Abstract
Presynaptic inhibition is a powerful mechanism for selectively and dynamically gating sensory inputs entering the spinal cord. We investigated how hindlimb mechanics influence presynaptic inhibition during locomotion using pioneering approaches in an in vitro spinal cord–hindlimb preparation. We recorded lumbar dorsal root potentials to measure primary afferent depolarization-mediated presynaptic inhibition and compared their dependence on hindlimb endpoint forces, motor output, and joint kinematics. We found that stance-phase force on the opposite limb, particularly at toe contact, strongly influenced the magnitude and timing of afferent presynaptic inhibition in the swinging limb. Presynaptic inhibition increased in proportion to opposite limb force, as well as locomotor frequency. This form of presynaptic inhibition binds the sensorimotor states of the two limbs, adjusting sensory inflow to the swing limb based on forces generated by the stance limb. Functionally, it may serve to adjust swing-phase sensory transmission based on locomotor task, speed, and step-to-step environmental perturbations.
Keywords: primary afferent depolarization, dorsal root potential, interlimb coupling, limb loading, ground reaction force
sensory feedback sculpts the spatiotemporal features of muscle activation during locomotion. Sensory signals can alter phase transition timing and flexor–extensor duty cycles (Hayes et al. 2009a; Pearson et al. 1998), modify extensor magnitude during stance (Donelan and Pearson 2004; Hayes et al. 2009a; Rossignol et al. 2006), reset locomotion (Kiehn et al. 1992; Quevedo et al. 2005; Rossignol et al. 2006), and reinforce weak locomotion (Hayes et al. 2009a; Pearson et al. 1998). Precisely because sensory feedback wields a strong influence on motor behavior, it must be tightly regulated to allow for refinement without unwanted interference. Presynaptic inhibition of intraspinal afferent terminals offers the first and highly selective central site for regulating sensory inflow (Cattaert et al. 1992; Eccles et al. 1962; Ménard et al. 2003; Rudomin 2009). One class of such inhibitory pathways activates γ-aminobutyric acid type A (GABAA) receptors at axo–axonic synapses on primary afferents, leading to a depolarization that depresses transmission for several hundred milliseconds (Cattaert et al. 1994; Eccles et al. 1962; Gossard and Rossignol 1990). This primary afferent depolarization (PAD) can be measured intracellular or out in the dorsal root as a dorsal root potential (DRP) (Duenas and Rudomin 1988; Gossard and Rossignol 1990; Kennedy et al. 1974; Ménard et al. 2003). PAD can be initiated by ongoing activity in numerous afferent fiber populations, including strong autogenic negative feedback from homonymous afferents, as well as convergent inputs from descending and spinal circuits (Clarac and Cattaert 1996; Rudomin 2009).
Due to the interactions between spinal and supraspinal locomotor circuits as well as afferent modalities, the effectiveness of specific afferents for generating inhibition or eliciting reflexes changes significantly across tasks and locomotor phase (Gossard and Rossignol 1990; Ménard et al. 2003). For example, spinal circuits can produce rhythmic PAD and DRPs during fictive locomotion that alter the effectiveness of afferents in generating PAD (e.g., Duenas and Rudomin 1988; El Manira et al. 1991; Gossard et al. 1991; Ménard et al. 2003). Given these complex interactions, it is vital to study presynaptic inhibition under behaviorally relevant conditions with the most natural afferent interactions possible. However, it has been difficult to study presynaptic inhibition during nonfictive vertebrate locomotion with intact sensory interactions due to the sensitivity of DRP recordings to cord movement (Beloozerova and Rossignol 2004; Ménard et al. 1999; Yakhnitsa et al. 1988).
The recently developed dorsal-up spinal cord hindlimb preparation (SCHP) (Hayes et al. 2009a) allows us to mechanically isolate the spinal cord from the limbs, providing sufficient stability for DRP recordings. The ability to stabilize the cord, while retaining sensory feedback and limb movement, make the SCHP a powerful model for studying presynaptic sensory regulation during locomotion. In this study, we characterized the patterns of presynaptic inhibition during nonfictive locomotion in the SCHP in relation to both ipsilateral and contralateral hindlimb mechanics. Studies on interlimb reflexes have shown that contralateral sensory inputs can elicit responses on the opposite limb (Eng et al. 1994; Forssberg 1979) and the sensorimotor state of one limb can affect the behavior of the other (Grillner and Rossignol 1978; Pang and Yang 2000; Ting et al. 1998). Despite the known importance of interlimb coordination, most studies in presynaptic inhibition have focused on ipsilateral effects and the role of the contralateral presynaptic inhibition during behavior remains unknown. Here, we hypothesized that contralateral limb movement and loading would influence presynaptic inhibition patterns on the ipsilateral limb and, thus, the magnitude and timing of sensory information entering the spinal cord. To test this hypothesis, we recorded DRP activity as a measure of PAD-mediated presynaptic inhibition and compared the spatiotemporal dependence of DRP patterns on ipsilateral and contralateral limb forces and kinematics. We performed mechanical perturbations on each limb to isolate the influence of individual limbs and distinguish between movement- and force-related feedback. Because both central circuits and sensory feedback can influence presynaptic inhibition, we also considered the dependence of presynaptic inhibition on motor output, as monitored at the ventral roots, and performed deafferentations to distinguish central and sensory sources. We found that stance-phase force on the opposite limb plays a pivotal role in regulating the strength of sensory transmission to the swing limb during locomotion. A portion of these results has been presented in abstract form (Hayes and Hochman 2009; Hayes et al. 2009b).
MATERIALS AND METHODS
All procedures were approved by the Emory University Institutional Animal Care and Use Committee. A total of 15 neonatal rats were used. Over 700 step cycles from 10 animals were analyzed to test the central hypothesis of DRP dependence on ipsilateral and contralateral limb loading and movement. Mechanical perturbations were then performed in 9 animals and neural perturbations in 7 animals. The sample size is reported below for each condition.
In Vitro Spinal Cord–Hindlimb Preparation
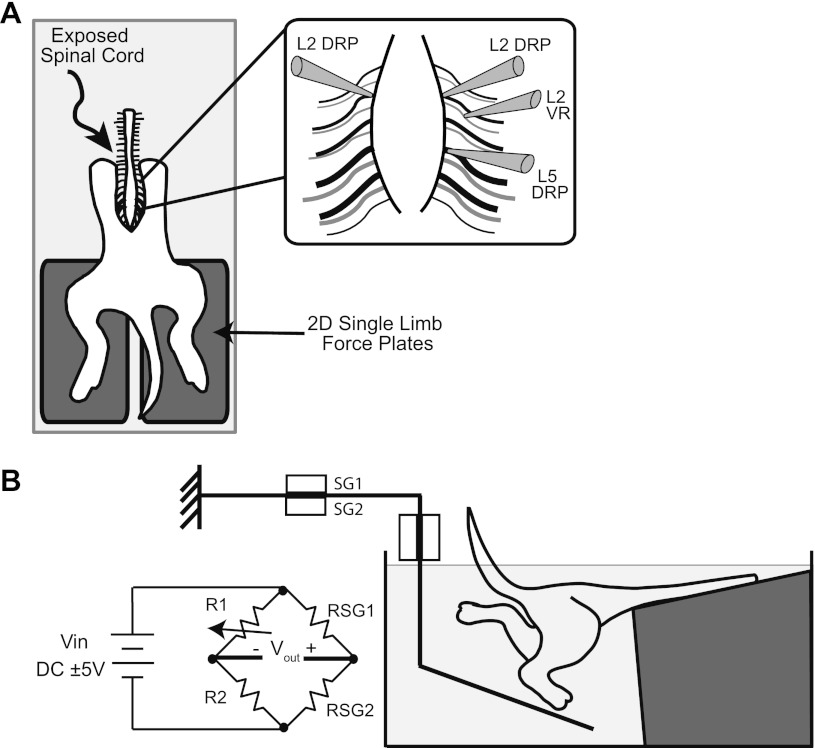
Studies were undertaken in the in vitro dorsal-up spinal cord–hindlimb preparation (SCHP), as described previously (Hayes et al. 2009a). Briefly, neonatal rats postnatal days 1–4 were decapitated and eviscerated. The spinal cord, caudal vertebral column, pelvis, and hindlimbs were isolated. All skin was removed except that covering the dorsal and plantar surfaces of the paws. The preparation was then transferred to a custom-built perfusion chamber. The cord was mounted dorsal-up on a Sylgard (Dow Corning) step and securely stabilized with insect pins through the ribs and remaining paraspinal tissues, with hindlimbs hanging pendant to step unrestrained on force platforms (described below, Fig. 1).
Fig. 1.
Experimental setup and methodology. A: overhead view of the in vitro spinal cord–hindlimb preparation (SCHP) with exposed spinal and each intact hindlimb free to walk on a separate 2D force platform. Inlay shows the recording configuration. Dorsal root potentials (DRPs) were recorded near the dorsal root entry zones of L2 and L5 dorsal roots using glass suction electrodes. Activity in the L2 ventral root (VR) was also recorded. B: sagittal view of hindlimb–force platform interaction and Wheatstone bridge circuitry. Strain produced by strain gauges SG1 and SG2 of Sensor 1 and fed into the Wheatstone bridge circuit. Strain sensed by SG3 and SG4 of Sensor 2 is fed into a separate but identical Wheatstone bridge circuit. Output voltages are amplified by a DC amplifier and then converted to vertical and fore–aft forces in offline analysis.
