Abstract
Autophagosomes may derive membrane from diverse sources, including the plasma membrane, Golgi, endoplasmic reticulum and mitochondria. The plasma membrane contributes membrane to ATG12–ATG5-ATG16L1-positive phagophore precursor vesicles (LC3-negative) by both clathrin-dependent and -independent routes. We recently observed that ARF6 regulates autophagy and that this could be explained, at least in part, by its role in the generation of phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2], which influences endocytic uptake of plasma membrane into autophagosome precursors. The subsequent maturation of these small phagophore precursors into phagophores (ATG12–ATG5-ATG16L1-positive and LC3-positive), is assisted by SNARE-mediated homotypic fusion that increase their size and enhance their ability to acquire LC3-II. It appears that a plasma membrane-derived pool of VAMP7 is a key mediator of these fusion events. Thus, events at the plasma membrane may regulate distinct steps in the biogenesis of phagophores.
Keywords: Arf6, ATG16L1, autophagy, phagophore, plasma membrane, SNARE
Macroautophagy (hereafter referred to as autophagy) is a major catabolic pathway that maintains cellular homeostasis, and thereby has an impact on several pathologies including infectious diseases, cancer, neurodegeneration and aging. One of the first events in autophagy that has been characterized is the formation of the phagophore, a cup-shaped isolation membrane. The edges of these phagophore membranes elongate and thereby engulf portions of cytoplasm. After the fusion of the membrane edges, the structure becomes a completed autophagosome. The fusion of a lysosome with an autophagosome leads to the formation of an autolysosome, a single membrane vesicle, where the content is degraded.
The early events in autophagosome biogenesis are still unclear. However, the recent identification of different cellular compartments, such as plasma membrane, Golgi, endoplasmic reticulum, and mitochondria, that may contribute membrane to autophagosome precursors, suggests that these structures may derive from many sites and also that there is probably not a unique source of membrane required for the formation of an autophagosome.
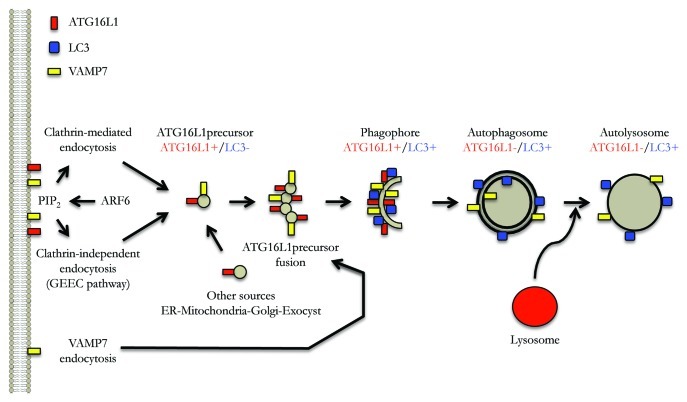
We recently demonstrated that clathrin-mediated endocytosis contributes membrane to phagophore precursors (ATG12–ATG5-ATG16L1-positive and LC3-negative), which mature to form phagophores (ATG12–ATG5-ATG16L1-positive and LC3-positive) and subsequently autophagosomes (ATG12–ATG5-ATG16L1-negative and LC3-positive) (Fig. 1). Subsequently, we showed that a form of clathrin-independent endocytosis, the GPI-Enriched Endocytic Compartments pathway (GEEC), may also contribute membrane to such precursors (Fig. 1).
Figure 1. The plasma membrane as a control center for autophagy. Model of autophagosome formation focusing on different routes of membrane acquisition from the plasma membrane, as well as the roles of endocytosed VAMP7 and plasma membrane PtdIns(4,5)P2. See text for details.
A role for the plasma membrane in the biogenesis of phagophore precursors was also compatible with the discovery that ARF6 is a critical autophagy regulator. ARF6, an ADP-ribosylation factor (ARF) family small G protein that is localized to the plasma membrane and the endocytic system, regulates the formation of phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] at the plasma membrane via its effect on phosphatidylinositol-4-phosphate 5-kinase (PIP5K). Both ARF6 and a PtdIns(4,5)P2 probe colocalize with ATG12–ATG5-ATG16L1 vesicles. ARF6 knockdown or strategies that decrease the levels of plasma membrane PtdIns(4,5)P2 impair autophagosome formation, reduce the numbers of ATG12–ATG5-ATG16L1 vesicles, and attenuate delivery of plasma membrane to ATG12-ATG5-ATG6L1-positive structures. While some of the effects of ARF6 appear to be related to its role as a modulator of endocytosis via PtdIns(4,5)P2, our data suggest that this small G protein may have other functions in autophagy, including roles via phospholipase D regulation.
Phagophore precursors, which we have operationally defined as ATG12–ATG5-ATG16L1-positive and LC3-negative vesicles, are small and appear to increase in size in live cell experiments as they mature into phagophores (ATG12–ATG5-ATG16L1-positive and LC3-positive). Recently, we showed that this step is regulated by homotypic fusions of the phagophore precursors (Fig. 1). The homotypic fusion appears to be important, as it enhances the ability of the phophagore precursors to acquire LC3, and thereby mature into phagophores and eventually into completed autophagosomes. Interestingly, we noted that some of the structures decorated by Atg16L1 have a tubulovesicular appearance that is consistent with this proposed process. These fusion events are SNARE-dependent and involve VAMP7 and partner SNARES.
Since at least some of these ATG16L1-positive structures derive membrane from the plasma membrane, we examined which pool of VAMP7 may be relevant for phagophore precursor homotypic fusion. A pool of VAMP7 located at the plasma membrane can be internalized via AGFG1/HRB (ArfGAP with FG repeats 1/Human immunodeficiency virus Rev-binding protein). VAMP7 interacts via its longin domain with AGFG1, which, in turn, interacts with the clathrin adaptor AP2 that is involved in clathrin-mediated endocytosis. Our data suggest that VAMP7 regulates autophagosome formation in an AGFG1- and longin-domain-dependent fashion, suggesting that endocytosis of the plasma membrane pool of this SNARE may be one route that affects its role in autophagy.
While our data add some pieces to the puzzle of autophagosome biogenesis, many questions remain. For instance, vesicles from multiple membrane sources may contribute to autophagosomes and these may fuse before autophagosome completion. How do these events occur and how are they regulated? Are these different routes all-or-none regulators of autophagy or can some autophagosomes be formed even if one route is completely obstructed? The identification of distinct endocytosis routes raises the questions of whether these play specific roles or whether they are a redundant safety net to allow autophagy to proceed effectively even when one of these routes is perturbed. However, we believe that the data described above implicate plasma membrane events as key regulators of different early stages leading to phagophore production. Plasma membrane uptake via different endocytic routes affects the ability to form phagophore precursors, and the endocytosis of VAMP7 is an important regulator of the subsequent homotypic fusion events that enable the maturation and expansion of these structures into phagophores.
Acknowledgments
We are grateful for funding from a Wellcome Trust Principal Research Fellowship. (D.C.R.).
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/20060