Abstract
Human infants can crawl using several very different styles; this diversity appears at first glance to contradict our previous findings from hands-and-knees crawling, which suggested that there were strict limitations on coordination, imposed either mechanically or by the developing nervous system. To determine whether coordination was similarly restricted across crawling styles, we studied free crawling overground in 22 infants who used a number of different locomotor strategies. Despite the wide variety in the use of individual limbs and even the number of limbs used, the duration of the stance phase increased with duration of cycle, whereas the duration of the swing phase remained more constant. Additionally, all infants showed organized, rhythmic interlimb coordination. Alternating patterns (e.g., trotlike) predominated (86% of infants). Alternatively, yet much less frequently, all limbs used could work in synchrony (14% of infants). Pacelike patterns were never observed, even in infants that crawled with the belly remaining in contact with the ground so that stability was not a factor. To explore the robustness of the interlimb coordination, a perturbation that prolonged swing of the leg was imposed on 14 additional infants crawling on hands and knees overground or on the treadmill. The perturbation led to a resetting of the crawling pattern, but never to a change in the coordination of the limbs. The findings concur with those regarding other infant animals, together suggesting that the nervous system itself limits the coordination patterns available at a young age.
Keywords: interlimb, intralimb, locomotion, gait, perturbation
in hands and knees crawling (henceforth called standard crawling), infants use interlimb coordination patterns in which homologous and ipsilateral limbs alternate: trotlike patterns, in which diagonal limbs tend to move together, and patterns with no limb pairing in which one limb is moved at a time in a rhythmic sequence (Burnside 1927; Hildebrand 1967; Patrick et al. 2009). This was what would be expected given the morphology and stability of the infants, as these same gait patterns are used by quadrupeds with relatively short limbs, wide stance, or poor stability (Hildebrand 1976, 1989; Walker 1979; Williams 1981). Infant quadrupeds also use these same patterns of coordination (Hildebrand 1967; Jamon and Clarac 1998; Peters 1983; Vilensky and Gankiewicz 1989). Thus, a role of mechanics is implicated in selection of interlimb coordination. However, in our previous study, human infants were still restricted to trotlike patterns even when they were supported and stability was no longer an issue, or when the functional length of the legs was increased by crawling on hands and feet, which induced more pacelike patterns in adults (Patrick et al. 2009). Further, investigations of neonatal and embryonic animals have demonstrated that the nervous system is capable of producing locomotor-like activity long before the animals themselves are mobile, and that the quadrupedal pattern exhibited is largely trotlike (Ballion et al. 2001; Jamon and Clarac 1998; Juvin et al. 2005; Pflieger et al. 1996; Ryu and Bradley 2009; Stehouwer et al. 1994). Together, these lines of evidence suggest that the nervous system itself may be limiting the coordination.
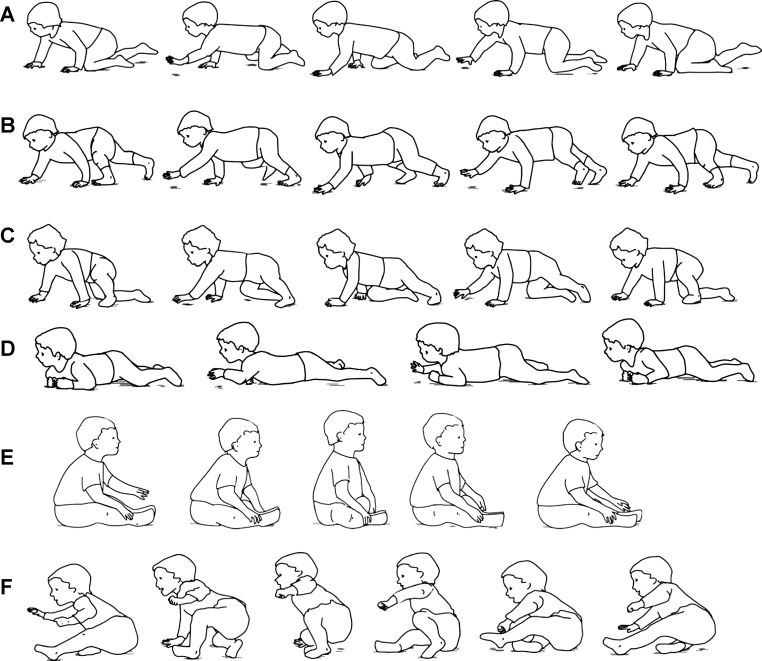
Crawling in human infants, however, is surprisingly diverse, not restricted to the most commonly reported style of hands-and-knees crawling (Fig. 1A; Bottos et al. 1989; Robson 1984). Several forms of crawling have been recognized in the literature, including crawling on hands and feet (Fig. 1B), creeping on the belly (Fig. 1D), scooting (Fig. 1E), and combinations of the different styles (Fig. 1, C and F; Adolph et al. 1998; Bottos et al. 1989; Burnside 1927; Freedland and Bertenthal 1994; McGraw 1941; Robson 1984). These different styles of crawling show great variation in both posture and the use of individual limbs; such diversity raises the question as to whether the more unusual forms of crawling are restricted to the same patterns of quadrupedal coordination as is hands-and-knees crawling (Patrick et al. 2009).
Fig. 1.
Illustration of example crawling styles. Each sequence depicts one cycle and reads from left to right. All sequences start with initiation of stance in the left leg. A: standard crawling. The infant crawls on hands and knees. Following initiation of stance of the left leg (1st panel), the left arm and right leg swing together (2nd panel). Likewise, following initiation of stance of the right leg (3rd panel), the right arm and left leg swing together (4th panel). The cycle ends with stance of the left leg (5th panel). B: hands-and-feet crawling. The pattern of stance and swing is the same as that in A, except that the infant always enters stance on the foot instead of the knee. C: step-crawl mix, using left foot and right knee. The left leg enters stance on the left foot (1st panel). The left arm and right leg swing together (2nd panel) and the right leg goes into stance on the knee (3rd panel). Swing of the right arm and left leg (4th panel) terminate with stance on the left foot (5th panel). D: creeping. The infant crawls with the belly in contact with the ground. As the left arm and right leg are in swing, the left leg extends to push against the ground and the right arm flexes against the ground to pull the infant forward (2nd panel). The reverse occurs in the 3rd panel: swing of the right arm and left leg is accompanied by pushing with the right leg and pulling with the left arm. E: scooting. The infant is seated and uses flexion of the legs to pull the body forward (panels 2, 3). Swing of the legs consists of knee extension (4th panel) to position legs for the start of the subsequence stance phase (5th panel). F: step-scoot mix using three limbs. The infant starts seated on one haunch (1st panel), then leans forward onto a tripod of one hand, one knee (ipsilateral to the arm), and one foot (2nd panel). The infant then swings the leg that was kneeling through (3rd and 4th panels), and finally swings the stepping leg (5th panel), resulting in a return to the seated position from which the cycle would repeat (6th panel).
Most previous studies that include less conventional means of crawling focus on motor development. Descriptions of crawling, especially of the more unique styles, are largely qualitative and often anecdotal. Quantitative data concerning interlimb coordination are sparse, and offered only for creeping (Adolph et al. 1998), hands-and-knees crawling (Adolph et al. 1998; Burnside 1927; Freedland and Bertenthal 1994; Hildebrand 1967; Patrick et al. 2009), and crawling on hands and feet (Patrick et al. 2009). However, study of the different crawling styles offers a unique opportunity to elucidate which aspects of coordination may be limited by the nervous system. For example, creeping, in which the abdomen stays in contact with the ground, removes the exigencies of stability.
In an effort to determine the limitations for coordination in the young nervous system, the current study describes quantitatively a number of uncommon forms of crawling, and identifies unifying features across the locomotor strategies of human and nonhuman infants, including: 1) the regulation of the duration of the stance phase, and 2) the coordination between limbs, regardless of the way in which mobility is achieved.
METHODS
Subjects
Data were obtained from a total of 36 infants recruited from New Mothers' groups of local public health clinics. Two infants that crawled on hands and feet were also included in our previous paper (Patrick et al. 2009) because this style of crawling is rare and, when present, exhibited for only a short period of the child's development. All experiments were performed with informed, written consent of the parent or guardian of the child in accordance with the Declaration of Helsinki Guidelines on Human Experimentation, and with the approval of the local ethics board.
Experimental Protocol
Infants crawling overground.
Infants were encouraged with voice, toys, or food to locomote by their method of choice across a limited area of the floor, or along a narrow corridor with Plexiglas walls, and videotaped. Crawling area provided was limited to ensure that the resolution of the video image was adequate for determination of stance and swing. Pauses, trips, and turning were omitted from analysis. First and last steps of each sequence (i.e., starting or stopping) were also omitted. In general, each analyzed sequence provided data for 2–5 steps (up to 12 steps if advancement was slow, e.g., for scooters and creepers). An effort was made to obtain at least 10 analyzable steps per infant.