Bathing Solutions
All bathing solutions were continuously oxygenated with 95% O2-5% CO2. The standard bathing solution was an artificial cerebral spinal fluid (aCSF) containing (in mM): 128 NaCl, 1.9 KCl, 1.2 KH2PO4, 26 NaHCO3, 2.4 CaCl2, 1.3 MgSO4, and 10 glucose at a pH of 7.4. For dissection and electrode placement, low-calcium, high-magnesium aCSF (same as normal aCSF except 0.85 mM CaCl2 and 6.5 mM MgSO4) was used to minimize movement. Solutions were provided through a gravity-fed perfusion system and recirculated by a peristaltic pump. To pharmacologically induce locomotion, 4–6 μM N-methyl-d-aspartate (NMDA) and 10–80 μM serotonin (5-HT) were added to the aCSF. In 13 experiments, 10–40 μM of dopamine (DA) was added as well.
Force Platforms for Monitoring Limb Endpoint Forces
In 10 experiments, limb endpoint forces were measured. Vertical and fore–aft forces were monitored using two 2D force platforms, one for each hindlimb (Fig. 1, designed after Chang et al. 1997; Heglund 1981). The platforms were composed of an acrylic-based photopolymer. Force was transduced via Omega Engineering SGD-1.5/120-LY11 uniaxial strain gauges oriented to sense longitudinal deformations created by vertical forces on the horizontal arm of the platform and by fore–aft forces on the vertical arm of the platform. The strain gauge outputs were fed into a Wheatstone bridge circuit (Fig. 1B) and DC amplifier. The output of the amplifier was then digitized at 5 kHz and recorded (Digidata 1322A 16-bit DAQ; Molecular Devices) for offline calibration and analysis.
The force platforms were calibrated by applying n known weights and the influence matrix [I] was calculated according to:
such that
where [V] is the voltage data in response to the known applied loads in μV, [L] is the known loads in mN, [I] is the influence matrix describing the relationship, and n is the number of known weights. After calibration, vertical and fore–aft forces from locomotor trials were computed from the recorded voltage traces according to:
where
where [F] is the force matrix that includes both vertical and fore–aft forces computed from the conversion matrix [I]−1(Chang et al. 1997).
Following collection, forces were exported to Matlab for calibration and analysis. Ground reaction forces during each locomotor cycle were initially identified by a threshold detector. Their onset and offset times were then more finely discriminated using their second derivatives to detect the maximum inflection points. Force magnitude was quantified by the area under the curve, peak-to-peak amplitude, and mean amplitude during each event, all relative to baseline. This study focuses on the relationship to vertical forces, defined as parallel to gravity. Thus, all subsequent references to limb endpoint force refer to vertical forces.
Two of 10 force experiments were carried out using a single 1D force platform shared by both hindlimbs. Each force event was discriminated as right or left using video data. A similar 1D calibration and conversion of voltage to vertical force was performed.
Kinematics
For sagittal plane kinematic analyses, joint centers were palpated and marked at the hip (greater trochanter), knee (lateral epicondyle), ankle (lateral malleolus), and 5th metatarsophalangeal joints using waterproof black ink. Video of hindlimb locomotion was collected in the sagittal plane using a digital video camera at a rate of 30 or 60 Hz. Video was synchronized with electrophysiological recordings using a trigger light in the field of view and a simultaneous voltage pulse sent to the data acquisition system. Following collection, joint positions were digitized using semiautomatic tracking (Dartfish Software) and joint angle trajectories computed for the right hindlimb. The ankle and knee angles were defined as included angles between the foot and shank segments and shank and thigh segments, respectively. Hip angle was defined as the angle between the thigh and the horizontal. In all cases, increasing values indicate extension (0° max flexion and 180° max extension). These trajectories were used as a measure of either ipsilateral or contralateral extension and flexion for comparison with the concurrent or subsequent DRP.
Ventral Root and Dorsal Root Potential Recordings
Activity in the right lumbar ventral root L2, whose bursting activity typically corresponds to flexor muscle activation (Kiehn and Kjaerulf 1996), was recorded as a monitor of spinal motor output using en passant glass suction electrodes (Fig. 1). This provided an approximate temporal marker for flexor phase along with joint kinematics. Ventral root recordings were passed through an AC-coupled differential amplifier, bandpass filtered (100 to 3,000 Hz), notch filtered (60 Hz), and digitized at 5 kHz (Digidata 1322A 16-bit DAQ; Molecular Devices). Following collection, ventral root recordings were rectified and low-pass Chebyshev filtered to create a burst envelope. Bursts were detected using a threshold detection graphical user interface in Matlab. Their onset, offset, and peak times, as well as the area under the low-passed envelope, were calculated.
DRPs were used to monitor both the timing and magnitude of presynaptic inhibition of primary afferent inflow (Duenas and Rudomin 1988; Gossard and Rossignol 1990; Ménard et al. 2003). Increases in DRP amplitude indicated increases in presynaptic inhibition and vice versa. DRPs were recorded with en passant glass suction electrodes at the entry zones of dorsal roots L2 (and occasionally L5). Recordings were collected through a DC amplifier or AC amplifier with a high-pass cutoff frequency ≤ 0.10 Hz and digitized at 5 kHz. Using custom software in Matlab, DRPs were initially identified using a threshold detector. Their onset and offset times were then more finely discriminated using their second derivatives to identify the maximum inflection points. DRP magnitude was then characterized by the area under the curve, peak-to-peak amplitude, and mean voltage deflection, all relative to a locally detrended baseline to account for DC drift.
Throughout this study, ipsilateral indicates the side of the recorded DRP (iDRP) and contralateral indicates the side contralateral to the DRP. In the figures, ipsilateral forces are represented in green, whereas contralateral forces are represented in red.
Statistical Analysis
All subsequent analyses were performed using custom software in Matlab, including the statistics and circular statistics toolboxes (Berens 2009). Differences were considered significant if P ≤ 0.05 unless otherwise stated.
Dependence of the DRP on ipsilateral and contralateral hindlimb forces.
To test the hypothesis that contralateral limb loading influences ipsilateral presynaptic inhibition, the spatiotemporal dependence of the L2 DRP on ipsilateral and contralateral force was compared. For each step cycle as delineated by ventral root or kinematic events, the DRP was detected. If no DRP or force occurred, a deletion was noted and the area, peak, and mean set to zero. The preceding and/or coincident ipsilateral and contralateral force events were then detected for each DRP or DRP deletion. Ipsilateral and contralateral force deletions were also noted.
To quantify the dependence of DRP magnitude on limb endpoint force, the DRP area, peak-to-peak amplitude, and mean amplitude were plotted against the corresponding ipsilateral and contralateral force values for each cycle. Linear regressions and the Pearson correlation coefficient (R) were computed. Student's t-tests were performed to test the significance of the correlations.
To examine the temporal relationship, the absolute time delay between force onset and DRP onset was measured for each cycle. The phase between DRP onset and force onset was defined as the delay divided by the cycle period and multiplied by 360°, such that 0° represented exactly in-phase and 180° represented out-of-phase. This temporal relationship could be graphically summarized on the unit circle by a vector at the mean phase angle θ̄ with length equal to r. The value of r indicates the concentration of cycle-phase angles about the mean phase angle and ranges from 0 to 1 (Zar 1974). Rayleigh's test for circular uniformity (Zar 1974) was then used to determine whether r was high enough to indicate a significant relationship between the DRP and ipsilateral and/or contralateral force. Phasing was also examined to determine whether the ipsilateral or contralateral force just preceded the DRP onset, indicating that the force could initiate the DRP.
In addition, the Wallraff procedure for comparing angular dispersion was used to compare the temporal coupling of the DRP to the ipsilateral force and contralateral force (Zar 1974). Angular distances for each cycle were computed as the cycle-phase angle minus the mean-phase angle. The angular distances were then pooled and two-sample Mann–Whitney tests applied to compare angular dispersion between the DRP-ipsilateral force phasing and the DRP-contralateral force phasing. Higher angular dispersions indicated less coupling between variables, whereas lower angular dispersions indicated tighter coupling.
Dependence of the DRP on motor output and kinematics.
Similar procedures were used to examine the spatiotemporal dependence of the L2 DRP on L2 ventral root motor output (n = 10). After identifying the DRP and L2 ventral root for each cycle, linear regressions and the Pearson correlation coefficient were used to characterize the relationship between DRP and ventral root areas and peaks. The temporal relationship was characterized by the phase angle, concentration about the mean angle (r), and angular dispersion. Again, high r and low angular dispersion for DRP-ventral root phasing indicated a tight coupling between the DRP and motor output, whereas low r and high angular dispersion indicated that the DRP timing was not dependent on motor output. As described earlier, the Wallraff procedure and Mann–Whitney tests were used to compare angular dispersion between DRP-force phasing and DRP-ventral root phasing to determine whether DRP timing was more dependent on limb endpoint force or motor output. To determine the relationship between the L2 DRP and ipsilateral and contralateral joint flexion and extension, the DRP area was plotted against the corresponding hip, knee, and ankle total range of motion, flexion range of motion, extension range of motion, and area under the joint angle trajectory for a given cycle. The temporal phasing between the DRP and these kinematics variables was also examined using the methods described earlier.
Mechanical and Neural Perturbations
In nine rats, force platforms were removed for 1 to 2 minutes to determine whether loss of ground contact force, either ipsilateral or contralateral, influenced the magnitude or consistency of the DRP. Ipsilateral and contralateral platforms were removed independently to isolate the effects of each limb force.
Neural cuts were performed in a total of seven rats. In four rats, DRPs were compared before and after lumbar dorsal root rhizotomy to distinguish afferent-evoked and centrally evoked DRPs. Care was taken to fully expose all dorsal roots of interest during the initial dissection. After collecting control data, aCSF was exchanged for low-calcium/high-magnesium aCSF to avoid central sensitization by noxious inputs. The dorsal roots were then completely transected and the preparation was returned to regular aCSF. Following a 30-minute wash in regular aCSF, deafferented data were collected and compared. To ensure that the effects were not simply due to low-calcium/high-magnesium aCSF, dorsal root rhizotomies were performed in regular aCSF in two experiments.
