Abstract
Angiogenesis is a critical component of mammalian brain adaptation to prolonged hypoxia. Hypoxia-induced angiogenesis is mediated by hypoxia inducible factor-1 (HIF-1) dependent transcriptional activation of growth factors, such as vascular endothelial growth factor (VEGF). Microvascular angiogenesis occurs over a three week period in the rodent brain. We have recently reported that HIF-1α accumulation and transcriptional activation of HIF target genes in the aged cortex of 24 month F344 rats is significantly attenuated during acute hypoxic exposure. In the present study, we show that cortical HIF-1α accumulation and HIF-1 activation remains absent during chronic hypoxic exposure in the aged rat brain (24 month F344). Despite this lack of HIF-1 activation, there is no significant difference in baseline or post-hypoxic brain capillary density counts between the young (3 month F344) and old age groups. VEGF mRNA and protein levels are significantly elevated in the aged cortex despite the lack of HIF-1 activation. Other HIF-independent mediators of hypoxia inducible genes could be involved during chronic hypoxia in the aged brain. PPAR-γ coactivator (PGC)-1α, a known regulator of VEGF gene transcription, is elevated in the young and aged cortex during the chronic hypoxic exposure. Overall, our results suggest a compensatory HIF-1 independent preservation of hypoxic-induced microvascular angiogenesis in the aged rat brain.
Keywords: Aging, Angiogenesis, Brain, Hypoxia, HIF-1, Microvessels, PGC-1α, VEGF
INTRODUCTION
The mammalian brain naturally functions in a low, but controlled, oxygen environment and is particularly sensitive to alterations in oxygen delivery [19, 23, 46, 47]. Metabolic demand and insufficient energy storage make the brain vitally dependent on a constant supply of oxygen and nutrients to allow adequate oxidative phosphorylation and ATP production. Metabolic stressors, such as tissue hypoxia, trigger compensatory mechanisms in attempt to restore the balance between local oxygen delivery and tissue oxygen consumption [8, 19]. A more detailed review of this has been described in LaManna et al (2004). Immediate systemic responses to acute imbalances in oxygen delivery include increased ventilation, cardiac output, and cerebral blood flow. Days later an increase in red blood cells aids in the attempt to restore oxygen tension. If the tissue hypoxia persists, mechanisms that promote angiogenesis, the formation of new blood vessels from preexisting vasculature, are activated thus increasing vascular density. In the rodent brain, adaptive microvascular angiogenesis is a multifaceted process requiring an orchestrated sequence of events and occurs over a three week period [5, 8, 18, 26, 51]. The end result of this response to chronic hypoxia is a decrease of intercapillary diffusional distances and normalization of tissue oxygen tension [8, 18].
Angiogenesis is a tightly regulated process that involves the proliferation, migration, differentiation, and organization of endothelial cells into new functional microvessels [10, 19, 28]. These mature microvessels, consisting of endothelial cells and adjacent pericytes, maintain a special structural relationship with glial astrocytes, neurons, and the surrounding extracellular matrix called the neurovascular unit (NVU) [18, 19]. Angiogenesis is an adaptive response to tissue hypoxia in a number of in vivo and in vitro models, including development and tumor models [27, 37, 38, 42]. A key mediator of this angiogenic response is hypoxia-inducible factor-1 (HIF-1), which is responsible for the transcriptional activation of a number of growth factors such as vascular endothelial growth factor (VEGF) [35, 36, 39–41]. Secreted VEGF, from astrocytes and pericytes, bind to VEGF receptors on the surface of endothelial cells activating receptor tyrosine kinases. HIF-1 has also been shown to have an essential role in development of systemic vessels and other brain cell types during embryogenesis with HIF-1 knockouts being embryonic lethals by E10-11 primarily due to circulatory system defects [39, 44]. These embryonic mice have also shown evidence of inadequate brain development likely due to impaired vascular development as well as massive cell death in the cephalic mesemchyme [1, 10, 15, 25, 33].
We have recently reported an age-dependent decline in cortical HIF-1α accumulation and transcriptional activation of HIF target genes in response to 72 hours of hypobaric hypoxic exposure [24]. In comparison to the response of younger Fischer 344 (F344) rats (3–12 months of age), the induction of cortical HIF-1α accumulation was completely attenuated in old F344 rats (24 months) following hypoxia and comparable to that of age-matched controls. Hypoxic-induced upregulation of HIF target gene mRNA, such as VEGF, was also completely attenuated in the aged cortex. This phenomenon appears to be post-translational with mRNA expression of HIF-1α intact in the aged cortex. This attenuated HIF-1α response was directly correlated with an increase in the cortical expression of the HIF regulatory enzymes, prolyl hydroxylase domain (PHD) containing proteins, in the aged rats relative to their younger rats. Attenuation of HIF-1α accumulation and transcriptional activation of growth factors such as VEGF might impair angiogenic responses in the brain of the aged rat. Diminished compensation for insufficient tissue oxygenation could make senescent rats more susceptible to ischemia and chronic hypoxic. This attenuated response might result in failure to maintain normal microvascular density, thus potentially affecting neuronal survival and plasticity-associated learning.