Perturbations to crawling.
Perturbations were administered to infants crawling on hands and knees overground or on a treadmill (Gaitway, Kistler Instruments, Amherst, NY), with belt speed set at its lowest setting of 0.22 m/s. Infants crawling on the treadmill wore a body harness, held by an experimenter, for safety. Although we could not guarantee that none of an infant's weight was supported by the harness (it was not instrumented with a force transducer), the experimenter holding the harness took great care to have the infant fully support its own weight. A baton instrumented with a force transducer was used to catch and briefly hold the left thigh soon after initiation of swing of the left leg (estimated visually; actual timing was confirmed with video and the digitized force transducer signal, and ill-timed perturbations were discarded). Successful perturbations were deemed as those that acted to prolong the swing phase of the left leg by at least 15%, but did not cause the infant to trip, fall, or cease crawling. Only successful perturbations with undisturbed cycles immediately before and immediately after were included in the analysis.
Data Collection
All trials were videotaped at 30 frames/s (Samsung Digital-Cam SC-D353; Samsung Electronics,); video was captured onto computer (Adobe Premiere 6.0, Adobe Systems) and deinterlaced off-line to 60 fields/s (VirtualDub, Avery Lee).
To facilitate identification of key anatomic landmarks on the video recording, infants wore snug-fitting clothing onto which were taped reflective markers over the wrist (ulnar styloid process), elbow (lateral epicondyle), shoulder (lateral to the acromion), trunk (lateral midline), hip (greater trochanter), knee (lateral joint line), and ankle (lateral malleolus). If the spherical reflective markers impeded the infant's movements (e.g., markers on the ankles of scooting infants), then flat paper markers were used instead.
Twin-axis electrogoniometers (Penny and Giles, Biometrics, Blackwood Gwent, UK) were fastened across the hip (bilaterally: 30 infants; unilaterally: 1 infant), knee (bilaterally: 16 infants, unilaterally: 1 infant), or shoulder (bilaterally: 2 infants). Baton (force transducer) and/or goniometer signals were amplified and filtered (low-pass 30 Hz) online using custom-made analog gain filters. Surface bipolar EMG recordings were obtained bilaterally from triceps brachii, quadriceps, and hamstrings from 29 infants. Electrode pairs (conductive diameter: 1 cm; Ag/AgCl Kendall Soft-E, Tyco Healthcare Group Canada, Pointe-Claire, Québec) were placed over the muscle belly about 2 cm apart center-to-center. Signals were amplified to achieve a final gain of 1 to 5K, and bandpass filtered from 10 to 1,000 Hz (Octopus AMT-8; Bortec Biomedical, Calgary, Alberta, Canada).
Analog (goniometer, EMG, baton) signals were digitized at 2000 s−1 (Axotape; Axon Instruments, Foster City, CA) and synchronized to the video recording using a digital counter. This counter controlled a light-emitting diode (LED) display viewable by the camera and with a resolution of 0.1 s, and emitted a 5-V pulse every second, which was digitized along with the other signals. All signals were recorded to Video Home System (VHS) tape for backup (A.R. Vetter, Rebersburg, PA).
Data Analysis
All data were analyzed off-line. Stance and swing of each limb were determined from video (Peak Motus, Peak Performance Technologies, or VirtualDub, Avery Lee). For overground crawling, initiation of swing was defined as the point of initiation of forward movement of the limb; initiation of stance was defined as the time at which forward progression ceased and the limb was in contact with the ground. For crawling on the treadmill, initiation of swing was the point at which the limb stopped moving backward with the treadmill; initiation of stance was the point at which the limb was in contact with the treadmill and started moving backward with the treadmill belt. Stance and swing of scooting (overground only) were defined slightly differently: initiation of stance was defined as the point when the ankle stopped moving downward to contact the ground, and initiation of swing was taken as the point in time when the infant's bottom stopped sliding forward and the leg began extending. For all styles of crawling, if a limb was obscured from view such that stance or swing could not be determined within a span of three frames, then that particular stance or swing event was omitted from analysis. One crawling cycle was defined as initiation of stance to initiation of stance of the left leg (or right leg if the left leg was not used by the infant). The rate of crawling was defined for each cycle as the inverse of the duration of the cycle.
Coordination between the limbs was expressed in terms of phasing between initiation of stance of the limbs over a single cycle. Thus, phasing between homologous limbs was expressed as the percentage of the cycle of the left limb at which the right homologous limb entered stance. Ipsilateral phasing was calculated as the percentage of the cycle of the reference leg (usually the left leg) at which the ipsilateral arm entered stance. When crawling is symmetrical (i.e., homologous limbs alternate and duration of stance of the arms was similar to that of the legs) then this ipsilateral phasing is sufficient to classify the gait used (Hildebrand 1966; Patrick et al. 2009). This classification is a continuum in which an ipsilateral phase lag of 0% or 100% indicates ipsilateral limbs are coupled (move together) as in a pace. A phase lag of 50% indicates ipsilateral limbs alternate and therefore diagonal limbs are coupled, as in a trot. A phase lag of 25% or 75% indicates no coupling of limbs, that is, one limb moves at a time in an alternating gait called singlefoot (Hildebrand 1966).
Statistics
Descriptive statistics include mean and SD, or median and interquartile (iq) range if the data were not normally distributed. In general, comparative statistics used parametric tests if data sets were normally distributed and of equal variance, or nonparametric tests otherwise; the use of specific tests is described in the following text. Spearman's rank coefficients (ρ) were used to determine correlation between the duration of stance or swing phases and cycle duration, because the data sets failed tests of normality and constant variance. Paired t-tests were used to compare ρ for stance and swing across styles of crawling. Slopes from linear regression for stance and swing phase duration vs. cycle duration were compared using ANCOVA within each style of crawling or for all data pooled. Paired t-tests were used to compare slopes for stance and swing duration across styles of crawling. Pearson's product moment correlation was used to determine the relationship between ipsilateral phase lag and rate of crawling (to compare with previous data), whereas differences in ipsilateral phase lag or rate across styles were tested for using Kruskal-Wallis ANOVA on ranks. Repeated-measures ANOVA (Bonferroni post hoc) or Kruskal-Wallis ANOVA on ranks (Tukey post hoc) were used to compare steps before, during, and after perturbation; comparisons between only the steps before and after perturbation used paired t-tests. Effects of perturbation overground vs. on the treadmill were compared using t-tests or Mann-Whitney rank-sum tests. Significance was set at 0.05. Statistical analysis was performed using commercially available software (SigmaPlot, Systat Software).
RESULTS
We characterized undisturbed crawling of various styles in 22 infants (9 male; mean age ± SD: 10.4 ± 1.5 mo). Despite substantial differences in the posture of the limbs and position of the body, the crawling styles shared a number of common features in terms of characteristics of stance and swing, and in terms of interlimb coordination. To further investigate the robustness of coordination between the limbs, we perturbed crawling by prolonging the swing phase of the left leg in infants crawling on hands and knees overground (7 infants, 3 male, 10.1 ± 1.4 mo) or on the treadmill (10 infants [3 also crawled overground], 7 male, 9.6 ± 0.9 mo).
Crawling Styles Vary in Use of Limbs and Body Position
The styles of crawling encountered differed in the use of the limbs and posture of the body: Standard crawling (Fig. 1A): crawling on hands and knees, supporting the body above the floor (n = 8; 10.5 ± 1.4 mo); Hands-and-feet crawling (Fig. 1B): similar to standard crawling except weight is supported on the feet instead of the knees (n = 2; 7.5 and 10.3 mo); Step-crawl mix (Fig. 1C): combination of the above-cited styles, using the knee of one leg and the foot of the other (n = 4; 9.6 ± 1.5 mo); Creeping (Fig. 1D): crawling with the belly in contact with the floor (n = 5; 10.3 ± 1.0 mo); Scooting (Fig. 1E): in a seated position, legs working together to pull the infant forward, whereas arms usually do not contribute to propulsion (n = 2; 12.1 and 13.5 mo). The majority of crawling styles used all four limbs, scooting being the main exception. However, other variations were observed. Two creepers used only three limbs (both arms but only one leg). Two infants (PBZ, JPR: 12.4 and 11.2 mo) demonstrated what could be considered a combination of stepping and scooting using three limbs (see Fig. 1F) Finally, one infant (SNA: 11.0 mo) displayed a variation on scooting, using only one leg for propulsion but entering a tripod of both hands and left foot during the propulsion phase. Infants that showed combinations of styles (step-crawl mix, step-scoot [PBZ, JPR], and infant SNA) were grouped together as “mixed crawlers.” Because two infants routinely demonstrated more than one form of crawling, two sets of data are presented for both of these infants, for a total of 24 sets of crawling data.
