Abstract
Interplay between posterior parietal cortex (PPC) and ipsilateral primary motor cortex (M1) is crucial during execution of movements. The purpose of the study was to determine whether functional PPC–M1 connectivity in humans can be modulated by sensorimotor training. Seventeen participants performed a sensorimotor training task that involved tapping the index finger in synchrony to a rhythmic sequence. To explore differences in training modality, one group (n = 8) learned by visual and the other (n = 9) by auditory stimuli. Transcranial magnetic stimulation (TMS) was used to assess PPC–M1 connectivity before and after training, whereas electroencephalography (EEG) was used to assess PPC–M1 connectivity during training. Facilitation from PPC to M1 was quantified using paired-pulse TMS at conditioning-test intervals of 2, 4, 6, and 8 ms by measuring motor-evoked potentials (MEPs). TMS was applied at baseline and at four time points (0, 30, 60, and 180 min) after training. For EEG, task-related power and coherence were calculated for early and late training phases. The conditioned MEP was facilitated at a 2-ms conditioning-test interval before training. However, facilitation was abolished immediately following training, but returned to baseline at subsequent time points. Regional EEG activity and interregional connectivity between PPC and M1 showed an initial increase during early training followed by a significant decrease in the late phases. The findings indicate that parietal–motor interactions are activated during early sensorimotor training when sensory information has to be integrated into a coherent movement plan. Once the sequence is encoded and movements become automatized, PPC–M1 connectivity returns to baseline.
Keywords: paired-pulse tms, EEG coherence, motor learning, functional connectivity
motor skills involve learning and performing movement sequences. Planning and execution of these complex sequences relies on incorporation of information from different sensory modalities into a movement plan. The posterior parietal cortex (PPC) has both sensory and motor properties, plays a pivotal role during sensorimotor integration, and acts as a sensorimotor interface (Andersen 1987; Buneo and Andersen 2006; Iacoboni 2006; Rushworth and Taylor 2006). Even though most studies have focused on the role of the PPC in visual-motor integration, there are several imaging studies suggesting that both auditory-motor and visual-motor integration during motor learning are reflected by activity in the PPC (Garraux et al. 2005; Karabanov et al. 2009; Lewis et al. 2004). Sensorimotor integration is critically important during hand motor control, and several EEG and functional magnetic resonance imaging (fMRI) studies have demonstrated PPC activity during a multitude of hand–motor tasks (Bohlhalter et al. 2009; Wheaton et al. 2005, 2009). The pivotal role of the PPC in both sensory integration and hand motor control makes it one of the key structures in manual tasks that rely heavily on sensorimotor integration such as synchronizing finger tapping to an external stimulus.
Anatomically, the PPC is heavily interconnected with motor and premotor areas (Makris et al. 2005; Petrides and Pandya 1984; Tanne-Gariepy et al. 2002). Noninvasive brain mapping has given researchers a wide range of tools to study functional connectivity between different brain regions in humans, such as paired-pulse transcranial magnetic stimulation (TMS) and electroencephalography (EEG) that allow the assessment of functional connections between motor and parietal areas.
Paired-pulse TMS can be used to assess pathways within the motor system and involves a conditioning-test paradigm in which a conditioning stimulus (CS) is delivered to a cortical site of interest followed by a suprathreshold test stimulus (TS) to M1 (Civardi et al. 2001; Ferbert et al. 1992; Hallett 2007; Sanger et al. 2001; Ziemann et al. 1996). Koch et al. (2007) used paired-pulse TMS to assess connections between the PPC and the ipsilateral M1 at rest. They found that a CS over the PPC facilitated the MEP elicited by the TS if pulses were given at specific intensities and ISIs. In subsequent studies Koch and colleagues demonstrated that the influence of the PPC on the ipsilateral M1 was modified during planning of reaching and grasping movements (Koch et al. 2008a, 2010).
EEG can also be used to investigate functional connectivity. Task-related coherence (TrCoh) is a measure of interregional coupling during a steady-state behavior that is computed by correlating oscillatory activity of different electrodes in the frequency domain. TrCoh is often complemented by task-related power (TrPow), which gives insights to regional activation by measuring the magnitude of neural oscillations during steady-state behavior. A wide array of studies have used EEG to investigate both functional coupling and regional activation during motor tasks (Andres et al. 1999; Gerloff et al. 1998; Manganotti et al. 1998; Rietschel et al. 2011). For example, Andres et al. (1999) investigated changes in TrCoh and TrPow before and after a bimanual motor sequence learning session. They showed that interregional connectivity between right and left M1 was high at the beginning of bimanual training and decreased at the end of the learning session. Additionally, they reported that parietal–motor connectivity was increased throughout the training session.
The primary purpose of our study was to determine whether functional connectivity between PPC and M1 can be modulated by sensorimotor training. A secondary purpose was to determine whether changes in functional connectivity would be dependent on the sensory modality used during motor training. Paired-pulse TMS was used to assess PPC–M1 functional connectivity before and after a sensorimotor training task that required participants to tap their finger synchronously with either a visual or an auditory stimulus. EEG was used to assess PPC–M1 connectivity during practice. TrPow and TrCoh were calculated for the first and the last minute of the training session to quantify functional connectivity during early and late phases of motor learning, respectively. In contrast to the EEG study done by Andres et al. (1999), we chose to use a temporal motor sequence (e.g., rhythmic structure) since the PPC has been shown to be equally involved in both spatial and temporal motor sequences (Garraux et al. 2005).
TrCoh between PPC and M1 was expected to be highest during early motor training since this period requires the greatest attention to the details of sensorimotor integration. In addition, it was hypothesized that modulations in the PPC–M1 pathway would outlast the training session and that the parietal–motor connectivity would be facilitated transiently by sensorimotor training.
MATERIALS AND METHODS
Population
Nineteen healthy volunteers (mean age 32.1 ± 8.3 years, 8 male) participated in the study. All participants were right-handed. They did not play an instrument, and had not received formal musical training for >5 years throughout their lives; this criterion was added to ensure that all participants were at a similar level in terms of rhythmic skills for the temporal sequence task. One participant had to be excluded due to failure to perform the task properly and one due to technical problems during TMS stimulation; therefore, only 17 participants were included in the TMS analysis. EEG was recorded for 14 of these participants. The study was approved by the Neuroscience Institutional Review Board (IRB) of the National Institutes of Health (NIH). All participants gave their informed oral and written consent before the experiments in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and the NIH guidelines.
Experimental Procedures
EMG setup.
Participants were seated in a comfortable armchair with both arms resting on a pillow placed on their laps during both training (including EEG) and TMS sessions. Electromyogram (EMG) activity of the right first dorsal interosseus muscle (FDI) was recorded throughout the experiment in a belly-tendon montage using Ag–AgCl surface electrodes. Impedances were kept <5 kΩ. EMG signals were collected using a Viking IV EMG machine (Nicolet Biomedical, Madison, WI), bandpass-filtered at 20 to 2,000 Hz. The amplified analog outputs from the Viking were digitized at 5 kHz using LabView software (National Instruments, Austin, TX), and stored for offline analysis.
