Abstract
This study investigated the behavior of motor units in the semispinalis cervicis muscle. Intramuscular EMG recordings were obtained unilaterally at levels C2 and C5 in 15 healthy volunteers (8 men, 7 women) who performed isometric neck extensions at 5%, 10%, and 20% of the maximal force [maximum voluntary contraction (MVC)] for 2 min each and linearly increasing force contractions from 0 to 30% MVC over 3 s. Individual motor unit action potentials were identified. The discharge rate and interspike interval variability of the motor units in the two locations did not differ. However, the recruitment threshold of motor units detected at C2 (n = 16, mean ± SD: 10.3 ± 6.0% MVC) was greater than that of motor units detected at C5 (n = 92, 6.9 ± 4.3% MVC) (P < 0.01). A significant level of short-term synchronization was identified in 246 of 307 motor unit pairs when computed within one spinal level but only in 28 of 110 pairs of motor units between the two levels. The common input strength, which quantifies motor unit synchronization, was greater for pairs within one level (0.47 ± 0.32) compared with pairs between levels (0.09 ± 0.07) (P < 0.05). In a second experiment on eight healthy subjects, interference EMG was recorded from the same locations during a linearly increasing force contraction from 0 to 40% MVC and showed significantly greater EMG amplitude at C5 than at C2. In conclusion, synaptic input is distributed partly independently and nonuniformly to different fascicles of the semispinalis cervicis muscle.
Keywords: motoneurons, synchronization
when the synaptic input is equally distributed among motoneurons, small-sized motoneurons are recruited before larger ones (Henneman 1957, 1985). However, motoneurons innervating muscle fibers within the same muscle but with different mechanical action may receive different synaptic input that depends on the biomechanical demands. Those fibers that have a mechanical action with greater advantage for the task may be preferentially activated (English et al. 1993; Hudson et al. 2009), so that the recruitment order is task dependent within a muscle. For example, the recruitment of motor units in different regions of the long head of the biceps brachii varies with the relative amount of elbow flexion and forearm supination torque (ter Haar Romeny et al. 1982, 1984). Moreover, a series of studies on inspiratory muscles showed that the recruitment pattern of motor units across inspiratory motoneuron pools follows the mechanical advantage for respiration (Butler 2007; Butler and Gandevia 2008). Other muscles with complex mechanical actions, such as the trapezius, also display a location-dependent modulation of motor unit discharge rate, likely reflecting spatial dependence in the control of motor units (Falla and Farina 2008a).
It may be expected that motor units are recruited according to their mechanical advantage for muscles with complex architecture and varying mechanical actions for different fascicles of the muscle. The deep spinal muscles are an example of such complexity. They attach directly to several vertebrae and span numerous articulations to control segmental movement and stability (Bergmark 1989).
In the cervical spine, the fascicles of the semispinalis cervicis muscle originate from the transverse processes of the upper five or six thoracic vertebrae and insert on the cervical spinous processes, from the axis to the seventh cervical vertebrae inclusive. Each fascicle spans four to six segments (Drake et al. 2010; Schuenke et al. 2006). The semispinalis cervicis contributes to extension, ipsilateral lateral flexion, and contralateral rotation of the cervical spine (Drake et al. 2010). These functions are applicable to each segment crossed by the muscle fibers. The mechanical load is higher in the lower cervical spine compared with the middle and upper cervical segments because of the longer moment arm; thus caudal fascicles of the semispinalis cervicis are expected to exert more force than cranial fascicles. Because of this difference in mechanical action, we hypothesized that different fascicles within the semispinalis cervicis receive different synaptic input at a given external extension force according to their mechanical advantage and that motor units innervating fascicles with a higher force demand during isometric neck extension are recruited earlier. To test these hypotheses, this study investigated the behavior of individual motor units in two fascicles of the semispinalis cervicis.
METHODS
Subjects.
Fifteen healthy subjects [7 women: age (mean ± SD): 24.1 ± 2.9 yr, height: 169.5 ± 4.2 cm, weight: 69.0 ± 7.1 kg, body mass index (BMI): 24.0 ± 3.34 kg/m2; 8 men: age: 24.2 ± 1.9 yr, height: 184.8 ± 7.2 cm, weight: 79.7 ± 13.0 kg, BMI: 23.2 ± 2.9 kg/m2] participated in the first experiment, which aimed to identify the discharge patterns of semispinalis cervicis motor units. In addition, a separate group of eight healthy women (age: 26.0 ± 2.7 yr, height: 167.3 ± 8.3 cm, weight: 58.3 ± 7.3 kg, BMI: 20.8 ± 2.1 kg/m2) participated in the second experiment, which aimed to measure the interference EMG of the same muscle at the same two locations (see Procedures for a detailed description of the 2 experiments). The data from experiment 2 were also used in a clinical study that compared activity of the semispinalis cervicis muscle in patients with chronic neck pain and healthy control subjects. Since chronic neck pain is more prevalent in women, the study was designed for female subjects only. For both experiments, subjects were included in the study if their age was between 18 and 45 yr and they were free of neck pain, had not had neck surgery, and had no history of neurological disorders.
Ethical approval for the study was granted by the Ethics Committee of Nordjylland, Denmark (ref. N-20090039). Informed written consent was obtained from all participants, and the procedures were conducted in accordance with the Declaration of Helsinki.
Electromyography.
Intramuscular EMG signals were recorded from the semispinalis cervicis muscle on the right side at the level of the second and fifth cervical vertebrae (C2 and C5, respectively) with wire electrodes made of Teflon-coated stainless steel (diameter 0.1 mm; A-M Systems, Carlsborg, WA). In the first experiment the recording end of the wire was cut to expose only the cross section in order to detect action potentials of individual motor units. In the second experiment the recording end of the wire was uninsulated for ∼3–4 mm. In this way the recordings provided an indication of the global intensity of activity in each fascicle, as opposed to the selective motor unit recordings in the first experiment that allowed the analysis of only a small portion of each fascicle.