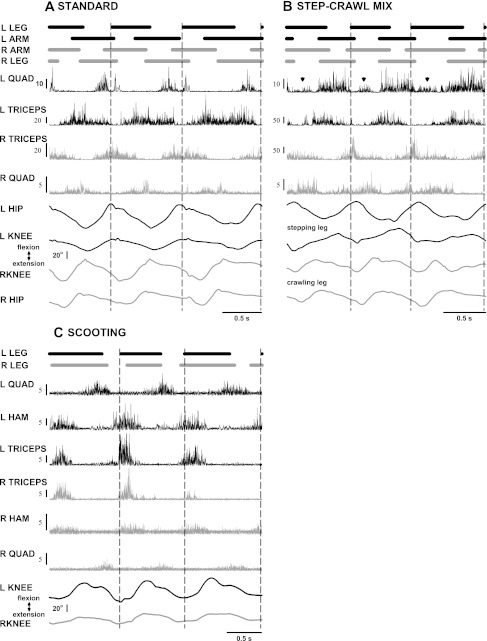
To highlight some of the different ways in which the limbs are used, Fig. 2 presents EMG and goniometer traces of three different styles of crawling in conjunction with footfall patterns (top plots) representing times of stance (solid lines) and swing (spaces between the lines). In standard crawling (Fig. 2A), the quadriceps (most likely the rectus femoris) are active mainly during swing of the leg and act to flex the hip, and triceps brachii are active throughout the stance phase of the arm. The knee and hip extend during stance and flex during the swing phase, with the left and right legs alternating. The mixed crawler (step-crawl mix, Fig. 2B) differs from the standard crawler in the use of one of the legs. In this example, the right leg of the mixed crawler crawls on the knee, demonstrating the same patterns as those for the standard crawler. The left leg, however, steps on the foot, and uses the quadriceps in stance (arrowheads) as well as in swing. The right and left hips show the same pattern as that for standard crawling, extending in stance and flexing in swing. However, the left knee shows the opposite, flexing during stance and extending during the swing phase. Knee flexion during stance of the stepping leg is one strategy for the step-crawl mix of crawling; the other strategy involves extension of both knee and hip during stance (Fig. 1C). The scooter (Fig. 2C) flexes both knees simultaneously during stance, the hamstrings presumably providing the propulsion to pull the body forward, with the quadriceps serving to extend the knee during swing; the hips (not shown) show little movement. It is interesting to note that although the scooter did not use the arms to progress, they were often pushing against the knees during the stance period, as is evident from the triceps activity. This activity could disappear for a cycle or two and then resume in alternation with the quadriceps and in synchrony with the hamstrings.
Fig. 2.
Use of individual limbs in three styles of crawling. Each set of traces consists of: footfall traces (top), indicating when each limb is in stance (solid line) and swing (spaces between the lines); EMG from the left quadriceps, left triceps, right triceps, and right quadriceps, and left and right hamstrings (scooting only); goniometer traces for hip and/or knee bilaterally. Left side traces are in black, whereas right side traces are in gray. A: standard crawling on hands and knees. B: step-crawl mix: this infant crawls on the knee of the right leg (crawling leg) but uses the foot of the left leg (stepping leg). C: scooting. Scale bars for EMG are in μV; each EMG trace was optimized to portray the pattern of activity and was not intended for comparison of amplitude. Upward deflection of the goniometer trace indicates flexion. See text for details.
Stance and Swing Phase Durations Show Similar Characteristics Between Crawling Styles
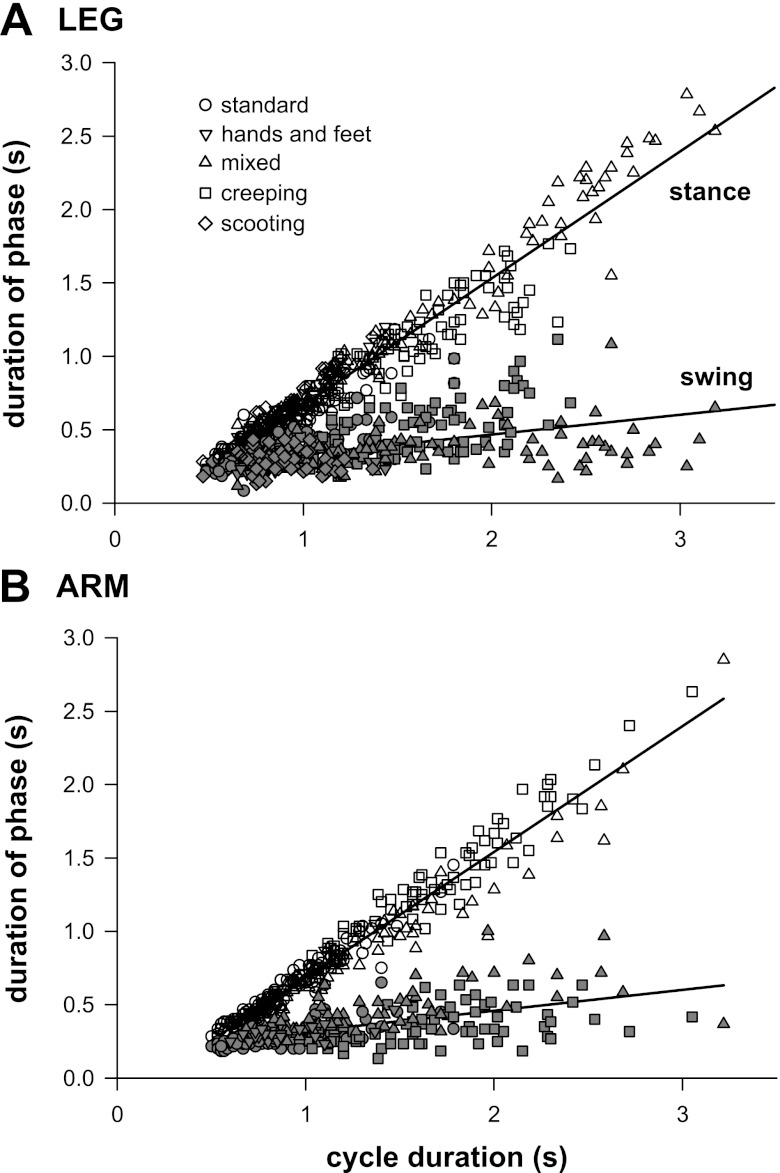
For all styles of crawling, the duration of the stance phase varied more with cycle duration than did the duration of the swing phase. Figure 3 plots the durations of the stance and swing phase against cycle duration for the leg (Fig. 3A) and the arm (Fig. 3B) for individual steps from all sequences of undisturbed overground crawling. The wide range of cycle duration was a result of variation in rate of crawling both within and between infants (see Table 1). Data from the left limbs are plotted for all infants except two, who used only one of the left limbs while crawling, and so for whom the right limbs are plotted. Data for the arm are not available for scooters, because they rarely used the arms in stance and swing, nor for one infant (SNA) who did not use arms for propulsion. Pooling data across styles of crawling, the slope of stance phase duration was significantly different from that of swing phase for both the leg (slope: stance = 0.865, swing = 0.134) and the arm (slope: stance = 0.858, swing = 0.142; ANCOVA), and duration of stance correlated more closely with duration of the crawling cycle than did duration of swing (Spearman's rank coefficient; see legend of Fig. 3). These same relationships held true grouping data by style of crawling: the slope for the stance phase was different from that for the swing phase both within (ANCOVA) and between (paired t-test) crawling styles (Table 1), and Spearman's rank coefficients were also significantly different for stance compared with swing across crawling styles (paired t-tests).
Fig. 3.
Duration of stance correlates more closely with cycle duration than does duration of swing. Data for the leg (A) and the arm (B) are individual cycles from all standard crawlers (circles; 113 cycles leg, 113 cycles arm), hands-and-feet crawlers (downward triangles, 29, 27), mixed crawlers (step-crawl and step-scoot; upward triangles, 113, 76), creepers (squares, 76, 77), and scooters (legs only, diamonds, 54). Open symbols represent stance phase, and filled symbols represent swing. Regression lines are for data pooled across all cycles for all styles. Spearman's rank coefficients (ρ): leg: stance = 0.962, swing = 0.562; arm: stance = 0.979, swing = 0.592; P < 0.05 for all correlations.
Table 1.
Rates of crawling and slopes of regression lines for individual crawling styles
| Slope of Regression Line |
|||||
|---|---|---|---|---|---|
| Leg |
Arm |
||||
| Crawling Style | Rate (cycles/s) | Stance | Swing | Stance | Swing |
| Standard | 1.2 ± 0.3 | 0.65 | 0.36 | 0.81 | 0.19 |
| Hands and feet | 1.2 ± 0.3 | 0.94 | (0.06) | 0.87 | 0.13 |
| Mixed | 0.8 ± 0.3 | 0.94 | 0.06 | 0.80 | 0.20 |
| Creeping | 0.6 ± 0.2 | 0.69 | 0.31 | 0.88 | 0.12 |
| Scooting | 1.2 ± 0.3 | 1.04 | (0.04) | — | — |
Values for crawling rate are means ± SD; steps were pooled for each crawling style. Regression lines are from linear regression of duration of phase (stance or swing) vs. duration of cycle. Parentheses indicate nonsignificance of regression line. Scooters did not use arms in stance and swing.
