Abstract
Oncolytic viruses (OV) are promising treatments for cancer, with several currently undergoing testing in randomised clinical trials. Measles virus (MV) has not yet been tested in models of human melanoma. This study demonstrates the efficacy of MV against human melanoma. It is increasingly recognised that an essential component of therapy with OV is the recruitment of host anti-tumour immune responses, both innate and adaptive. MV-mediated melanoma cell death is an inflammatory process, causing the release of inflammatory cytokines including type-1 interferons and the potent danger signal HMGB1. Here, using human in vitro models, we demonstrate that MV enhances innate antitumour activity, and that MV-mediated melanoma cell death is capable of stimulating a melanoma-specific adaptive immune response.
Keywords: Oncolytic, measles, melanoma, immunotherapy, HMGB1, interferon
Introduction
Measles virus is amongst the first viruses to have been recognised to cause spontaneous cancer remissions, and more recently has been studied both preclinically and clinically for its potential as a therapeutic oncolytic virus (OV)1-14. MV has not yet been studied in models of human melanoma, a disease with few therapeutic options, but one known to respond to other OV and immunomodulatory approaches 15-17.
Both the wild-type strain of measles and the Edmonston vaccine strain (MV) have tropism for the cell surface receptor CD150, commonly expressed on lymphocytes. The vaccine strain has additional tropism for CD46, a membrane cofactor protein 18,19. CD46 is ubiquitously expressed on all nucleated human cells and serves to downregulate the action of the complement pathway upon autologous cells. CD46 is known to be upregulated on several tumour types and is thought to be the predominant mechanism for the oncolytic preference of MV 20.
Around six OV have made progress into early phase clinical trials 21. MV, in addition to its long history of administration as a vaccine, has been safely delivered in patients with a variety of tumours by intravenous, intratumoural and intraperitoneal routes with no significant adverse events reported in a number of early phase clinical trials 13,14,22.
Though there is considerable interest in OV as agents directly toxic to cancer, we and others have also explored the potential for OV to trigger antitumour immune responses 18,23. Indeed in some models immunity, rather than obstructing OV efficacy, is required for therapy 24. We have demonstrated in preclinical melanoma models that immune responses against tumour targets are triggered by inflammatory responses to OV and are a vital component of successful treatment, capable of overcoming immunosuppressive tumour environments and clearing metastatic disease 25. Reovirus, a promising OV currently being explored in phase III clinical testing, has been shown to activate dendritic cells (DC) which in turn stimulated innate anti-tumour activity in both natural killer cells (NK) and T-cells 26, as well as adaptive T-cell responses 27. Here we investigate both the direct oncolytic activity of MV, and also the potential for MV to stimulate innate and adaptive antitumour immune responses in melanoma.
Results
MV has oncolytic activity against, and replicates in, human melanoma cell lines
CD46 is upregulated on tumour cells, and is one of the receptors for the Edmonston strain of MV. To test the potential of MV as an OV against melanoma, we therefore first confirmed expression of CD46 on four human melanoma cell lines, Mel888, Mel624, MeWo and SkMel28 (Figure 1a).
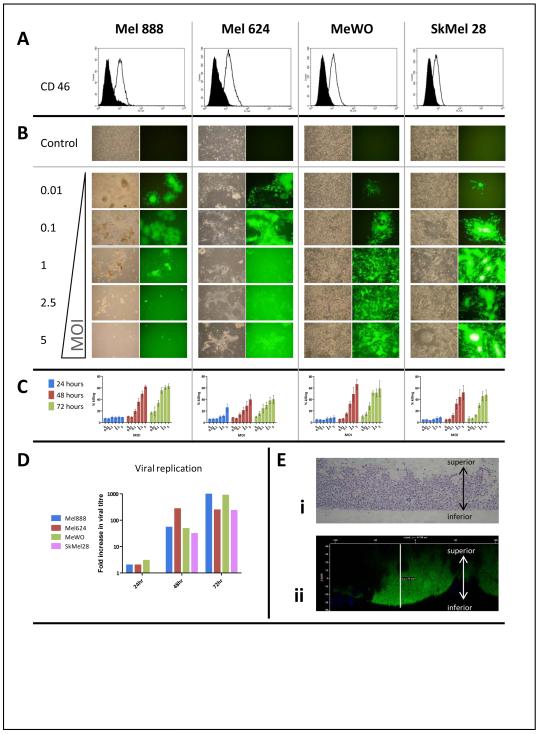
Figure 1.
Oncolytic activity of MV against human melanoma cell lines. A; Levels of surface expression of CD46, the MV receptor, were determined by flow cytometry in four human melanoma cell lines (Mel888, Mel624, MeWo, SkMel28). Filled histograms are isotype controls. Data shown are representative of three separate experiments. B; Melanoma cells were infected with GFP-expressing MV at a range of MOI. Photographs were taken 48hr after infection by phase contrast (left) and fluorescence (right) microscopy. C; Cytotoxicity was further measured in each cell line using the Live/Dead assay. Data shown are mean results from five separate experiments; bars demonstrate SE. D; Viral replication. Melanoma Cells were infected at an MOI of 0.1 and after 48 hours virus quantification was determined using the TCID50 method. Data shown are representative of 3 separate experiments. E; Mel624 were grown in a three dimensional model using transwell inserts for 5 days, then infected from the inferior (as indicated) surface with MV-GFP. i) Transverse sections of the multilayer model stained with H&E. ii) Confocal Z-stack image showing a lateral projection of a GFP-expressing syncytium.
The characteristic cytopathic effect (CPE) of MV is the formation of giant multinucleated cells, or syncytia, created as infected cells express fusogenic MV proteins on their surface 20. In all four melanoma cell lines tested, this distinctive CPE was evident even at low multiplicities of infection (MOI), from 48 hours after infection with a MV engineered to express GFP (MV-GFP - Figure 1b). MV-mediated killing of melanoma cell lines was quantified using a flow cytometry-based assessment of membrane integrity and cell viability (the Live/Dead assay), which demonstrated dose and time-dependent killing of melanoma targets by MV (Figure 1c). This oncolytic activity of MV was further confirmed using an MTT assay (data not shown).