Wires were inserted into the muscle via a 27-gauge hypodermic needle. Needle insertion was guided by ultrasound (Bexander et al. 2005) using a 10-MHz linear transducer (Acuson 128 Computed Sonography) (Lee et al. 2007). Ultrasound is a reliable tool to visualize the deep cervical extensors, as shown by measurements of cross-sectional area (Kristjansson 2004; Stokes et al. 2007). Participants were lying prone on a treatment table with the head resting in a neutral position. The spinous process of C2 was located by palpation as the first bony landmark caudal to the occiput (Lee et al. 2007). The seventh cervical vertebrae (C7) was palpated as the most prominent spinous process (Lee et al. 2007; Stokes et al. 2007). The spinous process of C5 was identified by palpation counting downwards from C2 and confirmed by counting upwards from C7.
Cutaneous landmarks were marked with a pen, and points 15 mm lateral to the midline of the C2 and C5 spinous processes were selected as the insertion points for intramuscular EMG recordings from the semispinalis cervicis (Kramer et al. 2003). For insertion of the wire electrode at the level of C2, the ultrasound transducer was placed transversally in the midline over C2 and moved laterally to image the extensor muscles. Identification of echogenic (bright, reflective) laminae and the spinous process provides the main bony landmarks for identifying the cervical extensors, which are separated by echogenic fascia layers (Stokes et al. 2007; Whittaker et al. 2007).
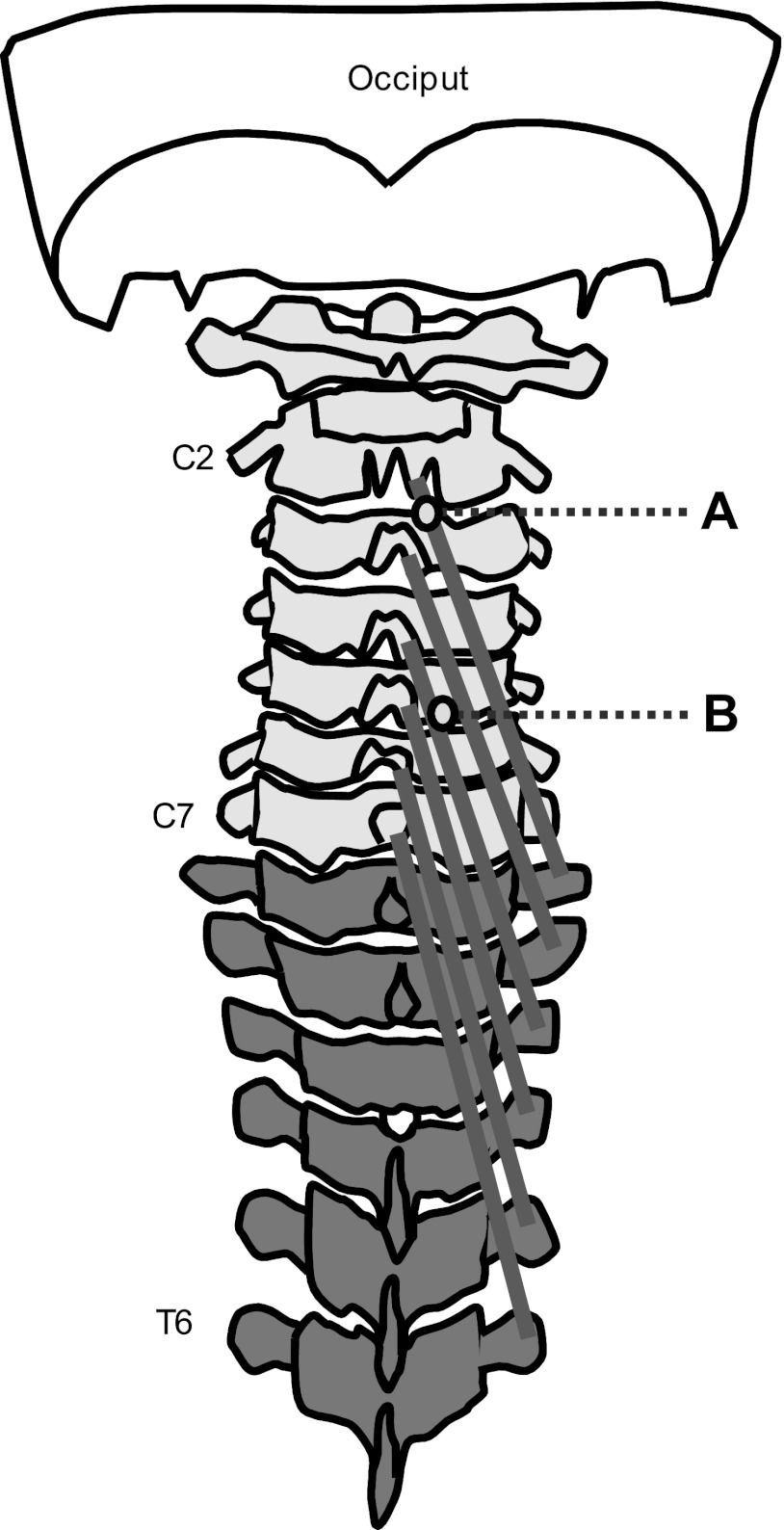
The needle was inserted after identification of the target muscle and disinfection of the skin. The needle containing the wire was inserted vertically into the muscle belly (Kramer et al. 2003), the location confirmed by ultrasonography, and the needle was removed immediately, leaving the wire in the muscle for the duration of the experiment. The end of the wire was hooked to ensure a stable position of the wire at the insertion point. The same procedure was repeated at C5. These procedures ensured that the location of the wires was within two distinct muscle fascicles (Fig. 1).
Fig. 1.
Schematic representation of the fascicles of the semispinalis cervicis muscle and the insertion points at spinal levels C2 (A) and C5 (B).
Intramuscular EMG signals were amplified (amplifier, EMG-USB2, 256-channel EMG amplifier, OT Bioelettronica, Torino, Italy; 500 Hz-5 kHz), sampled at 10,000 Hz, and converted to digital form by a 12-bit analog-to-digital converter. Common reference electrodes were placed around the right and left wrists.
