Abstract
A vital functional plasticity of humans is their ability to adapt to threats to posture stability. The purpose of this study was to investigate adaptations to repeated trips in walking. Sixteen young adults were recruited and exposed to the sudden (electronic-mechanical) release of an obstacle, 11-cm in height, in the path of over ground walking during the mid-to-late left swing phase. Although none of the subjects fell on the first of eight unannounced, consecutive trips, all of them had to rely on compensatory step with a step length significantly longer than their regular to reduce their instability. In the subsequent trials, they were able to rapidly make adaptive adjustments in the control of their center-of-mass (COM) stability both proactively and reactively (i.e., before and after hitting or crossing the obstacle), such that the need for taking compensatory step was substantially diminished. The proactive adaptations included a reduced forward COM velocity that lessened forward instability and an elevated toe clearance that reduced the likelihood of obstacle contact. The reactive adjustments were characterized by improved trunk control (by reducing its forward rotation) and limb support (by increasing hip height) and reduced forward instability (by both the posterior COM shift and the reduction in its forward velocity). These findings suggest that young adults can adapt appropriately to repeated trip perturbations and to reduce trip-induced excessive instability in both proactive and reactive manners.
Keywords: obstacle, stability, limb support, proactive, reaction, fall
1. Introduction
Trips account for 34% to 53% of falls in community-dwelling adults (Berg et al., 1997; Blake et al., 1988) with 20% of these falls resulting in hospitalization (Cumming and Klineberg, 1994; Nyberg et al., 1996). It is hence imperative to develop effective interventions for preventing trip-related falls. Because instability from a perturbation is often a precursor to falls, increasing stability control in the presence of a particular perturbation type might reduce the incidence of falls (Maki and McIlroy, 1997; Pai and Bhatt, 2007). The central nervous system (CNS) can adaptively improve both proactive and reactive controls of center of mass (COM) stability (i.e., controlling the dynamic relationship of the COM position and velocity with respect to its base of support (BOS) both before and after onset of perturbation) in repeated-slip training. Such adaptive control acquired via perturbation-based training has demonstrated its potential in fall prevention (Maki et al., 2008; Mansfield et al., 2010; Pai and Bhatt, 2007).
Recent studies have demonstrated rapid improvements resulting in the successful prevention of excessive forward instability following repeated treadmill-induced trips (Bieryla and Madigan, 2010; Bieryla et al., 2007; Owings et al., 2001). However, there are noticeable differences between the treadmill-induced trip and an obstacle-induced trip. First, it has been shown that the kinematics of the stepping response during a treadmill-induced trip are different from those in an obstacle-induced trip (e.g., a greater foot clearance over an obstacle-induced trip) (Troy and Grabiner, 2005). In addition, the simulated treadmill-trip in those studies was induced during standing rather than during walking (Bieryla et al., 2007; Owings et al., 2001). Because of those differences, it is unclear if recovery strategies for a treadmill-induced trip are different from those for an obstacle-induced trip. In addition, the training-induced improvements in dynamic stability control and its impact on behavioral outcomes are largely unknown.
The purpose of this study was to investigate the adaptation to obstacle-induced trips during walking. We hypothesized that after repeated-trip exposure, young adults would be able to adaptively improve their proactive (before trip onset) and reactive (after trip onset) control of their COM stability, to arrest excessive trunk flexion, and to provide sufficient limb support. We expected that those proactive and reactive adjustments observed before and after onset of trip perturbation would reduce the incidence of falls or reduce the need for taking a compensatory step to restore stability (Wu et al., 2007).
2. Methods
2.1 Subjects
Sixteen healthy young adults gave informed consent and participated in the research study (13 women; age 25±3 years; height 169±8 cm; mass 65±13 kg) approved by the Institutional Review Board. Subjects with histories of neurological or musculoskeletal disorders or any other injuries were excluded.
2.2 Protocol
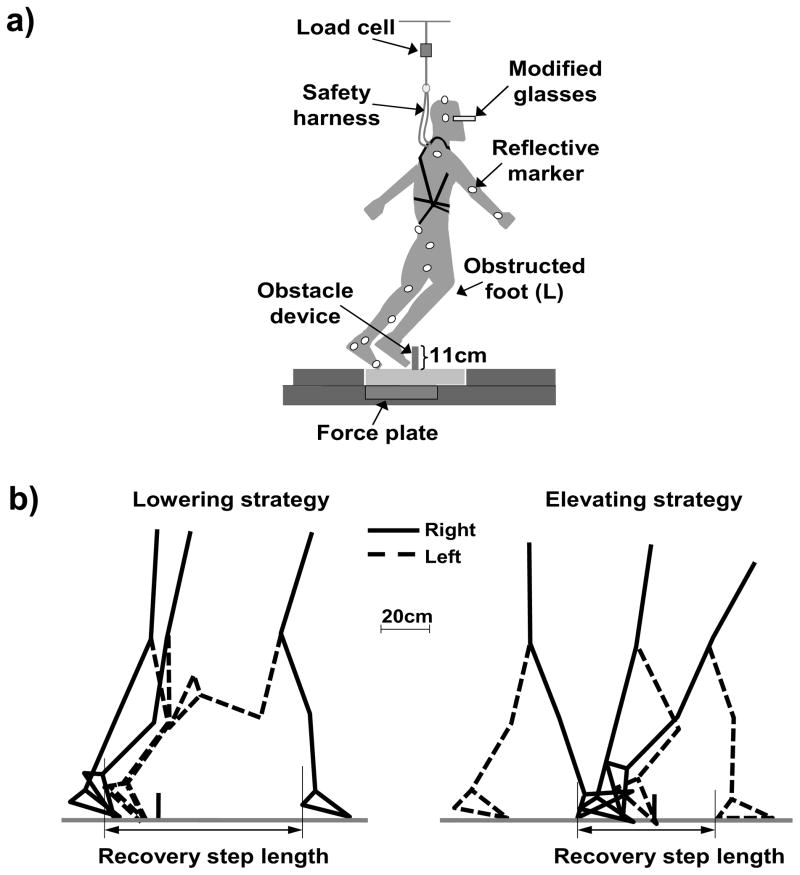
The obstacle device consists of a hinged metal plate 11-cm high, 27-cm wide and 0.5-cm thick. During regular walking, the plate was locked in a flat position by a pair of electromagnets. During the perturbed trials, the springs returned the plate to its upright position in less than 150ms to induce a trip after powering off the electromagnets (to unlock the plate) when the vertical ground reaction force (GRF) under the unperturbed (right) limb exceeded 80% of the body’s weight after the right heel strike. The subjects wore a pair of modified glasses that blocked the lower half of their visual field throughout the test (Fig. 1a) and a safety harness attached via shock-absorbing ropes to an overhead rail system through a load cell (Fig. 1a).
