Abstract
Work on schizophrenia demonstrates the involvement of the hippocampus in the disease and points specifically to hyperactivity of CA1. Many symptoms of schizophrenia can be mimicked by N-methyl-d-aspartate receptor (NMDAR) antagonist; notably, delta frequency oscillations in the awake state are enhanced in schizophrenia, an abnormality that can be mimicked by NMDAR antagonist action in the thalamus. Given that CA1 receives input from the nucleus reuniens of the thalamus, we sought to determine whether an NMDAR antagonist in the thalamus can affect hippocampal processes. We found that a systemic NMDAR antagonist (ketamine; 50 mg/kg) increased the firing rate of cells in the reuniens and CA1 in awake rats. Furthermore, ketamine increased the power of delta oscillations in both structures. The thalamic origin of the change in hippocampal properties was demonstrated in three ways: 1) oscillations in the two structures were coherent; 2) the hippocampal changes induced by systematic ketamine were reduced by thalamic injection of muscimol; and 3) the hippocampal changes could be induced by local injection of ketamine into the thalamus. Lower doses of ketamine (20 mg/kg) did not evoke delta oscillations but did increase hippocampal gamma power, an effect not dependent on the thalamus. There are thus at least two mechanisms for ketamine action on the hippocampus: a low-dose mechanism that affects gamma through a nonthalamic mechanism and a high-dose mechanism that increases CA1 activity and delta oscillations as a result of input from the thalamus. Both mechanisms may be important in producing symptoms of schizophrenia.
Keywords: reuniens, schizophrenia, ketamine
schizophrenia involves abnormalities in many brain regions, including the hippocampus (Heckers 2001; Tamminga et al. 2010). Changes in the hippocampus may account for memory abnormalities in the disease but may also affect other brain regions. This has been demonstrated using a major model of the disease, the neonatal injury model. Recent work shows that there is hyperactivity of CA1 in this model and that this hyperactivity affects downstream processes (Lodge and Grace 2007, 2011). Notably, the hippocampus can activate the dopamine system via polysynaptic pathways (Lodge and Grace 2011), which, in turn, affects prefrontal function (Tseng et al. 2006). Importantly, a high-resolution study of the hippocampal blood oxygenation level—dependent (BOLD) signal in schizophrenia shows a particularly strong activation in CA1 (Schobel et al. 2009). It would thus be important to understand potential causes of CA1 hyperactivity.
One possible source of CA1 activation is its thalamic input, which comes exclusively from the midline thalamic nucleus, the nucleus reuniens (Vertes et al. 2006). This nucleus specifically innervates the CA1 region (Vertes et al. 2006), but also activates layer 3 of the entorhinal cortex, a region that also specifically innervates CA1. The potential importance of the thalamic input is underscored by investigations of the NMDAR hypofunction model of schizophrenia (Coyle 1996; Javitt and Zukin 1991). The basis of this model is that NMDAR antagonists, when given to normal subjects, can mimic many of the behavioral symptoms of the disease (Krystal et al. 1994). Furthermore, in animal models, an NMDAR antagonist can mimic (Keilhoff et al. 2004; Zhang et al. 2008) many of the changes in interneurons seen in postmortem studies of schizophrenia (Woo et al. 2004; Zhang and Reynolds 2002). Other animal studies show that an NMDAR antagonist can mimic an electrophysiologic aspect of the disease: an increase in the power of delta frequency oscillations (1–4 Hz). Delta frequency bands (1–4 Hz) are generated within the thalamocortical system and normally predominate during slow-wave sleep (McCormick and Bal 1997). In schizophrenia (SZ), however, delta band power is increased in the awake state in the absence of a task (Clementz et al. 1994), an increase that is correlated with symptom scores of the disease (Czobor and Volavka 1992). The abnormality of delta has been confirmed by meta-analysis of both electroencephalography (EEG) (Boutros et al. 2008) and magnetoencephalography (MEG) studies (Fehr et al. 2001). Source imaging using MEG points to the medioventral prefrontal cortex (PFC) and the temporal lobe as sites of low-frequency oscillations (Llinás et al. 1999). According to some models (Lisman 2011; Lisman et al. 2010; Llinás et al. 1999), these abnormal low-frequency oscillations play a causal role in generating symptoms of the disease by putting the selectively affected subregions into a nonfunctional sleeplike state. Several lines of evidence in rats show that NMDAR antagonist action in the thalamus can stimulate thalamic activity (Sharp and Hendren 2007) and cause delta oscillations. Notably, NMDAR antagonists given systemically, strongly increase the power of delta frequency oscillations in the thalamus and cortex (Buzsáki 1991; Miyasaka and Domino 1968). Indeed, intrathalamic injection of ketamine, or of the more specific NMDAR antagonist, d-(−)-2-amino-5-phosphonovaleric acid (APV), can evoke delta oscillations in the thalamocortical system. Mechanistic studies of this effect (Zhang et al. 2009) point to a role of NR2C and T-type calcium channels in generating this effect. Given this evidence for abnormalities in thalamic function produced by an NMDAR antagonist and evidence implicating the thalamus and hippocampus in schizophrenia (Ferrarelli and Tononi 2011; Heckers 2001; Krause et al. 2003; Tamminga et al. 2010), it is important to understand whether thalamic abnormalities can be transmitted to the hippocampus. Given the importance of theta and gamma frequency oscillations in the normal function of the hippocampus, the introduction of aberrant oscillations could be highly disruptive (Lisman and Buzsáki 2008).
In this study, we sought to determine whether NMDAR antagonist action in the thalamus can induce abnormal activity in the nucleus reuniens, the thalamic nucleus that provides input to the hippocampus and in the hippocampus itself. We found evidence for such abnormalities and then conducted a series of studies to determine whether the abnormalities in the hippocampus resulted from thalamic input. Three types of signals were measured: 1) changes in delta frequency power; 2) changes in gamma frequency power; and 3) changes in cell firing rates. Because anesthesia can itself strongly affect rhythmogenesis in the thalamus (Hemmings and Hopkins 2005), experiments were conducted in unanesthetized animals.
MATERIALS AND METHODS
Subjects.
Male Long–Evans rats (Charles River, Wilmington, MA) were housed under a 12-h light/dark cycle in a temperature- and humidity-controlled environment with free access to food and water. All experimental protocols were approved by the institutional animal care and use committees at Brandeis University.
Surgery.