In this study, we examined microvascular angiogenesis in the aged F344 rat brain following three weeks of chronic hypobaric hypoxia and have compared it to that of younger (3 month) F344 rats. Multi-region analysis of the brain, including parietal cortex, corpus callosum, striatum, CA1 region of the hippocampus, was done to compare the microvascular angiogenic response of the young and aged rodent brain in response to prolonged hypoxia. Our results showed no significant difference in baseline capillary density between the young and aged rats in any of the four regions. The adaptive increase in brain microvasculature following three weeks of hypoxia is still intact in the aged brain and as robust as in the young rat brain. The increase in brain microvasculature is comparable to that of young rats and occurred despite the persistent attenuation of HIF-1α accumulation during the chronic hypoxic exposure. Although our previous study showed a lack of VEGF mRNA upregulation in the aged cortex following a 3 day hypoxic exposure, there appears to be a delayed increase in cortical mRNA and protein expression of VEGF in the aged cortex during chronic hypoxic exposure. The robust VEGF mRNA expression in the aged cortex suggests other mechanisms of transcriptional regulation that remain intact in the aged rats. PPAR-γ coactivator (PGC)-1α, a known regulator of VEGF gene transcription and angiogenesis, is elevated in the young and aged cortex during the chronic hypoxic exposure. This demonstrates HIF-independent regulaton of VEGF and angiogenesis that is maintained in the aged rat cortex.
RESULTS
Systemic Physiological Changes
The young and old F344 rats showed a significant elevation of blood hematocrit following chronic hypoxia relative to controls (increase from 50 +/− 2.8 to 78 +/− 2 in and 46 +/− 2.2 to 75 +/− 3.4 in the young and old rats respectively, figure 1A). Overall there was 56% and 64% increase in blood hematocrit on the young and old rats respectively. Despite a difference in control body weight, (292 +/− 23 in the young versus 405 +/− 30 in the old), both age groups displayed similar trends in body weight decline during the three week hypoxic exposure (figure 1B). In both age groups there was a decline in body weight over the first two weeks that stabilized by the third week. The maximal percent decline in body weight was 20% in both age groups.
Figure 1. Systemic changes during and following chronic hypoxia in the young and old F344 rats.
A, Increase in hematocrit following three weeks of chronic hypoxic exposure as a function of age. B, Body weight change during three week chronic hypoxic exposure as a function of age. Each value represents the mean +/− SD from at least three rats. *p < 0.05 compared with control (non-hypoxic) blood samples.
Increase in Cortical Microvascular Density Following Hypoxia as a Function of Age
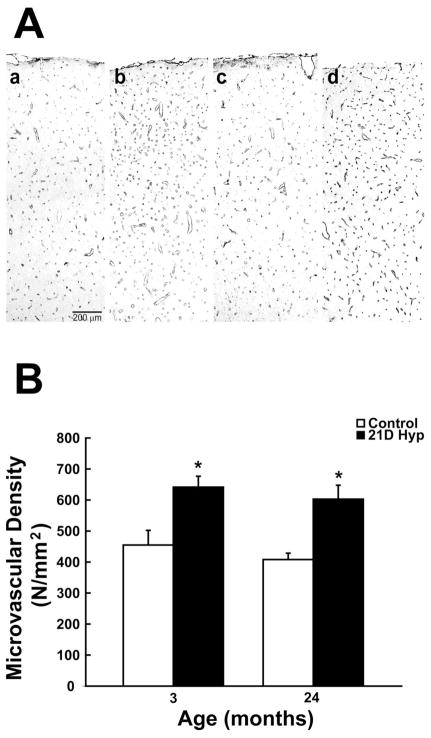
Cortical cerebral microvessels were identified by counting the number positive capillaries per unit area. Figure 2A shows representative photomicrographs of GLUT-1 stained sections in the parietal cortex of young and old F344 rats following three weeks of chronic hypoxia relative to controls. The capillary density increase as a function of age is shown in figure 2B. Following hypoxic exposure there was a 41% and 48% increase in cortical microvessels in the young and old rats respectively. There was an average increase in cortical microvessels from 455 +/− 47 to 642 +/− 35 in the young and from 408 +/− 21 to 603 +/− 45 in the old F344 rats. Although there was roughly a 10% drop in baseline capillaries in the aged cortex, it did not reach statistical significance. Cortical capillary counts were also made on microvessels greater than 25 um in diameter. Our results (not shown) suggest no differences as a function of age. In both age groups (control and hypoxic), there was less than one abnormally large cortical capillary per square millimeter representing less than 0.20% of total capillaries counted.
Figure 2. Hypoxia-induced cortical angiogenesis.
A, Representative images GLUT-1 positive stained sections showing microvascular density in the rat cerebral cortex following three weeks of chronic hypoxia compared to controls (a: 3 month control, b: 3 month hypoxic, c: 24 month control, d: 24 month hypoxic). B, Cortical microvascular density (microvessels per mm2) analysis of GLUT-1 stained sections before and following chronic hypoxia as a function of age. Each value represents the mean +/− SD from at least three rats with the individual values from each rat being the average of at least four different sections per region examined. Each quantified section was at least 250–300 um apart from the subsequent section. *p < 0.05 compared with control value.
HIF-1a Independent Increase in VEGF in the Aged Cortex During Hypoxia
We found an attenuation of HIF-1α accumulation after 10 days of hypoxic exposure. Consistent with previous results collected more acutely at 1 to 3 days of hypoxia [19, 24], this demonstrates that the HIF-1 attenuation persists. In contrast, whereas at 3 days VEGF mRNA was not upregulated in the aged cortex [24], at 10 days there was an apparent increase in VEGF message and protein levels relative to aged controls. Figure 3A shows a representative Western blot demonstrating the HIF-1α attenuation that still persists in the aged cortex during chronic hypoxic exposures. The Western analysis showed the characteristic hypoxia-inducible protein signal with a molecular mass of 120 kDa, in the young cortex following ten days of hypoxia. This signal was absent in the aged cortex following the same exposure. This absence had no effect on the transcriptional upregulation of vegf in the aged cortex. There was approximately a four-fold increase in vegf mRNA levels in the young and aged cortical samples relative to aged-matched controls (figure 3B). Although slightly less than that of the younger hypoxic rats, there was also an increase in protein expression of VEGF (approximate molecular weight in the 40–45 kDa range) in the aged cortex after 10 days of hypoxia despite the lack of HIF-1a accumulation (figure 3A). Optical density ratios (relative to β-actin) show a statistically significant increase in VEGF expression levels (8 to 9-fold) in both age groups during the chronic hypoxic exposure (figure 3C).
