Abstract
Purpose of review
Claudin-16 and claudin-19 play a major role in the regulation of magnesium reabsorption in the thick ascending limb (TAL). This review describes recent findings of the physiological function of claudin-16 and claudin-19 underlying normal transport function for magnesium reabsorption in the TAL.
Recent findings
Mutations in the genes encoding the tight junction proteins claudin-16 and claudin-19 cause the inherited human renal disorder familial hypomagnesemia with hypercalciuria and nephrocalcinosis. The cation selectivity of the tight junction is vital for generating the lumen positive transepithelial potential in the TAL, which drives paracellular absorption of magnesium. Claudin-16 and claudin-19 require each other for assembly into tight junctions in the TAL. Heteromeric claudin-16 and claudin-19 interaction forms a cation selective tight junction paracellular channel. Loss of either claudin-16 or claudin-19 in the mouse kidney abolishes the cation selectivity for the TAL paracellular pathway, leading to excessive renal wasting of magnesium.
Summary
Epithelial paracellular channels are increasingly understood to be formed from claudin oligomeric complexes. In the mouse TAL, claudin-16 and claudin-19 cooperate to form cation-selective paracellular channels required for normal levels of magnesium reabsorption. Different subsets of the claudin family of tight junction proteins are found distributed throughout the nephron, and understanding their roles in paracellular ion transport will be fundamental to understanding renal ion homeostasis.
Keywords: claudin, hypomagnesemia, thick ascending limb, tight junction, transepithelial voltage
Introduction
The thick ascending limb (TAL) of the loop of Henle is responsible for reabsorbing 25–40% of filtered Na+[1], 50–60% of filtered Mg2+[2] and 30–35% of filtered Ca2+[3]. The dissociation of salt and water reabsorption in the TAL serves both to dilute the urine and to establish the corticomedullary osmolality gradient [4]. Active transcellular salt reabsorption results in a lumen-positive transepithelial voltage that drives passive paracellular reabsorption of divalent cations [5,6]. Claudins are the key components of the paracellular channel. The paracellular channels in the tight junction have properties of ion selectivity, pH dependence and anomalous mole fraction effects, similar to conventional transmembrane channels [7]. Genetic mutations in claudin-16 [8] and claudin-19 [9] cause an inherited human renal disorder, familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC).
Transcellular and paracellular reabsorption of Na+
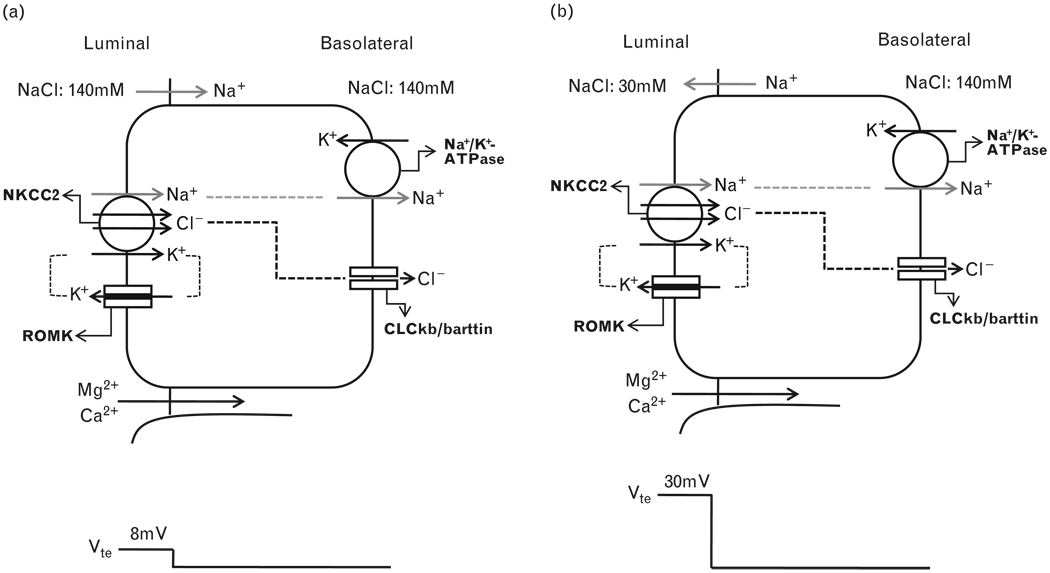
In the TAL of Henle’s loop, the epithelial cells form a water-impermeable barrier, actively transport Na+ and Cl− via the transcellular route, and provide a paracellular pathway for the selective absorption of cations [5,6]. Na+ K+ and Cl− enter the cell through the Na-K-2Cl cotransporter (NKCC2) in the luminal membrane. Na+ exits the cell through the Na+/K+-ATPase, in exchange for K+ entry. K+ is secreted into the lumen through the renal outer medullary potassium channel. Cl− leaves the cell through the basolateral Cl− channel, made up of two subunits, ClCKb and barttin. The polarized distribution of luminal K+ versus basolateral Cl− conductance generates a spontaneous voltage source (Vsp) of +7−8mV [10], depending on active transcellular NaCl reabsorption (Fig. 1a). With continuous NaCl reabsorption along the axis of the TAL segment, the luminal fluid is diluted to 30–60mmol/l [11] and a large NaCl transepithelial chemical gradient develops at the end of the TAL. Because the paracellular permeability of the TAL is cation-selective (with a PNa/PCl value between 2 and 4) [12,13], the diffusion voltage (Vdi) is superimposed onto the active transport voltage (Vsp) and becomes the major source of the transepithelial voltage (Vte), which now increases up to +30mV [11–13] (Fig. 1b).
