Abstract
Autophagy is an essential lysosomal degradation pathway in neurons, yet the basic mechanisms involved are poorly understood. Here, we discuss our recent study in which we define autophagosome dynamics in primary neurons. We find that autophagosomes initiate at the distal tip of the axon in primary neurons and undergo retrograde transport toward the cell soma. As they move distally to proximally along the axon, autophagosomes mature and become increasingly acidified, consistent with the formation of an autolysosomal compartment that may more effectively catalyze the degradation of enveloped cargo. Our findings suggest that autophagosome biogenesis and maturation is a constitutive process in primary neurons that is temporally and spatially regulated along the axon.
Keywords: autophagosome, axonal transport, dynein, lysosome, neurodegeneration
Neurons are particularly sensitive to the accumulation of misfolded protein and damaged organelles. This sensitivity reflects a post-mitotic state that affords no opportunity to dilute out proteotoxins by cell division. Consequently, neuronal survival depends heavily on efficient degradative pathways, such as autophagy, to eliminate deleterious components that have accumulated within the cytoplasm. Indeed, neuron-specific loss of autophagy is sufficient to cause neuron cell death. Further, defects in autophagy are apparent in various neurodegenerative diseases associated with protein misfolding and aggregation.
Neurons also face the additional challenge of maintaining this protein and organelle quality control over an extended landscape. Neurons have a highly polarized architecture with axons that can reach up to one meter in length. How degradative pathways respond to protein and organelle damage along the length of the axon is poorly understood. Where are autophagosomes generated in neurons? How do they mature into compartments capable of efficient degradation? Does autophagy require long-range transport along the axon or is it executed within a localized region? Most studies to date exploring the mechanisms of autophagy have focused on model systems that lack the extended and highly polarized processes that characterize neurons. Therefore, we set out to define the dynamics of autophagy along the axon of primary neurons in real time.
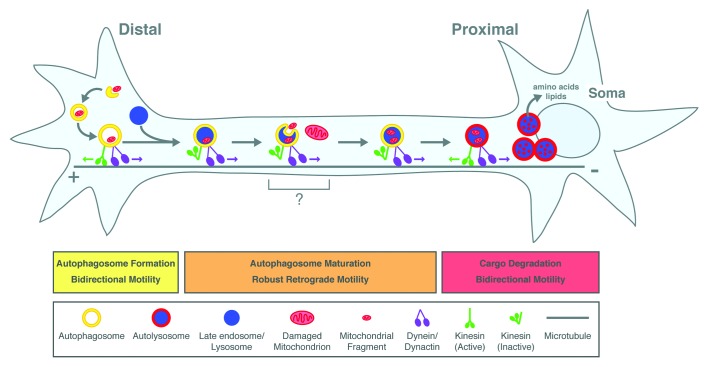
We found that autophagosomes are preferentially generated at the neurite tip via a constitutive mechanism. GFP-LC3-positive puncta appear and grow progressively into ring structures ~800 nm in diameter, a size and shape typical for autophagosomes. Following biogenesis, distal autophagosomes move bidirectionally. Occasionally autophagosomes escape from this distal pool and initiate unidirectional movement toward the cell soma. Along the axon, autophagosomes exhibit robust, primarily retrograde motility driven by the dynein/dynactin motor complex. However, kinesin motors remain bound while autophagosomes move processively in the retrograde direction. We hypothesize that the initial bidirectional motility exhibited by newly formed autophagosomes at the neurite tip is due to the competing activities of bound dynein and kinesin motors acting in a tug-of-war. Kinesin then becomes inactivated, possibly by adopting an autoinhibited conformation, allowing for robust dynein-driven transport.
As autophagosomes exit from the distal region, they acquire markers for late endosomes/lysosomes and become positive for the acidotropic probe LysoTracker-Red. Therefore, it is likely that fusion between autophagosomes and late endosomes/lysosomes occurs distally in the axon. Use of a dual-color LC3 reporter construct sensitive to compartment pH revealed that autophagosomes mature as they move distally to proximally along the axon as also shown by the Nixon laboratory. Proximal to the cell soma, they are fully acidified, consistent with the formation of an autolysosomal compartment that may more effectively degrade cargo. This maturation into autolysosomes is accompanied by a shift in dynamics to the primarily bidirectional motility characteristic of lysosomes.