Figure 3. HIF-1a independent upregulation of VEGF expression in the aged rat cortex during chronic hypoxic exposure.
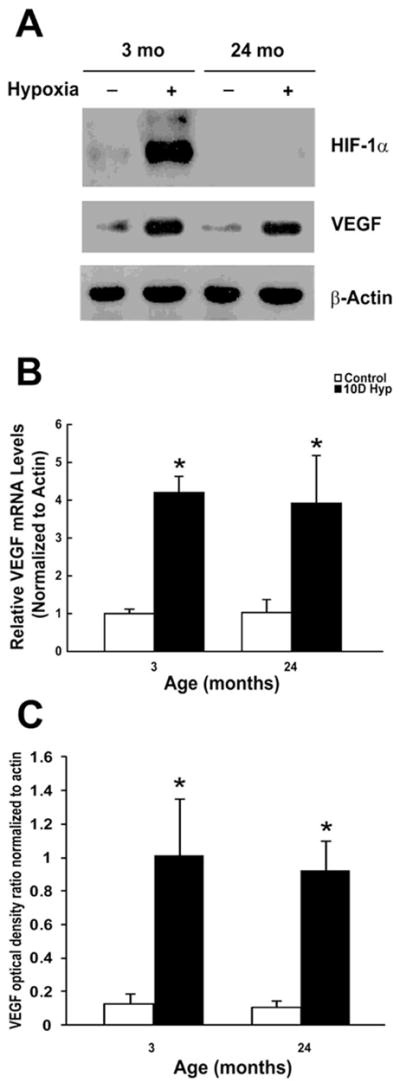
A, Representative Western blot analysis of HIF-1α, VEGF, & β-actin following 10 days of hypoxic exposure in the young and old F344 rats. Data are representative of at least 3 experiments. B, HIF-1 independent transcriptional activation of vegf in the aged rat cortex during chronic hypoxic exposure. Results were expressed as fold induction compared with cortical samples of brains exposed to normoxia and normalized to β-actin mRNA. Each individual value represents the mean +/− SD from three independent experiments from at least three different rats. *p < 0.05 compared with normoxia. C, Optical density ratios of VEGF normalized to β-actin before and after hypoxic exposure as a function of age. Each value represents the mean +/− SD from at least three rats. *p < 0.05 compared with control (non-hypoxic) cortical samples.
PGC-α Expression in the Young and Aged Cortex During Hypoxia
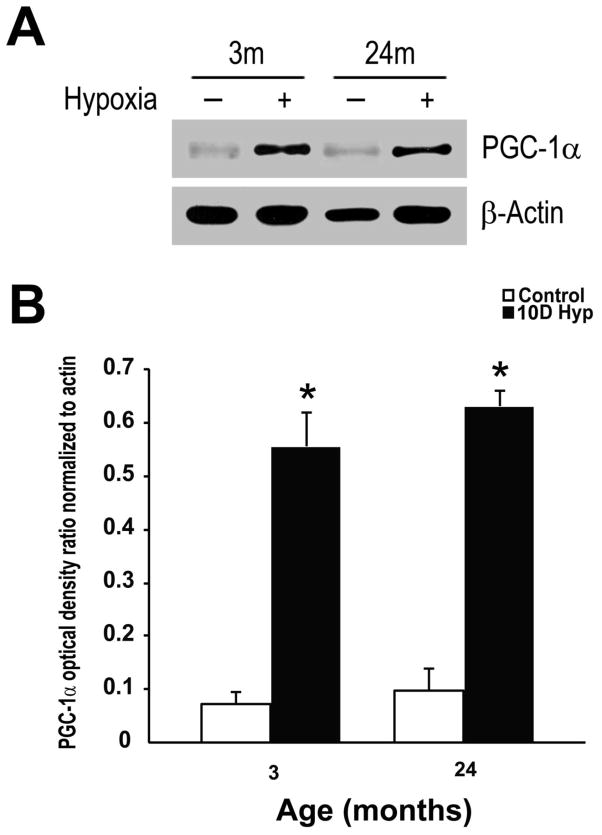
The robust VEGF mRNA expression in the aged cortex suggests that HIF-1 independent mechanisms of transcriptional upregulation remain intact in the aged rat brain during hypoxia. Peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α is a transcriptional coactivator that powerfully regulates oxidative and mitochondrial metabolism in a number of tissues particularly those where mitochondria are abundant and oxidative metabolism is active, such as the brain. PGC-1α, a known regulator of VEGF gene transcription and angiogenesis [2, 45], is significantly elevated in the young and aged cortex during chronic hypoxic exposure. Figure 4A shows a representative Western blot demonstrating an increase in PGC-1α expression in the cortex following hypoxia with a characteristic band approximately in the 90–95 kDa range. Optical density ratios (relative to β-actin) show a statistically significant increase in PGC-1α expression (7 to 8-fold) in the both age groups following ten days of hypoxic exposure (figure 4B).
Figure 4. Upregulation of PGC-1α expression in the young and aged rat cortex during chronic hypoxic exposure.
A, Representative Western blot analysis of PGC-1α, following 10 days of hypoxic exposure in the young and old F344 rats. Data are representative of at least 3 experiments. B, Optical density ratios of PGC-1α normalized to β-actin before and after hypoxic exposure as a function of age. Each value represents the mean +/− SD from at least three rats. *p < 0.05 compared with control (non-hypoxic) cortical samples.