Rats were anesthetized using an intraperitoneal injection of ketamine/xylazine/acepromozine mixture (100, 5.2, and 1 mg/kg, respectively), with supplemental intraperitoneal injections administered as needed. Each anesthetized rat was placed in a standard stereotaxic device, where its scalp was excised, and holes were bored in its skull for the insertion of five to six ground screws and electrode bundles. Multielectrode bundles (32 nichrome microwires) were inserted into dorsal hippocampal CA1 regions (rostral–caudal: −4.1 mm to bregma; medial–lateral: 2.5 mm to midline; dorsal–ventral: 2 mm to brain surface) or thalamic reuniens nuclei (rostral–caudal: −1.8 mm to bregma; medial–lateral: 1 mm to midline; dorsal–ventral: 6.8 mm to brain surface; 10° to vertical line). For local drug injection into the reuniens, a 27-G guiding cannula was implanted to guide a 30-G injecting cannula. Once in place, the assemblies were cemented to the skull. Rats were given 2 wk to recover from the surgery and to get familiar with the recording environments. Rats were 2.5 mo old at surgery and were about 3 mo old at recording.
Electrophysiological recording and data analysis.
The signal from each electrode was split: one channel (for spikes) was filtered at 300 to 5,000 Hz and sampled at 40,000 Hz; the other channel used to measure local field potentials (LFPs) was filtered at 0.1 to 200 Hz and sampled at 1,000 Hz. Plexon software was used for data recording and storage. Offline Sorter (v2.8.8; Plexon Inc., Dallas, TX) was used to do spike sorting. Single-unit spikes were sorted by a combination of waveform crossing and template methods, after which the data were imported to NeuroExplorer (v3.266) and MATLAB software for further analysis. Putative pyramidal neurons and fast-spiking interneurons were differentiated by their firing rates and distinct spike waveforms based on two parameters: time duration of valley to peak and the ratio of valley amplitude to peak amplitude. LFP traces were visually inspected to exclude movement artifacts and were band-pass filtered in the delta band (1–4 Hz) and gamma band (30–100 Hz) using a digital Chebyshev filter. Instantaneous LFP phase was computed by a Hilbert transform. Spike phase was assigned by their alignment with the LFP trace. Mean resultant length (MRL) was measured to quantify the strength of spike phase modulation as follows: z = Σ (cos θ + i × sin θ)/n, where θ is each spike phase in radians; n is the number of spikes; and MRL is absolute value of z. Student's t-test was used to calculate the P value. Because saline injection has no effects (see results), we have quantified ketamine effects by comparing before and after ketamine injection instead of saline and ketamine injection, unless otherwise noted. Each animal was used only once in each experiment. The sample number n refers to the animal number, which is the same as the session number. For those animals that were used in different experiments (e.g., different ketamine dose), the intervals between the experiments were at least 72 h to prevent possible interference.
Histology.
After the experimental sessions, all rats were deeply anesthetized and perfused through the heart with saline followed by 10% formalin in saline. Seven seconds of DC current (7 μA) were passed through selected microwires in preparation for staining. Brains were removed and immersed in a sucrose formalin mixture, where they remained, refrigerated, until fixed. Sections (40 μm) cut through the implanted areas on a freezing microtome were stained with Prussian blue for ferrous deposits blasted off of the electrode tips and were counterstained with cresyl violet for cell bodies. The animals with unsuccessful placement of the electrodes were excluded from data analysis.
RESULTS
To record local field potentials and spike discharges, electrodes were placed in the CA1 pyramidal cell layer of the dorsal hippocampus, in the nucleus reuniens of the thalamus, or both. Electrode positions were verified by post hoc histologic staining (Fig. 1A). Local field potentials were recorded for 10 min at baseline while the rat freely moved in a rectangular box. Measurements were then made after ketamine injection. The results on delta oscillations, pyramidal cell firing, and gamma oscillations are described separately in the next three sections.
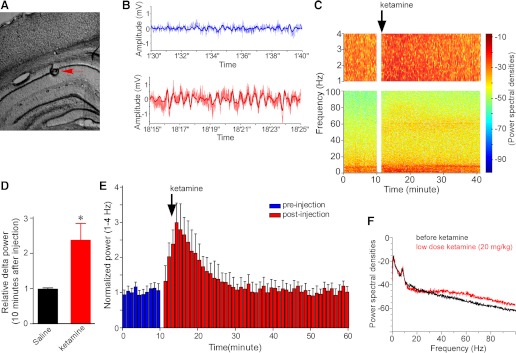
Fig. 1.
Systemic ketamine injection (50 mg/kg) enhanced delta and gamma oscillations in the LFP in the hippocampal CA1 region. A: stain section of the hippocampus shows the electrode position in the CA1 pyramidal cell body layer (red arrow). B: raw LFP traces (noisy) and band-pass filtered traces (smooth) before (top) and after (bottom) ketamine injection. C: power (in pseudocolor) at different frequencies as a function of time. Top: expanded power spectrum of delta band (1–4 Hz); delta and gamma power are increased by ketamine. D: summary data of relative increase in delta power (10 min after injection, n = 28, P < 0.05). E: time course of ketamine-induced increase in delta power; each bin is 1 min (n = 28). F: a power spectrum showing that, using the same animal 48 h later, low-dose ketamine (20 mg/kg) did not change delta power, whereas it increased gamma power.
Effect of ketamine on delta oscillations in CA1.
Raw and filtered traces (Fig. 1B) and plots of power spectra through time (Fig. 1C) show that systemic administration of ketamine (50 mg/kg) strongly enhanced delta oscillations in CA1. Group data reveal that ketamine increased delta power to 238 ± 45% of the baseline level within 10 min after injection (Fig. 1D, n = 28, P < 0.05). On average, the increase reached its maximum within 5 min after injection and returned to baseline within approximately 15 min (Fig. 1E). At this dose of ketamine, rats became sluggish but did not lose consciousness, as evidenced by their frequent locomotion (monitored via video camera). A lower dose of ketamine (20 mg/kg) did not increase delta power (Fig. 1F). Comparable injections of saline did not affect delta power (Fig. 1D). Because systemic ketamine produced a change in the behavioral state of the animal, there is the concern that the change in delta might not be a direct effect of ketamine, but rather secondary to the state change. As we will show later, this possibility can be rejected because local injection of ketamine into the reuniens, a procedure that does not cause changes in behavioral state, produces a similar increase in hippocampal delta power.
Effect of ketamine of delta oscillations in the reuniens.