Procedures.
For both the first and second experiments, the participants were seated with their head rigidly fixed in a device for the measurement of multidirectional neck force with their back supported, knees and hips in 90° of flexion, torso firmly strapped to the seat back, and hands resting comfortably in their lap (Falla et al. 2010). The device is equipped with eight adjustable contacts that are fastened around the head to stabilize the head and provide resistance during isometric contractions of the neck. The force device is equipped with force transducers (strain gauges) to measure force in the sagittal and coronal planes. The electrical signals from the strain gauges were amplified (OT Bioelettronica), and their output was displayed on an oscilloscope as visual feedback to the subject.
After a period of familiarization with the measuring device, the subjects performed three neck extension maximum voluntary contractions (MVCs) of 5 s each, separated by 1 min of rest. Verbal encouragement was provided to the subject to promote higher forces in each trial. The highest value of force recorded over the three maximum contractions was selected as the reference MVC. After the MVC contractions the electrodes were inserted as described above. The subject was then seated again in the measuring device with the head and body fixed as described above. In the first experiment the subjects were asked to perform sustained submaximal isometric neck extension contractions for 120 s at 5%, 10%, and 20% MVC. These submaximal force levels were determined in pilot trials as those that allowed identification of single motor unit action potentials with confidence. Each contraction was separated by rest periods of 2 min. After the sustained contractions, three ramp contractions were performed from 0% to 30% MVC over 3 s, with 1 min of rest between contractions.
For the second experiment, the MVCs were performed after wire insertion in order to be able to normalize the EMG amplitude in three subsequent ramped neck extension contractions from 0 to 50% MVC performed over 5 s. For all contractions, the subjects were provided with real-time visual feedback of force on an oscilloscope.
Signal analysis.
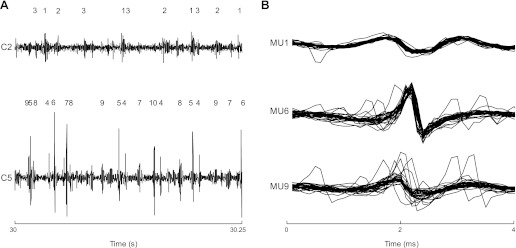
Single motor unit action potentials were identified from the intramuscular EMG signals of the first experiment with a decomposition algorithm described previously (McGill et al. 2005) (Fig. 2). The average discharge rate and discharge rate variability (coefficient of variation for interspike interval) were obtained. Motor units that were active for less than half of the duration of the contraction and motor units with repeated inactive periods of several seconds were discarded from the analysis.
Fig. 2.
Result of decomposition of EMG signals during a 10% maximal voluntary contraction (MVC) contraction for a representative subject. In this case, 3 motor units were identified from C2 and 7 from C5. The action potentials from each of these motor units are identified from the intramuscular EMG recording (A). B: superimposed motor unit action potentials for 3 of the detected motor units. In many cases, not all the identified motor units were included in the analysis since not all were consistently active throughout the contraction. In this example, motor unit (MU)5 and MU10 from C5 were excluded from further analysis since they were active for short periods.
From the ramp contractions of the first experiment, the recruitment threshold (expressed as % MVC) of each motor unit was estimated as the force level at which the motor unit began to discharge steadily (i.e., with separation between discharges in the range 20–200 ms). The estimated recruitment threshold values were averaged over the three ramp contractions to reduce variability in estimates. The same motor units were identified across the different recordings by comparing the shapes of the action potentials, obtained from spike-triggered averaging of the high-pass filtered (500 Hz) EMG recording. Action potentials were considered to be generated by the same motor unit if the mean square error between their shapes was <10%.
The level of common input to the motor unit population was assessed in both the time (short-term synchronization) and frequency (coherence) domains for pairs of motor units recorded in the first experiment. Synchronization was evaluated for pairs of units within the individual recording site and for pairs across recording sites. The quality of estimate of the strength of motor unit synchronization from motor units recorded in one single site strongly depends on the accuracy of the decomposition program. The applied decomposition software has been shown to be highly accurate, so that estimates of synchronization from a single electrode site are appropriate (Dideriksen et al. 2009), as recently discussed (Farina et al. 2012). The degree of motor unit synchronization was estimated by generating cross-histograms (±50 ms relative to the reference motor unit discharge; bin width 1 ms) of all combinations of motor unit pairs (Nordstrom et al. 1992; Semmler et al. 1997). Cross-histograms with an average bin count of <4 were excluded from the analysis (Semmler et al. 1997). The width of the synchronous peak in the cross-histogram was identified with the cumulative sum (Ellaway 1978). Synchronization was quantified by the common input strength (CIS) index (Nordstrom et al. 1992), which denotes the number of synchronous discharges in excess of chance per second. A significant synchronous peak in the cumulative sum function was defined as an increase of at least 3 standard deviations above the mean of the first 30 bins (Davey et al. 1986). The level of common input was also investigated by coherence analysis between spike trains. The coherence was estimated as the ratio of the squared magnitude of the cross-spectra of two spike trains and the product of their autospectra (Rosenberg et al. 1989). The peak value of coherence in the band 16–32 Hz was used to quantify the strength of common input in the beta band.
Since selective intramuscular EMG signals provide information on a very small muscle portion, we also measured global muscle activity in experiment 2. The interference EMG recordings from the second experiment were analyzed by estimating the average rectified value (ARV) from the EMG signals over 300-ms windows in which the average force was 5–40% MVC (5% MVC increments). The ARV computed at these force levels was normalized with respect to the ARV obtained during the MVC. These recordings served to compare the global intensity of muscle activity as a function of force at the two sites (C2 and C5).
Statistical analysis.