Increase in Brain Microvascular Density Following Hypoxia as a Function of Age
To see if the result is generalized, we also examined capillary density in the corpus callosum, striatum, and CA1 region of the hippocampus as a function of age. Although baseline microvascular density counts were typically lower in these regions versus the cerebral cortex, the values were fairly consistent across both age groups. Statistically significant increases in densities were observed in both age groups following chronic hypoxia. The baseline capillary density in the corpus callosum (188 +/− 31 in young versus 183 +/− 24 in the old) increased by 61% in the young (to 303 +/− 41) and 53% (279 +/− 16) in the old rats following chronic hypoxic exposure (figure 5A). Control capillary density counts in the striatum (325 +/− 29 in young versus 334 +/− 13 in the old) increased by 44% in the young (to 469 +/− 33) and 35% (451 +/− 19) in the old rats following chronic hypoxic exposure (figure 5B). Control capillary counts in the CA1 region of the hippocampus (243 +/− 8 in young versus 218 +/− 25 in the old) increased by 47% in the young (to 359 +/− 46) and 60% (349 +/− 51) in the old rats following chronic hypoxic exposure (figure 5C).
Figure 5. Hypoxia-induced angiogenesis in other brain regions.
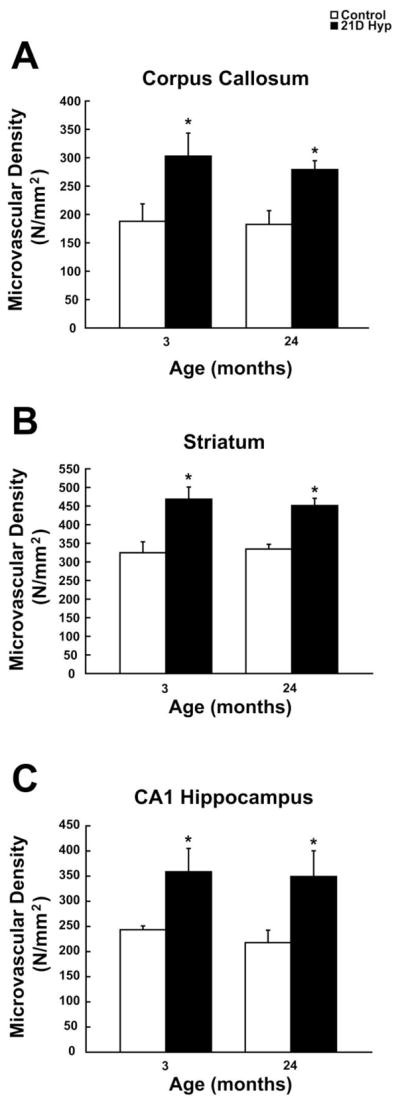
Callosal (A), striatal (B), and CA1 hippocampal (C) microvascular density (microvessels per mm2) analysis of GLUT-1 positive stained sections before and following chronic hypoxia as a function of age. Each value represents the mean +/− SD from at least three rats with the individual values from each rat being the average of at least four different sections per region examined. Each quantified section was at least 250–300 um apart from the subsequent section. *p < 0.05 compared with control value.
DISCUSSION
The vascular network of the mature rodent brain is relatively stable with microvascular capillary endothelial cells quiescent and tight junctions/basement membranes intact [18]. Metabolic stressors, such hypoxia [6, 10, 37, 38, 43, 51] and ischemia [4, 7, 44] are known to be strong inducers of microvascular remodeling in the brain. This remodeling during chronic hypoxia begins as early one week and is completed by three weeks resulting in an increase in capillary density [5, 8, 18, 19, 26, 51]. This remodeling in the brain allows for the restoration of normal oxygen tension profiles during chronic hypoxic exposure [8]. Cerebral tissue oxygen tensions measured in rats during acclimatization to chronic hypoxia (7 to 28 days) are double that of control rats (when breathing normoxic and normobaric air), suggesting an increase oxygen delivery following vascular remodeling [8].
In this study, we explored hypoxic-induced microvascular angiogenesis following three weeks of chronic hypoxia in the brain of F344 rats, an established model of aging. The systemic effects and adaptations to chronic hypoxia, such as blood hematocrit response and body weight decline, were similar between the young and old age groups. The results also demonstrate no significant difference in baseline capillary density counts or hypoxic-induced angiogenic response in the aged rat brain despite the attenuation of HIF-1α accumulation. In both age groups there was a range of 40–60% increase in microvascular density in the four regions studied. Similar to our previous report showing an absence of HIF-1α accumulation following 72 hours of continuous hypobaric hypoxia, the lack of HIF-1α accumulation in the aged cortex also persists during longer periods of hypoxia (10 days). Despite this lack of HIF-1 activation, relative mRNA levels of VEGF were significantly increased in the aged cortex at the 10 day intermediate time point and similar to that of the young cortex. These results suggest the appearance of a delayed compensatory response that preserves this adaptive response to hypoxic stress in the aged brain despite the absence of HIF-1 activation. The lack of HIF-1α accumulation and HIF-1 activation during prolonged chronic hypoxia in the aged cortex appears to have no negative effect on transcriptional upregulation or protein expression of VEGF. The other well described alpha isoform, HIF-2α, has been shown in some cell lines to not be a hypoxia-inducible factor and escapes oxygen-dependent protein degradation [25]. That report suggested that HIF-2α may be cytoplasmically sequestered and transcriptionally inactive while another report has also shown transcriptional inactivity due to a transcriptional repressor [13, 25]. There appears to be some sort of unknown modification to HIF-2α in certain cell types that effects its localization and activity. In support of these findings, our previous report showed increased protein expression of HIF-2α in the aged normoxic cortex [24]. This increased expression during normoxic and acute hypoxic conditions had no effect on the transcriptional activation of key target HIF genes, including erythropoietin (EPO) and VEGF, in the aged cortex [24]. Therefore, it is unlikely that HIF-2α is responsible for the increased VEGF mRNA levels during chronic hypoxia in the aged cortex.