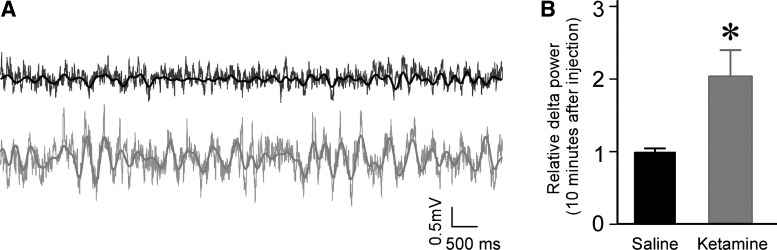
We next tested whether ketamine (50 mg/kg) produced delta oscillations in the nucleus reuniens. As shown in Fig. 2, this was the case (203 ± 35% of baseline, n = 9, P < 0.05). The time course of the elevation in delta power was similar to that found in the hippocampus-delta power in reuniens reached its peak at 5 min after injection and returned to baseline at 15 min after injection (averaged from nine experiments).
Fig. 2.
Systemic ketamine injection (50 mg/kg) enhanced delta oscillation in the nucleus reuniens. A: LFP traces before (black) and after (gray) ketamine injection; filtered and unfiltered traces are superposed. B: summary data showing that ketamine increased delta power in the reuniens (10 min after injection; normalized to before injection, n = 9, P < 0.05).
Causal role of the thalamus.
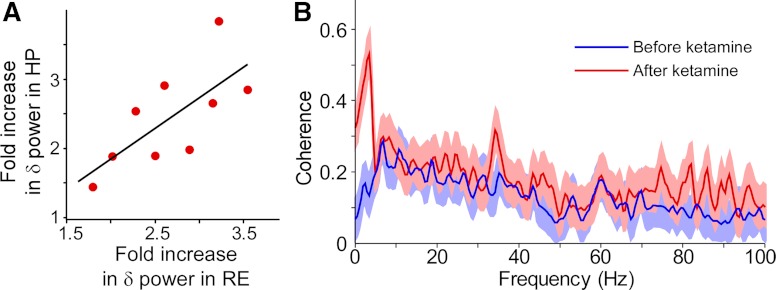
Given the fact that CA1 receives excitatory thalamic input from the nucleus reuniens (Vertes et al. 2006), it was reasonable to suppose that the ketamine-induced oscillations in CA1 might derive from oscillations in the reuniens. Initial experiments explored this possibility by making simultaneous thalamus/CA1 recordings and testing whether the amplitude changes in the two structures were correlated. The percentage increase in delta power varied from animal to animal, but the increase in the hippocampus and reuniens were correlated (r2 = 0.42, P < 0.05, Fig. 3A). Next, we performed coherence analysis of the signals in reuniens and hippocampus. Ketamine injection selectively increased interregional coherence in the delta frequency band (before ketamine: 0.145 ± 0.06; after ketamine: 0.444 ± 0.07, n = 9, P < 0.05, Fig. 3B). Although gamma power was enhanced in both regions, there was no overall coherence increase in this frequency band (Fig. 3B).
Fig. 3.
Relationship between delta oscillations in the reuniens and hippocampus. A: the fold increase in delta power in the hippocampus and reuniens in nine different experiments is correlated (r2 = 0.42, P < 0.05). B: the coherence of LFP between hippocampus and reuniens was selectively increased in the delta frequency range by ketamine. Solid lines are mean coherence; light background areas are 95% confidence intervals.
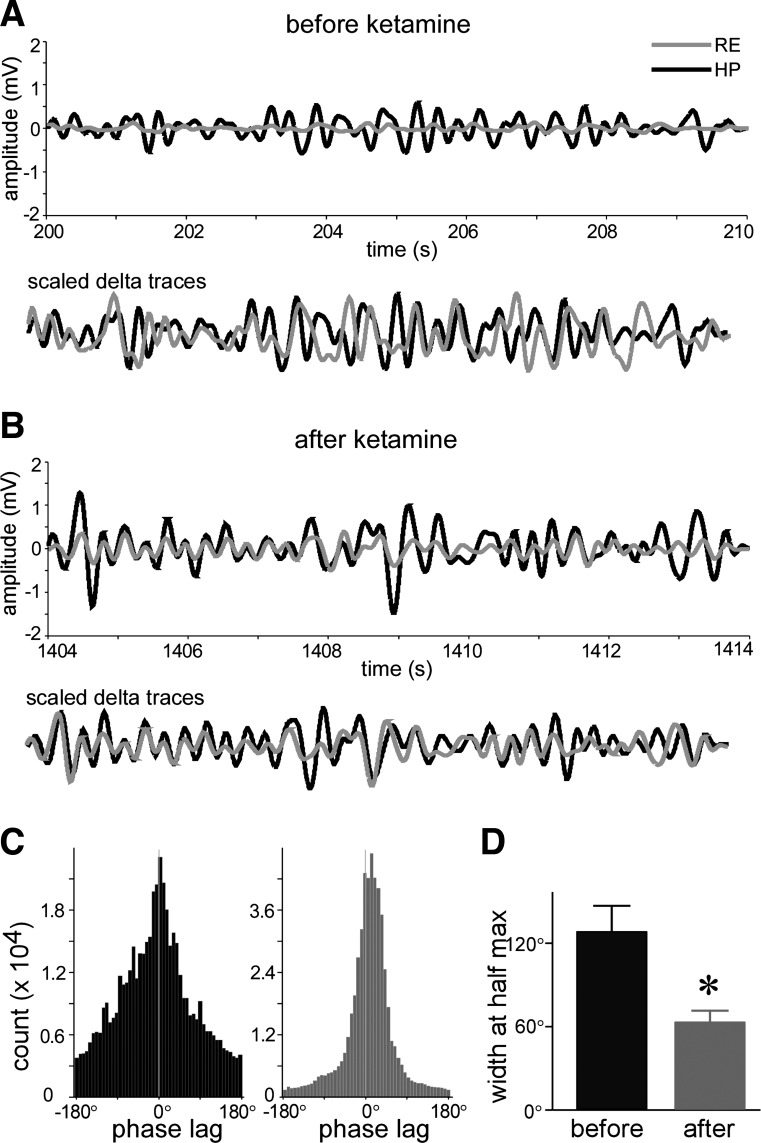
Increases in coherence often reflect greater coupling of instantaneous phase of LFP, which has been argued to reflect the temporal relationship between brain regions (Sehatpour et al. 2008). To explore this possibility, we band-pass filtered the LFP in the delta frequency range (1–4 Hz), computed the LFP phase at each data point, and then measured the phase difference at each time point between reuniens and hippocampus (reuniens phase minus hippocampus phase). The results are shown in Fig. 4. After ketamine injection, phase-locking of delta oscillations between the reuniens and hippocampus was higher. To further quantify the change in instantaneous phase difference, we computed the half-width of the histogram of phase difference (Fig. 4B). After ketamine injection, the half-width was reduced from 123 ± 18 to 64 ± 7.4° (n = 9, P < 0.05, Fig. 4C). This result provides further evidence that delta frequency oscillations in the two structures are more synchronized after ketamine injection.
