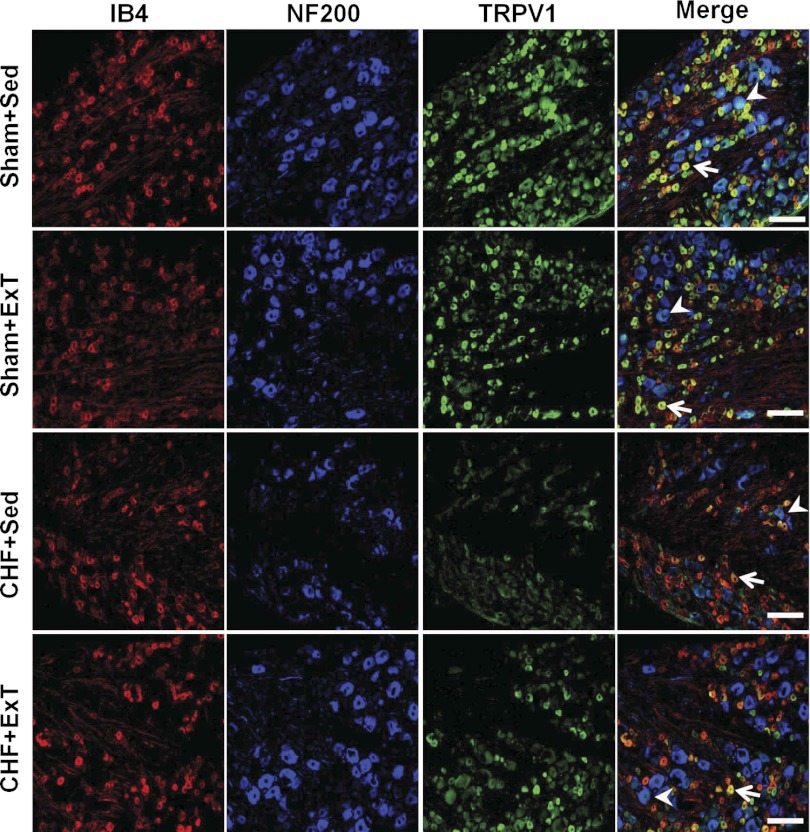
Immunohistochemical labeling was performed on DRG sections to assess which subpopulation of DRG cells exhibited altered expressions of P2X3 and TRPV1 receptors as indicated by the Western blot analysis. In the DRGs of all four groups, immunostaining of the P2X3 receptor was seen in both IB4-positive (C fiber) and NF200-positive (A fiber) neurons (Fig. 6). Compared with sham+Sed rats, the immunostaining was generally more intense in both A and C fiber neurons in DRG of CHF+Sed rats (Fig. 6, P2X3 panels, green). Furthermore, CHF+Sed rats had a greater percentage of DRG neurons positive for P2X3 against for A and C neurons compared with sham+Sed rats (Table 2). However, the increased immunostaining of P2X3 receptors in both A and C fiber DRG neurons of CHF+Sed rats was not seen in CHF+ExT rats, indicating that ExT prevents the upregulation of P2X3 receptors in the A and C fiber DRG neurons of CHF rats. Different with P2X3 receptor staining, immunostaining for the TRPV1 receptor was predominantly seen in IB4-positive (C fiber) neurons and a much smaller proportion of NF200-positive (A fiber) neurons (Fig. 7). Compared with sham+Sed rats, both immunostaining intensity of TRPV1 receptors was decreased in IB4-positive neurons in the DRG of CHF+Sed rats (Fig. 7, third panels, green), which was associated with a decreased percentage of DRG neurons positive for TRPV1 for C fiber neurons in CHF+Sed rats (Table 2). ExT only partially prevents the decreased immunostaining of TRPV1 receptors in C fiber DRG neurons of CHF rats.
Fig. 6.
Immunohistochemical data showing the protein expression of P2X3 receptors in L4/L5 DRG in Sham+Sed, Sham+ExT, CHF+Sed, and CHF+ExT rats. Isolectin B4 (IB4), a C-fiber neuron marker; NF200, an A-fiber neuron marker. White Bar = 100 μm. White arrow represents double staining of P2X3 with IB4; white arrowhead represents double staining of P2X3 with NF200.
Table 2.
