Abstract
This study utilized data from a prospective birth cohort study on 568 Indian children, to determine whether a longer duration of breastfeeding and later introduction of solid feeding were associated with a reduced higher body mass index (BMI) and less adiposity. Main outcomes were high BMI (>90th within‐cohort sex‐specific BMI percentile) and sum of skinfold thickness (triceps and subscapular) at age 5. Main exposures were breastfeeding (six categories from 1–4 to ≥21 months) and age of starting regular solid feeding (four categories from ≤3 to ≥6 months). Data on infant‐feeding practices, socio‐economic and maternal factors were collected by questionnaire. Birthweight, maternal and child anthropometry were measured. Multiple regression analysis that accounted for potential confounders demonstrated a small magnitude of effect for breastfeeding duration or introduction of solid feeds on the risk of high BMI but not for lower skinfold thickness. Breastfeeding duration was strongly negatively associated with weight gain (0–2 years) [adjusted β = –0.12 standard deviation, 95% confidence interval (CI): –0.19 to –0.05 per category change in breastfeeding duration, P = 0.001], and weight gain (0–2 years) was strongly associated with high BMI at 5 years (adjusted odds ratio = 3.8, 95% CI: 2.53–5.56, P < 0.001). In our sample, findings suggest that longer breastfeeding duration and later introduction of solids has a small reduction on later high BMI risk and a negligible effect on skinfold thickness. However, accounting for sampling variability, these findings cannot exclude the possibility of no effect at the population level.
Keywords: breastfeeding duration, complementary feeds, childhood body mass index, adiposity, infant weight gain, India
Introduction
Childhood overweight/obesity is an important public health issue for high‐income countries like Europe and North America. Despite India's high prevalence of cardiovascular and metabolic diseases, primary prevention, particularly during childhood and adolescence, has failed to become a priority. India is still striving to address the seemingly intractable problems of endemic infant and child undernutrition, micronutrient deficiencies and preventable infectious diseases (Chakravarty & Ghosh 2000; Nongkynrih et al. 2004; Boutayeb 2006; Arnold et al. 2009; Pasricha & Biggs 2010). However, its recent economic growth has resulted in urban areas undergoing a rapid nutrition transition, where increasing childhood adiposity and early infant undernutrition coexist (Sawaya et al. 1995; Popkin et al. 1996; Griffiths & Bentley 2001).
A recent study conducted in Delhi schoolchildren reported that in high‐income students, the prevalence of overweight and obesity was 6.8% and 15.3%, respectively (Kaur et al. 2008). Being overweight or obese in childhood increases the risk of being overweight/obese in adulthood (Garn & LaVelle 1985; Parsons et al. 1999; Power & Parsons 2000). Furthermore, higher levels of BMI in childhood are associated with higher prevalence of cardiometabolic risk factors (Chu et al. 1998; Freedman et al. 1999) and greater risk of developing atherosclerosis‐related vascular changes during childhood itself (Woo et al. 2004; Pena et al. 2006). Therefore, effective interventions are required, not only to lower the risk of higher BMI levels in childhood and the development of adverse cardiometabolic profiles, but also contribute to the lowering of the future burden of cardiometabolic disease in India (Wild et al. 2004; Reddy 2007) given the tracking of higher levels of BMI through to adolescence and adulthood.
Several systematic reviews and observational studies have suggested an association between breastfeeding and a later introduction of regular solid (complementary) feeds on the later risk of childhood overweight/obesity and adiposity (Kramer 1981; Wilson et al. 1998; Arenz et al. 2004; Burke et al. 2005; Harder et al. 2005; Owen et al. 2005b; Mayer‐Davis et al. 2006; Shields et al. 2006; Weyermann et al. 2006; Gillman et al. 2007). Additionally, there is some evidence of a dose–response effect with breastfeeding duration. However, these associations are reported from studies predominantly conducted in high‐income countries and could result from confounding by socio‐economic factors (Heck et al. 2006; Bolling & Grant 2007). In such settings, mothers who choose to initiate and continue breastfeeding tend to have higher incomes and greater educational attainment than those who do not breastfeed or ceased breastfeeding early (Hoddinott & Pill 1999). Therefore, a subsequent lower prevalence of higher BMI levels and adiposity in later childhood may reflect a ‘healthier’ environment, which avoids energy‐dense grains, fats and sugars and promotes physical activity, rather than an effect of breastfeeding. Studies conducted in low‐middle income (LMI) settings may help to resolve the confounding issues as in these populations, breastfeeding may have different relationships with socio‐economic status (SES) (Owen et al. 2005b).
For the last 15 years, India's average breastfeeding duration metrics has remained fairly constant. The median duration of exclusive breastfeeding is approximately 2 months, with predominant breastfeeding (either exclusive breastfeeding or receiving breast milk and plain water and/or non‐milk liquids only), median duration is slightly over 5 months (International Institute for Population Sciences (IIPS) and ORC Macro 2001). Median duration of any breastfeeding is approximately between 24 and 25 months (International Institute for Population Sciences (IIPS) and ORC Macro 2001; Arnold et al. 2009). Similar to other countries, India's infant‐feeding practices differ by socio‐economic position. Two recent nationally representative cross‐sectional surveys, the National Family Health Surveys (NFHS)‐2 (1998–1999) and NFHS‐3 (2005–2006), demonstrated that mothers residing in urban areas, having increasingly higher household income and greater years of schooling were more likely to stop breastfeeding within the first 2 years (Malhotra et al. 2008). The most recent survey demonstrated that the median duration of any breastfeeding decreases with maternal years of schooling from 24.1 months (mothers with 5–7 years of completed education) to 20.6 months (mothers with 12 or more years of completed education) and increasing household wealth index from 27.7 months (lowest quintile) to 20.8 months (highest wealth quintile) (Arnold et al. 2009).
We have examined the hypothesis that a longer duration of breastfeeding and later introduction of regular solid feeds are associated with a lower risk of high BMI and reduced adiposity at 5 years of age. This was due to the lack of prospective studies in settings undergoing the initial stages of the nutrition transition (Araújo et al. 2006). Based on growth studies during the early years of life (0–24 months) comparing breastfed and formula fed‐infants, it has been suggested that lower growth (Dewey 1998; Kramer et al. 2004; Baird et al. 2008; Robinson & Godfrey 2008) is a possible mechanism of breastfeeding duration effect on later childhood anthropometry. Therefore, we have also explored whether growth (from birth to 2 years) is a potential mediator of the proposed relationship between breastfeeding duration and child BMI and adiposity at 5 years.
Key messages
-
•
In a cohort of South Indian children per category increases in infant‐feeding practices (duration of breastfeeding, timing of the introduction of solid feeds) demonstrated a small effect on the risk of high BMI at 5 years.
-
•
Infant‐feeding practices were not associated with important sized effects on subcutaneous adiposity.
-
•
Assuming a causal association between the exposure and outcome, weight gain (from birth to 2 years) is likely to be a mediator of the proposed relationship between breastfeeding duration and risk of high BMI at 5 years.
-
•
Promoting and advocating for the maintenance of current WHO guidelines on infant‐feeding practices may have a small role in the prevention of higher levels of BMI in pre‐school children.
Materials and methods
Study design and participants
We used data from the Mysore Parthenon Study (initiated 1997–1998), a prospective birth cohort study of pregnancy‐related maternal risk factors and later infant and child health (Hill et al. 2005; Krishnaveni et al. 2005). Eight‐hundred thirty eligible mothers living in Mysore city or surrounding rural villages booking consecutively into the antenatal clinic of the Holdsworth Memorial Hospital (HMH) and those who satisfied the recruitment criteria (willingness to participate, gestational age <30 weeks and no history of diabetes prior to the pregnancy) were enrolled into the study. Further details of the study design methodology and HMH patient‐base are provided elsewhere (Krishnaveni et al. 2005). Six‐hundred sixty‐three women delivered normal live‐born babies at HMH. Follow‐up information was obtained for 585 children at 1, 2 and 5 years (Fig. 1). Study attrition was mainly due to refusal to participate and death. Five‐hundred sixty‐eight children had complete breastfeeding data and 5‐year outcomes.
Figure 1.
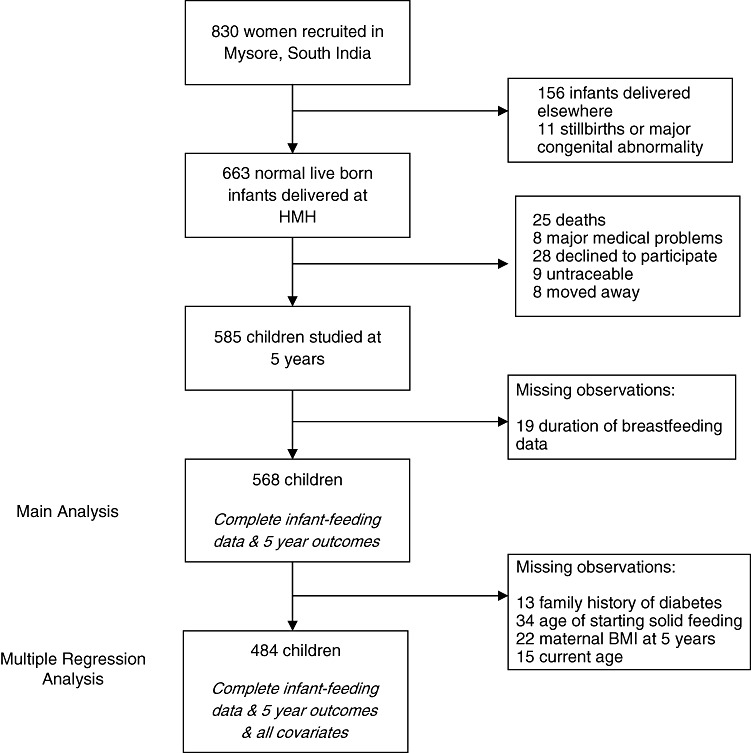
Cohort flow chart.