For the sustained contractions, two-way analysis of variance (ANOVA) was used to test for differences in motor unit discharge rate, coefficient of variation for interspike variability, and synchronization, with spinal level (C2 and C5) and force level (5%, 10%, and 20% MVC) as factors. A one-way ANOVA was used to evaluate differences in motor unit recruitment threshold, CIS, and coherence values within a spinal level compared with motor unit pairs between spinal levels. For comparisons between coherence values, the values were transformed (Amjad et al. 1989) as follows:
where Rxy(λ) 2 is the coherence value and N is the number of nonoverlapping signal intervals used for the calculation. Furthermore, a two-way ANOVA was used to examine differences in EMG ARV during the ramped contraction (experiment 2) with spinal level (C2 and C5) and force (5–40% MVC in 5% MVC increments) as factors.
Significant differences revealed by ANOVA were followed by post hoc Student-Newman-Keuls (SNK) pairwise comparisons. Results are reported as means and SD in text and SE in Figs. 5 and 6. Statistical significance was set at P < 0.05.
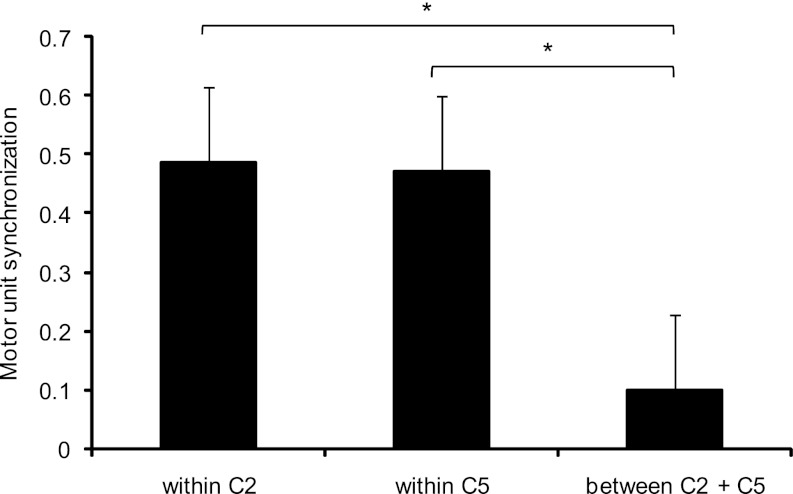
Fig. 5.
Mean and SE of the synchronization of motor unit pairs within each spinal level separately and between spinal levels. The level of synchronization was similar within spinal levels but differed from the synchronization between levels. *P < 0.05.
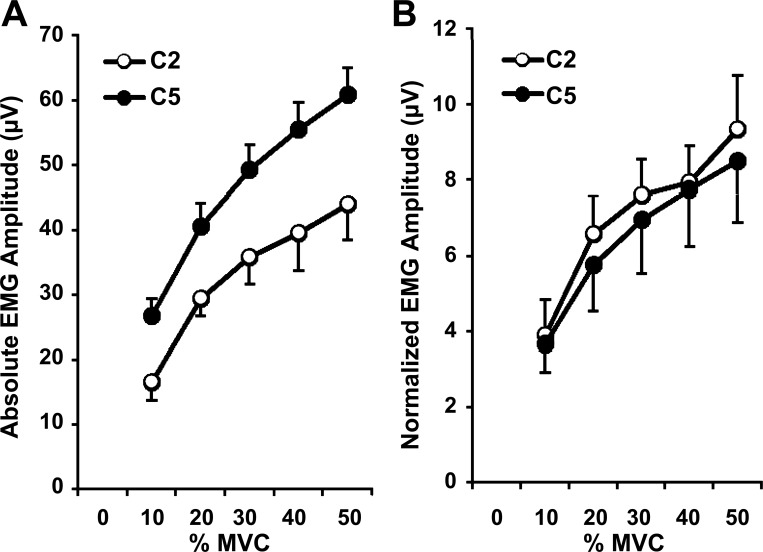
Fig. 6.
Mean and SE of the absolute (A) and normalized (B) EMG amplitude detected from the semispinalis cervicis muscle at the levels of C2 and C5 during a ramped neck extension contraction from 0 to 50% MVC.
RESULTS
Force.
Maximum neck extension force was 214.0 ± 45.0 N for women and 259.1 ± 61.9 N for men in the first experiment. In the second experiment the maximum neck extension force was 187.1 ± 46.1 N.
Motor unit behavior.
The discharge patterns of 98 individual motor units were identified across the three submaximal sustained contractions at C5 from the 15 subjects, whereas motor unit activity was detected in 5 of the 15 subjects at C2 (18 motor units in total). Many of these motor units could be tracked over more than one of the sustained contractions. Therefore, in comparisons of motor unit characteristics across force levels some motor units may contribute with values for more than one contraction (and thus the total number is greater than the number of individual motor units). In general, the number of motor units identified increased with increasing force (Table 1).
Table 1.
Number of motor units identified and mean discharge rate and coefficient of variation for interspike interval at three force levels
| 5% MVC | 10% MVC | 20% MVC | Total MUs | |
|---|---|---|---|---|
| Number of MUs | ||||
| C2 | 5 | 9 | 13 | 27 |
| C5 | 46 | 57 | 67 | 170 |
| Mean discharge rate, pps | ||||
| C2 | 11.3 ± 1.16 | 12.29 ± 1.91 | 13.25 ± 4.09 | 12.57 ± 3.10 |
| C5 | 9.41 ± 2.91 | 10.74 ± 4.03 | 13.80 ± 5.02 | 11.50 ± 4.54 |
| Coefficient of variation of interspike interval variability | ||||
| C2 | 23.82 ± 5.93 | 22.18 ± 4.61 | 23.67 ± 4.87 | 23.16 ± 4.84 |
| C5 | 21.97 ± 6.23 | 23.17 ± 5.99 | 25.42 ± 5.41 | 23.75 ± 5.97 |
Discharge rate and coefficient of variation for interspike interval values are means ± SD. MU, motor unit; MVC, maximal voluntary contraction; pps, pulses per second.
