Abstract
microRNAs (miRNAs) are a class of small regulatory RNAs that regulate gene expression at the post-transcriptional level. miRNAs play important roles in the regulation of development, growth, and metastasis of cancer, and in determining the response of tumor cells to anticancer therapy. In recent years, they have also emerged as important regulators of autophagy, a lysosomal-mediated pathway that contributes to degradation of a cell's own components. Imatinib, a targeted competitive inhibitor of the BCR-ABL1 tyrosine kinase, has revolutionized the clinical treatment of chronic myelogenous leukemia (CML). We demonstrate that MIR30A-mediated autophagy enhances imatinib resistance against CML including primary stem and progenitor cells. MIR30A, but not MIR101, is a potent inhibitor of autophagy by selectively downregulating BECN1 and ATG5 expression in CML cells. MIR30A mimics, as well as knockdown of BECN1 and ATG5, increases intrinsic apoptotic pathways. In contrast, the antagomir-30A increases autophagy and inhibits intrinsic apoptotic pathways, confirming that autophagy serves to protect against apoptosis. Taken together, these data clarify some of the underlying molecular mechanisms of tyrosine kinase inhibitor-induced autophagy.
Keywords: Atg5, autophagy, BCR-ABL tyrosine kinase, Beclin 1, chronic myelogenous leukemia, microRNA
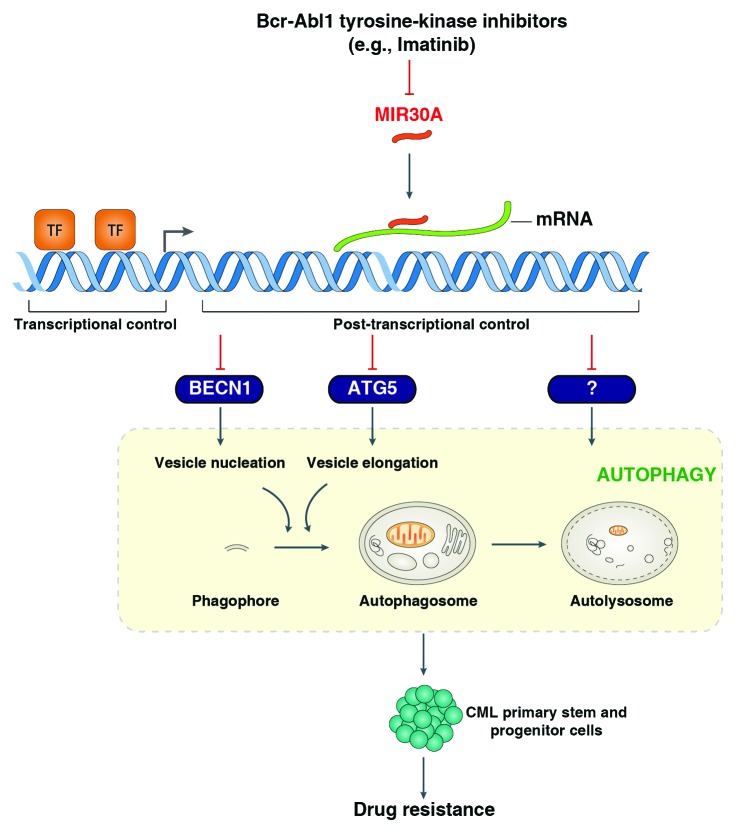
Autophagy is an evolutionarily conserved mechanism for degradation of cytoplasmic components including organelles and proteins, regulated primarily by autophagy-related (ATG) genes. Physiological and pathophysiological roles of autophagy have been linked with cellular homeostasis and a number of human diseases including cancer. Most cancer treatments induce autophagy in the remaining, resistant cancer cells, with inhibition of autophagy promoting sensitivity to therapy. The mode of activation of autophagy and its potential protective role remain incompletely understood. Our recent study indicates that MIR30A specifically regulates ATG protein expression during CML therapy, and that levels of miRNAs directly control the activity of autophagy and effectiveness of chemotherapy (Fig. 1). Thus miRNAs may represent an attractive therapeutic target, and warrant further development of agents to target MIR30A-mediated autophagy, which could have a significant therapeutic impact on CML.
Figure 1.MIR30A-mediated autophagy promotes imatinib resistance in CML cells. Imatinib, a member of the BCR-ABL1 tyrosine kinase inhibitors, inhibits MIR30A levels by unknown mechanisms. MIR30A selectively regulates ATG protein expression in CML cells. After transcription, MIR30A binds to cis-regulatory sites, usually in the 3′ UTR of the mRNA, and represses protein translation of BECN1, ATG5 and other unknown ATG genes. BECN1 and ATG5 are required for nucleation and elongation, respectively, of double-membraned phagophores to form vesicles named autophagosomes (also called autophagic vacuoles). Upregulated autophagy promotes survival of CML primary stem and progenitor cells, which are usually resistant to imatinib. TF, transcription factor.