The HMH Research Ethics Committee approved this study and verbal informed consent was obtained from parents and children. Applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed.
Measurements
Main exposures
Total duration of breastfeeding and age at starting regular solid foods in months were the two main exposures. Information on breastfeeding was obtained by interviewer‐administered questionnaire at 1, 2 and 3 years. The same set of questions was asked at each time point: ‘How was the baby fed from birth (breast, bottle, breast + bottle or other)?; If breastfed, was the baby still being breastfed?; If no longer breastfed, what was the age (months) at which breastfeeding stopped?’ At the age of 1 year, mothers were asked the following question regarding the introduction of solid foods to their infant at the age of 1 year: ‘Has the baby started taking solid foods regularly? If yes, at what age (months) was it started?’ This question ascertains the first time solid foods were introduced as a regular part of the infant's diet (our exposure of interest) rather than the time when the child first consumed solid food.
Breastfeeding duration was categorized as follows: 1–4, 5–8, 9–12, 13–16, 17–20 and ≥21 months. Categories were chosen based on (1) the need to have duration categories of equal length for the ease of interpretation of the regression analysis; (2) comparison with the other studies; and (3) statistical reasons, which included having sufficient observations in each bin, and the ability to adequately perform departure of linearity testing. Age at starting regular solid foods had four categories: ≤3, 4, 5, ≥6 months).
Outcome measures
The main study outcomes at 5 years were presence or absence of ‘high body mass index (BMI)’ (weight/height2) and sum of subscapular and triceps skinfold thickness. High BMI was defined as a sex‐specific BMI greater than the within‐cohort 90th percentile. Using this definition, 58/568 (10.2%) of children were classified as having a high BMI.
High BMI was chosen as a study outcome despite being below the International Obesity Task Force (IOTF) cut‐offs for overweight/obesity. In our sample, very few children were overweight (0.7%) or obese (0.2%). It is reasonable to use higher BMI levels in pre‐school children rather than IOTF cut‐offs, given that the population where the sample was obtained is undergoing the early stages of the nutrition transition, in addition to a high prevalence of infant and childhood undernutrition. Furthermore, using this outcome will include a broad group of pre‐school children who are likely to develop cardiometabolic risk factors in later childhood.
Anthropometry was performed by one of six trained field workers using standardized methods. Weight was measured to the nearest 100 g using a digital weighing scale (Seca, Hamburg, Germany). Standing height was measured to the nearest millimetre using a wall‐mounted stadiometer (Microtoise, CMS Instruments, London, UK). Triceps and subscapular skinfolds were measured to the nearest 1 mm using Harpenden callipers (CMS Instruments); the average of three readings was used. As skinfold thickness measurements were skewed, they were log transformed for analysis.
Other measures
At baseline, the family's SES and religion, family history of diabetes and maternal education attained were recorded. SES was assessed by the Kuppuswamy score, a standardized SES questionnaire for Indian urban populations (Kuppuswamy 1962). At 30 ± 2 weeks gestation, women had a 100‐g, 3‐h oral glucose tolerance test. Gestational diabetes mellitus (GDM) was diagnosed using the Carpenter and Coustan criteria (Carpenter & Coustan, 1982), the method chosen for clinical use in the hospital.
The babies were weighed to the nearest 10 g by one of four trained observers within 72 h of birth, using a digital weighing scale (Seca). The children were subsequently followed up annually. The 1‐year follow‐up was on the child's first birthday (± 4 weeks), for children born at term and on the anniversary of the expected date of delivery (± 4 weeks) for preterm children. Follow‐up visits from 2–5 years were on the child's birthday (± 4 weeks) for all children.
Weight gain (from birth to 2 years) was derived from conditional weight gain z‐scores, calculated as standardized residuals from the regression of weight at age 2 on birthweight and gestational age. Weight gain from birth to 2 years rather than 1 year was used as a proxy for child growth because a high proportion of children were breastfed beyond 1 year (64%, 363/568). Low BMI and stunting were defined as BMI and height two standard deviations (SDs) or more below the median, using the World Health Organization (WHO) reference (http://www.who.int/childgrowth). We defined indicators of household nutritional status at 5 years follow‐up, using maternal BMI constructed from maternal weight and height measurements and number of children in a household (proxied by parity at baseline) given by maternal report. These indicators are an imperfect measure of the average household nutritional status from birth to 5 years. However, previous research in LMI settings has demonstrated maternal nutritional status as a potential determinant of childhood nutritional status (Rahman et al. 1993), and number of children per household influencing childhood nutritional status by reducing scarce family resources for maintaining adequate nutrition (Bronte‐Tinkew & DeJong 2004; Heaton et al. 2005).
Statistical methods
We first examined bivariate associations of breastfeeding duration (six categories) and age at starting solid feeds (four categories) with covariates and outcomes. Associations between the main exposures and outcomes (high BMI, sum of skinfolds) were approximately linear across the categories [departure from linearity test, P = 0.515 (high BMI), P = 0.265 (sum of skinfolds)]; therefore, we assumed a linear trend across exposure categories for regression modelling (Fig. 2). Logistic and ordinary least squares regression (OLS) was used to examine the associations with high BMI and skinfold thickness, respectively. Additionally, OLS was used to conduct supplementary analysis on the effect of breastfeeding duration on the mean BMI of children at 5 years of age.
Figure 2.
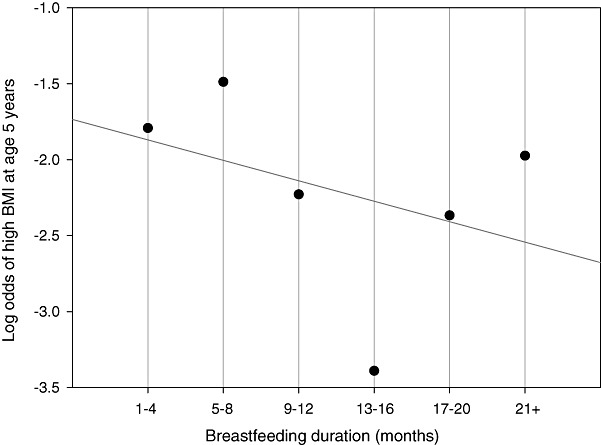
Observed and predicted log odds of high BMI at age 5. This plot shows the linearity of the relationship between breastfeeding and high BMI at age 5.
For the ease of interpretation, we present the results of the regression analyses for skinfold thickness (outcome), as the expected change, expressed as a percentage, in geometric mean sum of skinfold thickness (mm) per one‐unit category increase in exposure. This percentage change is estimated by the expression: 100(echange‐1), where ‘echange’ is the exponentiated regression coefficient for breastfeeding duration when a natural log transformation of the outcome has been used.
Multiple regression analysis was based on an a priori conceptual framework. This framework was constructed from subject matter knowledge on the relationships between covariates, main exposures and outcomes, organized in blocks of variables that followed a socio‐economic–biologic axis. Our conceptual framework provides a visual representation of the interrelationships among factors thought to influence 5‐year child outcomes (Fig. 3) (Victora et al. 1997). Individual‐level socio‐economic covariates are considered distal factors, which have a direct effect on individual behavioural/biological mechanisms. Additionally, our framework assumes that child outcomes at 5 years are affected by gender and current age at follow‐up.
Figure 3.
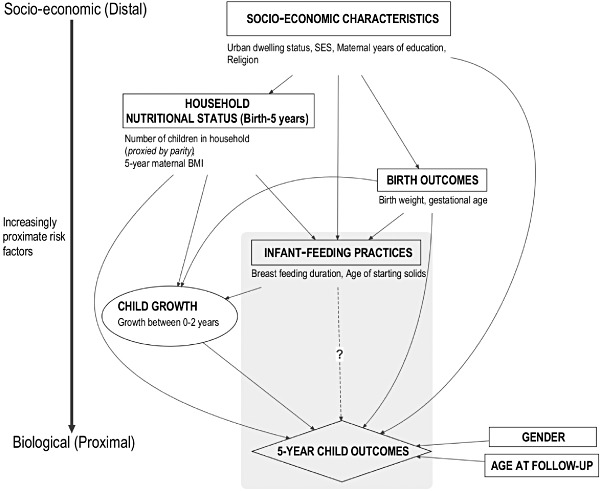
Conceptual framework for examining the potential association between infant‐feeding practices and 5‐year child study outcomes at 5 years, high BMI and adiposity, for multiple regression analyses.
There are four blocks of variables: socio‐economic characteristics, household nutritional status, birth outcomes and infant‐feeding practices. Blocks are used to enter specific covariates into regression models in chunks. Covariates were only entered as confounders in regression models if they were (1) considered a priori; or (2) had ≥5% change in estimate between the crude and adjusted estimates. For both exposures, we adjusted for urban dwelling, SES, maternal education, birthweight and gestational age (a priori confounders), maternal BMI at 5‐year follow‐up and family history of diabetes [confounders by criteria (2)]. Variables not included in the models were religion, GDM and parity. Six successive regression models were fitted based on the order of blocks, where later models included all variables from previous models. The first model assessed socio‐economic characteristics. Model 2 included socio‐economic characteristics and added household nutritional status at 5 years. This approach was continued until Model 6, the final model, which demonstrated the effect of infant‐feeding practices on 5‐year child outcomes controlling for socio‐economic characteristics, household nutritional status at 5 years, family history of diabetes, birth outcomes, other infant‐feeding practices, child age and gender.