Experimental Setup and Learning Task
The full experimental procedure is summarized in Fig. 1. The experiment started with a paired-pulse TMS session. After the initial TMS measurement participants performed 10 min of sensorimotor training that required tapping the right index finger in synchrony to a rhythmic sequence. The rhythmic sequences that each participant learned were nine intervals long, had a total duration of 6 s, and were composed of five 400-ms intervals, two 800-ms intervals, and two 1,200-ms intervals. All component intervals were thus low-integer multiples of 400 ms and were arranged to produce a metrical rhythm of a type commonly found in Western music. The sequence had the temporal structure 400–400-400-400-800-800-400–1,200–1,200. One group of participants (n = 8) learned the rhythmic sequence by visual stimuli (blinking square on a computer screen); the second group (n = 9) learned the identical sequence by an auditory stimulus (beep) presented via headphones. Of the 14 participants for which EEG was recorded during the sensorimotor training task 7 learned the sequence by auditory and 7 by visual stimuli. During the learning task, participants had their right hands placed on a four-key button box that was attached to the pillow supporting the right arm. They were instructed to use only their index finger and the corresponding key on the button box during the tapping task. All participants wore headphones (auditory stimulus) or earplugs (visual stimulus) to shield them from outside noises. After completing the learning task, participants had to perform a short dual task to test automaticity. During this 60-s task, the previously learned rhythm had to be performed from memory without any external cues. Automaticity was evaluated by having subjects perform a visual letter-counting task simultaneously. For the letter-counting task, letter sequences consisting of a random series of the letters A, G, L, and O were presented on a screen and subjects were asked to identify the number of times they saw a target letter (A) on the screen during the 60-s task. Directly after the training session, as well as 30, 60, and 180 min after, additional paired-pulse TMS sessions were done. All four posttraining TMS sessions were identical to the pretraining session and were done at rest. All TMS sessions were performed at rest with the participants' hands placed on their laps. Subjects were instructed not to type, write, text, or do any other manual work during the breaks.
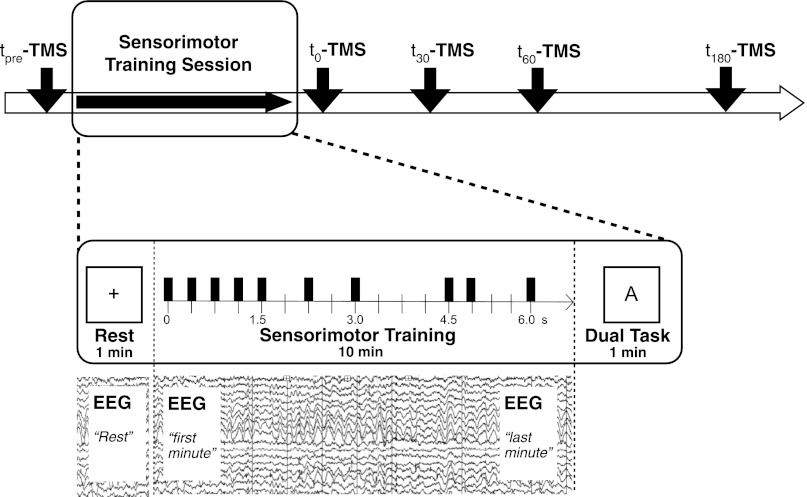
Fig. 1.
Displays the study full design. tpre-t180-TMS indicate the paired-pulse TMS sessions performed prior the rhythm tapping task (tpre) as well as at four time points after the tapping task (t1 = 0 min; t2 = 30 min; t3 = 60 min; t4 = 180 min). The motor training is divided into 1) 1 min of rest, used as baseline for the EEG analysis, 2) a 10-min-long rhythm learning task in which participants learn to synchronize index finger tapping to a rhythmic sequence, and 3) a “dual task” in which the same sequence has to be performed by memory while counting the occurrence of a specific letter on the computer screen. The bars in the “Sensorimotor Training” box indicate the structure of the sequence that was presented. Time is indicated in seconds on the arrow.
EEG Recording and Preprocessing
Directly after the initial TMS session the participants were prepared for the EEG recording. EEG was recorded first during a 1-min resting period and after that continuously throughout the 10-min sensorimotor training task (see Fig. 1). EEG signals were recorded from 32 surface electrodes mounted on a cap (Electro-Cap International, Eaton, OH) using the international 10–20 system referenced to the right earlobe electrode (A2). The left earlobe electrode (A1) was recorded as a separate channel, and we converted the EEG signals into the digitally linked earlobe reference before further analysis. Bipolar recordings of the vertical and horizontal electrooculogram (EOG) and surface EMG from the FDI muscles were simultaneously recorded to monitor eye and finger movements. Signals were amplified (Neuroscan, El Paso, TX), filtered (DC-100 Hz), and digitized with a sampling frequency 1 kHz.
Resting-state EEG was recorded for 1 min while the participants were looking at a fixation cross on a computer monitor. Resting-state EEG was followed by the 10-min sensorimotor training session during which EEG was constantly recorded. The first and last minutes of this recording were used for EEG analyses. The EEG analysis was focused on the electrodes corresponding roughly to stimulation spots during TMS (electrodes C3 and P3). Electrode C4 (right M1) and the interaction between electrodes C3 and C4 (bilateral primary motor cortices) were used in a separate analysis as a control for the specificity of the C3–P3 findings.
Epoching was done with a length of 1,024 ms, and linear trends were removed from the entire epoch. For artifact removal, an infomax independent component analysis (ICA) algorithm (Bell and Sejnowski 1995) implemented in EEGLAB (Delorme and Makeig 2004) was used. ICA-corrected EEG signals are assumed to be artifact-free data reflecting brain activity, and no influence of ICA on power and coherence estimation is expected.
Spectral power for the artifact-free EEG signals was calculated using a fast Fourier transformation (FFT) with Hamming windows. FFT were performed for each 1,024-ms epoch and all electrodes without overlapping between consecutive epochs. Spectral power was calculated for all frequency bins between 0.5 and 97.2 Hz, with 0.98 Hz of frequency resolution. FFT-based power for each epoch was averaged for each time period for the first and for the last minutes, respectively.
Normalized TrPow in an electrode was expressed as the percentage of spectral power during rest (Powrest) compared with the spectral power during task condition (Powtask) (Hummel et al. 2002; Jin et al. 2012). In this way, the effects of intersubject variation in absolute spectral power values were reduced (Gerloff et al. 1998; Hummel et al. 2002).
Since regional cortical activation disrupts neuronal oscillations and decreases regional power, negative TrPow values represent higher cortical activity during task than that at rest (Andres et al. 1999; Classen et al. 1998; Manganotti et al. 1998; Zhuang et al. 1997). Broad-band power changes were obtained by averaging the power values over all segments for alpha (8.3–12.2 Hz), low beta (15.1–20.0 Hz), and low gamma (29.9–44.6 Hz) bands.
The task-related coherence (TrCoh) was calculated in a similar fashion as calculating TrPow.
“Coh” denotes coherence between two EEG signals and was calculated using the cross-spectrum normalized by the auto-spectra. Positive values indicate stronger coherence during the task; negative values indicate higher coherence at rest. Broadband coherence changes were obtained by averaging the coherence values over all segments for each of the above-mentioned frequency bands. Due to volume conduction occipital alpha activity often contaminates the EEG signal in the alpha band (Andrew and Pfurtscheller 1996). Partial coherence can be used to control for this effect (Rosenberg et al. 1989). In the present study, partial coherence was calculated for the alpha band according to Mima et al. (2000). No contamination could be seen in the beta and gamma bands, so partial coherence was not used for these bands. ICA analysis was performed using EEG Lab. All additional preprocessing procedures, and the additional spectral power and coherence calculation were performed using the same homemade MATLAB (The MathWorks, Natick, MA) scripts as previously used in Bai et al. (2005).