Figure 1.
a) Diagrammatic representation of the experimental setup, and b) definition of the recovery step length. a) The obstacle device consisted of an 11-cm tall plate, which lay flat on the floor during level walking and became upright when triggered by turning off the electromagnets. The trip was induced by obstructing the subject’s left limb (L) during the mid-to-late swing phase. All subjects wore a pair of modified glasses that blocked the lower half of the visual field. Twenty-seven light-reflective markers were placed on the subject and the obstacle device. The subject wore a safety harness that was attached to shock-absorbing ropes and was individually adjusted to prevent a fall to the ground. A load cell connected to the rope was used to measure the force exerted on the harness by the subject. b) The recovery step length for the lowering/elevating strategy was calculated as the horizontal distance measured from the most posterior position of the left/right heel marker during the stance phase of the left/right foot (prior to obstacle-hit) to the most posterior position of the right/left heel marker during the stance phase of the right/left foot after an obstacle-hit. The thick black vertical line represents the location of the obstacle device.
Subjects were instructed to walk at their normal pace on a 7-m walkway and were told only that they might experience a trip later on. They were also told that they should try to recover their balance and continue walking upon a trip. Subjects walked for 8 trials as the baseline for walking performance. During which the starting position was adjusted to ensure that the upcoming trip trials were induced to consistently obstruct the subject’s left foot during the mid-to-late swing phase, such that a lowering strategy was likely implemented (Eng et al., 1994). Unannounced trip were induced in eight consecutive walking trials.
2.3 Data collection and reduction
An 8-camera motion analysis system (Motion Analysis Corporation, Santa Rosa, CA) recorded data from 27 reflective markers placed on bilateral upper and lower extremities, torso, and the plate at 120Hz. Marker displacement data were low-pass filtered at marker-specific cut-off frequencies (range 4.5–9Hz determined through a residual analysis, Winter, 2005) using fourth-order Butterworth filters. Force plate, load cell data, and trigger-release onset signal were synchronously collected at 600Hz. Both GRF and load cell force were low-pass filtered at 27Hz using fourth-order Butterworth filters.
Behavior outcome
A fall occurred after the trip onset when the subject was supported by the harness for more than 30% body weight (Yang and Pai, 2011). A compensatory step occurred when its length exceeded 6 standard deviations (SD) beyond the average, regular step length (SLbaseline) determined from the last 8 baseline trials prior to the trip (with four analyzable steps in each trial). A step length was considered compensatory when it exceeded SLbaseline+6SD after crossing over an obstacle. This threshold is rather conservative and it accounts for all step lengths that would likely fall outside of 99.9% variability observed during this individual’s regular walking. The outcome also included three strategies: 1) lowering-hit, the obstructed foot was quickly lowered to the ground and the contralateral unobstructed foot took a recovery step; 2) elevating-hit, the obstructed foot took a recovery step after hitting the obstacle (Eng et al., 1994); and 3) elevating-cross, subjects crossed over the obstacle without hitting it.
Pre-trip and post-trip events
Two instants (pre-trip and post-trip) that were respectively indicative of proactive and reactive adaptive performances (i.e., adaptive changes in behavior observed before and after onset of perturbation) were measured: 1) 30ms before the obstacle hit/cross (hit/cross, pre-trip) and 2) recovery-foot-touchdown (RecTD, post-trip). The instant of the obstacle-hit was defined as the time of minimum acceleration of the toe marker of the obstructed foot (i.e., left foot) in the walking direction (Pijnappels et al., 2004). For regular walking and in those trials in which subjects did not hit the plate, the time the obstacle crossing was defined as the instant when the left toe marker was immediately above the location of the plate. The step liftoff and touchdown were identified from the vertical GRF. The instant of recovery-foot-touchdown was the touchdown of the right or left foot for lowering and elevating strategies, respectively.