Fig. 4.
Ketamine increased LFP delta phase synchronization between the hippocampus and reuniens. A, top: filtered traces of delta oscillation in hippocampus and reuniens before ketamine injection (black: hippocampus; gray: reuniens). Bottom: scaled delta oscillations show phase relation. B: same as A, but after ketamine injection. C: histogram of phase difference between the hippocampus and reuniens (black: before ketamine; gray: after ketamine). D: summary data (n = 9) of width at half maximum in C.
A critical issue is whether the LFP of the reuniens and hippocampus reflects local neural activities or signals generated elsewhere and then volume conducted to the recording site. To investigate this issue, we analyzed single-unit spike activity in both the hippocampus and reuniens. Putative pyramidal neurons and fast-spiking interneurons were identified as described in previous studies (see materials and methods) (Maurer et al. 2006). Fast-spike interneurons were excluded from this analysis. To quantify spike phase modulation, we measured each spike phase referenced to the local delta oscillation in the field potential and then computed mean resultant length (MRL), a measure of the degree of phase modulation. As shown in Fig. 5, hippocampal neuronal spikes were weakly modulated by delta oscillation under basal conditions (MRL = 0.112 ± 0.008, n = 54, Fig. 5A). Ten minutes after ketamine injection, the MRL increased to 266 ± 65% of baseline (P < 0.05), indicating enhanced delta modulation. The spikes of reuniens showed a similar increase in delta modulation (increase in MRL = 217 ± 46% of baseline, n = 32, P < 0.05, Fig. 5B). Saline injection had no effects on either firing rate or delta phase modulation. The modulation of hippocampal and reuniens spiking by delta directly demonstrates that neurons in these regions are affected by delta oscillations during ketamine application.
Fig. 5.
Ketamine-induced delta oscillations in the reuniens and hippocampus can be seen in the spiking of single units. A, left: an individual hippocampal neuron shows weak delta-phase modulation before ketamine (black) and stronger delta-phase modulation after ketamine (gray). Right: the mean resultant length, a measure of delta phase-specific firing, is increased by ketamine (black: before ketamine; gray: after ketamine, n = 54 cells). B: same illustration as A, but in reuniens (n = 32 cells).
The above results suggest that oscillatory activity at delta frequency in the hippocampus might be caused by the delta activity of the thalamic reuniens nucleus. To test this hypothesis, we locally injected the γ-aminobutyric acid type A (GABAA) receptor agonist, muscimol (5 μg in 2 μL), into the reuniens to block the activity induced by systemic ketamine (Fig. 6A). Figure 6, B–D shows that such inactivation of the reuniens strongly attenuated the increase in LFP delta activity in the hippocampus caused by ketamine (122 ± 18% of baseline, n = 6, Fig. 6E, P > 0.05 in muscimol compared with 238 ± 45% without muscimol; Fig. 1). Local saline injections into the reuniens did not prevent the increase in delta power induced by systemic ketamine (251 ± 61%, n = 6, P < 0.05). We conclude that delta oscillations in the hippocampus are not generated by a local action of ketamine but, rather, depend on the projection from the nucleus reuniens.
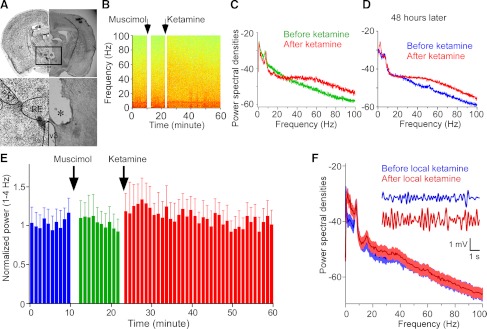
Fig. 6.
Tests of the causal role of the reuniens in increasing delta power in the hippocampus. A: picture shows the position of guiding canulae; ST, striatum; IC, internal capsule; nRT, nucleus reticularis of thalamus; RE, reuniens nucleus; V3, the third ventricle. B: power (pseudocolor) as a function of frequency and time. Injection of muscimol into reuniens prevented the subsequent elevation of delta power by systemic ketamine. C: power spectrum shows that, with pretreatment of muscimol, ketamine failed to increase delta power, whereas gamma power was still increased. D: ketamine was still able to increase delta power in the same animal 48 h later. E: time course of delta power. Injection of muscimol into reuniens prevents the large increase in delta power caused by a subsequent systemic injection of ketamine (n = 6). F: power spectrum shows that local injection of ketamine into the reuniens was sufficient to increase delta power in the hippocampus, whereas gamma oscillation was not affected (n = 5). Inset: filtered delta traces before (blue) and after (red) local ketamine injection into the reuniens.
A further test of the causal role of the reuniens in producing delta in the hippocampus is to locally injected ketamine (8 μg in 2 μL) into the reuniens. We found that this produced a large increase (208 ± 37% of baseline, n = 5, P < 0.05, Fig. 6F) in the delta power in the hippocampus. Because drugs can diffuse approximately 1 mm from the injection site in several minutes (Deniau and Chevalier 1985), ketamine may have spread beyond the reuniens to other thalamic nuclei. However, this spread is unlikely to be of importance because the reuniens provides the only thalamic input to the hippocampus (Vertes et al. 2006). Two important conclusions follow from these experiments. First, the results show that delta locally generated into the thalamus can produce delta in the hippocampus. Second, because the animal's behavior was not affected by ketamine injection into the thalamus, the delta oscillations in the hippocampus cannot be secondary to a change in behavioral state (with systemic ketamine, the animals are sluggish).
Effect of ketamine on firing rates.