Although VEGF expression represents only one component of the complex angiogenic response, it is still a critical activator of vascular endothelial cells. VEGF upregulation under hypoxic conditions is regulated at the transcriptional and translational level [10, 20, 35, 36, 49, 50]. HIF-1 is known to be a major transcriptional activator of VEGF but there are other mechanisms that may be involved in regulating VEGF expression. In vitro models have shown that other transcription factors, such as JunB, Sp1, STAT3, and Smad3, may also regulate VEGF transcription in connection with or independent of HIF-1 [10]. Hypoxic induction of VEGF expression and microvascular density increase are regulated by HIF-1 independent mechanisms in certain tumor cell lines [10, 21, 22, 34]. HIF-1α deficient tumors derived from H-ras transformed embryonic fibroblasts showed no difference in tumor vascularization [34]. Similarly, knockdown of HIF-1α in colon cancer cells had no effect on angiogenesis. Reduced tumor angiogensis was achieved through inhibition of NF-κB mediated IL-8 expression and K-rasVal12 dependent VEGF-A expression [10, 21, 22].
For almost two decades, research has focused on HIF-1 as a key regulator of the cellular oxygen-response system. More recently, evidence for other systems has also surfaced. One of these systems involves the PGC-1α coactivator, a powerful transcriptional regulator of mitochondrial and oxidative metabolic programs [2, 45]. PGC-1α can bind to and coactivate most nuclear receptors as well as many other transcription factors [2, 45]. PGC-1α interacts directly with the basal transcriptional machinery and also recruits chromatin-modifying enzymes, such as histone acetylase p300, to open chromatin and facilitate transcription [2, 45]. PGC-1α coactivates the transcription factor estrogen receptor related (ERR)-α on a number sites found both in the promoter and a novel conserved enhancer located in the first intron of the VEGF gene as demonstrated by chromatin immunoprecipitation assays [2, 45].
PGC-1α is involved in the cellular response to hypoxia and ischemia in vivo. Hypobaric hypoxia has been shown to induce PGC-1α and mitochondrial biogenesis in the mouse cerebral subcortex [11]. Under ischemic conditions, PGC-1α has been shown to regulate HIF-independent VEGF expression and angiogenesis in cultured muscle cells and skeletal muscle in vivo [2]. In that study, PGC-1α induced VEGF gene expression in cells deficient of ARNT (HIF-1β), demonstrating that HIF-1 activity was not needed. It still remains unclear how hypoxia and nutrient deprivation induces PGC-1α expression. It is thought that regulation of PGC-1α occurs at both the transcriptional and posttranslational level with candidate pathways including AMP kinase, reactive oxygen species, and nitric oxide [45].
Age-related alterations in neuronal and vascular density associated with pathology such as dementia and Alzheimer’s disease have been reported [16]. Normal aging in the absence of obvious pathology is not necessarily associated with the same structural and functional alterations. Studies over the years have reported varied results as far as age-associated changes in vascular density [3, 17, 30, 48]. Likewise, the reported changes in capillary density have varied from decreased to increased as a function of age [9, 16, 30], therefore there is no consensus on how aging affects microvascular density.
Age-related alterations in HIF-1 activation and angiogenesis have been reported using different models including hypoxia, ischemia, and wound healing. Despite this, very few studies have examined hypoxia-induced vascular plasticity as a function of age in the brain. Ingraham et al., 2008 reported an age related attenuation of hypoxia-induced microvascular response in the aged hippocampus (4 month vs. 30 month F344xBN rats) [14]. Similar to our results, their study showed no difference in baseline capillary density in any of the three hippocampal areas examined (CA1, CA3, DG) but demonstrated a diminished angiogenic response to hypoxia in all three regions in the aged versus the young. They report an increase of 35–40% in capillary density in the three regions of the young hippocampus. The increase in capillary density was only 20% (DG), 15% (CA3), and 7% (CA1) in the aged hippocampal regions. The CA3 & DG responses were statistically significant (versus aged baseline) but significantly lower than that of younger experimental rats. Experimental design differences between that study and ours include different rat age used, lower hypoxic stimulus, and restriction to the hippocampus only. The 30 month old rats used are significantly older than the 24 month old rats that we examined. In that study, the total experimental length was four weeks that included three weeks of exposure to 11% oxygen (versus 8% in ours). It is possible that a smaller hypoxic stimulus could have induced less of a response in the aged hippocampus. Lastly, that study only examined the hippocampus. Although our results differ from theirs with regard to the CA1 region, their results in the DG are somewhat similar our findings of significant microvascular angiogenesis in the aged rat brain.
Adaptive angiogenic responses to chronic hypoxia may be maintained despite an attenuated HIF-1 response, but the same is not necessarily in other models, such as wound healing and ischemia [29]. For example, old rabbits (4–5 years) and old mice (2 years) have impaired angiogenesis as a result of impaired endothelial function and lower VEGF expression in ischemic tissues (hind limb occlusion model) [31]. Also, HIF- 1α protein levels and DNA binding activity was significantly reduced in smooth muscle cells collected from old rabbits (4–5 years of age) versus younger rabbits (6–8 months of age) [32]. This attenuation of HIF-1 activity was correlated with a decrease in VEGF expression in the smooth muscle cells [32].
In conclusion, our study shows that cortical HIF-1a accumulation and HIF-1 activation remains attenuated during chronic hypoxic exposure in the aged rat. Despite this attenuation of HIF-1 activation, there is no significant difference in baseline or post-hypoxic brain capillary density counts between the young and old age groups. In a delayed manner, VEGF mRNA and protein levels are significantly elevated in the aged cortex despite the lack of HIF-1 activation. PPAR-γ coactivator (PGC)-1α, a known regulator of VEGF gene transcription, is elevated in the young and aged cortex during the chronic hypoxic exposure. Overall, our results suggest compensatory HIF-1 independent preservation of hypoxic-induced microvascular angiogenesis in the aged rat brain.
