Abstract
We investigated actin's function in vesicle recycling and exocytosis at lamprey synapses and show that FM1-43 puncta and phalloidin-labeled filamentous actin (F-actin) structures are colocalized, yet recycling vesicles are not contained within F-actin clusters. Additionally, phalloidin also labels a plasma membrane-associated cortical actin. Injection of fluorescent G-actin revealed activity-independent dynamic actin incorporation into presynaptic synaptic vesicle clusters but not into cortical actin. Latrunculin-A, which sequesters G-actin, dispersed vesicle-associated actin structures and prevented subsequent labeled G-actin and phalloidin accumulation at presynaptic puncta, yet cortical phalloidin labeling persisted. Dispersal of presynaptic F-actin structures by latrunculin-A did not disrupt vesicle clustering or recycling or alter the amplitude or kinetics of excitatory postsynaptic currents (EPSCs). However, it slightly enhanced release during repetitive stimulation. While dispersal of presynaptic actin puncta with latrunculin-A failed to disperse synaptic vesicles or inhibit synaptic transmission, presynaptic phalloidin injection blocked exocytosis and reduced endocytosis measured by action potential-evoked FM1-43 staining. Furthermore, phalloidin stabilization of only cortical actin following pretreatment with latrunculin-A was sufficient to inhibit synaptic transmission. Conversely, treatment of axons with jasplakinolide, which induces F-actin accumulation but disrupts F-actin structures in vivo, resulted in increased synaptic transmission accompanied by a loss of phalloidin labeling of cortical actin but no loss of actin labeling within vesicle clusters. Marked synaptic deficits seen with phalloidin stabilization of cortical F-actin, in contrast to the minimal effects of disruption of a synaptic vesicle-associated F-actin, led us to conclude that two structurally and functionally distinct pools of actin exist at presynaptic sites.
Keywords: synaptic vesicle release, cytoskeleton, exocytosis, endocytosis
actin appears ubiquitously at presynaptic terminals (Hirokawa et al. 1989; Landis et al. 1988; Wagner and Kelly 1979); however, its presynaptic functions remain elusive (Cingolani and Goda 2008). Actin exists in two states, monomeric G-actin, which is evenly distributed throughout axons at synaptic and extrasynaptic sites (Zhang and Benson 2002), and filamentous F-actin, which may surround vesicle pools (Kuromi and Kidokoro 1998; Richards et al. 2004; Sankaranarayanan et al. 2003; Shupliakov et al. 2002), with lower density within the pool (Bloom et al. 2003; Morales et al. 2000). Actin's association with presynaptic vesicle clusters has led to its proposed structural role. Actin filaments have been proposed to associate with synaptic vesicles via synapsin (Bahler and Greengard 1987; Li et al. 1995), which tethers vesicles (Landis et al. 1988; Li et al. 1995; Pieribone et al. 1995). However, F-actin disruption fails to disperse vesicle clusters (Bourne et al. 2006; Gaffield et al. 2006; Kuromi and Kidokoro 1998; Richards et al. 2004; Sankaranarayanan et al. 2003; Shupliakov et al. 2002), while disruption of synapsin results in a decrease in synaptic vesicle cluster size (Pieribone et al. 1995; Siksou et al. 2007).
Actin has also been proposed to play a dynamic role. Reorganization of F-actin can be induced by stimulation in many preparations (Bernstein and Bamburg 1989; Bloom et al. 2003; Sankaranarayanan et al. 2003; Shupliakov et al. 2002). However, G-actin has also been shown to incorporate into presynaptic F-actin clusters at rest, suggesting additional activity-independent reorganization (Bourne et al. 2006). Indeed, actin turnover is necessary for synaptic transmission because stabilization of F-actin by phalloidin (Vandekerckhove et al. 1985) inhibits neurotransmitter release (Bernstein and Bamburg 1989; Photowala et al. 2005), while agents that prevent actin polymerization or induce depolymerization result in only minimal enhancing effects on exocytosis (Kuromi and Kidokoro 1998; Sankaranarayanan et al. 2003) and synaptic transmission (Cole et al. 2000; Kuromi and Kidokoro 1998; Morales et al. 2000; Richards et al. 2004; Wang et al. 1996). Actin may also act as a barrier to exocytosis (Dillon and Goda 2005), for blockade of actin polymerization can enhance evoked release (Morales et al. 2000; Wang et al. 1996), However, this may not be true for all synapses (Sakaba and Neher 2003).
Finally, actin has been implicated in endocytosis. Actin has been visualized in association with clathrin-coated vesicles (Kaksonen et al. 2003; Merrifield et al. 1999) and as filaments attached to endocytosed vesicles in fibroblasts (Merrifield et al. 1999, 2005) and lamprey axons (Shupliakov et al. 2002). F-actin disruption causes deficits in vesicle recycling determined ultrastructurally, manifested as increases in clathrin-coated intermediaries, reduced vesicle recycling, vesicle pool depletion, and the appearance of recycling vesicles associated with filaments emanating from endocytic zones (Bloom et al. 2003; Shupliakov et al. 2002). However, actin polymerization inhibitors show little effect on vesicle recycling (Sankaranarayanan et al. 2003).
While the roles of actin at presynaptic sites remain contested, these discrepancies may be compounded by differences in synapse type (Dillon and Goda 2005) and synaptic activity (Cingolani and Goda 2008). However, discrepancies may also have arisen because of different approaches used and different access to the presynaptic terminal, which limit comparative analyses. In most synapses it is impossible to access the terminal directly. Thus we have utilized the unique accessibility of lamprey giant axon terminals to determine the effects of modification of actin polymerization and depolymerization on synaptic transmission and vesicle cycling. We present evidence that in lamprey giant axons destabilization of a dynamic actin pool associated with synaptic vesicle clusters does not modify synaptic transmission and leaves the entire synaptic vesicle cycle intact. However, alterations to the stability of a membrane-associated cortical F-actin pool (cortical actin) dramatically effects exocytosis and synaptic transmission.
MATERIALS AND METHODS
Experiments were performed on isolated spinal cords of 90 larval and young adult lampreys (Petromyzon marinus). All procedures on animals were reviewed and approved by the University of Illinois at Chicago Animal Care Committee. This committee adheres to guidelines set out by the National Institutes of Health and of the Association for Assessment and Accreditation of Laboratory Animal Care. The animals were anesthetized with tricaine methanesulfonate (MS-222; 100 mg/l; Sigma, St. Louis, MO) applied in the aquarium water. They were then killed by decapitation and dissected in a cold saline solution (Ringer) of the following composition (mM): 100 NaCl, 2.1 KCl, 2.6 CaCl2, 1.8 MgCl2, 4 glucose, and 5 HEPES, adjusted to a pH of 7.60.
Electrophysiology.
Lamprey reticulospinal axons were impaled and recorded from under current-clamp conditions with conventional sharp microelectrodes containing 1–3 M KCl. For those experiments in which phalloidin was injected into the presynaptic terminal, the phalloidin (labeled with Alexa Fluor 568 or 488; Invitrogen, Eugene, OR) was previously dissolved in deionized water at a concentration of 3 units/μl (final concentration 100 μM) and stored at −20°C for no longer than 4 wk. Prior to the experiment, the stock solution was added to an equal volume of 3 M KCl and the electrode was filled with this combination. Electrode impedances ranged from 20 to 50 MΩ. Phalloidin was applied to the interior of the recorded axon by pressure injection from the microelectrode. For experiments in which G-actin was injected into the presynaptic terminal, the G-actin (actin from rabbit muscle labeled with Alexa Fluor 488; Invitrogen) was stored at −20°C in buffer at a concentration of 11 mg/ml for no longer that 4 wk. Prior to the experiment the stock solution was further diluted in G-actin buffer [mM: 2 HEPES, 0.2 CaCl2, and 0.2 ATP, pH 8.0 (final concentration 1.1 mg/ml)] and the electrode was filled with this combination. Patch electrodes for whole cell postsynaptic recordings contained (mM) 102.5 K methanesulfonate, 1 NaCl, 1 MgCl2, 5 EGTA, and 5 HEPES. Osmolarity and pH were adjusted to physiological levels (240 mosmol/l and 7.2, respectively).
Imaging.
Confocal imaging was performed with a modified Bio-Rad MRC 600 confocal microscope. Two excitation wavelengths were used (488-nm argon ion laser and 568-nm krypton-argon laser) through the AOTF-coupled fiber-optic launch (Prairie Technologies, Madison, WI). Excitation was applied through a custom dichroic mirror with sharp excitation bands matching the two laser wavelengths (Omega Optical). Two detectors were placed after a second dichroic, with a transmission band from 500 to 560 nm and a long-pass reflection from 580 nm. Emission filters were band pass (500–560 nm) and long pass (above 580 nm). The photomultiplier outputs were amplified with low-noise current amplifiers (Stanford Instruments) and digitized to 12 bits with a National Instruments board and custom software written under MATLAB (MathWorks). The scan head mirrors were driven through the MRC 600 scan head amplifiers with the same custom software. This software is available on our website (http://alford.bios.uic.edu/Research/software.html).
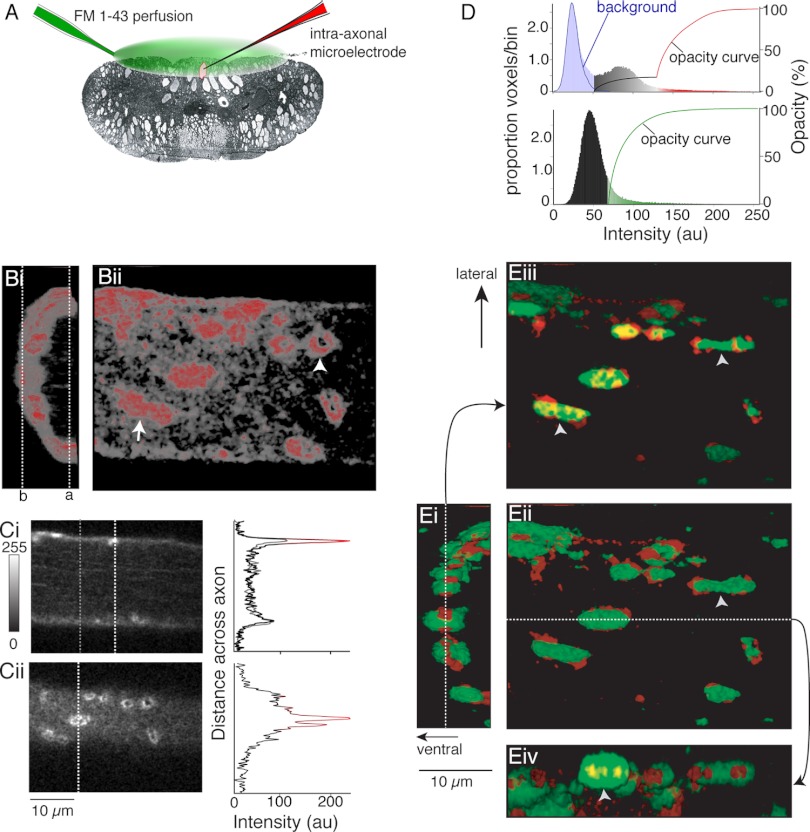
Volume data (Fig. 1, Fig. 2) were reconstructed with Voxblast software (Vaytech, Iowa City, IA). It is not possible to display volume data linearly; therefore, we have graphically represented voxel intensities, color, and opacity data (Fig. 1D) to demonstrate how different regions of axon labeling with phalloidin were displayed. Voxel intensity histograms from the data sets shown (Fig. 1, B and E) are displayed, overlaid with voxel opacity curves used to display data, to demonstrate that this cortical actin labeling was distinct from background fluorescence sampled outside of the labeled axons (Fig. 1D). A distinct population of low-intensity voxels are separable from this background. The opacity curve shown in this graph represents opacities applied to the three-dimensional (3D) voxel reconstructions (Fig. 1B, i and ii). The colors used in these histograms demonstrate the color coding of voxels in the images.
Fig. 1.