COM state stability
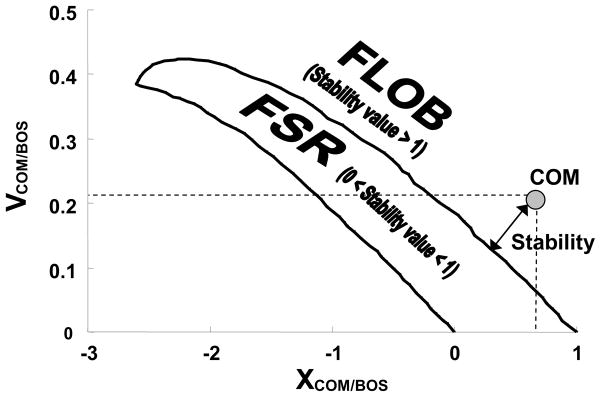
The COM kinematics in the anteroposterior direction were calculated using a 13-segment human model (de Leva, 1996). The COM position (XCOM/BOS) and velocity (VCOM/BOS) were calculated relative to the BOS and were normalized by foot length and the square root of (gh), respectively, where g was the gravitational acceleration and h, the body height. Stability (i.e., forward instability) was defined as the shortest distance between the instantaneous COM state (i.e., position and velocity) and predicted feasible stability limits for forward balance loss (Pai and Patton, 1997) (Fig. 2). A stability value greater than 1 indicated less stability against forward balance loss (or excessive forward instability).
Figure 2.
Shown is the instantaneous center-of-mass (COM) state (circle) with the computer model predicted boundary of the feasible stability region (FSR). The COM position (XCOM/BOS) was defined as the absolute COM position in anteroposterior direction relative to the rear of BOS and normalized by foot length. The COM velocity (VCOM/BOS) was calculated from the differentiation of the COM position and normalized to the square root of (gh), where g was the acceleration due to gravity and h was the body height. Stability (or forward instability) was measured as the shortest distance between the instantaneous COM state (i.e., in its position and velocity phase plane) and the FSR boundary against forward loss of balance (FLOB). A stability value of greater than 1 indicated FLOB, which is necessary during walking to achieve forward progression (but not excessive forward instability as in the case of a trip, which would require taking a compensatory step, Wu et al., 2007). A stability value between 0 and 1 indicated that the COM state was within the FSR, in which balance can be restored without the need to take extra step.
Kinematic variables
The following variables in a trip trial were analyzed: toe clearance, post-trip step lengths, trunk angle, hip height (as a measure to reflect control of limb support [Yang et al., 2009]), and COM angular momentum. The toe clearance was measured as the vertical distance from the ground to the left toe, and was obtained at pre-trip instant. The post-trip recovery step length was the fore-aft heel-to-heel distance measured during the stance phase after hitting the obstacle (Fig. 1b). The same measurement was taken for the follow-up step length (i.e., the step taken after the recovery step). All step lengths were normalized by height. Trunk angle was the angle between the trunk segment (defined by a line connecting the midpoint of the shoulders and the midpoint of the hips) and the vertical line. Hip height was assessed as the vertical distance from the ground to the midpoint of the hips and normalized by height. The maximum trunk flexion angle and minimum hip height during recovery (from obstacle-hit to RecTD) were calculated. The angular momentum of the COM with respect to the stance ankle at RecTD was calculated as:
where L⃗ is the angular momentum of the COM; r⃗, the displacement vector from the stance ankle to COM; v⃗, linear velocity vector of the COM; m, body mass.
2.4 Statistics
The Cochran’s Q tests with post-hoc Wilcoxon signed rank tests were performed to compare the changes in the behavioral outcome from the first (T1) to the eighth trips (T8). One-way repeated measures ANOVAs were performed to analyze the adaptation effects from T1 to T8 for the following variables: COM position and velocity, stability, step lengths, maximum trunk angle, and minimum hip height. Only 12 subjects with a complete data set were included in Cochran’s Q and ANOVA tests (data from 4 subjects with missing markers or triggering problems at certain trials were only included in the between-trial comparisons). For a significant main effect, planned paired-t tests were performed first by comparing T1 with all other trips (T2–T8) to identify at which trial a significant between-trial change emerged. Comparisons between consecutive trial pairs were then performed following the trial in which the significant change was first identified. A maximum of 3 comparisons were made for all variables other than toe clearance, for which 4 comparisons were made, with the significance level adjusted by Bonferroni corrections to account for them. Planned paired-t tests with an adjusted significance level were also performed to compare all of the continuous variables between the three trial pairs: T1-N (the regular walking trial prior to the first trip), T1–T8, and T8-N. All analyses were performed using SPSS (V.17.0, SPSS Inc., IL).
