Abstract
The mechanisms by which intermittent pneumatic leg compression (IPC) treatment effectively treats symptoms associated with peripheral artery disease remain speculative. With the aim of gaining mechanistic insight into IPC treatment, the purpose of this study was to investigate the effect of IPC frequency on limb hemodynamics, vascular function, and skeletal muscle gene expression. In this two study investigation, healthy male subjects underwent an hour of either high-frequency (HF; 2-s inflation/3-s deflation) or low-frequency (LF; 4-s inflation/16-s deflation) IPC treatment of the foot and calf. In study 1 (n = 11; 23.5 ± 4.7 yr), subjects underwent both HF and LF treatment on separate days. Doppler/ultrasonography was used to measure popliteal artery diameter and blood velocity at baseline and during IPC treatment. Flow-mediated dilation (FMD) and peak reactive hyperemia blood flow (RHBF) were determined before and after IPC treatment. In study 2 (n = 19; 22.0 ± 4.6 yr), skeletal muscle biopsies were taken from the lateral gastrocnemius of the treated and control limb at baseline and at 30- and 150-min posttreatment. Quantitative PCR was used to assess mRNA concentrations of genes associated with inflammation and vascular remodeling. No treatment effect on vascular function was observed. Cuff deflation resulted in increased blood flow (BF) and shear rate (SR) in both treatments at the onset of treatment compared with baseline (P < 0.01). BF and SR significantly diminished by 45 min of HF treatment only (P < 0.01). Both treatments reduced BF and SR and elevated oscillatory shear index compared with baseline (P < 0.01) during cuff inflation. IPC decreased the mRNA expression of cysteine-rich protein 61 from baseline and controls (P <0 .01) and connective tissue growth factor from baseline (P < 0.05) in a frequency-dependent manner. In conclusion, a single session of IPC acutely impacts limb hemodynamics and skeletal muscle gene expression in a frequency-dependent manner but does not impact vascular function.
Keywords: mechanical compression, reactive hyperemia, flow-mediated dilation, cysteine-rich protein 61, connective tissue growth factor
intermittent pneumatic compression (IPC) therapy of the calf is a home-based and cost-effective treatment for patients with peripheral artery insufficiency that is regarded as a promising alternative to pharmacological and surgical interventions. Regular IPC application has been shown to promote increases in claudication distance (7, 10, 12, 40) as well as marked improvements in limb hemodynamics (10, 12, 13, 28), ulcer healing (20, 35), and rates of limb salvage in cases of critical limb ischemia (20, 35). Surprisingly, despite growing clinical acceptance and prescription, the fundamental physiological adaptations responsible for these remarkable benefits have not been prospectively examined. Further, few efforts have been made to optimize the design and application of the IPC device to maximize the clinical impact of this therapy for patients with claudication.
Based on the observations of improved postexercise but not resting limb hemodynamics (10), it has been proposed that changes in vasomotor function of the arteries in the lower limb constitute the central adaptation underlying the changes in exercise capacity (7, 20). One prevailing hypothesis is that the brief hyperemic response to each compression improves endothelial function by transiently enhancing shear stress (7, 20). Although attractive, this notion is largely based on anecdotal evidence since no studies to date have comprehensively examined the dynamic changes in blood flow (BF) and shear rate that occur during IPC application. Previous studies (43) from our group demonstrated that IPC application in the forearm promotes a highly oscillatory shear rate pattern with low net BF. This is an important observation since this hemodynamic profile has been strongly associated with impairments in endothelial function and a proatherogenic endothelial phenotype (6). If also true for the lower limbs, it is conceivable that acute exposure to IPC impairs rather than improves endothelial function in the lower limbs. To date, however, the impact of IPC application on endothelial function in humans is unknown.
An alternative hypothesis for the mechanistic basis behind the beneficial effects of IPC is that the forceful compressions promote local structural adaptations in the compressed tissue. This idea originated from the influential observations of Tan et al. (47) that a single session of leg compressions increases endothelial nitric oxide (eNOS) expression in the compressed skeletal muscle. Recently, we (42) have also documented in rats that leg IPC triggers marked increases in monocyte chemoattractant protein 1 and vascular endothelial growth factor (VEGF) expression. Given the key role of these factors in inflammation and vascular growth, these studies gave rise to the notion that IPC can possibly promote vascular remodeling in skeletal muscle. However, these initial studies were conducted in animal models and there is no available evidence of whether comparable changes in gene expression exist in similarly treated human skeletal muscle.
One critical observation from our studies in rodents was the fact that a higher frequency of compression evoked more consistent and robust changes in gene expression in the muscle compared with the frequency commonly employed in commercially available devices (42). This finding led us to hypothesize that more frequent compressions of the leg could possibly magnify the documented clinical benefits associated with this therapy in humans. However, a higher compression frequency would in theory further increase the oscillation in BF and shear rate triggered by this therapy and therefore potentially negatively impact endothelial function in the leg. Aiming to gain insights into these issues, we examined in the present study the impact of a single session of IPC applied at the clinically used low-frequency (LF; 3 compression/min) and high-frequency (HF; 12 compression/min) on the hemodynamic profile and skeletal muscle gene expression in healthy young volunteers. We hypothesized that HF compressions would yield a higher mean BF but transiently impair endothelial function due to increased oscillatory shear patterns. Additionally, we anticipated that the HF compressions would result in an increased expression of genes involved in inflammation and vascular remodeling.
METHODS
Experimental Design
The protocol consisted of two studies. In the first study, ultrasound/Doppler imaging was used to characterize 1) the hemodynamic profile in the popliteal artery during IPC application, and 2) the impact of a single IPC session on endothelial function. In the second study, messenger ribonucleic acid (mRNA) concentrations were assessed before and following a single session of IPC therapy in the treated and control limbs using quantitative polymerase chain reaction (Q-PCR). Experimental protocols were approved by the Purdue University Institutional Review Board and the University of Missouri Health Sciences Board. Written informed consent was obtained from all subjects before participation.
Subjects
Eleven male subjects participated in study 1, and nineteen male subjects participated in study 2. Physical characteristics of the subjects for each study are reported in Table 1. In both studies, subjects reported to the laboratory having fasted and abstained from caffeine and multivitamins for ≥4 h and from alcohol and exercise for ≥12 h before participation. After arriving at the laboratory, subjects were initially seated in a comfortable chair, received detailed instructions about the experimental procedures, and completed a medical and exercise history questionnaire. Exclusion criteria included current smoking, hypertension (resting blood pressure of ≥140/90 mmHg), symptoms of metabolic or cardiovascular disease, and medications. Additionally, physical fitness was considered to be a potential confounding factor and subjects reporting regular exercise (defined as regular participation in exercise ≥2 days/wk) were excluded.
Table 1.
Subject characteristics
|
Study 2 |
|||||
|---|---|---|---|---|---|
| Study 1 (n = 11) | Control (n = 5) | HF (n = 7) | LF (n = 7) | Total (n = 19) | |
| Age | 23.5 ± 4.7 | 19.8 ± 1.9 | 23.1 ± 5.8 | 22.4 ± 4.8 | 22.0 ± 4.6 |
| BMI, kg/m2 | 23.5 ± 3.2 | 24.7 ± 2.7 | 25.0 ± 3.6 | 24.7 ± 5.3 | 24.7 ± 3.8 |
Data are means ± SD; HF, high-frequency treatment group; LF, low-frequency treatment group; BMI, body mass index.
IPC Device
A clinically used apparatus (ArtAssist 1000; ACI Medical, San Marcos, CA) was used to administer LF IPC to subjects in both studies. This device delivers three compressions per minute (3-s inflation/17-s deflation) at a pressure of 120 mmHg to the foot then calf with a 0.5-s delay. To provide HF IPC, the device was modified to deliver 12 compressions per min (2-s inflation/3-s deflation) at the same pressure and delay.