Next, cell lysates were prepared from each cell line 24-72 hours after infection with MV at an MOI of 0.1, and titrated for evidence of viral replication on VERO cells using the Reed-Muench method 28. As shown in figure 1d, viral replication was supported in all cell lines.
Cell morphology and behaviour differs between 2- and 3-dimensional culture models, with the latter better mimicking the complex cell-cell interactions occurring in vivo. In particular, viral penetration into tumours is likely to be a significant obstacle for therapy, not represented in conventional monolayer models 29. To address this concern, we used a multilayer model of Mel624 grown on transwell inserts, in which transverse sections taken through the multilayer demonstrate the thickness achieved (Figure 1e.i.) 30. Confocal microscopy of the multilayers 48 hours after infection with MV-GFP demonstrated susceptibility to infection, and significant penetration of large syncytia through the full thickness of the multilayer; Figure 1e.ii. shows a virtual slice through a GFP-expressing syncytium, looking from the side. DAPI-stained nuclei from uninfected cells are present around the syncytium, which is seen to extend all the way from the transwell membrane to the upper extent of the cellular multilayer. Hence cell-to-cell fusion, mediated by MV, is not a phenomenon restricted to two dimensional culture models, and in this 3D model leads to formation of large convoluted syncytia.
MV stimulates the release of an inflammatory profile of cytokines, chemokines and danger signals by infected melanoma cells
To investigate the inflammatory response to measles virus, cell-free supernatants collected from cells infected with varying MOI of MV were analysed by ELISA for the presence of cytokines and chemokines (Figure 2a)31. Although cytokine release varied between cell lines, certain patterns emerged. There was a dose dependent increase in the levels of the inflammatory cytokines IL6 (in 3 of 4 cell lines) and IL8 (in all cell lines) released by cells upon infection with MV. Moreover, type I interferons (IFN) α and/or β, and type III IFN λ were secreted to a variable degree by melanoma lines undergoing MV-induced killing. IFN have anti-proliferative effects on melanoma cells 32, as well as recruiting innate and adaptive immune responses 33,34
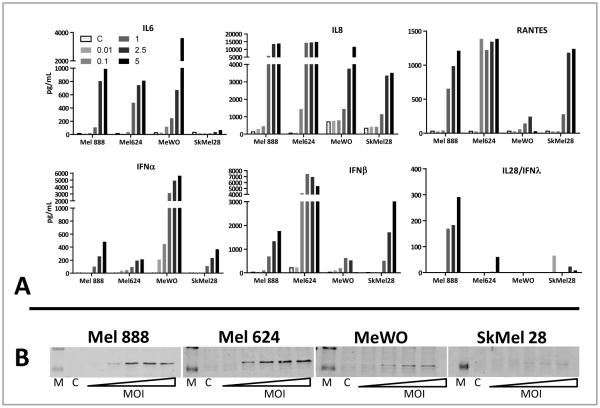
Figure 2.
The inflammatory response associated with MV infection. A; Cytokine/chemokine release. Cell-free supernatants were collected 48 hours after infection with MV and cytokine levels determined by ELISA. Data shown are representative of three independent experiments. B; HMGB1 release. Melanoma cells were treated with MV at MOI from 0.01 to 5. 48hours after infection cell-free supernatant was collected then analysed by western blot for HMGB1. Lanes with protein markers (M) and untreated controls (C) are indicated. Data shown are representative of two separate experiments.
Furthermore, significant levels of the chemokine RANTES were released by all cell lines except MeWo. In addition, western blotting also identified the presence of HMGB1 in cell-free supernatant from Mel888, Mel624 and MeWo (Figure 2b). HMGB1 is known to be a potent danger signal, acting upon dendritic cells (DC) and other cells through TLR4, and has been shown to be an obligatory mediator of antigen presentation by DC 35,36.
Primary melanoma cells are also susceptible to MV-oncolysis
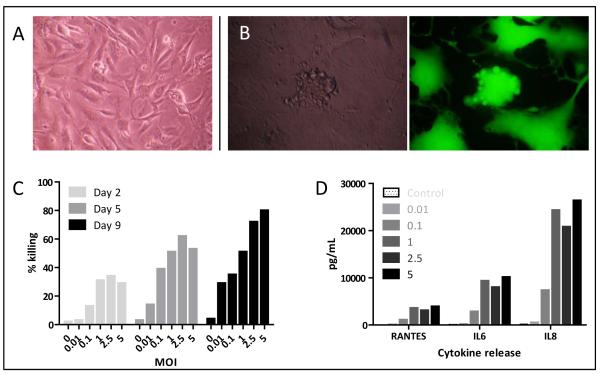
Metastatic deposits resected from three patients with histologically proven melanoma were used to prepare cell suspensions, maintained in culture prior to use at low passage numbers (Figure 3a)31. In keeping with the established melanoma cell lines, primary melanoma cells demonstrated the characteristic CPE of infection with either MV or MV-GFP treatment (Figure 3b). Live/Dead assay confirmed the susceptibility of cells to MV treatment, albeit at a prolonged time course relative to the immortalised cell lines (Figure 3c). Furthermore, ELISA demonstrated the release of RANTES, IL6 and IL8 from primary cells 72 hours after treatment with MV (Figure 3d).
Figure 3.
Effects of MV on primary melanoma cells. A; Primary cells from freshly explanted melanoma B; Characteristic CPE 48 hours following treatment with MV-GFP by phase contrast (left) and fluorescence (right) microscopy. C; Live/Dead assay 2, 5 and 9 days after treatment of primary cells with MV. Data shown are representative of primary cells from three donors. D; ELISA of supernatant from primary cells treated with MV 72 hours previously.