In a separate three rats, the medial and lateral plantar nerves were cut, removing most of the cutaneous innervation on the paw plantar surface. The plantar nerves are cutaneous nerves that innervate the plantar surface of the paw and digit pads as well as intrinsic foot musculature (Greene 1963). During the initial dissection, the medial and lateral plantar nerves were exposed by gently opening the space between the calcaneal tendon and tibia on the lateral aspect and removing any overlaying fascia. This sham exposure ensured that no additional biomechanical disturbances were made between control and cutaneous dennervation trials. After collecting control data, aCSF was exchanged for low-calcium/high-magnesium aCSF, and the plantar nerves were completely transected. Following a 30-minute wash in regular aCSF, dennervation data were collected and compared.
RESULTS
Rhythmic, GABAA-dependent DRPs Observed During In Vitro Locomotion
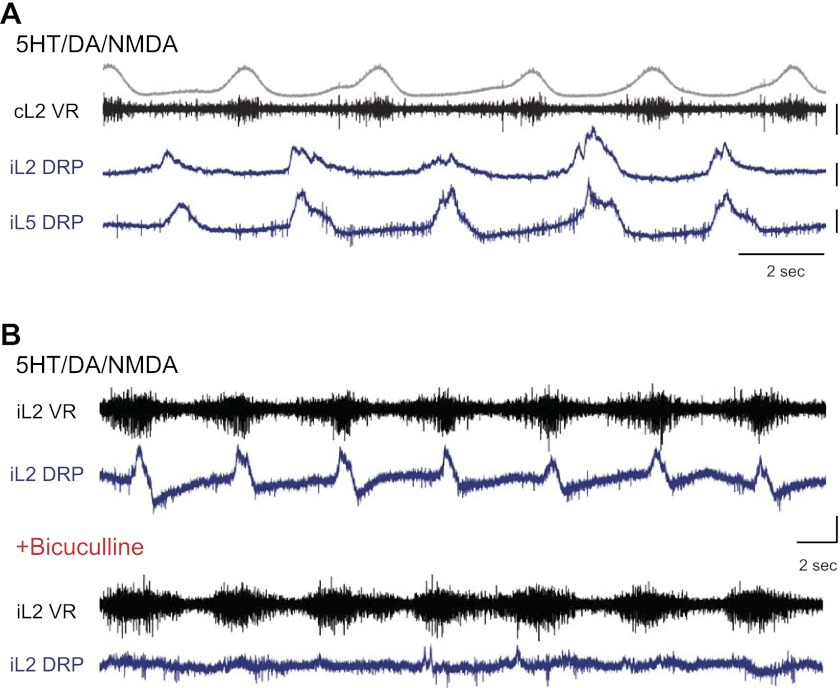
As seen during fictive locomotion, DRPs in dorsal root L2 were rhythmic during locomotion (n = 15), with the maximum depolarization occurring during the flexion phase (Fig. 2A). Often, extensor-phase depolarizations were also distinguishable in the L2 root, but they were significantly smaller and less consistent and, thus, not addressed here. In three experiments, DRPs were also recorded from dorsal root L5. L5 DRPs were always in-phase with the L2 DRP with their maximum during the flexion phase (Fig. 2A).
Fig. 2.
Multisegmental rhythmic DRPs during locomotion are γ-aminobutyric acid type A receptor(GABAAR) dependent. A: during locomotion induced by 5 μM N-methyl-d-aspartate (NMDA), 20 μM serotonin (5-HT), and 20 μM dopamine (DA), DRPs are rhythmic with peaks occurring during the ipsilateral flexion phase, or out of phase with the contralateral L2 ventral root (cL2 VR, black; rectified and integrated envelope, gray). L2 and L5 DRPs (iL2 and iL5 DRP, blue) were always in phase, suggesting that the patterns observed in L2 represented a distributed pattern of flexor-phase presynaptic inhibition in multiple lumbar segments. B, top: rhythmic DRPs from the L2 dorsal root relative to the ipsilateral L2 ventral root (iL2 VR) during locomotion induced by 4 μM NMDA, 60 μM 5-HT, and 40 μM DA. Bottom: application of 10 μM bicuculline nearly abolishes the DRPs. Scale bars are 100 μV and 2 s.
Application of 6–10 μM of bicuculline, a GABAA-receptor antagonist, abolished or greatly reduced the locomotor-related rhythmic DRPs (n = 3/3, Fig. 2B). This observation confirmed that the rhythmic DRP oscillations were mediated by GABAA-receptor activation, widely supported to be the predominant mechanism of PAD-mediated presynaptic inhibition (Eccles et al. 1962; Gossard and Rossignol 1990; Rudomin 2009). It is important to note that these doses of bicuculline did not cause observable changes in the locomotor pattern, including left–right and flexor–extensor alternation as well as both ipsilateral and contralateral forces. In one instance, changes in locomotion were observed because too much bicuculline was added, and the data were excluded.
DRPs Scale with Contralateral Limb Endpoint Force
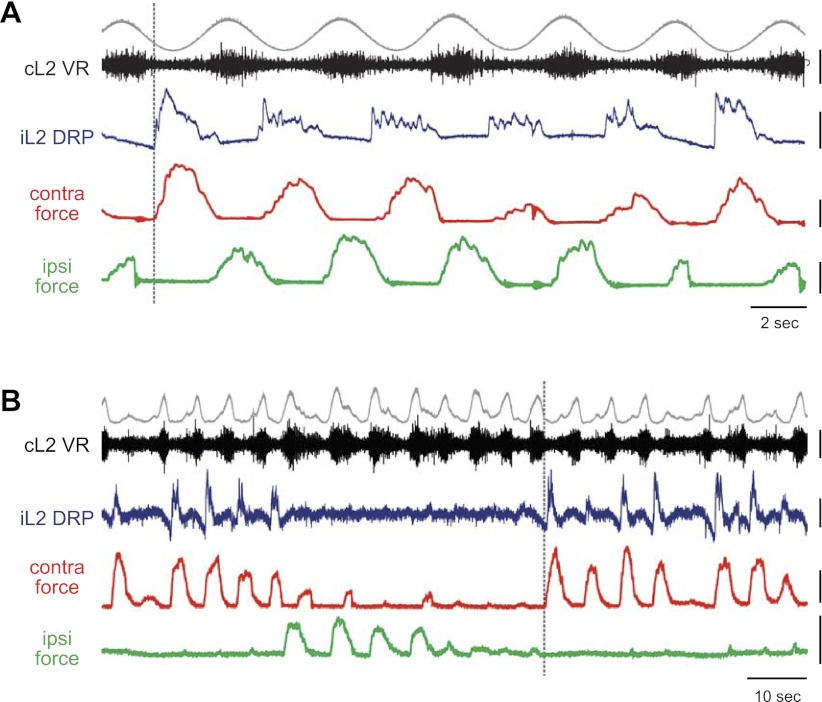
Figure 3A shows representative locomotor patterns of L2 DRPs in relationship to L2 ventral root and ipsilateral and contralateral forces. In all animals, variations in contralateral force predicted both the amplitude and timing of the ipsilateral DRP. When the contralateral force was small, the DRP was small; when the contralateral force increased, the DRP amplitude increased as well. In contrast, ipsilateral force magnitude and timing did not appear to affect the DRP.
Fig. 3.
Representative DRP patterns showing the relationship of L2 DRPs with motor output and limb endpoint forces during locomotion. Each panel shows representative patterns for L2 DRPs (iL2 DRP, blue) during locomotion relative to L2 ventral root activity (iL2 or cL2 VR, black; rectified and integrated envelope, gray) and ipsilateral (green) and contralateral (red) forces. Ipsilateral (i) always indicates the side of the iL2 DRP. Dashed vertical lines emphasize the relative timing. A: DRPs consistently occurred immediately following contralateral force onset and scaled with contralateral force amplitude, but were independent of ipsilateral force timing and amplitude. B: waxing and waning locomotor patterns were occasionally induced by dopamine (n = 3/13 with DA). DRPs occurred only during contralateral force bouts, independent of ipsilateral force and contralateral motor output. Force scale bars = 10 mN. VR and DRP scale bars = 400 μV in (A) and VR and DRP scale bars = 100 μV in (B).
In the presence of dopamine, the locomotor pattern occasionally waxed and waned (n = 3/13 experiments with DA), leading to locomotor bouts in which one limb reached peak strength, whereas the other limb weakened or exhibited deletions (Fig. 3B). During these bouts, the L2 DRP occurred only in cycles with a contralateral force, independent of ipsilateral force magnitude. Even in the absence of ipsilateral force, either due to a pause in locomotion or a lack of paw–plate interaction, an L2 DRP still occurred as long as a significant contralateral force was present. These bouts further highlighted the dependence of the L2 DRP on contralateral limb force and its independence from ipsilateral limb movement and force.
