The advent of general anesthesia remains one of the most important advances in the history of medicine. Although the first popular demonstration of general anesthesia occurred more than 150 years ago at the now-famous ether dome of the Massachusetts General Hospital, the mechanism by which it occurs is still debated (1). Surprisingly, among all of the questions asked about general anesthesia, the important issue of if there is an endogenous analogue of the process is rarely discussed. This situation is in distinction to the usual course of events when an exogenous natural product is found to have biological activity in that much activity is devoted to a search for the endogenous counterpart. Think, for example, about the opiates and the endorphins. As a formal proposition the question is if the brain or body makes compounds that have the properties and mode of action of general anesthetics. The issue reduces to what, if any, natural process are the general anesthetics mimicking. This question surfaces now because of the recent discovery of a sleep-inducing lipid, oleamide, in the cerebrospinal fluid (CSF) of sleep-deprived cats (2, 3). Oleamide since has been shown to affect diverse membrane proteins (4), has a structure expected to perturb the fluidity of membrane lipids, and is accompanied by a membrane-bound brain enzyme that rapidly inactivates the compound (3, 5). It is therefore possible that the way oleamide operates is unusual in that it perturbs lipid matrices either free or complexed to proteins and protein assemblies. This concept is worth considering because it would represent a new form of regulation distinct from the usual receptor-ligand interaction.
The main chemical facts about general anesthetics concern their diverse nature, hydrophobicity, and general lack of stereospecificity. The oldest correlate is called the Meyer–Overton rule, which states that the strength of a general anesthetic is proportional to its solubility in olive oil (6). This feature, which must be considered as a measure of general hydrophobicity of the anesthetic, has received much attention because of the remarkable precision of the correlation. The Meyer–Overton correlation and its modern counterparts together with the knowledge that stereospecificity often is lacking has led many to propose that general anesthetics operate by fluidizing the plasma membrane of brain cells. Indeed, experimental studies have shown that general anesthetics are potent fluidizers of natural and artificial membranes. One problem with the simple notion that general anesthetics operate by altering the bulk fluidity of membranes is that although large quantities of these compounds do fluidize membranes, no fluidity changes are observed in experiments that restrict the concentrations of these compounds to those expected to be present in the brain under actual conditions of anesthesia (7). This finding has led some to postulate that anesthetics directly interact with proteins (8), and others have speculated that they perturb specialized lipid matrices at the protein-lipid interface (9) (Fig. 1). If the latter postulate is true and there is an endogenous counterpart, then we can expect a new kind of transduction mechanism that, rather than operating in the usual ligand-receptor interaction, effects the function of membrane proteins by perturbing their environment. When the effect depends on altering membrane fluidity such compounds can be considered to be fluidity transmitters. This idea implies that some membrane proteins are sensitive to their lipid environment and that membrane alteration can change the conformations of these proteins in much the same way that secondary modifications of proteins and allosteric regulators operate. Interestingly, many lipids such as cholesterol esters form liquid crystals. If such ordered crystalline arrays were associated with membrane proteins or channels, then there is a marvelous opportunity for regulation by controlling the orientational order in the crystal-liquid transition. Such a system would constitute a new kind of molecular switch. Although little is known about this form of regulation, it is already clear that the fluidity of membranes is precisely regulated. For example, Escherichia coli precisely regulates the fluidity of their membranes by increasing the olefinic content of their lipids as the surrounding temperature is lowered. This regulation is accomplished, in part, by the induction of desaturase enzymes (10, 11). Recently, carp also have been found to possess an analogous process of desaturase-mediated increases in membrane fluidity in response to a drop in environmental temperature (12), indicating that mechanisms for the precise regulation of membrane lipid composition and structure have been conserved among both prokaryotes and eukaryotes. Additionally, the activity of several membrane receptors has been shown to be affected by changes in membrane fluidity (13–15), and in the 5HT1A receptor, the protein also has been found to copurify selectively with saturated phospholipids, supporting the notion that membrane receptors may indeed reside within particular lipid microdomain environments to preserve function (16).