MV treatment of peripheral blood mononuclear cells enhances innate antitumour immunity
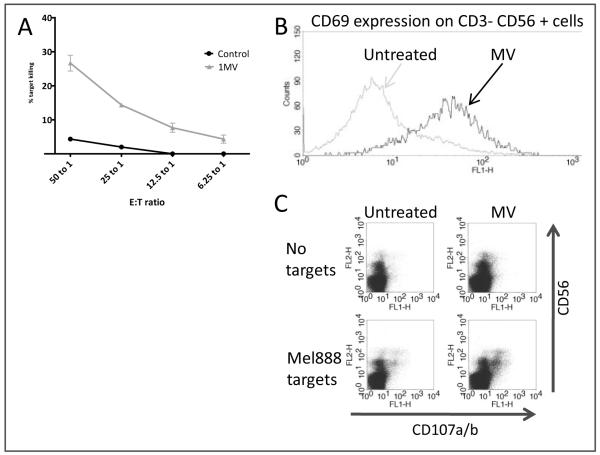
We have previously shown that another OV (reovirus) can stimulate innate anti-tumour immunity within PBMC effectors 37. Therefore, PBMC were prepared from fresh blood, treated overnight with MV at an MOI of 1, and assayed for their ability to kill chromium labelled tumour targets. As demonstrated in Figure 4a, MV treatment of PBMC markedly enhanced killing of melanoma targets over untreated PBMC, at a range of effector to target ratios. This effect was not melanoma-specific, as enhanced killing of the ovarian cancer cell line SkOV was also demonstrated (data not shown). Since innate cytotoxicity can be mediated by NK cells, we next looked for evidence of NK cell activation following treatment with MV. A panel of NK phenotypic activation markers was used, and CD69 was upregulated by MV treatment in all donors studied (Figure 4b); levels of expression of other markers (CD16, CCR7, DNAM1 and NKG2D) did not alter (data not shown). To further address whether NK cells were responsible for MV- induced innate activation, a CD107 assay was performed, which confirmed degranulation of CD3-CD56+ NK cells during MV-enhanced PBMC melanoma cell killing (Figure 4c).
Figure 4.
Activation of innate immunity by MV. PBMC were treated overnight with MV (MOI 1). A; Chromium release elicited from labelled Mel888 targets on 4 hour culture with MV-treated PBMC. B; Levels of CD69 on CD3-CD56+ NK cells with or without treatment with MV. C; CD107 upregulation on CD3-CD56+ NK cells within PBMC, following four hour co-culture with Mel888. All figures are representative of experiments in three healthy donors.
Dendritic cells are activated by MV-infected melanoma cells
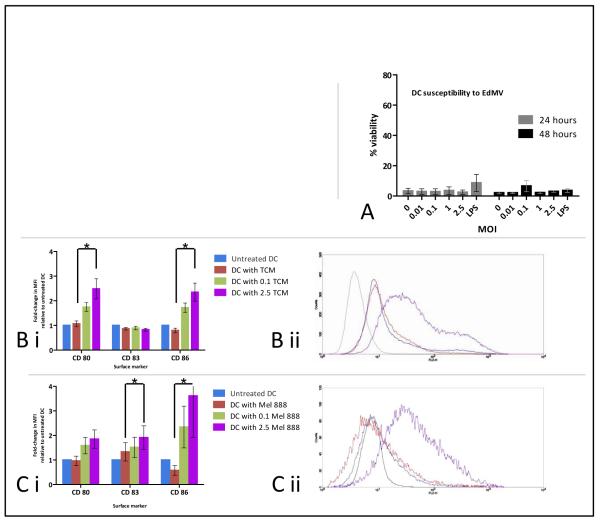
With regard to anti-tumour immune responses stimulated by OV, we have previously shown that adaptive immunity can also be primed in models of human melanoma, and that DC serve a critical role in that process 25,27,38. We therefore explored the impact of MV and MV-treated melanoma cells upon DC. The wild-type or pathogenic strain of measles is known to be toxic to human dendritic cells 39 and most of the deaths associated with the measles illness are due to secondary infections as a consequence of immunosuppression40. In contrast, and consistent with its long history of safe use as a vaccine and in keeping with previous reports18,39, we found that Edmonston strain oncolytic MV was not toxic to DC, as assessed both morphologically and by Live/Dead assay (Figure 5a). Indeed, DC from both healthy donors and patients with metastatic melanoma directly treated with MV upregulated the costimulatory surface activation markers CD80 and CD86 (data not shown).
Figure 5.
Effect of MV on dendritic cells. A; DC were directly treated with MV at a range of MOI, or LPS as an alternative maturation agent, and then analysed by Live/Dead. B; DC were cultured in filtered virus-free tumour conditioned media (TCM) from Mel888 infected with MV at MOI of 0, 0.1 or 2.5 for 48 hours, and expression of surface markers measured. B.i; shows levels of expression normalised to untreated DC, and are mean values from four donors. B.ii; is a representative histogram plot of CD86 expression from one donor; Black line is isotype control, blue line is untreated DC, red line is DC treated with TCM from untreated Mel888 and purple line is DC treated with TCM from Mel888 infected with MV at 2.5 MOI. C. DC were cultured with infected or uninfected Mel888 for 24 hours then levels of surface marker expression measured from non-adherent cells gated on class II positive populations. Data are mean values from four donors and bars indicate SEM throughout. Asterisks indicate statistically significant differences between MFI values for DC treated with Mel888 or Mel888 TCM, versus 2.5 Mel888 or 2.5 TCM
Tumours are known to generate an immunosuppressive environment capable of impeding the activation and function of DC 41. However, the inflammatory environment stimulated by MV infection of tumour may facilitate DC activation, alongside direct immunostimulatory recognition of the virus itself. To address this question, the tumour-conditioned media (TCM) from Mel888 and MV-treated Mel888 cells was collected and filtered to remove free virions 42, the efficacy of filtration having previously been confirmed by filtering neat virus stock and confirming the absence of CPE on Vero cells. Filtered TCM was added to DC in culture overnight, and the following day expression of DC activation/maturation markers were assessed by flow cytometry. As shown in Figure 5b, there was consistent upregulation of CD80 and CD86 in response to TCM from Mel888 treated with MV. Moreover, direct coculture of MV-infected Mel888 cells with DC (incorporating both cell-to-cell and direct virus effects) also led to phenotypic changes in DC with upregulation in CD80 and CD86 (Figure 5c). However, no significant additional cytokine production was elicited from DC on culture with MV-infected Mel888 cells (data not shown). Hence both the inflammatory milieu represented by TCM, and co-cultured virus-infected tumour cells, are capable of phenotypically activating DC for potential support of priming of adaptive anti-tumour immunity 42,43.