3. Results
3.1 Behavior outcome
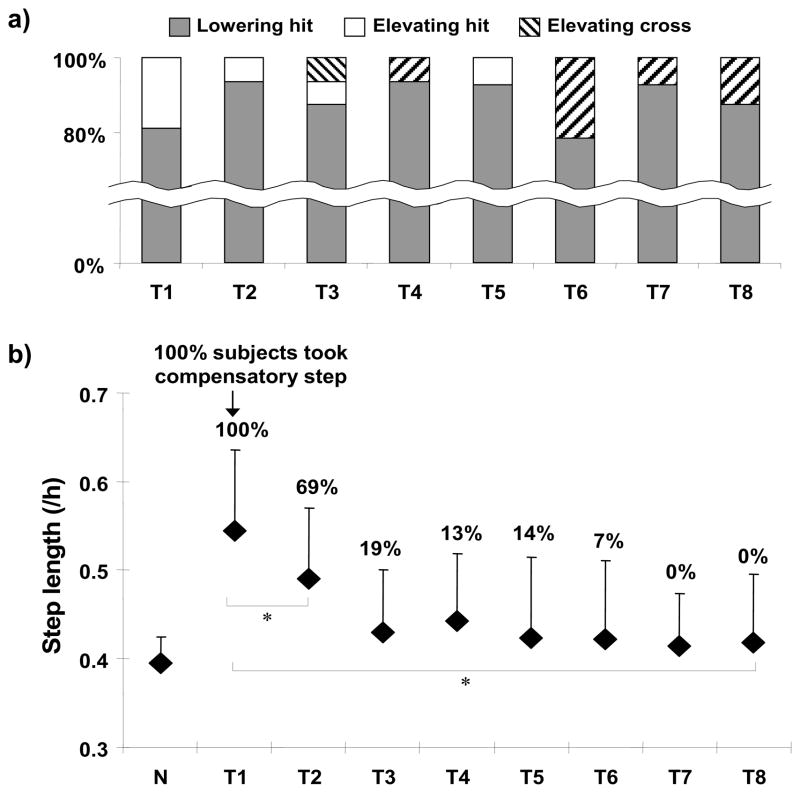
The perturbation occurred at the mid-to-late swing phase as intended (65% of the swing phase; 273±29ms after left foot liftoff). None of the subjects fell, but all of them hit the obstacle using either a lowering or elevating strategy during the first two trips, and thereafter some of the subjects demonstrated obstacle crossing (up to approximately 20% at T6) (Fig. 3a). Overall, most subjects (more than 75%) used the lowering strategy, which did not differ significantly from trial-to-trial over the course of 8 trips (Q(7, 14) = 8.037, p = .329, Fig. 3a). The subjects were able to successfully reduce the need for taking a compensatory step from 100% on T1 to 69% on T2 (p < .05) and to 0% on T8 (p < .001) with the decreases in their step length (T1–T2, p < .05; T1–T8, p < .05) (Fig. 3b).
Figure 3.
Shown are a) the percentage of the strategy used for trip recovery from each of the 8 trips (T1–T8), and b) the incidence of taking a compensatory step (indicated as a percentage on the top of each trial) and the average recovery step length across 8 trips. a) Three strategies were observed: lowering-hit strategy (filled), elevating-hit strategy (unfilled), and elevating-cross strategy (hatched). Overall, over 75% of the subjects used the lowering strategy to recover from a trip. b) The group means (±SD) of the recovery step length (normalized by subjects’ height [/h]) are shown for the 8 trip trials. A decrease in the recovery step length was associated with a decrease in the incidence of excessive forward instability with a compensatory step (the incidence is indicated on each trip trial). N represents the regular walking trial prior to the first trip. * p < 0.05.
3.2 Pre-trip adaptation
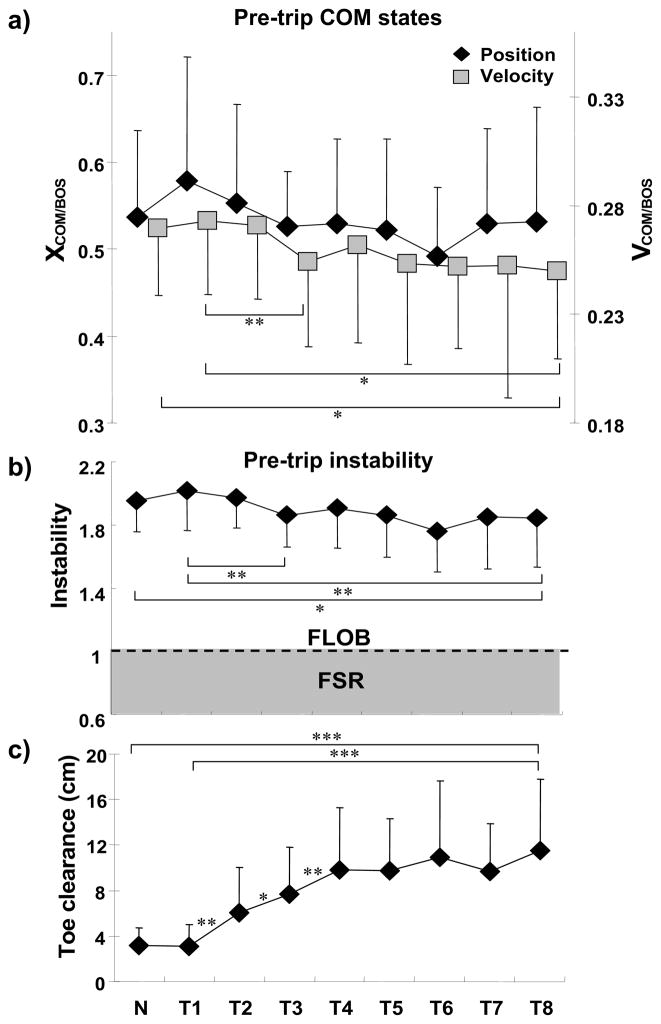
Pre-trip adaptations were most apparent in the COM velocity reduction (main effect: F8,88 = 2.945, p < .01) and the reduction of its forward instability (main effect: F8,88 = 4.215, p < .01) prior to the obstacle crossing during the mid-to-late swing phase (Fig. 4a & 4b). The COM velocity decreased from T1 to T3 by approximately 6.9% (p = .003, Fig. 4a), and remained constant thereafter (i.e., no trial-to-trial difference after T3, all p > .05). The COM velocity on T8 was still significantly lower than both on T1 (by approximately 8.4%, p = .013) and on N (by approximately 7.2%, p = .02) (Fig. 4a). There was no significant change in COM position (main effect: F8,88 = .582, p = .790, Fig. 4a), although a posterior shift trend was apparent (Fig. 4a). The changes in the COM velocity were sufficient to significantly reduce the forward COM instability from T1 to T3 by approximately 7.9% (p = .004, Fig. 4b). Likewise, the forward instability in T8 was lower than T1 by approximately 8.6% (p = .008) and lower than N by 5.6%, (p = .05) (Fig. 4b). Pre-trip adaptations were also evident in the modification of the toe clearance (main effect: F8,88 = 11.276, p < .001, Fig. 4c). It significantly increased from T1 to T2 by approximately 93.7% (p = .003, Fig. 4c), from T2 to T3 by 26.9% (p = .01), and from T3 to T4 by 28%, (p = .006). A higher toe clearance was observed on T8 in comparison to T1 by 270.5%, (p < .001) and to N by 260.3%, (p < .001) (Fig. 4c).