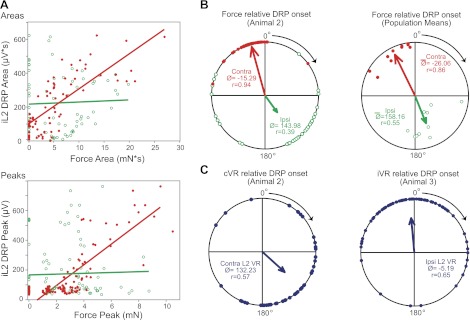
To quantify this relationship, L2 DRP area, peak amplitude, and mean amplitude were plotted against the corresponding values for the ipsilateral and contralateral forces for each cycle. In all preparations examined, DRP area correlated significantly with contralateral force (n = 10/10 at P < 0.05 with 8 at P < 0.001; mean R = 0.68 with 9/10 R values ranged from 0.57 to 0.84 and one weak correlation at 0.34), whereas none showed a significant positive correlation with ipsilateral force area. One preparation did exhibit a significant inverse relationship (R < 0) between DRP and ipsilateral force area, most likely due to the inverse relationship between ipsilateral and contralateral force magnitudes seen in that animal. Significant correlations were also observed between DRP and contralateral force peak (n = 9/10 at P < 0.05, with 6 at P < 0.001) and mean amplitude (n = 8/10 at P < 0.05, with 6 at P < 0.001) with no significant positive correlations to ipsilateral force peak or mean. Representative regressions are shown in Fig. 4A.
Fig. 4.
Spatiotemporal relationship of iDRP to force and ventral root. A, top: linear regression relating DRP area to ipsilateral (green open circles) and contralateral (red filled circles) force area from a single representative animal. The DRP scales with contralateral force but is independent of ipsilateral force. Each point represents a single cycle (n = 72 cycles). DRP area was strongly correlated with contralateral force (R = 0.77; P < 0.0001) but not ipsilateral force (R = 0.04; P = 0.72). Bottom: linear regression for peak values from another representative animal (n = 95 cycles). Again, DRP magnitude scales with contralateral force (R = 0.83, P < 0.0001) but not ipsilateral (R = 0.03; P = 0.79). When no contralateral force occurs (red points on the y-axis), the DRP is small or zero, but the absence of ipsilateral force (green points on the y-axis) does not affect DRP magnitude. B, C: phase relationships of force and ventral root onset relative to DRP onset. 0° represents the DRP onset and the cycle progresses clockwise from 0° (in-phase) to 180° (out-of-phase) to 360°/0°. Northwest points precede DRP onset, northeast points lag. Arrow length represents the concentration (r) about the mean angle (Ø). B, left: contralateral (red filled circles) and ipsilateral (green open circles) force onset relative to DRP onset. Contralateral force consistently precedes DRP onset. Ipsilateral force is typically out-of-phase with the DRP and contralateral force. When interlimb phasing varies, the DRP stays locked with contralateral force independent of ipsilateral force timing. Right: phasing for all animals. Each dot represents mean angle and r value for a single animal. Arrow length indicates the pooled r value and weighted mean angle for all animals. C, left: contralateral L2 ventral root burst onset relative to DRP onset for the same animal in (A). Right: ipsilateral L2 ventral root burst onset relative to DRP onset from another animal.
Contralateral Limb Force Precedes and Is Tightly Coupled with DRP Onset
For the contralateral limb to actually evoke or initiate the DRP, and thus influence the amount of presynaptic inhibition on ipsilateral limb afferents, contralateral force must precede the onset of the ipsilateral L2 DRP each cycle. To test this, the temporal phasing and coupling of the DRP to 1) contralateral force, 2) ipsilateral force, and 3) motor output were compared. Figure 4B shows the phasing of the ipsilateral and contralateral force onset relative to DRP onset for a representative bout of locomotion, with the contralateral force immediately preceding the DRP and the ipsilateral force showing a more varied and out-of-phase relationship. Examination of delays and phasing across all experiments confirmed that the mean onset of contralateral force always just preceded the onset of the DRP (n = 10/10, Fig. 4B, right). The mean phase angle of contralateral force onset relative to DRP onset was −26.06° (r = 0.86), with a mean delay of 411 ± 120 ms, whereas the ipsilateral mean phase angle was 158.16° (r = 0.55), with a mean delay of 2.90 ± 0.18 s.
As evidenced by low angular dispersions about the mean, L2 DRP onset was tightly coupled in time with contralateral force onset. Rayleigh's test confirmed significant coupling with contralateral force. The Wallraff procedure and Mann–Whitney tests revealed that the angular dispersion for contralateral force-DRP phasing was significantly lower than that for ipsilateral force (n = 10/10), reflecting a stronger temporal dependence on contralateral force.
The temporal relationship between L2 motor output and L2 DRP was also examined. As seen in Fig. 4C, ipsilateral L2 DRP onset exhibited a much weaker coupling with either L2 ventral root motor output onset [ipsilateral ventral root (n = 4/4), contralateral ventral root (n = 5/6)]. Rayleigh's test P-values for L2 motor output–DRP coupling were at least an order of magnitude smaller than that for contralateral force–DRP coupling. In 4/6 experiments with contralateral ventral root recordings, angular dispersion was significantly higher for contralateral L2 motor output–DRP phasing compared with contralateral force–DRP phasing. Angular dispersion for ipsilateral L2 motor output was also higher compared with contralateral force–DRP phasing in 4/4 experiments, but only statistically significant for 1/4.
In sum, neither ipsilateral force nor motor output timing appears to determine the timing of presynaptic inhibition during locomotion. Rather, the timing of presynaptic inhibition is tightly coupled to contralateral limb endpoint force.
Relationship to Hindlimb Kinematics
No consistent cycle-to-cycle relationships were observed between contralateral ankle, knee, and hip kinematics and the resulting L2 DRP pattern. Neither contralateral joint range of motion (all joints n = 4/6; mean P = 0.27), magnitude of flexion or extension (all joints n = 5/6; mean P = 0.40), nor area under the angular trajectory (ankle n = 5/6, knee n = 4/6, hip n = 5/6; mean P = 0.43) consistently influenced the magnitude of the DRP. As expected due to the relationship of the DRP to contralateral limb force, some relationships were significant but not consistently across animals, suggesting that these relationships were noncausal. Similarly, the magnitude of the concurrent ipsilateral joint kinematics showed no relationship to DRP magnitude (ankle n = 4/4, knee n = 3/4, hip n = 3/4; mean P = 0.44), further suggesting that the DRP was not related to flexor or extensor motor output.
Relationship to Locomotor Frequency
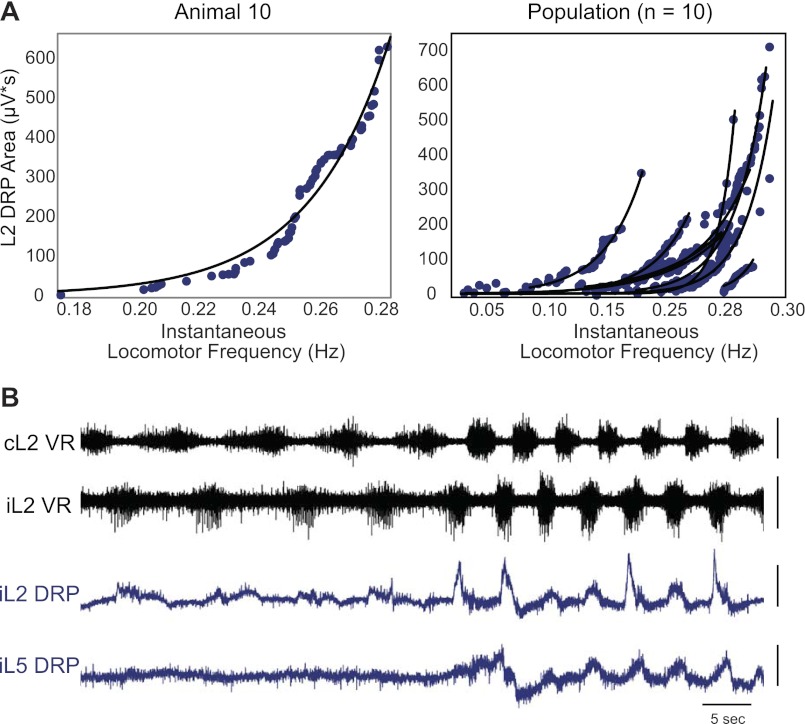
Figure 5 shows the relationship between L2 DRP area and instantaneous locomotor frequency. Locomotor frequency was defined as the L2 ventral root burst frequency in the corresponding cycle and is equivalent to stride frequency. DRP area consistently increased with locomotor frequency. The relationship was well fit with an exponential curve (n = 10/10 with R2 > 0.85, n = 6/10 with R2 > 0.94). A similar relationship was also found for limb force and frequency (n = 10/10 with R2 > 0.76, n = 5/10 with R2 > 0.94). An example of a sudden locomotor frequency increase leading to increased DRP amplitude is shown in Fig. 5B. Therefore, as locomotor frequency increases, the magnitude of the DRP increases, suggesting that central synaptic transmission is weakened in recruited afferent pathways during faster locomotion.
Fig. 5.
Relationship of DRP magnitude to locomotor frequency. A, left: DRP area versus instantaneous locomotor frequency fitted by an exponential curve (y = aebx, where y = DRP area, x = frequency) for a representative animal, whereas the right panel shows exponential fits for ten animals. As seen in both panels, DRP area increases with increasing locomotor frequency such that more swing-phase sensory inflow is inhibited at higher speeds. B: in another animal, the paw stuck to the plate in the middle of a locomotor bout. This natural perturbation resulted in a rapid increase in frequency and force. As a result, both the L2 and L5 DRP rapidly increased. This was an unusual and extreme case, but it highlights the increase in presynaptic inhibition with locomotor frequency and force. Scale bars: 10 s, 200 μV.
Perturbation Responses
Response to ipsilateral and contralateral plate removals.