Adaptive immune priming in response to MV infection of melanoma cells
Given the evidence that MV infection of melanoma cells results in the expression of inflammatory cytokines and danger signals (Figures 2 & 3), and that DC are activated by MV-infected melanoma (Figure 5c), we next investigated the ability of MV-infection to stimulate a functional adaptive immune response.
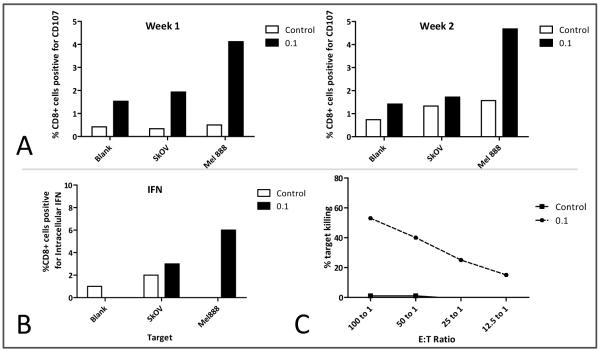
DC were cocultured for 24 hours with Mel888 that had been treated with MV for 48 hours. The Mel888 cells were allowed to adhere and the non-adherent tumour cell-loaded DC (MV-Mel-DC) collected and cocultured with autologous PBMC as previously described 27. One week later the PBMC were again restimulated with identically treated DC. A CD107 assay was performed after one and two weeks, to test CD8 T cell degranulation against Mel888 or an irrelevant tumour target (SkOV – Figure 6A); intracellular CD8 IFNγ was also assayed after two weeks(Figure 6B). To confirm a cytolytic T-cell response, a chromium release assay was performed, again against Mel888 and irrelevant SkOV targets (Figure 6C). Although results varied between donors, in 9 of 12 donors there was evidence of enhanced anti-melanoma activity in response to MV; figure 6 shows results from one representative donor. As seen in figure 6A, CD8 cytotoxic T cells cocultured with MV-Mel-DC degranulated specifically on recognition of melanoma targets. The same cell population was also positive for intracellular IFNγ, indicative of a Th1 cytokine response (Figure 6B). Finally, in a functional killing assay, CTL primed by MV-Mel-DC had more activity against uninfected melanoma targets than those primed by Mel-DC (Figure 6c).
Figure 6.
Priming an adaptive T cell immune response. CTL were primed from PBMC cocultured with autologous DC that had been loaded with either Mel888 (control) or Mel888 treated with Mv at an MOI of 0.1 (0.1). A; Degranulation of CTL following coculture with either Mel888, SkOV or an equal volume of media free of target cells. B; Intracellular IFNγ within CTL primed from PBMC following coculture with Mel888, SkOV or media control. C; Cr-51 release from Mel888 following coculture with CTL; no significant release of Cr51 was detected from irrelevant SkOV targets. Data shown are from one donor, representative of experiments in 12 donors, in 9 of which a specific anti-melanoma response was elicited.
Figure 6 shows that, in all 3 assays, MV-infected Mel888 cells were more effective than uninfected cells as an antigen source for loading of DC for priming of a specific anti-Mel888 immune response.
Discussion
In keeping with reports of the action of unmodified and modified strains of oncolytic MV in other tumour models, we have demonstrated the potential of MV as an OV for the treatment of melanoma (Figures 1 and 3). The presence of clear CPE in both 2 and 3D in vitro tumour models, and objective measures of cell viability, confirmed oncolytic activity to a magnitude, and within a timescale, similar to published studies in models of human prostate cancer 44,45, breast cancer 46, glioblastoma 47, myeloma 48, lymphoma 49 and mesothelioma 18,50. In addition to being susceptible to productive infection by MV, melanoma cells (both cell lines and primary) produced an inflammatory pattern of cytokines and chemokines following infection (Figures 2 and 3). Discussions about IFN in the context of OV normally centre around the need for therapeutic viruses to evade IFN, or on the loss of IFN responsiveness in tumour cells. Although melanoma cells have been demonstrated to have defective responses to IFN 51,52, our finding that melanoma cells retain the ability to release IFN in response to MV infection was not detrimental to viral killing 42. Moreover, this maintained IFN release could be beneficial to therapy, because IFN can protect normal tissues to enhance the therapeutic index of the virus 53, and IFN have direct anti-proliferative effects themselves and are used clinically in melanoma 32,54. Finally, IFN are known to activate DC 33 and enhance adaptive immune responses 55 as well as innate anti-tumour immunity in the context of OV 37. The presence of HMGB1 in the TCM of MV-infected melanoma cells is of note (Figure 2b), because HMGB1 is a potent danger signal capable of promoting antigen presentation, and is thought to be central to the efficacy of conventional treatments 35,56-59, as well as involved in responses to OV 36.