The mean firing rate of reuniens neurons was 5.62 ± 1.08 Hz (n = 32, P < 0.05). The rate increased to 286 ± 45% of baseline within 10 min after systemic ketamine (50 mg/kg) injection (n = 32 from 8 rats, P < 0.05, Fig. 5B). Axons from the reuniens innervate the stratum lacunosum moleculare of CA1 and target interneurons and distal dendrites of CA1 pyramidal cells (Vertes et al. 2006). Given that these axons become active in response to NMDAR, as judged by metabolic labeling (Miyamoto et al. 2000), it was of interest to determine whether the net effect of NMDAR antagonist would be excitatory or inhibitory. Under baseline condition, hippocampal pyramidal cells had a mean firing rate of 1.48 ± 0.12 Hz. This was increased to 256 ± 30% of baseline within 10 min after ketamine injection (n = 54, P < 0.05). The ketamine-induced increase in firing rate (baseline: 1.54 ± 0.28 Hz) was reduced, but not eliminated (178 ± 25% with muscimol injection into the reuniens, n = 22 vs. 250 ± 24%, n = 79 without muscimol, P < 0.05), indicating that at least part of the increase in firing rate in the hippocampus is driven by the reuniens input. Low-dose systemic ketamine (20 mg/kg), which did not induce delta oscillation, increased firing rate, but less so than the high dose (167 ± 24% of baseline, n = 52, P < 0.05).
To further test whether ketamine action in the thalamus can affect overall spiking activity in CA1, we monitored the firing of individual CA1 cells just before and 5 min after ketamine injection (10-min periods). Of 12 cells that could be followed (in 4 animals), 7 showed a statistically significant increase, 3 showed a decrease, and 2 were unchanged; taking all spikes together, ketamine injection into the reuniens increased CA1 spiking to 132% of baseline (P < 0.05).
Effect of ketamine of gamma oscillations.
Ketamine injections (50 mg/kg) that increased delta power in the hippocampus also increased gamma (30–100 Hz) power (308 ± 52% of baseline; n = 28, P < 0.05, Fig. 1C). Whereas delta power returned to baseline within an hour, gamma did not, suggesting that ketamine affects the two oscillations via different mechanisms. This is further supported by results with lower doses of ketamine (20 mg/kg); this produced an increase in gamma power, but not delta power (Fig. 1F).
Ketamine also increased gamma power (30–100 Hz) in the reuniens (258 ± 49% of baseline, n = 9, P < 0.05). To test whether the ketamine-induced increase in gamma in the hippocampus depended on the reuniens, we studied the effect of injecting muscimol into the reuniens. The ketamine-induced increase in gamma oscillation power (288 ± 55% of baseline, P < 0.05) was similar to that without muscimol (308 ± 52%; Fig. 6, B and C). Furthermore, injections of ketamine into the reuniens that increased delta did not affect gamma oscillations (106 ± 13% of baseline, n = 5, P < 0.05). These results indicate that the thalamus is not necessary or sufficient for ketamine-induced changes in hippocampal gamma oscillations.
DISCUSSION
Substantial previous work has implicated both the thalamus and hippocampus in schizophrenia (Clinton and Meador-Woodruff 2004; Ferrarelli and Tononi 2011; Heckers 2001; Tamminga et al. 2010), but there has been no previous indication that NMDAR antagonist acting in the thalamus can affect the hippocampus. Such processes might provide an explanation of why CA1, which is the only hippocampal subregion that receives thalamic input, is hyperactive in the disease (Schobel et al. 2009). Here, we show that an NMDAR antagonist increases the firing rate and delta frequency power in the reuniens, the thalamic nucleus that innervates CA1. We further show that this leads to an increase in the overall firing in CA1 and the imposition of delta rhythmicity on the normal CA1 theta oscillations that are necessary for hippocampal function (Shirvalkar et al. 2010). These effects require a high but still subanesthetic concentration of ketamine; lower concentrations produce elevation of gamma but not delta. Taken together, our findings point to three mechanisms by which NMDAR antagonist could affect hippocampal function: 1) at low doses, ketamine increases the power of gamma oscillations in the hippocampus; this effect is not dependent on the thalamic input from the reuniens; 2) at higher doses, ketamine increases delta power in the reuniens, which then drives delta in the hippocampus; 3) at both low and high doses, ketamine increases the overall cell firing in CA1; at least part of the increase is due to excitatory input from the reuniens. In the following sections, we will discuss these processes in more detail.
Elevated firing in CA1.
Ketamine increased the firing rate of both reuniens and CA1 cells. This is consistent with experiments in humans showing that NMDAR antagonist increases the BOLD signal in the thalamus and hippocampus (Deakin et al. 2008). One possibility is that the NMDAR antagonist increases pyramidal cell activity (Jackson et al. 2004) because NMDAR antagonist preferentially block NMDARs on local interneurons and thus disinhibits pyramidal cells (Homayoun and Moghaddam 2007). Our results point to an additional mechanism involving external excitation. Because we find that a substantial fraction of the ketamine-induced increase of firing in CA1 is prevented when the thalamus is inhibited by injection of muscimol, at least some of the ketamine-induced increase in firing rate is likely due to excitatory input from the thalamus.
These findings provide a potential explanation of the enhanced activation (imaging of cerebral blood volume) observed specifically in the CA1 region of schizophrenia patients (Schobel et al. 2009). This increase is correlated with the severity of psychosis. CA1 receives excitatory input from CA3, but this is unlikely to be what makes CA1 hyperactive because CA3 is not hyperactive according to the imaging data. CA1 is the only hippocampal subregion that receives input from the thalamus (specifically from the nucleus reuniens). Thus, our results suggest that the hyperactivity in CA1 seen in SZ could result from elevated reuniens activity. However, the reuniens also innervates layer 3 of the entorhinal cortex (Vertes et al. 2006), a region that is selectively activated by NMDAR antagonist (Vaisanen et al. 2004). Layer 3, in turn, provides input to CA1 in the stratum lacunosum moleculare (but not to dentate or CA3)(Ino et al. 1998). Thus, it is possible that CA1 is excited both by direct reuniens input and by layer 3 entorhinal cells that are excited by the reuniens. Indeed, 2-deoxyglucose labeling shows that axonal inputs in the stratum lacunosium, which arise from layer 3 and the reuniens, are strongly activated by systemic NMDAR antagonist (Miyamoto et al. 2000). Thus both the elevated firing of CA1 cells and elevated activity of input axons could be sources of the elevated CA1 BOLD signal observed in schizophrenia (Schobel et al. 2009).