Distribution of phalloidin labeling and FM-labeled vesicle clusters in axons. A: recording schematic. FM1-43 was superfused over the ventral spinal cord (green). Axons impaled with microelectrodes containing KCl (3 M) were loaded with FM1-43 by intracellular stimulation, and the tissue was cleared of dye with Advasep-7. Axons were reimpaled (electrode contained Alexa Fluor 568 phalloidin; red) for injection into the axon. Confocal z-sections were taken of live axons. B: phalloidin labels both synaptic structures and cortical actin. Bi: 3-dimensional (3D) reconstruction viewed along length of the axon (caudal-rostral) showing ventral half of axon and phalloidin labeling (ventral-left). Red, intense labeling at puncta; gray, dimmer, diffuse labeling under plasma membrane—cortical actin [see D for color look-up table (LUT)]. Bii: view of same axon from the spinal ventral surface. Ci: optical section from dashed line a in Bi. Graph shows intensity profile through the section at dashed lines [white, through active zone (graph color from same LUT as B); gray, outside synaptic regions]. This graph is in gray. Cii: optical section b in Bi. Graph of intensity profile along dashed line in image. Colors from LUT as for Ci. D, top: voxel intensity histogram from data set in B. Background noise sampled from outside axons (blue line). This matches background noise under the image (light blue bars). Other histogram bar colors represent the LUT used to generate 3D reconstructions. Black to red curve represents opacity values used in B. Bottom: voxel intensity histogram for FM1-43 labeling; opacity curve to display FM1-43 in E in green. au, Arbitrary units. Ei: 3D overlay of phalloidin structures (red) and FM1-43 labeling (green) (line, section in Eiii). Eii: axon from spinal ventral surface (view from left of Ei) (line, position of section in Eiv). Eiii: view as Ei but top sections removed (cut at dashed line in Ei) to reveal phalloidin and FM1-43 colocalization (arrows: phalloidin, red; FM-loaded vesicles, bright green) at the cut at dashed line in Ei. Yellow, colocalization at cut. Eiv: sagittal cut of FM1-43 and phalloidin seen from lateral spinal cord with lower half below dashed line in Eii removed. Vesicle clusters are associated with phalloidin, sometimes spanning more than 1 phalloidin structure (arrowheads in Eii, Eiii). FM/phalloidin colocalized (yellow) can be contained within an FM1-43 puncta (arrowhead in Eiv).
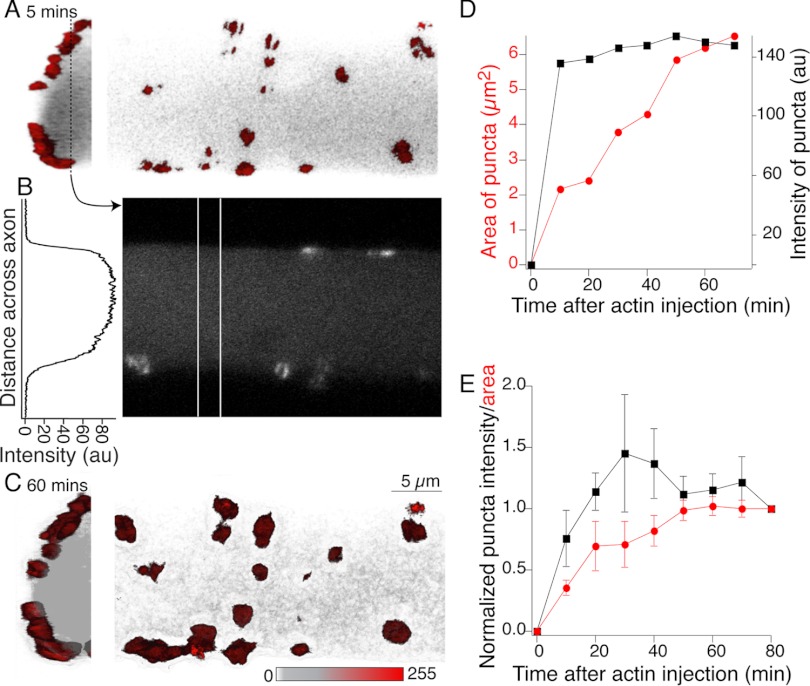
Fig. 2.
G-actin incorporates into presynaptic puncta but not cortical actin. Reticulospinal axon impaled with a microelectrode containing Alexa Fluor 488 G-actin (1.1 mg/ml) and buffer solution (2 mM HEPES, 0.2 mM CaCl2, 0.2 mM ATP, pH 8.0). This was pressure injected into the axon. Fluorescent actin structures formed within minutes of injection and increased in fluorescence over 80 min. A: 3D reconstruction of actin fluorescence close to the axon perimeter 5 min after injection. Hemi-axon is shown in transverse section (left) and from the ventral spinal surface (right). Red structures are bright actin labeling consistent with synaptic vesicle cluster colocalization; gray is the diffuse labeled G-actin within the axon. B: single optical section shown in linear grayscale taken from the dashed line in A. The graph is a profile plot of intensity taken from between the vertical white lines. There is no cortical actin signal. C: same views as A but after an hour of recording. D: mean intensity (black) and cross-sectional area (red) of all clusters in A plotted with time after injection. E: mean area and intensity (6 preparations) normalized to value 80 min after injection.
Application of FM dye and latrunculin-A.
FM1-43 (5 μM) was applied as a stream from a small pipette placed over the surface of the spinal cord (Fig. 1B). Constant flow was ensured with use of a syringe pump. Two thousand stimuli were applied to a microelectrode-recorded axon during the dye application, while the presence of the dye in the tissue surrounding the axons was confirmed with imaging. Dye application was subsequently terminated. During the staining protocol, synaptic activity was blocked with glutamate receptor antagonists 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and d-aminophosphonovalerate (AP-5) (10 and 100 μM, respectively). Excess dye was removed with Advasep-7 (Kay et al. 1999; 1 mM, 5 min; Cydex, Lenexa, KS) to reveal areas of stimulus-dependent staining. For experiments in which axons were treated with latrunculin-A (Invitrogen, Sigma), the latrunculin-A was previously dissolved in DMSO to a concentration of 24 mM and stored at −20°C for no longer than 4 wk. Prior to the experiment, the stock solution was added to 4 ml (final concentration 12 μM) of Ringer solution and superfused over the tissue in the same manner as FM application. Saturation of the tissue was monitored by adding Fast green to the solution.
All means of data are expressed as means ± SE. All statistical tests of significance used Student's t-test for difference in mean or mean of means.
RESULTS
Colocalization of actin and synaptic vesicles labeled with FM1-43.
Presynaptic actin has been proposed to surround a pool of vesicles in lamprey axons (Bourne et al. 2006; Shupliakov et al. 2002), although the localization of the active recycling pool of vesicles and presynaptic actin has not been shown in vivo in this preparation. Therefore, we determined the spatial relationship between the recycling synaptic vesicle pool and F-actin in the presynaptic terminal.
We investigated the location of F-actin in axons and presynaptic terminals with phalloidin. Axons were recorded from with electrodes containing KCl (1.5 M) and Alexa Fluor 598-labeled phalloidin (50 μM). Phalloidin was pressure ejected into axons from the microelectrode (Fig. 1A). Phalloidin has been shown to label ringlike structures associated with presynaptic periactive zones (Bourne et al. 2006; Shupliakov et al. 2002). However, we found that after phalloidin injection more complex bar-like structures were seen (Fig. 1, Bii and Cii) in addition to structures that sometimes resembled rings (Fig. 1Bii). In addition, we found that at lower fluorescence intensities a second distribution of phalloidin labeling is detectable above background signal (Fig. 1D) that labels the plasma membrane continuously. Thus in the 3D reconstruction (Fig. 1B) diffuse cortical staining is observed at the plasma membrane, punctuated by distinct points of phalloidin staining. The diffuse submembrane staining (Fig. 1B), which was not previously reported, is consistent with cortical actin (Trifaro et al. 1992), while the more intense structures (Fig. 1, B and E) we now further show (see below) to be associated with recycling synaptic vesicle clusters.
To quantify the relative locations and intensity of the cortical and vesicle cluster-associated fluorescence, phalloidin staining in two optical sections (from dashed lines in Fig. 1Bi) is shown separately (Fig. 1C). Absolute intensities in a cross section at each of these optical sections are plotted from lines that passed through phalloidin labeling associated with a synaptic vesicle cluster and through a region with no synaptic vesicle cluster (Fig. 1C). Labeling associated with the synaptic vesicle clusters is clearly distinct from the axon cortical actin signal, which is in turn distinct from background fluorescence.
To confirm that F-actin clusters and recycled vesicles colocalize, we labeled recycling vesicles with the styryl dye FM1-43 followed by injection of phalloidin into the axons (n = 8 axons). To label synaptic vesicles with FM1-43, we recorded intracellularly from reticulospinal axons and stimulated (2,000 action potentials, 5 Hz) while applying FM1-43 to the spinal cord through a perfusion pipette held immediately above the ventral surface of the spinal cord (Fig. 1A) (Photowala et al. 2005). Excess dye was cleared from the tissue with Advasep-7 (Kay et al. 1999) to reveal fluorescently labeled synaptic vesicle clusters (Fig. 1E). We then injected Alexa Fluor 598-labeled phalloidin into the same axon through the recording microelectrode. Using confocal microscopy, we imaged serial optical sections of the axons to reveal the location of F-actin relative to FM1-43-labeled vesicle clusters.
To demonstrate the association between vesicles and phalloidin labeling, the cortical, low-intensity phalloidin staining was thresholded out (voxels above a value of 120 are shown in red; see Fig. 1D for opacity settings and color look-up tables) to reveal presynaptic F-actin structures. The FM1-43 labeling appears as fluorescent puncta associated with the intensely labeled presynaptic F-actin. Phalloidin labeling of F-actin has been shown previously to form ringlike structures in lamprey giant axons that have been suggested to surround the presynaptic vesicle pool (Bourne et al. 2006; Shupliakov et al. 2002). Here we now show optical sections through phalloidin-labeled F-actin associated with the synaptic vesicle puncta that revealed complex structures that colocalized with FM-labeled vesicles but did not surround the pool of vesicles. In contrast, the F-actin structures were found both around and contained within the labeled vesicle pools (Fig. 1Eiv) and others where labeled vesicle clusters spanned across two independent F-actin structures (Fig. 1Eiii). Taken together, our results suggest that the full component of active vesicle pools is not contained within filamentous rings of F-actin and, furthermore, that two distinct pools of actin appear presynaptically.
Visualization of actin incorporation in axons.
In quiescent axons, monomeric G-actin has been shown to incorporate into clusters at presynaptic active sites (Bourne et al. 2006). To confirm the rate of G-actin incorporation into synaptic clusters and to determine whether this actin incorporates into the cortical actin pool, we microinjected Alexa Fluor 488-labeled monomeric G-actin into six axons in six preparations. The large electrodes used for injection were immediately removed, and the health of the axon was confirmed by reimpalement of the axons with a high-impedance KCl (1 M)-containing micropipette. Using confocal microscopy, we confirmed that monomeric G-actin clustered at synaptic sites (Fig. 2). Fluorescently labeled actin clusters appeared within minutes of injection (Fig. 2A). Their structure was visualized after reconstruction of confocal serial sections and revealed complex actin congregates. While some of these appeared as rings in optical sections (Fig. 2B), similar to phalloidin labeling, more common were barlike structures consistent with the phalloidin labeling (Fig. 1). We imaged these structures for up to 1 h after G-actin injection (Fig. 2C). Fluorescence intensity of the initially labeled structures reached its peak within 20 to 30 min (Fig. 2, D and E). However, the size of the structures measured as cross-sectional area continued to grow for up to 1 h after injection (Fig. 2, D and E), indicating continuous turnover of endogenous nonfluorescent actin with injected labeled G-actin.
However, in a marked difference from the images obtained after phalloidin injection, no cortical actin signal was observed during this period. Profile plots calculated from regions between labeled actin clusters in a single optical section (between vertical white lines in Fig. 2B) show simply uniform fluorescence in the axon volume. Comparison with those obtained after phalloidin labeling (Fig. 1, B and Ci) demonstrates an absence of fluorescent G-actin-labeled cortical actin, even though considerable diffuse actin fluorescence is visible throughout the cytosol (Fig. 2, A and C). It appears that during steady-state conditions actin associated with presynaptic vesicle clusters is dynamic with respect to a more stable cortical actin.