These data add to the growing body of evidence that a local combination of tumour target and oncolytic virus leads to death of cancer cells and the reversal of the immunosuppressive milieu propagated by tumours. The activation of innate anti-tumour immunity by OV (shown here for MV- Figure 4) can support therapy via a range of immune effectors, either systemically and/or within the tumour itself 26,60. Moreover, within an appropriate inflammatory tumour environment, DC may also become suitably activated (Figure 5) for acquisition of tumour associated antigens and effective priming of an adaptive anti-tumour immune response (Figure 6). The immune system has been viewed as likely to have a deleterious impact upon oncolytic virotherapy, by obstructing the systemic delivery of virus and limiting intratumoural viral replication and spread 23. Increasingly however, the evidence is mounting that OV may enhance both innate and adaptive antitumour responses 23, and that the net effect may be a benefit to therapy. In at least some preclinical models the bulk of the therapeutic effect of OV is in fact immune mediated, rather than due to direct oncolysis24,60, although the balance between the therapeutic and damaging effects of the immune response to any OV is likely to be complex and depend on multiple variables, including prior anti-viral immunity, route of administration and co-administration of immunomodulators such as chemotherapy. Hence, interesting new strategies are being pursued that recognise that the role of the immune system in OV therapy is not straightforward, and that accumulating preclinical and clinical findings need to be incorporated into novel strategies to maximise benefit for patients61.
The options to correlate these data with in vivo assays are limited as mice lack the CD46 receptor. Transgenic mice expressing CD46 are available only on IFN deficient backgrounds, so study of clinically relevant MV in immune competent murine models is, at present, problematic 62. The data presented here support the ongoing development of MV as an agent with both oncolytic and immunotherapeutic potential in the treatment of human melanoma. Recent clinical studies with ipilimumab, an anti-CTLA4 antibody 17 and ongoing trials using adoptive cell therapies 63, reiterate the potential for immunotherapeutic approaches to have profound benefits for patients with melanoma. As the immunogenic potential of OV therapy is increasingly recognised, it is important that future clinical trials with these agents seek to identify relevant translational immune end-points, in addition to conventional measures of safety and efficacy, in order to inform further preclinical investigation and maximise the potential impact of viral agents.
Materials and methods
Cell culture and reagents
Human melanoma cell lines Mel888, Mel624, SkMel28 and MeWo were all supplied by Cancer Research UK and were grown in DMEM (Invitrogen, Paisley, UK) supplemented with 10% (v/v) FCS (Biosera, Ringmer, UK) and 1% (v/v) L-glutamine (Invitrogen). Vero cells were supplied by the ATCC and grown in the same media. Cells were routinely tested and found to be negative for Mycoplasma infection.
Human melanoma explants, collected under the auspices of existing ethical approvals were cultivated in a complete tissue media comprising; DMEM containing 10% (v/v) FCS, 1% (v/v) L-glutamine, with the antimicrobials gentamicin (25mg/L), penicillin (1U/L) and streptomycin (1μg/L), amphotericin (0.1mg/L), and supplemented with 1% (v/v) non-essential amino acids (all Sigma-Aldrich, Dorset, UK), 1% (v/v) insulin, transferrin and selenium (Invitrogen) and 25mM HEPES buffer (Sigma-Aldrich). Single cell suspensions were prepared as previously described 31.
Human PBMC and human myeloid DC were prepared as previously described 27. PBMC were maintained in RPMI supplemented with 10% FCS and 1% L-glutamine. Immature DC were cultured in RPMI supplemented with 10% FCS, 1% glutamine 800 IU/mL GM-CSF (Peprotech, London, UK) and 0.05μg/mL IL-4 (R&D systems, Abingdon, UK).
Multilayer model and confocal microscopy
2×105 cells were seeded into 8μm pore size ThinCert™ tissue culture inserts for 24-well plates, (Greiner Bio-One, Stroudwater, UK). Virus was added to the underlying well around 5 days after cell seeding. 48 hours after infection transwells (TW) were fixed in 1% w/v PFA (Sigma) overnight and the following morning rinsed in PBS by gentle immersion in sequential containers of sterile PBS after cautious aspiration of residual PFA. The TW were immersed in 1% FCS in PBS and blocked for 2 hours. TW were then aspirated and immersed for 5 minutes in 300nM DAPI (Invitrogen) diluted in PBS, and thoroughly rinsed in serial changes of PBS as above. TW were imaged on glass-bottomed culture dishes using an Eclipse TE2000-E microscope and the data analysed using EZ-C1 FreeViewer software v3.5 (both from Nikon Instruments Europe, Kingston, UK).
Measles virus
Measles virus and measles virus expressing GFP were prepared as described previously49,64,65. Viral stocks were kept frozen at −80°C and thawed aliquots were used immediately. Viral stocks were bulked on Vero cells and titrated in a standard TCID50 assay as previously described 1. Viral treatment of cells was performed in a reduced volume of serum-free Optimem (Invitrogen). For experiments requiring virus-free tumour-conditioned media, supernatants were collected and passed through a 0.2 μm Acrodisc syringe filter (Pall Life Sciences, Portsmouth, UK) and then through a ViresolveNFR filter (Millipore, Watford, UK) to remove virus. In order to confirm the completeness of filtration neat viral stocks were filtered then the eluate titrated on Vero cells as above 42. Viral replication was quantified by infecting melanoma cells in 6 well plates at an MOI of 0.1 in triplicate. At the required time point cells and supernatant were harvested and subjected to 3 freeze-thaw cycles to release intracellular virus. The resulting product was titrated onto Vero cells in 96-well plates after serial tenfold dilutions in Optimem. 5 days later wells were inspected for CPE and viral titre calculated according to the method of Reed and Muench 28.
Flow cytometry
Antibodies to human CD3, CD16, CD46, CD56, CD80, CD86, CD150, DNAM1, CCR7 were supplied by Beckton-Dickinson Pharmingen (Oxford, UK). Anti-human CD40 was obtained from Invitrogen. Cells were acquired using a FACS Calibur and data analysed with CellQuest Pro software (both Beckton- Dickinson Biosciences, Oxford, UK). Differences between MFI values in DC experiments were compared using the paired T test and statistical significance defined where p values <0.05.
