Abstract
Muscles that are stretched during contraction (eccentric contractions) show deficits in force production and a variety of structural changes, including loss of antibody staining of cytoskeletal proteins. Extracellular Ca2+ entry and activation of calpains have been proposed as mechanisms involved in these changes. The present study used isolated mouse extensor digitorum longus (EDL) muscles subjected to 10 eccentric contractions and monitored force production, immunostaining of cytoskeletal proteins, and resting stiffness. Possible pathways for Ca2+ entry were tested with streptomycin (200 μM), a blocker of stretch-activated channels, and with muscles from mice deficient in the transient receptor potential canonical 1 gene (TRPC1 KO), a candidate gene for stretch-activated channels. At 30 min after the eccentric contractions, the isometric force was decreased to 75 ± 3% of initial control and this force loss was reduced by streptomycin but not in the TRPC1 KO. Desmin, titin, and dystrophin all showed patchy loss of immunostaining 30 min after the eccentric contractions, which was substantially reduced by streptomycin and in the TRPC1 KO muscles. Muscles showed a reduction of resting stiffness following eccentric contractions, and this reduction was eliminated by streptomycin and absent in the TRPC1 KO muscles. Calpain activation was determined by the appearance of a lower molecular weight autolysis product and μ-calpain was activated at 30 min, whereas the muscle-specific calpain-3 was not. To test whether the loss of stiffness was caused by titin cleavage, protein gels were used but no significant titin cleavage was detected. These results suggest that Ca2+ entry following eccentric contractions is through a stretch-activated channel that is blocked by streptomycin and encoded or modulated by TRPC1.
Keywords: stretch-induced damage, eccentric contractions, titin, cytoskeletal proteins, calpains
contractions in which the muscle is stretched (eccentric contractions) are particularly prone to cause muscle damage characterized by an immediate weakness and a more slowly developing stiffness, soreness, and swelling (12, 26). Eccentric contractions cause sarcomere inhomogeneity with the presence of long and short sarcomeres and loss of the normal continuity of Z lines (6). Eccentric contractions also increase membrane permeability, which allows soluble cytosolic proteins, such as creatine kinase, to leak out of the muscle and into the plasma (25), whereas extracellular proteins, such as albumin and fibronectin, can be detected inside the muscle (15, 21). A number of studies have shown that resting intracellular calcium concentration ([Ca2+]i) is elevated after eccentric contractions but not after isometric contractions (2, 32, 40).
Cytoskeletal protein changes following eccentric contractions were first described by Lieber et al. (15) who showed loss of desmin immunostaining in 20% of fibers 1 day after a bout of eccentric exercise. They also showed that many of the desmin-negative fibers were immunopositive for fibronectin, demonstrating an increase in membrane permeability. Subsequently many groups have confirmed these findings and shown that immunostaining changes in other cytoskeletal proteins, titin and dystrophin, are also present (16, 17, 42). Lieber et al. (16) proposed that Ca2+ entered the muscle either because of mechanical tears in the membrane (21) or because of opening of stretch-activated channels (9). The resulting rise in [Ca2+]i was proposed to activate calpain, which caused proteolysis of desmin and other susceptible proteins. Support for this hypothesis has come from the finding that calpains can be activated after eccentric exercise (23, 28). In addition, activation of calpains by physiological levels of Ca2+ have been shown to impair excitation-contraction coupling and to cleave titin with a resulting reduction in resting stiffness (36, 37).
An interesting and unresolved question is whether these cytoskeletal protein changes have functional consequences for the muscle. It is possible, on the basis that cytoskeletal proteins contribute to lateral transmission of force, that the reduction in active force production might be in part caused by changes in the mechanical properties of the cytoskeleton (17, 22, 28, 29). However, attempts to test this hypothesis have proved equivocal. For instance, desmin knockout animals generate less force than wild type but show less reduction in active force after eccentric contractions (31). In a previous study we used removal of extracellular calcium or application of the calpain inhibitor leupeptin to minimize the cytoskeletal protein changes caused by eccentric contractions. When cytoskeletal immunostaining changes were minimized in this way, the reduction of force after eccentric contractions showed a small but significant decrease (42). Thus our working hypothesis is that eccentric contractions lead to Ca2+ entry, activation of calpain and damage to a range of cytoskeletal proteins. We suggest that this cytoskeletal damage might affect both the developed force and the resting force.
The present study had two aims. 1) To determine the route of entry for Ca2+ involved in the cytoskeletal protein changes. We did this by using the stretch-activated channel blocker streptomycin (10) and a mouse lacking the transient receptor potential canonical 1 (TRPC1) gene (5) to test the hypothesis that the TRPC1 protein forms, or contributes to, the stretch-activated channel in skeletal muscle. (2) To identify whether the changes in immunostaining of the cytoskeletal proteins had any effect on either the reduction in active force after eccentric contractions or on the resting muscle stiffness. We focused particularly on titin, which connects the Z-discs to the M-lines of the thick filaments and is thought to bear much of the resting tension of muscle (11).
METHODS
Animals.
Male Balb/c mice (10–14 wk) were supplied by the Animal Resource Centre, Perth, WA, Australia, and used for the majority of experiments. TRPC1 KO mice (5) were supplied by Phillips-Universtät Marburg, Germany, and bred in Victor Chang Cardiac Research Institute, University of New South Wales. Male TRPC1 knockout (KO) and wild-type (C57BL) mice at 13–14 wk of age were supplied. The experiments were approved by the Animal Ethics Committee of the University of Sydney.
Muscle preparation.
Mice were killed by cervical dislocation. The extensor digitorum longus (EDL) muscles were dissected as previously described (42) from wild-type and TRPC1 KO mice. The muscle phenotype of TRPC1 KO mice was investigated by Zanou et al. (41), who reported that force/area during maximal tetani was reduced from 257 ± 13 mN/mm2 (wild type) to 209 ± 13 mN/mm2 (TRPC1 KO). The muscle with clips attached to the tendons was mounted in the experimental chamber between a force transducer and the lever of a motor (length controller, 300B, Aurora Scientific, Ontario, Canada). Two platinum electrodes, parallel to the muscle, provided stimulation. The muscle was superfused with a standard solution containing (in mM): 121 NaCl, 5 KCl, 1.8 CaCl2, 0.5 MgCl2, 0.4 NaH2PO4, 24 NaHCO3, and 5.5 glucose. This solution was bubbled with 95% O2-5% CO2, maintaining a pH of 7.4. All experiments were carried out at room temperature (∼22°C) because muscle performance is more stable at this temperature. The effect of the stretch-activated channel blocker, streptomycin (200 μM), was investigated. Aqueous stock solution of 200 mM streptomycin was prepared and diluted immediately before use.
Specific force was determined from the initial maximal tetanic force at Lo divided by the area determined from histological cross sections. For the Balb/c mice specific force was 281 ± 9 mN/mm2 (mean ± SE, n = 23). None of the experimental groups (isometric, eccentric, or streptomycin treated) were significantly different from each other. For the C57BL mice, the specific force was 270 ± 15, whereas for the TRPC1 KO the specific force was 294 ± 12 mN/mm2. These two groups were not significantly different (P = 0.2, unpaired t-test). Thus we did not confirm the reduced specific force in TRPC1 KO muscles reported by Zanou et al. (41).
Experimental protocol.