Disruption of dynamic actin cytoskeleton.
Presynaptic actin is believed to play a role in endocytosis (Merrifield et al. 2002) and recycling of vesicles to the vesicle cluster (Bloom et al. 2003; Bourne et al. 2006; Richards et al. 2004; Shupliakov et al. 2002). However, in cell culture blockade of actin polymerization does not prevent vesicle recycling (Sankaranarayanan et al. 2003). We sought to determine whether blockade of actin polymerization in the lamprey presynaptic terminal alters synaptic vesicle cycling.
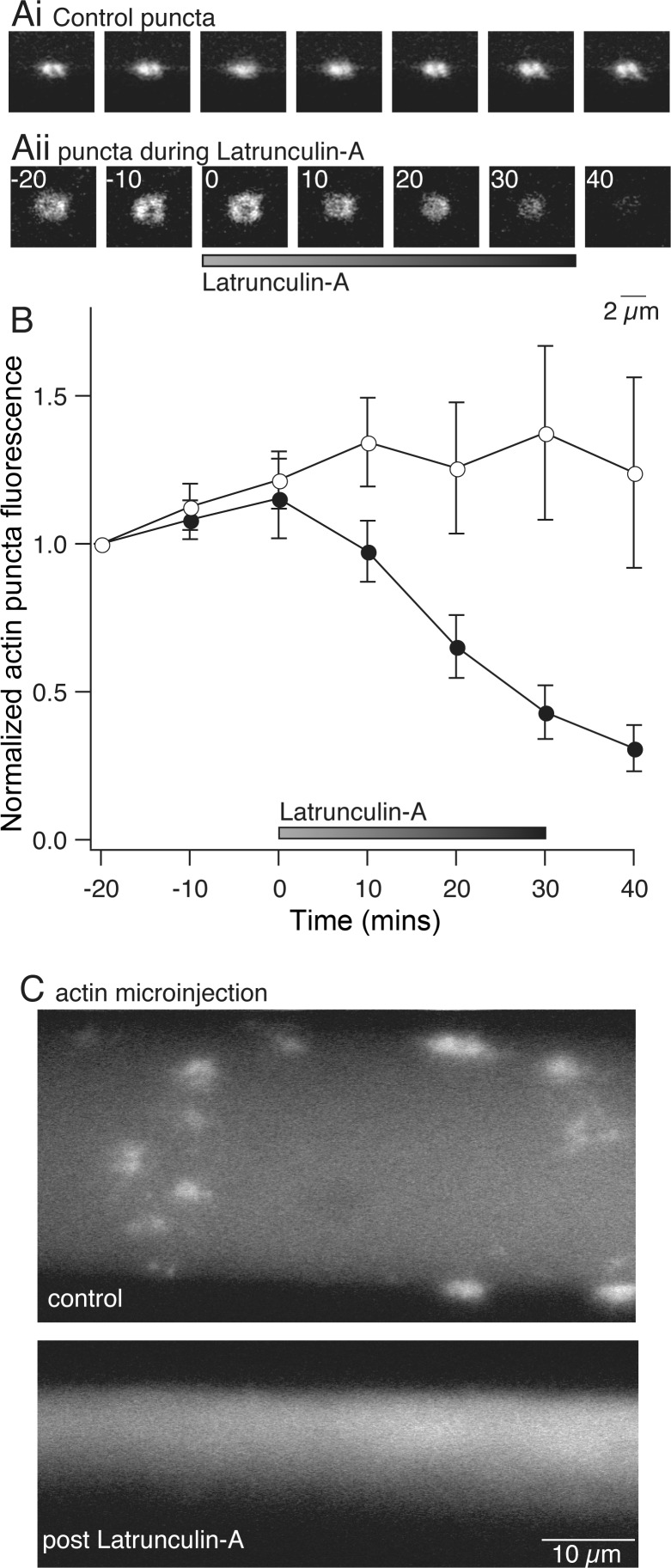
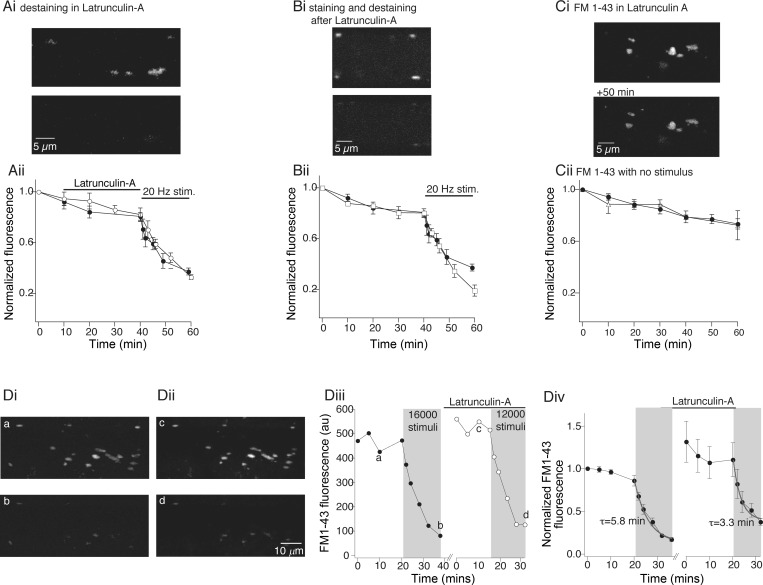
It was first necessary to demonstrate that F-actin structures can be disrupted by inhibiting actin polymerization. We used fluorescent G-actin labeling to measure the efficacy of the monomeric actin sequestering drug latrunculin-A. We injected axons with labeled G-actin followed by application of latrunculin-A (12 μM) by perfusion over the spinal cord (see materials and methods) and imaged the intensity of synaptic vesicle-associated fluorescent actin labeling (Fig. 3A). In control experiments, punctate fluorescent labeling appeared rapidly and increased in intensity over 40–50 min (Fig. 3Ai). During application of latrunculin-A, the labeled G-actin structures dispersed within 40 min of the start of treatment (to 30 ± 9% of control fluorescence measured at the same time after G-actin injection; Fig. 3, Aii and B). Furthermore, in axons pretreated with latrunculin-A for 30 min prior to labeled G-actin injection into the axons, labeled G-actin did not cluster into these punctate structures; the fluorescently labeled actin remained dispersed throughout the axon (Fig. 3C; n = 3). This inhibition of actin clustering was sustained for up to 1 h after removal of latrunculin-A treatment (data not shown). Our observation of labeled G-actin incorporation into synapse-associated structures within minutes of injection and their continued growth in size and intensity over 30 min, and the disruption of these structures within 30 min of latrunculin-A application, confirm that presynaptic vesicles are colocalized with a dynamic actin structure that is continuously turning over G-actin monomers (Bourne et al. 2006) and indicate that latrunculin-A treatment can be used to disperse these actin clusters.
Fig. 3.
Visualization of actin dynamics and disruption with latrunculin-A. Axons were labeled with fluorescent G-actin by pressure injection (as in Fig. 2). After injection, axons were reimpaled with an electrode containing 3 M KCl to monitor membrane potential. A: examples of G-actin labeling at synaptic vesicle clusters over time. Ai: in control axon, G-actin incorporated into presynaptic clusters, which increased in fluorescence and then stabilized within 40 min. Aii: to test latrunculin-A effects, G-actin was injected into presynaptic terminals. After 20 min, latrunculin-A was perfused onto the axon. G-actin clusters dispersed. B: graph of normalized mean fluorescence of puncta from control (○; n = 7) and latrunculin-A-treated (●; n = 7) axons. C: comparison of control G-actin staining and staining after pretreatment with latrunculin-A. Top: puncta form in control axons. Bottom: axons were pretreated with latrunculin-A and subsequently microinjected with G-actin. No G-actin clustering was observed after latrunculin-A, although diffuse fluorescence was observed.
However, while fluorescently labeled phalloidin and G-actin both label a punctate pool of actin at synaptic active sites, only phalloidin labels cortical actin. We wished to determine whether latrunculin-A also disrupts this actin structure. We therefore injected phalloidin into axons after 30 min of treatment with latrunculin-A (12 μM). Compared with control axons injected prior to latrunculin-A treatment (Fig. 4A), after latrunculin-A pretreatment phalloidin failed to label synapse-associated structures in any of the injected axons. However, phalloidin still labeled the cortical actin as in controls (Fig. 4B). This indicates that a stable F-actin cytoskeleton present along the plasma membrane of the axons is not dispersed after latrunculin-A treatment for 30 min under quiescent conditions, a time period sufficient to disperse actin clusters at synaptic sites. This conclusion is also supported by our finding that fluorescent G-actin fails to label the cortical actin structure (Fig. 2). Thus treatment with latrunculin-A can allow us to assess the role of the dynamic vesicle-associated actin in synaptic transmission and vesicle cycling.
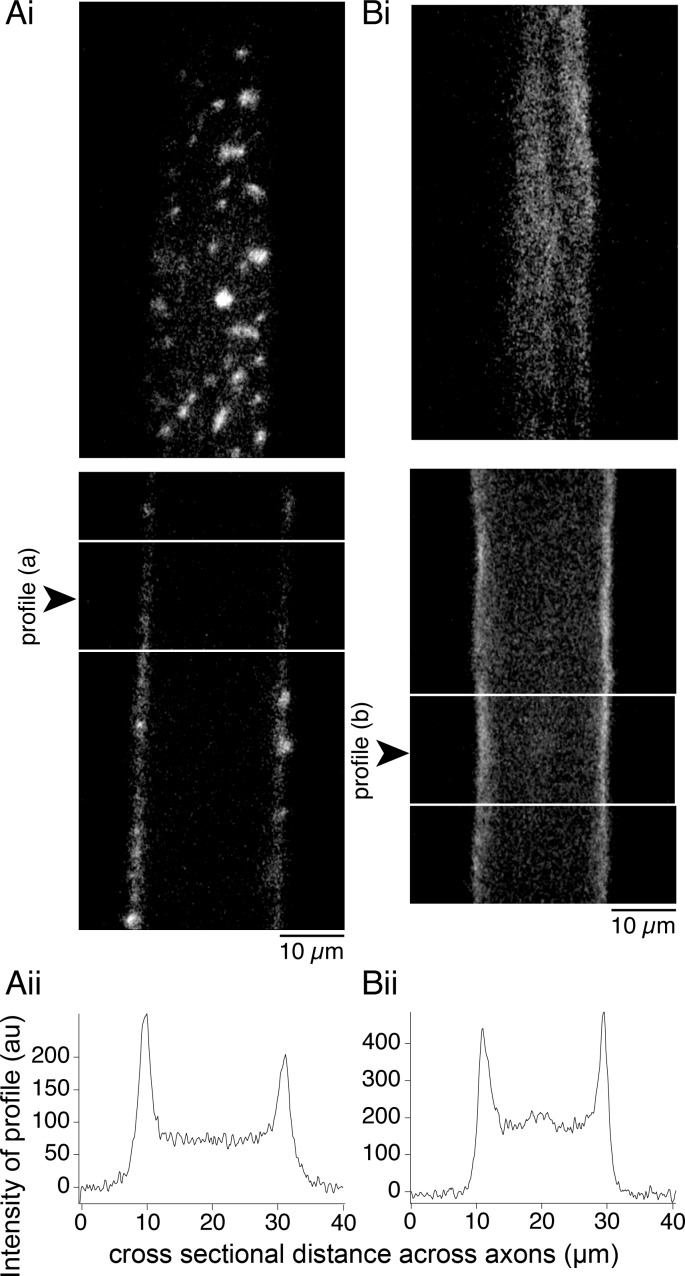
Fig. 4.