Interlimb Coordination Showed Similar Restrictions Across Styles of Crawling
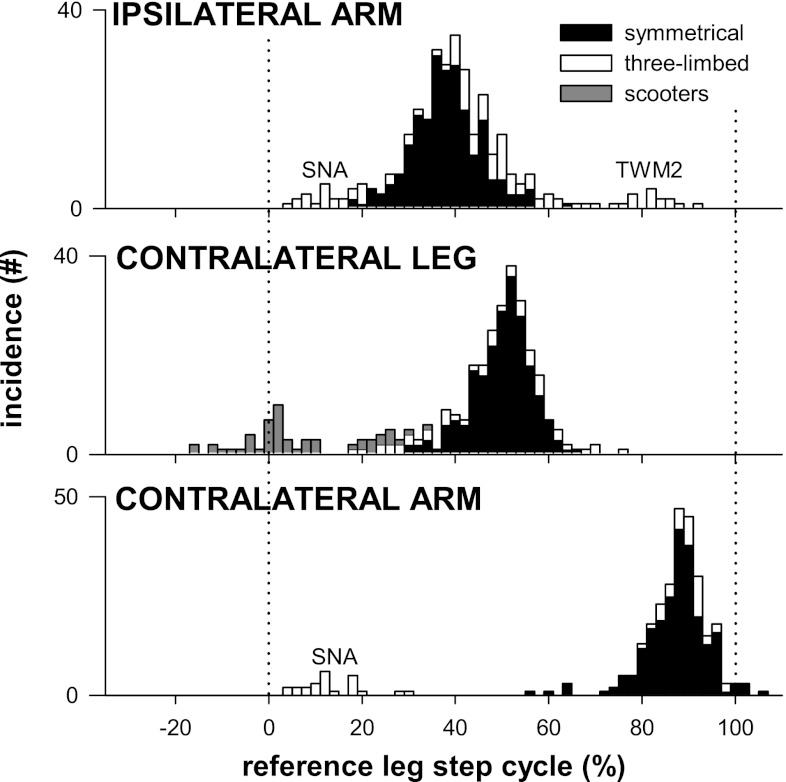
Coordination of the four limbs can be seen from the relative timing of stance phase initiation. Figure 4 presents the timing of the initiation of stance of three limbs with respect to the step cycle of a reference leg for all infants. The reference leg was the left leg for all infants except two, who did not use both the left arm and leg in crawling; in these cases, the right leg was used as the reference. Coordination is categorized as symmetrical, in which homologous limbs entered stance in alternation (Hildebrand 1966; n = 17, black bars), three-limbed, in which the infant used only three limbs to crawl (n = 5, open bars), or scooting (n = 2, gray bars).
Fig. 4.
Interlimb coordination is more typically alternating than synchronous. Each histogram plots the incidence, across all crawling cycles, of initiation of stance of the indicated limb with respect to the step cycle of the reference leg. Histograms for each crawling style are stacked. Whereas scooters (grey bars) and some three-limbed crawlers (open bars: sna, twm2) used limbs in synchrony, most infants showed alternation of homologous and ipsilateral limbs. Bin width: 2%.
Limbs of a homologous pair entered stance either alternately or synchronously. Alternate phasing for the legs was more common, as can be seen in Fig. 4 (middle plot): most instances of stance initiation of the contralateral leg occur around 50% of the cycle of the reference leg. Similarly, arm stance in alternation was also the norm, which can be seen by comparing the top and bottom plots (ipsilateral and contralateral arm, respectively): histogram peaks, at about 40% (top plot) and 90% (bottom plot), are about 50% out of phase with one another. Synchronous phasing between homologous limbs was much less common, but was observed in scooters (gray bars), one creeper (TWM2, arms enter stance together, open bars at far right of top and bottom plots), and a three-limbed crawler (SNA, arms enter stance together, open bars at far left of the top and bottom plots).
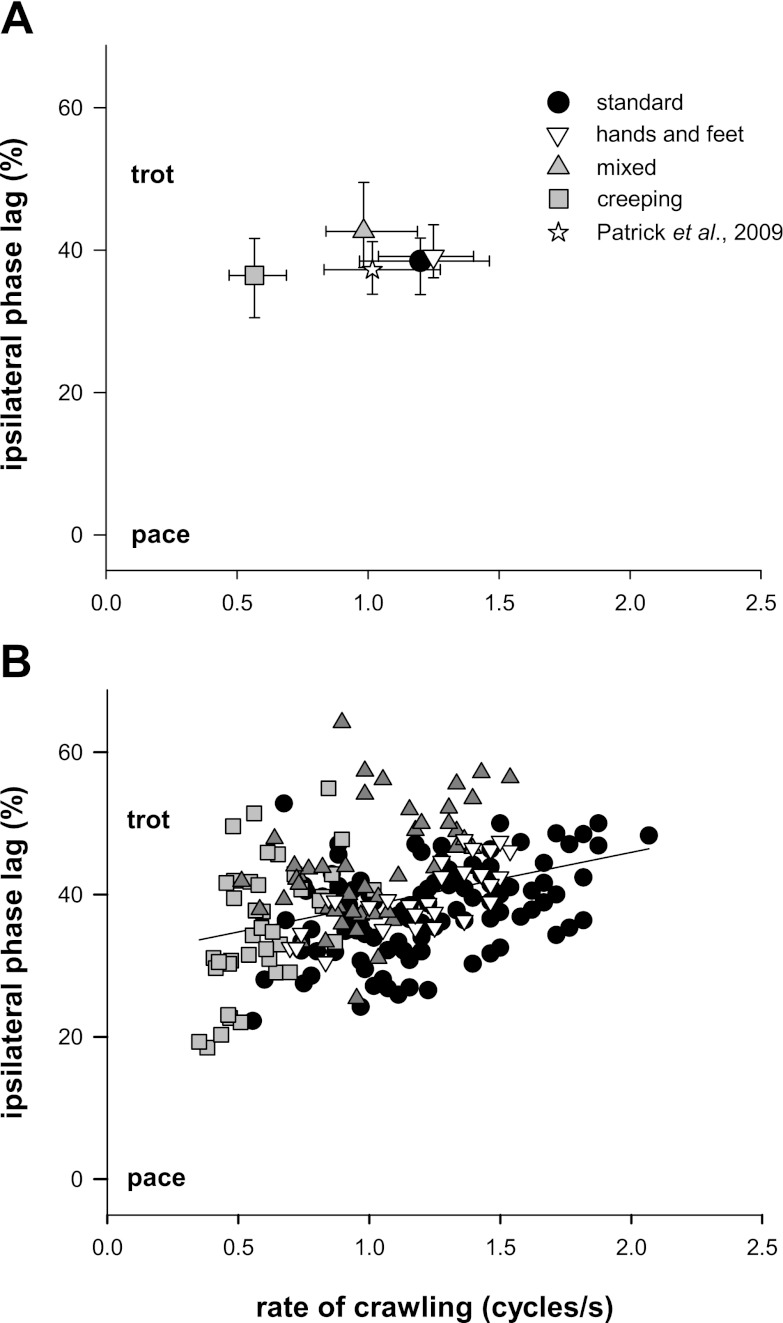
Ipsilateral limbs tended to enter stance roughly in alternation (Fig. 4, top plot, mode = 40%). A modified Hildebrand's method is used to classify symmetrical crawling based on phasing between ipsilateral limbs (Patrick et al. 2009). All patterns theoretically possible form a continuum between trotting, in which ipsilateral limbs move in alternation (phase lag = 50%), to pacing, in which ipsilateral limbs move in synchrony (phase lag = 0% or 100%). All symmetrical crawling styles observed in our infants maintained a coordination that is more trotlike in form than pacelike. Figure 5A presents median ± iq range for each style of crawling. Data from our previous report on standard crawling is superimposed for comparison (Patrick et al. 2009; star). Coordination was restricted to a limited range closer to the trotlike end of the continuum. Figure 5B shows individual cycle for all symmetrical crawlers. No infant used a pacelike form of locomotion. The regression line (slope = 7.459, r2 = 0.15) was remarkably similar to that of the set of 94 cycles of standard crawling we reported previously (Patrick et al. 2009; slope = 6.511, r2 = 0.17, not shown).
Fig. 5.