Weight gain (from birth to 2 years) was considered a mediator (intermediate variable) between the potential causal association of breastfeeding duration and 5‐year outcomes. Accordingly, weight gain was not adjusted for in the main analysis (Models 1–6). The presence of mediation was assessed by comparing the breastfeeding duration effect estimate for Model 6 before and after adjustment for weight gain. A reduction in the effect estimate was suggestive of mediation.
Differences in association by gender and prematurity status (a priori effect modifiers) as well as other covariates were investigated using Mantel–Haenszel stratified analysis, comparing stratum‐specific ORs and 95% CI, and inspecting for trends across strata. A χ 2 test of homogeneity was also used as statistical guidance for the presence of effect modification. There was no evidence of effect modification by either gender or prematurity status. Stata v10 (StataCorp, College Station, TX, USA) was used for all analyses.
Results
Characteristics of the children and parents are summarized in Table 1. Almost all mothers initiated breastfeeding, with 89% (504/568) of infants breastfed for at least 6 months. The median breastfeeding duration was 12 months, while the median age for starting solids was 4 months. The mean BMI of the children was 13.6 kg m–2 (SD 1.12). Low BMI prevalence (<–2SD, WHO reference) was 10.6% and 23.1% at 1 and 5 years of age, respectively. At 5 years, 10.1% of children were stunted (<–2SD for height, WHO reference). The prevalence of high BMI was not considered as this outcome is defined as a sex‐specific BMI greater than the within‐cohort 90th percentile. That is, 10% of children at 5 years with the greatest (sex‐specific) BMI was classified as having ‘high BMI’.
Table 1.
Characteristics of the sample in Mysore, South India, 1997–2003
| Variable | n | Boys (n = 272) | Girls (n = 296) | Both sexes |
|---|---|---|---|---|
| Level 1: socio‐economic characteristics | ||||
| Urban dwelling status | 568 | 195 (72) | 226 (76) | 421 (74) |
| Kuppuswamy score median (IQR) | 568 | 33 (29, 38) | 34 (30, 38) | 34 (30, 38) |
| Maternal education (years), n (%) | ||||
| Illiterate (0) | 568 | 5 (1.8) | 7 (2.4) | 12 (2.1) |
| Primary school (1–4) | 18 (6.6) | 10 (3.4) | 28 (4.9) | |
| Middle school (5–7) | 48 (17.7) | 36 (12.2) | 84 (14.8) | |
| High school (8–10) | 98 (36.0) | 131 (44.3) | 229 (40.3) | |
| High 2o school certificate (11–12) | 65 (23.9) | 60 (20.3) | 125 (22.0) | |
| Degree (13–15) | 31 (11.4) | 42 (14.2) | 73 (12.9) | |
| Professional (16+) | 7 (2.6) | 10 (3.4) | 17 (3.0) | |
| Religion, n (%) | ||||
| Hindu | 568 | 145 (53.3) | 175 (59.1) | 320 (56.3) |
| Muslim | 99 (36.4) | 99 (33.5) | 198 (34.9) | |
| Other | 28 (10.3) | 22 (7.4) | 50 (8.8) | |
| Level 2: family & maternal gestational diabetes | ||||
| Gestational diabetes, n (%) | 524 | 13 (4.9) | 22 (7.7) | 35 (6.3) |
| Family history of diabetes, n (%) | 555 | 52 (20) | 60 (21) | 112 (21) |
| Level 3: birth outcomes | ||||
| Birthweight (g) | 568 | 2909 (466) | 2834 (411) | 2870 (441) |
| Gestational age (weeks) median (IQR) | 568 | 39.1 (38.1, 40.0) | 39.4 (38.6, 40.1) | 39.3 (38.4, 40.1) |
| Prematurity (<37 weeks), n (%) | 568 | 26 (9.6) | 25 (8.5) | 51 (9.0) |
| Level 4: infant‐feeding practices | ||||
| Breastfeeding duration (months), n (%) | 568 | |||
| 1–4 | 20 (7.4) | 22 (7.4) | 42 (7.4) | |
| 5–8 | 42 (15.4) | 34 (11.5) | 76 (13.4) | |
| 9–12 | 109 (40.1) | 138 (46.6) | 247 (43.5) | |
| 13–16 | 44 (16.2) | 48 (16.2) | 92 (16.2) | |
| 17–20 | 33 (12.1) | 37 (12.5) | 70 (12.3) | |
| 21+ | 24 (8.8) | 17 (5.7) | 41 (7.2) | |
| Age started regular solids (months), n (%) | 532 | |||
| ≤3 | 46 (18.0) | 55 (19.9) | 101 (19.0) | |
| 4 | 93 (36.3) | 109 (39.5) | 202 (38.0) | |
| 5 | 79 (30.9) | 66 (23.9) | 145 (27.2) | |
| ≥6 | 38 (14.8) | 46 (16.7) | 84 (15.8) | |
| Level 5: household nutritional status | ||||
| Maternal BMI at 5 years (kg m–2) | 543 | 23.3 (4.4) | 23.8 (4.6) | 23.6 (4.5) |
| Parity, n (%) | 568 | |||
| No children | 135 (49.6) | 154 (52.0) | 289 (50.9) | |
| One child | 90 (33.1) | 90 (33.1) | 190 (33.5) | |
| Two or more children | 47 (17.3) | 47 (17.3) | 89 (15.7) | |
| Childhood outcomes measures at 1 and 2 years of age | ||||
| Weight (kg) at 1 year | 532 | 8.7 (1.1) | 8.1 (1.0) | 8.4 (1.1) |
| Weight (kg) at 2 years | 549 | 10.8 (1.2) | 10.2 (1.2) | 10.5 (1.2) |
| BMI <–2SD (WHO reference) at 1 year, n (%) | 529 | 33 (12.9) | 23 (8.4) | 56 (10.6) |
| BMI <–2SD (WHO reference) at 2 years, n (%) | 549 | 12 (4.5) | 9 (3.2) | 21 (3.8) |
| Childhood outcomes measures at 5 years of age | ||||
| Height (cm) | 568 | 106.4 (4.3) | 105.0 (4.3) | 105.7 (4.3) |
| Weight (kg) | 568 | 15.4 (1.9) | 15.0 (2.0) | 15.2 (2.0) |
| BMI <–2SD (WHO reference), n (%) | 568 | 60 (22.5) | 68 (23.7) | 128 (23.1) |
| Height <–2SD (WHO reference), n (%) | 568 | 21 (7.9) | 35 (12.2) | 56 (10.1) |
| BMI (kg m–2) | 568 | 13.6 (1.04) | 13.6 (1.21) | 13.6 (1.12) |
| High BMI, n (%) | 568 | 28 (10.3) | 30 (10.1) | 58 (10.2) |
| Skinfolds (mm) (median, IQR): | ||||
| Subscapular | 568 | 5.3 (4.8, 6.2) | 6.0 (5.1, 7.6) | 5.6 (4.9, 7.0) |
| Triceps | 568 | 7.2 (6.3, 8.4) | 8.1 (7.0, 9.8) | 7.7 (6.6, 8.9) |
| Sum of skinfolds | 568 | 12.5 (11.1, 14.4) | 14.4 (12.3, 17.3) | 13.5 (11.7, 15.8) |
IQR, interquartile range; BMI, body mass index; SD, standard deviation; WHO, World Heath Organization. Data are presented as mean and SD unless otherwise stated.
2, 3 present socio‐economic characteristics, history of diabetes, birth outcomes, household nutritional status and 5‐year child outcomes by the duration of breastfeeding and age of starting solids. Compared with children who were breastfed longer, infants having shorter breastfeeding duration were more likely to be urban residents, Muslim and live in households with two or more siblings (Table 2). No associations were observed between breastfeeding duration and SES, maternal education and maternal BMI at 5 years. While infants having either a short or long breastfeeding duration had higher birthweight, there was no association with prematurity status.
Table 2.