Figure 1. Transepithelial electrogenic ion transport and generation of lumen-positive potential.
(a) When similar salt concentrations are present at the luminal and basolateral sides, the luminal spontaneous potential Vsp is generated by the concerted action of luminal K+ channels, basolateral Cl− channels, the Na+2Cl−K+ cotransporter, and the Na+,K+-ATPase. Vsp drives Na+ absorption through the paracellular pathway. (b) When a dilute luminal fluid is present after NaCl absorption along the water-tight TAL, the luminal potential is now generated as a diffusion voltage Vdi by the ‘backleak’ of Na+. The diffusion voltage depends on the permselectivity of the tight junction.NKCC2, Na-K-Cl cotransporter; ROMK, renal outer medullary potassium.
Mechanism of Mg2+ transport
Early in-vivo micropuncture studies have shown that approximately 50–60% of the filtered Mg2+ is reabsorbed in the TAL [2]. The flux–voltage relationship indicates that Mg2+ is passively reabsorbed from the lumen to the peritubular space through the paracellular pathway in this segment, driven by a lumen positive Vte [14,15]. Vte is made of the sum of Vsp and Vdi. There are two prerequisites required for the paracellular Mg2+ reabsorption in the TAL: the lumen-positive Vte as the driving force and the paracellular permeability for the divalent cation Mg2+.
Claudin-16 and claudin-19 underlie familial hypercalciuric hypomagnesemia with nephrocalcinosis
FHHNC, OMIM #248250, is a rare autosomal recessive tubular disorder. As a consequence of excessive renal Mg2+ and Ca2+ wasting, patients develop the characteristic triad of hypomagnesemia, hypercalciuria and nephrocalcinosis [16–18]. Recurrent urinary tract infections and polyuria/polydipsia are frequent initial symptoms. Other clinical symptoms include nephrolithiasis, abdominal pain, convulsions, muscular tetany, and failure to thrive (Table 1). Additional laboratory findings include elevated serum parathyroid hormone levels before the onset of chronic renal failure, incomplete distal tubular acidosis, hypocitraturia, and hyperuricemia (Table 1). In contrast to hypomagnesemia and secondary hypocalcemia (HSH, OMIM #602014), FHHNC is generally complicated by end-stage renal failure in early childhood or adolescence.
Table 1.
Clinical and biochemical abnormalities in familial hypomagnesemia with hypercalciuria and nephrocalcinosis patients
| Symptoms | Frequencya(%) |
|---|---|
| Hypomagnesemia | 100 |
| Hypercalciuria | 100 |
| Nephrocalcinosis | 100 |
| Polyuria/polydipsia | 90 |
| Increased serum iPTH levels | 88 |
| Incomplete dRTA | 85 |
| Hypocitraturia | 80 |
| Urinary tract infection | 69 |
| Hyperuricemia | 64 |
| Muscular tetany | 33 |
| Nephrolithiasis | 32 |
dRTA, distal renal tubular acidosis; iPTH, intact parathyroid hormone.
[16].