Study 1
Protocol.
Study one was a randomized, paired design in which the treatment leg of each subject was exposed to the HF or LF treatment on 2 separate days. The contralateral leg served as an internal control. The time of day was similar between visits (visit 1: 11:28 AM ± 52 min; visit 2: 12:06 PM ± 69 min), and the treatment leg was consistent for each subject on both visits. Following completion of informed consent and medical/exercise history questionnaire, subjects were instructed to lay prone for 30 min. Resting blood pressure and heart rate were obtained using an automatic monitoring system (Suntech Tango; Suntech Medical, Morrisville, NC). A reactive hyperemia (RH) and flow-mediated dilation (FMD) measurement was performed on both the control and treatment legs in a randomized order. The subject was then transferred to a chair, and the IPC cuff was fitted on the intervention foot and leg. After 5 min of upright sitting, baseline hemodynamics were measured in the popliteal artery of the intervention limb. The subject then underwent IPC treatment at the designated frequency for 1 h. Hemodynamics were measured within the first 5 min and again at 45 min of treatment. Upon the completion of the compression session, the subject was transferred back to the examination table in the prone position for the posttreatment RH and FMD measurements. To detect transient changes in vascular function, the posttreatment order was not randomized; the intervention leg was always measured first after termination of treatment (occlusion occurred within 3.5 min on average). The same experienced sonographer completed all ultrasound/Doppler measurements in this investigation.
Reactive hyperemia.
During the 30-min acclimatization period, a cuff (Model SC5; Hokanson, Bellevue, WA) was fitted on the leg immediately distal to the tibial tuberosity and attached to a rapid inflation/deflation device (Hokanson). The popliteal artery was then imaged at 2–10 cm proximal to the popliteal fossa using a 5- to 12-MHz multifrequency linear array transducer attached to a high resolution ultrasound/Doppler system (Terason T3000; Teratech, Burlington, MA). Doppler velocity was measured with an isonation angle of 60° and sample volume was maximized to inner-lumen diameter. Transducer and cuff positions were marked to ensure consistent placement on subsequent measurements. Baseline vessel diameter and blood velocity were recorded continuously for 30 s, at which point the leg was occluded for 10 min by inflating the cuff to 220 mmHg. During occlusion, recording was paused but transducer placement was maintained to ensure image consistency. Recording was resumed within 10 s before cuff release and diameter and RH blood velocity were subsequently recorded continuously for 3 min following cuff deflation. Additionally, blood pressure and heart rate were noted within the first 10 s following cuff release.
Three-second rolling averages of vessel diameter and blood velocity were calculated at baseline and throughout the RH period. FMD, an indicator of endothelial function, was defined as the 3-s average diameter with the largest percent change from baseline diameter. BF (ml/min) was then calculated at 3-s intervals using the following formula:
where BV is blood velocity in cm/s, D is vessel diameter in cm, and 60 is used to convert to ml/min. Peak RH blood flow (RHBF) was considered to be the highest 3-s mean flow following cuff release. Postocclusions BF was calculated by summing the volume of BF during each 3-s interval for 2 min and was used to quantify the entire reactive hyperemic response. To account for possible changes in mean arterial pressure [MAP = diastolic + (systolic − diastolic)/3], vascular conductance was calculated as RHBF/MAP.
Hemodynamic profile during compressions.
With the subject seated upright and the treatment leg partially extended with the femur parallel with the ground and heel supported 4–6 cm above the floor, the popliteal artery was imaged at the same location marked during the RH measurement using the same ultrasound/Doppler system and settings described above. Measurements were taken at baseline, within the first 5 min of treatment, and again at 45 min of treatment. In an effort to minimize the motion artifact inherent to imaging in close proximity to the compression cuff, the technician's hand was stabilized by placing the wrist and forearm on an adjacent stationary object. Recording was maintained until at least four complete inflation/deflation cycles were recorded where both lumen diameter and velocity profile were clearly visible for the entire cycle. The calculations performed below used the mean of four cycles at a given time point. The small variation in vessel diameter (see Table 3) is an indication of the reliability of this method.
Table 3.
Mean antegrade and retrograde shear rate responses at baseline and during IPC treatment
| Baseline |
Minute 5 |
Minute 45 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inflation |
Deflation |
Inflation |
Deflation |
|||||||
| HF | LF | HF | LF | HF | LF | HF | LF | HF | LF | |
| Diameter, mm | 6.3 ± 0.2 | 6.4 ± 0.2 | 6.3 ± 0.2 | 6.4 ± 0.2 | 6.3 ± 0.2 | 6.4 ± 0.2 | 6.3 ± 0.2 | 6.3 ± 0.2 | 6.3 ± 0.2 | 6.3 ± 0.2 |
| MASR, s−1 | 175 ± 111 | 185 ± 13 | 313 ± 20* | 332 ± 29* | 434 ± 31*† | 338 ± 38* | 256 ± 17*§ | 279 ± 19*§ | 253 ± 15*‡ | 249 ± 22*‡ |
| MRSR, s−1 | −66.8 ± 2 | −71.7 ± 5.2 | −167 ± 11* | −161 ± 9.1* | −71.0 ± 11.1* | −81.7 ± 6.2* | −142 ± 8.1* | −133 ± 8.7* | −62.5 ± 4.3* | −61.2 ± 4.5* |
Data are means ± SE; n = 11. IPC, intermittent pneumatic leg compression; MASR, mean antegrade shear rate; MRSR, mean retrograde shear rate.
P < 0.01, different from baseline.
P < 0.05, different from LF.
P < 0.01, different from minute 5.
P < 0.05, different from minute 5.
To characterize the hemodynamic profile during compressions, peak antegrade, peak retrograde, and mean blood velocity were determined for both the inflated and deflated phases of each treatment and at each time point. The beginning and end of each phase were identified offline by examining the velocity tracing for nonphysiologic disturbances in flow (Fig. 1). The temporal length of each phase was measured for each observation to validate this method and ensure consistency. Vessel diameter was also measured. These variables were used to calculate BF, shear rate, and oscillatory shear index (OSI). Mean blood flow (MBF) during compressions was determined as follows:
where the total volume of BF during a single phase is TBV, i is inflation, d is delfation, and t / 60 represents the proportion of one min that the cuff is in each phase. Shear rate (SR, s−1), a useful estimator of shear stress that does not account for blood viscosity, was calculated using the formula SR = 4Vb / D, where Vb is blood velocity and D is vessel diameter (36). OSI is a dimensionless variable that ranges from 0 to 0.5, where zero is strictly antegrade flow and 0.5 is purely oscillatory (38). It was calculated as follows (38):
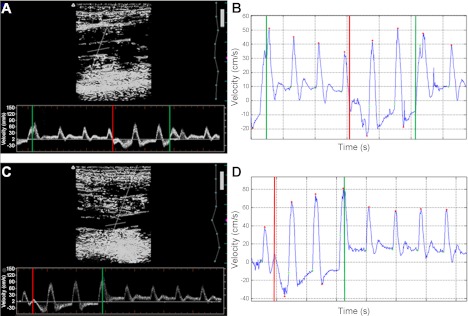
Fig. 1.
Representative samples of ultrasound/Doppler recordings and MATLAB output during high-frequency (HF; A and C, respectively) and low-frequency (LF; C and D, respectively) treatment. Cuff inflation and cuff deflation are represented by red lines and green lines, respectively.
It is important to note that OSI is typically used to quantify the oscillations in shear that the vessel experiences on average during each cardiac cycle. Due to the aperiodic nature of the blood velocity that results from IPC, the “peak” OSI (pOSI) was determined using the peak antegrade and peak retrograde shear rates experienced during an entire phase. Therefore, pOSI does not necessarily represent the oscillatory nature of a single cardiac cycle but rather the maximum oscillation throughout each inflation or deflation phase.