Relationship of breastfeeding duration by covariates and 5‐year child outcomes in Mysore, South India, 1997–2003
| Variable | n | Breastfeeding duration (months)* | P † | |||
|---|---|---|---|---|---|---|
| <6 (n = 64) | 6–11 (n = 141) | 12–17 (n = 253) | 18+ (n = 110) | |||
| Level 1: socio‐economic characteristics | ||||||
| Urban dwelling, n (%) | 568 | 55 (86.0) | 98 (69.5) | 178 (70.4) | 90 (81.8) | <0.001 ga |
| Kuppuswamy score | 568 | 33.6 (6.4) | 34.8 (5.6) | 33.9 (6.3) | 33.8 (7.3) | 0.83 |
| Maternal education (years) | 568 | 10.4 (3.5) | 10.9 (3.5) | 10.4 (3.4) | 10.3 (3.8) | 0.94 |
| Religion, n (%) | ||||||
| Hindu | 320 | 17 (26.6) | 86 (61) | 164 (64.8) | 53 (48.2) | |
| Muslim | 198 | 41 (64.1) | 46 (32.6) | 72 (28.5) | 39 (35.5) | <0.001 ga |
| Other | 50 | 6 (9.4) | 9 (6.4) | 17 (6.7) | 18 (16.4) | |
| Level 2: family & gestational history of diabetes | ||||||
| Family history of diabetes, n (%) | 555 | 16 (25.4) | 27 (19.7) | 46 (18.6) | 23 (21.5) | 0.15 |
| Gestational diabetes, n (%) | 538 | 4 (6.3) | 2 (1.4) | 15 (5.5) | 14 (12.7) | 0.01 |
| Level 3: birth outcomes | ||||||
| Birthweight (g) | 568 | 2913 (419) | 2805 (415) | 2837 (442) | 3005 (451) | 0.05 ga |
| Prematurity, n (%) | 568 | 4 (6.3) | 15 (10.6) | 23 (9.1) | 9 (8.2) | 0.95 |
| Level 4: infant‐feeding practices | ||||||
| Age of starting solids (months), n (%) | ||||||
| ≤3 | 101 | 13 (21.7) | 28 (21.0) | 43 (18.2) | 17 (16.7) | 0.16 ga |
| 4 | 202 | 32 (53.3) | 48 (35.8) | 88 (37.3) | 34 (33.3) | |
| 5 | 145 | 11 (18.3) | 37 (27.6) | 69 (29.2) | 28 (27.5) | |
| ≥6 | 84 | 4 (6.7) | 21 (15.7) | 36 (15.3) | 23 (22.6) | |
| Level 5: household nutritional status | ||||||
| Maternal BMI at 5 years (kg m–2) | 543 | 23.9 (4.9) | 23.1 (4.1) | 23.4 (4.3) | 24.5 (5.1) | 0.33 |
| Parity, n (%) | ||||||
| No children | 289 | 34 (53.1) | 80 (56.7) | 130 (51.4) | 45 (40.9) | |
| One child | 90 | 16 (25.0) | 45 (31.9) | 85 (33.6) | 44 (40.0) | 0.02 ga |
| Two or more children | 89 | 14 (21.9) | 16 (11.4) | 38 (15.0) | 21 (19.1) | |
| Main outcomes at 5 years of age | ||||||
| High BMI (>90th percentile), n (%) | 568 | 11 (17.2) | 19 (13.5) | 17 (6.7) | 11 (10.0) | 0.08 |
| Skinfolds (mm) geometric mean (+/– 1SD) | ||||||
| Subscapular | 568 | 6.3 (4.6, 8.5) | 6.0 (4.5, 8.0) | 5.9 (4.6, 7.6) | 5.9 (4.6, 7.6) | 0.07 |
| Triceps | 568 | 8.3 (6.2, 11.1) | 7.8 (6.1, 10.0) | 7.6 (6.2, 9.5) | 7.7 (6.0, 9.9) | 0.12 |
| Sum of skinfolds | 568 | 14.6 (11.0, 19.4) | 13.9 (10.8, 17.8) | 13.6 (11.0, 16.8) | 13.7 (10.8, 17.3) | 0.07 |
ga, general association; BMI, body mass index; SD, standard deviation. Data are presented as mean and SD unless otherwise stated. *The data are shown in four categories for economy of presentation, but all statistical tests used the six categories described in Methods. †Test of linear trend using breastfeeding duration in six categories, unless the test for a departure from linear trend was significant, in which case this is a likelihood ratio test or a χ 2 test of ga.
Table 3.
Relationship of age of starting regular solid feeding by covariates and 5‐year child outcomes in Mysore, South India, 1997–2003
| Variable | n | Age started regular solids (months) | P * | |||
|---|---|---|---|---|---|---|
| ≤3 (n = 100) | 4 (n = 201) | 5 (n = 145) | ≥6 (n = 83) | |||
| Level 1: socio‐economic characteristics | ||||||
| Urban dwelling, n (%) | 532 | 75 (74.3) | 166 (82.2) | 107 (73.8) | 56 (66.7) | 0.03 ga |
| Kuppuswamy score | 532 | 34.6 (6.4) | 34.5 (6.0) | 33.5 (6.3) | 33.2 (6.9) | 0.05 |
| Maternal education (years) | 532 | 10.5 (3.6) | 11.2 (3.1) | 10.0 (3.8) | 10.0 (3.7) | 0.01 ga |
| Religion, n (%) | ||||||
| Hindu | 294 | 57 (56.4) | 84 (41.6) | 101 (69.7) | 52 (18) | |
| Muslim | 188 | 26 (25.7) | 97 (48.0) | 39 (26.9) | 26 (14) | <0.001 ga |
| Other | 50 | 18 (17.8) | 21 (10.4) | 5 (3.5) | 6 (7.1) | |
| Level 2: family & gestational history diabetes | ||||||
| Family history of diabetes, n (%) | 521 | 22 (22.2) | 43 (21.1) | 25 (17.9) | 13 (15.7) | 0.61 ga |
| Gestational diabetes, n (%) | 503 | 5 (5.0) | 17 (7.9) | 4 (2.8) | 6 (7.1) | 0.76 ga |
| Level 3: birth outcomes | ||||||
| Birthweight (g) | 532 | 2946 (397) | 2901 (434) | 2797 (433) | 2854 (488) | 0.03 |
| Prematurity, n (%) | 532 | 7 (6.9) | 17 (8.4) | 13 (9.0) | 10 (11.9) | 0.25 |
| Level 4: infant‐feeding practices | ||||||
| Breastfeeding duration (months) | ||||||
| <6 | 60 | 13 (12.9) | 32 (15.8) | 11 (7.6) | 4 (4.8) | 0.16 ga |
| 6–11 | 134 | 28 (27.7) | 48 (23.8) | 37 (25.5) | 21 (25.0) | |
| 12–17 | 236 | 43 (42.6) | 88 (43.6) | 69 (47.6) | 36 (42.9) | |
| 18+ | 102 | 17 (16.8) | 34 (16.8) | 28 (19.3) | 23 (27.4) | |
| Level 5: household nutritional status | ||||||
| Maternal BMI at 5 years (kg m–2) | 509 | 23.5 (4.1) | 23.7 (4.1) | 23.1 (4.8) | 24.3 (5.2) | 0.58 |
| Parity, n (%) | ||||||
| No children | 267 | 60 (59.4) | 104 (51.5) | 67 (46.2) | 36 (42.9) | 0.32 ga |
| One child | 181 | 30 (29.7) | 67 (33.2) | 51 (35.2) | 33 (39.3) | |
| Two or more children | 84 | 11 (10.9) | 31 (15.4) | 27 (18.6) | 15 (17.9) | |
| Main outcomes at age 5 years | ||||||
| High BMI (>90th percentile), n (%) | 532 | 12 (11.9) | 24 (11.9) | 11 (7.6) | 7 (8.3) | 0.21 |
| Skinfolds (mm) geometric mean (+/– 1SD) | ||||||
| Subscapular | 532 | 6.1 (4.7, 8.0) | 6.0 (4.6, 7.9) | 5.8 (4.5, 7.7) | 5.9 (4.6, 7.7) | 0.19 |
| Triceps | 532 | 8.1 (6.1, 10.6) | 8.0 (6.2, 10.2) | 7.5 (6.0, 9.5) | 7.6 (6.1, 9.5) | 0.02 |
| Sum of skinfolds | 532 | 14.3 (11.1, 18.5) | 14.1 (11.1, 17.8) | 13.5 (10.7, 16.9) | 13.6 (10.9, 16.9) | 0.05 |
ga, general association; BMI, body mass index; SD, standard deviation. Data are presented as mean and SD unless otherwise stated. *Test of linear trend using age of starting regular solids in four categories, unless the test for a departure from linear trend was significant, in which case this is a likelihood ratio test for continuous variables or a χ 2 test for categorical variables, of a ga.
A later introduction of solid feeding was associated with urban residence, lower SES and maternal educational attainment (Table 3). Hindu mothers typically introduced solids later compared with mothers following Islam or ‘other’ religions.
Associations of breastfeeding duration with high BMI and sum of skinfold thickness at 5 years
The proportion of children having high BMI at 5 years was lower in children breastfed for a longer duration (≥18 months: 10%, 11/110) compared with children breastfed for a shorter duration (<6 months: 17%, 11/64), with weak statistical evidence of a dose–response effect (ptrend = 0.07) (Table 2). A longer duration of breastfeeding was associated with smaller sum of skinfold thickness at 5 years, with weak evidence of a dose–response effect (ptrend = 0.07).
Increasing breastfeeding duration (4‐month duration categories: 1–4, 5–8, 9–12, 17–20 and 21+) was weakly associated with a lower risk of high BMI at 5 years [odds ratio (OR) = 0.87, 95% confidence interval (CI): 0.69–1.10, P = 0.26, Table 4]. A one‐category increase in breastfeeding duration resulted in a 13% reduction in high BMI risk at 5 years. Successive introduction of confounding variables (Models 1–5) made negligible difference to the size of the unadjusted effect estimate. Adjusting for urban dwelling, Kuppuswamy score, maternal education, birthweight, gestational age, family history of diabetes and 5‐year maternal BMI, child gender and current age (Model 6) made no substantial difference to these findings, with the CIs too wide to exclude the possibility of no effect [adjusted OR (aOR) = 0.87, 95% CI: 0.68–1.11, P = 0.26). Multiple linear regression analysis estimated a reduction of mean BMI by 0.04 (kg m–2) per one‐category increase in breastfeeding duration, adjusting for an identical set of covariates included in Model 6, the final logistic regression model (95% CI: from –0.12 to 0.04, P = 0.33).