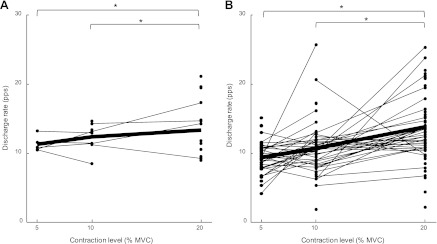
The discharge rate of the identified motor units was greater at 20% MVC compared with both 10% and 5% MVC (P < 0.05) (Table 1 and Fig. 3). The observed mean discharge rate did not differ between C2 and C5 at any force level (P > 0.05) (Table 1). The coefficient of variation for the interspike interval (Table 1) did not differ between the two spinal levels or across the three force levels (P > 0.05).
Fig. 3.
Average discharge rate for all motor units identified at each contraction force for C2 (A) and C5 (B), respectively. Lines connect the discharge rates for those motor units that were identified at more than 1 force level. Bold line represents the mean. *P < 0.05.
The motor unit recruitment thresholds identified from the ramp contractions were determined for 108 of the 116 individual motor units identified during the sustained contractions. The recruitment threshold was significantly greater at C2 (n = 16, 10.3 ± 6.0% MVC) compared with C5 (n = 92, 6.9 ± 4.3% MVC) (P < 0.01), indicating that motor units at C2 were recruited at greater forces than at C5. This result was in agreement with the smaller number of motor units identified at C2 with respect to C5 and with the global EMG amplitude (see below).
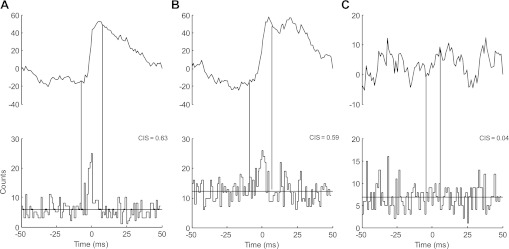
Figure 4 illustrates the cross-histograms and cumulative sum functions estimated during a sustained contraction at 10% MVC for a representative subject. In this example, the cross-histograms are shown for two motor unit pairs detected at the C2 level (Fig. 4A) and for two motor unit pairs at the C5 level (Fig. 4B). Figure 4C shows one motor unit pair across both levels and demonstrates a lower CIS compared with the motor unit pairs within the same spinal level. The peak of the cross-histogram obtained from motor units at different levels was not significant in this example. These observations were confirmed by the group data analysis. The cross-histograms showed significant peaks in 80% of the motor unit pairs (n = 307) when computed within the C2 and C5 levels (89.7% at C2 and 70.4% at C5) but only in 25% of the pairs (n = 110) when computed between levels. Moreover, for these pairs with significant peaks in the cross-histograms, the level of synchronization was significantly greater (P < 0.05) within levels (CIS = 0.48 ± 0.15 for C2; 0.47 ± 0.35 for C5) compared with pairs between levels (0.09 ± 0.07) (Fig. 5). The level of synchronization of these motor unit pairs did not differ between levels (P = 0.91).
Fig. 4.
Representative data showing the cross-histograms and cumulative sum (CuSum) for motor unit pairs in a representative subject during a 10% MVC contraction. The motor unit pairs are detected from the C2 level [A; common input strength (CIS): 0.63], the C5 level (B; CIS: 0.59), and between the 2 levels (C; CIS: 0.04). Vertical lines indicate the boundaries of the synchronous peaks as determined from the CuSum.
For the frequency band 16–32 Hz, the coherence was greater for motor unit pairs from the same level (nontransformed values 0.17 ± 0.13 and 0.19 ± 0.19, respectively) than for motor pairs from different levels (nontransformed value 0.04 ± 0.02) (P < 0.05). Significant coherence was found in 90% of the cases for motor unit pairs from the same level but only in 29% of the cases for motor unit pairs from different levels.
Interference EMG.
In the second experiment, the ARV of the interference EMG during the MVC was 555.5 ± 364.0 μV at C2 and 869.2 ± 388.3 μV at C5 for the eight women. The absolute amplitude increased with increasing force (significantly different between each force level; P < 0.0001) and was significantly greater for C5 than for C2 (P < 0.05; Fig. 6A). The normalized amplitude increased with increasing force (significantly different between each force level; P < 0.0001) but was not significantly different between levels (Fig. 6B).
DISCUSSION
This study investigated motor unit behavior in two fascicles of the semispinalis cervicis muscle. Although there are no previous data on motor unit behavior in this muscle, the discharge rates of the studied motor units were similar to those observed in other muscles (Christou et al. 2007; Falla and Farina 2008a, 2008b; Holobar et al. 2010; Kukulka and Clamann 1981). The coefficient of variation for the interspike interval was also within the physiological range previously observed in other muscles (e.g., first dorsal interosseus, Christou et al. 2007). The main finding of the study is the difference in the strength of synaptic input delivered to different fascicles of the semispinalis cervicis muscle during constant-force extensions. This may be partly due to the different mechanical advantage of the muscle fibers in different fascicles.
The greater recruitment threshold for motor units at the C2 spinal level compared with C5 indicate that the net excitatory input to motoneurons innervating the fibers at C5 is greater than for C2 at a given force level. The greater recruitment threshold at the C2 spinal level is supported by the observation that only 18 motor units were detected at the C2 level compared with the 98 motor units detected at C5. Finally, higher absolute interference EMG amplitude was detected at C5 compared with C2 during the ramped contraction from 0 to 50% MVC, which is consistent with the observed differences in recruitment thresholds between fascicles. It must be noted, however, that absolute EMG values are not directly associated to the number of active motor units, so that EMG amplitude and number of detected units remain partly independent measures of muscle activity. First, the number of motor units detected depends on the spatial distribution of muscle fibers. Second, the amplitude of the EMG is a poor indicator of the number of detected units because of amplitude cancellation that can be very high at the analyzed contraction levels (Keenan et al. 2005). Thus a larger number of active units does not necessarily imply a proportionally greater amplitude of the EMG. Indeed, it can be proven theoretically that surface EMG amplitude is relatively insensitive to changes in motor unit activity (or motor unit number) at relatively high levels of excitation (Farina et al. 2008). For these reasons, a discrepancy between the relative difference in amplitude of the interference EMG and the relative difference in number of motor units is expected.