Interlimb coordination is trotlike across all symmetrical styles of crawling. Ipsilateral phase lag is plotted against rate of crawling. A: median and iq range for each crawling style (symmetrical gaits only). The star represents standard crawling data from our previous paper (Patrick et al. 2009). B: individual cycles from all symmetrical crawlers. Regression line: r2 = 0.15. Data in Fig. 5 for infants crawling on hands and feet are from Patrick et al. (2009).
There were only two cases where ipsilateral limbs moved together (TWM2, SNA, open bars at extremes of top plot in Fig. 4): both were three-limbed crawlers using three limbs in synchrony, so this cannot be considered a pace. The remaining three-limbed crawlers (n = 3) used homologous and ipsilateral limbs in alternation; ipsilateral limb phasing in these cases would be described as trotlike.
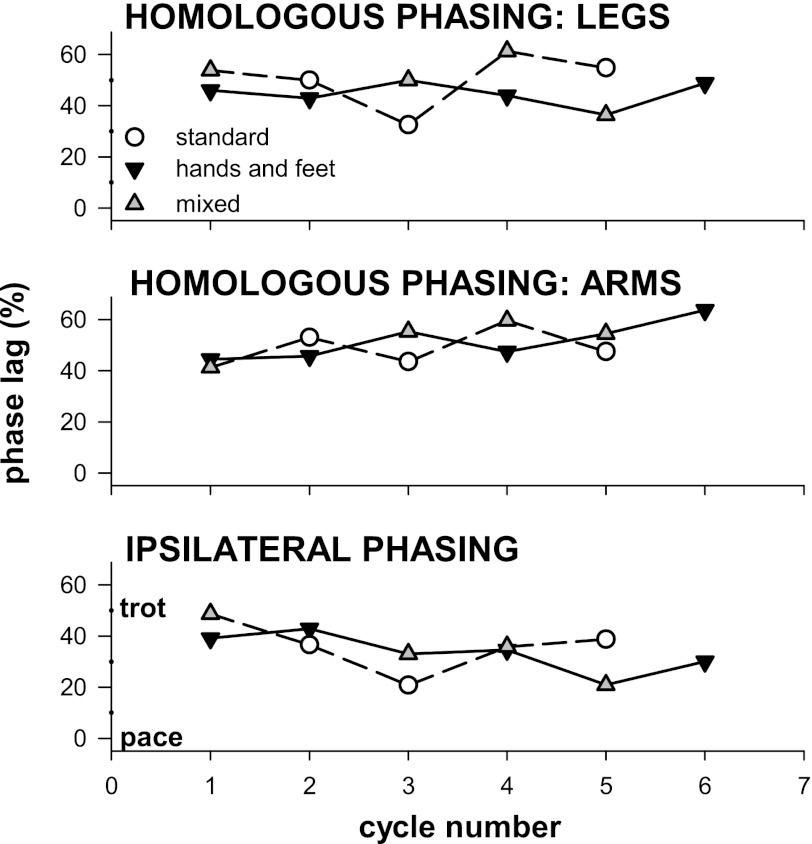
Some infants demonstrated more than one crawling style. In these infants, coordination was similar between styles. For example, one infant (TWM2) used legs together while scooting, and three limbs in synchrony during creeping (the fourth limb was not used). Another infant used four limbs in standard hands-and-knees crawling, or both legs and one arm in a step-scoot combination; in both cases, she used homologous and ipsilateral limb pairs in alternation. A couple of infants transitioned between hands-and-knees crawling and hands-and-feet crawling, or a mixed form that engaged the foot in stance on one leg and the knee on the other. Transitions were smooth and kept phasing between homologous and ipsilateral limbs constant (Fig. 6).
Fig. 6.
Transitions between crawling styles are smooth. Phasing between homologous and ipsilateral limb pairs remains similar despite variations in the use of the limbs over a crawling sequence. Each line represents one crawling sequence; data from two infants are shown. One infant (solid line) switched between hands-and-feet crawling (downward triangles) and a step-crawl mix (upward triangles). The other (broken line) switched between step-crawl mix and standard crawling (circles).
Prolongation of Leg Swing Leads to Resetting of Pattern
The great majority of infants used both homologous and ipsilateral limbs in alternation, in a trotlike coordination. To explore the robustness of this coordination, we recorded the effects of experimenter-induced perturbations in standard crawling.
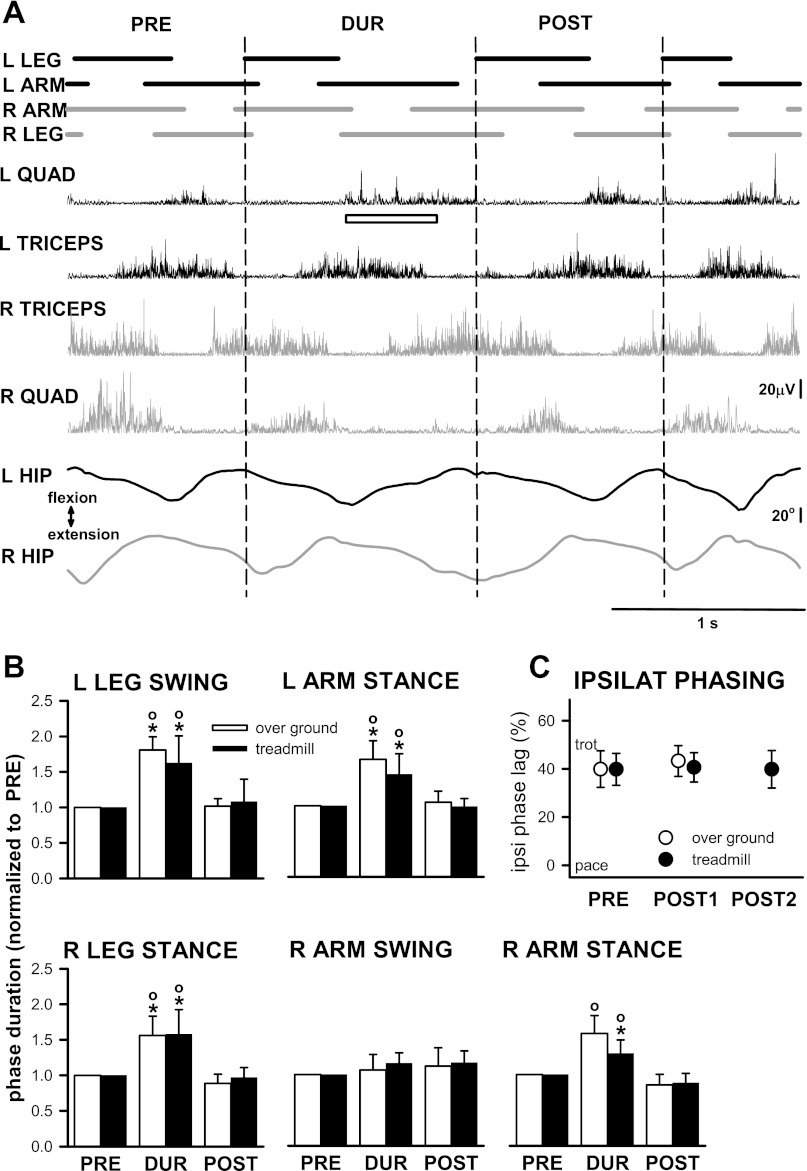
Prolongation of the swing phase of the left leg affected the durations of the stance phase of the other limbs. Successful leg-hold perturbations were achieved in 14 infants during standard crawling on a treadmill (10 infants, 21 perturbations) or overground (7 infants, 9 perturbations; three infants crawled both overground and on the treadmill). The perturbation occurred at a time when the right leg and left arm were in stance, and the right arm was in swing. The top of Fig. 7A shows an example of the stance (lines) and swing (spaces between lines) durations of steps prior to, during, and after the perturbation in an infant crawling overground. Corresponding rectified EMG signals and hip goniometer traces are shown below. The consequence of the perturbation (open horizontal bar) was prolongation of the stance phase of the right leg and left arm, both of which were in progress at the time the perturbation was applied. The right arm, in swing at the time of the perturbation, entered stance after the usual interval and the subsequent stance phase was prolonged instead. This stance phase spans the transition of the left leg from swing to stance, and is considered as belonging to the perturbed cycle.
Fig. 7.
Holding the left leg in swing prolongs stance of the other limbs and resets the crawling pattern. Traces, organized as in Fig. 2, show unperturbed (pre), perturbed (dur), and post (post) crawling cycles for a single perturbation (A). Group data from overground and treadmill perturbations are shown in B. Ongoing stance of the left arm and right leg were prolonged. The right arm, in swing at the time of the perturbation, enters stance at the regular time and the subsequent stance phase is perturbed instead (r arm stance in B). Interlimb coordination, as indicated by ipsilateral phasing, is unchanged by the perturbation (C). Bar plots: means ± SD across infants (B, overground: 6 infants [9 perturbations], treadmill: 9 infants [19 perturbations]); * indicates significant difference from PRE,° indicates significant difference from POST (repeated-measures ANOVA or Kruskal-Wallis ANOVA on ranks). Ipsilateral phasing from individual perturbations (C), no significant difference between groups (paired t-test [overground, 9 perturbations from 7 infants], or repeated-measures ANOVA [treadmill, POST 1: 21 perturbations from 9 infants, POST 2: 16 perturbations from 8 infants]).