Latrunculin-A prevents incorporation of phalloidin at presynaptic terminals but not the cortical actin. Axons were recorded from with a microelectrode and injected with phalloidin (Alexa Fluor 488). Ai: control axon injected with phalloidin reveals vesicle cluster-associated fluorescent structures. Top: optical section through the ventral axon surface. Bottom: section through the center of the same axon. Aii: to quantify this, a profile of fluorescence intensity was measured across the longitudinal sections from between the horizontal white lines in Ai. Profile was placed to avoid presynaptic puncta. A fluorescence peak is seen at the plasma membrane. Bi: axon injected with phalloidin after latrunculin-A treatment (30 min, 12 μM) displays fluorescence with no clustering of phalloidin. Top: optical section at the ventral surface. Bottom: section through the center. Although no presynaptic structures are seen, plasma membrane fluorescence is visible. Bii: profile of fluorescence intensity from between lines in phalloidin-labeled axon (Bi) after pretreatment with latrunculin-A. Fluorescence peaks are seen at the membrane.
Synaptic transmission is not blocked by latrunculin-A treatment.
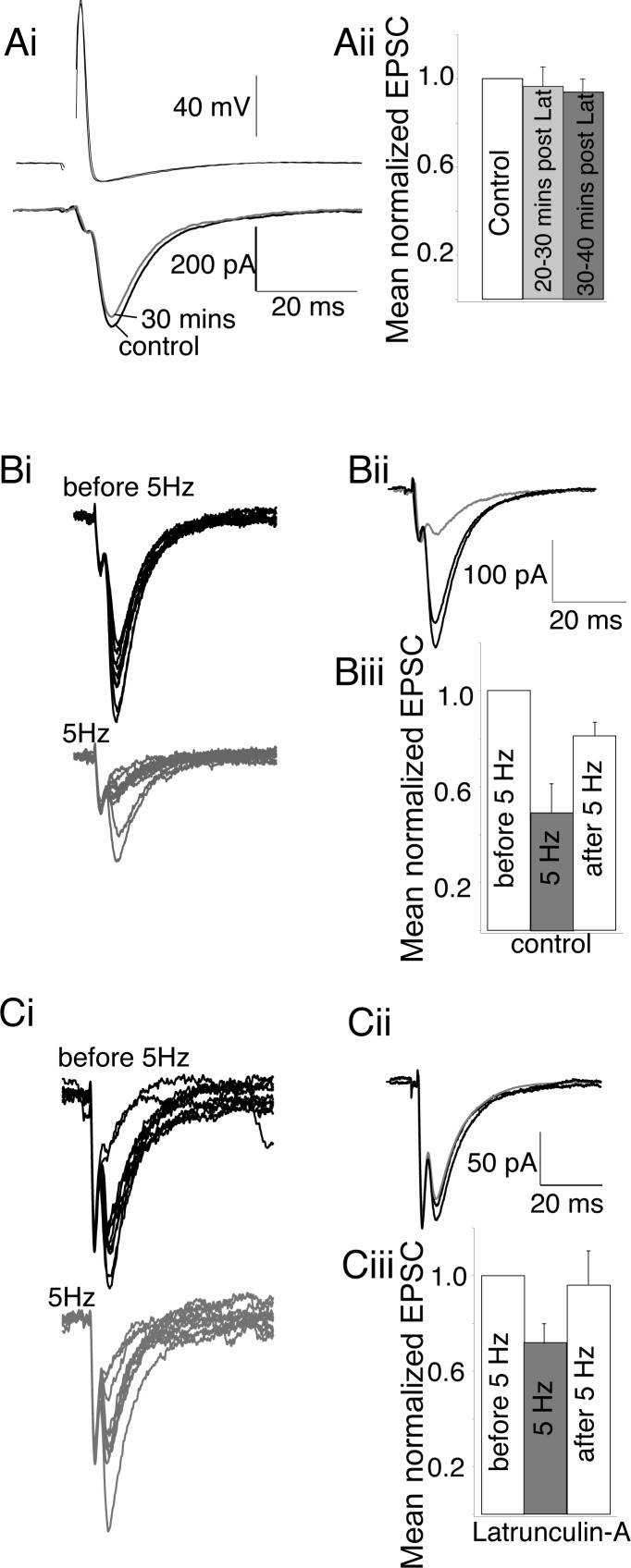
It has been suggested both that preventing F-actin formation with latrunculin-A has little effect on synaptic vesicle cycling (Sankaranarayanan et al. 2003) and, alternatively, that disruption of actin polymerization increases release probability (Morales et al. 2000). To determine the physiological effect of blocking actin polymerization on synaptic transmission at the lamprey presynaptic terminal, we conducted paired cell recordings between reticulospinal axons and their postsynaptic partners during and after treatment with latrunculin-A (30 min, 12 μM). Latrunculin-A treatment had no effect on the amplitude or kinetics of evoked excitatory postsynaptic currents (EPSCs) (Fig. 5; stimulated at 15-s intervals) during or after 30 min of treatment [mean EPSC amplitude was 97 ± 9% of control from 20 to 30 min of latrunculin-A treatment, and after wash of latrunculin-A responses remained at 92 ± 6% of control (n = 6); neither was significantly different from the control EPSC amplitude; Fig. 5A]. This is at a time point when actin-labeled structures at synapses are dispersed (Fig. 3, A and B).
Fig. 5.
Depolymerization of actin enhances synaptic responses to high frequency stimulation. A: at low frequencies of stimulation (1/15 Hz), latrunculin-A had no effect on excitatory postsynaptic current (EPSC) amplitude. Ai: means of representative traces of EPSCs before and after latrunculin-A treatment. Aii: mean normalized amplitudes of EPSCs during control, latrunculin-A treatment, and 30 min after latrunculin-A (6 preparations). B: periods of high-frequency stimulation (5 Hz) reduced the mean EPSC amplitude. Bi: representative traces (10 sequential EPSCs) before and during 5-Hz stimulation. Bii: means of these EPSCs before, during, and after 5-Hz stimulation. Biii: mean normalized amplitudes of EPSCs during 1/15 Hz stimulation, at 5 Hz, and returned to 1/15 Hz in controls (6 preparations). C: after latrunculin-A treatment 5-Hz stimulation had less effect. Ci: representative traces (10 sequential EPSCs) before, during, and after 5-Hz stimulation in latrunculin-A-treated preparations. Cii: means of these EPSCs before, during, and after 5-Hz stimulation. Ciii: mean normalized amplitudes of EPSCs during 1/15 Hz stimulation, at 5 Hz, and returned to 1/15 Hz in latrunculin-A-treated preparations (6 preparations).
Thus latrunculin-A treatment leaves low-frequency evoked EPSCs unaffected. However, disruption of actin polymerization has been shown to alter release probability (Morales et al. 2000) and to slightly enhance FM1-43 destaining (Sankaranarayanan et al. 2003). Consequently, 40 min after latrunculin-A treatment and stable recording of paired cell responses with no alteration in amplitude of synaptic response, the stimulation frequency was increased to 5 Hz for 2,000 stimulations to ensure that the readily releasable pool of primed vesicles was exhausted (Gerachshenko et al. 2005). High-frequency stimulation decreased evoked EPSC peak amplitudes in control (to 48 ± 10% of amplitude at low frequency, n = 6; Fig. 5B) and latrunculin-A-treated (to 71 ± 8% of amplitude at low frequency, n = 6; Fig. 5C) axons. Frequency-dependent inhibition during 5-Hz stimulation in control axons was significantly greater (P < 0.05) than in latrunculin-A-treated axons. In both conditions inhibition was released upon return to low-frequency stimulation (to 81 ± 6% and 96 ± 14% in control and latrunculin-A conditions, respectively; not significantly different). Thus latrunculin-A pretreatment reduces high-frequency-mediated short-term depression of synaptic responses but does not cause a loss of synaptic transmission following exhaustion of the readily releasable pool.
Vesicle recycling is possible after latrunculin-A treatment.
On the basis of electron microscopic (EM) evidence, it has been proposed that actin polymerization is required for endocytosis and recycling of endocytosed vesicles to the synaptic vesicle pool (Bourne et al. 2006; Richards et al. 2004; Shupliakov et al. 2002), although this may not be true for all central synapses (Morales et al. 2000; Sankaranarayanan et al. 2003). We therefore tested whether latrunculin-A treatment would diminish vesicle exocytosis, endocytosis, or recycling. Initially, we determined whether vesicle clusters prelabeled with FM1-43 could be destained after latrunculin-A treatment. Axons were first labeled with FM1-43 (Fig. 6A). The spinal cord was then treated with latrunculin-A (12 μM, 30 min) to disperse presynaptic actin clusters. During latrunculin-A treatment the fluorescence of FM1-43-labeled puncta was monitored and showed a slow decay not significantly different from controls (Fig. 6Aii). The axon was then stimulated to evoke destaining. After 16,000 stimuli, fluorescence was reduced to 33 ± 2% (Fig. 6Aii; n = 5) of fluorescence after labeling (Fig. 6Ai, bottom). This was not significantly different from control destaining with no latrunculin-A (Fig. 6Aii; destaining in control was to 37 ± 3%; n = 6).
Fig. 6.
Vesicle recycling is preserved in latrunculin-A. Ai: axons loaded with FM1-43 by stimulation (5 Hz, 2,000 stimuli) in FM1-43 as controls. Top: FM1-43 puncta in 1 axon. Bottom: destained axon after latrunculin-A treatment (12 μM, 30 min), then 12,000 stimuli (20 Hz). Aii: normalized puncta fluorescence with time. Axons labeled as in Ai. Fluorescence measured before and during latrunculin-A (○; n = 5) or control (●; n = 6) recordings for 40 min and then destained with 20-Hz stimulation. Bi: axons loaded with FM1-43 as in A 30 min after latrunculin-A (12 μM) treatment. Top: FM1-43 puncta in single axon. Bottom: destained axon after 12,000 stimuli. Bii: normalized fluorescence with time from all axons pretreated with latrunculin-A (□; n = 5) or left as untreated controls (●; n = 6), then labeled as in Bi. Control and latrunculin-A treated axons were monitored for 40 min, then destained. Ci: vesicle clusters in axons loaded with FM1-43 prior to treatment with latrunculin-A (top, after loading; bottom, after 1 h and latrunculin-A treatment). Cii: mean FM1-43 labeling during and after treatment with latrunculin-A (△; n = 4) was identical to untreated controls (●; n = 14) up to 1 h after treatment. D: latrunculin-A did not prevent endocytosis, recycling, or subsequent exocytosis. Di: axon showing FM1-43 labeling (top) and after 16,000 stimulus-evoked destaining (bottom). Dii: same axon after latrunculin-A treatment (12 μM, 30 min), then restained (top) and after destaining (with 16,000 stimuli) following latrunculin-A treatment (bottom). Diii: data from axon in Di and Dii. Fluorescence measured throughout cycles of staining and destaining in control and after latrunculin-A. Letters mark corresponding images. Div: pooled data from 4 axons treated as in Di and Dii. Exponentials (gray) fitted to the destaining data. Means of these exponentials obtained for these axons are shown. Gray denotes period of stimulation.
Actin has been proposed to have a role in the correct recycling of vesicles to synaptic pools (Brodin et al. 2000; Shupliakov et al. 2002) and vesicle mobility and transportation of vesicles between synaptic pools (Cole et al. 2000; Kuromi and Kidokoro 1998; Sakaba and Neher 2003). Thus we sought to determine whether vesicle clusters could be labeled after latrunculin-A treatment and subsequently destained. Axons were treated with latrunculin-A for 30 min and then stimulated in FM1-43. FM1-43 labeling was then measured after clearing the tissue with Advasep-7. It was clear that labeled vesicles were endocytosed (Fig. 6Bi) after latrunculin-A treatment. We then monitored vesicle cluster stability over 40 min after latrunculin-A treatment; vesicle clusters did not disperse and remained stable at active sites as seen in controls (Fig. 6Bii). A further period of stimulation of these labeled axons again caused destaining (to 19 ± 4% of initial fluorescence, Fig. 6Bii, n = 5; Fig. 6Bi, bottom). Indeed, this destaining was significantly more complete than control destaining (Fig. 6Bii; P < 0.01; to 37 ± 3%, n = 6). This is consistent with our electrophysiological data showing a reduced synaptic depression during high-frequency stimulation and earlier published work in neuronal culture (Sankaranarayanan et al. 2003). We confirmed that longer treatment with latrunculin-A for durations equivalent to our staining and destaining protocol failed to disrupt the pool of vesicles [Fig. 6C; fluorescence after 60 min was reduced to 73 ± 4% in control (n = 14) and to 72 ± 11% after latrunculin-A treatment (n = 4)].