P2X3 and TRPV1 receptor-positive DRG neurons to IB4- and NF200-positive neurons in intact ganglia in sham and CHF rats following exercise training
| Sham |
CHF |
|||
|---|---|---|---|---|
| Sed | ExT | Sed | ExT | |
| Total IB4-Positive | 2,213 ± 130 | 2,401 ± 131 | 2,079 ± 135 | 2,165 ± 131 |
| P2X3 with IB4 | 1,056 ± 112 | 1,179 ± 119 | 1,486 ± 128 | 1,083 ± 115 |
| % | 47.7 ± 2.0 | 49.1 ± 2.1 | 71.4 ± 2.3* | 50.0 ± 2.1# |
| Total NF200-Positive | 2,015 ± 122 | 2,212 ± 122 | 2,411 ± 132 | 2,141 ± 130 |
| P2X3 with NF200 | 1,009 ± 112 | 1,063 ± 115 | 1,885 ± 125 | 1,170 ± 116 |
| % | 50.1 ± 1.9 | 48.1 ± 1.8 | 78.2 ± 2.3* | 54.6 ± 2.1# |
| Total IB4-Positive | 2,343 ± 140 | 2,147 ± 125 | 2,293 ± 135 | 2,116 ± 131 |
| TRPV1 with IB4 | 1,567 ± 125 | 1,358 ± 128 | 746 ± 93 | 973 ± 98 |
| % | 66.9 ± 2.3 | 63.2 ± 2.0 | 32.5 ± 1.4* | 46.0 ± 1.5*# |
| Total NF200-Positive | 2,103 ± 131 | 2,350 ± 132 | 2,416 ± 127 | 2,213 ± 128 |
| TRPV1 with NF200 | 335 ± 38 | 382 ± 42 | 334 ± 39 | 320 ± 35 |
| % | 15.9 ± 1.1 | 16.3 ± 1.2 | 13.8 ± 1.0 | 14.5 ± 1.1 |
Values are means ± SE; n = 5/each group. IB4, isolectin; P2X3, purinergic 2X; TRPV1, transient receptor potential vanilloid 1.
P < 0.05 vs. sham+Sed.
P < 0.05 vs. CHF+Sed.
Fig. 7.
Immunohistochemical data showing the protein expression of TRPV1 receptors in L4/L5 DRG in sham and CHF rats. IB4, a C-fiber neuron marker; NF200, an A-fiber neuron marker. White bar = 100 μm. White arrow represents double staining of TRPV1 with IB4' white arrowhead represents double staining of TRPV1 with NF200.
Muscle tension produced by static contraction or passive stretch.
In the present study, muscle peak developed tension induced by static contraction ranged from 400 to 550 g in sham+Sed, sham+ExT, CHF+Sed, and CHF+ExT rats. There was no significant difference in TTI during static contraction in all four groups (Table 3). In the experiments in which passive stretch was performed, we used two levels of stretch to activate the stretch-sensitive group III afferents in sham and CHF rats. The low level of peak tension was ∼200 g and the high level was ∼500 g, which matched the peak tension developed by static contraction. Again, there were no significant differences in TTI during both levels of stretch in all four groups (Table 3).
Table 3.
Tension-time indexes for either static contraction or passive stretch in in sham+Sed, sham+ExT, CHF+Sed, and CHF+ExT rats
| TTI, kg×s |
|||
|---|---|---|---|
| Group | Contraction | Low stretch | High stretch |
| Sham+Sed | 15.9 ± 1.3 (37) | 7.0 ± 1.0 (18) | 16.2 ± 1.3 (18) |
| Sham+ExT | 16.2 ± 1.2 (35) | 6.9 ± 1.0 (17) | 16.3 ± 1.3 (17) |
| CHF+Sed | 15.7 ± 1.3 (37) | 7.0 ± 1.1 (23) | 16.2 ± 1.2 (23) |
| CHF+ExT | 15.7 ± 1.2 (38) | 6.8 ± 1.0 (22) | 16.4 ± 1.2 (22) |
Values are means ± SE; numbers in the bracket represent the number of tested fibers. TTI, tension-time index. There were no significant differences (P > 0.05) in TTI in all 4 groups.