Data analysis.
Analysis of both RH and during compression hemodynamics was completed offline by the same blinded, experienced technician. Diameter was assessed using commercially available software (brachial analyzer; Medical Imaging Applications, Coralville, IA) that automatically detects the inner-lumen diameter within a user-defined region of interest. The user is able to manually select the wall borders, although this practice was kept at a minimum to ensure consistency. Blood velocity was measured using a custom designed MatLab (Mathworks, Natick, MA) program that has been described and validated elsewhere (38, 43). Briefly, this program finds the average of all pixels of a given intensity range within a column of pixels in the Doppler velocity sweep so that each one-pixel width column provides one data point. This yields 100 data points per second of recorded data and allows for the determination of mean overall, mean antegrade, mean retrograde, peak antegrade, and peak retrograde velocities of each cardiac cycle. In the case of the during compression hemodynamics, this software enables the user to manually define the beginning and end of each phase.
Study 2
Protocol.
In study 2, 21 individuals were randomly allocated to one of three groups: HF, LF, and sham controls. Following 25–30 min of seated rest, the participants were positioned prone in a bed and the first biopsy was taken from the lateral gastrocnemius of the leg. Once recovered from the procedure, the subjects moved back to the chair and had the compression cuff firmly wrapped around the nondominant leg and foot. Compressions were applied continuously for 1 h following the protocol specification detailed above. Subjects in the sham-control group had the cuffs placed in their legs but did not undergo the treatment. Thirty and 150 min following the completion of the IPC session the biopsy procedure was repeated.
Muscle biopsies.
Biopsies were taken from the lateral gastrocnemius using a modified Bergstrom needle. While the subjects were under local anesthesia (3–5 ml of lidocaine), a small incision (∼1 cm) was made in the skin and muscle fascia and ∼30–50 mg of tissue were sampled. Subsequent biopsies were taken at sites separated by 2–3 cm. The specimens were promptly placed in RNA stabilization reagent (RNAlater; Ambion), incubated at 4°C for up to 48 h, and then stored at −80°C until further processing for RT-PCR analysis.
RNA isolation and RT-PCR analysis.
Approximately 30 mg of the muscle tissue were homogenized in a lysing solution (Buffer RLT; Qiagen, Valencia, CA) containing 14.3 M β-mercaptoethanol using a tissue homogenizer (Fisher Scientific, Waltham, MA) as previously described (42). Total RNA was isolated using an RNeasy fibrous tissue mini kit (Qiagen) and assayed using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE) to assess purity and concentration. First-strand cDNA was synthesized from total RNA by reverse transcription primed by a mixture of random hexamer and oligo (dT) primers (iScript cDNA synthesis kit; Bio-Rad, Hercules, CA). The reactions were incubated in a PCR Express Hybaid thermal cycler (Hybaid, Franklin, MA). Quantitative real-time PCR was performed using the ABI PRISM 7000 sequence detection system (Applied Biosystems, Foster City, CA). Primers for each target were purchased from IDT (Coralville, IA). Sequences used are outlined in Table 2. A 25-μl reaction mixture containing 24 μl of Power SYBR Green PCR MasterMix (Applied Biosystems) and the appropriate concentrations of gene-specific primers plus 1 μl of cDNA template was loaded in each well of a 96-well plate (duplicate samples). PCR was performed with thermal conditions as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. A dissociation curve analysis was performed after each run to verify the identity of the PCR products. The comparative cycle threshold (Ct) method was utilized to calculate the changes in expression of each target mRNA (32).
Table 2.
Primers
| Forward | Reverse | Reference | |
|---|---|---|---|
| VEGF | CTTGCTGCTCTACCTCCACCAT | ATGATTCTGCCCTCCTCCTTCT | (19) |
| eNOS | CGGCATCACCAGGAAGAAGA | CATGAGCGAGGCGGAGAT | (15) |
| MCP-1 | GCTGACCCCAAGCAGAAGTG | TCTTCGGAGTTTGGGTTTGC | (55) |
| CXCL12 | CCTGAGCTACAGATGCCCATG | TGAGATGCTTGACGTTGGCT | (21) |
| CYR61 | TGTGAAGAAATACCGGCCCA | AATGTCTCCCCATCTTCGCAG | (49) |
| CTGF | CCCTGCATCTTCGGTGGTA | GGCACGTGCACTGGTACTTG | (46) |
| PGC-1α | CAAGCCAAACCAACAACTTTATCTCT | CACACTTAAGGTGCGTTCAATAGTC | (39) |
| CXCL1 | AACCCCAAGTTAGTTCAATCTGGA | CATGTTGCAGGCTCCTCA GAA | (1) |
| IL-6 | AATTCGGTACATCCTCGACGG | GGTTGTTTTCTGCCAGTGCCT | (44) |
| GAPDH | CAGAACATCATCCCTGCCTCTA | CCAGTGAGCTTCCCGTTCA | (33) |
eNOS, endothelial nitric oxide synthase; MCP-1, monocyte chemoattractant protein-1; CXCL, chemokine C-X-C motif ligand; CYR61, cysteine-rich protein 61; CTGF, connective tissue growth factor; PGC-1α; proliferator-activated receptor-coactivator-α.
Statistical Analysis
Analysis was done using the MIXED procedure from the software SAS v9 (SAS Institute, Cary, NC). Initial analysis was done including all interaction terms with simpler models considered if interaction terms were decidedly nonsignificant. To account for the dependencies inherent in taking multiple measurements on the same individual, the subject was treated as a random effect in a mixed model ANOVA. Residuals from the fitted models were examined to determine if the assumption of normally distributed error terms was reasonable. Where the normality assumption was questionable, nonparametric methods appropriate for data collected in blocks were used. To account for the large heterogeneity of variances of the during compression hemodynamic measurements, the MIXED procedure in SAS v9 was used with REPEATED statement and appropriate options. This procedure also accounts for the fact that each subject is measured under all conditions. Significance level was set at α < 0.05. Data are presented as means ± SE unless otherwise noted.
RESULTS
Hemodynamics During Compressions
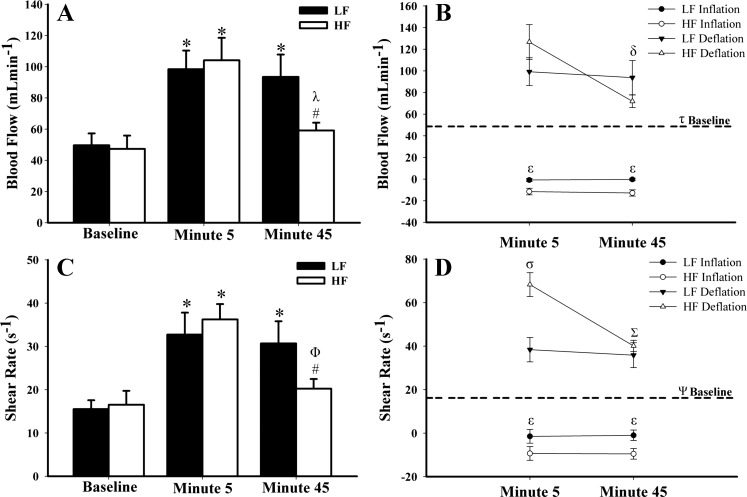
Hemodynamic responses to IPC treatment are displayed in Table 3. During the first 5 min of IPC application, HF and LF treatment frequencies resulted in a roughly twofold increase in mean BF compared with baseline (P ≤ 0.01; Fig. 2A). However, HF BF significantly decreased by 45 min and was no longer statistically different from baseline BF. Figure 2B demonstrates that this decrease in HF BF was driven by a significant decrease in BF during the deflation phase. Inflation BF was significantly lower throughout HF compared with LF treatment (P < 0.01; Fig. 2B). No changes in mean or phase BF responses were observed between 5 and 45 min during LF treatment.