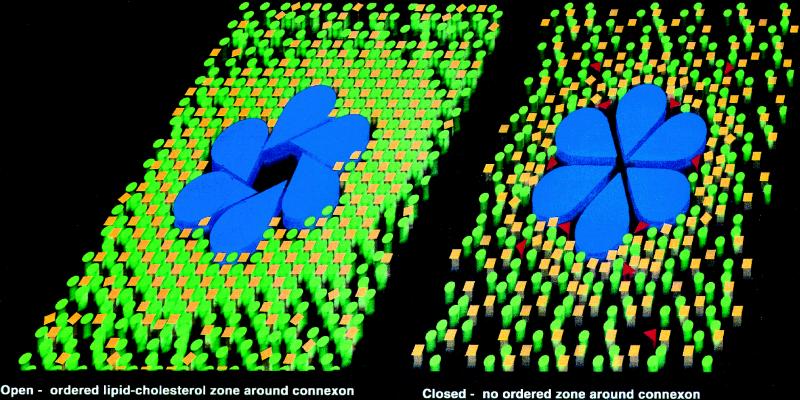
Figure 1.
A possible mechanism for oleamide-induced closure of gap junction membrane channels. A mixture of phospholipids (green circles) and cholesterol (yellow squares) are proposed to form a well-ordered lipid annulus surrounding the gap junction connexon (blue). On treatment with oleamide (red triangles), this lipid ring is fluidized and becomes disordered, promoting a conformational change in the connexon oligomer that leads to gap junction channel closure.
Oleamide is the primary amide of oleic acid. Oleamide was isolated from the CSF of sleep-deprived cats, and it and other fatty acid amides also have been found in the CSF and plasma of humans (3, 17). The molecular diversity that can be generated in this class of compounds in terms of the length of the alkane chain and the number, position, and configuration of the double bonds allows for specialization among effectors. Structurally, oleamide is not unlike some pheromones except that in the case of pheromones, a methyl ester replaces the amide functionality of oleamide (18). The presence of a long alkane chain with a centrally placed cis double bond and the presence of a primary amide with strong hydrogen bonding potential makes oleamide potentially very disruptive of membrane rigidity. The cis double bond is expected to perturb the packing of saturated alkanes in the membrane, whereas the amide should cause the formation of stacks of oleamide much like the β-sheets of proteins. In vivo, oleamide potentiates sleep and lowers temperature. Its effects on membrane proteins, so far, are to potentiate serotonin receptors (5) and close gap junction channels (19). In the case of the gap junction channel we now have an appreciation for the structural basis of the closure (V. M. Unger and M. Yeager, personal communication), but not how oleamide accomplishes this feat. The ability of oleamide to close the gap junction channel is of high interest given the suggestion of Gage and colleagues (20) that general anesthetics alter the stability of receptor-channel complexes. Likewise, Brett and colleagues (21) showed that the anesthetic Isoflurane caused “flickering” of the acetylcholine receptor.
The mode of synthesis and destruction of oleamide offers many points of potential regulation as might be expected for an important effector. Although not proven as the natural biosynthetic route, it has been shown that fatty acid amides can be enzymatically synthesized from their acyl-glycine precursors by a peptidylglycine-amidating monooxygenase (PAM)-dependent reaction, analogous to the biosynthesis of C terminally amidated peptide hormones (22). Oleamide is inactivated by a membrane-bound enzyme, fatty acid amide hydrolase (FAAH), which hydrolyses oleamide to oleic acid (3, 5, 23). In the rat brain, FAAH is predominantly expressed in neurons of the cortex, amygdala, hippocampus, thalamus, and confined regions of the hypothalamus, brain stem and cerebellum (24). FAAH is the first molecularly characterized mammalian member of a large family of amidase enzymes (5), and in both rat and human, apparently is derived from a single-copy gene (5, 23). The amidase family members share a common signature sequence of unknown significance, whereas FAAH has evolved a unique membrane spanning domain as well as a SH3-binding domain sequence.