DISCUSSION
The primary findings in the present study demonstrate that 8–10 wk of ExT started at an early stage of CHF prevents the sensitization of group III afferents and partially prevents the blunted sensitivity of group IV afferents in the CHF state. These data provide direct evidence showing that ExT has a beneficial effect on sensitized muscle afferents in the CHF state. Another important new finding of this study is that P2X and TRPV1 receptors are involved in the underlying molecular mechanisms by which ExT improves the abnormal sensitization of the group III and IV afferents in CHF.
Traditionally ExT was considered contraindicated for CHF patients. However, over the past decade numerous clinical trials and small randomized studies have demonstrated that long-term regular exercise is safe in stable CHF patients and increases the quality of life as well as survival (1, 17, 26). The beneficial effects of ExT include improved autonomic balance, reduced neurohumoral activation, an increase in exercise capacity, and ameliorated myopathy in CHF patients and animals (28, 33, 34). Furthermore, Piepoli et al. (31) first reported that 6-wk forearm training improved the abnormal exercise-evoked ventilation and cardiovascular responses in CHF patients, suggesting a beneficial effect of ExT on the abnormal muscle reflex function in CHF patients. In animal studies, our recent work (46) demonstrated that 1) ExT initiated at the third week of coronary ligation surgery prevents the exaggerated EPR as well as the excessive sympathoexcitation during static contraction in CHF rats; 2) ExT prevented the enhanced mechanoreflex function in CHF rats; and 3) ExT partially prevented the blunted metaboreflex function in CHF rats. However, these clinical and experimental studies above did not answer the question of whether ExT prevented the exaggerated EPR via either a central or peripheral mechanism. Previous studies (5, 23) have shown that peripheral skeletal myopathy develops in CHF (e.g., muscle atrophy, decreased peripheral blood flow, fiber-type transformation, and reduced oxidative capacity), which can be reversed by ExT (9, 14). Therefore, it is reasonable to speculate that the improvement of skeletal muscle abnormalites by ExT may subsequently affect muscle afferent function. This hypothesis has been supported by the current study showing that the abnormal sensitivity of group III and IV afferents was improved by ExT. On the other hand, the current study did not exclude the possibility that a central mechanism is also involved in the beneficial effect of ExT on the exaggerated EPR in the CHF state. This question needs to be clarified by additional experiments.
In contrast to a complete normalization of increased group III afferent sensitivity, ExT only partially prevented the blunted sensitivity of group IV afferents in response to either static contraction or capsaicin in CHF rats. This observation is consistent with our previous study (46) showing that ExT prevented the enhanced mechanoreflex function and only partially prevented the blunted metaboreflex function in CHF rats. These findings suggest that different molecular mechanisms may be involved in the beneficial effects of ExT on the abnormal sensitivity of group III and IV afferents in the CHF state. Previous studies reported that group III afferents can be sensitized by muscle metabolites during muscle contraction, such as prostaglandins and ATP (11, 19). Of these metabolites, ATP acts on the group III afferent ending via the P2 receptor (12). Kindig et al. (19) reported that PPADS, a P2 antagonist, attenuated the responses of group III muscle afferents to static contraction as well as to tendon stretch in decerebrate cats, suggesting that P2 activation sensitizes group III afferents in the normal state. Furthermore, our recent study (44) demonstrated that 1) PPADS attenuated the response of muscle group III afferents to either static contraction or passive stretch in CHF rats to a greater extent than in sham rats; 2) protein expression of P2X3 receptors in DRG was significantly increased in CHF rats compared with sham rats; and 3) increased protein expression of P2X3 receptors in DRG was located on both IB4-positive (C fiber marker) and NF200-positive (A fiber marker) neurons. These findings suggest that ATP and P2X receptors are involved in the mechanism underlying the sensitization of group III afferents in CHF state. In the present study, we found that 1) the greater antagonistic effect of PPADS on the sensitivity of group III afferents observed in CHF rats was prevented by ExT and 2) ExT prevented the upregulation of P2X3 receptors in both A- and C-fiber DRG neurons in CHF rats, indicating that ExT prevented the sensitization of group III afferents, at least in part, by the normalization of the upregulated P2X receptors in the CHF state.