Figure 4.
Shown are the group means (±SD) of adaptive changes in a) pre-trip center-of-mass (COM) position and velocity, b) pre-trip instability, and c) toe clearance for the regular walking trial (N) prior to the first trip and all 8 trips (T1–T8). The pre-trip was measured at 30 ms prior to the obstacle-hit. The dash line in figure 4b represents the threshold of the forward loss of balance (FLOB). Stability was defined as the shortest distance between the instantaneous COM motion state and the predicted feasible stability region (FSR) limits for FLOB. A stability value of greater than 1 indicated less stability against FLOB (excessive forward instability); a stability value between 0 and 1 indicated that the COM state was within the FSR (shade). The toe clearance was measured as the vertical distance from the ground to the left toe. * p < 0.05; ** p < 0.01; *** p < 0.001.
3.3 Post-trip adaptation
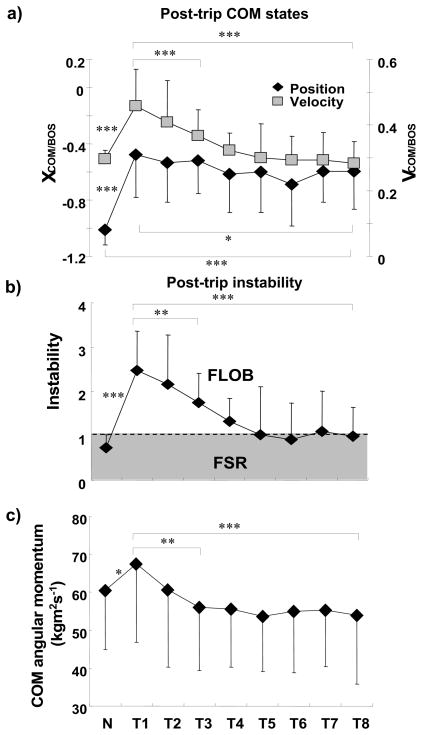
Post-trip adaptations in the control of COM stability included a posterior COM position shift (main effect: F8,88 = 8.848, p < .001) and a COM velocity reduction (F8,88 = 11.214, p < .001) at RecTD (Fig. 5a). Everyone was most unstable on T1, during which instability was approximately 237.7% greater than that on N (p < .001). However, this forward instability decreased from T1 to T3 by approximately 29.3% (p = .004) and from T1 to T8 by 597.7% (p < .001, Fig. 5b). Finally, forward instability was not significantly different between T8 and N (p = .187, Fig. 5b). The instability on T1 was due to the excessive forward COM position and forward velocity (i.e., the COM on T1 was 52.9% more forward and 54.6% faster than on N (both p < .001, Fig. 5a)). The COM was 25.5% more posterior and 38% slower on T8 than on T1 (both p < .05, Fig. 5a). Similarly, the forward rotating angular momentum was also 20.1% less on T8 than on T1 (p = .001, Fig. 5c).
Figure 5.
Shown are the group means (±SD) of adaptive changes in a) post-trip center-of-mass (COM) position and velocity, b) post-trip instability, and c) COM angular momentum at recovery-foot-touchdown for the regular walking trial (N) prior to the first trip and all 8 trips (T1–T8). The post-trip was measured at the recovery-foot-touchdown after the obstacle-hit. The dashed line in figure 5b represents the threshold of forward loss of balance (FLOB). A stability value of greater than 1 indicated less stability against FLOB (excessive forward instability); a stability value between 0 and 1 indicated that the COM state was within the feasible stability region (FSR, shade). The positive angular momentum corresponded to a clockwise rotation (or forward rotation). * p < 0.05; ** p < 0.01; *** p < 0.001.