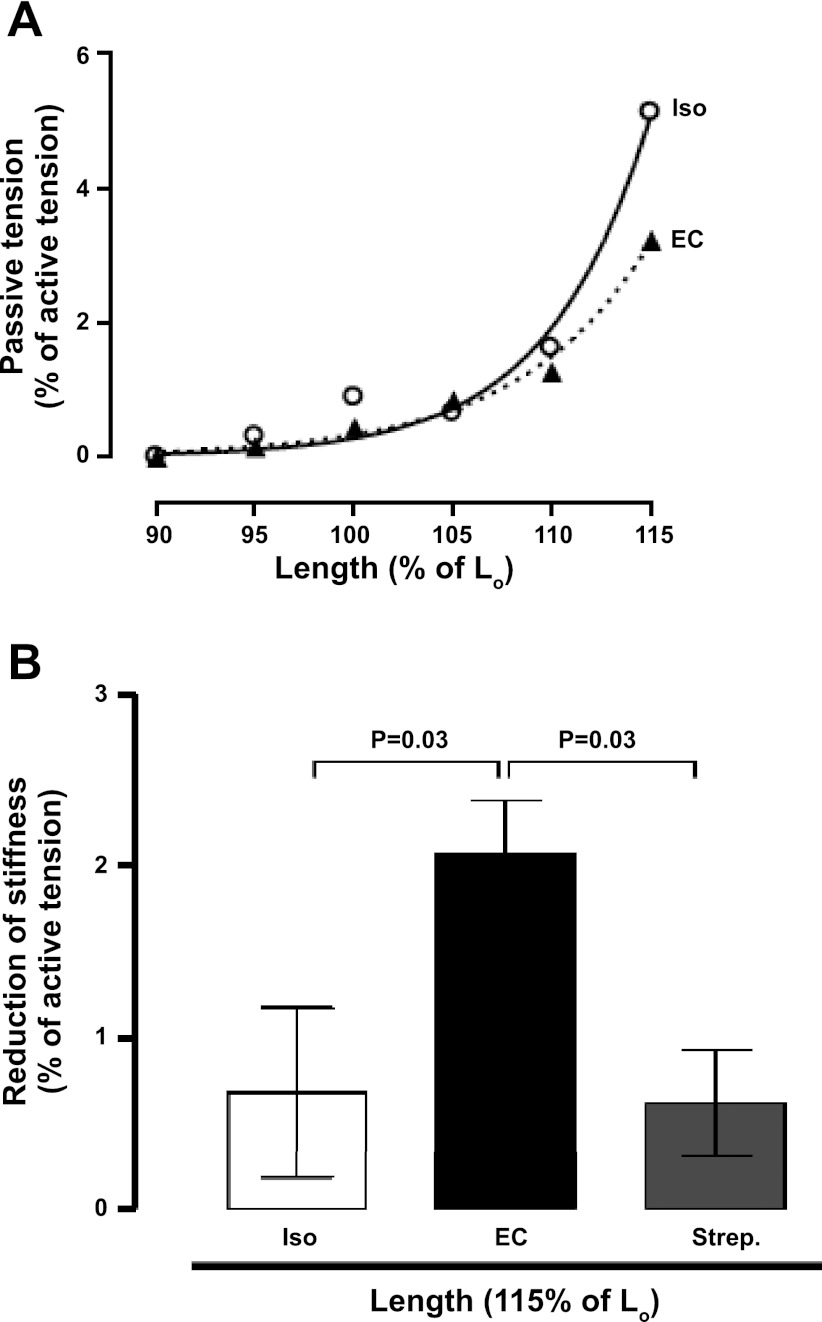
The standard stimulation protocol produced tetani of 400-ms duration using 100 Hz 0.5-ms supramaximal stimuli with a rest period of 9.6 s between tetani (cycle, 10 s). The muscle was adjusted to the length that produced maximal tetanic force (Lo). The resting stiffness of the muscle was determined from the resting force at 90%, 95%, 100%, 105%, 110%, and 115% of Lo. The muscle was stretched progressively, waiting 1 min at each length to allow resting tension to reach a steady state before measurement. The passive tension value when the muscle was visibly slack (90% of Lo) was set to zero, and passive tension at the longer lengths was determined from this baseline. The experimental resting force data were fitted to an exponential growth equation using a sum-of-squares minimization routine (see Figs. 2A and 3A). Resting stiffness was characterized by the force at 115% Lo derived from the fitted curve.
Fig. 2.
Effect of streptomycin on passive tension in isolated EDL muscle following eccentric contractions. A: representative experiments showing resting tension measured at different muscle length 30 min after isometric or eccentric contractions. Values expressed as a percentage of the tetanic force measured before the contractions. Lo, optimal length at which muscle produces maximal tetanic force; ○, following isometric contractions; ▴, following eccentric contractions. B: change of passive tension at 30 min after Iso or EC in standard solution or solution containing streptomycin. Values expressed as the reduction of passive tension after compared with before the contractions. Data are means ± SE.
Fig. 3.
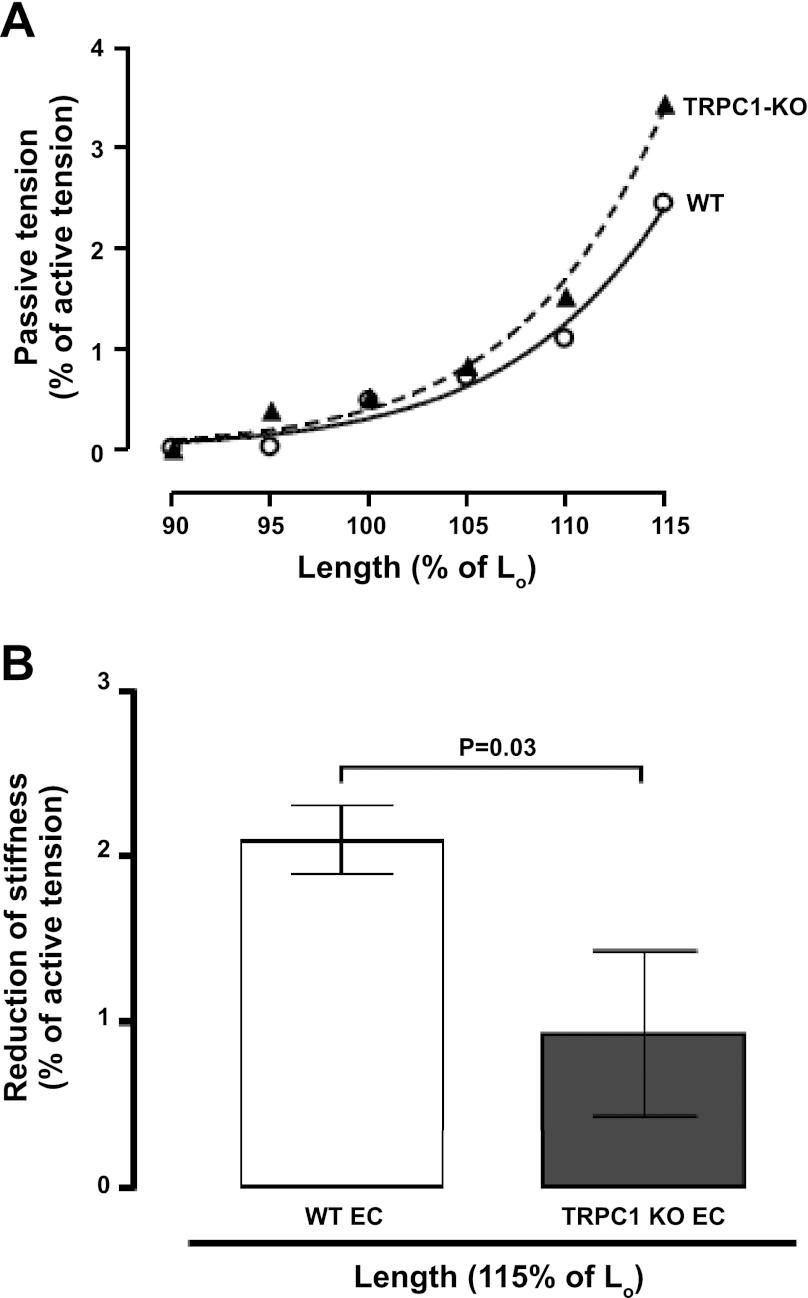
Effect of TRPC1 knockout on passive tension in mouse EDL muscle following eccentric contractions. A: representative experiments showing passive tension measured at different muscle lengths 30 min after eccentric contractions in TRPC1-KO or wild-type (WT) muscles (both groups C57BL mice). Values expressed as a percentage of the tetanic force measured before the contractions. Lo, optimal length at which muscle produces maximal tetanic force; ○, WT; ▴, TRPC1-KO. B: change of passive tension at 30 min after eccentric contractions in TRPC1-KO or WT muscles. Values expressed as the reduction of passive tension after compared with before the contractions. Data are means ± SE.