Figure 7B pools data across infants, comparing perturbations on the treadmill with those overground. Since without the constraints imposed by the treadmill perturbations could be considerably longer in overground crawling, perturbations included in the comparison were restricted to a range found both on the treadmill and overground (69.8 ± 32.2% prolongation of swing of the left leg, across infants). In both situations, prolongation of swing of the left knee produced prolongation of stance of the right leg and left arm. Swing of the right arm was not significantly affected, but the subsequent stance phase was prolonged. The step cycle following the perturbation was not significantly different from the cycle immediately prior to the perturbation (post). There were no differences between results for overground and treadmill (t-tests or Mann-Whitney rank-sum tests). The effects of the perturbation were the same when all overground or all treadmill perturbations were taken into account (not shown).
The perturbation led to a resetting of the coordination pattern. All infants used a trotlike pattern prior to the perturbation. Steps following the perturbation retained alternation of homologous limbs (not shown) and trotlike coordination patterns (Fig. 7C). Only one step after the perturbation (post1) could be obtained overground due to the limited area provided for crawling. However, data were obtained for up to 8 steps following the perturbation on the treadmill. The mean ipsilateral phase lag value for the second step following the perturbation (post2) is shown for treadmill crawling in Fig. 7C; all subsequent steps recorded also continued to use a trotlike coordination (repeated-measures ANOVA).
DISCUSSION
Herein, we characterized aspects of intra- and interlimb coordination in various styles of free crawling in infants. We found that despite the many different ways in which infants used their limbs for progression, several features remained constant. The duration of the stance phase was invariably more closely correlated to cycle duration than was the duration of the swing phase for all involved limbs in all forms of crawling. Phasing of homologous limbs was either alternating or, less commonly, synchronous. Ipsilateral phasing was alternating for all symmetrical crawlers. No infant was seen to pace. Perturbations that prolonged the swing phase of the left knee did not alter interlimb coordination, but instead led to a resetting of the crawling pattern. Thus, despite the diversity of limb use and posture, coordination is restricted across a wide range of crawling styles in human infants.
Duration of the Stance Phase Varies With the Cycle Duration Regardless of Crawling Style
Regardless of the use of individual limbs, the stance phase of each limb was found to correlate more strongly with cycle duration than did the swing phase for all styles of crawling. This relationship has been documented for bipedal walking (Grillner et al. 1979; Herman et al. 1976) and running (Grillner et al. 1979) in human adults, as well as for locomotion in many adult animals (Courtine et al. 2005; Cruse and Warnecke 1992; Earhart and Stein 2000; Górska et al. 1998; Halbertsma 1983; Pridmore 1992; Williams 1981). It appears to hold for infant animals as well (Jacobson and Hollyday 1982; Vilensky and Gankiewicz 1989), and has been described in bipedal stepping in human infants (Thelen 1986; Yang et al. 1998). Our recent work showed that in young children the modulation of phase duration during rhythmic leg movements such as kicking and stepping was related to load experienced during that phase, with increased load resulting in increased length of the phase duration (Musselman and Yang 2007). In this previous study, the durations of flexion and extension phases could be differentially modified by differential loading. During crawling in the present study, the stance phase could occur when the limb was undergoing extension (legs of creepers and standard, hands-and-feet, and some mixed crawlers), or flexion (arms of creepers, legs of scooters and some mixed crawlers) (see Figs. 1 and 2). In either case, the stance phase was undergoing load, and correlated better with cycle duration than did the swing phase. Thus, the concept that the loaded phase varies more closely with cycle duration holds for human infants across crawling styles.
All Children Showed Organized Rhythmic Interlimb Coordination
All of our infants demonstrated organized, rhythmic locomotor patterns, including our creepers. In contrast to our findings, other researchers have reported largely only random or inconsistent coordination in creeping (Adolph et al. 1998; Freedland and Bertenthal 1994; McGraw 1941). Careful scrutiny of the data presented by Adolph (1998, Figure 3), however, suggests that their creepers used a singlefoot coordination, an alternating coordination in which one limb is moved at a time in an invariant sequence. Our infants were also observed to use singlefoot and, additionally, all alternating creepers clearly showed use of trotlike coordination in 47–100% of their steps, including one infant who used only three limbs. Based on the low incidence of diagonal coupling observed prior to hands-and-knees crawling in their six infants, Freedland and Bertenthal (1994) speculated that the acquisition of the hands-and-knees posture is at least in part responsible for the emergence of the trotlike coordination pattern. Our data, however, suggest that the mechanism of producing a trotlike pattern of interlimb coordination is functioning before crawling styles that lift the belly off of the ground present. This is in agreement with observations that the nervous systems of neonates and even embryos of other animals are capable of well-coordinated locomotion patterns well before the animals normally display walking behaviors (Ballion et al. 2001; Jamon and Clarac 1998; Juvin et al. 2005; Pflieger et al. 1996; Ryu and Bradley 2009; Stehouwer et al. 1994).
Interlimb Coordination Largely Restricted to Alternating Patterns
Despite the different styles of crawling, most of our infants used a trotlike coordination pattern, in which both homologous and ipsilateral limbs work in alternation. An alternating coordination pattern appears to be the norm among neonatal vertebrates (Bekoff and Trainer 1979; Blumberg-Feldman and Eilam 1995; Cazalets et al. 1990; Eilam 1997; Ho 1997; Jamon and Clarac 1998; Johnston and Bekoff 1996; Nakano 1996; Peters 1983; Pridmore 1992; Stuurman and Van Hof 1979; Vilensky et al. 1989). Even developing anurans exhibit alternating then, later, synchronous activity of the hindlimbs during the transition from tadpole to frog (Hughes and Prestige 1967). The strong preference of the infants in the current study for alternating (86%) compared with synchronous (14%) coordination (Fig. 4) is in contrast to what was observed in bipedal locomotion, in which human infants showed an approximately equal preference for either coordination of the legs (alternate: 41%; synchronous: 51%; Musselman and Yang 2008). Interestingly, most of the cases of synchronous coordination seen in the current study were in children who scoot, during which the arms play a small role in progression. When more than two limbs are used, alternating use of the limbs is much more common than synchronous. Thus, it is possible that quadrupedal modes of locomotion place additional constraints on coordination of the limbs, forcing the limbs to alternate (see below).
As noted earlier, in very few cases, the infants used their limbs in synchrony instead of alternation. In these cases, either two homologous limbs were used in scooting, or three limbs worked together with the fourth contributing little. Occasionally, synchronous use of homologous (but not ipsilateral) limbs (Bradley and Smith 1988; Davenport 1987; Fayein and Viala 1976; Hughes and Prestige 1967; Jacobson and Hollyday 1982; Stuurman and Van Hof 1979) or synchronous activity of all four limbs (Davenport 1987) has also been reported in neonatal vertebrates. Thus, a synchronous pattern may be used by both human infants and neonate animals, although a trotlike pattern is much more common. In no case, however, do ipsilateral limbs as a pair work in synchrony; in other words, pacelike coordinations are never seen.
A few of the infants locomoted using more than one style of crawling. In these cases, the coordination pattern (trotlike or synchronous) remained the same across styles of crawling, suggesting a preference of the child for one type of interlimb coordination. There are several other examples of infant animals using the same interlimb coordination across varying motor activities. For example, in newly hatched chicks, stepping, swimming, and airstepping show the same alternation of limb movements (Johnston and Bekoff 1996) despite different kinematic (Johnston and Bekoff 1992) and EMG patterns of activity (Johnston and Bekoff 1996). Neonatal rats swimming (Bekoff and Trainer 1979; Cazalets et al. 1990; McEwen et al. 1997) and stepping (Jamon and Clarac 1998; Juvin et al. 2005) do so with the same trotlike coordination pattern. Hatchling leatherback turtles use synchronous coordination of the forelimbs to swim and of all four limbs to crawl (Davenport 1987). Overall, interlimb coordination patterns in infants appear limited across species to mainly trotlike or less commonly synchronous, with animals showing preferential use of one coordination across activities.