Thus, we find that autophagosome formation and maturation is spatially and temporally regulated along the axon, and the stages of maturation are accompanied by discrete changes in motility (Fig. 1). We propose that fusion between autophagosomes and late endosomes/lysosomes elicits a signal to initiate transport toward the cell soma. Upon arrival at the cell soma, autophagosomes are fully acidified. This acidification may be a continuous process that occurs during the journey and might entail fusion with additional lysosomes. Delivery to the cell soma ensures that efficient cargo degradation occurs in the region where components can be rapidly recycled in proximity to primary sites of protein synthesis.
Figure 1. Model for autophagosome formation and maturation along the axon of primary neurons. Autophagosomes are generated at the neurite tip in a constitutive process. Soluble and organelle cargoes become enveloped within newly forming autophagosomes. These autophagosomes initially move bidirectionally (green and purple arrows denote motor activity), but then switch to robust retrograde motility driven by dynein. This transition to retrograde motility is accompanied by fusion with late endosomes/lysosomes (gray arrows denote transitions between compartment maturation). En route to the cell soma, autophagosomes may collect additional cargoes along the axon. As they move distally to proximally, autophagosomes mature and become increasingly acidified as denoted by the change in color from yellow to red. This maturation into autolysosomes, a compartment that may more effectively degrade cargo, is accompanied by a switch back to the bidirectional motility characteristic of lysosomes. Delivery of autolysosomes to the cell soma ensures that digested contents are recycled to primary sites of protein and lipid synthesis.
We found that autophagosomes in the axon contain enveloped cargo such as mitochondria, ubiquitin and SOD1G93A. Soluble cargoes such as ubiquitin are present in nearly all distal as well as axonal autophagosomes and are likely engulfed within the autophagosome as it forms. We also observed the presence of mitochondrial fragments within autophagosomes. However, the frequency of this event is relatively low (~10–20% of autophagosomes along the axon). We estimate that this autophagosome-engulfed population represents ~1% of total mitochondrial mass consistent with low levels of organelle turnover for this model under basal conditions.
Under basal conditions, we find that autophagosome formation is spatially restricted to the neurite tip, suggesting that cargo envelopment is also likely to occur distally (Fig. 1). We have not observed the formation of autophagosomes along the axon, raising the question of how dysfunctional organelles along these processes may be cleared by autophagy. We speculate that additional cargoes may be engulfed during transit to the cell soma using mechanisms similar to those operating in late endosomal multivesicular bodies. Multivesicular bodies are generated by the invagination of late endosomal membrane to create intralumenal vesicles. Similarly, the inward budding of autophagosome membrane might capture proteins along the axon in a microautophagy-like process. Perhaps this uptake occurs during the time in which autophagosomes are paused along the axon before resuming transport to the cell soma; pausing accounts for ~12% of their journey. Thus, individual autophagosomes may make ‘stops’ to capture cargo en route to the cell soma. Furthermore, the constitutive formation of autophagosomes at the neurite tip followed by robust transit along the axon may serve as a surveillance mechanism for routine maintenance of the axon.
Do the mechanisms of autophagy under basal conditions differ from those elicited by cellular stress or disease? To begin to address this issue, we measured autophagosome dynamics in sensory neurons expressing SOD1G93A, an aggregation-prone variant of SOD1 that causes familial amyotrophic lateral sclerosis. Surprisingly, despite the formation of SOD1 aggregates along the axon, we did not observe alterations in autophagosome dynamics, density or flux, suggesting that this process is not efficiently upregulated in this system. In contrast, recent work from the Sheng laboratory suggests that autophagy within the cell soma is induced by a different cellular stress, which causes sustained global mitochondrial damage. Future work will be needed to determine how autophagy in either the axon or the cell soma responds to diverse cellular stresses. For example, does autophagosome initiation become less spatially restricted? Alternatively, is autophagosome biogenesis upregulated at the distal tip to increase flux along the axon to meet increased cargo demands? Further studies are needed to resolve these possibilities.
Acknowledgments
This work was supported by NIH NS060698 to E.L.F.H.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/20055