MATERIALS & METHODS
Exposure to hypobaric hypoxia
Male Fisher 344 (F344) rats aged 3 & 24 months were ordered from the National Institute of Aging (NIA). Animal housing and handling met IACUC standards. The aged-grouped rats were subjected to 10 or 21 days of chronic hypoxia in hypobaric chambers maintained at 380 Torr (0.5 ATM) for the first 24 hours (equivalent to 10% normobaric oxygen) and 290 Torr (0.4 ATM) for the subsequent 9 or 20 days (equivalent to 8% normobaric oxygen). The custom made steel chambers (with clear Plexiglas door), were connected to a vacuum source which reduced the internal atmospheric pressure [53]. Each experimental group of rats had an aged-matched control group, which was kept outside the hypobaric chambers but in the same location. After the chronic hypoxic exposure, experimental and control rats were briefly anesthetized and either decapitated or perfused. The brains and other organs of the decapitated rats were immediately removed and frozen in liquid nitrogen. Those tissues were stored at −80 degree Celsius until further processing. The non-decapitated rats were transcardially perfused with ice cold saline (pH 7.4) followed by 4% paraformaldehyde in saline. The organs of the perfused rats were stored in the 4% paraformaldehyde solution at 4 degree Celsius for at least 24 hours until further processing.
Real-time PCR Analysis
Total RNA was extracted from frozen brain cortex using an RNA extraction kit (RNAgents; Promega, Madison, WI). Complimentary DNA was synthesized from 2 ug of total RNA using the Superscript III system with an oligo-dT primer (Invitrogen, Carlsbad, CA). Real-time PCR analysis was performed with 0.5 ul of the final cDNA synthesis mix using commercially produced rat specific Taq-Man based gene expression assays (Applied Biosystems, Foster City, CA). The following assays were used: Beta-actin (Act b, catalog # Rn00667869_m1) and Vascular endothelial growth factor (Vegf, catalog # Rn00582935_m1). The PCR reactions were performed in an I-Cyler real-time PCR thermocycler (BioRad). All reactions were performed in triplicate using β-actin as an endogenous control.
Preparation of whole-cell lysates
Frozen brain cortical samples were dissected in a dish on dry ice and homogenized by standard procedures. A stainless steel motorized homogenizer was used to homogenize in ice-cold lysis buffer (NP-40, Boston Bioproducts: 50mM Tris-HCl, 150mM NaCl, 5mM EDTA, 1% NP-40) containing EDTA-free protease inhibitor tablet (Complete Mini, Roche Diagnostics, Indianapolis, IN). Homogenized samples were kept on ice for an hour and then centrifuged at 14,000 g for 30 min. Supernatants were collected, aliquoted and store at −20 degrees C. Protein concentrations of aliquots were determined by Bradford protein assay with bovine serum albumin (BSA) used as standards (BioRad).
Western blot analysis
Whole cell lysates (25 and/or 50 ug of protein) were denatured in lamelli buffer and electrophoresed on SDS-PAGE under reducing conditions. The proteins were transferred to nitrocellulose membranes (Bio-Rad) by standard procedures. Membranes were blocked with 10% nonfat dried milk in TBS containing 0.1 % Tween 20 (Bio-Rad). After blocking, membranes were incubated in 3% bovine serum albumin (BSA) in TBS with 0.1% Tween 20 containing primary antibodies. The specific primary antibodies of interest were mouse monoclonal antibodies for HIF-1α (R&D, 1:500), VEGF (Santa Cruz, 1:500), PGC-1α (Novus, 1:500) and goat polyclonal for β-actin (Santa Cruz, 1:2000). After a series of washes in TBS/Tween at room temperature for one hour, membranes were incubated with corresponding horseradish peroxidase-conjugated secondary antibodies for an hour. A final series of washes were done in TBS/Tween for at least another hour before Enhanced chemiluminescence was used to visualized specific protein-antibody complexes.
Immunohistochemistry & Microvessel Density
The fixed brains were removed from fixative and dehydrated before being embedded in paraffin. The parafinized brains were mounted and sectioned on a microtome into 5-micron coronal sections and plated. The plated sections were given at least 24 hours to dry before further processing. The sections were then deparafinized, hydrated, and subject to antigen retrival at boiling temperature for 20 minutes in a 10mM sodium citrate solution. The sections were washed and then incubated with blocking solutions including 10% normal horse serum in saline for one hour followed by overnight incubation in blocking serum and anti GLUT-1 antibody (Santa Cruz, 1:100). Following the overnight incubation, the sections were washed several times and then incubated for one hour in horse serum and a biotinylated secondary antibody (1:200). Color detection was carried out with the use avidin-biotin horseradish peroxidase solution and the diaminobenzidine peroxidase substrate kit. The sections were dehydrated and coversliped for microscopic analysis. Images spanning the full depth of the parietal cortex, corpus callosum, striatum, and CA1 region of the hippocampus were taken with a SPOT digital camera connected to a Nikon E600 Eclipse microscope with a x20 objective. GLUT-1 positive microvessels <20um were quantified and a computer-aided image analysis system (ImageProPlus) was used to determine the area of the images for microvascular density calculation. In all four brain regions examined in each rat, at least four different GLUT-1 stained sections were averaged for quantification. Each quantified section was at least 250–300 um apart from the subsequent quantified section.