Thus blockade of actin polymerization does not prevent endocytosis or exocytosis of synaptic vesicles and, consistent with results from hippocampal culture (Sankaranarayanan et al. 2003), slightly increases the pool of vesicles that can be mobilized. Because the intensity of FM1-43 loading between individual axons varies, it was not possible to compare control endocytosis directly with that recorded after latrunculin-A treatment in the above experiments. To overcome this limitation, in a further three preparations vesicle labeling with FM1-43 was tested at the same synapses in the same axons, before and after treatment with latrunculin-A. Axons were recorded with microelectrodes and labeled with FM1-43 under control conditions as above (Fig. 6Di). These axons were then destained with 16,000 stimuli (20 Hz; Fig. 6Di, bottom) to provide a control loading fluorescence intensity and a control rate of destaining. Data for the axon shown are plotted as a raw bit value (Fig. 6Diii) and for all axons are normalized to the initial staining intensity (Fig. 6Div). A single-exponential fit to the destaining curve reveals a time constant of 5.8 ± 0.8 min (6,960 ± 960 stimuli; Fig. 6Div). The axon was then treated with latrunculin-A (12 μM, 30 min). The tissue was again superfused with FM1-43 and the axon stimulated to induce recycling. Post-dye loading fluorescence was not significantly different from control loading (mean puncta intensity increased to 114 ± 23% of control loading after subtraction of the residual fluorescence from the first destaining stimulus). The axon was destained once more. Destaining rates were more rapid than control. A single-exponential fit to this destaining data reveals a time constant of 3.2 ± 0.4 min (3,840 ± 480 stimuli, significantly different from control, P < 0.05; Fig. 6Div). It is clear from these experiments that neither endocytosis nor exocytosis is prevented by blockade of actin polymerization. Indeed, these experiments demonstrate for the first time that the entire vesicle cycle from endocytosis to exocytosis remains intact after blockade of actin polymerization and dispersal of synaptic F-actin structures within the presynaptic vesicle pool. However, consistent with earlier work (Sankaranarayanan et al. 2003) and our electrophysiological data, latrunculin-A may slightly enhance release during periods of high-frequency stimulation.
Stabilization of polymerized actin disrupts vesicle recycling and synaptic transmission.
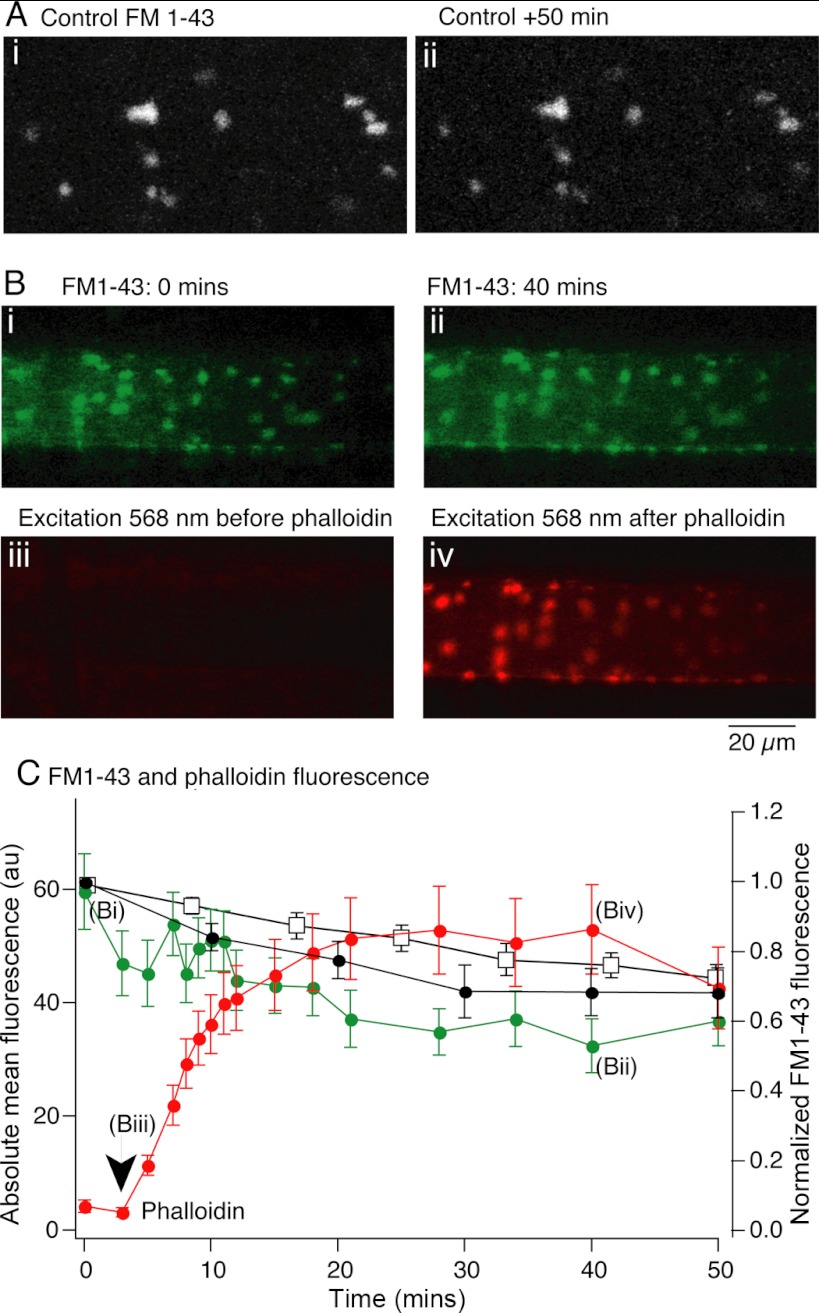
We previously showed that presynaptic injection of phalloidin inhibits chemical-evoked EPSCs but not presynaptic action potentials, evoked Ca2+ transients, or the electrical component of synaptic transmission (Photowala et al. 2005). This inhibition occurs rapidly (in <30 stimuli and <5 min) and acts faster than depletion of the readily releasable pool of vesicles in lamprey giant synapses, which requires between 170 and 400 presynaptic action potentials (Gerachshenko et al. 2005). Phalloidin might act to prevent exocytosis directly, or to disrupt the synaptic vesicle pool. To determine the effect of phalloidin on the stability of the FM1-43-labeled vesicle pool, presynaptic terminals were loaded with FM1-43 and the tissue was subsequently cleared of excess dye with Advasep-7 (1 mM). This revealed FM1-43-labeled vesicle clusters (Fig. 7A). In control conditions this fluorescence showed a slow decay in intensity to 75 ± 7% of initial fluorescence over 50 min (n = 5; Fig. 7, A, i and ii, Fig. 7C). In a further 12 axons, after FM1-43 loading and an initial measurement of FM1-43 fluorescence intensity (Fig. 7Bi), fluorescently labeled phalloidin was injected into the axon. FM1-43 fluorescence intensity was then measured as phalloidin incorporated into presynaptic F-actin to reveal punctate actin at synapses. Phalloidin incorporation was measured as an increase in fluorescence intensity at each synaptic site (Fig. 7B, iii and iv, Fig. 7C). This had no significant effect on the location or fluorescence intensity of FM1-43-labeled synaptic puncta (Fig. 7C, mean from 12 axons with phalloidin; green trace taken from axon in Fig. 7B). FM1-43 puncta fluorescence after 50 min was 68 ± 8% of initial fluorescence (not significantly different from control axons).
Fig. 7.
Presynaptic phalloidin injection leaves presynaptic vesicle clusters intact. A: presynaptic terminals were labeled with FM1-43 as for Fig. 1. These puncta remained labeled for over 50 min (Ai) although fluorescence was reduced to 70% of control after 50 min (□, C). B: axons were labeled with FM1-43 with presynaptic recording electrodes containing Alexa Fluor 568-labeled phalloidin. Bi: FM1-43 labeling prior to phalloidin microinjection. Bii: FM1-43 labeling 40 min after phalloidin microinjections demonstrates that FM1-43 labeling remains intact. Biii: no phalloidin fluorescence is visible prior to injection. Biv: vesicle cluster-associated F-actin visible after phalloidin injection colocalizes with FM1-43 puncta. C: FM1-43 and phalloidin fluorescence at puncta over time. Mean fluorescence of all FM1-43 puncta in the imaged axon (B, green) and of FM1-43 in all axons (●) subsequently labeled with phalloidin. The stability of this fluorescence signal did not differ significantly from control axons not labeled with phalloidin (□). Phalloidin binding to puncta after microinjection increases after injection (red; time of injection marked by arrow). Images in B taken at time points indicated.
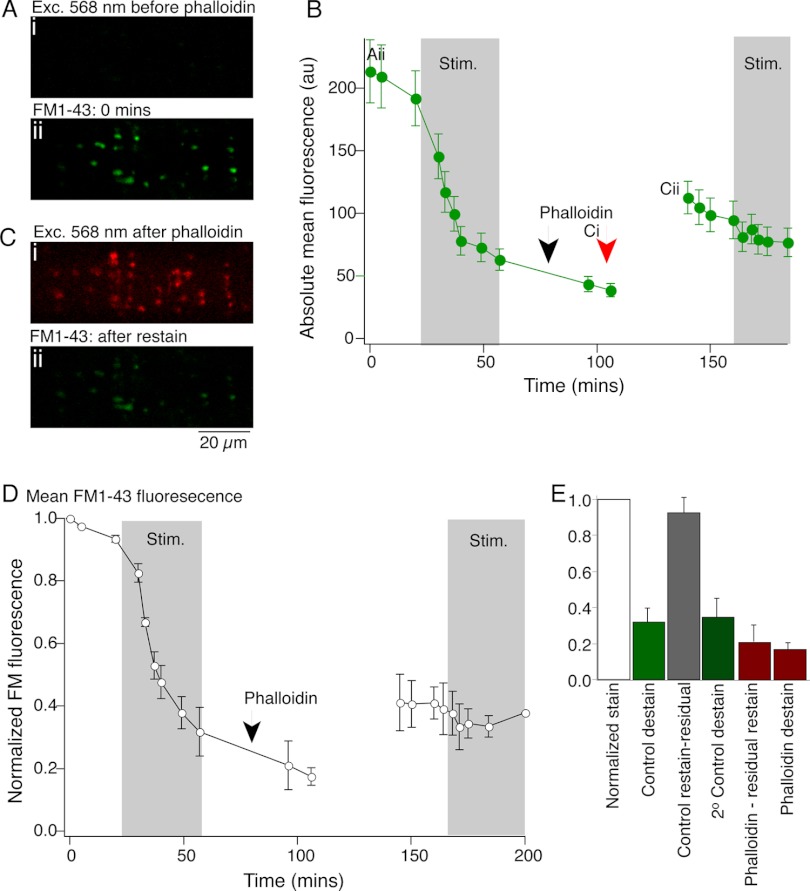
We then determined whether phalloidin altered presynaptic loading with FM1-43 or subsequent stimulus-evoked unloading of the dye. Axons were labeled with FM1-43 in three preparations as above to establish control fluorescence labeling (Fig. 8Aii). After excess dye was cleared with Advasep-7 (1 mM), the axon was stimulated at 5 Hz to destain the axon and measure control rates of destaining. After 16,000 stimuli, fluorescence was reduced to 31 ± 8% of prestimulus intensity (Fig. 8, B and D). After this period of action potential-evoked destaining, the axon was labeled with fluorescently tagged phalloidin by microinjection through the recording electrode (Fig. 8Ci). Phalloidin labeled the same structures that had previously been stained with FM1-43. After 20 min, to allow stabilization of phalloidin labeling of the presynaptic terminals (see Fig. 7C), the tissue was again superfused with FM1-43, stimulated as before to evoke FM1-43 dye labeling, and cleared once more with Advasep-7. After phalloidin labeling, the subsequent endocytosis and labeling of presynaptic vesicles was substantially reduced compared with control (staining was 41 ± 9% of initial stain, representing a relative fluorescence increase of only 21% of the control stain prior to phalloidin, after subtraction of the residual fluorescence, Fig. 8B, Cii, D, and E; this compares with control restaining intensities of 124 ± 8% of initial staining in control axons, which after subtraction of the residual labeling represents 93 ± 8% of initial staining, Fig. 8E). Additionally, a further period of stimulation to evoke exocytosis failed to destain the terminal fluorescence (staining reduced to 90 ± 9% of the fluorescence labeling after phalloidin, Fig. 8E, compared with a reduction to 35 ± 9% in control axons that had been stained, destained, restained, and destained once more).