Viability assays
Cells were harvested and washed with PBS then labelled with LIVE/DEAD® fixable dead cell stain (Invitrogen) according to the manufacturer’s protocol and incubated at room temperature for 30mins. Cells were subsequently washed twice, once with PBS and once with PBS supplemented with 1% FCS. They were then fixed in 1% PFA and analyzed by flow cytometry. Further assessment of oncolytic activity was performed using MTT assay after 24, 48 and 72 hours treatment with MV according to methodology previously described 31.
ELISA and Western blotting
IL6 and IL8 were detected using matched pair antibodies (BD Pharmingen). IFNβ was quantified using the Verikine™ Human Interferon Beta ELISA kit (PBL Interferon Source, Newmarket, UK). IFNα was quantified using the Mabtech Human ELISA kit (Mabtech Ab, Buro, Germany). IL28 and RANTES were measured using the respective R&D duosets.
HMGB1 was assayed by collecting cell-free supernatants, 48 hours after treatment with MV. The supernatants were diluted 1:1 with Laemmli buffer prior to loading 20μL of diluted supernatant on a 10% SDS page gel using standard protocols; staining for HMGB1 was performed using a monoclonal mouse anti-human HMGB1 antibody (R&D systems, Abingdon UK) used at 1μg/mL in a 1:1 mix of Odyssey blocking buffer (LiCor Biotechnology, Cambridge UK) and PBS/0.1% Tween. Goat anti-mouse conjugated with AlexaFluor 680 (Molecular probes, Invitrogen) was used as a secondary antibody at 0.2μg/mL for protein detection using the Odyssey Infrared imaging system (LiCor).
Priming assays
Cytotoxic T-lymphocytes were generated by loading Mel888 or MV-treated Mel888 onto DC at a ratio of 3:1. After 24 hours non-adherent DC were harvested then cocultured with autologous PBMC at ratios varying from 1:15 to 1:45 in CTL media (RPMI supplemented with 7.5% (v/v) human AB serum (Sera Laboratories Int., Hayward’s Heath, UK ), 1% (v/v) L-glutamine, 1% v/v sodium pyruvate (Invitrogen), 1% (v/v) non-essential amino acids, 1% (v/v) HEPES (Invitrogen), 20μM β-mercaptoethanol (Sigma)) and 5ng/ml IL-7 (R&D Systems). IL-2 (R&D Systems) was added at 30IU/ml on day 4 only. CTL were restimulated after one week in an identical fashion.
Cytotoxicity Assays
CD107 degranulation
Surface expression of CD107 was measured as follows. CTL and tumour targets were incubated at a 1:1 ratio in the presence of anti-CD107a and b-FITC (BD Pharmingen). Brefeldin A (Sigma)(10 μg/mL) was added after 1 hour. After a further 4 hours, cells were stained with anti–CD8, or anti-CD56 and anti-CD3, and analysis performed by flow cytometry.
Chromium release assay
The cytotoxic activity of CTL or PBMC was measured using the 51Chromium release assay as previously described 66. Percent lysis was calculated using the formula: % lysis = 100 × (cpm experiment − cpm spontaneous release)/ (cpm maximum release − cpm spontaneous release).
Intracellular IFNγ
CTL and tumour targets were incubated at a 1:1 ratio and brefeldin A added after 1 hour. After a further 4 hours cells were harvested and stained with anti-CD56 and anti-CD3 then fixed with 1% w/v PFA. Cells were subsequently permeabilised with 0.3% w/v saponin then stained with FITC-labeled goat anti-human IFNγ (BD Pharmingen), and analysis performed by flow cytometry.
Acknowledgements
The work described was supported by grants from the Medical Research Council (UK) and Cancer Research UK.
Footnotes
Conflict of interest Stephen J Russell is a named inventor on patents pertaining to the use of measles virus as an anticancer drug therapy. These patents are owned by Mayo Clinic. The authors otherwise declare no competing interests in relation to the work described.