Table 4.
Associations between breastfeeding duration and age of starting solid feeding with high BMI (sex‐specific BMI >90th centile) and sum of skinfolds at 5 years in Mysore, South India, 1997–2003
| Model* | High BMI | Sum of skinfolds † | ||
|---|---|---|---|---|
| OR (95% CI) | P‐value | % change | P‐value | |
| Breastfeeding duration | ||||
| Unadjusted association for breastfeeding duration effect | 0.87 (0.69 to 1.10) | 0.26 | –0.9 (–2.5 to 0.8) | 0.31 |
| Model 1: socio‐economic characteristics | 0.88 (0.69 to 1.11) | 0.27 | –0.8 (–2.5 to 0.8) | 0.33 |
| Model 2: model 1 + household nutritional status | 0.85 (0.67 to 1.07) | 0.17 | –1.0 (–2.6 to 0.7) | 0.24 |
| Model 3: model 2 + family history of diabetes | 0.85 (0.67 to 1.07) | 0.17 | –1.0 (–2.6 to 0.7) | 0.25 |
| Model 4: model 3 + birth outcomes | 0.84 (0.66 to 1.07) | 0.17 | –0.8 (–2.4 to 0.7) | 0.37 |
| Model 5: model 4 + age of starting solid feeds | 0.87 (0.68 to 1.11) | 0.25 | –0.6 (–2.2 to 1.1) | 0.48 |
| Model 6: model 5 + child gender and current age | 0.87 (0.68 to 1.11) | 0.26 | –0.6 (–2.2 to 1.0) | 0.47 |
| Age of starting solid feeds | ||||
| Unadjusted association for age of starting solid feeds effect | 0.79 (0.58 to 1.07) | 0.14 | –2.2 (–4.3 to 0.0) | 0.05 |
| Model 1: socio‐economic characteristics | 0.79 (0.58 to 1.08) | 0.15 | –1.8 (–3.9 to 0.4) | 0.10 |
| Model 2: model 1 + household nutritional status | 0.77 (0.56 to 1.06) | 0.10 | –1.9 (–4.1 to 0.2) | 0.08 |
| Model 3: model 2 + family history of diabetes | 0.77 (0.56 to 1.05) | 0.10 | –1.9 (–4.0 to 0.2) | 0.08 |
| Model 4: model 3 + birth outcomes | 0.75 (0.56 to 1.05) | 0.09 | –0.9 (–2.6 to 0.7) | 0.26 |
| Model 5: model 4 + breastfeeding duration | 0.77 (0.56 to 1.08) | 0.14 | –1.8 (–3.9 to 0.4) | 0.11 |
| Model 6: model 5 + child gender and current age | 0.78 (0.56 to 1.09) | 0.15 | –1.7 (–3.7 to 0.4) | 0.11 |
BMI, body mass index; OR, odds ratio; CI, confidence interval. OR and % change in sum of skinfolds are for a 1 category increase in breastfeeding (1–4, 5–8, 9–12, 13–16, 17–20 and 21+ months) and introduction of solids (≤3, 4, 5, 6+ months). To allow coefficients to be compared, all results are reported from the sample with complete data by each exposure (Final Model, n = 484). *Variables included in final regression models: socio‐economic characteristics (Block 1): urban dwelling status, Kuppuswamy score, maternal education; household nutritional status (Block 2): maternal BMI at 5‐year follow‐up; family history of diabetes; birth outcomes (Block 3): birthweight, gestational age; infant‐feeding practices (Block 4): either breastfeeding duration or age of starting solid feeds, child gender; current age at follow‐up. †Estimated percentage change in the geometric mean of sum of skinfold thickness (subscapular and triceps) for a per category increase in exposure.
There was no evidence of an important effect of breastfeeding duration on the geometric mean sum of skinfold thickness (mm) with weak statistical evidence of an association (% change –0.9, 95% CI: from –2.5 to 0.8, P = 0.31). This is interpretable as the percentage change in the expected geometric mean value of the sum of skinfolds, per category increase in breastfeeding duration. After adjustment for potential confounders, there was no change in the effect estimate and the lack of statistical evidence of an association remained.
Associations of age of starting regular solid feeding with high BMI and sum of skinfold thickness at 5 years
Children who were introduced to solids later tended to have a lower risk of high BMI, although there was weak evidence of a statistical association (P = 0.21, Table 3).
The regression analysis demonstrated (Table 4) that a later age of starting regular solid feeding was associated with a lower risk of high BMI (OR = 0.79, 95% CI: 0.58–1.07, P = 0.14). A one‐category increase in the age solids were introduced resulted in a 21% reduction in high BMI risk at 5 years. However, after accounting for sampling variability, there was weak statistical evidence of an association. After the adjustment for confounding factors in an identical manner to breastfeeding duration, there was negligible change in the effect estimate and the weak statistical evidence of the association remained. Multiple linear regression analysis, adjusting for an identical set of variables, estimated mean BMI decrease by 0.07 kg m–2 per one‐category increase in age of starting regular solid feeding (95% CI: –0.17 to –0.03, P = 0.15).
A one‐category increase in age of starting regular solid feeding was associated with a small reduction in the sum of skinfolds, compared with a lower category of duration (% difference: –2.2, 95% CI: –4.3 to 0.0, P = 0.05, Table 4). On adjustment for socio‐economic characteristics, this association was attenuated, and accounting for sampling variability, there was no statistical evidence of an association. The final model (Model 6) demonstrated that a one‐category increase in the age of starting regular solids resulted in a 1.7% reduction in the sum of skinfold thickness, with weak statistical evidence of an effect (95% CI: –3.7 to 0.4, P = 0.11).
Breastfeeding duration, weight gain (from birth to 2 years), high BMI and sum of skinfold thickness at 5 years
Breastfeeding duration was strongly negatively associated with weight gain (0–2 years) (β = –0.12 SD 95% CI: –0.19 to –0.05 per category change in breastfeeding duration, P = 0.001). After adjustment for breastfeeding duration and confounding variables (Model 6 variables), weight gain was strongly positively associated with high BMI at 5 years (aOR = 3.75, 95% CI: 2.53–5.56, P < 0.001) and sum of skinfolds (adjusted % change = 10.0, 95% CI: 7.9–12.2). The inclusion of weight gain attenuated the overall effect of breastfeeding duration on high BMI. The protective effect estimate (OR) of breastfeeding duration (per category increase) was reduced from 0.87 (95% CI: 0.69–1.10) to 1.02 (95% CI: 0.77–1.35). The skinfold association was attenuated from –0.9% (95% CI: –2.5 to 0.8) to 0.4% (95% CI: –1.1 to 1.9).
Discussion
We used data from a birth cohort study in South Indian children to evaluate whether a longer duration of breastfeeding, and the age an infant regularly starts solid feeding, reduced the risk of high BMI and skinfold thickness at 5 years of age. Based on our sample, there was a small magnitude of effect for the unadjusted association of a longer duration of breastfeeding on reducing the risk of high BMI. After adjusting for several important confounding variables, the breastfeeding effect estimate remained unchanged. The final model which included all potential confounding covariates (Model 6), estimated a 13% reduction in the risk of high BMI per duration of breastfeeding category. This finding suggests a potential public health benefit for promoting longer breastfeeding duration, but given the CI of the adjusted effect estimate, we cannot exclude the possibility of no effect. Additionally, our study found no evidence of an important size of exposure effect on skinfold thickness.
There is a paucity of literature in LMI countries investigating this potential association. In these settings, prospective observational studies with infant‐feeding data have tended to evaluate adult cardiometabolic risk factors as an outcome rather than childhood BMI (Fall et al. 2010). Our findings differ from two similar studies conducted in Brazil and Chile, which demonstrated no evidence of a protective effect of breastfeeding on overweight at 4 years of age (Araújo et al. 2006; Corvalan et al. 2009). There have been mixed results from systematic reviews and recent studies in high‐income countries (Hediger et al. 2001; Arenz et al. 2004; Burke et al. 2005; Harder et al. 2005; Owen et al. 2005b; Mayer‐Davis et al. 2006; Shields et al. 2006; Weyermann et al. 2006) and the few studies conducted on direct adiposity measures (Baranowski et al. 1990; Neutzling et al. 2009). A North American study directly evaluating childhood adiposity using Dual‐energy X‐ray absorptiometry (DXA) measurements in 5‐year‐old children demonstrated no association with breastfeeding duration (Burdette et al. 2006). Furthermore Promotion of Breastfeeding Intervention trial (PROBIT), a cluster randomized control trial in Belarus evaluated whether a breastfeeding promotion intervention to increase breastfeeding duration and exclusivity would result in reduced later childhood anthropometry. Their findings indicated there was little evidence of a reduction in BMI or skinfold thickness in children at 6 years of age (Kramer et al. 2007).
