Abstract
Homeostatic synaptic plasticity remains an enigmatic form of synaptic plasticity. Increasing interest on the topic has fuelled a surge of recent studies that have identified key molecular players and the signaling pathways involved. However, the new findings also highlight our lack of knowledge concerning some of the basic properties of homeostatic synaptic plasticity. In this review we address how homeostatic mechanisms balance synaptic strengths between the presynaptic and the postsynaptic terminals and across synapses that share the same postsynaptic neuron.
Introduction
The nervous system constantly undergoes structural and functional changes to acquire and store information while adapting to changes in the environment. The human brain contains an estimated 100 billion neurons making together roughly 100 trillion synapses. Understanding how single synapses at specific locations develop and work in concert with other synapses to fine-tune neural circuitry across the brain is an enduring challenge for modern biology. The behavior and the organization of neural circuits can be modified by adjusting the number and/or the strength of synapses in a process collectively referred to as synaptic plasticity. Although synaptic plasticity alters neural activity on relatively short time scales, the mean neuronal firing rates remain highly stable over long time periods. How do neurons and circuits maintain stability? This might be a confounding task given that most aspects of neuronal physiology are subject to activity-dependent modifications. In a growing idea, expression of two opposing forms of synaptic plasticity is thought to allow dynamic changes in neural networks while maintaining their stability [1]. On the one hand, Hebbian plasticity, such as long-term potentiation (LTP) that is generated rapidly, is durable and is strengthened with repetition, can be seen as a source of instability. On the other hand, homeostatic synaptic plasticity that operates over hours to days, is suggested to promote network stability by adjusting global synaptic strength when neural activity deviates from permissive levels of activity [1]. In other words, homeostatic plasticity might restrict the neural network from reaching excessive excitation due to ongoing LTP.
In this review, we address the question of cross-talk between neurons for ensuring both adaptability and integrity of the synaptic networks they form. In light of recent studies on the topic of homeostatic synaptic plasticity, we will focus on the role for homeostatic mechanisms in modulating synaptic strengths across a synaptic network, including the co-ordination between the presynaptic and the postsynaptic neurons at single synapses as well as among distant synapses in a same network.
Matching of the presynaptic and the postsynaptic strengths
Both presynaptic and postsynaptic changes are involved in homeostatic compensation [2-5]. Almost all studies in which network activity is altered for a few hours or days have detected compensatory changes in excitatory quantal amplitude in vitro [6-9] and in vivo [10-12]. The occurrence of compensatory changes in presynaptic function is more controversial, although, in general, manipulations that block synaptic activity induce an increase in release probability (pr) at central synapses and at the Drosophila neuromuscular junction (NMJ) [6,8,13,14]. This change in presynaptic strength often accompanies an increase in postsynaptic receptor accumulation [6,8], suggesting a functional co-ordination between the presynaptic and the postsynaptic compartments in homeostatic adaptation of synaptic strength.
The relationship between the presynaptic and the postsynaptic strengths was directly addressed in hippocampal cultures using FM1-43 and surface GluA2 labeling to optically estimate pr and the postsynaptic receptor abundance, respectively [15•]. No correlation was detected under basal conditions, and only manipulations that enhanced synaptic activity but not the ones that blocked activity, revealed a positive correlation between pr and surface GluA2 abundance. This led to the idea that synaptic activity drove a functional correlation across the two sides of the synapse. Interestingly, the correlation likely resulted from adjusting (reducing) GluA2 levels to match pr rather than the opposite as only the levels of GluA2 showed a significant change. Therefore, the postsynaptic strength may be more responsive in such activity-dependent co-ordination, and in turn, this raises questions of how the postsynaptic cell adjusts its synaptic receptor abundance to match presynaptic function. Another recent study in hippocampal cultures reported of a correlation between recycling vesicle pool size labeled by FM dye and postsynaptic responses elicited by local glutamate uncaging under basal conditions in mature but not in young cultures, suggesting that co-ordinated synaptic strengths emerge in an activity-dependent manner during synapse maturation [16]. Again, no functional correlation was detected after activity blockade [15•,16]. Collectively, these studies highlight the importance of synaptic activity in shaping and driving a co-ordination between presynaptic and postsynaptic strengths. Notably, in acute rat neocortical slices a strong correlation between pr and quantal amplitude at excitatory synapses has also been observed, but whether this correlation is intrinsically fixed or driven by synaptic activity has not been addressed [17].