Fig. 2.
Mean and phase blood flow and shear rate responses at baseline and during intermittent pneumatic leg compression (IPC) application. Mean blood flow and shear rate during each treatment are represented in A and C, respectively. Mean blood flow and shear rate responses during each phase are represented in B and D, respectively. Dashed horizontal line in B and D represents grouped baseline values for each treatment. *P < 0.01, different from baseline ; λP < 0.001, different from minute 5; #P < 0.05, different from LF minute 45; ΦP < 0.01, different from minute 5; δP < 0.01, HF deflation minute 45 different from HF deflation minute 5; εP < 0.01, LF inflation different from HF inflation; τP < 0.0001, all treatments at all time points and phases significantly different from baseline except HF deflation minute 45 (P = 0.08); σP < 0.0001, HF deflation different from LF deflation; ΣP < 0.0001, HF deflation minute 45 different from HF deflation minute 5; ΨP < 0.0001, all treatments at all time points and phases different from baseline.
Shear rate within the first 5 min of treatment was significantly greater in HF than LF (P < 0.01; Fig. 2C). Similar to BF, HF shear rate decreased significantly by 45 min (Fig. 2C) and was driven by a decrease in shear rate during the deflation phase (Fig. 2D). Retrograde shear was more negative during inflation throughout HF treatment compared with LF treatment.
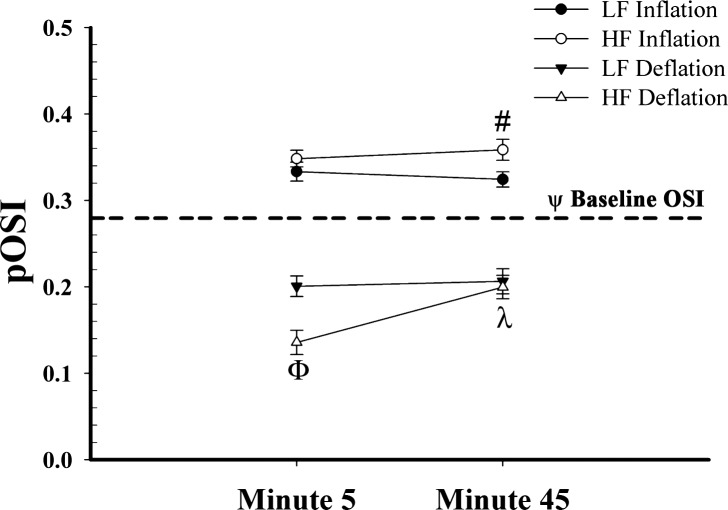
Peak OSI responses to IPC are illustrated in Fig. 3. Both treatments yielded a pOSI that was significantly higher during inflation and significantly lower during deflation (P < 0.005) than baseline OSI. By 45 min of HF treatment, pOSI during deflation was significantly increased compared with 5 min (P < 0.0001) and the pOSI during inflation was significantly higher than LF (P < 0.02).
Fig. 3.
Peak oscillatory shear index (pOSI) responses to IPC application. Dashed horizontal line represents grouped baseline OSI for both treatments. ΨP < 0.01, all treatments at all time points and phases significantly different from baseline. ΦP < 0.0001, HF deflation different from LF deflation; #P < 0.05, HF inflation different from LF inflation; λP < 0.0001, HF deflation minute 45 different from HF deflation minute 5.
Vascular Function
The reactive hyperemia measurements of one subject were excluded due to poor image quality. Thus 10 subjects (n = 10) were included in the analysis of vascular function. Baseline diameter, velocity, and BF and postocclusion FMD, peak velocity, peak BF, MAP, and conductance are displayed in Table 4. No changes were observed in FMD. Despite a decrease in RHBF and 2-min BF in the treated limbs following treatment, no treatment effect was detected due to a similar response in the control limbs.
Table 4.
Reactive hyperemia variables pre- and posttreatment
| HF |
LF |
|||||||
|---|---|---|---|---|---|---|---|---|
| Treated Limb |
Control Limb |
Treated Limb |
Control Limb |
|||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Baseline variables | ||||||||
| Diameter, mm | 6.36 ± 0.20 | 6.33 ± 0.23 | 6.35 ± 0.20 | 6.32 ± 0.18 | 6.35 ± 0.19 | 6.19 ± 0.18 | 6.39 ± 0.18 | 6.35 ± 0.21 |
| Velocity, cm/s | 4.01 ± 0.59 | 3.16 ± 0.37†‡ | 3.82 ± 0.55 | 1.33 ± 0.20* | 4.21 ± 0.49 | 3.30 ± 0.42†‡ | 3.52 ± 0.46 | 1.79 ± 0.38* |
| Blood flow, ml/min | 77.23 ± 14.80 | 57.21 ± 7.54†‡ | 75.53 ± 12.62 | 26.15 ± 4.33* | 82.61 ± 12.65 | 58.59 ± 8.5†‡ | 69.05 ± 11.31 | 28.76 ± 6.25* |
| Reactive hyperemia variables | ||||||||
| FMD | 2.45 ± 0.50 | 2.76 ± 0.32 | 2.82 ± 0.45 | 2.91 ± 0.46 | 3.16 ± 0.36 | 3.56 ± 0.52 | 3.05 ± 0.43 | 2.68 ± 0.54 |
| Peak velocity, cm/s | 48.71 ± 3.61 | 42.91 ± 2.20† | 48.51 ± 2.46 | 41.07 ± 1.73* | 44.93 ± 1.80 | 40.16 ± 2.71 | 40.88 ± 2.68 | 37.56 ± 2.43 |
| Peak blood flow, ml/min | 938.6 ± 98.5 | 820.8 ± 60.2† | 918.8 ± 44.7 | 772.1 ± 43.3† | 852.6 ± 53.7 | 724.0 ± 50.9† | 788.9 ± 57.0 | 729.0 ± 75.1 |
| 2-min blood flow, ml | 1201.7 ± 142.3 | 949.9 ± 72.2* | 1118.9 ± 57.8 | 801.7 ± 74.4* | 1119.3 ± 68.2 | 877.8 ± 63.3* | 1017.7 ± 104.1 | 781.1 ± 72.6* |
| MAP, mmHg | 77.60 ± 2.02 | 82.83 ± 1.41* | 82.03 ± 1.69 | 84.37 ± 2.07 | 76.97 ± 2.16 | 80.63 ± 1.92† | 77.77 ± 2.14 | 81.33 ± 1.57* |
| Conductance, ml · mmHg−1 · min−1 | 12.50 ± 1.53 | 9.84 ± 0.86† | 11.32 ± 0.74 | 9.68 ± 0.67* | 11.56 ± 0.82 | 9.24 ± 0.71* | 10.50 ± 0.88 | 9.42 ± 0.86 |
| Heart rate, beats/min | 64.8 ± 3.4 | 64.2 ± 3.1 | 63.2 ± 3.7 | 63.7 ± 3.9 | 65.3 ± 2.9 | 66.9 ± 2.9 | 67.4 ± 3.5 | 69.1 ± 3.9 |
Data are means ± SE; n = 10. FMD, flow-mediated dilation; MAP, mean arterial pressure.
P < 0.01, different from preintervention.
P < 0.05, different from preintervention.
P < 0.01, different from control.