Simon et al. [8] used the positional cloning strategy and identified claudin-16 (formerly known as paracellin-1), which is mutated in patients with FHHNC. Most mutations reported to date in claudin-16 are missense mutations clustering in the first extracellular loop composing the putative ion selectivity filter [19•]. Konrad et al. [9] have found mutations in another tight junction gene encoding claudin-19 from new cohorts of FHHNC patients (OMIM #248190). The renal tubular phenotypes are indistinguishable between patients with mutations in claudin-16 and those with mutations in claudin-19. Although claudin-16 and claudin-19 underlie FHHNC and paracellular Mg2+ reabsorption in the TAL, the transient receptor potential channel melastatin 6 (TRPM6) regulates the apical entry of Mg2+ into the distal convoluted tubule epithelia [20]. Mutations in TRPM6 cause the HSH syndrome [21].
Claudin-16 is a cation channel
These above data suggested the hypothesis that claudin-16 and/or claudin-19 forms a selective paracellular Mg2+/Ca2+ channel, which was tested in a number of in-vitro studies. Ikari et al. [22] transfected low-resistance Madin-Darby canine kidney (MDCK) cells with claudin-16 and reported that the Ca2+ flux in these cells was increased in the apical to basolateral direction, whereas the Ca2+ flux in the opposite direction remained unchanged. The Mg2+ flux was without any noticeable change. Kausalya et al. [23] transfected the high-resistance MDCK-C7 cell line and found that claudin-16 only moderately increased Mg2+ permeability without any directional preference. The effects of claudin-16 on Mg2+/Ca2+ permeation appeared so small (<50%) that the Mg2+/Ca2+ channel theory incompletely explains the dramatic effect of mutations in claudin-16 on Mg2+ and Ca2+ homoeostasis in FHHNC patients.
In contrast to these studies, Hou et al. [24] transfected the anion-selective LLC-PK1 cell line with claudin-16 and found a large increase in Na+ permeability (PNa) accompanied by a moderately enhanced Mg2+ permeability (PMg). The permeability of claudin-16 to other alkali metal cations was found to be: K+ > Rb+ > Na+ ≈ Li+. This sequence is quite different from the sequence of their free-solution mobilities and resembles the Eisenman selectivity sequence V–VIII. The Eisenman selectivity sequence reflects the electrostatic interaction strength (the Coulomb forces) in the claudin-16 paracellular channel and suggests that permeating cations have to dehydrate to enter the channel where negatively charged interaction sites stabilize the permeating cation [25,26].The increase in PNa was not affected by a Na+/K+-ATPase inhibitor (1mmol/l ouabain) but was partially reduced or completely lost in all FHHNC relevant claudin-16 mutants (Table 2). In an elegant linkage study, Konrad et al. [27] had correlated the PNa levels of claudin-16 mutations identified in FHHNC to the phenotypes of FHHNC patients. They found that the patients who had mutations resulting in complete loss of PNa of both alleles were significantly younger at the onset of FHHNC symptoms than the patients who had one wild-type allele providing partial function. In addition, those patients with complete loss of PNa had a more rapid decline in the glomerular filtration rate.
Table 2.
Mutations affecting the function of claudin-16
| Construct | Mutation | Localization | PNa (10-6cm/s) | PCl (10-6cm/s) | Function |
|---|---|---|---|---|---|
| Vector | – | – | 6.381 ± 0.107 | 21.857 ± 0.107 | − |
| WT | – | TJ | 25.750 ± 0.092 | 21.310 ± 0.092 | + |
| D97S | Charge removal | ER | 9.065 ± 0.530 | 34.633 ± 0.530 | − |
| D104S | Charge removal | TJ | 19.260 ± 0.206 | 31.723 ± 0.208 | Partial − |
| D105S | Charge removal | TJ | 22.683 ± 0.223 | 26.917 ± 0.223 | Partial − |
| E108T | Charge removal | TJ | 22.937 ± 0.203 | 21.830 ± 0.200 | + |
| E119T | Charge removal | TJ | 22.917 ± 0.101 | 28.070 ± 0.098 | Partial − |
| D126S | Charge removal | TJ | 18.563 ± 0.047 | 24.120 ± 0.050 | Partial − |
| D132S | Charge removal | TJ | 28.983 ± 0.497 | 22.003 ± 0.500 | + |
| E133T | Charge removal | TJ | 23.307 ± 0.103 | 22.583 ± 0.103 | + |
| D135S | Charge removal | TJ | 25.003 ± 0.053 | 20.880 ± 0.050 | + |
| E140T | Charge removal | TJ | 17.063 ± 0.165 | 24.650 ± 0.162 | Partial − |