The preferential recruitment of motoneurons innervating fibers in specific fascicles might be explained by a nonuniform distribution of motoneuron size innervating the different fascicles, although no studies could be found in this regard. However, according to similar studies on other muscles, such as respiratory muscles (Butler and Gandevia 2008; Hudson et al. 2011), the mechanical demands of semispinalis cervicis fascicles at different spinal levels could explain a nonuniform synaptic input to motoneurons in different fascicles.
A nonuniform activation of motor units within muscle regions as found in this study has also been observed in other human muscles, such as the extensor digitorum (Keen and Fuglevand 2004), the flexor digitorum profundus (Reilly et al. 2004), the flexor digitorum superficialis (McIsaac and Fuglevand 2006), and the upper trapezius muscle (Falla and Farina 2008a). Furthermore, in the external intercostal muscles, a cranial-caudal (Hudson et al. 2011) and dorsal-ventral (De Troyer et al. 2003) gradient of activation has been observed. As for the semispinalis cervicis, these recruitment patterns could not be explained by a specific spatial arrangement of muscle fibers. For example, the caudal-cranial gradient for expiratory activity and the medio-lateral gradient for inspiratory activity of the internal intercostalis muscle are not due to fiber distribution, which is similar in different muscle regions (De Troyer et al. 2005). The distribution of synaptic input can diminish the influence of size in recruitment, so that other principles may be prominent. For example, motor units may be recruited according to the mechanical advantage of the muscle fibers (Butler and Gandevia 2008). This neuromechanical principle observed in inspiratory muscles appears to be preset, since it persists when all feedback possibilities are removed in experimental animals (Butler and Gandevia 2008). Similar to the observations for the intercostal muscles, different fascicles of the semispinalis cervicis muscle may be recruited to different degrees depending on the force demand for each fascicle.
Analysis of the correlation between spike trains in the time and frequency domains indicated that the input to motoneurons innervating different fascicles of the semispinalis cervicis muscle is almost independent. The low degree of synchronization between pairs of motor units detected in the present study from two individual fascicles of the semispinalis cervicis muscle indicates a different neural input to semispinalis cervicis at these levels for their independent control. This is further supported by the observation that the coherence in the 16–32 Hz band was highest for pairs of motor units from the same fascicle of the semispinalis cervicis muscle. Independent input to the two fascicles is in agreement with the observation that recruitment thresholds were significantly different for motor units at C2 and C5.
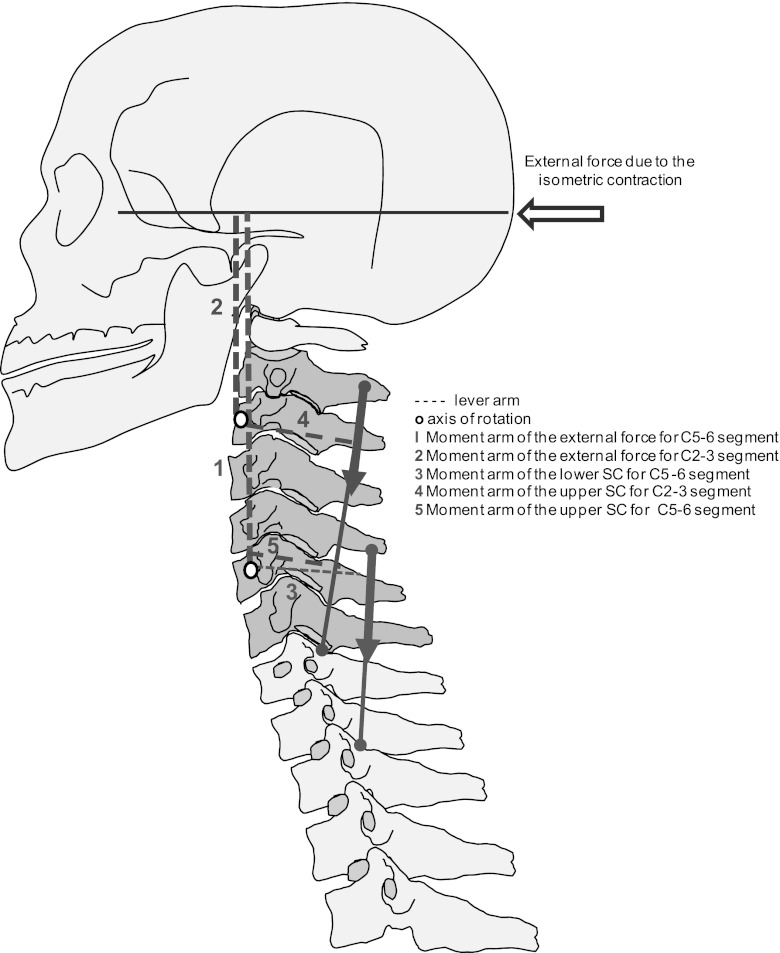
The earlier recruitment of motor units in the caudal with respect to the cranial spinal segments can be explained by different moments exerted by the fascicles of the semispinalis cervicis muscle. The force-generating capacity of a muscle can be deduced from its architectural parameters, such as physiological cross-sectional area, fascicle length, tendon length, pennation angle, and moment arm (Vasavada et al. 1998). These parameters, however, are difficult to assess for the deep cervical muscles because of their complexity (Mayoux-Benhamou et al. 1989; Vasavada et al. 1998) and, except for multifidus (Anderson et al. 2005), are largely unknown. Nevertheless, simple modeling allows a qualitative assessment of the distribution of forces for different fascicles. Figure 7 shows a schematic model describing the mechanical action of the semispinalis cervicis fascicles during isometric extension of the head. To have equilibrium, the external force has to be balanced by muscles and passive structures surrounding the cervical segments from C0 to C7. For example, the external moment to be balanced around C5–6 is larger than the external moment around C2–3, because the moment arm for the C5–6 segment is larger than at C2–3. Therefore, the fascicles spanning the joint C5–6 need to create a higher extension moment than the fascicles spanning C2–3. These conclusions are in agreement with the moment arms for extension of the different fascicles of multifidus, which decrease from ∼1.4 to ∼0.9 and ∼0.3 cm from C6–7 to C5–6 and C4–5, respectively, for the superficial fascicles and from ∼0.7 to ∼0.6 and ∼0.4 cm for the deep fascicles (Anderson et al. 2005).