References
- 1.Grote D, et al. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood. 2001;97:3746–3754. doi: 10.1182/blood.v97.12.3746. [DOI] [PubMed] [Google Scholar]
- 2.Peng KW, et al. Systemic therapy of myeloma xenografts by an attenuated measles virus. Blood. 2001;98:2002–7. doi: 10.1182/blood.v98.7.2002. [DOI] [PubMed] [Google Scholar]
- 3.Peng K-W, et al. Intraperitoneal Therapy of Ovarian Cancer Using an Engineered Measles Virus. Cancer Res. 2002;62:4656–4662. [PubMed] [Google Scholar]
- 4.Phuong LK, et al. Use of a Vaccine Strain of Measles Virus Genetically Engineered to Produce Carcinoembryonic Antigen as a Novel Therapeutic Agent against Glioblastoma Multiforme. Cancer Research. 2003;63:2462–2469. [PubMed] [Google Scholar]
- 5.Peng K-W, et al. Biodistribution of oncolytic measles virus after intraperitoneal administration into Ifnar-CD46Ge transgenic mice. Hum. Gene Ther. 2003;14:1565–1577. doi: 10.1089/104303403322495070. [DOI] [PubMed] [Google Scholar]
- 6.Dingli D, et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103:1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- 7.Langfield KK, et al. 77. Production and Purification of Measles Virus for Oncolytic Virotherapy Clinical Trials. Mol Ther. 2004;9:S31. [Google Scholar]
- 8.Kunzi V, Oberholzer PA, Heinzerling L, Dummer R, Naim HY. Recombinant Measles Virus Induces Cytolysis of Cutaneous T-Cell Lymphoma In Vitro and In Vivo. J Invest Dermatol. 2006;126:2525–2532. doi: 10.1038/sj.jid.5700529. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa K, et al. Dual therapy of ovarian cancer using measles viruses expressing carcinoembryonic antigen and sodium iodide symporter. Clin. Cancer Res. 2006;12:1868–1875. doi: 10.1158/1078-0432.CCR-05-1803. [DOI] [PubMed] [Google Scholar]
- 10.Blechacz B, et al. Engineered measles virus as a novel oncolytic viral therapy system for hepatocellular carcinoma. Hepatology. 2006;44:1465–1477. doi: 10.1002/hep.21437. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann M, et al. Human precision-cut liver tumor slices as a tumor patient-individual predictive test system for oncolytic measles vaccine viruses. Int. J. Oncol. 2009;34:1247–1256. [PubMed] [Google Scholar]
- 12.Penheiter AR, et al. Sodium Iodide Symporter (NIS)-Mediated Radiovirotherapy for Pancreatic Cancer. Am. J. Roentgenol. 2010;195:341–349. doi: 10.2214/AJR.09.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinzerling L, et al. Oncolytic measles virus in cutaneous T-cell lymphomas mounts antitumor immune responses in vivo and targets interferon-resistant tumor cells. Blood. 2005;106:2287–2294. doi: 10.1182/blood-2004-11-4558. [DOI] [PubMed] [Google Scholar]
- 14.Msaouel P, Dispenzieri A, Galanis E. Clinical testing of engineered oncolytic measles virus strains in the treatment of cancer: an overview. Curr. Opin. Mol. Ther. 2009;11:43–53. [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufman HL, et al. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann. Surg. Oncol. 2010;17:718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 16.Senzer NN, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J. Clin. Oncol. 2009;27:5763–5771. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- 17.O’Day S, et al. A phase III, randomized, double-blind, multicenter study comparing monotherapy with ipilimumab or gp100 peptide vaccine and the combination in patients with previously treated, unresectable stage III or IV melanoma. J Clin Oncol (Meeting Abstracts) 2010;28:4. [Google Scholar]
- 18.Gauvrit A, et al. Measles Virus Induces Oncolysis of Mesothelioma Cells and Allows Dendritic Cells to Cross-Prime Tumor-Specific CD8 Response. Cancer Res. 2008;68:4882–4892. doi: 10.1158/0008-5472.CAN-07-6265. [DOI] [PubMed] [Google Scholar]
- 19.Kemper C, Atkinson JP. Measles virus and CD46. Curr. Top. Microbiol. Immunol. 2009;329:31–57. doi: 10.1007/978-3-540-70523-9_3. [DOI] [PubMed] [Google Scholar]
- 20.Anderson BD, Nakamura T, Russell SJ, Peng K-W. High CD46 Receptor Density Determines Preferential Killing of Tumor Cells by Oncolytic Measles Virus. Cancer Res. 2004;64:4919–4926. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- 21.Donnelly OG, Errington-Mais F, Prestwich R, Harrington K, Pandha H, Vile R, et al. Recent Clinical Experience With Oncolytic Viruses. Curr Pharm Biotechnol. doi: 10.2174/138920112800958904. ePub ahead of print. 2011.ISN 1873-4316. [DOI] [PubMed] [Google Scholar]
- 22.Galanis E, et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010;70:875–882. doi: 10.1158/0008-5472.CAN-09-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prestwich RJ, et al. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum. Gene Ther. 2009;20:1119–1132. doi: 10.1089/hum.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wongthida P, et al. VSV Oncolytic Virotherapy in the B16 Model Depends Upon Intact MyD88 Signaling. Mol Ther. 2011;19:150–158. doi: 10.1038/mt.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prestwich RJ, et al. Immune-Mediated Antitumor Activity of Reovirus Is Required for Therapy and Is Independent of Direct Viral Oncolysis and Replication. Clinical Cancer Research. 2009;15:4374–4381. doi: 10.1158/1078-0432.CCR-09-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Errington F, et al. Reovirus activates human dendritic cells to promote innate antitumor immunity. J Immunol. 2008;180:6018–26. doi: 10.4049/jimmunol.180.9.6018. [DOI] [PubMed] [Google Scholar]
- 27.Prestwich RJ, et al. Tumor Infection by Oncolytic Reovirus Primes Adaptive Antitumor Immunity. Clinical Cancer Research. 2008;14:7358–7366. doi: 10.1158/1078-0432.CCR-08-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. American Journal of Epidemiology. 1938;27:493. [Google Scholar]
- 29.Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Meth. 2007;4:359–365. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans CJ, et al. A mathematical model of doxorubicin penetration through multicellular layers. Journal of Theoretical Biology. 2009;257:598–608. doi: 10.1016/j.jtbi.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 31.Errington F, et al. Inflammatory tumour cell killing by oncolytic reovirus for the treatment of melanoma. Gene Ther. 2008;15:1257–1270. doi: 10.1038/gt.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubo H, et al. Interferon-β therapy for malignant melanoma: the dose is crucial for inhibition of proliferation and induction of apoptosis of melanoma cells. Arch Dermatol Res. 2008;300:297–301. doi: 10.1007/s00403-008-0841-6. [DOI] [PubMed] [Google Scholar]