| L145P | FHHNC | TJ | 13.977 ± 0.174 | 29.723 ± 0.174 | Partial − |
| R149L | FHHNC | ER | 7.830 ± 0.186 | 26.157 ± 0.187 | − |
| L151F | FHHNC | TJ | 17.107 ± 0.093 | 24.603 ± 0.093 | Partial − |
| L167P | FHHNC | – | 7.183 ± 0.038 | 27.450 ± 0.040 | − |
| G191R | FHHNC | TJ | 17.070 ± 0.192 | 21.980 ± 0.192 | Partial − |
| G198D | FHHNC | – | 6.656 ± 0.090 | 24.453 ± 0.091 | − |
| A209T | FHHNC | TJ | 20.513 ± 0.156 | 31.927 ± 0.156 | Partial − |
| R216T | FHHNC | – | 10.023 ± 0.159 | 29.873 ± 0.156 | − |
| F232C | FHHNC | TJ | 31.570 ± 0.070 | 31.713 ± 0.073 | + |
| G233D | FHHNC | – | 8.015 ± 0.106 | 28.690 ± 0.106 | − |
| S235P | FHHNC | ER | 7.273 ± 0.366 | 26.717 ± 0.364 | − |
| G239R | FHHNC | Golgi | 8.906 ± 0.094 | 23.297 ± 0/094 | − |
−, abolishing the function of claudin-16; +, showing the function of claudin-16; ECL, extracellular loop; ER, endoplasmic reticulum; FHHNC, familial hypomagnesemia with hypercalciuria and nephrocalcinosis; Golgi, Golgi apparatus; TJ, tight junction; TMD, transmembrane domain; WT, wild type.
In general, functional studies of paracellular permeability have shown that there are at least two types of paracellular channels: a high-capacity pathway with an estimated pore radius of about 4 Å and a low-capacity pathway with a pore radius of at least 7 Å. The low-capacity pathway permits paracellular permeation on a slower timescale (minutes to hours) and may involve dynamic rearrangements of the tight junction structure [28]. In studies of claudin-2 expression in MDCK cells, paracellular permeability of the two types of paracellular channels was observed [29,30••]. The rapid, high-capacity paracellular channels show claudin-specific selectivity. The selectivity is mediated by the electrostatic interaction of partially dehydrated permeating ions with charged sites within the channel. It is generally accepted that the charges on the extracellular loops (ECL) of claudins line the channel and electrostatically influence passage of dehydrated ions [31,32].The first ECL of claudin-16 is enriched with 10 negatively charged amino acids. Hou et al. [24] have systematically mutated each of the negatively charged amino acids to serine or threonine to study the effects of charge upon the function of claudin-16 in LLC-PK1 cells. A summary of the physiological changes resulting from these mutations is shown in Table 2. Mutational analysis identified a locus of acidic amino acids (D104S, D105S, E119T, D126S and E140T) that affected its cation selectivity and were interspersed with other acidic residues that had no effect. Mutation of each of the five functionally important residues had a modest effect (11–33% reduction in PNa),and combining these mutations appeared to be additive [24]. However, as emphasized by Yu et al. [30••], these residue replacements can influence protein structures that may have impacts on ion permeability independent of amino acid charge.
Heteromeric claudin-16 and claudin-19 interaction generates cation selectivity of the tight junction
Claudins interact with each other both intracellularly and intercellularly: they copolymerize linearly within the plasma membrane of the cell, together with the integral protein occludin, to form the classical intramembrane fibrils or strands visible in freeze-fracture replicas. These intramembrane interactions (side-to-side) can involve one claudin protein (homomeric or homopolymeric) or different claudins (heteromeric or heteropolymeric) [33]. In the formation of the intercellular junction, claudins may interact head-to-head with claudins in an adjacent cell, generating both homotypic and heterotypic claudin–claudin interactions [33]. Using the split-ubiquitin yeast 2-hybrid assay, Hou et al. [34] found strong claudin-16 and claudin-19 heteromeric interaction. The point mutations in claudin-16 (L145P, L151F, G191R, A209T, and F232C) or claudin-19 (L90P and G123R) that are known to cause human FHHNC disrupted the claudin-16 and claudin-19 heteromeric interaction. In mammalian cells such as the human embryonic kidney 293 cells, claudin-16 can be coimmunoprecipitated with claudin-19 [34]. Freeze-fracture replicas revealed the assembly of tight junction strands in L cells coexpressing claudin-16 and claudin-19, supporting their heteromeric interaction [34].