Mounting evidence points to the hippocampus as a site of dysfunction in schizophrenia (Heckers 2001). The hippocampal abnormalities that have been reported include reduced hippocampal volume, elevated basal perfusion, and impaired activation during cognitive tasks (Heckers 2001; Lisman et al. 2008; Tamminga et al. 2010). Studies in animal models also show that hippocampal memory functions are interfered with by NMDAR antagonist (Chrobak et al. 2008). The functional abnormalities produced by abnormal hippocampal activity are probably due both to local effects on hippocampal processing and to effects of elevated hippocampal activity on downstream brain regions (Deakin et al. 2008). As an example of the latter mechanism, hyperactivity in the hippocampus can increase dopamine release via a polysynaptic pathway (Legault and Wise 2001; Lisman and Grace 2005). Notably, inactivation of hippocampus can completely reverse the elevated dopamine neuron activity in an animal model that raises hippocampal firing rates (Lodge and Grace 2011).
Delta oscillations.
Delta power is elevated in schizophrenia, as summarized in two meta-analyses (Boutros et al. 2008; Fehr et al. 2001). Recent work shows that it is also increased in an animal model of SZ (Sigurdsson et al. 2010). We found that 50 mg/kg ketamine generates delta oscillations in both the thalamus and hippocampus. Similar enhancement of delta has been reported in cortex, in response to both APV and ketamine (50 mg/kg)(Buzsáki 1991; Miyasaka and Domino 1968). The results with APV are important because the high selectivity of this agent argues that the molecular target is the NMDAR. The dose required to induce delta is relatively high, but the animals, although sluggish, are not anesthetized. Furthermore, we found that delta can be induced by injection of ketamine into the thalamus, which has no obvious behavioral effect. Thus, the delta oscillations induced by ketamine are not secondary to a change in behavioral state.
We provide the first evidence that delta originating in the thalamus is imposed on the hippocampus. Three lines of evidence support this conclusion: 1) the delta in the two structures is correlated in amplitude and is coherent; 2) local infusion of ketamine into the reuniens is sufficient to generate delta oscillation in the hippocampus; and 3) inactivation of the reuniens by muscimol substantially attenuates the delta oscillations in hippocampus produced by systemic ketamine injection. Previous work had shown that NMDA antagonist injected into the thalamus could cause cell distress in regions of cortex (Tomitaka et al. 2000). The abnormal electrical activity generated in the thalamus by an NMDAR antagonist is transmitted to the hippocampus and cortex and may be responsible for such damage to target tissues.
Gamma oscillations.
Ketamine also increased gamma power in the hippocampus, consistent with previous reports on gamma in the cortex of awake rats (Kocsis 2011; Ma and Leung 2000; Phillips et al. 2011; Pinault 2008). The effect of a ketamine injection on hippocampal gamma has a much longer duration than the effects on delta, suggesting that different processes are involved. Indeed, we find that unlike delta oscillations, gamma oscillations do not depend on interactions between the hippocampus and thalamus; the ketamine-induced increase in gamma was not blocked by muscimol injection into reuniens. Finally, ketamine induced gamma at concentrations lower than required to induce reliable delta oscillations. Given that NMDAR antagonist applied to hippocampal slices can increase gamma oscillations (Roopun et al. 2008), it seems likely that local processes within the hippocampus produce the increase in gamma frequency power that we observed in vivo.
Implications for the development of symptoms of schizophrenia.
We found that spiking in the hippocampus, which is normally phase coupled to theta oscillations, becomes phase coupled to delta. Thus, the normal synchronization processes crucial for representation of information (Lisman and Buzsáki 2008; Shirvalkar et al. 2010) are altered. This may explain the abnormalities in hippocampal-dependent memory produced by NMDAR antagonist (Chrobak et al. 2008) and the hippocampal-dependent memory disorders in schizophrenia (Heckers et al. 1998). As noted before, we found that only high concentrations of ketamine produce delta, whereas lower concentrations can produce gamma. Consistent with this, low-dose ketamine does not elevate delta in humans and may even reduce it (Hong et al. 2010). Ketamine in moderate dose can produce forms of psychosis, but the incidence and severity of psychosis increases with dose (Krystal et al. 1994). Our finding that gamma, but not delta, is produced by low-dose ketamine suggests that the abnormalities produced by low-dose ketamine are due to gamma rather than to delta, whereas the more severe psychosis produced by high-dose ketamine may be due to delta.
It is thought that gamma abnormalities in schizophrenia are related to interneuron malfunction (Gonzalez-Burgos et al. 2010), a malfunction that is widespread in cortex (Hashimoto et al. 2008) and that may contribute to general cognitive and perceptual abnormalities (Javitt and Zukin 1991). Gamma abnormalities could also contribute to psychosis by disruption of neural codes that depend on gamma (Lisman and Buzsáki 2008; Uhlhaas and Singer 2010).
Elevation of delta (and theta) power in the awake state is consistently observed in schizophrenia (Boutros et al. 2008; Llinás et al. 1999). The severe psychosis of the disease may be related to the severe psychosis caused by high-dose ketamine and result from the delta oscillations produced by high-dose ketamine. According to one model (Lisman et al. 2010), the abnormal delta state in schizophrenia arises from positive feedback in the thalamus-hippocampus-VTA-thalamus loop. It was previously known that hippocampal hyperactivity can excite the VTA (Blaha et al. 1997) and cause dopamine release (Floresco et al. 2001; Lodge and Grace 2007) and that dopamine in the thalamus can promote delta frequency bursting in the thalamus (Zhang et al. 2009). There was, however, no direct evidence that NMDAR action in the thalamus would lead to excitation of the hippocampus. Our results show this to be the case and thus strengthen the overall hypothesis.