Fig. 8.
Presynaptic phalloidin injection inhibits exocytosis and compensatory endocytosis. A: an axon was first impaled with an electrode containing KCl (Ai; no phalloidin signal) and stimulated (2,000 stimuli) in FM1-43 to label presynaptic vesicle clusters (Aii). B: FM1-43 fluorescence at puncta in Aii. Axon stimulated intracellularly (16,000 stimuli, 20 Hz) and intensity of puncta declined. The presynaptic electrode was removed and replaced with one containing phalloidin, which was pressure injected (black arrow). Intracellular phalloidin application was confirmed by imaging its Alexa Fluor 568 label (Ci; image at red arrow in B), which marked the same puncta previously labeled with FM1-43 (Aii). The tissue was again superfused with FM1-43 and the axon stimulated (2,000 stimuli). This revealed the same puncta (labeled with FM1-43) but with significantly reduced fluorescence than in Aii. A further 16,000 stimuli failed to destain these puncta. Ci: phalloidin labels structures associated with vesicle clusters in the same axon as A. Cii: FM1-43 puncta labeled in same axon as A but after destaining and restaining following phalloidin injection. D: mean normalized data from 3 preparations treated as for B. E: summary of staining and destaining data from 3 preparations; control destaining (green) and control restaining (grey) of the same puncta following the destaining protocol. After phalloidin injection, restaining was minimal (1st red bar). Subsequent destaining (2nd red bar) was also minimal.
From these data showing a stronger effect of phalloidin on exocytosis than endocytosis, and from our previous results demonstrating a very rapid loss of evoked synaptic transmission following presynaptic phalloidin injection (Photowala et al. 2005), phalloidin appears to directly inhibit exocytosis. FM1-43 restaining after phalloidin application is reduced but not eliminated. This is consistent with reduced compensatory endocytosis leading from inhibition of exocytosis. One might expect a proportionate loss of endocytosis following block of exocytosis; however, these process can be uncoupled (Neale et al. 1999; Smith and Neher 1997; Yao et al. 2012), although we cannot rule out an effect of phalloidin stabilization of F-actin on vesicle endocytosis.
Phalloidin inhibition of synaptic transmission does not act at dynamic F-actin puncta.
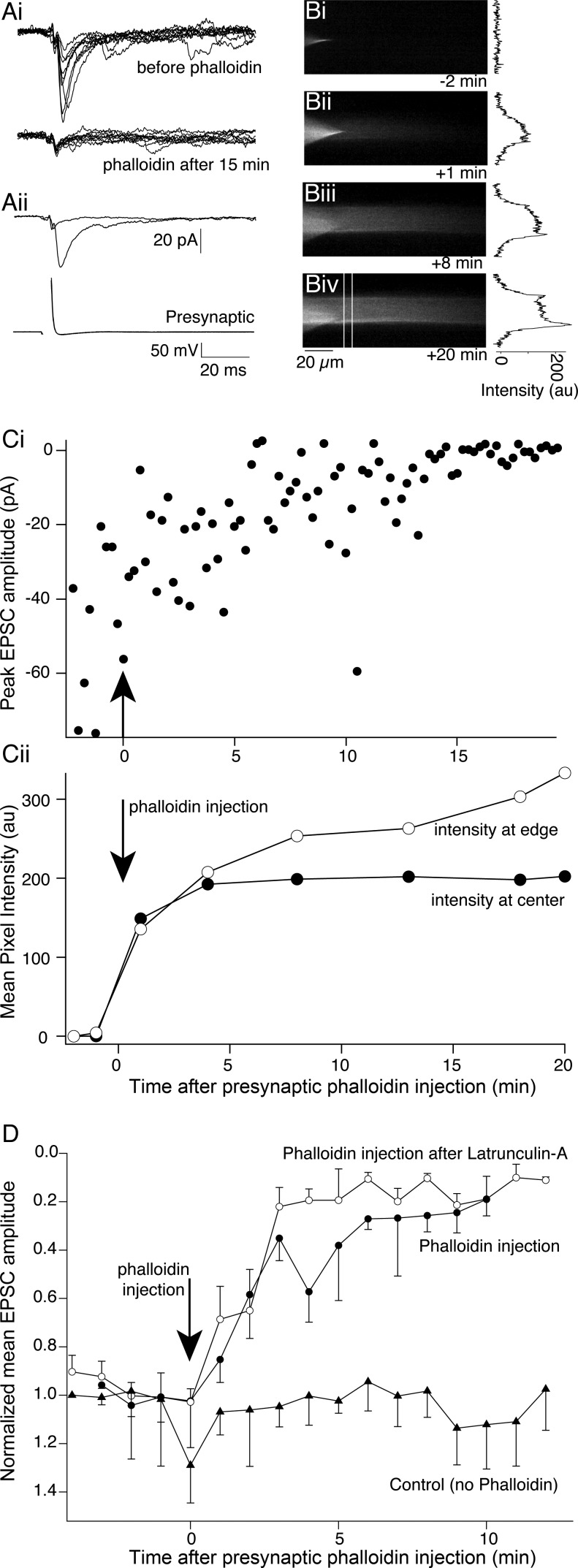
Previous studies have indicated that perisynaptic actin seen at the EM level is necessary for vesicle recycling (Bloom et al. 2003; Bourne et al. 2006; Shupliakov et al. 2002). However, we have now demonstrated that, in common with cultured hippocampal neurons (Morales et al. 2000), dispersal of this actin leaves synaptic transmission and vesicle cycling intact. Because phalloidin injection is possible in lamprey giant axons, we have also been able to demonstrate that blockade of actin depolymerization blocks synaptic transmission and arrests vesicle recycling. We therefore hypothesize that this effect of phalloidin acts at a different F-actin pool, and because the cortical actin remains after treatment with latrunculin-A including at synaptic active sites, it or a component of it at the synaptic active zone is a possible candidate.
We therefore determined what effect stabilization of only the cortical actin had on synaptic transmission. We pretreated spinal cords with latrunculin-A (12 μM, 30 min) to disperse synaptic actin clusters but leave cortical actin intact. Paired cell recordings were then performed (Fig. 9). EPSCs were readily recorded after pretreatment with latrunculin-A (Fig. 9Ai, top). After recording of evoked EPSCs, labeled phalloidin was injected into the axon. Confirming that pretreatment with latrunculin-A dispersed punctate F-actin, the phalloidin did not label any presynaptic active sites (Fig. 9B), yet it still accumulated immediately under the axon membrane to label cortical actin (Fig. 9, B and Cii). During this time, EPSCs were inhibited, corresponding to an increase in phalloidin intensity at the axonal edge (Fig. 9, C and D; 15 min after phalloidin injection EPSCs were reduced to 7.3 ± 4.2% of control amplitudes, n = 4 preparations). Phalloidin clearly inhibits synaptic transmission in the absence of any labeled presynaptic F-actin clusters.
Fig. 9.
Phalloidin blocks synaptic transmission after dispersal of actin at vesicle clusters by latrunculin-A. A: after pretreatment with latrunculin-A (12 μM; 30 min) a paired cell recording was made between a reticulospinal axon and a postsynaptic neuron. Synaptic transmission was not prevented by latrunculin-A pretreatment. In these recordings the presynaptic electrode contained phalloidin labeled with Alexa Fluor 488. Ai, top: 10 sequentially evoked EPSCs following presynaptic action potentials. Bottom: 10 EPSCs recorded 15 min after phalloidin injection. The remaining response is the electrical component. Aii: means of control traces and traces after phalloidin injection from Ai: postsynaptic (top) and presynaptic (bottom) action potentials. B: Alexa Fluor 488 phalloidin-labeled axons. Bi: prior to injection, only the recording microelectrode is visible. Bii: 1 min after injection, dye is visible in the axon. Biii: 10 min after injection the axon outline is visible. Biv: 20 min after injection. In no cases are presynaptic active zone vesicle clusters labeled by phalloidin, indicating that these F-actin clusters were dispersed by pretreatment with latrunculin-A. Graphs to right of each image are intensity profiles from each image from region indicated by white lines in Biv. Cortical actin is revealed after 10–20 min of phalloidin. Ci: peak EPSC amplitude before and after phalloidin injection. Cii: intensity of phalloidin Alexa Fluor 488 fluorescence was measured at the center of the axon and at its edge over time. The increase in phalloidin fluorescence at the axon border correlates well with the reduction in EPSC amplitude in Ci. D: means of EPSC amplitudes before and after phalloidin injection after treatment with latrunculin-A (n = 4), with no pretreatment (phalloidin injection, n = 5), or after latrunculin-A but with no phalloidin injection (control, n = 5) (NB: vertical axis inverted to reflect inward currents recorded).
Jasplakinolide disruption of actin enhances neurotransmission and blocks cortical actin stabilization with phalloidin.
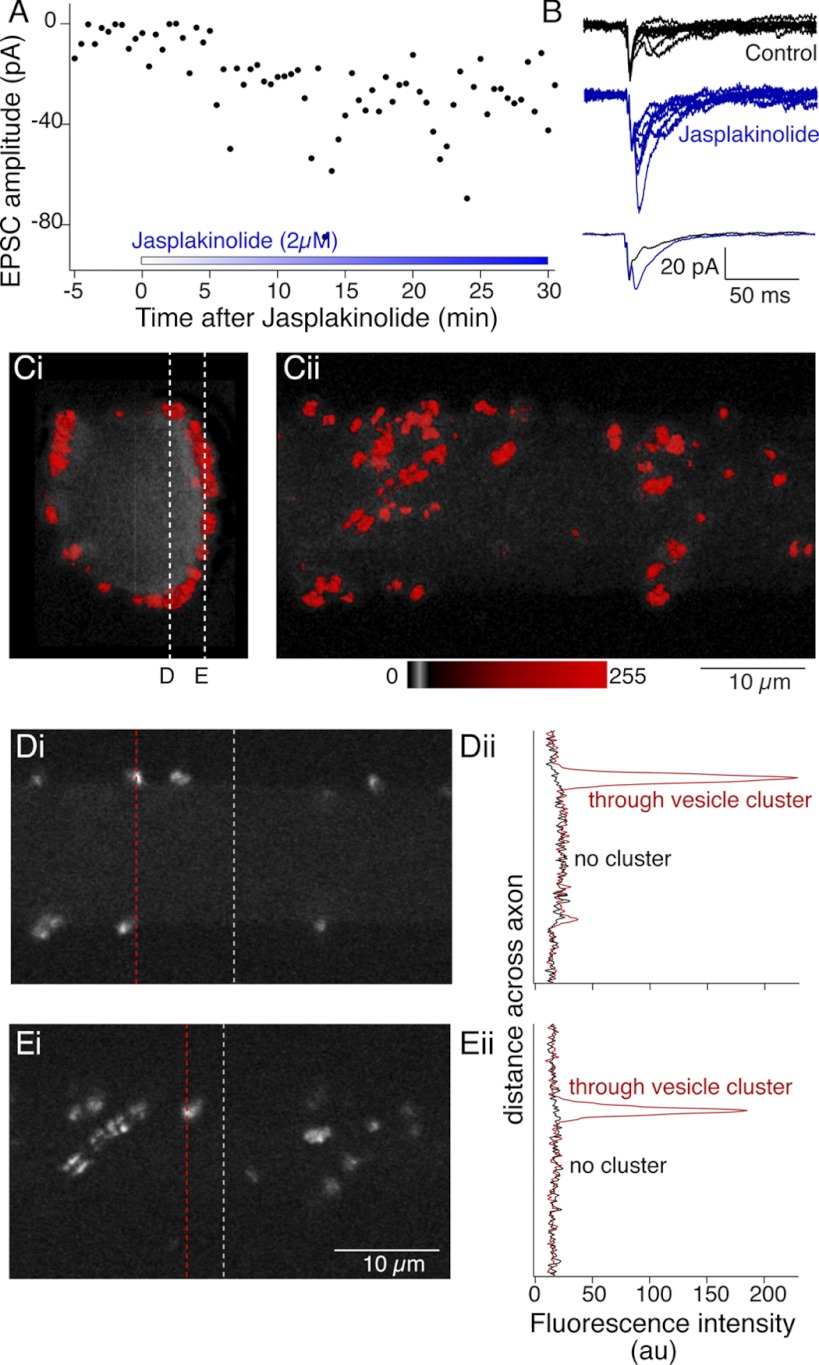
While phalloidin stabilization of cortical actin alone was sufficient to block synaptic transmission, we wished to rule out a possible nonspecific result of blockade of actin depolymerization. Thus we performed paired cell recordings and treated the spinal cord with jasplakinolide, a known blocker of actin depolymerization, which can also enhance polymerization and disrupt F-actin structures in vivo by favoring F-actin nucleation (Bubb et al. 2000). Evoked EPSCs were recorded for 5 min (at 30-s intervals), after which jasplakinolide (2 μM) was applied to the superfusate. During jasplakinolide application EPSC amplitudes increased (to 328 ± 109% of control) over 40 min of recording in jasplakinolide (Fig. 10, A and B; n = 4). This result was in marked contrast to that observed after phalloidin injection (Fig. 9). We thus determined the effect of jasplakinolide treatment on presynaptic F-actin by pretreating axons with jasplakinolide and subsequently visualizing F-actin by injecting labeled phalloidin. Jasplakinolide was superfused over the spinal cord for a minimum of 20 min, after which we pressure injected labeled phalloidin into single axons (Fig. 10, C and D). While phalloidin was still found to cluster at presynaptic sites, we were not able to detect any cortical labeling (Fig. 10E). We do not believe that this is solely the result of competitive binding of actin by phalloidin and jasplakinolide (Bubb et al. 1994), because jasplakinolide did not prevent phalloidin labeling of synaptic vesicle-associated actin clusters and because its application after phalloidin treatment did not reduce cortical actin labeling by phalloidin (data not shown). Thus a jasplakinolide-induced disruption of cortical actin appears to correlate well with an enhancement in evoked neurotransmitter release.