Gene Expression
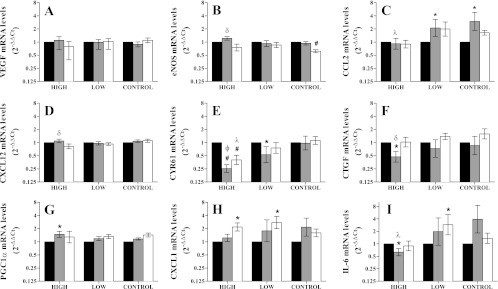
The results for two control subjects were excluded due to difficulties in obtaining a quality biopsy in one subject and misinformation provided by the other. Thus the control group represents five subjects (n = 5). Skeletal muscle mRNA concentrations were assessed at baseline and at 30 and 150 min posttreatment in subjects exposed to cuff placement only (controls), HF IPC, and LF IPC. All changes are expressed as relative change from baseline levels. Decreased cysteine-rich protein 61 (CYR61) mRNA concentrations were observed following HF IPC compared with baseline (P < 0.01) and control (P < 0.05) at both 30 and 150 min, whereas LF IPC elicited a more modest decrease that was only significantly different from baseline at 30 min (P < 0.05; Fig. 4E). Connective tissue growth factor (CTGF) mRNA concentrations decreased significantly compared with baseline at 30 min after HF IPC (P < 0.05), whereas LF IPC resulted in no changes (Fig. 4F). No significant changes were revealed in VEGF expression (Fig. 4A). eNOS was significantly higher in HF at 30 min compared with 150 min (P < 0.01), and there was a decrease observed in the controls at 150 min compared with baseline (P < 0.01; Fig. 4B). CCL2 was decreased in HF at 30 min (P < 0.05) but elevated at 30 min in LF and controls compared with baseline (P < 0.05; Fig. 4C). Chemokine C-X-C motif ligand (CXCL)12 mRNA concentrations were elevated (P < 0.05) compared with 150 min in HF only (Fig. 4D). HF IPC increased proliferator-activated receptor-coactivator-α expression at 30 min compared with baseline (P < 0.01; Fig. 4G). A significant increase in CXCL1 expression was observed at 150 min following both LF and HF treatments but not in the controls (P < 0.05; Fig. 4H). Concentration of IL-6 mRNA decreased at 30 min following HF treatment compared with baseline and controls (P < 0.05) and was elevated following LF at 150 min compared with baseline (P < 0.05; Fig. 4I).
Fig. 4.
Relative change from baseline (black bars) in mRNA concentrations at 30 (grey bars) and 150 min (white bars) post-IPC treatment of vascular endothelial growth factor (VEGF; A), endothelial nitric oxide synthase (eNOS; B), chemokine C-C motif ligand 2 (CCL2; C), chemokine C-X-C motif ligand 12 (CXCL12; D), cysteine rich protein 61 (CYR61; E) and connective tissue growth factor (CTGF; F), PPAR γ coactivator-1α (PGC-1α; G), chemokine C-X-C motif ligand 1 (CXCL1; H), and interleukin-6 (IL-6; I).*P < 0.05, different from baseline (P < 0.05); #P < 0.01, different from baseline; фP < 0.01, different from controls; λP < 0.05, different from controls; δP < 0.05, different from 150 min.
DISCUSSION
Extensive evidence supports the efficacy of IPC therapy in the treatment of symptoms associated with peripheral artery disease (7, 8, 10, 12, 28, 40, 52, 53). Surprisingly, the physiologic consequences of this treatment have not been examined and, as a result, it is unknown whether the existing IPC paradigm is optimized for clinical outcomes. To this end, we sought to describe in humans the physiologic response to a single bout of IPC treatment. The results of this investigation revealed three novel findings. First, IPC treatment results in periodic alternation between hyperemia during cuff deflation and a high oscillatory/low net shear rate pattern during cuff inflation. Consistent with our hypothesis, HF treatment causes both a greater magnitude and occurrence of oscillatory shear compared with LF. Second and contrary to our hypothesis, IPC does not appear to acutely affect endothelial function as assessed by FMD and/or RHBF. Third, we observed in the compressed skeletal muscle a frequency-dependent modulation of the expression of some genes (CYR61 and CTGF) but not others.
Hemodynamic Profile During IPC Application
The hyperemic response to cuff deflation during IPC has been well described (10, 11, 13, 28, 29). However, this is the first study to 1) document shear rate during IPC treatment, 2) describe the BF response to both phases of treatment, and 3) determine the time course of these local hemodynamic responses. Our results indicate that IPC elevates both shear rate and BF and lowers pOSI during cuff deflation whereas cuff inflation has the opposite effect. Further, HF IPC results in a reduction in mean BF and shear rate by 45 min of treatment to a point no longer statistically different from baseline BF.
Elevated shear stress is known to acutely induce the release of vasodilatory substances such as nitric oxide and prostacyclin (24, 50) from the endothelium and regular exposure imparts atheroprotection by altering endothelial cell phenotype (34). However, previous reports of the hemodynamic response to IPC treatment have failed to take into account the consequences of cuff inflation. The results from the current study indicate that cuff inflation intermittently exposes the vasculature to a low net/high oscillatory shear profile. This is consistent with previous work in rodents (42) and in the forearm of humans (43). Acute exposure to low net/ high oscillatory shear pattern has been reported to impair endothelial cell function (17, 48, 57), increase the expression of genes associated with inflammation and cell adhesion (16, 17), and induce vasoconstriction via increased endothelin-1 expression (57). IPC frequency had a profound impact on the extent to which the vasculature is exposed to this detrimental shear profile. HF IPC resulted in a greater pOSI and lower net shear during inflation, and this occurred for a greater proportion of every min during HF treatment (24 s/min) compared with LF treatment (9 s/min). Thus it is important to recognize that any adaptations to IPC therapy resulting from hemodynamic alterations during treatment represent a balance between the opposing effects of these two distinct hemodynamic profiles.
The importance of the balance between high and low/oscillatory shear is supported by the unexpected observation that BF and shear rate were considerably decreased from 5 to 45 min of HF IPC treatment. This decrease appears to have been driven solely by attenuation of blood velocity during the deflated phase. One potential explanation is that elevated exposure of the vasculature to low and oscillatory shear during HF treatment induced vasoconstriction in the resistance vessels. That a similar effect was not observed during LF treatment possibly indicates that the increased temporal exposure to high shear during deflation was sufficient to counteract the vasoconstrictive tendencies of low/oscillatory shear during inflation. Further examination is warranted to determine the precise mechanism responsible for this observation.
Acute Effects of IPC on FMD and Reactive Hyperemia BF
Enhanced endothelial function secondary to increased hemodynamic shear is thought to be a primary adaptation to IPC treatment (3, 7, 10, 53). Reports (10) of increased postexercise ankle-brachial index but not resting ankle-brachial index following treatment certainly lend support to this theory. Yet surprisingly, IPC induced alterations in endothelial function have not been directly measured. As discussed above, the endothelium is highly adaptive to the hemodynamic environment and exposure to oscillatory shear for as little as 30 min can acutely impair its function (48). In the current study, endothelial function was assessed in 1) the popliteal artery proximal to the compression site via FMD, and 2) the downstream vasculature via RHBF following an hour of IPC therapy. RHBF is indicative of the capacity of the downstream vasculature to accommodate flow and is used to assess structural and vasodilatory adaptations to training protocols (43, 45). In the present study, we reasoned that a 1-h intervention is unlikely to induce structural changes and, therefore, we interpreted changes in RHBF to indicate transient alterations in vasodilatory ability of the downstream vasculature. Despite increased exposure to a detrimental shear profile and contrary to our hypothesis, IPC did not transiently impair vascular function (Table 4). This finding can be interpreted in two ways. First, upright posture chronically exposes the leg vasculature to increased hydrostatic pressure and OSI that appears to confer a resistance to temporary changes in vasomotor function (37). If this is the case, then a chronic repeated stimulus may be required to alter endothelial function after IPC therapy. Second, a balance may exist due to the alternation between hyperemic and oscillatory hemodynamic responses to each phase of treatment, thus resulting in no net effect on vascular function. Nonetheless, endothelial function needs to be examined as an outcome variable to chronic IPC therapy to determine definitively if endothelium-dependent dilation is a key adaptation to treatment.