How do the presynaptic and the postsynaptic compartments co-ordinate their changes in strengths to activity alterations? The sufficiency of chronic postsynaptic activity blockade in triggering presynaptic changes suggests that a trans-synaptic signaling is important for presynaptic homeostasis [6,18]. But is the presynaptic terminal limited to playing a passive role, waiting for instructions from its postsynaptic partner? Or, does it play a more active role in this process by directing its postsynaptic partner to allow the correlation? We now turn to a discussion on the mechanisms of presynaptic adaptation.
Presynaptic adaptation
Chronic activity blockade induces an enhancement of presynaptic function as suggested by the enlargement of presynaptic terminals, an increase in the frequency of excitatory quantal responses, an enhancement of synaptic vesicle turnover, and an increase in pr, which are paralleled by an increase in quantal size [8,13,19,20]. Although homeostatic changes in quantal size are generally ascribed to the postsynaptic component, an additional presynaptic component attributed to activity-dependent modulation of vesicular glutamate transporter expression has been described [20]. In particular, presynaptic mechanisms that underlie homeostatic changes in pr are poorly understood. Given the strong dependence of neurotransmitter release on Ca2+, Ca2+-dependent signaling pathways are likely to be involved. This is indeed supported by studies at the Drosophila NMJ showing that changes in Ca2+ entry through P/Q type Ca2+ channels account at least partially for activity-dependent compensatory changes of the presynaptic strength [21-23]. Similarly, at vertebrate central synapses, presynaptic homeostasis has been suggested to occur by changing the amount of Ca2+ entry into the terminal in response to a spike [24]. How is the Ca2+ influx modified by synaptic activity? Recent studies show that the Ca2+ sensor synaptotagmin, the synaptic vesicle protein SV2B implicated in facilitating pr [25], and the pore forming subunit Cav2.1 of P/Q type Ca2+ channels become enriched at presynaptic terminals upon activity blockade [26]. Taken together, these findings raise the possibility that pr is homeostatically regulated by controlling the number of voltage sensitive Ca2+ channels mediating the Ca2+ influx into the presynaptic terminal and by tuning the Ca2+ responsiveness of the neurotransmitter release machinery.
A second crucial issue is how the synaptic vesicle cycle is homeostatically regulated to support the adjustment of release. RIM and its interacting proteins Rab are possible molecular candidates involved in the process. They are essential for basal and activity-dependent neurotransmitter release, and their levels show a steep correlation with synaptic activity [25,27]. Furthermore, a study suggests that Rab3-GAP, by binding to a yet unidentified homeostatic repressor, regulates the progression of the synaptic vesicle cycle to provide additional control on the activity-dependent regulation of release [28•].
Postsynaptic scaling and trans-synaptic co-ordination
The first and the most commonly studied form of homeostatic plasticity is the global synaptic scaling that involves a cell-wide increase or decrease of postsynaptic AMPARs [2,4]. There are many well-documented reviews on different aspects of this phenomenon [2-5]. Here we discuss the retrograde signaling initiated during postsynaptic scaling and its importance in modifying presynaptic function. That adaptation to inactivity involves a presynaptic increase in vesicle turnover in a manner dependent on postsynaptic changes, has been convincingly demonstrated recently [29,30•,31]. How might postsynaptic activity blockade produce changes in presynaptic function? This presynaptic enhancement seems to be mediated by a βCAMKII-dependent-upregulation of surface GluA1 in postsynaptic neurons and by at least two retrograde messengers that work in series along the same signaling pathway: BDNF and NO [29,30•,31] (Figure 1c). Interestingly, BDNF application by itself has no effect on surface GluA1 levels, indicating that BDNF acts downstream of GluA1. Furthermore, BDNF has been previously suggested to regulate both the organization of N type and P/Q type calcium channels and neurotransmitter release under basal conditions [32]. Taken together, BDNF could be a key player in initiating the presynaptic changes in concert with changes at the postsynaptic side, and it would be of interest to study whether BDNF and NO could modify Ca2+ influx to mediate the enhancement of presynaptic function during homeostatic synaptic adaptation.