Fig. 7.
Schematic representation of the moment system for the upper and lower regions of the semispinalis cervicis (SC) during isometric neck extension. The force moment of the reaction force of the head is greater for C5 than for C2. The required force to stabilize C5 is consequently higher than for C2.
As a limitation of this study, it must be noted that motor units were detected from only a single location within each spinal level, which, as explained above, may lead to errors in the estimation of the strength of motor unit synchronization within the fascicles. This was because of the difficulties of needle insertion medially and anterior to the fascia separating semispinalis capitis and semispinalis cervicis, in order to avoid 1) puncture of the laterally lying arteria cervicalis profunda (Kramer et al. 2003) and 2) penetration into the multifidus muscle, which is not always clearly separated from semispinalis cervicis by an echogenic fascia, especially at levels C2 and C5 (Kristjansson 2004; Stokes et al. 2007). Nonetheless, as discussed above, the single site insertions in each fascicle did not influence the results.
In conclusion, this study shows that individual fascicles within the semispinalis cervicis muscle are activated partly independently and with nonuniform synaptic input that allows preferential recruitment of motor units in caudal with respect to cranial fascicles.
GRANTS
This work was partly supported by the ERC Advanced Research Grant DEMOVE (“Decoding the Neural Code of Human Movements for a New Generation of Man-machine Interfaces”; no. 267888).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.S., J.L.D., D. Farina, and D. Falla conception and design of research; J.S., J.L.D., D. Farina, and D. Falla performed experiments; J.S., J.L.D., D. Farina, and D. Falla analyzed data; J.S., J.L.D., D. Farina, and D. Falla interpreted results of experiments; J.S., J.L.D., D. Farina, and D. Falla prepared figures; J.S., J.L.D., D. Farina, and D. Falla drafted manuscript; J.S., J.L.D., D. Farina, and D. Falla edited and revised manuscript; J.S., J.L.D., D. Farina, and D. Falla approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Mark de Zee, Aalborg University, for the useful discussion on the mechanical action of the semispinalis cervicis muscle.
REFERENCES
- Amjad AM, Breeze P, Conway BA, Halliday DM, Rosenberg JR. A framework for the analysis of neuronal networks. Prog Brain Res 80: 243–255, 1989 [DOI] [PubMed] [Google Scholar]
- Anderson JS, Hsu AW, Vasavada AN. Morphology, architecture, and biomechanics of human cervical multifidus. Spine 30: E86–E91, 2005 [DOI] [PubMed] [Google Scholar]
- Bergmark A. Stability of the lumbar spine. A study in mechanical engineering. Acta Orthop Scand 230: 1–54, 1989 [DOI] [PubMed] [Google Scholar]
- Bexander CSM, Mellor R, Hodges PW. Effect of gaze direction on neck muscle activity during cervical rotation. Exp Brain Res 167: 422–432, 2005 [DOI] [PubMed] [Google Scholar]
- Butler JE. Drive to the human respiratory muscles. Respir Physiol Neurobiol 159: 115–126, 2007 [DOI] [PubMed] [Google Scholar]
- Butler JE, Gandevia SC. The output from human inspiratory motoneurone pools. J Physiol 586: 1257–1264, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou EA, Rudroff T, Enoka JA, Meyer F, Enoka RM. Discharge rate during low-force isometric contractions influences motor unit coherence below 15 Hz but not motor unit synchronization. Exp Brain Res 178: 285–295, 2007 [DOI] [PubMed] [Google Scholar]
- Davey NJ, Ellaway PH, Stein RB. Statistical limits for detecting change in the cumulative sum derivative of the peristimulus time histogram. J Neurosci Methods 17: 153–166, 1986 [DOI] [PubMed] [Google Scholar]
- De Troyer A, Gorman RB, Gandevia SC. Distribution of inspiratory drive to the external intercostal muscles in humans. J Physiol 546: 943–954, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Kirkwood PA, Wilson TA. Respiratory action of the intercostal muscles. Physiol Rev 85: 717–756, 2005 [DOI] [PubMed] [Google Scholar]
- Dideriksen JL, Falla D, Baekgaard M, Mogensen ML, Steimle KL, Farina D. Comparison between the degree of motor unit short-term synchronization and recurrence quantification analysis of the surface EMG in two human muscles. Clin Neurophysiol 120: 2086–2092, 2009 [DOI] [PubMed] [Google Scholar]
- Drake RL, Vogl WA, Mitchell AWM. Gray's Anatomy. Philadelphia, PA: Churchill Livingstone Elsevier, 2010 [Google Scholar]
- Ellaway PH. Cumulative sum technique and its application to the analysis of peristimulus time histograms. Electroencephalogr Clin Neurophysiol 45: 302–304, 1978 [DOI] [PubMed] [Google Scholar]
- English AW, Wolf SL, Segal RL. Compartmentalization of muscles and their motor nuclei: the partitioning hypothesis. Phys Ther 73: 857–867, 1993 [DOI] [PubMed] [Google Scholar]
- Falla D, Farina D. Motor units in cranial and caudal regions of the upper trapezius muscle have different discharge rates during brief static contractions. Acta Physiol (Oxf) 192: 551–558, 2008a [DOI] [PubMed] [Google Scholar]
- Falla D, Farina D. Non-uniform adaptation of motor unit discharge rates during sustained static contraction of the upper trapezius muscle. Exp Brain Res 191: 363–370, 2008b [DOI] [PubMed] [Google Scholar]
- Falla D, Lindstrøm R, Rechter L, Farina D. Effect of pain on modulation in discharge rate of sternocleidomastoid motor units with force direction. Clin Neurophysiol 121: 744–753, 2010 [DOI] [PubMed] [Google Scholar]