Although there was no effect of IPC treatment on RHBF, an interesting and unexpected result of these measurements warrants mention. Following IPC treatment, there was a significant decrease in baseline BF and velocity in both the treated and control limbs. This appears to be maintained throughout the reactive hyperemia period following cuff occlusion. Additionally, that MAP increased and conductance decreased following treatment while heart rate remained unchanged may suggest that local increases in leg vascular resistance contributed to the increase in MAP without sympathetic engagement. Although speculative, it is possible that this provides evidence of a local adaptive mechanism to maintain blood pressure during upright posture. Interestingly, similar observations were not made by Padilla et al. (37) following 3 h of upright sitting. The reasons for this discrepancy are not immediately clear. Further, the decrease in baseline BF was measured in the control limb roughly 20 min after the hydrostatic stimulus was removed. This is an important observation since 20–30 min is used as the acclimatization period for subjects to reach a baseline state in many investigations, including the current one.
IPC Frequency Differentially Effects Skeletal Muscle Gene Expression
Our previous work in rodents (42) led us to test the hypothesis that IPC would induce local alterations in skeletal muscle gene expression in humans. We selected genes that are both known to impact either inflammation or vascular remodeling and display evidence of being rapidly expressed in response to mechanical stimuli. The novel findings were 1) that IPC transiently alters gene expression of the compressed tissue in a frequency-dependent manner, and 2) that our results are inconsistent with other models previously used to investigate the impacts of IPC on gene expression.
The most robust changes in mRNA concentrations in response to IPC treatment arose from the CCN gene family: CYR61/CCN1 and CTGF/CCN2 (Fig. 4). Elevated mRNA concentrations of CYR61 and CTGF have been reported 30 min after an exercise bout with high mechanical load (23). Additionally, chronically elevated expression of these genes is characteristic of certain pathologies that involve neovascularization such as atherosclerosis and some metastatic cancers. From these observations, we anticipated that IPC treatment would locally enhance CYR61 and CTGF expression. However, CYR61 and CTGF mRNA concentrations following HF IPC were robustly decreased from baseline and CYR61 was also decreased compared with controls. LF treatment yielded a more modest decrease in CYR61 and no significant effect on CTGF expression.
The precise reasons for these discrepant findings are elusive. CYR61 and CTGF play a significant regulatory role in the angiogenic process through chemotactic signaling of vascular endothelial cell migration and adhesion in the extracellular matrix (27). Additionally, these genes regulate the transcription and posttranscriptional processing of other important angiogenic factors. For example, VEGF expression is directly related to concentrations of CYR61 protein (56), whereas CTGF forms a complex with VEGF that renders it ineffective (18). Due to the complex regulatory role of CYR61 and CTGF in vascular remodeling (for review, see Ref. 2), the implications of the transient decrease of the expression of these genes observed in the current investigation are unclear. One possibility is that IPC elicits a hormetic response such that repeated exposure to an acute down-regulation results in a compensatory chronic upregulation.
This is the first investigation to examine gene expression responses to external limb compression in humans. A noteworthy observation is that our results are largely contradictory to previous work in both rodents and in vitro cultured human umbilical vein endothelial cells. We have previously reported in rodents that 2.5 h of high frequency (2-s inflation/2-s deflation, 120 mmHg) IPC increases the relative expression of VEGF and CCL2 compared with both low (4 s/16 s, 120 mmHg) frequency and control groups (42). Tan et al. (47) reported a 180% increase in eNOS mRNA in the hindlimbs of rodents exposed to IPC at a low rate of compression (5-s inflation/25-s deflation) at 55 mmHg. Inherent experimental differences between rodents and humans (i.e., rodents must be anesthetized during treatment) or species differences (i.e., 120 mmHg is not the same stimulus between species) may account for our dissimilar findings. Further, Dai et al. (5) reported a twofold increase in eNOS mRNA concentration in cultured human umbilical vein endothelial cells in response to an in vitro system designed to mimic external limb compression by applying both tube compression and pulsatile shear stress for 5 s once per minute. Other stimuli present during IPC, such as low net/oscillatory shear during cuff inflation, were neither applied nor considered. Our conflicting results highlight that the many stimuli of IPC need to be considered collectively and that translating observations in animal and in vitro IPC models to in vivo responses in humans must be done cautiously.
Impact of Compression Frequency
Commercially available IPC devices typically apply pressures ranging from 65 to 120 mmHg at a cycle rate of three compressions per minute (30). The design of these pumps appears to be based on two major premises: first, it is believed that the hyperemic response during the deflation phase is the primary mechanism underlying the clinical benefits associated with this therapy (9, 10, 29). Second, it has been hypothesized that the enhanced arterial-venous pressure difference during IPC application is the major driving force for the temporary increases in BF (10). Thus the aforementioned pressures and timing of compressions were chosen to provide optimal emptying of the venous circulation and maximize the hyperemia following each compression cycle (9). Although insightful, the design of the current IPC application protocols ignores the fact that the change in arterial-venous pressure during compressions only partially explains the hyperemic response during the deflation phase of IPC (54). In fact, it is now known that a fast vasodilatory response ensues after compression of a limb irrespective of the venous pressure (22, 51). Further, the design of these pumps does not take into account that factors other than BF could also modulate the adaptations to this therapy. Alterations in circumferential wall strain as well as the direct impact of forceful compressions on skeletal muscle (41) are a few of a vast array of mechanical events that could underlie the benefits associated with IPC. If both active vasodilation and other mechanical forces are also important, it is conceivable that more frequent compressions could provide superior benefits compared with the currently employed protocols. In that regard, this is the first study in humans to examine the impact of compression frequency on clinically relevant outcomes. Taken together, our findings encourage additional studies aimed at defining the optimal protocols of application for IPC therapy.
Potential Systemic Mechanism
The focus of the current study was to examine acute local responses in the treated limb. However, evidence exists that indicates that external limb compression is capable of inducing systemic effects as well. Bilateral leg IPC in rats has been shown to have effects in the cremaster muscle by increasing eNOS expression (4) and inducing NO-mediated vasodilation in the microvasculature (4, 31). Additionally, ischemic preconditioning protocols provide evidence of altering gene expression in both circulating leukocytes (25) and the myocardium (26). Although significant differences exist in stimulation characteristics (i.e., compression pressure, frequency, and duration) of these studies compared with the current one, the potential for IPC to induce important systemic effects warrants further consideration.
Experimental Considerations
Location of hemodynamic measurements.
In the present study, the hemodynamic profile during IPC applications was characterized in the popliteal artery proximal to the compression site. This measurement location is that previously used to assess the BF response to IPC (14, 28, 29, 54). Ultrasound/Doppler imaging of conduit arteries in the compressed region was not possible 1) because the IPC cuff covers the majority of the lower leg, and 2) because of the significant movement artifact in that region due to rapid cuff inflation. This required us to make the major assumption that this hemodynamic profile was mirrored downstream in the compressed vasculature. While it is likely that this holds true during cuff deflation because inflow to the limb is unimpeded, it is less certain as to what occurs during cuff inflation. Both treatments yielded negative mean BF and shear rate values during inflation. This indicates that each compression fully, albeit briefly, occluded the limb. Therefore, it is fair to assume that in the compressed region during inflation there is a low-net shear profile and that this profile is disturbed by the external compression. A low-net/disturbed hemodynamic profile is characteristic of highly atherosusceptible bifurcating regions of the arterial tree. Thus, although the current study was unable to directly measure hemodynamics in the compressed vasculature, extrapolation from the observed flow proximal to the compressed site suggests that a detrimental flow profile is likely to exist in this region during inflation.
Subjects.