TMS
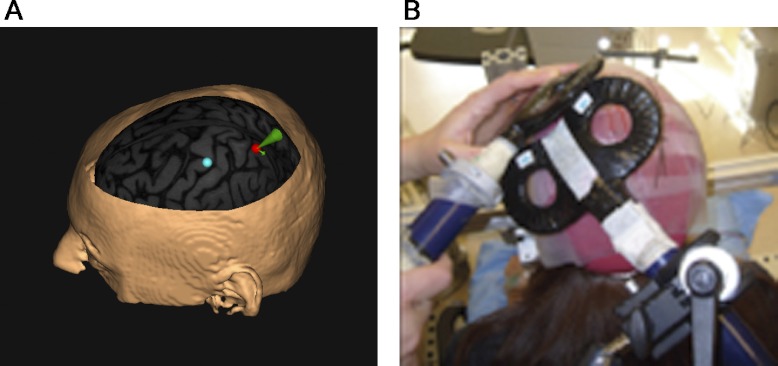
Magnetic stimulation was delivered using two custom-made figure-of-eight coils (external diameter: 50 mm), connected to two high-power Magstim 200 stimulators (Magstim Ltd, Whitland, Dyfed, UK). Stimulations with the test coil were applied over the scalp at the point that evoked the largest MEP in the contralateral FDI (“motor hotspot”). The M1 coil was held tangentially to the scalp, at a 45° angle from the anteroposterior axis and with the handle pointing posterolaterally. Resting motor threshold of the FDI (RMTFDI) was measured for each subject and defined as the lowest intensity that induced a 50-μV peak-to-peak amplitude MEP in at least five of ten trials. The conditioning coil was positioned over the left PPC, more precisely over the most posterior part of the intraparietal sulcus (pIPS). The center of the coil was positioned over pIPS tangentially to the skull, with the handle pointing downward and slightly medial (10°) to induce a posterior–anterior-directed current in the underlying cortical tissue. This orientation was chosen based on a previous description by Koch et al. (2007). Neuronavigation (Brainsight, Magstim Ltd) was used for precise positioning of the conditioning coil. Magnetic resonance imaging (MRI) data specific to each participant were used to ensure correct placement of the coil over the pIPS. Each individual MRI was normalized a posteriori onto the Montreal Neurological Institute (MNI) brain template using the same software. pIPS stimulation coordinates were then expressed with respect to the MNI standard space. The mean normalized MNI coordinates of the pIPS stimulation sites were 15.94 ± 1.97, −60.74 ± 2.83, and 60.24 ± 1.83 (mean + SE). The mean coordinates belong to Brodmann area 7 according to the Talairach atlas. The mean location of the conditioning coil is shown in Fig. 2A. The positions of the M1 coil were marked on a tight-fitting cap to ensure proper coil placement throughout the experiment; the position of the PPC coil was monitored using Brainsight. Figure 2B shows the placement of both coils on the head.
Fig. 2.
Coil coordinates and the coil placement. A: posterior parietal cortex (PPC) is shown with mean coordinates averaged across all subjects; the placement of M1 is estimated since no neuronavigation coordinates were available for this site. B: the placement of both coils on the head.
TMS-evoked MEPs were recorded at five different time points. A baseline recording (tpre) was done prior to a 10-min motor training session and four posttraining recordings were done at 0 (t0), 30 (t30), 60 (t60), and 180 (t180) min after training. In all recordings the conditioning stimulus was applied at 90% RMTFDI. Four interstimulus intervals (ISIs) between conditioning and test pulses were tested for each participant (in ms): 2, 4, 6, and 8 (Koch et al. 2007). The test stimulus was applied over the motor hotspot at an intensity set to evoke an MEP of 1 mV over the FDI at rest. Sixty stimuli were applied for each of the five recordings (12 test pulses only and 12 for each of the four ISIs). The presentation of stimuli was pseudorandomized and the intertrial interval between stimuli was set to 5 s, and each TMS session took 5 min. During TMS sessions, EMG from FDI was monitored. MEP size was determined by averaging peak-to-peak amplitudes.
Behavioral Measures
Performance on the rhythmic learning task was measured by calculating the correctly pressed (CP) intervals within each sequence repetition. A tap was considered to be correct if it was made within one third of the onset-to-onset duration before or after the pacing stimulus and the raw score of CP intervals for each rhythm repetition was calculated. Performance on the dual task was measured by recording the interresponse intervals (IRIs) between button presses, and a mean IRI for each element of the sequence was computed in each individual. The mean IRIs were compared with the “target intervals” of the rhythm as it was presented during the learning task to assess whether participants could correctly repeat the rhythm from memory.
Statistical Analysis
To stabilize the variance of the EEG data prior to further statistical assessment (Halliday et al. 1995; Rosenberg et al. 1989) spectral power and coherence were transformed using a logarithmic (log) transformation (Halliday et al. 1995) and a Fisher z-transformation (tanh−1) (Rosenberg et al. 1989).
All data were checked for normality distribution using the Kolmogorov–Smirnov test. If the data were not normally distributed (this was only in the case of C3–C4 TrCoh) a logarithmic transformation was performed to normalize the data. Since TrCoh contains both positive and negative values a constant of 100 was added to get all values over zero before the log transformation. To test for changes and group differences in the behavioral, TMS and EEG data several repeated-measures ANOVAS were done: rhythmic learning task data were analyzed with an ANOVA, with CP as the dependent variable and both modality (visual/auditory) and repetition (1–98) as independent measures. Dual-task data were analyzed with an ANOVA, with IRI as the dependent and modality as the independent variable. PPC–M1 connectivity at rest was analyzed with an ANOVA, with MEP size as the dependent and ISI (TS alone, 2 ms, 4 ms, 6 ms, and 8 ms) as the independent variable. Training-induced changes in the PPC–M1 connection were investigated with an ANOVA with MEP size as the dependent and ISI (2 ms, 4 ms, 6 ms, 8 ms), Training (tpre, t0, t30, t60, t180), and Modality (visual/auditory) as the independent variables and, after that, with an ANOVA in which the factors modality and ISI were collapsed. The main EEG analysis focused on the electrodes P3 and C3. TrPow was analyzed with an ANOVA with the repeated factors Site (C3, P3), Band (α, low β, low γ), Time (early, late), and Modality (auditory, visual) as the between-groups factor and TrCoh changes were investigated with an ANOVA with the independent factors Band (α, β, γ) and Time (early, late) and Modality (visual, auditory). Post hoc comparisons were done using the Fisher's Least Significant Differences (LSD) test. To check for specificity of TrPow and TrCoh results two additional ANOVAs were done for the control site C4. TrPow in C4 was analyzed using Band (α, low β, low γ) and Time (early, late) as the repeated factors and Modality (auditory, visual) as the between-groups factor. TrCoh between C3 and C4 was analyzed with an ANOVA with the independent factors Band (α, β, γ) and Time (early, late) and Modality (visual, auditory).