With regard to the underlying molecular mechanism by which ExT improves the blunted group IV afferent sensitivity in CHF, we investigated the role of TRPV1 receptors in mediating the desensitization of group IV afferents. TRPV1 receptors are primarily localized to metabolically sensitive afferent fibers (group IV) in skeletal muscle (41), which is believed to be closely related to the group IV afferent activation. TRPV1 receptors are stimulated by capsaicin and hydrogen ions, the latter being a by-product of muscular contraction (15, 35). A recent study of Smith et al. (37) reported that TRPV1 blockade attenuated the pressor response to static contraction in decerebrate rats, indicating that TRPV1 plays an important role in evoking the exercise pressor reflex in the decerebrate rat model. In CHF rats, several other studies by this group (37, 39, 41) reported that 1) TRPV1 activation by capsaicin caused a blunted cardiovascular response in CHF rats compared with sham rats, which was confirmed by our recent study (46); 2) chronic deletion of TRPV1 receptors in normal rats recaptures the exaggerated EPR observed in CHF rats; and 3) the mRNA level of TRPV1 in DRG and skeletal muscle was decreased in CHF rats compared with sham rats. Furthermore, our recent study (44) demonstrated that 1) the response of group IV afferents to exogenous TRPV1 activation by capsaicin was blunted in CHF rats compared with sham rats; and 2) protein expression of TRPV1 receptor in DRG was significantly decreased in C-fiber DRG neurons of CHF rats. These findings suggest that the TRPV1 receptor plays an important role in causing the blunted group IV sensitivity in the CHF state. In the present study, we found that ExT partially prevents the blunted sensitivity of group IV afferents in response to either static contraction or to administration of capsaicin in CHF rats. This was associated with an improvement in the decrease in protein expression of TRPV1 receptors in C-fiber DRG neurons of CHF+ExT rats. We believe that the current finding provides a mechanism by which ExT improves the blunted sensitivity of group IV afferents in the CHF state.
Limitations.
We acknowledge that a potential limitation is that although PPADS antagonizes most P2X receptors, it also antagonizes P2Y receptors. Even for P2X receptors, there are several different subtypes, including P2X1–7 receptors. Although we found that ExT prevented the increased protein expression of P2X3 receptors in the DRG of CHF rats, we cannot exclude the possibility that protein expression of other P2 receptor subtypes in the DRG were affected by ExT and contribute to the beneficial effect of ExT on the sensitization of group III afferents in the CHF state. In addition, considering that 1) direct quantitative measurement of P2X3 and TRPv1 protein expression in afferent terminals is technically unavailable and 2) all proteins can only be synthesized in the neuronal cell body and delivered to the afferent terminal, we measured proteins in the DRG soma as an alternative. Nonetheless, we acknowledge that P2X3 and TRPv1 expression in the DRG may not be reflective of protein expression at the afferent nerve terminals in muscle.
Perspectives and Significance
An exaggerated EPR-induced sympathoexcitation can potentially increase cardiovascular risk and contributes to exercise intolerance during physical activity in CHF patients (8, 32, 39). A therapeutic strategy for preventing or slowing the progression of the exaggerated EPR may be beneficial in CHF patients. The present study provides direct evidence that long-term exercise training has a beneficial effect on the abnormal sensitization of group III and IV afferents in the CHF state. In addition, we found that the protective effect of ExT on the abnormal sensitization of muscle afferents in CHF rats is associated with the improvement of the P2X and TRPV1 receptors dysfunction respectively. These findings have broad implications for understanding the mechanisms by which ExT improves exercise intolerance and the exaggerated sympathoexcitation during exercise in CHF. Given that we started ExT in the early stages of heart failure (2 wk after coronary ligation) in which the exaggerated EPR has not been developed (38), the current training strategy indicates a “protective” effect rather than a “curative” effect on the exaggerated EPR and on the abnormal sensitization of muscle afferents in CHF rats. Whether ExT can improve the more established exaggerated EPR in the latter stages of CHF remains unclear.
GRANTS
This work was supported, in part, by a grant from the National Heart, Lung, and Blood Institute (PO1 HL-62222) and from The American Heart Association (11GRNT7530022). H.-J. Wang was supported by a postdoctoral fellowship from the American Heart Association, Heartland Affiliate.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.-J.W., I.H.Z., and W.W. conception and design of research; H.-J.W. performed experiments; H.-J.W., Y.-L.L., I.H.Z., and W.W. analyzed data; H.-J.W., Y.-L.L., I.H.Z., and W.W. interpreted results of experiments; H.-J.W. prepared figures; H.-J.W. drafted manuscript; H.-J.W., I.H.Z., and W.W. edited and revised manuscript; H.-J.W., Y.-L.L., I.H.Z., and W.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Kurtis G. Cornish, Johnnie F. Hackley, and Richard Robinson for expert technical assistance.