The experimental protocol was to first measure resting stiffness. Muscles were then returned to Lo for 10 min and subjected to either 10 isometric contractions (control group) or 10 eccentric contractions (eccentric group). Stretch was applied 250 ms after the start of stimulation, and the muscle was stretched from Lo to Lo + 20% over 150 ms (stretching velocity, 1.3 × Lo/s). The muscle was returned to Lo between tetani. In some experiments, the muscle was perfused with streptomycin (200 μM) 30 min before tetanic stimulation and continued for a further 30 min after isometric or eccentric contractions. The same stretch protocol was carried out on the muscles from TRPC1 KO and their wild-type mice (C57BL/6J). Because it has been shown that the magnitude of the extra force in an eccentric contraction is one determinant of the subsequent force decline (20), we measured the increase in force above the isometric level in eccentric contractions. In the eccentric control muscles (Balb/c), the increase in force during eccentric contractions was 54.0 ± 2.2% isometric force (mean ± SE, n = 6). In the streptomycin group the increase in force was 47.7 ± 1.7% (n = 6), in the WT for the TRPC1 KO (C57BL) the increase was 44.7 ± 2.3% (n = 4), and in the TRPC1 KO the increase was 51.0 ± 2.8% (n = 5). These differences were marginally significant (P = 0.06 on a one-way ANOVA). The biggest difference was between the two strains of mice and possibly reflects differences between these strains.
Following the series of 10 eccentric or isometric contractions, the isometric tension was measured at Lo immediately and again after 30 min after the series. The resting stiffness of the muscle was reassessed 30 min after the isometric or eccentric protocols. All mechanical data are presented as a percentage of the initial isometric force.
Immunohistochemistry of muscle cytoskeletal proteins.
After the mechanical protocol, muscle cytoskeletal proteins were evaluated by immunohistochemistry at 30 min posttetanic stimulation. Each EDL muscle was embedded in OCT medium and snap-frozen in isopentane cooled in liquid nitrogen and stored at −80°C for further analysis.
The following primary antibodies were used in this study: mouse monoclonal anti-dystrophin (Dy8/6C5, Novocastra Laboratories), mouse monoclonal anti-desmin (DE-R-11, Novocastra Laboratories), mouse monoclonal anti-titin (9D10, Developmental Studies Hybridoma Bank), and rabbit monoclonal anti-fibronectin (FN-1, Sigma) antibodies. All primary antibodies were diluted to 1:50 concentration just before use.
Muscle cryosections (6 μm) were fixed in cold acetone (−20°C) for 10 min. Following three washes in PBS, sections were permeabilized with 0.1% Triton X-100 in PBS for 5 min at room temperature, washed twice, and blocked in 1% BSA/PBS for 30 min. Then sections were incubated overnight at 4°C with primary antibodies against either dystrophin (mouse), desmin (mouse), titin (mouse), or fibronectin (rabbit). After three washes in PBS, sections were incubated with Alexa Fluor 555 goat anti-mouse or Alexa Fluor 488 goat anti-rabbit IgG (H+L) (1:300 dilution; Invitrogen) for 1 h. The sections were again washed in PBS and mounted in ProLong Gold antifade reagent with DAPI (Invitrogen). The cover slip was sealed with nail polish for microscopic analysis.
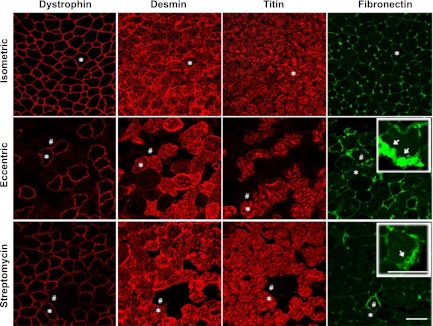
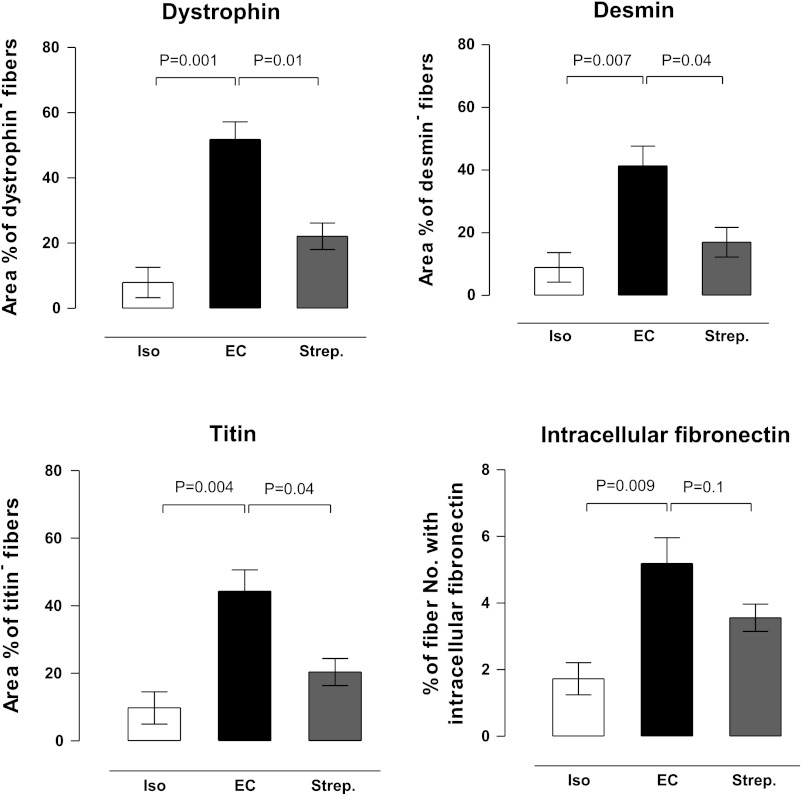
Sections were imaged with a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss). All sections were imaged using fixed optical parameters, filters, and magnification (EC Plan-Neofluar 20× 0.75 NA dry objective) to ensure comparable levels of background fluorescence. All cells were included in the analysis (∼1,100 fibers in the EDL muscle cross section), and the acquired images were evaluated using a digital image morphometry program (ImageJ 1.32j, NIH). Fibers were counted as abnormal if the staining intensity was less than (desmin and titin) or exceeded (fibronectin) a predetermined threshold, determined from the fluorescence intensity of negative control staining. Fibronectin staining was only measured where clearly intracellular (Fig. 4, arrows in insets); when the fibronectin staining was close to the membrane so that intracellular or extracellular location was uncertain (# in Fig. 4), these fibers were not included in the analysis. In the case of dystrophin, abnormal fibers were identified by discontinuous or patchy dystrophin labeling. The area of reduced staining for dystrophin, desmin, or titin was calculated as a percentage of the entire muscle cross-sectional area. The change in fibronectin was evaluated by scoring the percentage of abnormal fibers (intracellular staining) to the total number of fibers per muscle cross section. All image analysis was performed by an experienced observer blinded to the treatment.