Pearson product–moment correlations were used to liberally explore if changes in PPC–M1 connectivity correlated with learning. Correlations between different behavioral measures (CP early training, CP late training, CP improvement during training) and EEG measures (TrPow and TrCoh during early and late training as well as TrCoh and TrPow changes over training) were explored in all investigated frequency bands (α, β, γ). All statistical analyses were performed using Statistica 9.1 (Statsoft, Tulsa, OK).
RESULTS
Behavioral Data
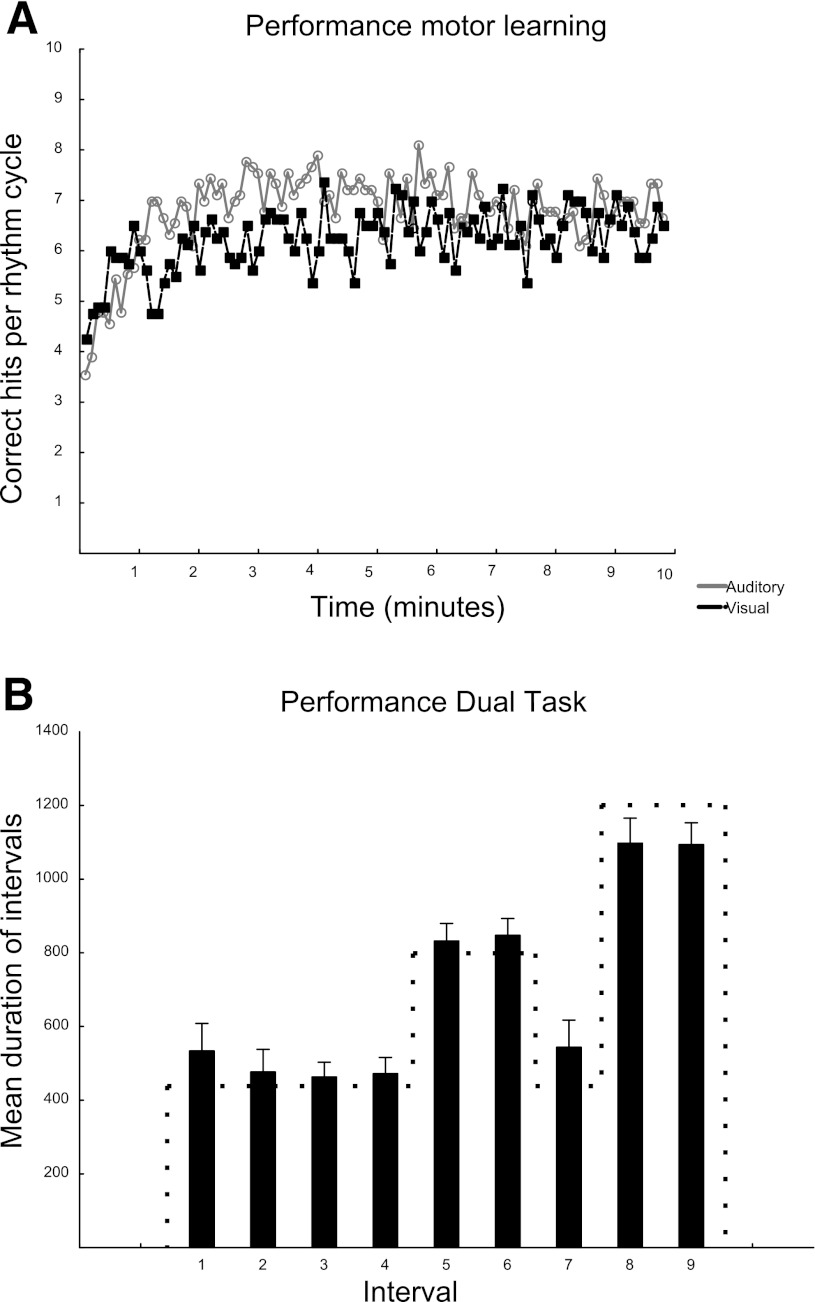
Mean performance (averaged over all 9 sequence intervals and all participants) for the rhythmic learning task is shown in Fig. 3A. A repeated-measures ANOVA with CP as the dependent variable and both modality (visual/auditory) and repetition (1–98) as independent measures showed a significant main effect for repetition [F(97) = 3.09; P < 0.001] but not for modality [F(1) = 1.27; P = 0.27]. An interaction effect could be seen between modality and repetition [F(92) = 1.36; P < 0.01].
Fig. 3.
Behavioral results. A: performance during sensory training. The number of correctly pressed (CP) intervals for each rhythm cycle is displayed for the visual (black) and the auditory (gray) learners separately. B: the performance during the dual task across all participants. Each bar shows the mean duration in milliseconds (ms) of one of the intervals of the rhythmic sequence (mean + SE). The dotted line indicates target durations of each interval.
The performance during the dual task is shown in Fig. 3B. To check for differences in IRIs between auditory and visual learners a repeated-measures ANOVA with IRI as the dependent and modality as the independent variable was performed and revealed no significant main effect for modality [F(1) = 0.06; P < 0.8]. No interaction effect could be observed between modality and IRI. Mean answer for the letter-counting task was 13.22 ± 0.57 in the auditory and 13.88 ± 0.35 (mean + SE) in the visual group (the correct answer was 15). A Student's t-test showed no difference between groups.
TMS Results
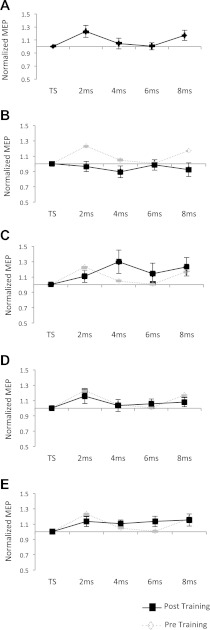
To investigate the PPC–M1 connectivity at rest a repeated-measures ANOVA with MEP size as the dependent and ISI (TS alone, 2 ms, 4 ms, 6 ms, and 8 ms) as the independent variable was performed and revealed a significant main effect for ISI [F(4) = 3.76; P = 0.018]. Post hoc tests confirmed that there was a significant facilitation of MEPs when the CS was given 2 ms prior to the TS (P = 0.01). Figure 4A shows the M1–PPC interaction at rest.
Fig. 4.
A: transcranial magnetic stimulation (TMS) curve before training. Each data point represents the mean ± SE for the test stimulus alone as well as for all four paired-pulse interstimulus intervals (ISIs; 2, 4, 6, 8 ms). MEPs are normalized to the test stimulus (TS). B–E: The normalized MEP data after sensorimotor training. B: the normalized MEPs directly after the tapping session. C: the normalized MEPs 30 min after sensorimotor training. D: the normalized MEPs 60 min after sensorimotor training. E: the normalized MEPs 180 min after sensorimotor training. All post-training data are plotted in black. The pretraining results are displayed in B–E in gray for comparison.
To check whether motor training had an effect on the excitability of M1 in general, a repeated-measures ANOVA was performed using the stimulator intensity used to evoke an MEP of 1 mV for the TS as a dependent variable and Training time point (tpre, t0, t30, t60, t180) as the independent variable. No significant effect of training on M1 excitability was observed [F(4) = 1.49; P = 0.21].