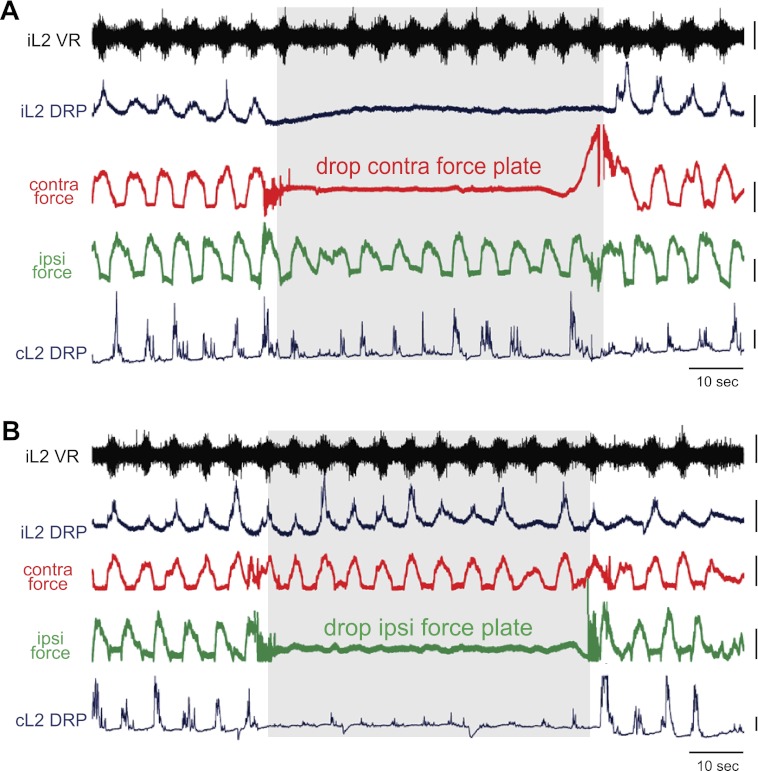
To determine whether significant contralateral force was necessary for cycle-to-cycle generation of the large L2 DRPs observed during locomotion, ipsilateral and contralateral force plates were removed separately. Ipsilateral DRPs continued unchanged when the ipsilateral plate was removed (n = 6/7, Fig. 6B), but were greatly reduced or abolished immediately upon contralateral plate removal (n = 7/8, Fig. 6A). Importantly, these changes were seen without any significant (or even slight) changes in locomotor frequency, excluding frequency as a confounding factor. In the absence of the force platform, the limb still experienced some force-feedback as it stepped through the aqueous medium, but the endpoint force experienced by the limb was necessarily greatly reduced in the absence of the plate and resulted in a loss of ipsilateral L2 DRP. It should be noted that small DRPs could sometimes be seen following contralateral plate drop (e.g., Fig. 6B). These smaller DRPs likely reflect primary afferent depolarizations generated centrally, as reported during fictive locomotion (Gossard et al. 1989, 1991) or spontaneous activity (Bos et al. 2011), or generated by other afferent inputs, but the largest component is dependent on interaction with the contralateral plate. Thus, the responses to plate removal confirm an essential role of contralateral endpoint force in ipsilateral DRP generation.
Fig. 6.
Response to contralateral and ipsilateral plate removals. Ipsilateral and contralateral DRPs shown with ipsilateral ventral root activity, contralateral force (red), and ipsilateral force (green). Gray boxes highlight the period of contralateral (A) and then ipsilateral (B) plate removals. Note that there was not a significant change in locomotor frequency before, during, or after plate removal. A: when the contralateral plate was removed, reducing contralateral limb loading, the ipsilateral L2 DRP was nearly abolished. The DRPs returned as soon as the contralateral plate was restored. This result demonstrates that sufficient contralateral limb loading is required to generate the large DRPs typically seen during nonfictive locomotion. The contralateral L2 DRP was largely unaffected by the plate removal, as its opposite force remained. B: when the ipsilateral plate was removed, the contralateral DRP was greatly reduced while the ipsilateral DRP was largely unchanged. Note that small contralateral DRPs remained, which were likely centrally generated as in fictive locomotor literature or generated by residual ipsilateral and/or contralateral input. Yet, the largest contralateral force-sensitive component of the DRP required significant contralateral force. Scale bars are 10 mN, 200 μV, and 10 s.
Contralateral lumbar dorsal root rhizotomy, but not plantar nerve transection, abolishes rhythmic DRPs.
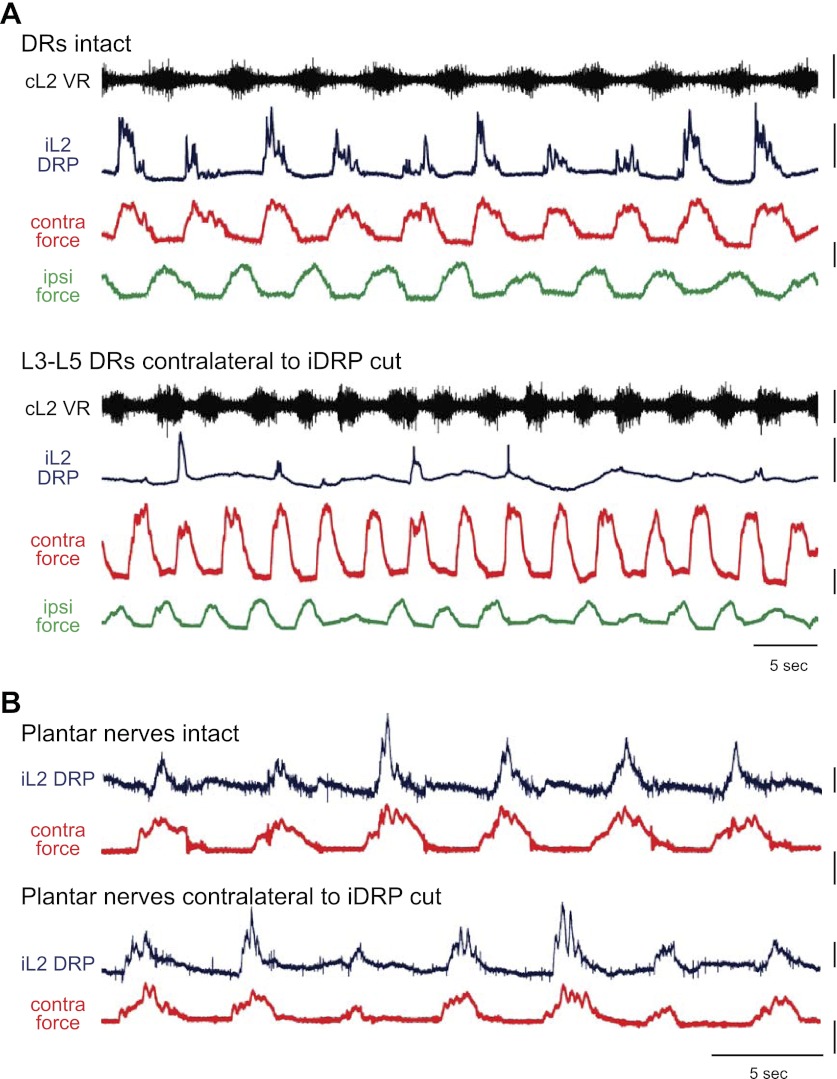
Because central circuits are capable of producing rhythmic presynaptic inhibition in the absence of afferent activity (Dubuc et al. 1988; Gossard et al. 1991), contralateral lumbar dorsal roots were rhizotomized to confirm that contralateral afferents were responsible for evoking the force-related DRPs observed during locomotion. In 3/4 experiments, the force-related DRPs were abolished or reduced in number and consistency when a minimum of contralateral L3–L5 dorsal roots were rhizotomized (Fig. 7A). DRPs that persisted were inconsistent and unrelated to contralateral force.
Fig. 7.
Response to contralateral dorsal root rhizotomy and plantar nerve transection. A: L2 DRP relative to ipsilateral ventral root, contralateral force, and ipsilateral force during locomotion with dorsal roots intact. Following rhizotomy of contralateral L3–5 dorsal roots, the DRP was significantly reduced and inconsistent despite high contralateral forces. Note that the increase in frequency results from the addition of 2 μM NMDA to induce locomotion without intact roots and did not occur when drug concentrations were held constant. Scale bars are 10 mN, 400 μV, and 5 s. B: L2 DRP and contralateral force before and after plantar nerve transection. DRP persists following transection of the contralateral medial and lateral plantar nerves and continues to scale with contralateral force. Scale bars are 20 mN, 800 μV, and 5 s.
In the fourth experiment, which exhibited dopaminergic waxing and waning locomotor-like activity, the prerhizotomy L2 DRPs were less consistent and occurred only in the presence of the maximal contralateral limb endpoint forces. Postrhizotomy, larger, highly consistent L2 DRPs emerged independent of the presence or absence of contralateral force. Although removing contralateral lumbar afferent input did not abolish the DRPs in this animal, their nature was clearly altered.
In contrast to dorsal root rhizotomy, a more selective removal of only paw plantar surface cutaneous afferents by contralateral plantar nerve transection did not substantially affect ipsilateral DRP generation (Fig. 7B). L2 DRPs persisted and continued to scale with contralateral force (n = 3/3). The failure of plantar nerve transection to block force-dependent DRPs suggests that the majority of cutaneous input from the paw plantar surface is not required. However, because the cutaneous branch of the deep peroneal nerve has a small cutaneous receptive field on digits two and three (Bouyer and Rossignol 2003; Holmberg and Schouenborg 1996), a cutaneous contribution from toe contact cannot be ruled out (see the following text). Contributions from the sural and saphenous nerves are unlikely because their receptive fields in the rat lie largely on the medial and lateral sides of the paw or dorsal surface (Swett and Woolf 1985).