Fig. 4.
Effect of streptomycin on stretch-induced changes in the antibody staining patterns of muscle proteins. Cross sections of EDL muscle immunostained for different cytoskeletal proteins 30 min after isometric or eccentric contractions in standard solution or streptomycin-containing solution. *,#Same fiber in serial sections. Arrows indicate representative staining of intracellular fibronectin. Scale bar, 50 μm.
Western blotting.
EDL muscles were dissected and subjected to a series of eccentric contractions as described above. The contralateral muscle was used as a control. Muscles were immediately frozen after the experiment and kept at −80°C until the immunoblotting was performed. Frozen EDL muscles were lysed with a polytron PT 1200 homogenizer (Kinematica, Littau/Lucerne, Switzerland) using ice-cold lysis buffer containing (in mM): 50 Tris pH 7.5, 150 NaCl, 25 EDTA, 25 EGTA, 1% Triton X-100, protease inhibitor cocktail, and phosphatase inhibitor (Sigma). After 30 min of incubation on ice, homogenates were centrifuged at 15,800 g for 30 min at 4°C and the supernatant was removed. Protein concentration in the supernatant was determined using the Bradford assay (Bio-Rad, Hercules, CA).
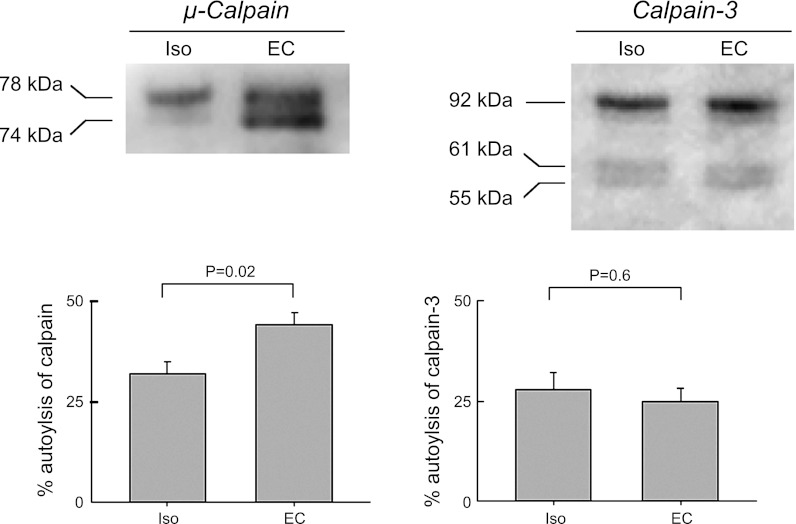
A total of 20 μg of protein per well was loaded into a 4% (stacking)-12% (resolving) polyacrylamide gel. Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane using a Mini Trans-Blot Transfer Cell (Bio-Rad). Membranes were blocked for 1 h with 5% skim milk powder in PBS-Tween 20 at room temperature and immunoblotted using the following antibodies: mouse anti-μ-calpain (1:1,000; Sigma, clone 15C10) or mouse anti-calpain-3 (1:200; Novocastra monoclonal 12A2, Newcastle, UK) overnight at 4°C. Primary antibodies were detected using HRP-conjugated anti-mouse IgG (1:1,000, 1 h at room temperature; Santa Cruz, CA). The protein bands were developed using an ECL Plus detection kit (Amersham Pharmacia Biotech) and visualized with an Alpha Innotech (San Leandro, CA) FluoChem SP Imaging System. Protein band quantification was obtained using Image J (National Institutes of Health). μ-calpain (calpain-1) was visualized at 78 kDa with one autolytic product at 74 kDa. Calpain-3 was visualized at 92 kDa with two autolytic products at 61 and 51 kDa. The activation of each calpain was calculated as a percentage of the autolytic products relative to the total amount of protein detected (24).
Titin extraction and analysis.
Immediately following contractions, the EDL was placed in 140 μl of a buffer containing; 10% SDS, 100 mM Tris (pH 8.8), 5 mM EGTA, 50 mM DTT, and a protease/phosphatase inhibitor cocktail (Sigma). The muscle was minced in the buffer using a pair of fine-tipped scissors and heated to 65°C for 5 min and then spun down at 13,000 g for 5 min at room temperature. Supernatant (100 μl) was removed, added to 50% glycerol in PBS with bromophenol blue (100 μl), and stored at −20°C for analysis. Positive controls were also performed to enhance the Ca2+-induced calpain activation (24). For these controls, EGTA and the inhibitor cocktail were omitted from the buffer and the homogenate was heated to 35°C for 30 min prior to the 65°C treatment. All samples were analyzed within 1 wk of collection.
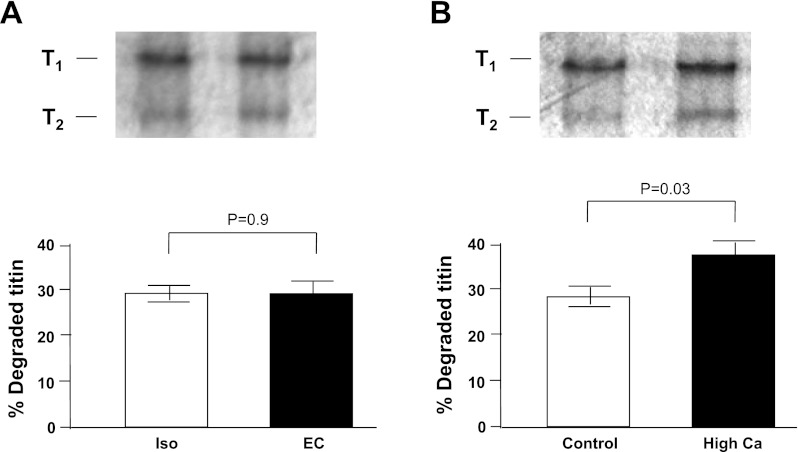
Low acrylamide (2.8%) gels with silver staining were used to determine whether titin was degraded following eccentric contractions as reported elsewhere (24). Briefly, electrophoresis was performed at room temperature (10 mA for 30 min, 20 mA for 2 h, and 30 mA for 1 h) and then silver-stained using a commercially available kit (SilverSNAP Stain Kit II; Pierce, IL). The protein bands were visualized with an Alpha Innotech FluoChem SP Imaging System. Protein band quantification was obtained using Image J (National Institutes of Health) and presented as the fraction of degraded titin (T2) to total titin (T1 + T2).
Genotyping.
To distinguish the TRPC1 −/− (KO) from the TRPC1 +/+ (WT) mice, 3 μg of mouse tail genomic DNA was added to a PCR mix containing: 2 μl of 10 × PCR reaction buffer, 4 μl of 5 × GC-Rich Buffer, 1.5 mM MgCl2, 200 μM dNTPs (Bioline), 300 μM of both forward and reverse primers (Sigma-Aldrich), 0.4–0.8 Units of FastStart Taq DNA Polymerase. The following primers were used to identify WT mice (forward) tccctttacttggcaaccttt and (reverse) ttggcaaaatgaggataatga and KO mice (forward) tctatggcttctgaggcgga and (reverse) gcattattaatatctgagtcattttcttattggcaaaatgagc. PCR was initiated with a preincubation step at 94°C (3 min), followed by 35 cycles of denaturation at 94°C (20 s), annealing at 55°C (30 s). and elongation at 72°C (60 s). A final step followed at 72°C (8 min).