Before comparing pre- and posttraining, MEPs for each participant were normalized to the test pulse for each ISI to express change in MEP in percentage, with respect to the baseline. To test for training-induced changes in the PPC–M1 connection a 4 × 5 × 2 repeated-measures ANOVA with MEP size as the dependent variable and ISI (2 ms, 4 ms, 6 ms, 8 ms), Training (tpre, t0, t30, t60, t180), and Modality (visual/auditory) as the independent variables was performed. The factor Training showed a trend [F(4) = 2.23; P = 0.076], but none of the other main effects or interactions was significant. Figure 4, B–E shows all posttraining curves compared with the pretraining TMS. To further focus on the effect of Training and to increase the power of our ANOVA the nonsignificant factors modality and ISI were collapsed. In a second repeated-measures ANOVA with only the independent factor Training (tpre, t0, t30, t60, t180) a significant training effect [F(4) = 0.55; P = 0.0004] was observed. Post hoc tests confirmed that directly after training was concluded (to) there was a significant reduction in MEP size compared with pretraining (P = 0.007).
EEG Results
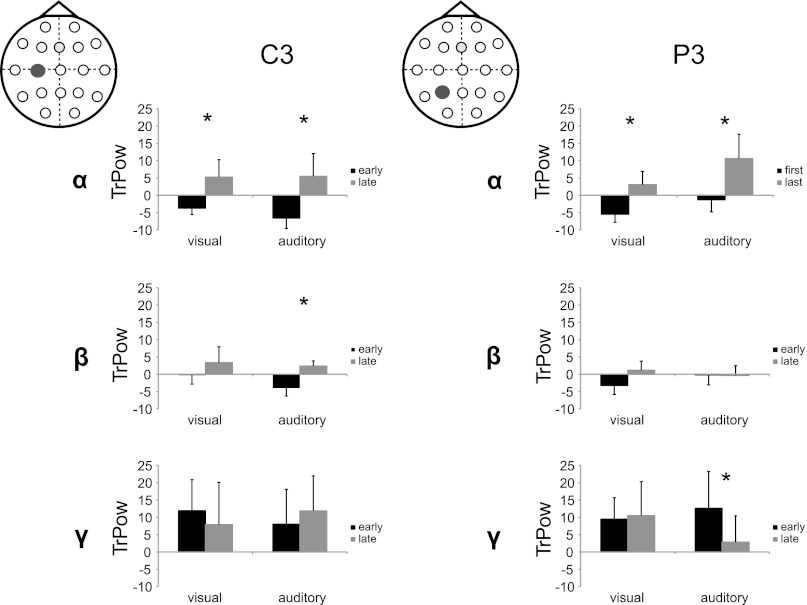
Analysis of TrPow and TrCoh was focused on the electrodes corresponding roughly to stimulation spots during TMS (electrodes C3 and P3). To test PPC–M1 connectivity TrCoh was calculated for the electrode pair P3–C3. TrPow and TrCoh were calculated for early (first minute) and late (last minute) training. To investigate changes in TrPow during learning a 2 × 3 × 2 × 2 repeated-measures ANOVA was used with the repeated factors Site (C3, P3), Band (α, low β, low γ), Time (early, late), and Modality (auditory, visual) as the between-groups factor. No significant main effects were found. However, there was a significant Site × Time × Modality and a significant Band × Time × Site × Modality interaction [F(1) = 5.50; P = 0.037 and F(2) = 7.74; P = 0.002, respectively]. Due to the large number of comparisons in this interaction and to minimize false positive P values, only Bonferroni-corrected comparisons are reported as significant (P = 0.005/24). Post hoc testing confirmed significant power increases during learning in the α band for both sites (C3 and P3) and both learning groups (visual and auditory). β showed the same general pattern even though power increases were not significant, except for C3 in the auditory learning group. In the γ-band no general trend for a power increase could be observed; however, a significant decrease in TrPow in P3 was seen for the auditory learning group. For TrPow results see Fig. 5. To check that the observed changes in TrPow were specific to P3 and C3 an additional test was run for the control electrode (C4). A 3 × 2 × 2 ANOVA with the within-factors Band (α, low β, low γ) and Time (early, late) and the between factor Modality (auditory, visual) was done and revealed no significant main effects or interactions [Main effects: Modality: F(1) = 0.09; P = 0.77; Band: F(2) = 2.43; P = 0.11; Time: F(1) = 0.79; P = 0.39].
Fig. 5.
Log-transformed task-related power (TrPow) changes in C3 and P3 in the alpha (α), beta (β), and gamma (γ) bands (mean ± SE) for the visual and auditory training groups separately. TrPow during early learning is displayed in black and during late learning in gray. Asterisks indicate which differences were significant (after Bonferroni correction).
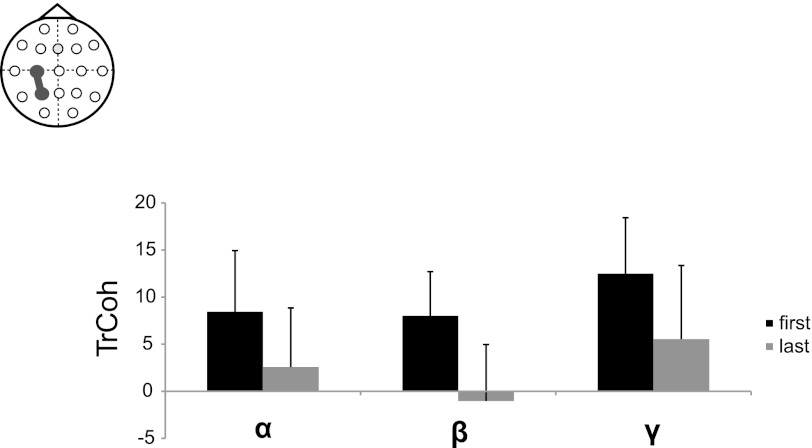
To investigate changes in TrCoh during training a second 3 × 2 × 2 repeated-measures ANOVA was used, with the independent factors Band (α, β, γ), Time (early, late), and Modality (visual, auditory). The analysis showed a significant main effect for Time [F(1) = 5.99; P = 0.046]. TrCoh data are shown in Fig. 6. No other significant main effects or interactions were seen. To check if the changes in TrCoh were specific to the P3–C3 connection, an ANOVA was done for the interaction between the left and the right motor cortex (C3–C4). As in the P3–C3 ANOVA the independent factors were Band (α, β, γ), Time (early, late), and Modality (visual, auditory). No significant main effects or interactions were seen in this comparison [Main effects: Modality: F(1) = 0.56; P = 0.466; Band: F(2) = 2.42; P = 0.11; Time: F(1) = 0.73; P = 0.41].
Fig. 6.
Z-Fischer transformed task-related coherence (TrCoh) changes in C3–P3 in the alpha (α), beta (β), and gamma (γ) bands (mean ± SE) during early (black) and late (gray) learning.
Correlations
No significant correlations were observed between behavioral measures and EEG results in any of the tested comparisons.