Analysis/Statistics
All results are shown as means ± S.D., and in all cases p < 0.05 was considered statistically significant. Image J was used to quantify the densitometry of antigen-antibody complexes and normalized to that of β-actin (optical density ratios). Statistical analysis was carried out using SPSS software. Student’s t-test was used to assess difference between two groups, such as control to hypoxic optical density ratios.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Acker T, Acker H. Cellular oxygen sensing need in CNS function: physiological and pathological implications. J Exp Biol. 2004;207(Pt 18):3171–3188. doi: 10.1242/jeb.01075. [DOI] [PubMed] [Google Scholar]
- 2.Arany Z, Foo SY, Ma Y, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451(7181):1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 3.Bell MA, Ball MJ. Morphometric comparison of hippocampal vasculature in ageing and demented people: diameters and densities. Acta Neuropathol. 1981;53:299–318. doi: 10.1007/BF00690372. [DOI] [PubMed] [Google Scholar]
- 4.Bernaudin M, Nedelec AS, Divoux D, MacKenzie ET, Petit E, Schumann-Bard P. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J Cereb Blood Flow Metab. 2002;22(4):393–403. doi: 10.1097/00004647-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Boero JA, Ascher J, Arregui A, Rovainen C, Woolsey TA. Increased brain capillaries in chronic hypoxia. J Appl Physiol. 1999;86(4):1211–1219. doi: 10.1152/jappl.1999.86.4.1211. [DOI] [PubMed] [Google Scholar]
- 6.Chavez JC, Agani F, Pichiule P, LaManna JC. Expression of hypoxia-inducible factor-1alpha in the brain of rats during chronic hypoxia. J Appl Physiol. 2000;89(5):1937–1942. doi: 10.1152/jappl.2000.89.5.1937. [DOI] [PubMed] [Google Scholar]
- 7.Chavez JC, LaManna JC. Activation of hypoxia-inducible factor-1 in the rat cerebral cortex after transient global ischemia: potential role of insulin-like growth factor-1. J Neurosci. 2002;22(20):8922–8931. doi: 10.1523/JNEUROSCI.22-20-08922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn JF, Grinberg O, Roche M, Nwaigwe CI, Hou HG, Swartz HM. Noninvasive assessment of cerebral oxygenation during acclimation to hypobaric hypoxia. J Cereb Blood Flow Metab. 2000;20(12):1632–1635. doi: 10.1097/00004647-200012000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- 10.Fong GH. Mechanisms of adaptive angiogenesis to tissue hypoxia. Angiogenesis. 2008;11(2):121–140. doi: 10.1007/s10456-008-9107-3. [DOI] [PubMed] [Google Scholar]
- 11.Gutsavea DR, Carraway MS, Sulliman HB, et al. Transient hypoxia stimulates mitochondrial biogenesis in brain subcortex by neuronal nitric oxide synthase-dependent mechanism. J Neurosci. 2008;28:2015–2024. doi: 10.1523/JNEUROSCI.5654-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23(24):9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu CJ, Iyer S, Sataur A, Covello KL, Chodosh LA, Simon MC. Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells. Mol Cell Biol. 2006;26(9):3514–3526. doi: 10.1128/MCB.26.9.3514-3526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingraham JP, Forbes ME, Riddle DR, Sonntag WE. Aging reduces hypoxia-induced microvascular growth in the rodent hippocampus. J Gerontol A Biol Sci Med Sci. 2008;63(1):12–20. doi: 10.1093/gerona/63.1.12. [DOI] [PubMed] [Google Scholar]
- 15.Iyer NV, Kotch LE, Agani F, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12(2):149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalaria RN. Cerebral vessels in ageing and Alzheimer’s disease. Pharmacol Ther. 1996;72(3):193–214. doi: 10.1016/s0163-7258(96)00116-7. [DOI] [PubMed] [Google Scholar]
- 17.Knox CA, Oliveira A. Brain aging in normotensive and hypertensive strains of rats: a quantitative study of cerebral vasculature. Acta Neuropathol. 1980;52:17–25. doi: 10.1007/BF00687224. [DOI] [PubMed] [Google Scholar]
- 18.LaManna JC. Rat brain adaptation to chronic hypobaric hypoxia. Adv Exp Med Biol. 1992;317:107–114. doi: 10.1007/978-1-4615-3428-0_9. [DOI] [PubMed] [Google Scholar]
- 19.LaManna JC, Chavez JC, Pichiule P. Structural and functional adaptation to hypoxia in the rat brain. J Exp Biol. 2004;207(Pt 18):3163–3169. doi: 10.1242/jeb.00976. [DOI] [PubMed] [Google Scholar]
- 20.Levy AP, Levy NS, Wegner S, Goldberg MA. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995;270(22):13333–13340. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- 21.Mizukami Y, Jo WS, Duerr EM, et al. Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nat Med. 2005;11(9):992–997. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- 22.Mizukami Y, Kohgo Y, Chung DC. Hypoxia inducible factor-1 independent pathways in tumor angiogenesis. Clin Cancer Res. 2007;13(19):5670–5674. doi: 10.1158/1078-0432.CCR-07-0111. [DOI] [PubMed] [Google Scholar]
- 23.Ndubuizu O, LaManna JC. Brain tissue oxygen concentration measurements. Antioxid Redox Signal. 2007;9(8):1207–1219. doi: 10.1089/ars.2007.1634. [DOI] [PubMed] [Google Scholar]