Statistics.
The change in active and passive tension, as well as cytoskeletal staining, among isometric, eccentric, and streptomycin-treated groups was analyzed by one-way ANOVA with post hoc test for multiple comparisons. Differences in the above parameters between TRPC1 KO and wild-type mice after stretch were compared with unpaired t-test. The values were expressed as means ± SE. The significance level was set at P < 0.05.
RESULTS
Effects of stretch-activated channel blocker and TRPC1 KO on tetanic force in isolated EDL muscle.
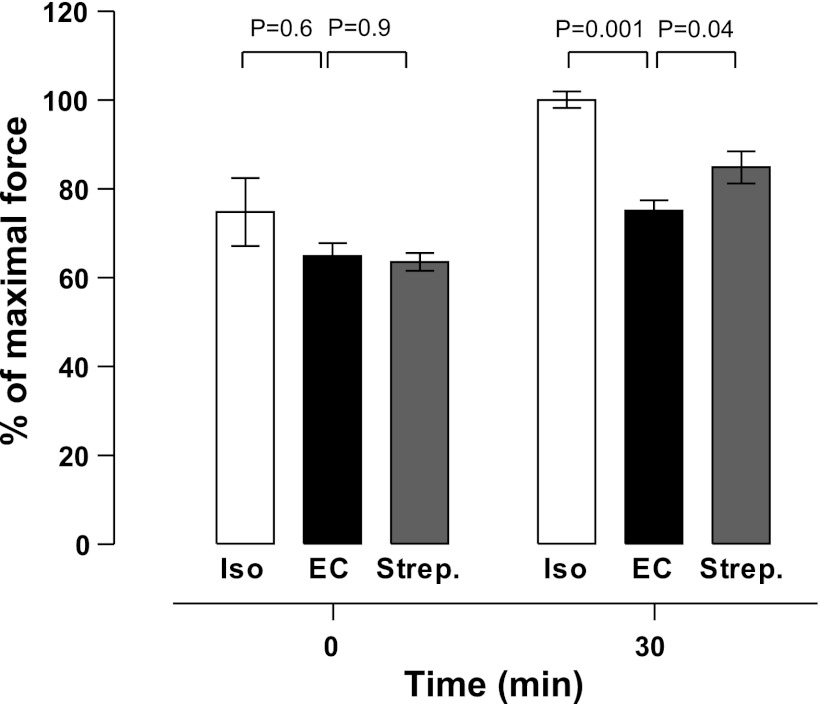
The changes in active force following isometric and eccentric contractions, with or without streptomycin treatment, were determined (Fig. 1). Tetanic force was transiently depressed to 75 ± 8% of control level immediately after 10 isometric contractions, due to muscle fatigue, but recovered completely by 30 min (n = 5). In separate muscles (n = 6), following 10 eccentric contractions, the force declined to 65 ± 3% of prestretch level immediately (P = 0.3 vs. isometric) and partially recovered, reaching 75 ± 3% at 30 min (P < 0.001 vs. isometric). To address the question of whether the force deficit induced by eccentric contractions can be prevented by blocking Ca2+ influx through a stretch-activated channel, eccentric contractions were performed in the presence of 200 μM streptomycin, a blocker of stretch-activated channels. In the presence of streptomycin the mean force at 30 min poststretch was increased (85 ± 4%) and was significantly greater than in its absence (n = 6, one-sided unpaired t-test, P = 0.04; Fig. 1). To address the question as to whether the stretch-activated channel might be encoded by TRPC1 we used muscle from TRPC1 KO mice. However, there was no significant difference between the force deficit 30 min after the eccentric contractions in wild-type (C57BL) vs. TRPC1 KO muscles (WT 77 ± 4%, n = 4; TRPC1 KO 76 ± 2%, n = 5; P = 0.7).
Fig. 1.
Effect of streptomycin on tetanic force in mouse extensor digitorum longus (EDL) muscle following eccentric contractions. Maximal isometric force measured immediately and 30 min after isometric (Iso) or eccentric contractions (EC) in standard solution or solution containing streptomycin (Strep.). Values expressed as a percentage of the force measured before the contractions. Data are means ± SE.
Effects of stretch-activated channel blocker and TRPC1 KO on resting stiffness.
We determined resting stiffness after isometric and eccentric contractions, with or without streptomycin treatment. Data from representative experiments (Fig. 2A) demonstrates a reduction in resting stiffness following eccentric contractions. To reduce variability between muscles, we looked at the change in muscle stiffness before and after the contraction series (Fig. 2B). There was no significant change in resting stiffness after isometric contractions but a significant reduction in stiffness after eccentric contractions (n = 6, P = 0.03 vs. isometric). This reduction in resting stiffness is not simply due to stress relaxation because it was not observed after isometric contractions or after cyclic passive stress (not shown). The eccentric contraction-induced reduction in resting stiffness was largely prevented by streptomycin (n = 6, P = 0.03; Fig. 2B). This suggests that the reduced stiffness was mediated by Ca2+ entry through a stretch-activated channel. To test the hypothesis that the stretch-activated channel requires the TRPC1 protein, we also performed eccentric contractions on TRPC1 KO muscles and measured the resulting muscle stiffness (Fig. 3A). In support of this hypothesis, the TRPC1 KO muscles showed a significantly smaller reduction in muscle stiffness after eccentric contractions compared with the wild-type muscle (n = 6, P = 0.03; Fig. 3B).
Effects of stretch-activated channel blocker and TRPC1 KO on the levels of cytoskeletal proteins.
Representative examples of the distribution of dystrophin, desmin, titin, and fibronectin immunostaining after isometric and eccentric contractions are shown in Fig. 4. As previously demonstrated (16, 42) after eccentric contractions, dystrophin, desmin, and titin show patchy loss of immunostaining and there is entry of fibronectin into some cells. Figure 4, bottom, shows that streptomycin provides a substantial measure of protection against these changes in the protein staining. Quantitative analysis and statistics supporting these observations are shown in Fig. 5.
Fig. 5.
Quantification of changes in protein immunogenicity. Stretch-induced changes in Balb/c mouse cytoskeletal proteins (dystrophin, desmin, and titin), expressed as the percentage area of muscle fibers affected (area of affected fibers/total area of cross section). Change in fibronectin staining was quantified by scoring the percentage of fibres stained with intracellular fibronectin (affected fibers/total no. of fibers per section). Data are mean ± SE.
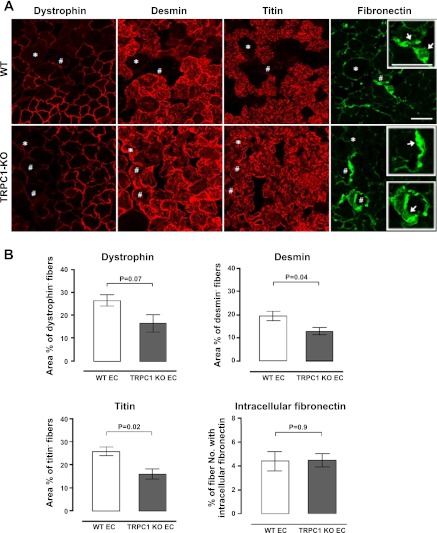
We investigated the changes in immunostaining after TRPC1 KO muscles were subjected to eccentric contractions; note that strain of mice was C57BL/6 for both wild type and TRPC1 KO. It is apparent from Fig. 6A that the immunostaining changes caused by eccentric contractions were somewhat reduced in the TRPC1 KO muscle compared with the wild type. Quantification and statistics for these changes are shown in Fig. 6B. The changes in dystrophin staining in the TRPC1 KO were statistically marginal (P = 0.07), whereas the desmin and titin changes were statistically significant (P = 0.04 and 0.02, respectively). Fibronectin staining following eccentric contractions was unaffected in the TRPC1 KO. These results suggest that the protein encoded by TRPC1 has a contributory role in the cytoskeletal damage following eccentric contractions, perhaps as one component of the stretch-activated channel.