DISCUSSION
The purpose of the study was to determine whether the functional interactions between PPC and M1 could be modulated by a short period of sensorimotor training. The study produced two main findings on the influence of short-term sensorimotor training on the modulation of PPC–M1 interactions. First, TrCoh between PPC and M1 was higher during the early compared with the late phase of sensorimotor training. Second, the facilitation in the PPC–M1 pathway at rest was abolished immediately following sensorimotor training, but had returned toward baseline at 30, 60, and 180 min after training. Taken together, the results indicate that PPC–M1 interactions are modulated during the early phase of motor training when sensory information has to be integrated into a coherent movement plan. However, once the new learned sequence is encoded and the movement becomes automated, PPC–M1 interactions become less important and stay down-regulated for a short period beyond the training session.
Sensorimotor Training
Participants improved their performance of tapping in synchrony to the presented rhythms mainly during the first minute of practice and performance plateaued for the remainder of the 10 min of training. Participants were also able to reproduce the rhythm freely and without paying attention to the sequence during the dual task at the end of the training session. Taken together these data confirmed that behavior was fairly automated by the end of the sensorimotor training session. During the mid-training phase a significant performance difference between auditory and visual learners was observed (approximately around minutes 2 to 5) but no changes in performance could be seen during early and late training. Slight differences in performance are in line with earlier behavioral studies reporting more accurate performance during auditory than during visual synchronization tasks (Kolers and Brewster 1985; Repp and Penel 2002). However, small performance differences seem to have little effect on neural activity once the rhythms are encoded. Karabanov (2008) showed that trained rhythms activated the same network independent of training modality. Therefore, we do not expect that the small performance differences observed in the mid-learning phase interact with our TMS and EEG findings concerning the early and late training phases.
Parietal–Motor Connectivity During Sensorimotor Training
The EEG results indicate that both intraregional activity in motor and parietal regions and interregional parietal–motor coherence were enhanced during the early phase of sensorimotor training, but returned to baseline when the task had become well-trained and automated. Intraregional activity in the PPC and M1 during training was assessed by calculating TrPow. In the α band, cortical activation was high during early training, but returned to baseline toward the end of the training session in both the parietal and primary motor cortices. A similar pattern of activation was observed in the β band, although most of these differences there did not reach significance. These findings are consistent with earlier observations that have suggested that both α and low β band activity are important for motor learning and sensorimotor processing (Andres et al. 1999; Gerloff et al. 1998; Stancak and Pfurtscheller 1996). For example, Andres et al. (1999) found that both the PPC and M1 regions were especially active during early practice of a bimanual task, but decreased their activity throughout training. In addition, several functional imaging studies (Honda et al. 1998; Shadmehr and Holcomb 1997) support the idea that neural activity, especially in the parietal areas, is especially important during early motor learning. In contrast, neither a decrease in TrPow during early learning nor a general trend for decreasing regional activity during learning was observed in the γ band. No training-related intraregional changes could be observed in the right motor cortex.
Interregional connectivity between PPC and M1, measured by TrCoh, was greater during early (first minute) sensorimotor training in the α, β, and γ bands compared with the end (last minute) of sensorimotor training. These results support the findings of previous studies (Andres et al. 1999) that the functional connectivity between various brain regions can undergo rapid use-dependent changes that are related to behavioral performance. Additionally, there was no significant difference in interregional M1–PPC connectivity depending on visual or auditory training. Interregional connectivity between left and right primary motor cortex did not significantly decrease during training. This shows that the observed TrCoh changes were not due to a nonspecific whole-brain effect. The finding also relates to earlier studies on TrCoh changes during motor learning. Anders et al. (1999) showed that, whereas TrCoh between motor cortices decreased with training of bimanual tasks, there was no significant decrease in interhemispheric M1 connectivity when training a unimanual task.
In general TrPow and TrCoh measures indicate that regional activity in parietal and motor areas as well as interregional communication between these areas are important during the acquisition of a new motor routine but decrease once the motor sequence is well trained and automated.
PPC–M1 Connectivity at Rest
In a series of studies, Koch and colleagues identified the existence of a facilitatory pathway between PPC–M1 pathway using paired-pulse TMS. In the present study, a CS at an intensity of 90% of RMT applied to the PPC facilitated the MEP evoked over the ipsilateral M1 at rest, which is similar to previous studies that have investigated this pathway (Koch et al. 2007, 2008a,b). However, Koch et al. (2007) found that the facilitation of M1 was greatest at an ISI of 4 ms, whereas the facilitation was greatest at 2 ms in the current study. Both of those intervals are relatively short. One possible explanation for such short ISIs is that the CS activates not only polysynaptic but also monosynaptic connections: The superior longitudinal fasiculus (SLF) is the main fiber tract connecting the PPC and frontal areas and, even though most of its fibers terminate in prefrontal areas (Battaglia-Mayer et al. 2003; Petrides and Pandya 1984; Picard and Strick 1996), it has been suggested that a fraction of these fibers terminate directly in M1. Anatomically, the SLF is divided into three subdivisions (Makris et al. 2005; Petrides and Pandya 1984; Schmahmann et al. 2007): The SLF-I whose tracts start in the white matter of the superior parietal lobule and lead through the pre- and postcentral gyrus to dorsal premotor and dorsolateral frontal areas; the SLF-II, which links the angular gyrus via the post- and precentral gyrus with caudal–lateral prefrontal regions; and the SLF-III that connects the supramarginal gyrus to ventral premotor and prefrontal regions (Makris et al. 2005). Especially the SLF-1 tracts are thought to have some direct projections to M1 (Johnson et al. 1996; Koch et al. 2010). Potentially, these direct projections are responsible for the effects observed at short ISIs. Furthermore, a recent study by Groppa et al. (2012) provides evidence that even polysynaptic connections via the premotor cortex could be responsible for the facilitation effect: They demonstrated that a CS over the premotor cortex given 2–4 ms after a test pulse over M1 was able to facilitate MEPs evoked by the test stimulus. This is possible because the MEP elicited by a TMS pulse is not caused by direct excitation of the corticospinal fibers (called direct wave or D-wave) but appears to arise via intracortical circuits (called indirect waves or I-waves) (Di Lazzaro et al. 1999). The presence of the I-waves indicates that there is a window of 2–4 ms after the TS is given, in which a conditioning pulse can still modulate the MEP elicited by the TS. The existence of these short-latency premotor–motor connections would allow enough time for a polysynaptic PPC–premotor–motor pathway to be activated, even with the short ISIs that were observed in this study.
The slight differences in the time course of PPC–M1 connectivity between this study and the work by Koch et al. (2007) could be due to differences in coil placement relative to the specific PPC subdivision targeted by the CS. In the present study, the mean stimulation site lay in Brodmann area 7, which is part of the superior parietal lobule and therefore likely to activate the white matter tracts of the SLF-I. In contrast, in the study by Koch et al. (2007) the location of the conditioning coil was closer to the angular gyrus, which most likely stimulated the underlying white matter tracts of the SLF-II. It has been shown that TMS is sufficiently selective to activate different subdivisions of the PPC, which can lead to different effects of CS on the TS elicited over M1. For example, there is evidence that stimulation of the aIPS, results in an inhibition of the ipsilateral and the contralateral M1 instead of a facilitatory effect (Koch et al. 2007, 2009) and that anterior and the posterior sites are differently modulated by different hand-motor tasks (Koch et al. 2010). In summary, coil placement seems to be critical in detecting PPC–M1 connectivity.