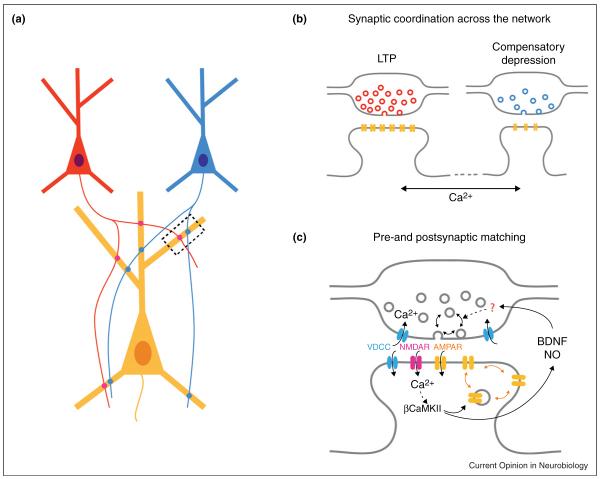
Figure 1.
Co-ordination of synaptic strengths across a network. (a) Representative scheme of three connected neurons. A postsynaptic neuron (yellow) receives inputs from two presynaptic neurons (red and blue) across its dendritic arbor. How heterosynaptic interactions between the red and the blue inputs occur at the level of individual synapses is not known. The scheme in (b) illustrates, at the synapse resolution, a possible interaction between a synapse belonging to the red input undergoing LTP and another (near or distant) synapse from the blue input showing compensatory depression. One possible mechanism involved in the heterosynaptic interaction is the spreading of Ca2+-dependent signaling molecules. (c) Retrograde signaling involved in matching of the presynaptic and the postsynaptic strengths under chronic synaptic activity blockade. This mechanism involves the postsynaptic accumulation of β-CaMKII and surface GluA1-containing AMPARs, followed by the postsynaptic release of BDNF and NO, and the subsequent increase in synaptic vesicle recycling.
Global versus local: how specific is homeostatic plasticity to individual synapses?
The spatial extent over which neurons perceive activity changes and implement the homeostatic response remains unclear. Are the activity changes sensed at each individual synapse, the single neuron or the entire network? How do synapses co-ordinate their response to normalize global network activity? These questions are difficult to address as the degree of compartmentation of homeostatic plasticity is likely to depend on a variety of parameters including the connectivity, the state of synaptic maturation and, perhaps most importantly, the induction stimulus.
A popular paradigm used to elicit homeostatic plasticity is the global and chronic pharmacological treatment of cultured neurons to either enhance or block neuronal activity [4]. Do all synapses respond uniformly to such global activity manipulations? TTX and bicuculline treatments, for instance, have been shown to induce multiplicative postsynaptic scaling up or down that is suggestive of a uniform effect on all synapses [4]. This in turn implicates the operation of a cell-wide mechanism to scale all postsynaptic sites in response to a global activity challenge. Recent studies support the existence of such presumed cell-wide mechanisms involving signaling cascades triggered by Ca2+ influx, and requiring molecules such as Arc, Homer1a, retinoic acid, and Polo-like kinase2, to produce the uniform scaling of synapses on a same neuron [33-38]. Furthermore, a growing idea stipulates that a single neuron can ‘sense’ activity changes and engage a cell-autonomous homeostatic response [6,36,39•]. Nevertheless, should activity changes be heterogenous across different subcellular regions, it is not clear how a single neuron would integrate local differences to elicit a global response. Notably, astrocytes also appear to be important players in this global homeostatic response and might control the spatial extent of the scaling by releasing TNFα in the extracellular space [7]. A better understanding of the three dimensional spatial configuration of astrocyte–neuron interaction and the mode of release of TNFα should help to clarify how TNFα signaling is embedded in the mechanism of global scaling. The uniform adjustment of synaptic strengths is potentially important as it allows to conserve the relative differences between synaptic weights, which are likely to be crucial for the storage and processing of specific information. How this global multiplicative scaling is translated at single synapses with differing initial strengths and the mechanism by which each synapses insert the proportionate number of postsynaptic receptors to scale their strengths by some uniform factor remain unknown.
This prevailing view of global multiplicative scaling may be challenged by the observed scaling of presynaptic strengths in some experimental conditions, where it remains unclear whether this presynaptic adaptation is achieved uniformly in co-ordination with postsynaptic scaling. For instance, a non-uniform scaling of both presynaptic and postsynaptic functions in response to prolonged AMPARs blockade or chronic TTX treatment has been shown in hippocampal neurons in dissociated and organotypic cultures and also in vivo [8,11,40]. These studies suggest that the pattern of synaptic connectivity and activation may be a divisive factor in enabling distant synapses on a same postsynaptic neuron to undergo co-ordinated changes. The importance of synaptic organization is further highlighted by work in organotypic hippocampal and cortical cultures showing that different types of synaptic inputs within a same circuit adapt differently to global activity deprivation [41,42].