MIR30A selectively regulates ATG gene expression in CML cells. In CML, BCR-ABL1 tyrosine kinase appears activated, resulting in excessive proliferation of white blood cells. Imatinib binds directly to the ATP binding pocket within the kinase, preventing its activity, and thereby causing tumor cell death. Emergence of resistance to imatinib has become a significant clinical problem. Recent studies indicate autophagy regulates imatinib resistance in CML cells including the CML stem cells. Moreover, work on miRNAs has helped us to understand the mechanism of dysfunction of autophagy in normal and tumor cells. There is a great deal of interest in investigating whether and how miRNAs regulates effectiveness of imatinib therapy in CML cells. We demonstrate that MIR30A, but not other miRNAs such as MIR101, is dose-dependently downregulated in K562 and primary CML cells. Moreover, imatinib induces protein and mRNA expression of BECN1 and ATG5, but not other ATGs such as ULK1 and ATG7 in K562 and primary CML cells. Importantly, downregulation of MIR30A expression by antagomir-30A promotes mRNA and protein expression of BECN1 and ATG5 in K562 cells with or without imatinib treatment. Conversely, upregulation of MIR30A expression by MIR30 mimics inhibits both mRNA and protein expression of BECN1 and ATG5, but not ULK1 and ATG7 in CML cells. Individual mRNAs may also be targeted by other miRNAs in various cell types. Thus global miRNA expression profiling and evaluation of MIR30A target genes using microarray technology is required in future studies.
MIR30A regulates autophagy in CML cells. BECN1 and ATG5 have been previously characterized as key ATGs to regulate sequential steps in the autophagy process, although BECN1-independent and ATG5-independent autophagic pathways exist. MIR30A negatively regulates BECN1 as well as ATG5 expression, and thus we propose that MIR30A is a potential inhibitor of autophagy. To address this hypothesis, we used a MIR30 mimic and antagomir-30A to modulate cellular levels of MIR30A in K562 cells and analyzed autophagy using three different strategies. The formation of the autophagosome was monitored by endogenous microtubule-associated protein 1 light chain 3 (LC3) aggregation revealed by staining with a specific LC3 antibody. The protein level of LC3 was determined by western blotting with or without combination of the lysosomal protease inhibitors E64d and pepstatin A. The ultrastructure of autophagic vesicles was assessed by transmission electron microscopy. We demonstrated that treatment with MIR30 mimic inhibits imatinib-induced expression of LC3-II, formation of LC3 puncta and autophagic vesicles. In contrast, treatment with antagomir-30A increases imatinib-induced autophagic flux. These findings suggest that MIR30A is a negative regulator of imatinib-induced autophagy in CML cells. Interestingly, MIR30A has no effects on rapamycin-induced expression of LC3-II, suggesting that MIR30A regulates autophagy in an MTOR-independent manner.
MIR30A-mediated autophagy limits the effectiveness of BCR-ABL1 tyrosine kinase inhibitors. BCR-ABL1 tyrosine kinase inhibitors (TKIs) treatment induces autophagy, and inhibition of autophagy enhances the effect of TKIs on CML cells. MIR30A mimics significantly decrease cell viability and enhance imatinib as well as other TKIs (e.g., nilotinib and dasatinib) induce apoptosis in CML cells, suggesting a wide role for MIR30A in regulating the effectiveness of TKIs. In contrast, antagomir-30A increases autophagy and decreases apoptosis in K562 and primary CML cells after treatment with these TKIs. Similar with other studies, knockdown of BECN1 and ATG5 by specific shRNA transfection increases apoptosis in K562 cells following imatinib treatment. Notably, knockdown of BECN1 and ATG5 restores the sensitivity to imatinib-induced apoptosis following treatment with antagomir-30A, suggesting that BECN1 and ATG5 are downstream target genes in antagomir-30A-mediated drug resistance.
MIR30A-mediates autophagy and limits intrinsic apoptosis. Imatinib induces mitochondria-dependent intrinsic apoptosis of CML cells, but how the drug promotes this process is only partially understood. There is an extremely complex relationship between autophagy and apoptosis because they share common stimuli, cellular signaling mechanisms, and regulators. We demonstrated that MIR30A mimics, as well as knockdown of BECN1 and ATG5, increases imatinib-induced intrinsic apoptotic events such as loss of mitochondrial membrane potential (ΔΨm), increased BAX mitochondrial translocation, increased cytochrome c release and activation of CASP9. Moreover, knockdown of BECN1 and ATG5 increases imatinib-induced loss of ΔΨm and activation of CASP9 in antagomir-30A-transfected K562 cells. These findings suggest that MIR30A-mediated expression of BECN1 and ATG5 is required for imatinib-induced resistance to mitochondria-dependent intrinsic apoptosis. It is unclear whether MIR30A regulates mitochondrial autophagy, namely mitophagy, which has long been proposed to remove damaged and dysfunctional mitochondria, and control mitochondrial quality.
Effects of MIR30A-mediated autophagy on drug resistance in CML stem cells. CML is a stem cell disease, and any curative therapy must eradicate the stem cell, which is usually resistant to imatinib. Previous studies have shown that TKIs in combination with inhibition of autophagy results in near complete eradication of CML stem cells. We find that the expression of MIR30A is lower in CML CD34+ stem and progenitor cells compared with CD34- cells. Decrease of MIR30A is observed following imatinib treatment in CD34+ cells. As expected, the level of BECN1 is increased in CD34+ cells with or without imatinib treatment, suggesting that autophagy is upregulated in CML stem cells. Inhibition of BECN1 or treatment with a MIR30A mimic restores sensitivity of CML stem cells to imatinib. Thus, targeting MIR30A-mediated autophagy is a novel anticancer strategy with therapeutic implications for imatinib resistance in patients with CML targeting the primary stem/progenitor cells. Additional work is necessary to explore the molecular characterization of MIR30A-mediated autophagy and the upstream mechanism regarding how imatinib regulates MIR30A expression.
Acknowledgments
This work was supported by grants from The National Natural Sciences Foundation of China (30973234 and 31171328 to L.C.) and funding from the Department of Surgery, at the University of Pittsburgh (D.T.).
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/20053