Parietal–Motor Connectivity After Sensorimotor Training
Paired-pulse TMS assessments of PPC–M1 connectivity were performed before and at 0, 30, 60, and 180 min after sensorimotor training was concluded. The facilitating effect of the PPC CS on the ipsilateral M1 MEP that was observed before sensorimotor training was abolished directly after training. However, this effect was transient and facilitation returned 30 min after the training and remained for all subsequent time points. The suppressed facilitation observed directly after training was contrary to our initial hypothesis, but complements the changes observed in parietal–motor connectivity during sensorimotor training and demonstrates that parietal–motor connectivity remained down-regulated even shortly after training ended. This effect was independent of the modality that was used for sensorimotor training.
We suggest that both the decreasing interregional connectivity during sensorimotor training and the suppressed facilitation seen after training are due to the specific nature of the sensorimotor training task: During early training when integration of sensory information in the motor plan is crucial, connectivity between parietal and motor areas is increased; however, once the sequence is successfully encoded and the motor plan automated, participants no longer rely on the external sensory information to guide their movements but use their internal movement plan instead. During this automatic stage when the relevant sensory information has already been incorporated in a movement plan, parietal–motor connectivity might be even suppressed to avoid unnecessary and potentially conflicting sensory inputs. Additionally, the data suggest that parietal–motor connectivity is important for both auditory–motor and visual–motor integration, since we were not able to show any differences in functional parietal–motor connectivity based on the training modality.
Some studies have shown that sensorimotor training can increase primary motor excitability (Jensen et al. 2005; Kolers and Brewster 1985; Ljubisavljevic 2006; Pascual-Leone et al. 1995). In this study we did not observe a direct effect of sensorimotor training on primary motor cortex excitability. This can be explained by several factors: First, significant changes in primary motor cortex excitability often require repeated training sessions (Jensen et al. 2005; Pascual-Leone et al. 1995). Second, in all experiments that show increased M1 excitability the trained movement directly engaged the target muscle where MEPs were recorded. We used the FDI muscle to get a general read-out of changes in the effectiveness of the PPC–M1 interaction but the FDI was not the primarily engaged muscle during finger tapping.
Study Limitations
The conclusion that parietal–motor connectivity is increased during early learning is based on our EEG data. For further validation of the role of parietal–motor connectivity during early learning it would be of interest to perform TMS studies testing parietal–motor connectivity during the early phase of motor learning. Based on our EEG results, TMS measures of parietal–motor connectivity should increase after the early training phase. In the future, experiments that closely investigate the time course of parietal–motor connectivity during different learning phases will be of great importance to further demonstrate the role of parietal–motor integration during early learning and to add proof for the comparability of different measures of functional connectivity.
A possible caveat of this study is that it does not address the possibility that the changes in observed interregional connectivity could be due to motor activity rather than to sensorimotor integration and motor training. First, it is important to emphasize that we are not claiming that the observed changes are sequence learning specific, but that modulations in parietal–motor connectivity might be expected to occur in any task requiring training of a motor–sensory synchronization task. It is therefore difficult to find an experimental control since any repetitive motor activity, such as isometric tapping at a fixed frequency, will have a practice component and most often require sensorimotor integration (e.g., if the tapping is externally paced). Completely free movements on the other hand will not necessarily be comparable in terms of the amount of movement.
Other studies that have attempted to control for the effect of movement in similar experiments also support the claim that changes in intra- and interregional activity are dependent on motor practice rather than on motor activity alone. Pascual-Leone and colleagues studied plastic changes in primary motor cortex during a 5-day training period of a motor sequence and could show that both motor and mental practice of the sequence but not movement alone had a significant effect on motor plasticity (Pascual-Leone et al. 1995). Also studies on interregional connectivity suggest that the observed changes are likely due to learning and training and not to movement. Andres et al. (1999) demonstrated that TrCoh and TrPow did not significantly decrease over a 30-min training session if participants performed overlearned motor sequences for which performance did not change during training. Significant TrCoh and TrPow changes were seen accompanied only by changes in motor performance. Taken together these findings suggest that the observed findings are indeed due to sensorimotor training and not caused by movement alone.
GRANTS
This work was supported by the National Institute of Neurological Disorders and Stroke Intramural Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.N.K. and M.H. conception and design of research; A.N.K., A.J., and B.P. performed experiments; A.N.K., S.-H.J., and J.A. analyzed data; A.N.K., S.-H.J., A.J., B.P., A.E., and M.H. interpreted results of experiments; A.N.K. prepared figures; A.N.K. and S.-H.J. drafted manuscript; A.N.K., S.-H.J., A.J., B.P., A.E., and M.H. edited and revised manuscript; A.N.K., S.-H.J., A.J., B.P., J.A., A.E., and M.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Dr. Elise Houdayer and Dr. Silvina Horovitz for insightful discussion and S. Vornbach for technical assistance.
REFERENCES
- Andersen RA, Essick GK, Siegel RM. Neurons of area 7a activated by both visual and oculomotor behavior. Exp Brain Res 67: 316–322, 1987 [DOI] [PubMed] [Google Scholar]
- Andres FG, Mima T, Schulman AE, Dichgans J, Hallett M, Gerloff C. Functional coupling of human cortical sensorimotor areas during bimanual skill acquisition. Brain 122: 855–870, 1999 [DOI] [PubMed] [Google Scholar]
- Andrew C, Pfurtscheller G. Event-related coherence as a tool for studying dynamic interaction of brain regions. Electroencephalogr Clin Neurophysiol 98: 144–148, 1996 [DOI] [PubMed] [Google Scholar]
- Bai O, Mari Z, Vorbach S, Hallett M. Asymmetric spatiotemporal patterns of event-related desynchronization preceding voluntary sequential finger movements: a high-resolution EEG study. Clin Neurophysiol 116: 1213–1221, 2005 [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer A, Caminiti R, Lacquaniti F, Zago M. Multiple levels of representation of reaching in the parieto-frontal network. Cereb Cortex 13: 1009–1022, 2003 [DOI] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation, and blind deconvolution. Neural Comput 7: 1129–1159, 1995 [DOI] [PubMed] [Google Scholar]
- Bohlhalter S, Hattori N, Wheaton L, Fridman E, Shamim EA, Garraux G, Hallett M. Gesture subtype-dependent left lateralization of praxis planning: an event-related fMRI study. Cereb Cortex 19: 1256–1262, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buneo CA, Andersen RA. The posterior parietal cortex: sensorimotor interface for the planning, and online control of visually guided movements. Neuropsychologia 44: 2594–2606, 2006 [DOI] [PubMed] [Google Scholar]
- Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal, and medial cortex in humans. Neuroimage 14: 1444–1453, 2001 [DOI] [PubMed] [Google Scholar]