Deletion of Limb Use in Crawling
Several infants crawled using only three limbs. This is in concordance with other human infant studies that have reported three-limb locomotion (Adolph et al. 1998; Burnside 1927; McGraw 1941) or creeping using only two diagonal (Burnside 1927) or two homologous limbs (Adolph et al. 1998; Bottos et al. 1989; Freedland and Bertenthal 1994). Rhythmic locomotor activity using fewer than four limbs has also been observed in infant quadrupeds (Bekoff and Trainer 1979; McCrea et al. 1994; McEwen et al. 1997). The ability of neonatal rats to use one to four limbs in swimming was cited as evidence of independent pattern generators for each limb (Bekoff and Trainer 1979). The ability of human infants to step with just one limb (Pang and Yang 2001), or to step forward with one leg while stepping backward with the other (Yang et al. 2005) suggests this is also the case in human infants. Infants crawling using two, three, or four limbs now offer further evidence.
Is the Absence of Pacing Due to Mechanical or Neurological Factors?
Although it is physically possible for the nervous system to use ipsilateral limbs together (as demonstrated by infants using three limbs in synchrony), pacelike gaits were never observed. Is this restriction of interlimb coordination a result of mechanics or the state of the nervous system? Singlefoot and trotlike gaits offer greater stability than that of pacelike patterns, and are thus commonly used by animals with relatively shorter limbs (and therefore wider stance), slower gaits, or poorer balance (Hildebrand 1976, 1989; Walker 1979; Williams 1981). We previously reported that infants persisted in using a trotlike gait while unloaded during hands-and-knees crawling, despite no mechanical need to maintain stability (Patrick et al. 2009). In the current study, infants creeping on their bellies also used a trotlike gait, or else used three limbs in synchrony; again, a pacelike gait was never seen. Thus, stability does not appear to be the reason for the absence of a pacelike coordination. Pharmacological treatment of intact animals has indicated that the capabilities of the nervous system may be greater than that exhibited in normal behavior. For example, whereas only trotlike and singlefoot coordinations are seen in neonatal rats (Bekoff and Trainer 1979; Cazalets et al. 1990), l-DOPA (but not vehicle) administration to these animals can produce air-galloping (McCrea et al. 1994; McEwen et al. 1997). However, trotlike patterns appear the default (Ballion et al. 2001; Juvin et al. 2005), and pacelike patterns have not been shown even with pharmacologic manipulations in reduced preparations (note variability in in vitro preparations; c.f., Figure 8A in Ballion et al. 2001). Thus, it appears that the young nervous system may not yet be capable of expressing pacelike forms of coordination. This is consistent with the hypothesis that there exist at birth basic, “primitive” patterns of locomotion, possibly common across species, to which are added more complexity over the course of development (Dominici et al. 2011).
Perturbations Reset the Crawling Pattern
Holding the left leg in swing in standard crawling prolonged the stance phase of the other limbs in agreement with other studies examining two limbs in locomotion (Dietz et al. 1986; Duysens and Stein 1978; Matsukawa et al. 1982; Pang and Yang 2001), prolongation of stance being consistent with maintenance of stability. It could be argued that in the current study, holding the leg interfered with the forward progression of the infant, thus resulting in all of the legs stopping; however, the effect was the same on the treadmill, suggesting the perturbation itself affected the neural network controlling timing of the locomotor pattern. The lack of a change in gait pattern in all steps subsequent to the perturbation is further evidence of a resetting.
Why Do Infants Use Different Crawling Styles?
Although there may be developmental and genetic factors involved (Bottos et al. 1989; Robson 1970, 1984), it is also possible that these different forms of crawling are infants' different solutions to a problem: how to get somewhere. Bottos (1989) suggests a number of factors may be involved in the “locomotor choices” of prewalking infants. Indeed, it has been suggested that in the late 1800s and early 1900s, less conventional crawling forms may have been encouraged by period gownlike dress that precluded crawling on hands and knees (Burnside 1927). Similarly, one of our infants crawled with hands and knees indoors, but with hands and feet on the grass. Sloped substrates induced some young (8- to 9-mo-old) infants to modify their style of crawling (Adolph et al. 1993), and more experienced crawlers to use a number of descent strategies including sliding in a seated or prone position (Adolph et al. 1997). Crawling infants have been noted to descend stairs by crawling backward or scooting (Berger et al. 2007), and even 3-mo-old infants can use different solutions to trigger a sensor-driven mobile (Angulo-Kinzler et al. 2002). It would not be unreasonable to expect that the problem-solving abilities of infants come into play in producing the early stages of locomotion. Regardless, the infants are limited as to the coordination patterns available.
Conclusion
Despite the quite varied means by which infants may crawl, several characteristics of intra- and interlimb coordination remain consistent. Most notably, a trotlike gait pattern is predominant, even in infants that use only three limbs. Synchronous use of the limbs appears the only other (and less common) alternative. Pacelike patterns appear to require a more mature nervous system in both humans and other species.
GRANTS
J. F. Yang was funded by the Canadian Institutes of Health Research and Natural Sciences and Engineering Research Council of Canada. J. A. Noah was funded by a fellowship from the National Institute for Health Research, USA.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.K.P., J.A.N., and J.F.Y. conception and design of research; S.K.P., J.A.N., and J.F.Y. performed experiments; S.K.P. and J.A.N. analyzed data; S.K.P. and J.F.Y. interpreted results of experiments; S.K.P. prepared figures; S.K.P. drafted manuscript; S.K.P. and J.F.Y. edited and revised manuscript; S.K.P., J.A.N., and J.F.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Keir Pearson and Simon Gosgnach for helpful comments on the manuscript, and R. Vishram for excellent technical assistance.
Present address: J. A. Noah, ADAM Center, Long Island University, Brooklyn, NY 11201.
REFERENCES
- Adolph KE, Bertenthal BI, Boker SM, Goldfield EC, Gibson EJ. Learning in the development of infant locomotion. Monogr Soc Res Child Dev 62: I–VI, 1–140, 1997 [PubMed] [Google Scholar]
- Adolph KE, Eppler MA, Gibson EJ. Crawling versus walking infants' perception of affordances for locomotion over sloping surfaces. Child Dev 64: 1158–1174, 1993 [PubMed] [Google Scholar]
- Adolph KE, Vereijken B, Denny MA. Learning to crawl. Child Dev 69: 1299–1312, 1998 [PubMed] [Google Scholar]
- Angulo-Kinzler RM, Ulrich B, Thelen E. Three-month-old infants can select specific leg motor solutions. Motor Control 6: 52–69, 2002 [DOI] [PubMed] [Google Scholar]
- Ballion B, Morin D, Viala D. Forelimb locomotor generators and quadrupedal locomotion in the neonatal rat. Eur J Neurosci 14: 1727–1738, 2001 [DOI] [PubMed] [Google Scholar]
- Bekoff A, Trainer W. The development of interlimb co-ordination during swimming in postnatal rats. J Exp Biol 83: 1–11, 1979 [DOI] [PubMed] [Google Scholar]
- Berger SE, Theuring C, Adolph KE. How and when infants learn to climb stairs. Infant Behav Dev 30: 36–49, 2007 [DOI] [PubMed] [Google Scholar]
- Blumberg-Feldman H, Eilam D. Postnatal development of synchronous stepping in the gerbil (Gerbillus dasyurus). J Exp Biol 198: 363–372, 1995 [DOI] [PubMed] [Google Scholar]
- Bottos M, Barba BD, Stefani D, Pettenà G, Tonin C, D'Este A. Locomotor strategies preceding independent walking: prospective study of neurological and language development in 424 cases. Dev Med Child Neurol 31: 25–34, 1989 [DOI] [PubMed] [Google Scholar]
- Bradley NS, Smith JL. Neuromuscular patterns of stereotypic hindlimb behaviors in the first two postnatal months. I Stepping in normal kittens Brain Res 466: 37–52, 1988 [DOI] [PubMed] [Google Scholar]
- Burnside LH. Coordination in the locomotion of infants. Genet Psychol Monogr 2: 279–372, 1927 [Google Scholar]
- Cazalets JR, Menard I, Cremieux J, Clarac F. Variability as a characteristic of immature motor systems: an electromyographic study of swimming in the newborn rat. Behav Brain Res 40: 215–225, 1990 [DOI] [PubMed] [Google Scholar]