How might the presence of delta oscillations produce symptoms of schizophrenia? The reuniens carries information from the PFC to the temporal lobe. Communication between these regions could be disrupted by the presence of delta oscillations in the reuniens and this could potentially account for the growing evidence for a disconnection between these cortical regions in schizophrenia (Marenco et al. 2012; Mitelman et al. 2005; Ragland et al. 2006). One specific interpretation of such disconnection provides a potential explanation of the first rank symptoms of schizophrenia (Ford et al. 2002). According to this class of hypotheses, corollary discharge is sent from action/motor regions in the PFC to more posterior sensory regions, thereby setting expectations of the sensory consequences of one's own actions. It is postulated that such corollary discharge is compromised in schizophrenia, leading to deficits in source monitoring and the resulting feeling that actions are not one's own (this and related symptoms are called “first rank”). Recent work has shown that the corollary discharge information about eye movements is transmitted to the cortex through the thalamus (Sommer and Wurtz 2006). The reuniens might similarly relay corollary discharge from the medial prefrontal cortex (Vertes et al. 2006) (in monkey, this is from areas 25 and 32) (Hsu and Price 2007), a region that represents actions and goals (Matsumoto and Tanaka 2004), to targets in the medial temporal region. Corollary discharge may allow these targets to recognize the sensory consequences of action as self-generated (Poulet and Hedwig 2007). Consistent with this hypothesis, the theta synchronization between prefrontal and temporal areas that is thought to be mediated by corollary discharge is greatly reduced in schizophrenia, particularly in patients with hallucinations (Ford et al. 2002). Our results suggest two possible reasons for this loss of synchronization: 1) desynchronization could occur because delta oscillations interfere with transmission through the thalamus (Steriade et al. 1993) or 2) it could occur because of disruption of the normal oscillatory pattern by imposition of delta on the hippocampus.
Further investigation of the role of the reuniens and other midline thalamic nuclei in schizophrenia is warranted. Among thalamic nuclei, midline nuclei in rats are preferentially affected by antipsychotic drugs (Ma et al. 2003) and by NMDAR antagonist (Vaisanen et al. 2004). In humans, the thalamus is activated by an NMDAR antagonist (Deakin et al. 2008), but the degree to which the midline nuclei are differentially activated is not known. Similarly, it would be important to determine whether this region is preferentially affected in schizophrenia.
GRANTS
This work was supported by National Institutes of Health/National Institute of Mental Health Conte Center Grant 5P50 MH-060450 and National Institutes of Health/National Institute of Mental Health Grant R01 MH-086518.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Y.Z., D.B.K., and J.E.L. conception and design of research; Y.Z. performed experiments; Y.Z. and T.Y. analyzed data; Y.Z., D.B.K., and J.E.L. interpreted results of experiments; Y.Z. prepared figures; Y.Z. and J.E.L. drafted manuscript; Y.Z., D.B.K., and J.E.L. edited and revised manuscript; Y.Z. and J.E.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Caitlin Piette for technical assistance and Nonna Otmakhova and Edwin Richard for helpful comments.
REFERENCES
- Blaha CD, Yang CR, Floresco SB, Barr AM, Phillips AG. Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. Eur J Neurosci 9: 902–911, 1997 [DOI] [PubMed] [Google Scholar]
- Boutros NN, Arfken C, Galderisi S, Warrick J, Pratt G, Iacono W. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophr Res 99: 225–237, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. The thalamic clock: emergent network properties. Neuroscience 41: 351–364, 1991 [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Hinman JR, Sabolek HR. Revealing past memories: proactive interference and ketamine-induced memory deficits. J Neurosci 28: 4512–4520, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, Sponheim SR, Iacono WG, Beiser M. Resting EEG in first-episode schizophrenia patients, bipolar psychosis patients, and their first-degree relatives. Psychophysiology 31: 486–494, 1994 [DOI] [PubMed] [Google Scholar]
- Clinton SM, Meador-Woodruff JH. Thalamic dysfunction in schizophrenia: neurochemical, neuropathological, and in vivo imaging abnormalities. Schizophr Res 69: 237–253, 2004 [DOI] [PubMed] [Google Scholar]
- Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry 3: 241–253, 1996 [DOI] [PubMed] [Google Scholar]
- Czobor P, Volavka J. Level of haloperidol in plasma is related to electroencephalographic findings in patients who improve. Psychiatry Res 42: 129–144, 1992 [DOI] [PubMed] [Google Scholar]
- Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry 65: 154–164, 2008 [DOI] [PubMed] [Google Scholar]
- Deniau JM, Chevalier G. Disinhibition as a basic process in the expression of striatal functions. II. The striato-nigral influence on thalamocortical cells of the ventromedial thalamic nucleus. Brain Res 334: 227–233, 1985 [DOI] [PubMed] [Google Scholar]
- Fehr T, Kissler J, Moratti S, Wienbruch C, Rockstroh B, Elbert T. Source distribution of neuromagnetic slow waves and MEG-delta activity in schizophrenic patients. Biol Psychiatry 50: 108–116, 2001 [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Tononi G. The thalamic reticular nucleus and schizophrenia. Schizophr Bull 37: 306–315, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci 21: 4915–4922, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry 51: 485–492, 2002 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep 12: 335–344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry 165: 479–489, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus 11: 520–528, 2001 [DOI] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci 1: 318–323, 1998 [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Hopkins PM. Foundations of Anesthesia: Basic Sciences for Clinical Practice. Maryland Heights, MO: Reed Elsevier Mosby, 2005, p. 848 [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27: 11496–11500, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, Buchanan RW, O'Donnell P, Thaker GK, Weiler MA, Lahti AC. Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology 35: 632–640, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DT, Price JL. Midline and intralaminar thalamic connections with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol 504: 89–111, 2007 [DOI] [PubMed] [Google Scholar]
- Ino T, Kaneko T, Mizuno N. Direct projections from the entorhinal cortical layers to the dentate gyrus, hippocampus, and subicular complex in the cat. Neurosci Res 32: 241–265, 1998 [DOI] [PubMed] [Google Scholar]
- Jackson ME, Homayoun H, Moghaddam B. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci USA 101: 8467–8472, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148: 1301–1308, 1991 [DOI] [PubMed] [Google Scholar]
- Keilhoff G, Becker A, Grecksch G, Wolf G, Bernstein HG. Repeated application of ketamine to rats induces changes in the hippocampal expression of parvalbumin, neuronal nitric oxide synthase and cFOS similar to those found in human schizophrenia. Neuroscience 126: 591–598, 2004 [DOI] [PubMed] [Google Scholar]