In our study, the effect of a longer duration of breastfeeding on mean BMI was very small, with no strong evidence of a statistical association. Notwithstanding small sample size considerations, this finding is consistent with other studies that suggest that breastfeeding may lower the risk of higher BMI levels rather than shifting down (movement to the left along the x‐axis) the entire BMI distribution (Owen et al. 2005a; Toschke et al. 2008). Similarly, the effect of longer duration of breastfeeding on the sum of skinfolds was very small, with weak statistical evidence of an effect. Studies that have measured skinfold thickness in children have demonstrated mixed results (Zive et al. 1992; Bergmann et al. 2003; Bogen et al. 2004; Kramer et al. 2007). The lack of an association of either public health or statistical importance may reflect that the long lag period between exposure and outcome is outside the exposure's biological range of effect. That is, infant‐feeding practices are more likely to be biologically associated to subcutaneous adiposity in the earlier years of life rather than at pre‐school age. Furthermore, as South Asian populations are known to have increased abdominal fat deposition compared with white Caucasians, (McKeigue et al. 1991) the lack of an observed effect observed could potentially be because of breastfeeding influencing central adiposity (e.g. intra‐abdominal fat) more than subcutaneous fat, which would not be assessed with skinfold measurements.
Both infant‐feeding exposure effects had a similar low magnitude of association, that is, protective ORs in the range of 0.77–0.91 (corresponding to risk ORs: 1.1–1.3). However, compared with an increase in breastfeeding duration, a later age for starting regular solid feeding had a greater sized reduction in high BMI risk, with the CIs too wide to exclude the possibility of no effect. Additionally, there was no evidence of an association between a later start of solids and skinfold thickness at 5 years. Previous findings regarding our results are mixed. While some studies have reported lower rates of obesity in children who started solids later (Wilson et al. 1998; Reilly et al. 2005), others have reported no association (Kramer 1981; Zive et al. 1992; Burdette et al. 2006) or greater adiposity associated with delayed introduction of solids (Agras et al. 1990). Future research could be used to help resolve this issue. Firstly, the age of starting regular solid feeding is a simple exposure and ignores the dynamics of nutrition during the period of 6–24 months, which is likely to influence later child anthropometry. Firstly, utilizing detailed infant‐feeding measures such as the WHO Infant and Young Complementary Feeding (IYCF) indicators (WHO 2008) in a longitudinal study design may help. Secondly, studies need to be conducted in settings of relatively high child overweight/obesity prevalence, in order to describe changing dietary patterns during infancy and its relation to later body composition.
There are only a few studies that report the effect of adjusting for weight gain during the early years of life on the associations between infant feeding and later BMI or adiposity (Lamb et al. 2010; van Rossem et al. 2010). Our qualitative mediational analysis agrees with these studies by suggesting that weight gain (from birth to 2 years) is a mediator of the relationship between breastfeeding duration and risk of high BMI. We observed that a longer breastfeeding duration is strongly associated with slower weight gain between 0–2 years and that weight gain is strongly associated with high BMI. When weight gain (0–2 years) is controlled for, the breastfeeding duration effect estimate was attenuated. Within the literature, robust findings are that breastfeeding is associated with lower weight gain compared with formula‐feeding infants (Dewey et al. 1992; Kramer et al. 2007; Griffiths et al. 2009) and that lower weight gain during the early years of life is associated with a lower later BMI (Ong et al. 2002; Baird et al. 2005; Monteiro & Victora 2005; Wells et al. 2005; Stettler 2007; Griffiths et al. 2010). Primary studies evaluating the size of the mediated effect using path analysis will provide further insight into the potential mechanism between breastfeeding duration and levels of pre‐school BMI.
In this sample, undernutrition (low BMI and stunting) had a greater prevalence than high BMI at 5 years (23% had a BMI of <–2 SDs below WHO median, 10.1% had a height of <–2 SDs below WHO median). There was no association with these outcomes and infant‐feeding practices (results not shown). The lower prevalence of overweight/obesity in this population may undermine our decision to investigate high BMI risk. However, in an Indian population where overweight and obesity emerges from a context of infant undernutrition, we think it is important to examine the role of infant‐feeding practices on later childhood adiposity. Underscoring this approach is that (1) BMI tracks through the life course such that children with higher BMI levels are more likely to be overweight/obese adolescents and adults compared with thinner children (Singh et al. 2008) and (2) upward crossing of BMI percentiles during childhood, even within the normal range of BMI, is associated with an increased risk of adult type 2 diabetes and metabolic syndrome (Fall et al. 2008; Sachdev et al. 2009).
Particular strengths of our study include the use of skinfold thickness, a direct measure of adiposity, as well as BMI, and that it adds to the small number of studies on this subject from LMI countries. Additionally, a conceptually designed analysis was used to adjust for potential confounding and identify potential mediating factors. However, in interpreting our findings, a few methodological issues require consideration. This was a relatively small observational study. As such, it may lack the statistical power to detect small‐sized infant‐feeding effects on the outcome. Given that infant‐feeding practices were not randomly assigned to children, there is likely to be an unobserved systematic allocation of exposure assignment. So while adjusting for several important confounding variables, we cannot exclude the possibility that there were remaining systematic differences in children with varying infant‐feeding practices. For example, even within SES strata maternal infant‐feeding practices and related child‐care attitudes may vary (Bentley et al. 2003; Sharps et al. 2008).
Because of exposure misclassification and the absence of exclusive breastfeeding data, the magnitude of the protective effect of increasing breastfeeding duration could have been underestimated (towards null results). The ascertainment of breastfeeding duration based on maternal recall at 1 year is considered to be reasonably accurate (Kark et al. 1984). However, because we ascertained breastfeeding data at 12, 24, 36 months follow‐up, this may have increased the tendency of ‘heaping’ (digit preference) – a specific form of recall bias, which is suggested by observation of a large peak in breastfeeding duration at 12 months. Alternatively, such a peak may represent true breastfeeding duration behaviour because of local breastfeeding norms. The effect of starting solid feeding may also be underestimated, as the lack of information on the type and quality of feeds may be more strongly related to its long‐term effects on body composition rather than the age at which solids was started. We observed a lack of association between infant‐feeding practices and SES. As diet and physical activity is typically related to SES, it is unlikely that the absence of this covariate data for children at 5 years will strongly confound the association between infant‐feeding practices and 5‐year outcomes.
In a sample of South Indian pre‐school children, this cohort study demonstrates findings suggestive that a longer duration of breastfeeding is associated with a small reduction in the risk of high BMI at 5 years. Additionally, an earlier introduction of solid feeding may slightly increase the risk of high BMI. After accounting for sampling variability, these findings cannot exclude no effect as a possibility at the population level. We have shown qualitatively that a longer duration of breastfeeding is associated with slower weight gain from birth to 2 years, which in turn is associated with a reduced risk of high BMI. Consideration of both our own findings that infant‐feeding practices have a low magnitude of effect and previous meta‐analyses (Harder et al. 2005; Owen et al. 2005b), the promotion of current WHO guidelines on infant feeding may offer only a fairly small role in the prevention of overweight/obesity in pre‐school children. Rather, for India to effectively avoid the alarming trends of child overweight/obesity prevalence seen in other LMI countries with fast growing economies (Luo & Hu 2002; Jiang et al. 2006; Wang & Lobstein 2006), will depend on how the country develops its own long‐term, multilevel strategies to address the major determinants of child overweight/obesity at the population level: the increasing consumption of high energy‐dense diets and reduced physical activity.
Sources of funding
The study was funded by the Parthenon Trust, Switzerland, the Wellcome Trust, UK, and the MRC Lifecourse Epidemiology Unit, UK.
Conflict of interest
The authors declare that they have no conflicts of interest.
Contributions
AC had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design were done by AC, AKW, JH and CHDF. GVK, SRV, SCK and JH collected data. Data was analysed and interpreted by AC, CHDF and AKW. AC and CHDF drafted the manuscript. Critical revision of the manuscript for important intellectual content was done by AC, AKW and CHDF. Statistical analysis was done by AKW and AC.
Acknowledgements
We are tremendously grateful to the women and children who participated, to Dr BDR Paul, former director of HMH, and the obstetrics and paediatric consultants. We thank Jayakumar, Geetha, Saroja, Chachyamma, Tony Gerald, Tony Clifford, Shobha, Gopal Singh, Kiran, Jane Pearce and Patsy Coakley for their substantial contributions. We also thank Sneha‐India for its support.