- Farina D, Cescon C, Negro F, Enoka RM. Amplitude cancellation of motor-unit action potentials in the surface electromyogram can be estimated with spike-triggered averaging. J Neurophysiol 100: 431–40, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina D, Negro F, Gizzi L, Falla D. Low-frequency oscillations of the neural drive to the muscle are increased with experimental muscle pain. J Neurophysiol 107: 958–965, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman E. Relation between size of neurons and their susceptibility to discharge. Science 126: 1345–1347, 1957 [DOI] [PubMed] [Google Scholar]
- Henneman E. The size-principle: a deterministic output emerges from a set of probabilistic connections. J Exp Biol 115: 105–112, 1985 [DOI] [PubMed] [Google Scholar]
- Holobar A, Minetto MA, Botter A, Negro F, Farina D. Experimental analysis of accuracy in the identification of motor unit spike trains from high-density surface EMG. IEEE Trans Neural Syst Rehabil Eng 18: 221–229, 2010 [DOI] [PubMed] [Google Scholar]
- Hudson AL, Gandevia SC, Butler JE. Common rostrocaudal gradient of output from human intercostal motoneurones during voluntary and automatic breathing. Respir Physiol Neurobiol 175: 20–28, 2011 [DOI] [PubMed] [Google Scholar]
- Hudson AL, Taylor JL, Gandevia SC, Butler JE. Coupling between mechanical and neural behaviour in the human first dorsal interosseous muscle. J Physiol 587: 917–926, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen DA, Fuglevand AJ. Common input to motor neurons innervating the same and different compartments of the human extensor digitorum muscle. J Neurophysiol 91: 55–62, 2004 [DOI] [PubMed] [Google Scholar]
- Keenan KG, Farina D, Maluf KS, Merletti R, Enoka RM. Influence of amplitude cancellation on the simulated surface electromyogram. J Appl Physiol 98: 120–131, 2005 [DOI] [PubMed] [Google Scholar]
- Kramer M, Schmid I, Sander S, Högel J, Eisele R, Kinzl L, Hartwig E. Guidelines for the intramuscular positioning of EMG electrodes in the semispinalis capitis and cervicis muscles. J Electromyogr Kinesiol 13: 289–295, 2003 [DOI] [PubMed] [Google Scholar]
- Kristjansson E. Reliability of ultrasonography for the cervical multifidus muscle in asymptomatic and symptomatic subjects. Man Ther 9: 83–88, 2004 [DOI] [PubMed] [Google Scholar]
- Kukulka CG, Clamann HP. Comparison of the recruitment and discharge properties of motor units in human brachial biceps and adductor pollicis during isometric contractions. Brain Res 219: 45–55, 1981 [DOI] [PubMed] [Google Scholar]
- Lee JP, Tseng WY, Shau YW, Wang CL, Wang HK, Wang SF. Measurement of segmental cervical multifidus contraction by ultrasonography in asymptomatic adults. Man Ther 12: 286–294, 2007 [DOI] [PubMed] [Google Scholar]
- Lord S, Barnsley L, Wallis BJ, Bogduk N. Chronic cervical zygapophysial joint pain after whiplash. Spine 21: 1737–1745, 1996 [DOI] [PubMed] [Google Scholar]
- Mayoux-Benhamou MA, Wybier M, Revel M. Strength and cross-sectional area of the dorsal neck muscles. Ergonomics 32: 513–518, 1989 [DOI] [PubMed] [Google Scholar]
- McGill KC, Lateva ZC, Marateb HR. EMGLAB: an interactive EMG decomposition program. J Neurosci Methods 149: 121–133, 2005 [DOI] [PubMed] [Google Scholar]
- McIsaac TL, Fuglevand AJ. Motor-unit synchrony within and across compartments of the human flexor digitorum superficialis. J Neurophysiol 97: 550–556, 2006 [DOI] [PubMed] [Google Scholar]
- Nordstrom MA, Fuglevand AJ, Enoka RM. Estimating the strength of common input to human motoneurons from the cross-correlogram. J Physiol 453: 547–574, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly KT, Nordstrom MA, Schieber MH. Short-term synchronization between motor units in different functional subdivisions of the human flexor digitorum profundus muscle. J Neurophysiol 92: 734–742, 2004 [DOI] [PubMed] [Google Scholar]
- Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM. The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog Biophys Mol Biol 53: 1–31, 1989 [DOI] [PubMed] [Google Scholar]
- Schuenke M, Schulte E, Schumacher U. Thieme Atlas of Anatomy, General Anatomy and Musculoskeletal System. Stuttgart, Germany: Thieme, 2006 [Google Scholar]
- Semmler JG, Nordstrom MA, Wallace CJ. Relationship between motor unit short-term synchronization and common drive in human first dorsal interosseous muscle. Brain Res 767: 314–320, 1997 [DOI] [PubMed] [Google Scholar]
- Stokes M, Hides J, Elliott JM, Kiesel K, Hodges PW. Rehabilitative ultrasound imaging of the posterior paraspinal muscles. J Orthop Sports Phys Ther 37: 581–595, 2007 [DOI] [PubMed] [Google Scholar]
- ter Haar Romeny BM, Denier van der Gon JJ, Gielen CC. Changes in recruitment order of motor units in the human biceps muscle. Exp Neurol 78: 360–368, 1982 [DOI] [PubMed] [Google Scholar]
- ter Haar Romeny BM, van der Gon JJ, Gielen CC. Relation between location of a motor unit in the human biceps brachii and its critical firing levels for different tasks. Exp Neurol 85: 631–650, 1984 [DOI] [PubMed] [Google Scholar]
- Vasavada AN, Siping L, Scott D. Influence of muscle morphometry and moment arms on the moment-generating capacity of human neck muscles. Spine 23: 412–422, 1998 [DOI] [PubMed] [Google Scholar]
- Whittaker JL, Teyhen DS, Elliott JM, Cook K, Langevin HM, Dahl HH, Stokes M. Rehabilitative ultrasound imaging: understanding the technology and its applications. J Orthop Sports Phys Ther 37: 434–449, 2007 [DOI] [PubMed] [Google Scholar]