REFERENCES
- 1. Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation 99: 1173–1182, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Coats AJ, Adamopoulos S, Radaelli A, McCance A, Meyer TE, Bernardi L, Solda PL, Davey P, Ormerod O, Forfar C. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation 85: 2119–2131, 1992 [DOI] [PubMed] [Google Scholar]
- 3. Cohn JN. Abnormalities of peripheral sympathetic nervous system control in congestive heart failure. Circulation 82: I59–I67, 1990 [PubMed] [Google Scholar]
- 4. Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation 85: 1751–1759, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol 10: 248–253, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Francis GS. Neurohumoral mechanisms involved in congestive heart failure. Am J Cardiol 55: 15A–21A, 1985 [DOI] [PubMed] [Google Scholar]
- 8. Grassi G, Mancia G. Sympathetic overactivity and exercise intolerance in heart failure: a cause-effect relationship. Eur Heart J 20: 854–855, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Hambrecht R, Fiehn E, Yu J, Niebauer J, Weigl C, Hilbrich L, Adams V, Riede U, Schuler G. Effects of endurance training on mitochondrial ultrastructure and fiber type distribution in skeletal muscle of patients with stable chronic heart failure. J Am Coll Cardiol 29: 1067–1073, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Hayes SG, Kindig AE, Kaufman MP. Comparison between the effect of static contraction and tendon stretch on the discharge of group III and IV muscle afferents. J Appl Physiol 99: 1891–1896, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Hayes SG, Kindig AE, Kaufman MP. Cyclooxygenase blockade attenuates responses of group III and IV muscle afferents to dynamic exercise in cats. Am J Physiol Heart Circ Physiol 290: H2239–H2246, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Hayes SG, McCord JL, Kaufman MP. Role played by P2X and P2Y receptors in evoking the muscle chemoreflex. J Appl Physiol 104: 538–541, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Hoheisel U, Reinohl J, Unger T, Mense S. Acidic pH and capsaicin activate mechanosensitive group IV muscle receptors in the rat. Pain 110: 149–157, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Howald H, Hoppeler H, Claassen H, Mathieu O, Straub R. Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflügers Arch 403: 369–376, 1985 [DOI] [PubMed] [Google Scholar]
- 15. Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983 [DOI] [PubMed] [Google Scholar]
- 16. Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984 [DOI] [PubMed] [Google Scholar]
- 17. Khan MH, Sinoway LI. Muscle reflex control of sympathetic nerve activity in heart failure: the role of exercise conditioning. Heart Fail Rev 5: 87–100, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Kiilavuori K, Naveri H, Salmi T, Harkonen M. The effect of physical training on skeletal muscle in patients with chronic heart failure. Eur J Heart Fail 2: 53–63, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Kindig AE, Hayes SG, Hanna RL, Kaufman MP. P2 antagonist PPADS attenuates responses of thin fiber afferents to static contraction and tendon stretch. Am J Physiol Heart Circ Physiol 290: H1214–H1219, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Koba S, Xing J, Sinoway LI, Li J. Sympathetic nerve responses to muscle contraction and stretch in ischemic heart failure. Am J Physiol Heart Circ Physiol 294: H311–H321, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Li J, Sinoway AN, Gao Z, Maile MD, Pu M, Sinoway LI. Muscle mechanoreflex and metaboreflex responses after myocardial infarction in rats. Circulation 110: 3049–3054, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Lynn B, Carpenter SE. Primary afferent units from the hairy skin of the rat hind limb. Brain Res 238: 29–43, 1982 [DOI] [PubMed] [Google Scholar]
- 23. Mancini DM, Walter G, Reichek N, Lenkinski R, McCully KK, Mullen JL, Wilson JR. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation 85: 1364–1373, 1992 [DOI] [PubMed] [Google Scholar]