It is important to recognize that the benefits of IPC therapy have been reported only in an aging clinical population with peripheral artery disease. It is therefore plausible that this pathologic condition is necessary for IPC to be effective. Thus physiologic responses to IPC may be vastly different in the young, healthy male population examined in the current investigation. Further, the effect of everyday locomotion inherent in our subject population undoubtedly exposes the leg to a similar stimulus as provided by IPC and thus may have limited the response to treatment observed in our subjects. Nonetheless, elucidating the response to treatment in a healthy population provides critical first step towards understanding of the physiologic effects of IPC therapy.
Conclusions
This is the first investigation to describe the acute physiologic responses to a single session of IPC therapy in humans. Our findings indicate that IPC treatment induces a significant perturbation to the hemodynamic profile of the compressed limb. Surprisingly, the periodic exposure to low net/oscillatory shear during inflation does not appear to acutely impair vasodilatory ability. This may be due to the counteracting effects of elevated shear consequent of cuff deflation. Additionally, a single session of IPC treatment causes local gene expression changes in the compressed skeletal muscle. Notably, the frequency-dependent decrease in the expression of CYR61 and CTGF expression suggests that mechanical compressions may influence extracellular matrix stability and vascular remodeling. The current study challenges the traditional assumptions regarding the mechanisms of action of IPC therapy. Longitudinal research is necessary to determine whether chronic exposure to the acute responses we describe herein are responsible for the beneficial clinical effects of IPC treatment.
GRANTS
This work was funded by the American College of Sports Medicine Research Endowment (to S. C. Newcomer) and National Heart, Lung, and Blood Institute Grant HL-36088 (to M. H. Laughlin and B. T. Roseguini). B. T. Roseguini is a Fulbright/Brazilian Ministry of Education (Capes) Fellow.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.D.S., B.T.R., M.H.L., and S.C.N. conception and design of research; R.D.S., B.T.R., J.P.T., and B.D.C. performed experiments; R.D.S., B.T.R., and J.P.T. analyzed data; R.D.S., B.T.R., J.P.T., B.D.C., M.H.L., and S.C.N. interpreted results of experiments; R.D.S. and B.T.R. prepared figures; R.D.S. drafted manuscript; R.D.S., B.T.R., J.P.T., B.D.C., M.H.L., and S.C.N. edited and revised manuscript; R.D.S., B.T.R., J.P.T., B.D.C., M.H.L., and S.C.N. approved final version of manuscript.
ACKNOWLEDGMENTS
R. D. Sheldon and B. T. Roseguini contributed equally to this work. We thank Dr. Richard Madsen for aid with statistical analysis, Ann Melloh for invaluable technical assistance, and ACI Medical for generously providing the IPC devices used in this study.
REFERENCES
- 1. Bieche I, Chavey C, Andrieu C, Busson M, Vacher S, Le Corre L, Guinebretiere JM, Burlinchon S, Lidereau R, Lazennec G. CXC chemokines located in the 4q21 region are up-regulated in breast cancer. Endocr Relat Cancer 14: 1039–1052, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Brigstock DR. Regulation of angiogenesis and endothelial cell function by connective tissue growth factor (CTGF) and cysteine-rich 61 (CYR61). Angiogenesis 5: 153–165, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Chen AH, Frangos SG, Kilaru S, Sumpio BE. Intermittent pneumatic compression devices–physiological mechanisms of action. Eur J Vasc Endovasc Surg 21: 383–392, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Chen LE, Liu K, Qi WN, Joneschild E, Tan X, Seaber AV, Stamler JS, Urbaniak JR. Role of nitric oxide in vasodilation in upstream muscle during intermittent pneumatic compression. J Appl Physiol 92: 559–566, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Dai G, Tsukurov O, Chen M, Gertler JP, Kamm RD. Endothelial nitric oxide production during in vitro simulation of external limb compression. Am J Physiol Heart Circ Physiol 282: H2066–H2075, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Davies PF, Spaan JA, Krams R. Shear stress biology of the endothelium. Ann Biomed Eng 33: 1714–1718, 2005 [DOI] [PubMed] [Google Scholar]
- 7. de Haro J, Acin F, Florez A, Bleda S, Fernandez JL. A prospective randomized controlled study with intermittent mechanical compression of the calf in patients with claudication. J Vasc Surg 51: 857–862, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Delis KT. The case for intermittent pneumatic compression of the lower extremity as a novel treatment in arterial claudication. Perspect Vasc Surg Endovasc Ther 17: 29–42, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Delis KT, Azizi ZA, Stevens RJ, Wolfe JH, Nicolaides AN. Optimum intermittent pneumatic compression stimulus for lower-limb venous emptying. Eur J Vasc Endovasc Surg 19: 261–269, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Delis KT, Nicolaides AN. Effect of intermittent pneumatic compression of foot and calf on walking distance, hemodynamics, and quality of life in patients with arterial claudication: a prospective randomized controlled study with 1-year follow-up. Ann Surg 241: 431–441, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Delis KT, Nicolaides AN, Labropoulos N, Stansby G. The acute effects of intermittent pneumatic foot vs. calf versus simultaneous foot and calf compression on popliteal artery hemodynamics: a comparative study. J Vasc Surg 32: 284–292, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Delis KT, Nicolaides AN, Wolfe JH, Stansby G. Improving walking ability and ankle brachial pressure indices in symptomatic peripheral vascular disease with intermittent pneumatic foot compression: a prospective controlled study with one-year follow-up. J Vasc Surg 31: 650–661, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Delis KT, Slimani G, Hafez HM, Nicolaides AN. Enhancing venous outflow in the lower limb with intermittent pneumatic compression. A comparative haemodynamic analysis on the effect of foot vs calf vs foot and calf compression. Eur J Vasc Endovasc Surg 19: 250–260, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Eze AR, Comerota AJ, Cisek PL, Holland BS, Kerr RP, Veeramasuneni R, Comerota AJ., Jr Intermittent calf and foot compression increases lower extremity blood flow. Am J Surg 172: 130–134; discussion 135, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Hellsten Y, Nielsen JJ, Lykkesfeldt J, Bruhn M, Silveira L, Pilegaard H, Bangsbo J. Antioxidant supplementation enhances the exercise-induced increase in mitochondrial uncoupling protein 3 and endothelial nitric oxide synthase mRNA content in human skeletal muscle. Free Radic Biol Med 43: 353–361, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Hsiai TK, Cho SK, Wong PK, Ing M, Salazar A, Sevanian A, Navab M, Demer LL, Ho CM. Monocyte recruitment to endothelial cells in response to oscillatory shear stress. FASEB J 17: 1648–1657, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hwang J, Ing MH, Salazar A, Lassegue B, Griendling K, Navab M, Sevanian A, Hsiai TK. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res 93: 1225–1232, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Inoki I, Shiomi T, Hashimoto G, Enomoto H, Nakamura H, Makino K, Ikeda E, Takata S, Kobayashi K, Okada Y. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J 16: 219–221, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Jensen L, Pilegaard H, Neufer PD, Hellsten Y. Effect of acute exercise and exercise training on VEGF splice variants in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 287: R397–R402, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Kavros SJ, Delis KT, Turner NS, Voll AE, Liedl DA, Gloviczki P, Rooke TW. Improving limb salvage in critical ischemia with intermittent pneumatic compression: a controlled study with 18-month follow-up. J Vasc Surg 47: 543–549, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Keeton EK, Brown M. Cell cycle progression stimulated by tamoxifen-bound estrogen receptor-alpha and promoter-specific effects in breast cancer cells deficient in N-CoR and SMRT. Mol Endocrinol 19: 1543–1554, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol 583: 861–874, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kivela R, Kyrolainen H, Selanne H, Komi PV, Kainulainen H, Vihko V. A single bout of exercise with high mechanical loading induces the expression of Cyr61/CCN1 and CTGF/CCN2 in human skeletal muscle. J Appl Physiol 103: 1395–1401, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Koller A, Huang A, Sun D, Kaley G. Exercise training augments flow-dependent dilation in rat skeletal muscle arterioles. Role of endothelial nitric oxide and prostaglandins. Circ Res 76: 544–550, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Konstantinov IE, Arab S, Kharbanda RK, Li J, Cheung MM, Cherepanov V, Downey GP, Liu PP, Cukerman E, Coles JG, Redington AN. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics 19: 143–150, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Konstantinov IE, Arab S, Li J, Coles JG, Boscarino C, Mori A, Cukerman E, Dawood F, Cheung MM, Shimizu M, Liu PP, Redington AN. The remote ischemic preconditioning stimulus modifies gene expression in mouse myocardium. J Thorac Cardiovasc Surg 130: 1326–1332, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Kubota S, Takigawa M. CCN family proteins and angiogenesis: from embryo to adulthood. Angiogenesis 10: 1–11, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Labropoulos N, Leon LR, Jr, Bhatti A, Melton S, Kang SS, Mansour AM, Borge M. Hemodynamic effects of intermittent pneumatic compression in patients with critical limb ischemia. J Vasc Surg 42: 710–716, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Labropoulos N, Watson WC, Mansour MA, Kang SS, Littooy FN, Baker WH. Acute effects of intermittent pneumatic compression on popliteal artery blood flow. Arch Surg 133: 1072–1075, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Labropoulos N, Wierks C, Suffoletto B. Intermittent pneumatic compression for the treatment of lower extremity arterial disease: a systematic review. Vasc Med 7: 141–148, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Liu K, Chen LE, Seaber AV, Johnson GW, Urbaniak JR. Intermittent pneumatic compression of legs increases microcirculation in distant skeletal muscle. J Orthop Res 17: 88–95, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Ludwig A, Berkhout T, Moores K, Groot P, Chapman G. Fractalkine is expressed by smooth muscle cells in response to IFN-gamma and TNF-alpha and is modulated by metalloproteinase activity. J Immunol 168: 604–612, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282: 2035–2042, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Montori VM, Kavros SJ, Walsh EE, Rooke TW. Intermittent compression pump for nonhealing wounds in patients with limb ischemia. The Mayo Clinic Experience (1998–2000). Int Angiol 21: 360–366, 2002 [PubMed] [Google Scholar]
- 36. Newcomer SC, Sauder CL, Kuipers NT, Laughlin MH, Ray CA. Effects of posture on shear rates in human brachial and superficial femoral arteries. Am J Physiol Heart Circ Physiol 294: H1833–H1839, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Padilla J, Sheldon RD, Sitar DM, Newcomer SC. Impact of acute exposure to increased hydrostatic pressure and reduced shear rate on conduit artery endothelial function: a limb-specific response. Am J Physiol Heart Circ Physiol 297: H1103–H1108, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Padilla J, Young CN, Simmons GH, Deo SH, Newcomer SC, Sullivan JP, Laughlin MH, Fadel PJ. Increased muscle sympathetic nerve activity acutely alters conduit artery shear rate patterns. Am J Physiol Heart Circ Physiol 298: H1128–H1135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol 546: 851–858, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ramaswami G, D'Ayala M, Hollier LH, Deutsch R, McElhinney AJ. Rapid foot and calf compression increases walking distance in patients with intermittent claudication: results of a randomized study. J Vasc Surg 41: 794–801, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Rivilis I, Milkiewicz M, Boyd P, Goldstein J, Brown MD, Egginton S, Hansen FM, Hudlicka O, Haas TL. Differential involvement of MMP-2 and VEGF during muscle stretch- versus shear stress-induced angiogenesis. Am J Physiol Heart Circ Physiol 283: H1430–H1438, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Roseguini BT, Mehmet Soylu S, Whyte JJ, Yang HT, Newcomer S, Laughlin MH. Intermittent pneumatic leg compressions acutely upregulate VEGF and MCP-1 expression in skeletal muscle. Am J Physiol Heart Circ Physiol 298: H1991–H2000, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roseguini BT, Sheldon R, Stroup A, Bell JW, Maurer D, Crist BD, Laughlin MH, Newcomer SC. Impact of chronic intermittent external compressions on forearm blood flow capacity in humans. Eur J Appl Physiol 111: 509–519, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ross ML, Halson SL, Suzuki K, Garnham A, Hawley JA, Cameron-Smith D, Peake JM. Cytokine responses to carbohydrate ingestion during recovery from exercise-induced muscle injury. J Interferon Cytokine Res 30: 329–337, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Sinoway LI, Shenberger J, Wilson J, McLaughlin D, Musch T, Zelis R. A 30-day forearm work protocol increases maximal forearm blood flow. J Appl Physiol 62: 1063–1067, 1987 [DOI] [PubMed] [Google Scholar]
- 46. Strassburg S, Richardson SM, Freemont AJ, Hoyland JA. Co-culture induces mesenchymal stem cell differentiation and modulation of the degenerate human nucleus pulposus cell phenotype. Regen Med 5: 701–711 [DOI] [PubMed] [Google Scholar]
- 47. Tan X, Qi WN, Gu X, Urbaniak JR, Chen LE. Intermittent pneumatic compression regulates expression of nitric oxide synthases in skeletal muscles. J Biomech 39: 2430–2437, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 53: 986–992, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Tullai JW, Schaffer ME, Mullenbrock S, Kasif S, Cooper GM. Identification of transcription factor binding sites upstream of human genes regulated by the phosphatidylinositol 3-kinase and MEK/ERK signaling pathways. J Biol Chem 279: 20167–20177, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Uematsu M, Ohara Y, Navas JP, Nishida K, Murphy TJ, Alexander RW, Nerem RM, Harrison DG. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol Cell Physiol 269: C1371–C1378, 1995 [DOI] [PubMed] [Google Scholar]
- 51. Valic Z, Buckwalter JB, Clifford PS. Muscle blood flow response to contraction: influence of venous pressure. J Appl Physiol 98: 72–76, 2005 [DOI] [PubMed] [Google Scholar]
- 52. van Bemmelen P, Char D, Giron F, Ricotta JJ. Angiographic improvement after rapid intermittent compression treatment [ArtAssist] for small vessel obstruction. Ann Vasc Surg 17: 224–228, 2003 [DOI] [PubMed] [Google Scholar]
- 53. van Bemmelen PS, Gitlitz DB, Faruqi RM, Weiss-Olmanni J, Brunetti VA, Giron F, Ricotta JJ. Limb salvage using high-pressure intermittent compression arterial assist device in cases unsuitable for surgical revascularization. Arch Surg 136: 1280–1285; discussion 1286, 2001 [DOI] [PubMed] [Google Scholar]
- 54. van Bemmelen PS, Mattos MA, Faught WE, Mansour MA, Barkmeier LD, Hodgson KJ, Ramsey DE, Sumner DS. Augmentation of blood flow in limbs with occlusive arterial disease by intermittent calf compression. J Vasc Surg 19: 1052–1058, 1994 [DOI] [PubMed] [Google Scholar]
- 55. Wolf SC, Sauter G, Jobst J, Kempf VA, Risler T, Brehm BR. Major differences in gene expression in human coronary smooth muscle cells after nebivolol or metoprolol treatment. Int J Cardiol 125: 4–10, 2008 [DOI] [PubMed] [Google Scholar]
- 56. Zhou D, Herrick DJ, Rosenbloom J, Chaqour B. Cyr61 mediates the expression of VEGF, alphav-integrin, and alpha-actin genes through cytoskeletally based mechanotransduction mechanisms in bladder smooth muscle cells. J Appl Physiol 98: 2344–2354, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Ziegler T, Bouzourene K, Harrison VJ, Brunner HR, Hayoz D. Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arterioscler Thromb Vasc Biol 18: 686–692, 1998 [DOI] [PubMed] [Google Scholar]