Studies in which activity is locally manipulated have provided compelling support to the spatial compartmentation of homeostatic plasticity [43,44]. These studies show that dendrites, independently from the cell body, can homeostatically respond to prolonged activity blockade through the local synaptic incorporation of newly synthesized GluA1-containing AMPARs. Such a confined homeostatic response may have a presynaptic component as presynaptic boutons also adapt by adjusting their pr to a local and prolonged dendritic stimulations [18]. Recent work using single synapse manipulation has provided a direct evidence of a synapse-autonomous homeostatic response. Silencing single inputs by overexpressing the inwardly rectifying potassium channel Kir2.1 in cultured hippocampal neurons induces an accumulation of GluA2-lacking AMPARs at corresponding postsynaptic sites but not at neighboring synapses [45,46•]. Intriguingly, this scaling relies on Arc-dependent signaling [46•], suggesting that global and local homeostatic regulations share similar mechanisms. Collectively, these studies highlight the ability of single synapses/inputs to sense and integrate local activity in an autonomous manner. As a consequence, these findings raise the question of the balance between autonomy and co-operation amongst synapses: how do autonomous synapses co-ordinate their synaptic strength changes with each other to maintain the stability of global network activity? We consider this issue below.
Interplay between homeostatic and Hebbian plasticity: role of heterosynaptic interactions
Computational modeling has shown that long-term changes in synaptic weights are difficult to achieve without a ‘normalizing’ mechanism to regulate total synaptic strength or excitability [47-49]. In this regard, compensatory heterosynaptic changes might provide a useful mechanism for synaptic homeostasis and for optimizing the lifetime of memory traces during ongoing learning. Electrophysiological studies in well-characterized systems, including the amygdala and the hippocampus, have provided direct evidence for such heterosynaptic interactions between identified inputs: long-term plasticity elicited at one set of inputs (homosynapses) can be balanced by opposite, presumably homeostatic, changes at other synaptic inputs (heterosynapses) on the same postsynaptic neuron [50,51] (Figure 1a and b). Although electrophysiology allows manipulating and monitoring specific functional connections, postsynaptic response of each connection represents the behavior of a synapse population and it gives no information about the spatial distribution of individual synaptic weights contributing to the connection. The inability to observe the behavior of individual synapses is however overcome by recent advances in imaging techniques with high spatial resolution, which has provided important clues concerning the extent of spatial confinement of plasticity elicited at single synapses. For instance, a recent report using two photon imaging and glutamate uncaging to trigger LTP at single spines highlights the importance of Ca2+-dependent local interactions involving Ras signaling in promoting the spread of plasticity over very short distances along a dendrite [52]. Despite an incomplete spatial description of changes affecting the global distribution of synaptic weights, this study suggests that fine-tuned interactions can occur postsynaptically at least between nearby synapses.
Finally, activity-dependent heterosynaptic interactions are believed to be particularly important during critical periods in the developing nervous system, when synaptic afferents undergo experience-dependent competition to refine circuits. This competition serves to stabilize some connections and eliminate others, and in several central and peripheral systems the underlying mechanism has been shown to rely on Hebbian forms of synaptic plasticity [53]. Recent work suggests that synaptic competition also involves homeostatic plasticity. Manipulating visual experience during developmental critical periods by dark rearing or monocular deprivation (MD) induces a compensatory scaling of synaptic responses in the visual cortex and thalamus deprived of input activity [12,54-56]. Interestingly, two recent studies indicate that occular dominance plasticity in the visual cortex where the competition between synaptic inputs from the two eyes drives the refinement, may result from the interplay between Hebbian and homeostatic forms of plasticity [56,57]. In particular, the occular dominance shift following MD is likely to emerge from first, the fast and lasting depression of deprived-eye responses and second, the delayed TNFα-dependent, and presumably homeostatic, potentiation of open-eye responses [57]. Collectively these studies establish the in vivo functional relevance of homeostatic plasticity in the developing visual system.