- 24. McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mense S, Meyer H. Different types of slowly conducting afferent units in cat skeletal muscle and tendon. J Physiol 363: 403–417, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mueller PJ. Exercise training and sympathetic nervous system activity: evidence for physical activity dependent neural plasticity. Clin Exp Pharmacol Physiol 34: 377–384, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Mueller PJ. Exercise training attenuates increases in lumbar sympathetic nerve activity produced by stimulation of the rostral ventrolateral medulla. J Appl Physiol 102: 803–813, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Negrao CE, Middlekauff HR. Adaptations in autonomic function during exercise training in heart failure. Heart Fail Rev 13: 51–60, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Negrao CE, Middlekauff HR. Exercise training in heart failure: reduction in angiotensin II, sympathetic nerve activity, and baroreflex control. J Appl Physiol 104: 577–578, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Pan YX, Gao L, Wang WZ, Zheng H, Liu D, Patel KP, Zucker IH, Wang W. Exercise training prevents arterial baroreflex dysfunction in rats treated with central angiotensin II. Hypertension 49: 519–527, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJ. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation 93: 940–952, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Piepoli M, Ponikowski P, Clark AL, Banasiak W, Capucci A, Coats AJ. A neural link to explain the “muscle hypothesis” of exercise intolerance in chronic heart failure. Am Heart J 137: 1050–1056, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Pliquett RU, Cornish KG, Patel KP, Schultz HD, Peuler JD, Zucker IH. Amelioration of depressed cardiopulmonary reflex control of sympathetic nerve activity by short-term exercise training in male rabbits with heart failure. J Appl Physiol 95: 1883–1888, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Rondon E, Brasileiro-Santos MS, Moreira ED, Rondon MU, Mattos KC, Coelho MA, Silva GJ, Brum PC, Fiorino P, Irigoyen MC, Krieger EM, Middlekauff HR, Negrao CE. Exercise training improves aortic depressor nerve sensitivity in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol 291: H2801–H2806, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol 64: 2306–2313, 1988 [DOI] [PubMed] [Google Scholar]
- 36. Sinoway LI, Li J. A perspective on the muscle reflex: implications for congestive heart failure. J Appl Physiol 99: 5–22, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Smith SA, Leal AK, Williams MA, Murphy MN, Mitchell JH, Garry MG. The TRPv1 receptor is a mediator of the exercise pressor reflex in rats. J Physiol 588: 1179–1189, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith SA, Mammen PP, Mitchell JH, Garry MG. Role of the exercise pressor reflex in rats with dilated cardiomyopathy. Circulation 108: 1126–1132, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol 91: 89–102, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Smith SA, Mitchell JH, Naseem RH, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation 112: 2293–2300, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Smith SA, Williams MA, Mitchell JH, Mammen PP, Garry MG. The capsaicin-sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation 111: 2056–2065, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation 81: 518–527, 1990 [DOI] [PubMed] [Google Scholar]
- 43. Wang H, Rivero-Melian C, Robertson B, Grant G. Transganglionic transport and binding of the isolectin B4 from Griffonia simplicifolia I in rat primary sensory neurons. Neuroscience 62: 539–551, 1994 [DOI] [PubMed] [Google Scholar]
- 44. Wang HJ, Li YL, Gao L, Zucker IH, Wang W. Alteration in skeletal muscle afferents in rats with chronic heart failure. J Physiol 588: 5033–5047, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang HJ, Li YL, Zhang LB, Zucker IH, Gao L, Zimmerman MC, Wang W. Endogenous reactive oxygen species modulates voltage-gated sodium channels in dorsal root ganglia of rats. J Appl Physiol 110: 1439–1447, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang HJ, Pan YX, Wang WZ, Gao L, Zimmerman MC, Zucker IH, Wang W. Exercise training prevents the exaggerated exercise pressor reflex in rats with chronic heart failure. J Appl Physiol 108: 1365–1375, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang HJ, Pan YX, Wang WZ, Zucker IH, Wang W. NADPH oxidase-derived reactive oxygen species in skeletal muscle modulates the exercise pressor reflex. J Appl Physiol 107: 450–459, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wilson JR. Exercise intolerance in heart failure. Importance of skeletal muscle. Circulation 91: 559–561, 1995 [DOI] [PubMed] [Google Scholar]