- 33.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 34.Pichlmair A, Sousa CR. e Innate Recognition of Viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Apetoh L, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 36.Huang B, Sikorski R, Kirn DH, Thorne SH. Synergistic anti-tumor effects between oncolytic vaccinia virus and paclitaxel are mediated by the IFN response and HMGB1. Gene Ther. 2011;18:164–172. doi: 10.1038/gt.2010.121. [DOI] [PubMed] [Google Scholar]
- 37.Prestwich RJ, et al. Reciprocal Human Dendritic Cell–Natural Killer Cell Interactions Induce Antitumor Activity Following Tumor Cell Infection by Oncolytic Reovirus. The Journal of Immunology. 2009;183:4312–4321. doi: 10.4049/jimmunol.0901074. [DOI] [PubMed] [Google Scholar]
- 38.White CL, et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Ther. 2008;15:911–920. doi: 10.1038/gt.2008.21. [DOI] [PubMed] [Google Scholar]
- 39.Ohgimoto K, et al. Difference in production of infectious wild-type measles and vaccine viruses in monocyte-derived dendritic cells. Virus Research. 2007;123:1–8. doi: 10.1016/j.virusres.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Schneider-Schaulies S, Schneider-Schaulies J. Measles virus-induced immunosuppression. Curr. Top. Microbiol. Immunol. 2009;330:243–269. doi: 10.1007/978-3-540-70617-5_12. [DOI] [PubMed] [Google Scholar]
- 41.Yang L, Carbone DP. Tumor-host immune interactions and dendritic cell dysfunction. Adv. Cancer Res. 2004;92:13–27. doi: 10.1016/S0065-230X(04)92002-7. [DOI] [PubMed] [Google Scholar]
- 42.Steele L, et al. Pro-inflammatory cytokine/chemokine production by reovirus treated melanoma cells is PKR/NFkB mediated and supports innate and adaptive anti-tumour immune priming. Molecular Cancer. 2011;10:20. doi: 10.1186/1476-4598-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galivo F, et al. Single-cycle viral gene expression, rather than progressive replication and oncolysis, is required for VSV therapy of B16 melanoma. Gene Ther. 2009;17:158–170. doi: 10.1038/gt.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu C, Hasegawa K, Russell SJ, Sadelain M, Peng K-W. Prostate-specific membrane antigen retargeted measles virotherapy for the treatment of prostate cancer. Prostate. 2009;69:1128–1141. doi: 10.1002/pros.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Msaouel P, et al. Noninvasive imaging and radiovirotherapy of prostate cancer using an oncolytic measles virus expressing the sodium iodide symporter. Mol. Ther. 2009;17:2041–2048. doi: 10.1038/mt.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iankov ID, et al. Demonstration of anti-tumor activity of oncolytic measles virus strains in a malignant pleural effusion breast cancer model. Breast Cancer Res. Treat. 2010;122:745–754. doi: 10.1007/s10549-009-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myers R, et al. Toxicology study of repeat intracerebral administration of a measles virus derivative producing carcinoembryonic antigen in rhesus macaques in support of a phase I/II clinical trial for patients with recurrent gliomas. Hum. Gene Ther. 2008;19:690–698. doi: 10.1089/hum.2008.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dingli D, et al. Dynamics of multiple myeloma tumor therapy with a recombinant measles virus. Cancer Gene Ther. 2009;16:873–882. doi: 10.1038/cgt.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haralambieva I, et al. Engineering oncolytic measles virus to circumvent the intracellular innate immune response. Mol Ther. 2007;15:588–97. doi: 10.1038/sj.mt.6300076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H, Peng K-W, Dingli D, Kratzke RA, Russell SJ. Oncolytic measles viruses encoding interferon beta and the thyroidal sodium iodide symporter gene for mesothelioma virotherapy. Cancer Gene Ther. 2010;17:550–558. doi: 10.1038/cgt.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Critchley-Thorne RJ, et al. Impaired interferon signaling is a common immune defect in human cancer. Proceedings of the National Academy of Sciences. 2009;106:9010–9015. doi: 10.1073/pnas.0901329106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stojdl DF, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 53.Willmon CL, et al. Expression of IFN-{beta} Enhances Both Efficacy and Safety of Oncolytic Vesicular Stomatitis Virus for Therapy of Mesothelioma. Cancer Res. 2009;69:7713–7720. doi: 10.1158/0008-5472.CAN-09-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mocellin S, Pasquali S, Rossi CR, Nitti D. Interferon Alpha Adjuvant Therapy in Patients With High-Risk Melanoma: A Systematic Review and Meta-analysis. Journal of the National Cancer Institute. 2010;102:493–501. doi: 10.1093/jnci/djq009. [DOI] [PubMed] [Google Scholar]
- 55.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nature immunology. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 56.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 57.Bianchi ME, Manfredi AA. High□mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunological Reviews. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 58.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 59.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: Endogenous Danger Signaling. Mol Med. 2008;14:476–484. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wongthida P, et al. Type III IFN Interleukin-28 Mediates the Antitumor Efficacy of Oncolytic Virus VSV in Immune-Competent Mouse Models of Cancer. Cancer Research. 2010;70:4539–4549. doi: 10.1158/0008-5472.CAN-09-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Banaszynski LA, Sellmyer MA, Contag CH, Wandless TJ, Thorne SH. Chemical control of protein stability and function in living mice. Nat Med. 2008;14:1123–1127. doi: 10.1038/nm.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ungerechts G, et al. An immunocompetent murine model for oncolysis with an armed and targeted measles virus. Mol. Ther. 2007;15:1991–1997. doi: 10.1038/sj.mt.6300291. [DOI] [PubMed] [Google Scholar]
- 63.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Current Opinion in Immunology. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duprex WP, McQuaid S, Hangartner L, Billeter MA, Rima BK. Observation of Measles Virus Cell-to-Cell Spread in Astrocytoma Cells by Using a Green Fluorescent Protein-Expressing Recombinant Virus. J. Virol. 1999;73:9568–9575. doi: 10.1128/jvi.73.11.9568-9575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Radecke F, et al. Rescue of measles viruses from cloned DNA. EMBO J. 1995;14:5773–5784. doi: 10.1002/j.1460-2075.1995.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Errington F, et al. Fusogenic membrane glycoprotein-mediated tumour cell fusion activates human dendritic cells for enhanced IL-12 production and T-cell priming. Gene Ther. 2005;13:138–149. doi: 10.1038/sj.gt.3302609. [DOI] [PubMed] [Google Scholar]