Coexpression of claudin-16 and claudin-19 in LLC-PK1 cells resulted in a dramatic upregulation of PNa and down-regulation of PCl, generating a highly cation-selective paracellular pathway [34]. Certain FHHNC mutations in claudin-16 (L145P, L151F, G191R, A209T, and F232C) or claudin-19 (L90P and G123R) that disrupted their heteromeric interaction abolished this physiological change [34]. As claudin-16 colocalizes with claudin-19 in the TAL epithelia of the kidney [9], claudin-16 and claudin-19 association through heteromeric interactions confers cation selectivity to the tight junction in the TAL. Human FHHNC mutations in claudin-16 or claudin-19 that abolish the cation selectivity diminish the lumen-positive Vdi as the driving force for Mg2+ and Ca2+ reabsorption, readily explaining the devastating phenotypes in FHHNC patients.
Claudin-16 is required for renal Mg2+ reabsorption
Hou et al. [35] generated claudin-16 deficient mouse models using lentiviral transgenesis of siRNA to knock down claudin-16 expression by more than 99% in mouse kidneys. Claudin-16 knockdown mice show significantly reduced plasma Mg2+ levels and excessive urinary excretions (approximately four-fold) of Mg2+ and Ca2+. Calcium deposits are observed in the basement membranes of the medullary tubules and the interstitium in the kidney of claudin-16 knockdown mice. These phenotypes of claudin-16 knockdown mice recapitulate the symptoms in human FHHNC patients.
Claudin-16 is required for paracellular cation selectivity in the thick ascending limb
The paracellular reabsorption of Mg2+ and Ca2+ is driven by a lumen-positive Vte made up of two components: Vsp and Vdi. When isolated TAL segments were perfused ex vivo with symmetrical NaCl solutions, there was no difference in Vsp between claudin-16 knockdown and wild-type mice, indicating Vsp was normal in claudin-16 knockdown [35]. Blocking the NKCC2 channel with furosemide (thus dissipating VSP), the cation selectivity (PNa/PCl) was significantly reduced from3.1 ± 0.3 in wild type to 1.5 ± 0.1 in claudin-16 knockdown, resulting in the loss of Vdi. When perfused with a NaCl gradient of 145mmol/l (bath) versus 30mmol/l (lumen), the resulting Vdi was +18mV in wild type, but only +6.6mV in claudin-16 knockdown [35]. Thus, the reduction in Vdi accounted for a substantive loss of the driving force for Mg2+ and Ca2+ reabsorption.
Claudin-16 is required for paracellular Na+ absorption in the thick ascending limb
Renal handling of Na+ in claudin-16 knockdown mice is more complex. In the early TAL segment, the transcellular and paracellular pathways form a current loop in which the currents traversing the two pathways are of equal size but opposite direction [5–6]. Net luminal K+ secretion and basolateral Cl− absorption polarize the TAL epithelium and generate Vsp. As the paracellular pathway is cation selective (PNa/PCl=2–4 [12,13]), the majority of the current driven by Vsp through the paracellular pathway is carried by Na+ moving from the lumen to the interstitium (Fig. 1a). Hebert et al. [36] estimated that, for each Na+ absorbed through the trans-cellular pathway, one Na+ is absorbed through the paracellular pathway. With the loss of claudin-16 and the concomitant loss of paracellular cation selectivity, Na+ absorption through the paracellular pathway is reduced.
In the late TAL segment, dilution of NaCl in the luminal space creates an increasing chemical transepithelial gradient; back diffusion of Na+ through the cation-selective tight junction generates a lumen-positive Vdi across the epithelium (Fig. 1b). The paracellular absorption of Na+ will be diminished when Vdi equals Vsp, and reversed when Vdi exceeds Vsp. As an equilibrium potential, Vdi blocks further Na+ backleak into the lumen. Without claudin-16, Vdi will be markedly reduced well below normal, providing a driving force for substantial Na+ secretion. Indeed, claudin-16 knockdown mice had increased fractional excretion of Na+ (FENa) and developed hypotension and secondary hyperaldosteronism [35]. The observed Na+ and volume loss are consistent with human FHHNC phenotypes. For example, polyuria and polydipsia are the most frequently reported symptoms from FHHNC patients [16].
Claudin-19 deficient animals phenocopy claudin-16 deficient animals
By using the same transgenic siRNA strategies to developing the claudin-16 knockdown mouse lines, Hou et al. [37••] generated claudin-19 deficient knockdown mice. Claudin-19 knockdown mice also develop the FHHNC symptoms of reduced plasma Mg2+ levels and excessive renal wasting of Mg2+ and Ca2+. Secondary hyperaldosteronism in claudin-19 knockdown indicates increases in TAL luminal NaCl concentration, similar to the findings of claudin-16 knockdown mice.
Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions of the thick ascending limb
In vitro, claudin-16 and claudin-19 interact and form a cation-selective paracellular channel [34]. In vivo, either claudin knockdown animal model phenocopies the other, suggesting claudin-16 and claudin-19 interaction is a critical component of normal TAL tight junction function. Hou et al. [37••] have shown that siRNA knockdown of claudin-19 causes a loss of claudin-16 from tight junctions in the TAL without a decrease in claudin-16 expression level, whereas siRNA knockdown of claudin-16 produces a similar effect on claudin-19. Thus, knockdown of either claudin results in the loss of both claudins from the junctional structure, explaining the similarity between the phenotypes. In both mouse lines, claudin-10, claudin-18, occludin, and ZO-1, normal constituents of TAL tight junctions, remain correctly localized. Based on these data, Hou et al. [37••] proposed a three-stage hypothesis of claudin assembly in the TAL tight junction. First, claudins 16 and 19 cis associate within the plane of the membrane into dimers, or higher oligomeric states prior to junctional assembly. If this association does not occur, neither claudin can traffic correctly to the cell junction. This requirement is unique to the cells of the TAL, as the claudins will traffic to junctional structures in cultured transfected cell lines. Second, trans interactions between claudins in adjacent cells take place. Third, additional cis interactions occur elaborating the tight junction strands. The intracellular location of the cooligomerization of claudin-16 and claudin-19 is currently not known.
Conclusion
The knockdown of claudin-16 and claudin-19 in the mouse has highlighted a role of the paracellular pathway in kidney function. Accumulating evidence from both in-vitro and in-vivo studies indicates that the primary role of claudin-16 and claudin-19 is to cogenerate cation selectivity of the tight junction in the TAL underlying normal transport function for Mg2+ reabsorption in the TAL. However, the molecular nature of this pathway is far from clear. First, the oligomeric structure of the claudin-16 and claudin-19 complex is not known. Mitic et al. [38] have studied claudin oligomerization on the basis of claudin-4 expression in insect cells using the perfluorooctanoic acid (PFO) as detergent to solubilize claudin-4 oligomers revealing a hexameric quaternary structure for claudin-4. A similar study using PFO solubilization followed by sucrose gradient sedimentation might allow determination of the molecular structure for claudin-16 and claudin-19. Second, the molecular basis for the charge selectivity of the claudin-16 and claudin-19 heteromeric channel is not known. It is generally accepted that the charges on the first extracellular loops of claudins line the channel pores and electrostatically influence passage of soluble ions. In a preliminary study, Hou et al. [24] found that charge removal in the first extracellular loop of claudin-16 reduces its cation selectivity. However, claudin-19 shares limited homology with claudin-16 in this domain. In any case, the mutational analysis was performed on cells in culture in the absence of claudin-19 expression. The coordinated interaction of claudin-16 and claudin-19 in vivo suggests that meaningful conclusions about the molecular basis of charge selectivity will require studies on cells expressing both claudins. Third, the identity of the paracellular Mg2+ channel in the TAL is not known. Hou et al. [37••] have shown that the loss of neither claudin-16 nor claudin-19 affected the junctional localization of claudin-10 and claudin-18, normal constituents of TAL tight junctions, suggesting that claudin-10 and claudin-18 continue to participate in the barrier function of TAL tight junctions. The barrier function of claudins has been shown recently in human colon where aldosterone enhances Na+ uptake by both inducing transcellular passage of sodium while decreasing the paracellular ion flux by increasing claudin-8 expression [39•]. Although cation selectivity is lost in the absence of claudin-16 and claudin-19, the TAL epithelia maintain high levels of paracellular permeability for cations such as Mg2+. A reasonable hypothesis is that claudin-10 and/or claudin-18 consitute the paracellular Mg2+ channel.
Acknowledgements
This work was supported by National Institutes of Health grants EY02430 (to D.A.G.) and by American Heart Association grant 0930050N (to J.H.).
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 509).