Fig. 10.
Jasplakinolide enhances synaptic transmission and prevents phalloidin labeling of cortical actin. A: paired cell recordings between reticulospinal axons and their whole cell clamped postsynaptic target neurons. Control responses were recorded to single presynaptic action potentials at 30-s intervals for 5 min. Jasplakinolide (2 μM) was added to the superfusate. Graph shows single EPSC peak amplitudes against time after addition of jasplakinolide. B: examples of 6 sequential EPSCs from control (black) and in jasplakinolide after 20 min of application (blue). Overlaid traces at bottom, averages of the EPSCs shown at top. C: after treatment with jasplakinolide (2 μM) for a minimum of 20 min, phalloidin was microinjected into axons through the recording pipette. Fluorescence was imaged confocally to reconstruct labeling in 3D 40 min after injection. Vesicle cluster-associated phalloidin was readily labeled. However, no cortical actin was seen. Ci: view along the length of the axon. Cii: view from ventral spinal cord. D and E. single optical sections from positions indicated by the dashed lines in Ci through the axon (Di) and at the ventral surface (Ei). Intensity profiles (Dii, Eii) are shown through these optical sections at the dashed lines in Di and Ei including clusters (red) and regions with no clusters (black). No cortical actin signal is seen at the axon membrane.
DISCUSSION
Actin is found throughout presynaptic terminals; however, its role in the physiology of synaptic transmission remains unclear (Doussau and Augustine 2000). Indeed, published works on presynaptic actin propose contradictory roles for its physiological functions. While there is evidence for its active role in endocytosis and vesicle recycling (Bloom et al. 2003; Bourne et al. 2006; Brodin and Shupliakov 2006; Pieribone et al. 1995; Rosahl et al. 1995; Shupliakov et al. 2002) and in exocytosis (Lang et al. 2000; Vitale et al. 1995), others have proposed a structural function with no strong role in vesicle cycling (Sankaranarayanan et al. 2003). Beyond synapses, a role for actin has been proposed during clathrin-mediated endocytosis (Kaksonen et al. 2003, 2006; Merrifield et al. 2002, 2005), although this may not be true of all cells (Fujimoto et al. 2000). However, these conclusions are drawn from a wide variety of preparations and conditions, and thus it is possible that actin subserves many of these roles (Cingolani and Goda 2008).
The presynaptic actin cytoskeleton has previously been envisaged as a ringlike structure in lamprey (Bourne et al. 2006), surrounding a vesicle pool labeled with anti-synapsin antibodies (Shupliakov et al. 2002). Similarly, an association of actin with synaptic vesicles has been observed in cultured hippocampal neurons (Sankaranarayanan et al. 2003) and the frog neuromuscular junction (Richards et al. 2004). We demonstrated that vesicle clusters loaded with FM1-43 are colocalized with phalloidin-decorated F-actin. However, the phalloidin-labeled actin at synapses does not surround vesicle clusters as previously described but is contained within them. Additionally, our injections of phalloidin or G-actin into axons often revealed complex actin congregates in addition to rings. Furthermore, vesicle clusters were often associated with more than one phalloidin-labeled presynaptic site. This localization of single vesicle clusters with multiple active sites has also been observed in EM reconstructions in lamprey (Gustafsson et al. 2002) and is suggestive of the dynamic sharing of vesicles between active zones (Cingolani and Goda 2008). The discrepancy between our findings and previous results in which F-actin was found to surround the vesicle pool could be a result of our stimulation protocol, which may label a larger proportion of the vesicle cluster with FM dye than inferred from anti-synapsin antibody labeling. It is known that anti-synapsin antibodies disrupt the distal portion of the vesicle pool (Pieribone et al. 1995), causing a loss of reserve pool vesicles. In addition, previous ultrastructural EM studies investigating presynaptic actin localization have identified filaments either as comet tails associated with recycling vesicles or as gold particle-labeled anti-actin antibodies (Shupliakov et al. 2002). It is possible that smaller actin filaments are not detectable by these methods (Siksou et al. 2011). Clearly though, the actin clusters labeled by either phalloidin or G-actin are unnecessary to retain synaptic vesicle clusters, as has been shown previously (Bourne et al. 2006; Gaffield et al. 2006; Kuromi and Kidokoro 1998; Richards et al. 2004; Sankaranarayanan et al. 2003; Shupliakov et al. 2002).
Actin's association with the periphery of presynaptic active sites, its colocalization with endocytic proteins (Bloom et al. 2003; Morgan et al. 2004), and the disruption of clathrin-mediated endocytosis upon disruption of actin polymerization (Bourne et al. 2006) have led to an hypothesis that actin is necessary for synaptic vesicle recycling from the endocytic region during stimulation (Brodin and Shupliakov 2006). Stimulation has been shown to alter actin polymerization within synaptosomes (Bernstein and Bamburg 1989) and its distribution in presynaptic terminals of hippocampal neurons in culture (Sankaranarayanan et al. 2003) and in lamprey presynaptic terminals (Bloom et al. 2003; Shupliakov et al. 2002). However, in lamprey activity-independent actin turnover at this region was also seen in axons in both depleted and physiological extracellular Ca2+ concentration (Bourne et al. 2006). We also show that actin dynamically clusters at presynaptic sites under quiescent conditions (Figs. 2 and 3) yet does not incorporate into cortical actin. Furthermore, we have now shown that disruption of this more dynamic actin by sequestration of G-actin monomers with latrunculin-A does not inhibit neurotransmission or vesicle cycling. We also observed a slight enhancement in neurotransmitter release during high-frequency stimulation. While this latter effect is not profound, similar effects have also been recorded in other systems (Bernstein and Bamburg 1989; Cole et al. 2000; Richards et al. 2004; Wang et al. 1996), although under conditions of prolonged high-frequency stimulation blockade of actin polymerization may inhibit synaptic transmission (Kuromi and Kidokoro 1998; Richards et al. 2004) or prolong recovery of synaptic transmission (Cole et al. 2000). It is difficult to reconcile our results with those seen in previous studies utilizing latrunculin and other stronger actin depolymerizing agents, although the greater effects seen with depolymerizing agents known to sever actin filaments may have additional effects on cortical actin not previously examined (see below).
Within presynaptic terminals, actin filaments are closely associated with synaptic vesicles (Gotow et al. 1991) but do not contact vesicles directly; rather, they are thought to associate with synapsin filaments, which might tether vesicles in a phosphorylation-dependent manner (Shupliakov et al. 2011). Indeed, disruption of synapsin depletes synaptic vesicles at synapses and inhibits vesicle cycling during high-frequency stimulation (Pieribone et al. 1995). Because of actin's association with synapsin (Bloom et al. 2003) and the physiological deficits in vesicle cycling seen with actin disruption (Cole et al. 2000; Morales et al. 2000; Wang et al. 1996), it has been proposed that actin may also help to anchor vesicles at the terminal and play a role in exocytosis.
In the lamprey reticulospinal axon, preventing actin depolymerization with phalloidin rapidly blocks synaptic transmission (Photowala et al. 2005), and we now show that this treatment also disrupts vesicle recycling. Intriguingly, phalloidin labeling of cortical actin specifically blocks synaptic transmission. Indeed, EPSCs are inhibited much faster than can be accounted for by loss of the primed vesicle pool determined with botulinum B toxin (Gerachshenko et al. 2005). Similarly, phalloidin markedly reduced vesicle labeling by FM1-43 and completely blocked destaining. However, as it does so, it does not alter EPSC kinetics—synaptic delay, rise time, or decay. Phalloidin, like latrunculin-A, though, does not disperse vesicle clusters. Thus it seems unlikely that phalloidin alters synaptic structure. There is limited information on such treatment in other synapses because it is difficult to apply phalloidin to presynaptic terminals, although in other preparations the membrane-permeant agent jasplakinolide, a known stabilizer and active promoter of actin filaments (Bubb et al. 2000), has been used to determine the effects of inhibiting actin depolymerization on synaptic transmission and vesicle cycling. However, in hippocampal cultures, application of jasplakinolide had no effect on spontaneous synaptic transmission (Morales et al. 2000), nor did it affect the rate of destaining of FM-labeled synaptic vesicles (Sankaranarayanan et al. 2003). We observed a marked increase in EPSC amplitude during evoked synaptic transmission, which was accompanied by a loss of the ability of phalloidin to label cortical actin. We do not believe that this is merely a competition between jasplakinolide and phalloidin as has been demonstrated (Bubb et al. 1994) because phalloidin was still able to bind to presynaptic clusters of actin and, furthermore, injection of phalloidin prior to jasplakinolide treatment still labeled cortical actin. A possible explanation for our results is that, in contrast to in vitro conditions, jasplakinolide in vivo may disrupt existing F-actin filaments and induce anomalous accumulations of actin clusters (Bubb et al. 2000). Nevertheless, in jasplakinolide or latrunculin-A combined with phalloidin treatments we have observed that alterations to the cortical actin result in more pronounced deficits in synaptic transmission and vesicle cycling.
We propose that this cortical actin, or a subcomponent at the active zone that unfortunately cannot be separately resolved with these techniques, is involved in vesicle cycling, because its labeling with phalloidin and its stabilization alone is associated with a blockade of synaptic transmission even after the disruption of synaptic vesicle cluster-associated actin by pretreatment with latrunculin-A. The location of this actin is reminiscent of cortical actin, which has been proposed to play a necessary role in various forms of nonneuronal exocytosis (Eitzen 2003; Vitale et al. 1995) and has been implicated in large dense core vesicle docking (de Wit 2010). It is also possible that a more stable pool of actin provides a framework for myoglobin, which may also play an important role in vesicle recycling (Mochida 1995; Srinivasan et al. 2008; Takagishi et al. 2005). However, our proposal would also agree with the recent concept of a matrix of filaments within the active zone that might alter vesicular release and motility (Pechstein and Shupliakov 2010). We conclude that there exist, at minimum, dual pools of actin at the presynaptic terminal. These pools have different rates of turnover from G- to F-actin, and the two pools have unique roles during vesicle recycling. The stable pool that is resistant to treatment with latrunculin-A may play a direct role in exocytosis, such that its further stabilization with phalloidin entirely blocks synaptic transmission.