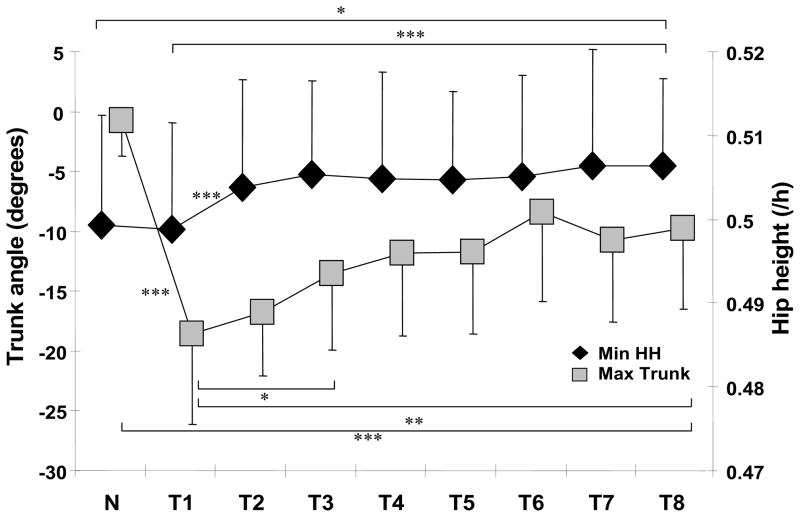
Post-trip adaptations also included improvements in trunk control and in limb support. The maximum trunk flexion and minimum hip height showed significant main effects (F8,88 = 11.585, p < .001 and F8,88 = 2.629, p < .05, respectively). After a significant increase in maximum trunk flexion was observed on T1 in comparison to N (p < .001, Fig. 6), it decreased significantly from T1 to T3 by approximately 5 degrees (p = .035). It was 8 degrees less on T8 than on T1 (p = .006), but 9 degrees greater than on N (p < .001) (Fig. 6). The minimum hip height increased significantly from T1 to T2 by approximately 1% (p = .001) without further change thereafter, such that it was still higher on T8 by approximately 1.5% than on T1 (p < .001, Fig. 6), and by approximately 1% than on N (p = .027, Fig. 6).
Figure 6.
Shown are group means (±SD) of adaptive changes in the post-trip a) trunk angle and b) hip height for the regular walking trial (N) prior to the first trip and all 8 trips (T1-T8). The maximum trunk flexion and minimum hip height during recovery (from the obstacle-hit to the recovery-foot-touchdown) were obtained. Trunk angle was defined as the angle between the trunk segment and the vertical axis (+: extension; −: flexion). Hip height was calculated as the vertical distance from the ground to the midpoint of the hips and normalized by subjects’ height (/h). * p < 0.05; ** p < 0.01; *** p < 0.001.
4. Discussion
The results revealed young adults’ ability to adaptively improve gait stability for the successful negotiation of an obstacle-induced trip. After repeated trip, they were able to proactively improve toe clearance, both proactively and reactively improve the control of their COM stability against excessive perturbation-related forward instability, while reactively improve trunk control (to arrest excessive forward rotation) and limb support (to prevent collapse). Such successful adaptations led to the reduction or elimination of the need for taking compensatory step.
4.1 Pre-trip (proactive) adaptation
The results indeed supported our hypothesis that subjects were able to proactively decrease excessive forward instability following repeated trips and to successfully resume walking without resorting to a compensatory step after hitting or crossing the obstacle. Evidence from computational simulations indicates that the ability to avoid a balance loss during a dynamic task is partly determined by the initial COM motion state, e.g., a more forward COM position and a greater forward velocity increase forward instability (Pai and Patton, 1997; Wu et al., 2007). The adaptive improvements in pre-trip stability control from a reduction in the forward COM velocity prior to obstacle hit or cross were consistent with the theoretical model prediction against forward instability. The rapidity in the reduction of compensatory stepping from 100% to only 19% in just two trials could be better explained by the recalibration of the motor commands to reduce excessive instability rather than by other means, such as improving strength (Wu et al., 2007).
Pre-trip adaptation was also evident via the trajectory adjustment of the obstructed limb to increase toe clearance. An increase in toe clearance immediately after the first trip reflected an adaptive strategy that the CNS adopted to reduce or even eliminate the possibility of subsequent obstacle contact. However, raising the limb more than necessary may require an additional energy cost (Patla et al., 1991). In addition, because the exact dimensions of the obstacle were unknown, there would still be a chance of destabilization upon obstacle impact if the preplanned foot trajectory was too low. Furthermore, the additional time required to raise the foot might increase the extent of the forward rotation of the body (Troy and Grabiner, 2005), which could further destabilize the subject. Those problems could be solved by increasing the COM stability proactively or reactively to minimize the extent of balance disturbances in the case of a deleterious contact with the obstacle.
4.2 Post-trip (reactive) adaptation
Although none of the subjects in the present study fell on the first trip, an unexpected obstacle contact seriously disturbed their control of stability demonstrated by the excessive forward instability at recovery-foot-touchdown. Consequently, all of them had to take a compensatory step (Fig. 3b). A decrease in post-trip instability that reduced the need for taking a compensatory step was observed after the first few trips and indicated that the subjects became better at responding to a trip with the appropriate reactive responses (Fig. 3b). Such improvements in the control of stability could result from a reduced forward velocity and a posterior shift of the COM (Fig. 5a).
This improvement in the control of COM stability was coupled with the stabilization of angular motion in which the forward angular momentum of the COM was sufficiently neutralized (Fig. 5c). To successfully regain stability and prevent falls from an unintended obstacle contact requires the reduction of the trip-induced forward angular momentum in the trunk rotation (Grabiner et al., 1993; Pijnappels et al., 2004) as well as providing sufficient limb support for executing an efficient recovery step and retarding the fall of the upper body (Pavol et al., 2001; Pijnappels et al., 2004). Adaptive improvements in both aspects of reactive control (i.e., trunk rotation and limb support) were apparent during adaptation (Fig. 6), similar to previous studies with treadmill training (Bieryla et al., 2007; Owings et al., 2001).