- 1.Giebisch G, Klose RM, Windhager EE. Micropuncture study of hypertonic sodium chloride loading in the rat. Am J Physiol. 1964;206:687–693. doi: 10.1152/ajplegacy.1964.206.4.687. [DOI] [PubMed] [Google Scholar]
- 2.Cole DEC, Quamme GA. Inherited disorders of renal magnesium handling. J Am Soc Nephrol. 2000;11:1937–1947. doi: 10.1681/ASN.V11101937. [DOI] [PubMed] [Google Scholar]
- 3.Hebert SC. Calcium and salinity sensing by the thick ascending limb: a journey from mammals to fish and back again. Kidney Int. 2004;91:S28–S33. doi: 10.1111/j.1523-1755.2004.09105.x. [DOI] [PubMed] [Google Scholar]
- 4.Han JS, Thompson KA, Chou CL, Knepper MA. Experimental tests of three-dimensional model of urinary concentrating mechanism. J Am Soc Nephrol. 1992;2:1677–1688. doi: 10.1681/ASN.V2121677. [DOI] [PubMed] [Google Scholar]
- 5.Hebert SC, Culpepper RM, Andreoli TE. NaCl transport in mouse medullary thick ascending limbs. I: functional nephron heterogeneity and ADH-stimulated NaCl cotransport. Am J Physiol. 1981;241:F412–F431. doi: 10.1152/ajprenal.1981.241.4.F412. [DOI] [PubMed] [Google Scholar]
- 6.Hebert SC, Culpepper RM, Andreoli TE. NaCl transport in mouse medullary thick ascending limbs. II: ADH enhancement of transcellular NaCl cotransport; origin of transepithelial voltage. Am J Physiol. 1981;241:F432–F442. doi: 10.1152/ajprenal.1981.241.4.F432. [DOI] [PubMed] [Google Scholar]
- 7.Tang VW, Goodenough DA. Paracellular ion channel at the tight junction. Biophys J. 2003;84:1660–1673. doi: 10.1016/S0006-3495(03)74975-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon DB, Lu Y, Choate KA, et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 9.Konrad M, Schaller A, Seelow D, et al. Mutations in the tight-junction gene claudin-19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet. 2006;79:949–957. doi: 10.1086/508617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandon B, Siga E, Roinel N, et al. Ca2+, Mg2+ and K+ transport in the cortical and medullary thick ascending limb of the rat nephron: influence of transepithelial voltage. Pflügers Arch. 1993;424:558–560. doi: 10.1007/BF00374924. [DOI] [PubMed] [Google Scholar]
- 11.Burg M, Good D. Sodium chloride coupled transport in mammalian nephrons. Annu Rev Physiol. 1983;45:533–547. doi: 10.1146/annurev.ph.45.030183.002533. [DOI] [PubMed] [Google Scholar]
- 12.Burg MB, Green N. Function of the thick ascending limb of Henle’s loop. Am J Physiol. 1973;224:659–668. doi: 10.1152/ajplegacy.1973.224.3.659. [DOI] [PubMed] [Google Scholar]
- 13.Greger R. Cation selectivity of the isolated perfused cortical thick ascending limb of Henle’s loop of rabbit kidney. Pflügers Arch. 1981;390:30–37. doi: 10.1007/BF00582707. [DOI] [PubMed] [Google Scholar]
- 14.Di Stefano A, Roinel N, De Rouffignac C, et al. Transepithelial Ca2+ and Mg2+transport in the cortical thick ascending limb of Henle’s loop of the mouse is a voltage-dependent process. Renal Physiol Biochem. 1993;16:157–166. doi: 10.1159/000173762. [DOI] [PubMed] [Google Scholar]
- 15.Hebert JC, Andreoli TE. Ionic conductance pathways in the mouse medullary thick ascending limb of Henle. J Gen Physiol. 1986;87:567–590. doi: 10.1085/jgp.87.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber S, Schneider L, Peters M, et al. Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol. 2001;12:1872–1881. doi: 10.1681/ASN.V1291872. [DOI] [PubMed] [Google Scholar]
- 17.Manz F, Schärer K, Janka P, Lombeck J. Renal magnesium wasting, incomplete tubular acidosis, hypercalciuria and nephrocalcinosis in siblings. Eur J Pediatr. 1978;128:67–79. doi: 10.1007/BF00496992. [DOI] [PubMed] [Google Scholar]
- 18.Praga M, Vara J, Gonzalez-Parra E, et al. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis. Kidney Int. 1995;47:1419–1425. doi: 10.1038/ki.1995.199. [DOI] [PubMed] [Google Scholar]
- 19. Günzel D, Haisch L, Pfaffenbach S, et al. Claudin function in the thick ascending limb of Henle’s loop. Ann N Y Acad Sci. 2009;1165:152–162. doi: 10.1111/j.1749-6632.2009.04051.x.. This review summarizes recent advances in claudin distribution, function and mutation in the TAL of the kidney.