- Kocsis B. Differential role of NR2A and NR2B subunits in N-methyl-D-aspartate receptor antagonist-induced aberrant cortical gamma oscillations. Biol Psychiatry (November 2, 2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M, Hoffmann WE, Hajos M. Auditory sensory gating in hippocampus and reticular thalamic neurons in anesthetized rats. Biol Psychiatry 53: 244–253, 2003 [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51: 199–214, 1994 [DOI] [PubMed] [Google Scholar]
- Legault M, Wise RA. Novelty-evoked elevations of nucleus accumbens dopamine: dependence on impulse flow from the ventral subiculum and glutamatergic neurotransmission in the ventral tegmental area. Eur J Neurosci 13: 819–828, 2001 [DOI] [PubMed] [Google Scholar]
- Lisman J. Excitation, inhibition, local oscillations, or large-scale loops: what causes the symptoms of schizophrenia? Curr Opin Neurobiol November 2, 2011. Online [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Buzsáki G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr Bull 34: 974–980, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci 31: 234–242, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron 46: 703–713, 2005 [DOI] [PubMed] [Google Scholar]
- Lisman JE, Pi HJ, Zhang Y, Otmakhova NA. A thalamo-hippocampal-ventral tegmental area loop may produce the positive feedback that underlies the psychotic break in schizophrenia. Biol Psychiatry 68: 17–24, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci USA 96: 15222–15227, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci 27: 11424–11430, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci 32: 507–513, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Leung LS. Relation between hippocampal gamma waves and behavioral disturbances induced by phencyclidine and methamphetamine. Behav Brain Res 111: 1–11, 2000 [DOI] [PubMed] [Google Scholar]
- Marenco S, Stein JL, Savostyanova AA, Sambataro F, Tan HY, Goldman AL, Verchinski BA, Barnett AS, Dickinson D, Apud JA, Callicott JH, Meyer-Lindenberg A, Weinberger DR. Investigation of anatomical thalamo-cortical connectivity and FMRI activation in schizophrenia. Neuropsychopharmacology 37: 499–507, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Tanaka K. The role of the medial prefrontal cortex in achieving goals. Curr Opin Neurobiol 14: 178–185, 2004 [DOI] [PubMed] [Google Scholar]
- Maurer AP, Cowen SL, Burke SN, Barnes CA, McNaughton BL. Phase precession in hippocampal interneurons showing strong functional coupling to individual pyramidal cells. J Neurosci 26: 13485–13492, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci 20: 185–215, 1997 [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Brickman AM, Shihabuddin L, Newmark R, Chu KW, Buchsbaum MS. Correlations between MRI-assessed volumes of the thalamus and cortical Brodmann's areas in schizophrenia. Schizophr Res 75: 265–281, 2005 [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Leipzig JN, Lieberman JA, Duncan GE. Effects of ketamine, MK-801, and amphetamine on regional brain 2-deoxyglucose uptake in freely moving mice. Neuropsychopharmacology 22: 400–412, 2000 [DOI] [PubMed] [Google Scholar]
- Miyasaka M, Domino EF. Neural mechanisms of ketamine-induced anesthesia. Int J Neuropharmacol 7: 557–573, 1968 [DOI] [PubMed] [Google Scholar]
- Phillips KG, Cotel MC, McCarthy AP, Edgar DM, Tricklebank M, O'Neill MJ, Jones MW, Wafford KA. Differential effects of NMDA antagonists on high frequency and gamma EEG oscillations in a neurodevelopmental model of schizophrenia. Neuropharmacology 62: 1359–1370, 2012 [DOI] [PubMed] [Google Scholar]
- Pinault D. N-Methyl-d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry 63: 730–735, 2008 [DOI] [PubMed] [Google Scholar]
- Poulet JF, Hedwig B. New insights into corollary discharges mediated by identified neural pathways. Trends Neurosci 30: 14–21, 2007 [DOI] [PubMed] [Google Scholar]
- Ragland JD, Valdez JN, Loughead J, Gur RC, Gur RE. Functional magnetic resonance imaging of internal source monitoring in schizophrenia: recognition with and without recollection. Schizophr Res 87: 160–171, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopun AK, Cunningham MO, Racca C, Alter K, Traub RD, Whittington MA. Region-specific changes in gamma and beta2 rhythms in NMDA receptor dysfunction models of schizophrenia. Schizophr Bull 34: 962–973, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, Small SA. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry 66: 938–946, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehatpour P, Molholm S, Schwartz TH, Mahoney JR, Mehta AD, Javitt DC, Stanton PK, Foxe JJ. A human intracranial study of long-range oscillatory coherence across a frontal-occipital-hippocampal brain network during visual object processing. Proc Natl Acad Sci USA 105: 4399–4404, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp FR, Hendren RL. Psychosis: atypical limbic epilepsy versus limbic hyperexcitability with onset at puberty? Epilepsy Behav 10: 515–520, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirvalkar PR, Rapp PR, Shapiro ML. Bidirectional changes to hippocampal theta-gamma comodulation predict memory for recent spatial episodes. Proc Natl Acad Sci USA 107: 7054–7059, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature 464: 763–767, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Influence of the thalamus on spatial visual processing in frontal cortex. Nature 444: 374–377, 2006 [DOI] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Curro Dossi R, Nunez A. The slow (<1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci 13: 3284–3299, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry 167: 1178–1193, 2010 [DOI] [PubMed] [Google Scholar]
- Tomitaka S, Tomitaka M, Tolliver BK, Sharp FR. Bilateral blockade of NMDA receptors in anterior thalamus by dizocilpine (MK-801) injures pyramidal neurons in rat retrosplenial cortex. Eur J Neurosci 12: 1420–1430, 2000 [DOI] [PubMed] [Google Scholar]
- Tseng KY, Amin F, Lewis BL, O'Donnell P. Altered prefrontal cortical metabolic response to mesocortical activation in adult animals with a neonatal ventral hippocampal lesion. Biol Psychiatry 60: 585–590, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci 11: 100–113, 2010 [DOI] [PubMed] [Google Scholar]
- Vaisanen J, Ihalainen J, Tanila H, Castren E. Effects of NMDA-receptor antagonist treatment on c-fos expression in rat brain areas implicated in schizophrenia. Cell Mol Neurobiol 24: 769–780, 2004 [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Do Valle AC, Sherman A, Rodriguez JJ. Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J Comp Neurol 499: 768–796, 2006 [DOI] [PubMed] [Google Scholar]
- Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry 61: 649–657, 2004 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Behrens MM, Lisman JE. Prolonged exposure to NMDAR antagonist suppresses inhibitory synaptic transmission in prefrontal cortex. J Neurophysiol 100: 959–965, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Llinás RR, Lisman JE. Inhibition of NMDARs in the nucleus reticularis of the thalamus produces delta frequency bursting. Front Neural Circuits 3: 20, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res 55: 1–10, 2002 [DOI] [PubMed] [Google Scholar]