Contralateral Toe Contact Linked to DRP Generation
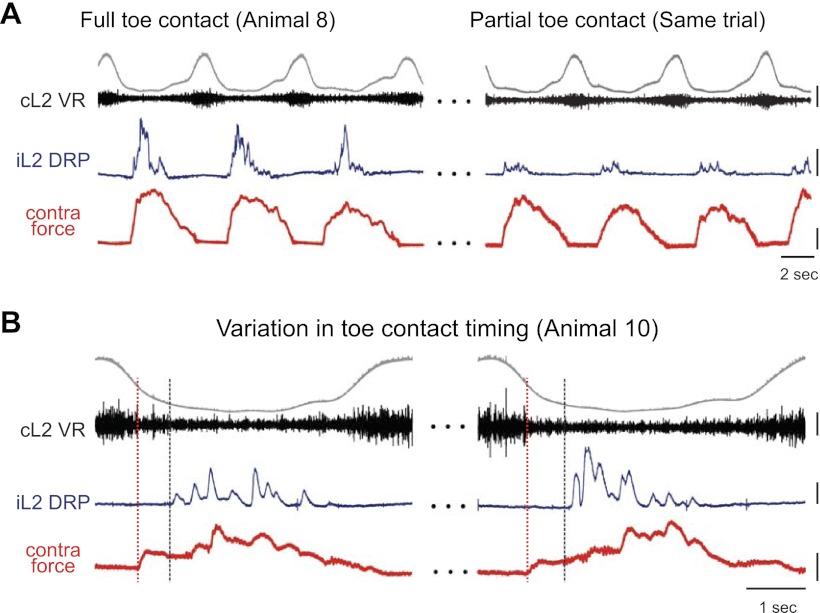
Contralateral toe ground contact, defined as contact at or distal to the metatarsophalangeal joint, was required for significant DRP generation. Occasionally during locomotion (n = 3), a portion of the paw proximal to the metatarsophalangeal joint (i.e., the mid-foot) rather than the toe would strike the front edge of the plate. When limited or no toe contact occurred, DRPs were reduced or absent (Fig. 8A).
Fig. 8.
Role of contralateral toe contact in DRP generation. A: L2 DRP relative to ipsilateral ventral root during locomotion with full contralateral toe contact (MTP to toe tip, left); 130 s later in the same locomotor bout (right), the toe moved to the front of the force platform such that only a small portion of the toe contacted the plate. The reduction in toe contact, and likely toe afferent input, resulted in reduced DRPs. Scale bars are 400 μV, 5 mN, and 2 s. B: in another animal, toe contact occurred after force onset due to mid-foot contact. Two examples of this are shown. Black dashed vertical lines indicate toe contact time. The DRP occurred immediately after the toe contacted the plate rather than at force onset as indicated by red dashed line. Scale bars are 200 μV, 2 mN, and 1 s.
The timing of contralateral toe contact also influenced DRP onset. When a more proximal portion of the paw struck first, followed by the toe, the onset of the DRP was delayed relative to the initial force (Fig. 8B). Together, these results suggest that afferents about the toe activated by limb loading contribute contralateral afferent signals that evoke the DRP and, thereby, influence DRP magnitude and timing.
DISCUSSION
Most investigations on afferent-evoked presynaptic inhibition give little attention to contralateral afferents. A small number of early studies found that stimulation of group I muscle and flexor reflex afferents produced a contralateral DRP along with the larger ipsilateral DRP (Devanandan et al. 1965; Gossard and Rossignol 1990; Jankowska et al. 1966). Several behavioral observations in humans, particularly from studies on H-reflex modulation, support a strong influence of contralateral sensory feedback, purportedly via presynaptic inhibition, on ipsilateral sensorimotor state (McIlroy et al. 1992; Ting et al. 2000). Further, sagittal hemisection of the in vitro isolated mouse spinal cord leads to increased probability of eliciting a monosynaptic reflex, leading the authors to suggest possible contralateral presynaptic inhibition of these reflexes (Jiang et al. 1999). However, despite these findings, no literature has addressed the contribution of contralaterally derived presynaptic inhibition to sensory regulation during locomotion.
Our results reveal a previously unstudied mechanism for presynaptic inhibition during locomotion, demonstrating that the contralateral limb plays a pivotal role in regulating ipsilateral sensory inflow at the presynaptic terminal. Specifically, stance-phase force experienced by one limb influences both the extent and timing of swing-phase presynaptic inhibition on the other limb. Here we discuss the potential underlying circuitry and the functional implications of these findings.
The L2 DRPs were abolished by bicuculline, a GABAA-receptor antagonist, suggesting that the contralateral afferents act through a GABAergic pathway, the hallmark of PAD-mediated presynaptic inhibition. It should be noted that the contribution of other nonspecific pharmacological actions of bicuculline cannot be discounted, and may also contribute (Hochman et al. 2010).
As shown in Fig. 4, the magnitude of the DRP strongly correlates with contralateral limb endpoint force, but is independent of ipsilateral force. On a step-to-step basis, if contralateral stance-phase force increases, presynaptic inhibition increases such that less sensory feedback can enter the spinal cord through the inhibited afferent pathways; if contralateral force decreases, more feedback is allowed through. In this way, contralateral limb force can dynamically shape sensory inflow to match environmental conditions by selectively reducing specific inputs and potentially altering the balance between various sensory pathways.
The plate removal experiments confirmed that contralateral force not only influences presynaptic inhibition but is necessary for its generation. When the contralateral plate was removed and ground contact force was absent, the large L2 DRPs were abolished. The much smaller DRPs that sometimes remain following contralateral plate removal likely reflect rhythmic PADs generated by central circuits, as reported previously during fictive locomotion (Gossard et al. 1989, 1991). Alternatively, they could result from spontaneous activity (Bos et al. 2011) or other afferent inputs. The largest component of the locomotor presynaptic inhibition, however, appears to be dependent on contralateral force.
For contralateral force–responsive afferents to evoke this presynaptic inhibition, this force must occur immediately before the DRP. This was true in all experiments. The strong temporal coupling (Fig. 4) suggests that contralateral stance onset determines when sensory transmission is maximally inhibited during ipsilateral flexion. In contrast, ipsilateral force exhibited significantly weaker coupling and exerted little influence on the timing of presynaptic inhibition. Motor output also exhibited weak coupling, further confirming that afferent input, not motor output, is largely responsible for the majority of presynaptic inhibition during locomotion. Thus, contralateral stance-phase force influences not only the extent but also the timing of presynaptic inhibition during ipsilateral swing.
Interestingly, the spatiotemporal profile of presynaptic inhibition agrees with previous assertions from humans. First, studies on the soleus H-reflex suggest that the monosynaptic reflex gain is highest during ipsilateral stance and lowest during swing (e.g., Brooke et al. 1997; Stein 1995). Here we showed that contralaterally derived presynaptic inhibition of multisegmental afferents reaches maximum during swing, providing direct evidence that this form of presynaptic inhibition could explain the reflex modulation patterns consistently observed in humans. Further, in humans, loading is essential for inhibition of Ib afferent pathways (Faist et al. 2006) and cycling studies have repeatedly shown the importance of contralateral limb loading and interlimb phasing in sculpting the ipsilateral sensorimotor state (Alibiglou et al. 2009; Brooke et al. 1997; Ting et al. 1998, 2000). Thus, our studies provide a physiological mechanism to explain the behavioral observations made in humans.
Potential Afferent Modalities Involved
As demonstrated by contralateral dorsal root rhizotomy and force plate removal, force-sensitive swing-phase presynaptic inhibition requires contralateral afferent signaling. Although the specific afferent modality was not directly identified, several observations suggest that Ib afferents of extrinsic toe flexors may be involved. First, removing substantial cutaneous innervation of the paw plantar surface by plantar nerve transection did not reduce the DRPs, suggesting that the majority of toe cutaneous afferents are not necessary. It should be noted the cutaneous branch of the deep peroneal nerve has a small cutaneous receptive field on digits two and three (Bouyer and Rossignol 2003b; Holmberg and Schouenborg 1996). Contributions from these afferents cannot be ruled out. Second, intrinsic toe muscles are also primarily innervated by the plantar nerves and thus not required. Additionally, DRP magnitude did not scale with contralateral range of motion (muscle stretch) or slope (rate of stretch) at the ankle, knee, or hip, making a strong contribution from these muscle spindles unlikely (Prochazka et al. 1989).
Because toe contact was required for DRP generation, toe afferents constitute one potential source of the contralateral presynaptic inhibition. The extrinsic toe flexor muscles, flexor hallucis longus (FHL) and flexor digitorum longus (FDL), are active during stance with other ankle extensors in the rat (Kiehn and Kjaerulf 1996; O'Donovan et al. 1982). In cat, FHL and FDL group Ia and group Ib afferents both fire during stance, particularly at toe contact (Loeb and Duysens 1979; Prochazka and Gorassini 1998; Prochazka et al. 1976), making their peak firing well-timed to evoke the observed presynaptic inhibition. Additionally, Ib afferents, but not Ia or group II afferents, have been shown to produce contralateral presynaptic inhibition in the anesthetized cat (Devanandan et al. 1965). Ia presynaptic actions tended to be strictly ipsilateral, mirroring the largely ipsilateral postsynaptic actions of Ia afferents (Harrison and Zytnicki 1984; Holmqvist 1961). Additionally, increased contralateral limb endpoint force would likely lead to increased Ib firing as ankle extensor/toe flexor activity increased (Donelan and Pearson 2004; Loeb and Duysens 1979), readily explaining the scaling with contralateral force. Toe afferents are also well positioned to sense ground stability and unexpected toe or ankle perturbations during stance because most quadrupeds walk with digitigrade limb posture (Cunningham et al. 2010). Joint afferents may also contribute because some joint afferents signal joint angle changes in the midrange, and toe joint afferents are involved in proprioception (Ferrell 1980). In sum, Ib afferents from FHL and FDL, and potentially toe joint afferents or nonplantar cutaneous afferents, are the most likely sources of contralateral force–sensitive presynaptic inhibition.