- 20.Alexander RT, Hoenderop JG, Bindels RJ. Molecular determinants of magnesium homeostasis: insights from human disease. J Am Soc Nephrol. 2008;19:1451–1458. doi: 10.1681/ASN.2008010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlingmann KP, Weber S, Peters M, et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6: a new member of the TRPM gene family. Nat Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- 22.Ikari A, Hirai N, Shiroma M, et al. Association of paracellin-1 with ZO-1 augments the reabsorption of divalent cations in renal epithelial cells. J Biol Chem. 2004;279:54826–54832. doi: 10.1074/jbc.M406331200. [DOI] [PubMed] [Google Scholar]
- 23.Kausalya PJ, Amasheh S, Günzel D, et al. Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of claudin-16. J Clin Invest. 2006;116:878–891. doi: 10.1172/JCI26323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou J, Paul DL, Goodenough DA. Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci. 2005;118:5109–5118. doi: 10.1242/jcs.02631. [DOI] [PubMed] [Google Scholar]
- 25.Diamond JM, Wright EM. Biological membranes: the physical basis of ion and nonelectrolyte selectivity. Annu Rev Physiol. 1969;31:581–646. doi: 10.1146/annurev.ph.31.030169.003053. [DOI] [PubMed] [Google Scholar]
- 26.Eisenman G, Horn R. Ionic selectivity revisited: the role of kinetic and equilibrium processes in ion permeation through channels. J Membr Biol. 1983;76:197–225. doi: 10.1007/BF01870364. [DOI] [PubMed] [Google Scholar]
- 27.Konrad M, Hou J, Weber S, et al. CLDN16 genotype predicts renal decline in familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol. 2008;19:171–181. doi: 10.1681/ASN.2007060709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol. 2010;20:142–149. doi: 10.1016/j.tcb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Van Itallie CM, Holmes J, Bridges A, et al. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci. 2008;121:298–305. doi: 10.1242/jcs.021485. [DOI] [PubMed] [Google Scholar]
- 30. Yu AS, Cheng MH, Angelow S, et al. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J Gen Physiol. 2009;133:111–127. doi: 10.1085/jgp.200810154.. This study uses several sophisticated electrophysiological approaches to characterize the claudin-2 channel and suggests a single-pore cylinder model for paracellular channels.
- 31.Colegio OR, Van Itallie C, Rahner C, et al. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol. 2003;284:C1246–C1254. doi: 10.1152/ajpcell.00547.2002. [DOI] [PubMed] [Google Scholar]
- 32.Van Itallie CM, Fanning AS, Anderson JM , Reversal of charge selectivity in cation or anion selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol. 2003;285:F1078–F1084. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- 33.Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol. 1999;147:891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou J, Renigunta A, Konrad M, et al. Claudin-16andclaudin-19 interactandforma cation-selective tight junction complex. J Clin Invest. 2008;118:619–628. doi: 10.1172/JCI33970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou J, Shan Q, Wang T, et al. Transgenic RNAi depletion of claudin-16 and the renal handling of magnesium. J Biol Chem. 2007;282:17114–17122. doi: 10.1074/jbc.M700632200. [DOI] [PubMed] [Google Scholar]
- 36.Hebert SC, Reeves WB, Molony DA, et al. The medullary thick limb: function and modulation of the single-effect multiplier. Kidney Int. 1987;31:580–589. doi: 10.1038/ki.1987.38. [DOI] [PubMed] [Google Scholar]
- 37. Hou J, Renigunta A, Gomes AS, et al. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci U S A. 2009;106:15350–15355. doi: 10.1073/pnas.0907724106.. This paper for the first time reveals the fundamental unit making a paracellular channel within tight junction strands and highlights the role of claudin interaction in tight junction assembly.
- 38.Mitic LL, Unger VM, Anderson JM. Expression, solubilization, and biochemical characterization of the tight junction transmembrane protein claudin-4. Protein Sci. 2003;;12:218–227. doi: 10.1110/ps.0233903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Amasheh S, Milatz S, Krug SM, et al. Tight junction proteins as channel formers and barrier builders. Ann NY Acad Sci. 2009;1165:211–219. doi: 10.1111/j.1749-6632.2009.04439.x.. This review discusses the new concept of epithelial barrier function coupled to transcellular changes in ion absorption.