Concluding remarks
The last several years have seen a surge of interest in homeostatic forms of synaptic plasticity. Nevertheless, our understanding not only of the underlying molecular mechanisms but also of the basic properties of homeostatic synaptic plasticity remains rudimentary. For instance, we do not know how many different forms of homeostatic adaptive mechanisms operate in parallel at individual synapses. Here we have attempted to highlight the co-ordination of adaptation of synaptic strengths across the two sides of a synapse and amongst synapses shared by a neuron. Given that the original appeal of homeostatic synaptic plasticity has been its counter relationship to Hebbian processes, further characterization of homeostatic alterations in association with Hebbian plasticity will be important.
Acknowledgements
Research in the authors laboratory is supported by the Medical Research Council, the European Union 7th Framework Programme under grant agreement no. HEALTH-F2-2009-241498 (‘EUROSPIN’ project), and the RIKEN Brain Science Institute.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol. 2000;10:358–364. doi: 10.1016/s0959-4388(00)00091-x. [DOI] [PubMed] [Google Scholar]
- 2.Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66:337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu Rev Neurosci. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- 4.Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu LM, Goda Y. Dendritic signalling and homeostatic adaptation. Curr Opin Neurobiol. 2009;19:327–335. doi: 10.1016/j.conb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Burrone J, O’Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- 7.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 8.Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 9.Wierenga CJ, Ibata K, Turrigiano GG. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J Neurosci. 2005;25:2895–2905. doi: 10.1523/JNEUROSCI.5217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Islas C, Wenner P. Spontaneous network activity in the embryonic spinal cord regulates AMPAergic and GABAergic synaptic strength. Neuron. 2006;49:563–575. doi: 10.1016/j.neuron.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Echegoyen J, Neu A, Graber KD, Soltesz I. Homeostatic plasticity studied using in vivo hippocampal activity-blockade: synaptic scaling, intrinsic plasticity and age-dependence. PLoS ONE. 2007;2:e700. doi: 10.1371/journal.pone.0000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- 13.Murthy VN, Schikorski T, Stevens CF, Zhu Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- 14.Wierenga CJ, Walsh MF, Turrigiano GG. Temporal regulation of the expression locus of homeostatic plasticity. J Neurophysiol. 2006;96:2127–2133. doi: 10.1152/jn.00107.2006. [DOI] [PubMed] [Google Scholar]
- 15.Tokuoka H, Goda Y. Activity-dependent coordination of presynaptic release probability and postsynaptic GluR2 abundance at single synapses. Proc Natl Acad Sci U S A. 2008;105:14656–14661. doi: 10.1073/pnas.0805705105. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study in dissociated hippocampal cultures shows for the first time that the correlation between presynaptic and postsynaptic strengths can be driven by neuronal activity.
- 16.Kay L, Humphreys L, Eickholt BJ, Burrone J. Neuronal activity drives matching of pre- and postsynaptic function during synapse maturation. Nat Neurosci. 2011;14:688–690. doi: 10.1038/nn.2826. [DOI] [PubMed] [Google Scholar]
- 17.Hardingham NR, Read JC, Trevelyan AJ, Nelson JC, Jack JJ, Bannister NJ. Quantal analysis reveals a functional correlation between presynaptic and postsynaptic efficacy in excitatory connections from rat neocortex. J Neurosci. 2010;30:1441–1451. doi: 10.1523/JNEUROSCI.3244-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Branco T, Staras K, Darcy KJ, Goda Y. Local dendritic activity sets release probability at hippocampal synapses. Neuron. 2008;59:475–485. doi: 10.1016/j.neuron.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bacci A, Coco S, Pravettoni E, Schenk U, Armano S, Frassoni C, Verderio C, De Camilli P, Matteoli M. Chronic blockade of glutamate receptors enhances presynaptic release and downregulates the interaction between synaptophysin–synaptobrevin-vesicle-associated membrane protein 2. J Neurosci. 2001;21:6588–6596. doi: 10.1523/JNEUROSCI.21-17-06588.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Gois S, Schafer MK, Defamie N, Chen C, Ricci A, Weihe E, Varoqui H, Erickson JD. Homeostatic scaling of vesicular glutamate and GABA transporter expression in rat neocortical circuits. J Neurosci. 2005;25:7121–7133. doi: 10.1523/JNEUROSCI.5221-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank CA, Kennedy MJ, Goold CP, Marek KW, Davis GW. Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron. 2006;52:663–677. doi: 10.1016/j.neuron.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frank CA, Pielage J, Davis GW. A presynaptic homeostatic signaling system composed of the Eph receptor, ephexin, Cdc42, and CaV2.1 calcium channels. Neuron. 