- Classen J, Gerloff C, Honda M, Hallett M. Integrative visuomotor behavior is associated with interregionally coherent oscillations in the human brain. J Neurophysiol 79: 1567–1573, 1998 [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21, 2004 [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Rothwell JC, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P. Intracortical origin of the short latency facilitation produced by pairs of threshold magnetic stimuli applied to human motor cortex. Exp Brain Res 129: 494–499, 1999 [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol 453: 525–546, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraux G, McKinney C, Wu T, Kansaku K, Nolte G, Hallett M. Shared brain areas but not functional connections controlling movement timing, and order. J Neurosci 25: 5290–5297, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff C, Richard J, Hadley J, Schulman AE, Honda M, Hallett M. Functional coupling, and regional activation of human cortical motor areas during simple, internally paced and externally paced finger movements. Brain 121: 1513–1531, 1998 [DOI] [PubMed] [Google Scholar]
- Groppa S, Schlaak BH, Münchau A, Werner-Petroll N, Dünnweber J, Bäumer T, van Nuenen BF, Siebner HR. The human dorsal premotor cortex facilitates the excitability of ipsilateral primary motor cortex via a short latency cortico-cortical route. Hum Brain Mapp 33: 419–430, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron 55: 187–199, 2007 [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data: theory, and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol 64: 237–278, 1995 [DOI] [PubMed] [Google Scholar]
- Honda M, Deiber MP, Ibanez V, Pascual-Leone A, Zhuang P, Hallett M. Dynamic cortical involvement in implicit, and explicit motor sequence learning. A PET study. Brain 121: 2159–2173, 1998 [DOI] [PubMed] [Google Scholar]
- Hummel F, Andres F, Altenmuller E, Dichgans J, Gerloff C. Inhibitory control of acquired motor programmes in the human brain. Brain 125: 404–420, 2002 [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Visuo-motor integration, and control in the human posterior parietal cortex: evidence from TMS and fMRI. Neuropsychologia 44: 2691–2699, 2006 [DOI] [PubMed] [Google Scholar]
- Jensen JL, Marstrand PC, Nielsen JB. Motor skill training, and strength training are associated with different plastic changes in the central nervous system. J Appl Physiol 99: 1558–1568, 2005 [DOI] [PubMed] [Google Scholar]
- Jin SH, Lin P, Hallett M. Reorganization of brain functional small-world networks during finger movements. Hum Brain Mapp 33: 861–872, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PB, Ferraina S, Bianchi L, Caminiti R. Cortical networks for visual reaching: physiological, and anatomical organization of frontal and parietal lobe arm regions. Cereb Cortex 6: 102–119, 1996 [DOI] [PubMed] [Google Scholar]
- Karabanov A, Blom O, Forsman L, Ullen F. The dorsal auditory pathway is involved in performance of both visual, and auditory rhythms. Neuroimage 44: 480–488, 2009 [DOI] [PubMed] [Google Scholar]
- Koch G, Cercignani M, Pecchioli C, Versace V, Oliveri M, Caltagirone C, Rothwell J, Bozzali M. In vivo definition of parieto-motor connections involved in planning of grasping movements. Neuroimage 51: 300–312, 2010 [DOI] [PubMed] [Google Scholar]
- Koch G, Fernandez Del Olmo M, Cheeran B, Ruge D, Schippling S, Caltagirone C, Rothwell JC. Focal stimulation of the posterior parietal cortex increases the excitability of the ipsilateral motor cortex. J Neurosci 27: 6815–6822, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Fernandez Del Olmo M, Cheeran B, Schippling S, Caltagirone C, Driver J, Rothwell JC. Functional interplay between posterior parietal, and ipsilateral motor cortex revealed by twin-coil transcranial magnetic stimulation during reach planning toward contralateral space. J Neurosci 28: 5944–5953, 2008a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Cheeran B, Ruge D, Lo Gerfo E, Salerno S, Torriero S, Marconi B, Mori F, Driver J, Rothwell JC, Caltagirone C. Hyperexcitability of parietal-motor functional connections in the intact left-hemisphere of patients with neglect. Brain 131: 3147–3155, 2008b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolers PA, Brewster JM. Rhythms and responses. J Exp Psychol Hum Percept Perform 11: 150–167, 1985 [DOI] [PubMed] [Google Scholar]
- Lewis PA, Wing AM, Pope PA, Praamstra P, Miall RC. Brain activity correlates differentially with increasing temporal complexity of rhythms during initialisation, synchronisation, and continuation phases of paced finger tapping. Neuropsychologia 42: 1301–1312, 2004 [DOI] [PubMed] [Google Scholar]
- Ljubisavljevic M. Transcranial magnetic stimulation, and the motor learning-associated cortical plasticity. Exp Brain Res 173: 215–222, 2006 [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex 15: 854–869, 2005 [DOI] [PubMed] [Google Scholar]
- Manganotti P, Gerloff C, Toro C, Katsuta H, Sadato N, Zhuang P, Leocani L, Hallett M. Task-related coherence, and task-related spectral power changes during sequential finger movements. Electroencephalogr Clin Neurophysiol 109: 50–62, 1998 [DOI] [PubMed] [Google Scholar]
- Mima T, Matsuoka T, Hallett M. Functional coupling of human right, and left cortical motor areas demonstrated with partial coherence analysis. Neurosci Lett 287: 93–96, 2000 [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol 74: 1037–1045, 1995 [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol 228: 105–116, 1984 [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location, and functional activation. Cereb Cortex 6: 342–353, 1996 [DOI] [PubMed] [Google Scholar]
- Repp BH, Penel A. Auditory dominance in temporal processing: new evidence from synchronization with simultaneous visual, and auditory sequences. J Exp Psychol Hum Percept Perform 28: 1085–1099, 2002 [PubMed] [Google Scholar]
- Rietschel JC, Goodman RN, King BR, Lo LC, Contreras-Vidal JL, Hatfield BD. Cerebral cortical dynamics, and the quality of motor behavior during social evaluative challenge. Psychophysiology 48: 479–487, 2011 [DOI] [PubMed] [Google Scholar]
- Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM. The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog Biophys Mol Biol 53: 1–31, 1989 [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Taylor PC. TMS in the parietal cortex: updating representations for attention, and action. Neuropsychologia 44: 2700–2716, 2006 [DOI] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol 530: 307–317, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, de Crespigny AJ, Wedeen VJ. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging, and autoradiography. Brain 130: 630–653, 2007 [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Holcomb HH. Neural correlates of motor memory consolidation. Science 277: 821–825, 1997 [DOI] [PubMed] [Google Scholar]
- Stancak A, Jr, Pfurtscheller G. Event-related desynchronisation of central beta-rhythms during brisk and slow self-paced finger movements of dominant and nondominant hand. Brain Res Cogn Brain Res 4: 171–183, 1996 [DOI] [PubMed] [Google Scholar]
- Tanne-Gariepy J, Rouiller EM, Boussaoud D. Parietal inputs to dorsal versus ventral premotor areas in the macaque monkey: evidence for largely segregated visuomotor pathways. Exp Brain Res 145: 91–103, 2002 [DOI] [PubMed] [Google Scholar]
- Wheaton L, Fridman E, Bohlhalter S, Vorbach S, Hallett M. Left parietal activation related to planning, executing, and suppressing praxis hand movements. Clin Neurophysiol 120: 980–986, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton LA, Yakota S, Hallett M. Posterior parietal negativity preceding self-paced praxis movements. Exp Brain Res 163: 535–539, 2005 [DOI] [PubMed] [Google Scholar]
- Zhuang P, Toro C, Grafman J, Manganotti P, Leocani L, Hallett M. Event-related desynchronization (ERD) in the alpha frequency during development of implicit, and explicit learning. Electroencephalogr Clin Neurophysiol 102: 374–381, 1997 [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition, and facilitation in human motor cortex. J Physiol 496: 873–881, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]