Fig. 6.
Effect of TRPC1 knockout on stretch-induced changes in the antibody staining of muscle proteins. A: cross sections of EDL muscle immunostained for different cytoskeletal proteins 30 min after eccentric contractions in WT or TRPC1-KO muscles (both groups C57BL mice). *,#Same fiber in serial sections. Arrows indicate representative staining of intracellular fibronectin. Scale bar, 50 μm. B: stretch-induced changes in mouse cytoskeletal proteins (dystrophin, desmin, and titin), expressed as the percentage area of muscle fibers affected (area of affected fibers/total area of cross section). Change in fibronectin staining was quantified by scoring the percentage of fibers stained with intracellular fibronectin (affected fibers/total no. of fibers per section). Data are means ± SE.
Activation of calpains following eccentric contractions.
A popular hypothesis (16, 42) is that Ca2+ entry activates calpains, which then degrade susceptible proteins. Activation of calpains involves autolysis and can be quantified by the percentage of the total protein autolyzed (24). We examined μ-calpain and the muscle-specific calpain-3, as both have been proposed as possible candidates. Activation of μ-calpain leads to a lower molecular weight autolysis product (74 kDa) as seen in Fig. 7, left. In the gel shown, the low molecular weight product was minimal following isometric contractions but substantial after eccentric contractions. The percentage autolysis averaged 32 ± 3% in the isometric controls and was significantly greater at 44 ± 3% (n = 4, P = 0.02, unpaired t-test) following eccentric contractions. In contrast calpain-3 showed no significant difference between the isometric controls and the eccentric contractions (P = 0.6).
Fig. 7.
Eccentric contractions induced activation of μ-calpain, but not calpain-3. EDL muscles were subject to a series of eccentric contractions and were analyzed by Western blotting. Membranes were immunoblotted for μ-calpain and calpain-3, and the activation of each calpain was measure. Left: μ-calpain was detected as a nonautolyzed band at 78 kDa and an autolytic product at 74 kDa. Activation of μ-calpain was calculated as a percentage of the autolytic band relative to the total amount of μ-calpain. Right: relative autolysis of calpain-3 (61 and 55 kDa bands) relative to the total amount of the protein (i.e., calpain activation) was not statistically different between groups. Representative Western blots are shown for each calpain. Graphs show mean and SE of 4 different EDL muscles in each group from 2 independent experiments.
Absence of increased titin degradation after eccentric contractions.
The loss in resting stiffness following eccentric contractions may be the result of altered titin protein conformation or degradation, as the immunohistochemical data suggests (Figs. 4A and 6A). To test this hypothesis, muscle homogenates from eccentric and isometric contractions were stained for titin, which can be recognized by its high molecular weight. Figure 8A demonstrates that titin degradation was not significantly different between groups (P = 0.9), suggesting that eccentric contractions do not cause a significant increase in protein degradation. The loss in passive force following eccentric contractions may therefore be the result of altered titin protein conformation rather than gross titin degradation. In contrast, Fig. 8B shows that when the muscle homogenate was subjected to conditions designed to increase titin degradation (35°C for 30 min with a high Ca2+), an increase in titin degradation was present (24).
Fig. 8.
Eccentric contractions did not induce titin degradation. Following contractions, muscles homogenates underwent electrophoresis using low acrylamide gels. Gels were silver-stained to determine if eccentric contractions induce titin degradation. Full-length titin has a molecular weight of 3,700 kDa and is the only protein to appear in this region of a low acrylamide gel. Degradation product of titin (2,500 kDa, T2) is presented as a fraction of the total titin (T1 + T2). A: no significant difference is observed between isometric and eccentric contractions (P = 0.9). B: control experiments demonstrate that titin can degrade under high Ca2+ conditions (positive control), possibly by Ca2+-induced calpain activation (P = 0.03; see methods). Graphs show data from 4 independent experiments.
DISCUSSION
Changes in contractile performance following eccentric contractions.
The present data confirm that eccentric contractions cause a larger decrease in force than isometric contractions when measured 30 min after contractions (15, 26, 42). The greater loss in force following eccentric contractions might be attributable to damaged and/or modified cytoskeletal proteins (17, 29). We explored this possibility in a previous study and demonstrated that the changes in immunostaining of cytoskeletal proteins could be reduced by either removal of extracellular Ca2+ or the calpain inhibitor leupeptin (42). The reduction in cytoskeletal staining produced by these interventions was associated with a small but significant improvement in the developed force. The present study partly confirms this conclusion; here we could reduce the immunostaining changes of the cytoskeletal proteins by either streptomycin or the TRPC1 KO but only the streptomycin intervention was accompanied by a significant improvement in isometric force. Thus the force deficit following eccentric contractions seems to show a small and variable association with the cytoskeletal changes. There are many possible causes of this association. One possibility is that desmin and dystrophin are necessary for alignment and lateral force transmission between myofibrils and the membrane and hence through the extracellular connective tissue to neighboring fibers. When these cytoskeletal elements are damaged there will be less effective transmission of force laterally, which will tend to reduce the developed force (29). Another possibility is that damage to the desmin cytoskeleton increases the variability of sarcomere lengths, which tends to reduce the tension development (22). One well known consequence of this variability of sarcomere lengths is that Lo is increased (33) and some of the reduction of force that we observed in our experiments might have been reduced by stretching the muscles to the new and longer Lo. We conclude that there is some evidence that damage to the cytoskeletal elements contributes to the decline of force after eccentric contractions but this mechanism is likely to be only one of a number of mechanisms that contribute to the decline (for review see Ref. 1).
In the present study we also studied resting stiffness stimulated by the observations that elevating intracellular Ca2+ in a skinned fiber could activate calpain, cleave titin, and reduced resting stiffness (36, 37). We found that resting stiffness was reduced by eccentric contractions but not by isometric contractions. This does not seem to be caused by mechanical changes induced by the eccentric contractions because it was prevented by treatment with streptomycin (Fig. 2B). Previous studies of this topic in intact muscles have given variable results. Many in vivo studies have found that joint stiffness or muscle stiffness increased after eccentric contractions (4, 13, 38) which probably result from the inflammatory response, swelling and local contractures seen in intact tissues. However, there are reports of reduce joint stiffness following eccentric stretches even in intact animals muscles (27). In the present study utilizing isolated muscle at room temperature, inflammation will not occur and contractures are not common (40), and, perhaps for these reasons, we have been able to detect a small reduction in resting stiffness. In skinned fibers, most resting stiffness is attributable to titin (11) and Verburg et al. (36, 37) further demonstrated that elevating intracellular Ca2+ to activate calpain was capable of reducing stiffness. Verburg et al. (37) confirmed that this arose from titin cleavage by demonstrating products of titin cleavage in extracts from the muscle. However, in the present study we could not detect such products (Fig. 8A) and nor did Ochi et al. (27). This negative result could mean that titin cleavage did not occur; alternatively we may not have had sufficient sensitivity to detect the cleaved products. A similar dissociation between immunostaining and cleavage studies exists for desmin; there is loss of desmin immunostaining after eccentric contractions (16) but Barash et al. (3) were unable to detect desmin cleavage products on Western blotting. Our interpretation of these findings is that Ca2+ activation of calpain leads to some titin cleavage, sufficient to cause detectable loss of stiffness but not sufficient to be detectable on the titin gels.