The identity of the afferents receiving contralateral presynaptic inhibition may include Ia, Ib, and cutaneous afferents, with Ib afferents being the most likely. Ib afferents receive crossed presynaptic inhibition from contralateral Ib afferents (Devanandan et al. 1965). In contrast, cutaneous and Ia afferents have not been shown to receive crossed presynaptic inhibition from low-threshold muscle afferents (Baldissera et al. 1981; Devanandan et al. 1965). However, cutaneous afferents do receive crossed presynaptic inhibition from higher-threshold muscle afferents and cutaneous afferents (Eccles 1964; Eccles et al. 1964). In the presence of l-DOPA, Ia afferents also receive crossed presynaptic inhibition from higher-threshold muscle afferents (Jankowska et al. 1966). Thus, although we cannot exclude the possibility of contralateral presynaptic inhibition of cutaneous or Ia afferents, Ib afferents most likely receive crossed presynaptic inhibition from low-threshold muscle afferents, including Ib afferents that may respond to limb loading during stance.
Functional Implications
Contralateral force–sensitive presynaptic inhibition has numerous functional implications for locomotor sensory regulation. First, like all forms of presynaptic inhibition, contralateral inhibition can reduce task-irrelevant or counterproductive inputs and thereby focus attention on behaviorally relevant sensory information during the swing phase, such as those necessary for directing limb placement or adjusting to perturbations (e.g., Bouyer and Rossignol 2003a; Forssberg 1979; Van Wezel et al. 1997). Once the contralateral limb is loaded, it is safe for the ipsilateral limb to enter swing; contralateral presynaptic inhibition may serve to reduce inputs that would otherwise impede flexion progression, such as negative force feedback onto flexor motoneurons during the peak of flexor activity. In fact, previous work in the cat suggests that Ib inhibition of flexor motoneurons may be replaced by disynaptic excitation during locomotion (Quevedo et al. 2000) and work in humans suggests that loading may be necessary for inhibition of Ib afferents (Faist et al. 2006). Contralateral presynaptic inhibition of Ib afferents supports both of these findings. Additionally, contralateral presynaptic inhibition neuromechanically couples the sensorimotor state of the two limbs; the force experienced by one limb regulates sensory transmission in the other limb. In this way, interlimb coordination is achieved not only through rhythm-generating circuits, supraspinal commands, and crossed reflex pathways, but also through contralateral presynaptic inhibition. Finally, by responding to limb loading, contralateral presynaptic inhibition can dynamically modulate sensory transmission depending on task-specific force profiles, speed, and even step-to-step perturbations to ground contact and stability. Altering sensory transmission in response to such peripheral cues allows animals or humans to shape the sensitivity of their motor program to specific sensory cues (Prochazka 1989).
Due to the loss or damage of descending systems, presynaptic inhibition is typically reduced after spinal cord injury and stroke (Calancie et al. 1993; Faist et al. 1999; Stein 1995; Yang et al. 1991), possibly contributing to sensory dysfunctions such as spasticity. This work demonstrates that contralateral limb loading may be an important variable for reestablishing appropriate sensory regulation during locomotion. Much research has focused on how ipsilateral manipulations can restore regulation after injury (Fung and Barbeau 1994; Knikou 2010), but the present results emphasize that contralateral limb loading may be an even more powerful input. Contralateral manipulations during body-weight–-supported treadmill training, such as phasic loading at the foot or electrical stimulation, may help restore presynaptic inhibition on the ipsilateral limb. Contralateral manipulations may be particularly useful in hemiparesis seen in stroke and certain spinal cord injuries because the unimpaired limb could be used as a regulatory gateway to the paretic limb.
GRANTS
This work was funded by National Institute of Neurological Disorders and Stroke Grants NS-45248, NS-65949, and National Science Foundation (NSF) Grant 0745164 to S.H., National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-054760 to Y.H.C., and NSF Graduate Research Fellowship Program fellowship to H.B.H.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.B.H., Y.-H.C., and S.H. conception and design of research; H.B.H. performed experiments; H.B.H. analyzed data; H.B.H., Y.-H.C., and S.H. interpreted results of experiments; H.B.H. prepared figures; H.B.H. drafted manuscript; H.B.H., Y.-H.C., and S.H. edited and revised manuscript; H.B.H., Y.-H.C., and S.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Bill Goolsby for contributions to the force platform instrumentation.
REFERENCES
- Alibiglou L, Lopez-Ortiz C, Walter CB, Brown DA. Bilateral limb phase relationship and its potential to alter muscle activity phasing during locomotion. J Neurophysiol 102: 2856–2865, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems. In: Handbook of Physiology. The Nervous System. Motor Control. Bethesda, MD: Am. Physiol. Soc., 1981, p. 509–595 [Google Scholar]
- Beloozerova I, Rossignol S. Antidromic discharges in dorsal roots of decerebrate cats. II. Studies during treadmill locomotion. Brain Res 996: 227–236, 2004 [DOI] [PubMed] [Google Scholar]
- Berens P. CircStat: a Matlab toolbox for circular statistics. J Stat Softw 31: 1–21, 2009 [Google Scholar]
- Bos R, Brocard F, Vinay L. Primary afferent terminals acting as excitatory interneurons contribute to spontaneous motor activities in the immature spinal cord. J Neurosci 31: 10184–10188, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer LJG, Rossignol S. Contribution of cutaneous inputs from the hindpaw to the control of locomotion. I. Intact cats. J Neurophysiol 90: 3625–3639, 2003a [DOI] [PubMed] [Google Scholar]
- Bouyer LJG, Rossignol S. Contribution of cutaneous inputs from the hindpaw to the control of locomotion. II. Spinal cats. J Neurophysiol 90: 3640–3653, 2003b [DOI] [PubMed] [Google Scholar]
- Brooke JD, Cheng J, Collins DF, McIlroy WE, Misiaszek JE, Staines WR. Sensori-sensory afferent conditioning with leg movement: gain control in spinal reflex and ascending paths. Prog Neurobiol 51: 393–421, 1997 [DOI] [PubMed] [Google Scholar]
- Calancie B, Broton JG, Klose KJ, Traad M, Difini J, Ayyar DR. Evidence that alterations in presynaptic inhibition contribute to segmental hypo- and hyperexcitability after spinal cord injury in man. Electroencephalogr Clin Neurophysiol 89: 177–186, 1993 [DOI] [PubMed] [Google Scholar]
- Cattaert D, El Manira A, Clarac F. Direct evidence for presynaptic inhibitory mechanisms in crayfish sensory afferents. J Neurophysiol 67: 610–624, 1992 [DOI] [PubMed] [Google Scholar]
- Cattaert D, El Manira A, Clarac F. Chloride conductance produces both presynaptic inhibition and antidromic spikes in primary afferents. Brain Res 666: 109–112, 1994 [DOI] [PubMed] [Google Scholar]
- Chang YH, Bertram JEA, Ruina A. A dynamic force and moment analysis system for brachiation. J Exp Biol 200: 3013–3302, 1997 [DOI] [PubMed] [Google Scholar]
- Clarac F, Cattaert D. Invertebrate presynaptic inhibition and motor control. Exp Brain Res 112: 163–180, 1996 [DOI] [PubMed] [Google Scholar]
- Cunningham CB, Schilling N, Anders C, Carrier DR. The influence of foot posture on the cost of transport in humans. J Exp Biol 213: 790–797, 2010 [DOI] [PubMed] [Google Scholar]
- Devanandan MS, Holmqvist B, Yokota T. Presynaptic depolarization of group I muscle afferents by contralateral afferent volleys. Acta Physiol Scand 63: 46–54, 1965 [DOI] [PubMed] [Google Scholar]
- Donelan JM, Pearson KG. Contribution of force feedback to ankle extensor activity in decerebrate walking cats. J Neurophysiol 92: 2093–2104, 2004 [DOI] [PubMed] [Google Scholar]
- Dubuc R, Cabelguen JM, Rossignol S. Rhythmic fluctuations of dorsal root potentials and antidromic discharges of primary afferents during fictive locomotion in the cat. J Neurophysiol 60: 2014–2036, 1988 [DOI] [PubMed] [Google Scholar]
- Duenas SH, Rudomin P. Excitability changes of ankle extensor group Ia and Ib fibers during fictive locomotion in the cat. Exp Brain Res 70: 15–25, 1988 [DOI] [PubMed] [Google Scholar]
- Eccles JC. Presynaptic inhibition in the spinal cord. Prog Brain Res 12: 65–91, 1964 [DOI] [PubMed] [Google Scholar]
- Eccles JC, Schmidt RF, Willis WD. Presynaptic inhibition of the spinal monosynaptic reflex pathway. J Physiol 161: 282–297, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles RM, Holmqvist B, Voorhoeve PE. Presynaptic depolarization of cutaneous afferents by volleys in contralateral muscle afferents. Acta Physiol Scand 62: 474–484, 1964 [DOI] [PubMed] [Google Scholar]
- El Manira A, DiCaprio RA, Cattaert D, Clarac F. Monosynaptic interjoint reflexes and their central modulation during fictive locomotion in crayfish. Eur J Neurosci 3: 1219–1231, 1991 [DOI] [PubMed] [Google Scholar]