References
- Agras W.S., Kraemer H.C., Berkowitz R.I. & Hammer L.D. (1990) Influence of early feeding style on adiposity at 6 years of age. The Journal of Pediatrics 116, 805–809. [DOI] [PubMed] [Google Scholar]
- Araújo C.L., Victora C.G., Hallal P.C. & Gigante D.P. (2006) Breastfeeding and overweight in childhood: evidence from the Pelotas 1993 birth cohort study. International Journal of Obesity (2005) 30, 500–506. [DOI] [PubMed] [Google Scholar]
- Arenz S., Rückerl R., Koletzko B. & von Kries R. (2004) Breast‐feeding and childhood obesity – a systematic review. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 28, 1247–1256. [DOI] [PubMed] [Google Scholar]
- Arnold F., Parasuraman S., Arokiasamy P. & Kothari M. (2009) Nutrition in India. National Family Health Survey (NFHS‐3), India, 2005–06. International Institute for Population Sciences: Mumbai; Calverton, Maryland, USA: ICF Macro. [Google Scholar]
- Baird J., Fisher D., Lucas P., Kleijnen J., Roberts H. & Law C. (2005) Being big or growing fast: systematic review of size and growth in infancy and later obesity. British Medical Journal 331, 929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird J., Poole J., Robinson S., Marriott L., Godfrey K., Cooper C. et al (2008) Milk feeding and dietary patterns predict weight and fat gains in infancy. Paediatric and Perinatal Epidemiology 22, 575–586. [DOI] [PubMed] [Google Scholar]
- Baranowski T., Bryan G.T., Rassin D.K., Harrison J.A. & Henske J.C. (1990) Ethnicity, infant‐feeding practices, and childhood adiposity. Journal of Developmental and Behavioral Pediatrics 11, 234–239. [PubMed] [Google Scholar]
- Bentley M.E., Dee D.L. & Jensen J.L. (2003) Breastfeeding among low income, African‐American women: power, beliefs and decision making. The Journal of Nutrition 133, 305S–309S. [DOI] [PubMed] [Google Scholar]
- Bergmann K.E., Bergmann R.L., Von Kries R., Böhm O., Richter R., Dudenhausen J.W. et al (2003) Early determinants of childhood overweight and adiposity in a birth cohort study: role of breast‐feeding. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 27, 162–172. [DOI] [PubMed] [Google Scholar]
- Bogen D.L., Hanusa B.H. & Whitaker R.C. (2004) The effect of breast‐feeding with and without formula use on the risk of obesity at 4 years of age. Obesity Research 12, 1527–1535. [DOI] [PubMed] [Google Scholar]
- Boutayeb A. (2006) The double burden of communicable and non‐communicable diseases in developing countries. Transactions of the Royal Society of Tropical Medicine and Hygiene 100, 191–199. [DOI] [PubMed] [Google Scholar]
- Bolling K. & Grant C. (2007) Infant Feeding Survey 2005. London: The Information Centre. [Google Scholar]
- Bronte‐Tinkew J. & DeJong G. (2004) Children's nutrition in Jamaica: do household structure and household economic resources matter? Social Science & Medicine 58, 499–514. [DOI] [PubMed] [Google Scholar]
- Burdette H.L., Whitaker R.C., Hall W.C. & Daniels S.R. (2006) Breastfeeding, introduction of complementary foods, and adiposity at 5 y of age. The American Journal of Clinical Nutrition 83, 550–558. [DOI] [PubMed] [Google Scholar]
- Burke V., Beilin L.J., Simmer K., Oddy W.H., Blake K.V., Doherty D. et al (2005) Breastfeeding and overweight: longitudinal analysis in an Australian birth cohort. The Journal of Pediatrics 147, 56–61. [DOI] [PubMed] [Google Scholar]
- Carpenter M.W. & Coustan D.R. (1982) Criteria for screening tests for gestational diabetes. American Journal of Obstetrics and Gynecology 144, 768–773. [DOI] [PubMed] [Google Scholar]
- Chakravarty I. & Ghosh K. (2000) Micronutrient malnutrition – present status and future remedies. Journal of the Indian Medical Association 98, 539–542. [PubMed] [Google Scholar]
- Chu N.F., Rimm E.B., Wang D.J., Liou H.S. & Shieh S.M. (1998) Clustering of cardiovascular disease risk factors among obese schoolchildren: the Taipei Children Heart Study. The American Journal of Clinical Nutrition 67, 1141–1146. [DOI] [PubMed] [Google Scholar]
- Corvalan C., Kain J., Weisstaub G. & Uauy R. (2009) Impact of growth patterns and early diet on obesity and cardiovascular risk factors in young children from developing countries. The Proceedings of the Nutrition Society 68, 327–337. [DOI] [PubMed] [Google Scholar]
- Dewey K.G. (1998) Growth characteristics of breast‐fed compared to formula‐fed infants. Neonatology 74, 94–105. [DOI] [PubMed] [Google Scholar]
- Dewey K.G., Heinig M.J., Nommsen L.A., Peerson J.M. & Lonnerdal B. (1992) Growth of breast‐fed and formula‐fed infants from 0 to 18 months: the DARLING study. Pediatrics 89 (6 Pt 1), 1035–1041. [PubMed] [Google Scholar]
- Fall C.H.D., Sachdev H.S., Osmond C., Lakshmy R., Biswas S.D., Prabhakaran D. et al (2008) Adult metabolic syndrome and impaired glucose tolerance are associated with different patterns of BMI gain during infancy: data from the New Delhi brth cohort. Diabetes Care 31, 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall C.H.D., Borja J.B., Osmond C., Richter L., Bhargava S.K., Martorell R. et al & the COHORTS Group (2010) Infant‐feeding patterns and cardiovascular risk factors in young adulthood: data from five cohorts in low‐ and middle‐income countries. International Journal of Epidemiology 40, 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman D.S., Dietz W.H., Srinivasan S.R. & Berenson G.S. (1999) The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics 103, 1175–1182. [DOI] [PubMed] [Google Scholar]
- Garn S.M. & LaVelle M. (1985) Two‐decade follow‐up of fatness in early childhood. American Journal of Diseases of Children (1960) 139, 181–185. [DOI] [PubMed] [Google Scholar]
- Gillman M.W., Barker D., Bier D., Cagampang F., Challis J., Fall C. et al (2007) Meeting report on the 3rd International Congress on Developmental Origins of Health and Disease (DOHaD). Pediatric Research 61 (5 Pt 1), 625–629. [DOI] [PubMed] [Google Scholar]
- Griffiths P.L. & Bentley M.E. (2001) The nutrition transition is underway in India. The Journal of Nutrition 131, 2692–2700. [DOI] [PubMed] [Google Scholar]
- Griffiths L.J., Smeeth L., Hawkins S.S., Cole T.J. & Dezateux C. (2009) Effects of infant feeding practice on weight gain from birth to 3 years. Archives of Disease in Childhood 94, 577–582. [DOI] [PubMed] [Google Scholar]
- Griffiths L.J., Hawkins S.S., Cole T.J., Dezateux C. & Millennium Cohort Study Child Health Group (2010) Risk factors for rapid weight gain in preschool children: findings from a UK‐wide prospective study. International Journal of Obesity (2005) 34, 624–632. [DOI] [PubMed] [Google Scholar]
- Harder T., Bergmann R., Kallischnigg G. & Plagemann A. (2005) Duration of breastfeeding and risk of overweight: a meta‐analysis. American Journal of Epidemiology 162, 397–403. [DOI] [PubMed] [Google Scholar]
- Heaton T.B., Forste R., Hoffmann J.P. & Flake D. (2005) Cross‐national variation in family influences on child health. Social Science and Medicine (1982) 60, 97–108. [DOI] [PubMed] [Google Scholar]
- Heck K.E., Braveman P., Cubbin C., Chávez G.F. & Kiely J.L. (2006) Socioeconomic Status and Breastfeeding Initiation Among California Mothers. Public Health Reports 121, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hediger M.L., Overpeck M.D., Kuczmarski R.J. & Ruan W.J. (2001) Association between infant breastfeeding and overweight in young children. JAMA: The Journal of the American Medical Association 285, 2453–2460. [DOI] [PubMed] [Google Scholar]
- Hill J., Krishnaveni G.V., Annamma I., Leary S.D. & Fall C.H.D. (2005) Glucose tolerance in pregnancy in South India: relationships to neonatal anthropometry. Acta Obstetricia Et Gynecologica Scandinavica 84, 159–165. [DOI] [PubMed] [Google Scholar]
- Hoddinott P. & Pill R. (1999) Qualitative study of decisions about infant feeding among women in east end of London. BMJ 318, 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Institute for Population Sciences (IIPS) and ORC Macro (2001) National Family Health Survey (NFHS‐2), India, 1998–99. IIPS, Mumbai: Mumbai. [Google Scholar]
- Jiang J., Rosenqvist U., Wang H., Greiner T., Ma Y. & Toschke A.M. (2006) Risk factors for overweight in 2‐ to 6‐year‐old children in Beijing, China. International Journal of Pediatric Obesity: IJPO: An Official Journal of the International Association for the Study of Obesity 1, 103–108. [DOI] [PubMed] [Google Scholar]
- Kark J.D., Troya G., Friedlander Y., Slater P.E. & Stein Y. (1984) Validity of maternal reporting of breast feeding history and the association with blood lipids in 17 year olds in Jerusalem. Journal of Epidemiology and Community Health 38, 218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S., Sachdev H.P., Dwivedi S.N., Lakshmy R. & Kapil U. (2008) Prevalence of overweight and obesity amongst school children in Delhi, India. Asia Pacific Journal of Clinical Nutrition 17, 592–596. [PubMed] [Google Scholar]
- Kramer M.S. (1981) Do breast‐feeding and delayed introduction of solid foods protect against subsequent obesity? The Journal of Pediatrics 98, 883–887. [DOI] [PubMed] [Google Scholar]
- Kramer M.S., Guo T., Platt R.W., Vanilovich I., Sevkovskaya Z., Dzikovich I. et al (2004) Feeding effects on growth during infancy. The Journal of Pediatrics 145, 600–605. [DOI] [PubMed] [Google Scholar]
- Kramer M.S., Matush L., Vanilovich I., Platt R.W., Bogdanovich N., Sevkovskaya Z. et al (2007) Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: evidence from a large randomized trial. The American Journal of Clinical Nutrition 86, 1717–1721. [DOI] [PubMed] [Google Scholar]