The term “adaptation in reactive control” is reflected in the changes that occurred after the onset of the perturbation, which could result from reactive adjustments made through feedback-error-based mechanisms (e.g., spinal or subcortical reflex and triggered cortical response) and from proactive adjustments made in absence or in advance of any perturbation. Adaptation can be defined as a process of adjusting movements to meet new demands, and it is characterized by a shift from a reliance on feedback mechanisms for error corrections to being influenced by feedforward mechanisms over the course of practice (Bastian, 2008; Shadmehr and Mussa-Ivaldi, 1994). An elevating-cross strategy observed later in practice could be indicative of an adaptive process in which the control strategy shifted from feedback reliant to feedforward dominant.
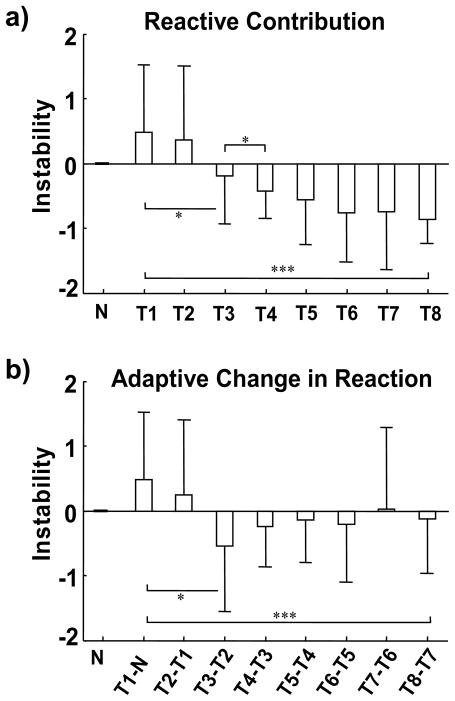
To further estimate the net effect of the reactive control of stability, we subtracted pre-trip stability from post-trip stability in a post-hoc analysis (Fig. 7a). While this may not be the most accurate approach, it provided estimation on reactive contribution made after trip onset (Fig. 7a). As shown in this analysis, subjects relied heavily on reactive feedback control to correct their movement instability on the first trial (Fig. 7a). The trial-to-trial change in reaction would then indicate the adaptive adjustments made in the control of stability (Fig. 7b). The need for such corrections was substantially reduced over the course of 8 trips (Fig. 7b). Although by then many subjects may not actually have hit the obstacle, they nonetheless could not “voluntarily” trigger or control the appearance of the obstacle. Rather, their success often came from negotiating the obstacle without hitting it after its appearance, hence attributable to their reactive adjustments. Individuals who have developed an appropriate feedforward motor command, accompanied by accurately predicted sensory consequences in an updated efference copy would in fact lessen (or may even eliminate) the need for feedback correction, although these feedback-error-correction mechanisms are all intact, active, and ready to work during their reaction to the rise of the obstacle (Witney et al., 1999; Wolpert et al., 1995).
Figure 7.
Estimated (a) reactive contribution and (b) its adaptive change to reduce trip-related instability (from the first trip trial, T1, to the last, T8). a) The pre-trip instability (which reflects the effect of feedforward control) is subtracted from the post-trip instability to estimate the net reactive contribution in each one of these trials. b) The adaptive change in reactive control of stability is quantified as the trial-to-trial changes in the reactive contribution (*** p < 0.001; * p < 0.05). In comparison, the need for reactive response and the trial-to-trial changes during regular walking trials are minimum and negligible during preceding walking trials (N and the trial before). Positive means an increase and negative, a reduction in instability.
4.3 Limitation of the study
The present study has limitations. It cannot determine the extent to which the observed strategies were specific to the phase of the gait cycle when the trip was induced and the factors contributing to variations in the strategy selection. Further, the obstacle-trip in the current study was not entirely unexpected, and anticipation may play a role in recovery that is beyond the scope of the present investigation. Finally, older adults who are more vulnerable to falls are not included in this study.
In summary, adaptive improvements in the control of stability following repeated trips led to a significant reduction in the need for or the magnitude of compensation (Fig. 3b). It is the net effect of the aforementioned adaptive improvements, made in both proactive and reactive control, which led to the successful resumption of a regular gait pattern even in the presence of an obstacle-hit or merely its appearance.
Acknowledgments
The authors thank Daniel Phelps, Natalie Menick, Hyosub Kim, and Noman Khan for assistance with data collection. This work was funded by NIH 2RO1-AG16727 and RO1-AG029616.