2009;61:556–569. doi: 10.1016/j.neuron.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Engisch KL, Li Y, Pinter MJ, Cope TC, Rich MM. Decreased synaptic activity shifts the calcium dependence of release at the mammalian neuromuscular junction in vivo. J Neurosci. 2004;24:10687–10692. doi: 10.1523/JNEUROSCI.2755-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao C, Dreosti E, Lagnado L. Homeostatic synaptic plasticity through changes in presynaptic calcium influx. J Neurosci. 2011;31:7492–7496. doi: 10.1523/JNEUROSCI.6636-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Custer KL, Austin NS, Sullivan JM, Bajjalieh SM. Synaptic vesicle protein 2 enhances release probability at quiescent synapses. J Neurosci. 2006;26:1303–1313. doi: 10.1523/JNEUROSCI.2699-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazarevic V, Schone C, Heine M, Gundelfinger ED, Fejtova A. Extensive remodeling of the presynaptic cytomatrix upon homeostatic adaptation to network activity silencing. J Neurosci. 2011;31:10189–10200. doi: 10.1523/JNEUROSCI.2088-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, Schmitz F, Malenka RC, Sudhof TC. RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002;415:321–326. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- 28.Muller M, Pym EC, Tong A, Davis GW. Rab3-GAP controls the progression of synaptic homeostasis at a late stage of vesicle release. Neuron. 2011;69:749–762. doi: 10.1016/j.neuron.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]; • In this study, an electrophysiology-based genetic screen has led to defining a role for Rab3-GAP and Rab3 in homeostatic synaptic plasticity at the Drosophila NMJ. A mechanism that acts to oppose the progression of synaptic homeostasis at a late stage of synaptic vesicle cycle is described.
- 29.Jakawich SK, Nasser HB, Strong MJ, McCartney AJ, Perez AS, Rakesh N, Carruthers CJ, Sutton MA. Local presynaptic activity gates homeostatic changes in presynaptic function driven by dendritic BDNF synthesis. Neuron. 2010;68:1143–1158. doi: 10.1016/j.neuron.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindskog M, Li L, Groth RD, Poburko D, Thiagarajan TC, Han X, Tsien RW. Postsynaptic GluA1 enables acute retrograde enhancement of presynaptic function to coordinate adaptation to synaptic inactivity. Proc Natl Acad Sci U S A. 2010;107:806–811. doi: 10.1073/pnas.1016399107. [DOI] [PMC free article] [PubMed] [Google Scholar]; • By combining electrophysiology and imaging approaches in hippocampal dissociated cultures, the authors show that adaptation to inactivity involves a GluA1-dependent enhancement of presynaptic function in both spontaneous and evoked release. This retrograde mechanism could require a postsynaptic rise in intracellular Ca2+ levels and retrograde messengers such as BDNF and NO.
- 31.Groth RD, Lindskog M, Thiagarajan TC, Li L, Tsien RW. Beta Ca2+/CaM-dependent kinase type II triggers upregulation of GluA1 to coordinate adaptation to synaptic inactivity in hippocampal neurons. Proc Natl Acad Sci U S A. 2011;108:828–833. doi: 10.1073/pnas.1018022108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tyler WJ, Perrett SP, Pozzo-Miller LD. The role of neurotrophins in neurotransmitter release. Neuroscientist. 2002;8:524–531. doi: 10.1177/1073858402238511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aoto J, Nam CI, Poon MM, Ting P, Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008;60:308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evers DM, Matta JA, Hoe HS, Zarkowsky D, Lee SH, Isaac JT, Pak DT. Plk2 attachment to NSF induces homeostatic removal of GluA2 during chronic overexcitation. Nat Neurosci. 2010;13:1199–1207. doi: 10.1038/nn.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu JH, Park JM, Park S, Xiao B, Dehoff MH, Kim S, Hayashi T, Schwarz MK, Huganir RL, Seeburg PH, et al. Homeostatic scaling requires group I mGluR activation mediated by Homer1a. Neuron. 2010;68:1128–1142. doi: 10.1016/j.neuron.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron. 2008;57:819–826. doi: 10.1016/j.neuron.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 37.Seeburg DP, Feliu-Mojer M, Gaiottino J, Pak DT, Sheng M. Critical role of CDK5 and Polo-like kinase 2 in homeostatic synaptic plasticity during elevated activity. Neuron. 2008;58:571–583. doi: 10.1016/j.neuron.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goold CP, Nicoll RA. Single-cell optogenetic excitation drives homeostatic synaptic depression. Neuron. 2010;68:512–528. doi: 10.1016/j.neuron.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Using optogenetic tools and molecular and pharmacological manipulations this study shows that hippocampal neurons can homeostatically adjust synaptic strength in response to cell-autonomous increases in activity. Neuromodulation turns out to be mediated by a compensatory synaptic depression of AMPARs and NMDARs which is sensitive to the postsynaptic calcium influx and requires the activation of CaMKK and its downstream target CaMK4.