- Krishnaveni G.V., Hill J.C., Leary S.D., Veena S.R., Saperia J., Saroja A. et al (2005) Anthropometry, glucose tolerance, and insulin concentrations in Indian children: relationships to maternal glucose and insulin concentrations during pregnancy. Diabetes Care 28, 2919–2925. [DOI] [PubMed] [Google Scholar]
- Kuppuswamy (1962) Manual of Socioeconomic Status Scale. Manasayan Publication: Delhi. [Google Scholar]
- Lamb M.M., Dabelea D., Yin X., Ogden L.G., Klingensmith G.J., Rewers M. et al (2010) Early‐life predictors of higher body mass index in healthy children. Annals of Nutrition & Metabolism 56, 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J. & Hu F.B. (2002) Time trends of obesity in pre‐school children in China from 1989 to 1997. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 26, 553–558. [DOI] [PubMed] [Google Scholar]
- Malhotra R., Noheria A., Amir O., Ackerson L.K. & Subramanian S.V. (2008) Determinants of termination of breastfeeding within the first 2 years of life in India: evidence from the National Family Health Survey‐2. Maternal & Child Nutrition 4, 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer‐Davis E.J., Rifas‐Shiman S.L., Zhou L., Hu F.B., Colditz G.A. & Gillman M.W. (2006) Breast‐feeding and risk for childhood obesity. Diabetes Care 29, 2231–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeigue P.M., Shah B. & Marmot M.G. (1991) Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet 337, 382–386. [DOI] [PubMed] [Google Scholar]
- Monteiro P.O.A. & Victora C.G. (2005) Rapid growth in infancy and childhood and obesity in later life – a systematic review. Obesity Reviews: An Official Journal of the International Association for the Study of Obesity 6, 143–154. [DOI] [PubMed] [Google Scholar]
- Neutzling M.B., Hallal P.R.C., Araújo C.L.P., Horta B.L., Vieira M.F.A., Menezes A.M.B. et al (2009) Infant feeding and obesity at 11 years: prospective birth cohort study. International Journal of Pediatric Obesity: IJPO: An Official Journal of the International Association for the Study of Obesity 4, 143–149. [DOI] [PubMed] [Google Scholar]
- Nongkynrih B., Patro B.K. & Pandav C.S. (2004) Current status of communicable and non‐communicable diseases in India. The Journal of the Association of Physicians of India 52, 118–123. [PubMed] [Google Scholar]
- Ong K.K., Preece M.A., Emmett P.M., Ahmed M.L., Dunger D.B. & ALSPAC Study Team (2002) Size at birth and early childhood growth in relation to maternal smoking, parity and infant breast‐feeding: longitudinal birth cohort study and analysis. Pediatric Research 52, 863–867. [DOI] [PubMed] [Google Scholar]
- Owen C.G., Martin R.M., Whincup P.H., Davey‐Smith G., Gillman M.W. & Cook D.G. (2005a) The effect of breastfeeding on mean body mass index throughout life: a quantitative review of published and unpublished observational evidence. The American Journal of Clinical Nutrition 82, 1298–1307. [DOI] [PubMed] [Google Scholar]
- Owen C.G., Martin R.M., Whincup P.H., Smith G.D. & Cook D.G. (2005b) Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics 115, 1367–1377. [DOI] [PubMed] [Google Scholar]
- Parsons T.J., Power C., Logan S. & Summerbell C.D. (1999) Childhood predictors of adult obesity: a systematic review. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 23 (Suppl. 8), S1–107. [PubMed] [Google Scholar]
- Pasricha S.‐R. & Biggs B.‐A. (2010) Undernutrition among children in South and South‐East Asia. Journal of Paediatrics and Child Health 46, 497–503. [DOI] [PubMed] [Google Scholar]
- Pena A.S., Wiltshire E., MacKenzie K., Gent R., Piotto L., Hirte C. et al (2006) Vascular Endothelial and Smooth Muscle Function Relates to Body Mass Index and Glucose in Obese and Nonobese Children. The Journal of Clinical Endocrinology and Metabolism 91, 4467–4471. [DOI] [PubMed] [Google Scholar]
- Popkin B.M., Richards M.K. & Montiero C.A. (1996) Stunting is associated with overweight in children of four nations that are undergoing the nutrition transition. The Journal of Nutrition 126, 3009–3016. [DOI] [PubMed] [Google Scholar]
- Power C. & Parsons T. (2000) Nutritional and other influences in childhood as predictors of adult obesity. The Proceedings of the Nutrition Society 59, 267–272. [DOI] [PubMed] [Google Scholar]
- Rahman M., Roy S.K., Ali M., Mitra A.K., Alam A.N. & Akbar M.S. (1993) Maternal nutritional status as a determinant of child health. Journal of Tropical Pediatrics 39, 86–88. [DOI] [PubMed] [Google Scholar]
- Reddy K.S. (2007) India wakes up to the threat of cardiovascular diseases. Journal of the American College of Cardiology 50, 1370–1372. [DOI] [PubMed] [Google Scholar]
- Reilly J.J., Armstrong J., Dorosty A.R., Emmett P.M., Ness A., Rogers I. et al (2005) Early life risk factors for obesity in childhood: cohort study. British Medical Journal 330, 1357–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S.M. & Godfrey K.M. (2008) Feeding practices in pregnancy and infancy: relationship with the development of overweight and obesity in childhood. International Journal of Obesity (2005) 32 (Suppl. 6), S4–10. [DOI] [PubMed] [Google Scholar]
- van Rossem L., Taveras E.M., Gillman M.W., Kleinman K.P., Rifas‐Shiman S.L., Raat H. et al (2010) Is the association of breastfeeding with child obesity explained by infant weight change? International Journal of Pediatric Obesity 6, e415–e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev H.P., Osmond C., Fall C.H., Lakshmy R., Ramji S., Dey Biswas S.K. et al (2009) Predicting adult metabolic syndrome from childhood body mass index: follow‐up of the New Delhi birth cohort. Arch Dis Child 94, 768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaya A.L., Dallal G., Solymos G., de Sousa M.H., Ventura M.L., Roberts S.B. & Sigulem D.M. (1995) Obesity and malnutrition in a Shantytown population in the city of Sao Paulo, Brazil. Obesity Research 3 (Suppl. 2), 107s–115s. [DOI] [PubMed] [Google Scholar]
- Sharps P.W., El‐Mohandes A.A.E., Nabil El‐Khorazaty M., Kiely M. & Walker T. (2008) Health beliefs and parenting attitudes influence breastfeeding patterns among low‐income African‐American women. Journal of Perinatology: Official Journal of the California Perinatal Association 23, 414–419. [DOI] [PubMed] [Google Scholar]
- Shields L., O'Callaghan M., Williams G.M., Najman J.M. & Bor W. (2006) Breastfeeding and obesity at 14 years: a cohort study. Journal of Paediatrics and Child Health 42, 289–296. [DOI] [PubMed] [Google Scholar]
- Singh A.S., Mulder C., Twisk J.W.R., van Mechelen W. & Chinapaw M.J.M. (2008) Tracking of childhood overweight into adulthood: a systematic review of the literature. Obesity Reviews: An Official Journal of the International Association for the Study of Obesity 9, 474–488. [DOI] [PubMed] [Google Scholar]
- Stettler N. (2007) Nature and strength of epidemiological evidence for origins of childhood and adulthood obesity in the first year of life. International Journal of Obesity (2005) 31, 1035–1043. [DOI] [PubMed] [Google Scholar]
- Toschke A.M., von Kries R., Beyerlein A. & Rückinger S. (2008) Risk factors for childhood obesity: shift of the entire BMI distribution vs. shift of the upper tail only in a cross sectional study. BMC Public Health 8, 115–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora C.G., Huttly S.R., Fuchs S.C. & Olinto M.T. (1997) The role of conceptual frameworks in epidemiological analysis: a hierarchical approach. International Journal of Epidemiology 26, 224–227. [DOI] [PubMed] [Google Scholar]
- Wang Y. & Lobstein T. (2006) Worldwide trends in childhood overweight and obesity. International Journal of Pediatric Obesity: IJPO: An Official Journal of the International Association for the Study of Obesity 1, 11–25. [DOI] [PubMed] [Google Scholar]
- Wells J.C.K., Hallal P.C., Wright A., Singhal A. & Victora C.G. (2005) Fetal, infant and childhood growth: relationships with body composition in Brazilian boys aged 9 years. International Journal of Obesity (2005) 29, 1192–1198. [DOI] [PubMed] [Google Scholar]
- Weyermann M., Rothenbacher D. & Brenner H. (2006) Duration of breastfeeding and risk of overweight in childhood: a prospective birth cohort study from Germany. International Journal of Obesity (2005) 30, 1281–1287. [DOI] [PubMed] [Google Scholar]
- WHO (2008) Indicators for assessing infant and young child feeding practices : conclusions of a consensus meeting held 6–8 November 2007 in Washington D.C., USA [Online]. Available at: http://extranet.who.int/iris/handle/123456789/604
- Wild S., Roglic G., Green A., Sicree R. & King H. (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27, 1047–1053. [DOI] [PubMed] [Google Scholar]
- Wilson A.C., Forsyth J.S., Greene S.A., Irvine L., Hau C. & Howie P.W. (1998) Relation of infant diet to childhood health: seven year follow up of cohort of children in Dundee infant feeding study. BMJ (Clinical Research Ed.) 316, 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K.S., Chook P., Yu C.W., Sung R.Y.T., Qiao M., Leung S.S.F. et al (2004) Overweight in children is associated with arterial endothelial dysfunction and intima‐media thickening. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 28, 852–857. [DOI] [PubMed] [Google Scholar]
- Zive M.M., McKay H., Frank‐Spohrer G.C., Broyles S.L., Nelson J.A. & Nader P.R. (1992) Infant‐feeding practices and adiposity in 4‐y‐old Anglo‐ and Mexican‐Americans. The American Journal of Clinical Nutrition 55, 1104–1108. [DOI] [PubMed] [Google Scholar]