- 24.Ndubuizu O, Chavez JC, LaManna JC. Increased prolyl 4-hydroxylase expression and differential regulation of hypoxia-inducible factors in the aged rat brain. Am J Physiol Regul Integr Comp Physiol. 2009;297(1):R158–R165. doi: 10.1152/ajpregu.90829.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SK, Dadak AM, Haase VH, Fontana L, Giaccia AJ, Johnson RS. Hypoxia-induced gene expression occurs solely through the action of hypoxia-inducible factor 1alpha (HIF-1alpha): role of cytoplasmic trapping of HIF-2alpha. Mol Cell Biol. 2003;23(14):4959–4971. doi: 10.1128/MCB.23.14.4959-4971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pichiule P, LaManna JC. Angiopoietin-2 and rat brain capillary remodeling during adaptation and deadaptation to prolonged mild hypoxia. J Appl Physiol. 2002;93(3):1131–1139. doi: 10.1152/japplphysiol.00318.2002. [DOI] [PubMed] [Google Scholar]
- 27.Pugh CW, Ratcliffe PJ. The von Hippel-Lindau tumor suppressor, hypoxia-inducible factor-1 (HIF-1) degradation, and cancer pathogenesis. Semin Cancer Biol. 2003;13(1):83–89. doi: 10.1016/s1044-579x(02)00103-7. [DOI] [PubMed] [Google Scholar]
- 28.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9(6):677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 29.Reed MJ, Edelberg JM. Impaired angiogenesis in the aged. Sci Aging Knowl Environ. 2004;7:7–17. doi: 10.1126/sageke.2004.7.pe7. [DOI] [PubMed] [Google Scholar]
- 30.Riddle DR, Sonntag WE, Lichtenwalner RJ. Microvascular plasticity in aging. Ageing Res Rev. 2003;2:149–168. doi: 10.1016/s1568-1637(02)00064-8. [DOI] [PubMed] [Google Scholar]
- 31.Rivard A, Fabre JE, Silver M, et al. Age-dependent impairment of angiogenesis. Circulation. 1999;99(1):111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 32.Rivard A, Berthou-Soulie L, Principe N, et al. Age-dependent defect in vascular endothelial growth factor expression is associated with reduced hypoxia-inducible factor 1 activity. J Biol Chem. 2000;275(38):29643–29647. doi: 10.1074/jbc.M001029200. [DOI] [PubMed] [Google Scholar]
- 33.Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17(11):3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan HE, Poloni M, McNulty W, et al. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60(15):4010–4015. [PubMed] [Google Scholar]
- 35.Semenza GL, Agani F, Booth G, et al. Structural and functional analysis of hypoxia- inducible factor 1. Kidney Int. 1997;51(2):553–555. doi: 10.1038/ki.1997.77. [DOI] [PubMed] [Google Scholar]
- 36.Semenza GL. Oxygen-regulated transcription factors and their role in pulmonary disease. Respir Res. 2000;1(3):159–162. doi: 10.1186/rr27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Semenza GL. Surviving ischemia: adaptive responses mediated by hypoxia- inducible factor 1. J Clin Invest. 2000;106(7):809–812. doi: 10.1172/JCI11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88(4):1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 39.Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13(2):167–171. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 40.Semenza GL. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell. 2001;107(1):1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- 41.Semenza GL. Life with oxygen. Science. 2007;318(5847):62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- 42.Semenza GL. Hypoxia and cancer. Cancer Metastasis Rev. 2007;26(2):223–224. doi: 10.1007/s10555-007-9058-y. [DOI] [PubMed] [Google Scholar]
- 43.Sharp FR, Bergeron M, Bernaudin M. Hypoxia-inducible factor in brain. Adv Exp Med Biol. 2001;502:273–291. doi: 10.1007/978-1-4757-3401-0_18. [DOI] [PubMed] [Google Scholar]
- 44.Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci. 2004;5(6):437–448. doi: 10.1038/nrn1408. [DOI] [PubMed] [Google Scholar]
- 45.Shoage J, Arany Z. Regulation of hypoxia-inducible genes by PGC-1α. Arterioscler Thromb Vasc Biol. 2010;30:662–666. doi: 10.1161/ATVBAHA.108.181636. [DOI] [PubMed] [Google Scholar]
- 46.Sick TJ, Lutz PL, LaManna JC, Rosenthal M. Comparative brain oxygenation and mitochondrial redox activity in turtles and rats. J Appl Physiol. 1982;53(6):1354–1359. doi: 10.1152/jappl.1982.53.6.1354. [DOI] [PubMed] [Google Scholar]
- 47.Silver I, Erecinska M. Oxygen and ion concentrations in normoxic and hypoxic brain cells. Adv Exp Med Biol. 1998;454:7–16. doi: 10.1007/978-1-4615-4863-8_2. [DOI] [PubMed] [Google Scholar]
- 48.Sonntag WE, Lynch CD, Cooney PT, et al. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- 49.Stein I, Itin A, Einat P, Skaliter R, Grossman Z, Keshet E. Translation of vascular endothelial growth factor mRNA by internal ribosome entry: implications for translation under hypoxia. Mol Cell Biol. 1998;18(6):3112–3119. doi: 10.1128/mcb.18.6.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Storkebaum E, Lambrechts D, Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays. 2004;26(9):943–954. doi: 10.1002/bies.20092. [DOI] [PubMed] [Google Scholar]
- 51.Ward NL, Moore E, Noon K, et al. Cerebral angiogenic factors, angiogenesis, and physiological response to chronic hypoxia differ among four commonly used mouse strains. J Appl Physiol. 2007;102(5):1927–1935. doi: 10.1152/japplphysiol.00909.2006. [DOI] [PubMed] [Google Scholar]
- 52.Warnecke C, Zaborowska Z, Kurreck J, et al. Differentiating the functional role of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2alpha target gene in Hep3B and Kelly cells. FASEB J. 2004;18(12):1462–1464. doi: 10.1096/fj.04-1640fje. [DOI] [PubMed] [Google Scholar]
- 53.Wright BM. Aparatus for exposing animals to reduced atmospheric pressure for long periods. Br J Haematol. 1964;10:75–77. doi: 10.1111/j.1365-2141.1964.tb00680.x. [DOI] [PubMed] [Google Scholar]