- 40.Cingolani LA, Goda Y. Differential involvement of beta3 integrin in pre- and postsynaptic forms of adaptation to chronic activity deprivation. Neuron Glia Biol. 2008;4:179–187. doi: 10.1017/S1740925X0999024X. [DOI] [PubMed] [Google Scholar]
- 41.Bartley AF, Huang ZJ, Huber KM, Gibson JR. Differential activity-dependent, homeostatic plasticity of two neocortical inhibitory circuits. J Neurophysiol. 2008;100:1983–1994. doi: 10.1152/jn.90635.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J, Tsien RW. Synapse-specific adaptations to inactivity in hippocampal circuits achieve homeostatic gain control while dampening network reverberation. Neuron. 2008;58:925–937. doi: 10.1016/j.neuron.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci. 2004;7:244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- 44.Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 45.Hou Q, Zhang D, Jarzylo L, Huganir RL, Man HY. Homeostatic regulation of AMPA receptor expression at single hippocampal synapses. Proc Natl Acad Sci U S A. 2008;105:775–780. doi: 10.1073/pnas.0706447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beique JC, Na Y, Kuhl D, Worley PF, Huganir RL. Arc-dependent synapse-specific homeostatic plasticity. Proc Natl Acad Sci U S A. 2011;108:816–821. doi: 10.1073/pnas.1017914108. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study reveals that individual synapses can autonomously increase AMPAR function after a prolonged reduction of glutamate release. This adjustment of the postsynaptic strength does not occur on neighbouring spines and it is mediated by the immediate early gene Arc/Arg3.1.
- 47.Chistiakova M, Volgushev M. Heterosynaptic plasticity in the neocortex. Exp Brain Res. 2009;199:377–390. doi: 10.1007/s00221-009-1859-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rabinowitch I, Segev I. Two opposing plasticity mechanisms pulling a single synapse. Trends Neurosci. 2008;31:377–383. doi: 10.1016/j.tins.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Thiagarajan TC, Lindskog M, Malgaroli A, Tsien RW. LTP and adaptation to inactivity: overlapping mechanisms and implications for metaplasticity. Neuropharmacology. 2007;52:156–175. doi: 10.1016/j.neuropharm.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 50.Royer S, Pare D. Conservation of total synaptic weight through balanced synaptic depression and potentiation. Nature. 2003;422:518–522. doi: 10.1038/nature01530. [DOI] [PubMed] [Google Scholar]
- 51.Yasuda H, Huang Y, Tsumoto T. Regulation of excitability and plasticity by endocannabinoids and PKA in developing hippocampus. Proc Natl Acad Sci U S A. 2008;105:3106–3111. doi: 10.1073/pnas.0708349105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harvey CD, Yasuda R, Zhong H, Svoboda K. The spread of Ras activity triggered by activation of a single dendritic spine. Science. 2008;321:136–140. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lichtman JW, Colman H. Synapse elimination and indelible memory. Neuron. 2000;25:269–278. doi: 10.1016/s0896-6273(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 54.Gao M, Sossa K, Song L, Errington L, Cummings L, Hwang H, Kuhl D, Worley P, Lee HK. A specific requirement of Arc/Arg3.1 for visual experience-induced homeostatic synaptic plasticity in mouse primary visual cortex. J Neurosci. 2010;30:7168–7178. doi: 10.1523/JNEUROSCI.1067-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krahe TE, Guido W. Homeostatic plasticity in the visual thalamus by monocular deprivation. J Neurosci. 2011;31:6842–6849. doi: 10.1523/JNEUROSCI.1173-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mrsic-Flogel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, Hubener M. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54:961–972. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 57.Kaneko M, Stellwagen D, Malenka RC, Stryker MP. Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron. 2008;58:673–680. doi: 10.1016/j.neuron.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]