Changes in immunogenicity of titin.
The present study shows that the immunogenicity of titin using the antibody 9D10 was reduced following eccentric stretches. This antibody binds to the PEVK region of titin, which unravels and lengthens when the muscle is stretched (34). μ-Calpain can bind to various sites in muscle including membranes and two sites on the titin: N1, which is very close to the Z-line, and N2, which is adjacent to the PEVK region (30). However, most of the μ-calpain seems to be freely diffusible in muscle (24) so the sites of titin cleavage might either be close to the calpain binding sites on titin (30) or the preferred cleavage sites for calpain on titin, which are close to the Z-line, the PEVK region, and close to the M protein binding site (14, 30). Thus the idea that activation of μ-calpain would simultaneously lead to loss of immunogenicity and reduced stiffness seems consistent with cleavage at the PEVK site. More puzzling is the fact that the previously used titin antibody, SC-8724 (Santa Cruz), which is a polyclonal antibody raised to an epitope close to the COOH terminus of titin, showed an increase in binding after eccentric contractions (16, 42). Perhaps the calpain cleavage site near the COOH terminus causes a structural change that allows better access of the antibody to its epitope.
Damage pathway.
The hypothesis under consideration to explain muscle damage following eccentric exercise is as follows: eccentric exercise triggers extracellular Ca2+ to enter the cell, which increases [Ca2+]i. The elevated [Ca2+]i activates calpains, which in turn cause cleavage of susceptible proteins resulting in reduced force production and reduced resting stiffness. In support of this pathway prevention of muscle damage by reduction of extracellular Ca2+ has been demonstrated (39, 42), and several studies have measured a rise in [Ca2+]i (2, 18, 32). Activation of calpain-3 after eccentric exercise has been detected but only 24 hr after the activity (23), whereas a recent study (28) found a threefold increase in calpain activity 30 min after eccentric exercise in humans. Our study extends these findings by showing that μ-calpain is activated at 30 min, whereas calpain-3 is not. This finding supports a recent study in skinned fibers in which Ca2+-induced excitation-contraction uncoupling and titin degradation could be produced normally in calpain-3-deficient muscles (36). These authors showed that μ-calpain was activated and thought to underlie the Ca2+-dependent muscle damage.
Another aspect of this hypothesis is whether the rise in [Ca2+]i that follows eccentric contractions is sufficient to activate μ-calpain. Recent studies in skinned fibers with careful control of [Ca2+]i levels show that unautolyzed μ-calpain is just detectably activated (autolyzed) by 2 min of 2 μM [Ca2+]i (24). Higher [Ca2+]i or longer periods lead to more substantial activation. Importantly, once μ-calpain has been activated, it requires a substantially lower [Ca2+]i to maintain its activity (7, 24). Although elevated [Ca2+]i has been observed following eccentric contractions (2, 18, 32), the increases were quite modest and we estimate that [Ca2+]i rises by ∼200–400 nM. Several proposal have been offered to explain this discrepancy. The localized [Ca2+]i near the stretch-activated channels will transiently be much higher than the mean [Ca2+]i and these localized elevations may initiate calpain autolysis. Another possibility is that the modest elevations of [Ca2+]i lead to some change in the regulation of calpain leading to activation, as proposed by Goll et al. (7).
Route of Ca2+ entry of following eccentric stretch.
The route of Ca2+ entry involved in the damage pathway has long been a source of interest. One possibility is that the high forces and stretch lead to membrane disruption or tears (21). Although this hypothesis is intuitively attractive, the increasing number of studies in which damage can be prevented by channel blocking drugs suggest that channel opening may be of greater importance. Muscle contains stretch-activated channels (9) that are Ca2+ permeable and have been implicated in stretch-induced muscle damage. Several groups have now shown that blockers of stretch-activated channels, which include streptomycin, can prevent various aspects of stretch-induced muscle damage (32, 39, 40).
However, the gene that encodes for stretch-activated channels remains controversial. Maroto et al. (19) provided strong evidence that TRPC1 encoded the nonspecific cationic stretch-activated channel; however, a later publication from the same group casts doubt on parts of the earlier work (8). Other studies have also implicated TRPC1 as a stretch-activated channel in mammalian muscle (35). However, in a recent study on TRPC1 KO mouse muscles, Zanou et al. (41) found that although a small conductance Ca2+ channel was absent in the KO, the stretch-activated channel was still present in the KO muscle, suggesting that TRPC1 does not encode the stretch-activated channel. Our data support the idea that TRPC1 encodes or modulates a Ca2+ permeable channel that contributes to the activation of calpains and subsequent events.
Conclusion.
The present study used pharmacological and genetic approaches to test the hypothesis that stretch-induced muscle damage involves Ca2+ entry from the extracellular space, activation of calpain, and cleavage of susceptible proteins. Our study showed that the loss of immunogenicity of cytoskeletal proteins following eccentric contractions was reduced in muscles treated with the stretch-activated channel blocker streptomycin and was also reduced in TRPC1 KO muscles. We also demonstrated that μ-calpain, but not the muscle-specific calpain-3, was activated following eccentric contractions. This suggests that μ-calpain may have been responsible for the reduction in immunogenicity following eccentric contractions. Titin is also a known target of calpain, and our results suggest that titin cleavage probably occurs after eccentric contraction, although we were unable to detect increased titin cleavage products under these conditions. These data support the idea that TRPC1 either encodes or contributes to the Ca2+ entry pathway triggered by eccentric contractions.
GRANTS
We acknowledge the support of a Endeavour Australia Cheung Kong Research Fellowship (to B. T. Zhang) and research funds from a National Health and Medical Research Council Program Grant and an Australian Research Council Discovery Grant (to D. G. Allen). The 9D10 monoclonal antibody developed by M. Greaser was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute for Child Health and Development and maintained by the University of Iowa.
Current address of B. T. Zhang: School of Chinese Medicine, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: B.T.Z., N.P.W., D.F., A.D., E.W.Y., and D.G.A. conception and design of research; B.T.Z., N.P.W., O.L.G., T.F.R., and M.V. performed experiments; B.T.Z., N.P.W., and T.F.R. analyzed data; B.T.Z., N.P.W., and D.G.A. interpreted results of experiments; B.T.Z., O.L.G., and T.F.R. prepared figures; B.T.Z., N.P.W., O.L.G., T.F.R., M.V., D.F., and D.G.A. edited and revised manuscript; B.T.Z., N.P.W., O.L.G., T.F.R., M.V., D.F., A.D., E.W.Y., and D.G.A. approved final version of manuscript; D.G.A. drafted manuscript.