- Courtine G, Roy RR, Hodgson J, McKay H, Raven J, Zhong H, Yang H, Tuszynski MH, Edgerton VR. Kinematic and EMG determinants in quadrupedal locomotion of a non-human primate (Rhesus). J Neurophysiol 93: 3127–3145, 2005 [DOI] [PubMed] [Google Scholar]
- Cruse H, Warnecke H. Coordination of the legs of a slow-walking cat. Exp Brain Res 89: 147–156, 1992 [DOI] [PubMed] [Google Scholar]
- Davenport J. Locomotion in hatchling leatherback turtles Dermochelys coriacea. J Zool Lond 212: 85–101, 1987 [Google Scholar]
- Dietz V, Quintern J, Boos G, Berger W. Obstruction of the swing phase during gait: phase-dependent bilateral leg muscle coordination. Brain Res 384: 166–169, 1986 [DOI] [PubMed] [Google Scholar]
- Dominici N, Ivanenko YP, Cappellini G, d'Avells A, Mondì V, Cicchese M, Fabiano A, Silei T, Di Paolo A, Giannini C, Poppele RE, Lacquaniti F. Locomotor primitives in newborn babies and their development. Science 334: 997–999, 2011 [DOI] [PubMed] [Google Scholar]
- Duysens J, Stein RB. Reflexes induced by nerve stimulation in walking cats with implanted nerve cuff electrodes. Exp Brain Res 32: 213–224, 1978 [DOI] [PubMed] [Google Scholar]
- Earhart GM, Stein PSG. Step, swim, and scratch motor patterns in the turtle. J Neurophysiol 84: 2181–2190, 2000 [DOI] [PubMed] [Google Scholar]
- Eilam D. Postnatal development of body architecture and gait in several rodent species. J Exp Biol 200: 1339–1350, 1997 [DOI] [PubMed] [Google Scholar]
- Fayein NA, Viala D. Development of locomotor activities in young chronic spinal rabbits. Neurosci Lett 3: 329–333, 1976 [DOI] [PubMed] [Google Scholar]
- Freedland RL, Bertenthal BI. Developmental changes in interlimb coordination: transition to hands-and-knees crawling. Psychol Sci 5: 26–32, 1994 [Google Scholar]
- Górska T, Majczyński H, Zmysłowski W. Overground locomotion in intact rats: contact electrode recording. Acta Neurobiol Exp (Wars) 58: 227–237, 1998 [DOI] [PubMed] [Google Scholar]
- Grillner S, Halbertsma JM, Nilsson J, Thorstensson A. The adaptation to speed in human locomotion. Brain Res 165: 177–182, 1979 [DOI] [PubMed] [Google Scholar]
- Halbertsma JM. The stride cycle of the cat: the modeling of locomotion by computerized analysis of automatic recordings. Acta Physiol Scand Suppl 521: 1–75, 1983 [PubMed] [Google Scholar]
- Herman R, Wirta R, Bampton S, Finley FR. Human solutions for locomotion: single limb analysis. In: Neural Control of Locomotion, edited by Herman RM, Grillner S, Stein PSG, Stuart DG. New York: Plenum Press, 1976, p. 13–49 [Google Scholar]
- Hildebrand M. Analysis of the symmetrical gaits of tetrapods. Folio Biotheoretica 6: 9–22, 1966 [Google Scholar]
- Hildebrand M. Symmetrical gaits of primates. Am J Phys Anthropol 26: 119–130, 1967 [Google Scholar]
- Hildebrand M. Analysis of tetrapod gaits: general considerations and symmetrical gaits. In: Neural Control of Locomotion, edited by Herman RM, Grillner S, Stein PSG, Stuart DG. New York: Plenum Press, 1976, p. 203–236 [Google Scholar]
- Hildebrand M. The quadrupedal gaits of vertebrates. BioScience 39: 766–775, 1989 [Google Scholar]
- Ho SM. Rhythmic motor activity and interlimb co-ordination in the developing pouch young of a wallaby (Macropus eugenii). J Physiol 501: 623–636, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A, Prestige MC. Development of behaviour in the hindlimb of Xenopus laevis. J Zool (Lond) 152: 347–359, 1967 [Google Scholar]
- Jacobson RD, Hollyday M. A behavioral and electromyographic study of walking in the chick. J Neurophysiol 48: 238–256, 1982 [DOI] [PubMed] [Google Scholar]
- Jamon M, Clarac F. Early walking in the neonatal rat: a kinematic study. Behav Neurosci 112: 1218–1228, 1998 [DOI] [PubMed] [Google Scholar]
- Johnston RM, Bekoff A. Constrained and flexible features of rhythmical hindlimb movements in chicks: kinematic profiles of walking, swimming and airstepping. J Exp Biol 171: 43–66, 1992 [DOI] [PubMed] [Google Scholar]
- Johnston RM, Bekoff A. Patterns of muscle activity during different behaviors in chicks: implications for neural control. J Comp Physiol A Sens Neural Behav Physiol 179: 169–184, 1996 [DOI] [PubMed] [Google Scholar]
- Juvin L, Simmers J, Moring D. Propriospinal circuitry underlying interlimb coordination in mammalian quadrupedal locomotion. J Neurosci 25: 6025–6035, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa K, Kamei H, Minoda K, Udo M. Interlimb coordination in cat locomotion investigated with perturbation. Exp Brain Res 46: 425–437, 1982 [DOI] [PubMed] [Google Scholar]
- McCrea AE, Stehouwer DJ, Van Hartesveldt C. L-DOPA-induced air-stepping in preweanling rats. I Effects of dose and age Brain Res Dev Brain Res 82: 136–142, 1994 [DOI] [PubMed] [Google Scholar]
- McEwen ML, Van Hartesveldt C, Stehouwer DJ. A kinematic comparison of L-DOPA-induced air-stepping and swimming in developing rats. Dev Psychobiol 30: 313–327, 1997 [DOI] [PubMed] [Google Scholar]
- McGraw MB. Development of neuro-muscular mechanisms as reflected in the crawling and creeping behavior of the human infant. J Genet Psychol 58: 83–111, 1941 [Google Scholar]
- Musselman KE, Yang JF. Interlimb coordination in rhythmic leg movements: spontaneous and training-induced manifestations in human infants. J Neurophysiol 100: 2225–2234, 2008 [DOI] [PubMed] [Google Scholar]
- Musselman KE, Yang JF. Loading the limb during rhythmic leg movements lengthens the duration of both flexion and extension in human infants. J Neurophysiol 97: 1247–1257, 2007 [DOI] [PubMed] [Google Scholar]
- Nakano Y. Footfall patterns in the early development of the quadrupedal walking of Japanese macaques. Folia Primatol 66: 113–125, 1996 [DOI] [PubMed] [Google Scholar]
- Pang MYC, Yang JF. Interlimb co-ordination in human infant stepping. J Physiol 533: 617–625, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick SK, Noah JA, Yang JF. Interlimb coordination in human crawling reveals similarities in development and neural control with quadrupeds. J Neurophysiol 101: 603–613, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SE. Postnatal development of gait behaviour and functional allometry in the domestic cat (Felis catus). J Zool (Lond) 199: 461–486, 1983 [Google Scholar]
- Pflieger JF, Cassidy G, Cabana T. Development of spontaneous locomotor behaviors in the opossum, Monodelphis domestica. Behav Brain Res 80: 137–143, 1996 [DOI] [PubMed] [Google Scholar]
- Pridmore PA. Trunk movements during locomotion in the marsupial Monodelphis domestica (Didelphidae). J Morphol 211: 137–146, 1992 [DOI] [PubMed] [Google Scholar]
- Robson P. Shuffling, hitching, scooting or sliding: some observations in 30 otherwise normal children. Dev Med Child Neurol 12: 608–617, 1970 [DOI] [PubMed] [Google Scholar]
- Robson P. Prewalking locomotor movements and their use in predicting standing and walking. Child Care Health Dev 10: 317–330, 1984 [DOI] [PubMed] [Google Scholar]
- Ryu YU, Bradley NS. Precocious locomotor behavior begins in the egg: development of leg muscle patterns for stepping in the chick. PLoS One 4: e6111, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehouwer DJ, McCrea AE, Van Hartesveldt C. L-DOPA-induced air-stepping in preweanling rats. II Kinematic analyses Brain Res Dev Brain Res 82: 143–151, 1994 [DOI] [PubMed] [Google Scholar]
- Stuurman PM, Van Hof MW. The postnatal development of swimming behavior in the rabbit. Physiol Behav 23: 185–186, 1979 [DOI] [PubMed] [Google Scholar]
- Thelen E. Treadmill-elicited stepping in seven-month old infants. Child Dev 57: 1498–1506, 1986 [PubMed] [Google Scholar]
- Vilensky JA, Gankiewicz E. Early development of locomotor behavior in vervet monkeys. Am J Primatol 17: 11–25, 1989 [DOI] [PubMed] [Google Scholar]
- Vilensky JA, Wilson P, Gankiewicz E. An analysis of air-stepping in normal infant vervet monkeys. J Mot Behav 21: 429–456, 1989 [DOI] [PubMed] [Google Scholar]
- Walker WF. Locomotion. In: Turtles, Perspectives and Research, edited by Harless M, Morlock H. New York: Wiley, 1979, p. 435–454 [Google Scholar]
- Williams TL. Experimental analysis of the gait and frequency of locomotion in the tortoise, with a simple mathematical description. J Physiol 310: 307–320, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JF, Lamont EV, Pang MYC. Split-belt treadmill stepping in infants suggests autonomous pattern generators for the left and right leg in humans. J Neurosci 25: 6869–6876, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JF, Stephens MJ, Vishram R. Infant stepping: a method to study the sensory control of human walking. J Physiol 507: 927–937, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]