Footnotes
Conflict of interest statement
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bastian AJ. Understanding sensorimotor adaptation and learning for rehabilitation. Current Opinion in Neurology. 2008;21:628–633. doi: 10.1097/WCO.0b013e328315a293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg WP, Alessio HM, Mills EM, Tong C. Circumstances and consequences of falls in independent community-dwelling older adults. Age and Ageing. 1997;26:261–268. doi: 10.1093/ageing/26.4.261. [DOI] [PubMed] [Google Scholar]
- Bieryla KA, Madigan ML. Proof of concept for perturbation-based balance training in older adults at a high risk for falls. Archives of Physical Medicine and Rehabilitation. 2010;92:841–843. doi: 10.1016/j.apmr.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Bieryla KA, Madigan ML, Nussbaum MA. Practicing recovery from a simulated trip improves recovery kinematics after an actual trip. Gait & Posture. 2007;26:208–213. doi: 10.1016/j.gaitpost.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Blake AJ, Morgan K, Bendall MJ, Dallosso H, Ebrahim SB, Arie TH, Fentem PH, Bassey EJ. Falls by elderly people at home: prevalence and associated factors. Age and Ageing. 1988;17:365–372. doi: 10.1093/ageing/17.6.365. [DOI] [PubMed] [Google Scholar]
- Cumming RG, Klineberg RJ. Fall frequency and characteristics and the risk of hip fractures. Journal of the American Geriatric Society. 1994;42:774–778. doi: 10.1111/j.1532-5415.1994.tb06540.x. [DOI] [PubMed] [Google Scholar]
- de Leva P. Adjustments to Zatsiorsky-Seluyanov’s segment inertia parameters. Journal of Biomechanics. 1996;29:1223–1230. doi: 10.1016/0021-9290(95)00178-6. [DOI] [PubMed] [Google Scholar]
- Eng JJ, Winter DA, Patla AE. Strategies for recovery from a trip in early and late swing during human walking. Experimental Brain Research. 1994;102:339–349. doi: 10.1007/BF00227520. [DOI] [PubMed] [Google Scholar]
- Grabiner MD, Koh TJ, Lundin TM, Jahnigen DW. Kinematics of recovery from a stumble. Journal of Gerontology. 1993;48:M97–102. doi: 10.1093/geronj/48.3.m97. [DOI] [PubMed] [Google Scholar]
- Maki BE, McIlroy WE. The role of limb movements in maintaining upright stance: the “change-in-support” strategy. Physical Therapy. 1997;77:488–507. doi: 10.1093/ptj/77.5.488. [DOI] [PubMed] [Google Scholar]
- Mansfield A, Peters AL, Liu BA, Maki BE. Effect of a perturbation-based balance training program on compensatory stepping and grasping reactions in older adults: a randomized controlled trial. Physical Therapy. 2010;90:476–491. doi: 10.2522/ptj.20090070. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Gustafson Y, Berggren D, Brannstrom B, Bucht G. Falls leading to femoral neck fractures in lucid older people. Journal of the American Geriatric Society. 1996;44:156–160. doi: 10.1111/j.1532-5415.1996.tb02432.x. [DOI] [PubMed] [Google Scholar]
- Owings TM, Pavol MJ, Grabiner MD. Mechanisms of failed recovery following postural perturbations on a motorized treadmill mimic those associated with an actual forward trip. Clinical Biomechanics. 2001;16:813–819. doi: 10.1016/s0268-0033(01)00077-8. [DOI] [PubMed] [Google Scholar]
- Pai YC, Bhatt TS. Repeated-slip training: an emerging paradigm for prevention of slip-related falls among older adults. Physical Therapy. 2007;87:1478–1491. doi: 10.2522/ptj.20060326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai YC, Patton J. Center of mass velocity-position predictions for balance control. Journal of Biomechanics. 1997;30:347–354. doi: 10.1016/s0021-9290(96)00165-0. [DOI] [PubMed] [Google Scholar]
- Patla AE, Prentice SD, Robinson C, Neufeld J. Visual control of locomotion: strategies for changing direction and for going over obstacles. Journal of Experimental Psychology: Human Perception and Performance. 1991;17:603–634. doi: 10.1037//0096-1523.17.3.603. [DOI] [PubMed] [Google Scholar]
- Pavol MJ, Owings TM, Foley KT, Grabiner MD. Mechanisms leading to a fall from an induced trip in healthy older adults. Journal of Gerontology Series A, Biological Sciences and Medical Sciences. 2001;56:M428–437. doi: 10.1093/gerona/56.7.m428. [DOI] [PubMed] [Google Scholar]
- Pijnappels M, Bobbert MF, van Dieen JH. Contribution of the support limb in control of angular momentum after tripping. Journal of Biomechanics. 2004;37:1811–1818. doi: 10.1016/j.jbiomech.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. Journal of Neuroscience. 1994;14:3208–3224. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy KL, Grabiner MD. The presence of an obstacle influences the stepping response during induced trips and surrogate tasks. Experimental Brain Research. 2005;161:343–350. doi: 10.1007/s00221-004-2078-8. [DOI] [PubMed] [Google Scholar]
- Winter DA. Biomechanics and motor control of human movement. 3. John Wiley & Sons; Hoboken, NJ: 2005. [Google Scholar]
- Witney AG, Goodbody SJ, Wolpert DM. Predictive motor learning of temporal delays. Journal of Neurophysiology. 1999;82:2039–2048. doi: 10.1152/jn.1999.82.5.2039. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269:1880–1882. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- Wu M, Ji L, Jin D, Pai YC. Minimal step length necessary for recovery of forward balance loss with a single step. Journal of Biomechanics. 2007;40:1559–1566. doi: 10.1016/j.jbiomech.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Bhatt T, Pai YC. Role of stability and limb support in recovery against a fall following a novel slip induced in different daily activities. Journal of Biomechanics. 2009;42:1903–1908. doi: 10.1016/j.jbiomech.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Pai YC. Automatic recognition of falls in gait-slip training: Harness load cell based criteria. Journal of Biomechanics. 2011;44:2243–2249. doi: 10.1016/j.jbiomech.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]