Given the properties of general anesthetics and what is known about oleamide, it raises the intriguing possibility that both compounds could operate in a similar fashion. One could envision that a single or many membrane-spanning proteins or channels are surrounded by an annulus of specialized lipids that when perturbed by oleamide change their conformation (Fig. 1). This hypothesis is identical to that suggested by Lee (9) and others (8) except that now an additional postulate is made that there is an endogenous effector leading to a unique method of cell regulation. Indeed, Ordway and colleagues (25) suggested that there is a direct regulation of ion channels by fatty acids. As suggested above, however, the fatty acid amides would be expected to be more powerful perturbants of membrane than the free acids. Perhaps consistent with this notion, oleic acid has been shown to have no effect on either the closure of the gap junction channel (19) or on the potentiation of 5-HT receptors (4). Interestingly, the structural homology of oleamide to pheromones might suggest a primitive regulatory device for basal functions such as sleep and temperature. Later, more complex lipids such as steroids and prostaglandins evolved along with their companion-specific receptors to regulate more differentiated functions such as immunity and inflammation.
There are many weaknesses in this argument. First, it does not necessarily follow that there is an endogenous counterpart to every effector that perturbs the human condition. After all, what is the endogenous counterpart of an auto accident? Second, the true endogenous role of oleamide has yet to be determined, nor has it been ruled out that oleamide functions via a more traditional receptor. In this context some scientists have come to the conclusion that general anesthetics operate principally by interacting with proteins (26, 27). If this process was how oleamide functioned in the closure of the gap junction channel, for example, the compound then would be thought of as an allosteric regulator that binds to pockets in proteins via its hydrophobic component. The binding might be stabilized by hydrogen bonding interactions between the relatively polar amide and molecules that solvate the protein surface. If oleamide acted in this way it still could be a mimic of general anesthetics although the mechanistic implications would differ. Finally, there is little doubt that, as for other organisms, mammals must regulate the fluidity of their membranes with extreme precision. The question is whether this general function has been specialized by the evolution of an endogenous fluidity transmitter that regulates some of the proteins that span the membrane. It would be this process that general anesthesia is mimicking.
References
- 1.Collins V J. Physiologic and Pharmacologic Bases of Anesthesia. Baltimore: Williams & Wilkins; 1996. [Google Scholar]
- 2.Lerner R A, Siuzdak G, Prospero-Garcia O, Henriksen S J, Boger D L, Cravatt B F. Proc Natl Acad Sci USA. 1994;91:9505–9508. doi: 10.1073/pnas.91.20.9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cravatt B F, Prospero-Garcia O, Siuzdak G, Gilula N B, Henriksen S J, Boger D L, Lerner R A. Science. 1995;268:1506–1509. doi: 10.1126/science.7770779. [DOI] [PubMed] [Google Scholar]