- Eng JJ, Winter DA, Patla AE. Strategies for recovery from a trip in early and late swing during human walking. Exp Brain Res 102: 339–349, 1994 [DOI] [PubMed] [Google Scholar]
- Faist M, Ertel M, Berger W, Dietz V. Impaired modulation of quadriceps tendon jerk reflexes during spastic gait: differences between spinal and cerebral lesions. Brain 122: 567–579, 1999 [DOI] [PubMed] [Google Scholar]
- Faist M, Hoefer C, Hodapp M, Dietz V, Berger W, Duysens J. In humans Ib facilitation depends on locomotion while suppression of Ib inhibition requires loading. Brain Res 1076: 87–92, 2006 [DOI] [PubMed] [Google Scholar]
- Ferrell WR. The adequacy of stretch receptors in the cat knee joint for signalling joint angle throughout a full range of motion. J Physiol 299: 85–99, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssberg H. Stumbling corrective reaction: a phase-dependent compensatory reaction during locomotion. J Neurophysiol 42: 936–953, 1979 [DOI] [PubMed] [Google Scholar]
- Fung J, Barbeau H. Effects of conditioning cutaneomuscular stimulation on the soleus H-reflex in normal and spastic paretic subjects during walking and standing. J Neurophysiol 72: 2090–2104, 1994 [DOI] [PubMed] [Google Scholar]
- Gossard JP, Cabelguen JM, Rossignol S. Intra-axonal recordings of cutaneous primary afferents during fictive locomotion in the cat. J Neurophysiol 62: 1177–1188, 1989 [DOI] [PubMed] [Google Scholar]
- Gossard JP, Cabelguen JM, Rossignol S. An intracellular study of muscle primary afferents during fictive locomotion in the cat. J Neurophysiol 65: 914–926, 1991 [DOI] [PubMed] [Google Scholar]
- Gossard JP, Rossignol S. Phase-dependent modulation of dorsal root potentials evoked by peripheral nerve stimulation during fictive locomotion in the cat. Brain Res 537: 1–13, 1990 [DOI] [PubMed] [Google Scholar]
- Greene EC. Anatomy of the Rat. New York: Hafner Publishing, 1963 [Google Scholar]
- Grillner P, Rossignol S. Contralateral reflex reversal controlled by limb position in the acute spinal cat injected with clonidine iv. Brain Res 144: 411–414, 1978 [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Zytnicki D. Crossed actions of group I muscle afferents in the cat. J Physiol 356: 263–273, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes HB, Chang YH, Hochman S. An in vitro spinal cord-hindlimb preparation for studying behaviorally relevant rat locomotor function. J Neurophysiol 101: 1114–1122, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes HB, Chang YH, Hochman S. Modulation of sensory input and interneuronal activity during non-fictive locomotion in the in vitro spinal cord-hindlimb rat preparation. Program No. 564.10. Chicago, IL: Society for Neuroscience, 2009b. Online [Google Scholar]
- Hayes HB, Hochman S. Sensory processing by spinal neurons during non-fictive locomotor behavior. Cellular and Network Functions in the Spinal Cord. Madison, WI: University of Wisconsin-Madison, June 2009 [Google Scholar]
- Heglund NC. A simple design for a force-plate to measure ground reaction forces. J Exp Biol 93: 333–338, 1981 [Google Scholar]
- Hochman S, Shreckengost J, Kimura H, Quevedo J. Presynaptic inhibition of primary afferent by depolarization: observations supporting nontraditional mechanisms. Ann NY Acad Sci 1198: 140–152, 2010 [DOI] [PubMed] [Google Scholar]
- Holmberg H, Schouenborg J. Developmental adaptation of withdrawal reflexes to early alteration of peripheral innervation in the rat. J Physiol 495: 399–409, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmqvist B. Crossed spinal reflex actions evoked by volleys in somatic afferents. Acta Physiol Scand Suppl 52: 1–66, 1961 [PubMed] [Google Scholar]
- Jankowska E, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 4. Depolarization evoked in the contralateral terminals of contralateral Ia afferent terminals by volleys in the flexor reflex afferents. Acta Physiol Scand 68: 337–341, 1966 [Google Scholar]
- Jiang Z, Carlin KP, Brownstone RM. An in vitro functionally mature mouse spinal cord preparation for the study of spinal motor networks. Brain Res 816: 493–499, 1999 [DOI] [PubMed] [Google Scholar]
- Kennedy D, Calabrese RL, Wine JJ. Presynaptic inhibition: primary afferent depolarization in crayfish neurons. Science 186: 451–454, 1974 [DOI] [PubMed] [Google Scholar]
- Kiehn O, Iizuka M, Kudo N. Resetting from low threshold afferents of N-methyl-D-aspartate-induced locomotor rhythm in the isolated spinal cord-hindlimb preparation from newborn rats. Neurosci Lett 148: 43–46, 1992 [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulf O. Spatiotemporal characteristics of 5-HT and dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat. J Neurophysiol 75: 1472–1482, 1996 [DOI] [PubMed] [Google Scholar]
- Knikou M. Plantar cutaneous afferents normalize the reflex modulation patterns during stepping in chronic human spinal cord injury. J Neurophysiol 103: 1304–1314, 2010 [DOI] [PubMed] [Google Scholar]
- Loeb GE, Duysens J. Activity patterns in individual hindlimb primary and secondary muscle spindle afferents during normal movements in unrestrained cats. J Neurophysiol 42: 420–440, 1979 [DOI] [PubMed] [Google Scholar]
- McIlroy WE, Collins DF, Brooke JD. Movement features and H-reflex modulation. II. Passive rotation, movement velocity and single leg movement Brain Res 582: 85–93, 1992 [DOI] [PubMed] [Google Scholar]
- Ménard A, Leblond H, Gossard JP. The modulation of presynaptic inhibition in single muscle primary afferents during fictive locomotion in the cat. J Neurosci 19: 391–400, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard A, Leblond H, Gossard JP. Modulation of monosynaptic transmission by presynaptic inhibition during fictive locomotion in the cat. Brain Res 964: 67–82, 2003 [DOI] [PubMed] [Google Scholar]
- O'Donovan MJ, Pinter MJ, Dum RP, Burke RE. Actions of the FDL and FHL muscles in intact cats: functional dissociation between anatomical synergists. J Neurophysiol 47: 1126–1143, 1982 [DOI] [PubMed] [Google Scholar]
- Pang MYC, Yang JF. The initiation of the swing phase in human infant stepping: importance of hip position and leg loading. J Physiol 528: 389–404, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KG, Misiaszek JE, Fouad K. Enhancement and resetting of locomotor activity by muscle afferents. Ann NY Acad Sci 860: 203–215, 1998 [DOI] [PubMed] [Google Scholar]
- Prochazka A. Sensorimotor gain control: a basic strategy of motor systems? Prog Neurobiol 33: 281–307, 1989 [DOI] [PubMed] [Google Scholar]
- Prochazka A, Gorassini MA. Ensemble firing of muscle afferents recorded during normal locomotion in cats. J Physiol 507: 293–304, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Trend P, Hulliger M, Vincent S. Ensemble proprioceptive activity in the cat step cycle: towards a representative look-up chart. Prog Brain Res 80: 61–74, 1989 [DOI] [PubMed] [Google Scholar]
- Prochazka A, Westerman RA, Ziccone SP. Discharges of single hindlimb afferents in the freely moving cat. J Neurophysiol 39: 1090–1104, 1976 [DOI] [PubMed] [Google Scholar]
- Quevedo J, Fedirchuk B, Gosgnach S, McCrea DA. Group I disynaptic excitation of cat hindlimb flexor and bifunctional motoneurones during fictive locomotion. J Physiol 525: 549–564, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo J, Stecina K, McCrea DA. Intracellular analysis of reflex pathways underlying the stumbling corrective reaction during fictive locomotion in the cat. J Neurophysiol 94: 2053–2062, 2005 [DOI] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev 86: 89–154, 2006 [DOI] [PubMed] [Google Scholar]
- Rudomin P. In search of lost presynaptic inhibition. Exp Brain Res 196: 139–151, 2009 [DOI] [PubMed] [Google Scholar]
- Stein RB. Presynaptic inhibition in humans. Prog Neurobiol 47: 533–544, 1995 [DOI] [PubMed] [Google Scholar]
- Swett JE, Woolf CJ. The somatotopic organization of primary afferent terminals in the superficial laminae of the dorsal horn of the rat spinal cord. J Comp Neurol 231: 66–77, 1985 [DOI] [PubMed] [Google Scholar]
- Ting LH, Christine RC, Brown DA, Kautz SA, Zajac FE. Sensorimotor state of the contralateral leg affects ipsilateral muscle coordination of pedaling. J Neurophysiol 80: 1341–1351, 1998 [DOI] [PubMed] [Google Scholar]
- Ting LH, Kautz SA, Brown DA, Zajac FE. Contralateral movement and extensor force generation alter flexion phase muscle coordination in pedaling. J Neurophysiol 83: 3351–3365, 2000 [DOI] [PubMed] [Google Scholar]
- Van Wezel BMH, Ottenhoff FAM, Duysens J. Dynamic control of location-specific information in tactile cutaneous reflexes from the foot during human walking. J Neurosci 17: 3804–3814, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakhnitsa IA, Pilyavskii AI, Bulgakova NV. Phase-dependent changes in dorsal root potential during actual locomotion in rats. Neirofiziologiya 20: 333–340, 1988 [PubMed] [Google Scholar]
- Yang JF, Fung J, Edamura M, Blunt R, Stein RB, Barbeau H. H-reflex modulation during walking in spastic paretic subjects. Can J Neurol Sci 18: 443–452, 1991 [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. Englewood Cliffs, NJ: Prentice Hall, 1974 [Google Scholar]