REFERENCES
- 1. Allen DG. Eccentric muscle damage: mechanisms of early reduction of force. Acta Physiol Scand 171: 311–319, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Balnave CD, Allen DG. Intracellular calcium and force in single mouse muscle fibres following repeated contractions with stretch. J Physiol 488: 25–36, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barash IA, Peters D, Friden J, Lutz GJ, Lieber RL. Desmin cytoskeletal modifications after a bout of eccentric exercise in the rat. Am J Physiol Regul Integr Comp Physiol 283: R958–R963, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Chleboun GS, Howell JN, Conatser RR, Giesey JJ. Relationship between muscle swelling and stiffness after eccentric exercise. Med Sci Sports Exerc 30: 529–535, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Dietrich A, Kalwa H, Storch U, Mederos YS, Salanova B, Pinkenburg O, Dubrovska G, Essin K, Gollasch M, Birnbaumer L, Gudermann T. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflügers Arch 455: 465–477, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Fridén J, Sjöström M, Ekblom B. A morphological study of delayed muscle soreness. Experientia 37: 506–507, 1981 [DOI] [PubMed] [Google Scholar]
- 7. Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev 83: 731–801, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, Bowman C, Bichet D, Patel A, Sachs F, Martinac B, Hamill OP, Honore E. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflügers Arch 455: 1097–1103, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol 352: 685–701, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamill OP, McBride DW., Jr The pharmacology of mechanogated membrane ion channels. Pharmacol Rev 48: 231–252, 1996 [PubMed] [Google Scholar]
- 11. Horowits R, Kempner ES, Bisher ME, Podolsky RJ. A physiological role for titin and nebulin in skeletal muscle. Nature 323: 160–164, 1986 [DOI] [PubMed] [Google Scholar]
- 12. Hough T. Ergographic studies in muscular soreness. Am J Physiol 7: 76–92, 1902 [Google Scholar]
- 13. Jones DA, Newham DJ, Clarkson PM. Skeletal muscle stiffness and pain following eccentric exercise of the elbow flexors. Pain 30: 233–242, 1987 [DOI] [PubMed] [Google Scholar]
- 14. Kramerova I, Kudryashova E, Tidball JG, Spencer MJ. Null mutation of calpain 3 (p94) in mice causes abnormal sarcomere formation in vivo and in vitro. Hum Mol Genet 13: 1373–1388, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Lieber RL, Schmitz MC, Mishra DK, Friden J. Contractile and cellular remodeling in rabbit skeletal muscle after cyclic eccentric contractions. J Appl Physiol 77: 1926–1934, 1994 [DOI] [PubMed] [Google Scholar]
- 16. Lieber RL, Thornell LE, Friden J. Muscle cytoskeletal disruption occurs within the first 15 min of cyclic eccentric contraction. J Appl Physiol 80: 278–284, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Lovering RM, De Deyne PG. Contractile function, sarcolemma integrity, and the loss of dystrophin after skeletal muscle eccentric contraction-induced injury. Am J Physiol Cell Physiol 286: C230–C238, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lynch GS, Fary CJ, Williams DA. Quantitative measurement of resting skeletal muscle [Ca2+]i following acute and long-term downhill running in mice. Cell Calcium 22: 373–383, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol 7: 179–185, 2005 [DOI] [PubMed] [Google Scholar]
- 20. McCully KK, Faulkner JA. Characteristics of lengthening contractions associated with injury to skeletal muscle fibers. J Appl Physiol 61: 293–299, 1986 [DOI] [PubMed] [Google Scholar]
- 21. McNeil PL, Khakee R. Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. Am J Pathol 140: 1097–1109, 1992 [PMC free article] [PubMed] [Google Scholar]
- 22. Meyer GA, Kiss B, Ward SR, Morgan DL, Kellermayer MS, Lieber RL. Theoretical predictions of the effects of force transmission by desmin on intersarcomere dynamics. Biophys J 98: 258–266, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murphy RM, Goodman CA, McKenna MJ, Bennie J, Leikis M, Lamb GD. Calpain-3 is autolyzed and hence activated in human skeletal muscle 24 h following a single bout of eccentric exercise. J Appl Physiol 103: 926–931, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Murphy RM, Verburg E, Lamb GD. Ca2+-activation of diffusible and bound pools of μ-calpain in rat skeletal muscle. J Physiol 576: 595–612, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Newham DJ, Jones DA, Edwards RH. Plasma creatine kinase changes after eccentric and concentric contractions. Muscle Nerve 9: 59–63, 1986 [DOI] [PubMed] [Google Scholar]
- 26. Newham DJ, Mills KR, Quigley BM, Edwards RH. Pain and fatigue after concentric and eccentric muscle contractions. Clin Sci 64: 55–62, 1983 [DOI] [PubMed] [Google Scholar]
- 27. Ochi E, Nakazato K, Ishii N. Effects of eccentric exercise on joint stiffness and muscle connectin (titin) isoform in the rat hindlimb. J Physiol Sci 57: 1–6, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Raastad T, Owe SG, Paulsen G, Enns D, Overgaard K, Crameri R, Kiil S, Belcastro A, Bergersen L, Hallen J. Changes in calpain activity, muscle structure, and function after eccentric exercise. Med Sci Sports Exerc 42: 86–95, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Ramaswamy KS, Palmer ML, van der Meulen JH, Renoux A, Kostrominova TY, Michele DE, Faulkner JA. Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. J Physiol 589: 1195–1208, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raynaud F, Fernandez E, Coulis G, Aubry L, Vignon X, Bleimling N, Gautel M, Benyamin Y, Ouali A. Calpain 1-titin interactions concentrate calpain 1 in the Z-band edges and in the N2-line region within the skeletal myofibril. FEBS J 272: 2578–2590, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Sam M, Shah S, Friden J, Milner DJ, Capetanaki Y, Lieber RL. Desmin knockout muscles generate lower stress and are less vulnerable to injury compared with wild-type muscles. Am J Physiol Cell Physiol 279: C1116–C1122, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Sonobe T, Inagaki T, Poole DC, Kano Y. Intracellular calcium accumulation following eccentric contractions in rat skeletal muscle in vivo: role of stretch-activated channels. Am J Physiol Regul Integr Comp Physiol 294: R1329–R1337, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Talbot JA, Morgan DL. The effects of stretch parameters on eccentric exercise-induced damage to toad skeletal muscle. J Muscle Res Cell Motil 19: 237–245, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Trombitas K, Greaser M, French G, Granzier H. PEVK extension of human soleus muscle titin revealed by immunolabeling with the anti-titin antibody 9D10. J Struct Biol 122: 188–196, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Vandebrouck C, Martin D, Colson-Van Schoor M, Debaix H, Gailly P. Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J Cell Biol 158: 1089–1096, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Verburg E, Murphy RM, Richard I, Lamb GD. Involvement of calpains in Ca2+-induced disruption of excitation-contraction coupling in mammalian skeletal muscle fibers. Am J Physiol Cell Physiol 296: C1115–C1122, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Verburg E, Murphy RM, Stephenson DG, Lamb GD. Disruption of excitation-contraction coupling and titin by endogenous Ca2+-activated proteases in toad muscle fibres. J Physiol 564: 775–790, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Whitehead NP, Weerakkody NS, Gregory JE, Morgan DL, Proske U. Changes in passive tension of muscle in humans and animals after eccentric exercise. J Physiol 533: 593–604, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Willems ME, Stauber WT. Streptomycin and EDTA decrease the number of desmin-negative fibers following stretch injury. Muscle Nerve 32: 310–315, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Yeung EW, Whitehead NP, Suchyna TM, Gottlieb PA, Sachs F, Allen DG. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J Physiol 562: 367–380, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zanou N, Shapovalov G, Louis M, Tajeddine N, Gallo C, Van SM, Anguish I, Cao ML, Schakman O, Dietrich A, Lebacq J, Ruegg U, Roulet E, Birnbaumer L, Gailly P. Role of TRPC1 channel in skeletal muscle function. Am J Physiol Cell Physiol 298: C149–C162, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang BT, Yeung SS, Allen DG, Qin L, Yeung EW. Role of the calcium-calpain pathway in cytoskeletal damage after eccentric contractions. J Appl Physiol 105: 352–357, 2008 [DOI] [PubMed] [Google Scholar]