GRANTS
This work was supported by National Institutes of Health Grants R01 MH-084874 and R01 NS-052699 to S. Alford.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.B., H.P., and S.A. conception and design of research; A.B., H.P., and S.A. performed experiments; A.B. and S.A. analyzed data; A.B. and S.A. interpreted results of experiments; A.B. and S.A. prepared figures; A.B. and S.A. drafted manuscript; A.B. and S.A. edited and revised manuscript; A.B. and S.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Timm Schubert, Michael Alpert, Andy Seymour, and Edaeni Hamid for critical reading of the manuscript and invaluable discussions.
Present address of A. Bleckert: Dept. of Biological Structure, University of Washington, 1959 NE Pacific St., Box 357420, Seattle, WA 98195.
REFERENCES
- Bahler M, Greengard P. Synapsin I bundles F-actin in a phosphorylation-dependent manner. Nature 326: 704–707, 1987 [DOI] [PubMed] [Google Scholar]
- Bernstein BW, Bamburg JR. Cycling of actin assembly in synaptosomes and neurotransmitter release. Neuron 3: 257–265, 1989 [DOI] [PubMed] [Google Scholar]
- Bloom O, Evergren E, Tomilin N, Kjaerulff O, Low P, Brodin L, Pieribone VA, Greengard P, Shupliakov O. Colocalization of synapsin and actin during synaptic vesicle recycling. J Cell Biol 161: 737–747, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne J, Morgan JR, Pieribone VA. Actin polymerization regulates clathrin coat maturation during early stages of synaptic vesicle recycling at lamprey synapses. J Comp Neurol 497: 600–609, 2006 [DOI] [PubMed] [Google Scholar]
- Brodin L, Low P, Shupliakov O. Sequential steps in clathrin-mediated synaptic vesicle endocytosis. Curr Opin Neurobiol 10: 312–320, 2000 [DOI] [PubMed] [Google Scholar]
- Brodin L, Shupliakov O. Giant reticulospinal synapse in lamprey: molecular links between active and periactive zones. Cell Tissue Res 326: 301–310, 2006 [DOI] [PubMed] [Google Scholar]
- Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem 269: 14869–14871, 1994 [PubMed] [Google Scholar]
- Bubb MR, Spector I, Beyer BB, Fosen KM. Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations. J Biol Chem 275: 5163–5170, 2000 [DOI] [PubMed] [Google Scholar]
- Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci 9: 344–356, 2008 [DOI] [PubMed] [Google Scholar]
- Cole JC, Villa BR, Wilkinson RS. Disruption of actin impedes transmitter release in snake motor terminals. J Physiol 525: 579–586, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Molecular mechanism of secretory vesicle docking. Biochem Soc Trans 38: 192–198, 2010 [DOI] [PubMed] [Google Scholar]
- Dillon C, Goda Y. The actin cytoskeleton: integrating form and function at the synapse. Annu Rev Neurosci 28: 25–55, 2005 [DOI] [PubMed] [Google Scholar]
- Doussau F, Augustine GJ. The actin cytoskeleton and neurotransmitter release: an overview. Biochimie 82: 353–363, 2000 [DOI] [PubMed] [Google Scholar]
- Eitzen G. Actin remodeling to facilitate membrane fusion. Biochim Biophys Acta 1641: 175–181, 2003 [DOI] [PubMed] [Google Scholar]
- Fujimoto LM, Roth R, Heuser JE, Schmid SL. Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic 1: 161–171, 2000 [DOI] [PubMed] [Google Scholar]
- Gaffield MA, Rizzoli SO, Betz WJ. Mobility of synaptic vesicles in different pools in resting and stimulated frog motor nerve terminals. Neuron 51: 317–325, 2006 [DOI] [PubMed] [Google Scholar]
- Gerachshenko T, Blackmer T, Yoon EJ, Bartleson C, Hamm HE, Alford S. Gbetagamma acts at the C terminus of SNAP-25 to mediate presynaptic inhibition. Nat Neurosci 8: 597–605, 2005 [DOI] [PubMed] [Google Scholar]
- Gotow T, Miyaguchi K, Hashimoto PH. Cytoplasmic architecture of the axon terminal: filamentous strands specifically associated with synaptic vesicles. Neuroscience 40: 587–598, 1991 [DOI] [PubMed] [Google Scholar]
- Gustafsson JS, Birinyi A, Crum J, Ellisman M, Brodin L, Shupliakov O. Ultrastructural organization of lamprey reticulospinal synapses in three dimensions. J Comp Neurol 450: 167–182, 2002 [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Sobue K, Kanda K, Harada A, Yorifuji H. The cytoskeletal architecture of the presynaptic terminal and molecular structure of synapsin 1. J Cell Biol 108: 111–126, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaksonen M, Sun Y, Drubin DG. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell 115: 475–487, 2003 [DOI] [PubMed] [Google Scholar]
- Kaksonen M, Toret CP, Drubin DG. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 7: 404–414, 2006 [DOI] [PubMed] [Google Scholar]
- Kay AR, Alfonso A, Alford S, Cline HT, Holgado AM, Sakmann B, Snitsarev VA, Stricker TP, Takahashi M, Wu LG. Imaging synaptic activity in intact brain and slices with FM1–43 in C. elegans, lamprey, and rat. Neuron 24: 809–817, 1999 [DOI] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Two distinct pools of synaptic vesicles in single presynaptic boutons in a temperature-sensitive Drosophila mutant, shibire. Neuron 20: 917–925, 1998 [DOI] [PubMed] [Google Scholar]
- Landis DM, Hall AK, Weinstein LA, Reese TS. The organization of cytoplasm at the presynaptic active zone of a central nervous system synapse. Neuron 1: 201–209, 1988 [DOI] [PubMed] [Google Scholar]
- Lang T, Wacker I, Wunderlich I, Rohrbach A, Giese G, Soldati T, Almers W. Role of actin cortex in the subplasmalemmal transport of secretory granules in PC-12 cells. Biophys J 78: 2863–2877, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chin LS, Shupliakov O, Brodin L, Sihra TS, Hvalby O, Jensen V, Zheng D, McNamara JO, Greengard P, Andersen P. Impairment of synaptic vesicle clustering and of synaptic transmission, and increased seizure propensity, in synapsin I-deficient mice. Proc Natl Acad Sci USA 92: 9235–9239, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield CJ, Feldman ME, Wan L, Almers W. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat Cell Biol 4: 691–698, 2002 [DOI] [PubMed] [Google Scholar]
- Merrifield CJ, Moss SE, Ballestrem C, Imhof BA, Giese G, Wunderlich I, Almers W. Endocytic vesicles move at the tips of actin tails in cultured mast cells. Nat Cell Biol 1: 72–74, 1999 [DOI] [PubMed] [Google Scholar]
- Merrifield CJ, Perrais D, Zenisek D. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell 121: 593–606, 2005 [DOI] [PubMed] [Google Scholar]
- Mochida S. Role of myosin in neurotransmitter release: functional studies at synapses formed in culture. J Physiol (Paris) 89: 83–94, 1995 [DOI] [PubMed] [Google Scholar]
- Morales M, Colicos MA, Goda Y. Actin-dependent regulation of neurotransmitter release at central synapses. Neuron 27: 539–550, 2000 [DOI] [PubMed] [Google Scholar]
- Morgan JR, Di Paolo G, Werner H, Shchedrina VA, Pypaert M, Pieribone VA, De Camilli P. A role for talin in presynaptic function. J Cell Biol 167: 43–50, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale EA, Bowers LM, Jia M, Bateman KE, Williamson LC. Botulinum neurotoxin A blocks synaptic vesicle exocytosis but not endocytosis at the nerve terminal. J Cell Biol 147: 1249–1260, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechstein A, Shupliakov O. Taking a back seat: synaptic vesicle clustering in presynaptic terminals. Front Synaptic Neurosci 2: 143, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Photowala H, Freed R, Alford S. Location and function of vesicle clusters, active zones and Ca2+ channels in the lamprey presynaptic terminal. J Physiol 569: 119–135, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieribone VA, Shupliakov O, Brodin L, Hilfiker-Rothenfluh S, Czernik AJ, Greengard P. Distinct pools of synaptic vesicles in neurotransmitter release. Nature 375: 493–497, 1995 [DOI] [PubMed] [Google Scholar]
- Richards DA, Rizzoli SO, Betz WJ. Effects of wortmannin and latrunculin A on slow endocytosis at the frog neuromuscular junction. J Physiol 557: 77–91, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosahl TW, Spillane D, Missler M, Herz J, Selig DK, Wolff JR, Hammer RE, Malenka RC, Sudhof TC. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature 375: 488–493, 1995 [DOI] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Involvement of actin polymerization in vesicle recruitment at the calyx of Held synapse. J Neurosci 23: 837–846, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan S, Atluri PP, Ryan TA. Actin has a molecular scaffolding, not propulsive, role in presynaptic function. Nat Neurosci 6: 127–135, 2003 [DOI] [PubMed] [Google Scholar]
- Shupliakov O, Bloom O, Gustafsson JS, Kjaerulff O, Low P, Tomilin N, Pieribone VA, Greengard P, Brodin L. Impaired recycling of synaptic vesicles after acute perturbation of the presynaptic actin cytoskeleton. Proc Natl Acad Sci USA 99: 14476–14481, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shupliakov O, Haucke V, Pechstein A. How synapsin I may cluster synaptic vesicles. Semin Cell Dev Biol 22: 393–399, 2011 [DOI] [PubMed] [Google Scholar]
- Siksou L, Rostaing P, Lechaire JP, Boudier T, Ohtsuka T, Fejtova A, Kao HT, Greengard P, Gundelfinger ED, Triller A, Marty S. Three-dimensional architecture of presynaptic terminal cytomatrix. J Neurosci 27: 6868–6877, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Neher E. Multiple forms of endocytosis in bovine adrenal chromaffin cells. J Cell Biol 139: 885–894, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan G, Kim JH, von Gersdorff H. The pool of fast releasing vesicles is augmented by myosin light chain kinase inhibition at the calyx of Held synapse. J Neurophysiol 99: 1810–1824, 2008 [DOI] [PubMed] [Google Scholar]
- Takagishi Y, Futaki S, Itoh K, Espreafico EM, Murakami N, Murata Y, Mochida S. Localization of myosin II and V isoforms in cultured rat sympathetic neurones and their potential involvement in presynaptic function. J Physiol 569: 195–208, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifaro JM, Vitale ML, Rodriguez Del Castillo A. Cytoskeleton and molecular mechanisms in neurotransmitter release by neurosecretory cells. Eur J Pharmacol 225: 83–104, 1992 [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J, Deboben A, Nassal M, Wieland T. The phalloidin binding site of F-actin. EMBO J 4: 2815–2818, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale ML, Seward EP, Trifaro JM. Chromaffin cell cortical actin network dynamics control the size of the release-ready vesicle pool and the initial rate of exocytosis. Neuron 14: 353–363, 1995 [DOI] [PubMed] [Google Scholar]
- Wagner JA, Kelly RB. Topological organization of proteins in an intracellular secretory organelle: the synaptic vesicle. Proc Natl Acad Sci USA 76: 4126–4130, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XH, Zheng JQ, Poo MM. Effects of cytochalasin treatment on short-term synaptic plasticity at developing neuromuscular junctions in frogs. J Physiol 491: 187–195, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Kwon SE, Gaffaney JD, Dunning FM, Chapman ER. Uncoupling the roles of synaptotagmin I during endo- and exocytosis of synaptic vesicles. Nat Neurosci 15: 243–249, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Benson DL. Developmentally regulated changes in cellular compartmentation and synaptic distribution of actin in hippocampal neurons. J Neurosci Res 69: 427–436, 2002 [DOI] [PubMed] [Google Scholar]