- 4.Huidobro-Toro J P, Harris R A. Proc Natl Acad Sci USA. 1996;93:8078–8082. doi: 10.1073/pnas.93.15.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cravatt B F, Giang D K, Mayfield S P, Boger D L, Lerner R A, Gilula N B. Nature (London) 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 6.Meyer H H. Arch Exp Pathol Pharmakol. 1899;42:109–118. [Google Scholar]
- 7.Franks N P, Lieb W R. J Mol Biol. 1979;133:469–500. doi: 10.1016/0022-2836(79)90403-0. [DOI] [PubMed] [Google Scholar]
- 8.Franks N P, Lieb W R. Nature (London) 1981;292:248–251. doi: 10.1038/292248a0. [DOI] [PubMed] [Google Scholar]
- 9.Lee A G. Nature (London) 1976;262:545–548. doi: 10.1038/262545a0. [DOI] [PubMed] [Google Scholar]
- 10.Fulco A J. Annu Rev Biochem. 1974;43:215–241. doi: 10.1146/annurev.bi.43.070174.001243. [DOI] [PubMed] [Google Scholar]
- 11.Okuyama H, Saito M, Vasudev J, Gunsberg S, Wakil S. J Biol Chem. 1979;254:12281–12284. [PubMed] [Google Scholar]
- 12.Tiku P E, Gracey A Y, Macartney A I, Beynon R J, Cossins A R. Science. 1996;271:815–818. doi: 10.1126/science.271.5250.815. [DOI] [PubMed] [Google Scholar]
- 13.Heron D S, Shinitzky M, Hershkowitz M, Samuel D. Proc Natl Acad Sci USA. 1980;77:7463–7467. doi: 10.1073/pnas.77.12.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong K, Qiu Y, Hyun W, Nixon R, VanCleff J, Sanchez-Salazar J, Prusiner S B, DeArmond S J. Neurology. 1996;47:741–750. doi: 10.1212/wnl.47.3.741. [DOI] [PubMed] [Google Scholar]
- 15.Ma Z, Lee S S, Meddings J B. J Hepatol. 1997;26:904–912. doi: 10.1016/s0168-8278(97)80259-0. [DOI] [PubMed] [Google Scholar]
- 16.Banerjee P, Dawson G, Dasgupta A. Biochem Biophys Acta. 1992;1110:65–74. doi: 10.1016/0005-2736(92)90295-w. [DOI] [PubMed] [Google Scholar]
- 17.Arafat E S, Trimble J W, Andersen R N, Dass C, Desiderio D M. Life Sci. 1989;45:1679–1687. doi: 10.1016/0024-3205(89)90278-6. [DOI] [PubMed] [Google Scholar]
- 18.Rasmussen L E, Lee L, Roelofs T D, Zhang W L, Daves A G D., Jr Nature (London) 1996;379:684. doi: 10.1038/379684a0. [DOI] [PubMed] [Google Scholar]
- 19.Guan, X. G., Cravatt, B. F., Ehring, G. R., Hall, J. E., Boger, D. L., Lerner, R. A. & Gilula, N. B. (1997) J. Cell Biol., in press. [DOI] [PMC free article] [PubMed]
- 20.Gage P W, McKinnon D, Robertson B. In: Molecular and Cellular Mechanisms of Anesthetics. Roth S H, Miller K W, editors. New York: Plenum; 1986. pp. 139–154. [Google Scholar]
- 21.Brett R S, Dilger J P, Yland K F. Anesthesiology. 1988;69:161–170. doi: 10.1097/00000542-198808000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Merkler D J, Merkler K A, Stern W, Fleming F F. Arch Biochem Biophys. 1996;333:430–343. doi: 10.1006/abbi.1996.0272. [DOI] [PubMed] [Google Scholar]
- 23.Giang D K, Cravatt B F. Proc Natl Acad Sci USA. 1997;94:2238–2242. doi: 10.1073/pnas.94.6.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas E A, Cravatt B F, Danielson P E, Gilula N B, Sutcliffe J G. J Neurosci Res. 1997;50:1–6. doi: 10.1002/(SICI)1097-4547(19971215)50:6<1047::AID-JNR16>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 25.Ordway R W, Singer J J, Walsh J V., Jr Trends Neurosci. 1991;14:96–100. doi: 10.1016/0166-2236(91)90069-7. [DOI] [PubMed] [Google Scholar]
- 26.Franks N P, Lieb W R. Nature (London) 1978;274:339–342. doi: 10.1038/274339a0. [DOI] [PubMed] [Google Scholar]
- 27.Franks N P, Lieb W R. Nature (London) 1982;300:487–493. doi: 10.1038/300487a0. [DOI] [PubMed] [Google Scholar]