Abstract
Background
The prevention of mother-to-child transmission (PMTCT) of HIV has been focused mainly on women who are HIV-positive at their first antenatal visit, but there is uncertainty regarding the contribution to overall transmission from mothers who seroconvert after their first antenatal visit and before weaning.
Method
A mathematical model was developed to simulate changes in mother-to-child transmission of HIV over time, in South Africa. The model allows for changes in infant feeding practices as infants age, temporal changes in the provision of antiretroviral prophylaxis and counselling on infant feeding, as well as temporal changes in maternal HIV prevalence and incidence.
Results
The proportion of MTCT from mothers who seroconverted after their first antenatal visit was 26% (95% CI: 22-30%) in 2008, or 15 000 out of 57 000 infections. It is estimated that by 2014, total MTCT will reduce to 39 000 per annum, and transmission from mothers seroconverting after their first antenatal visit will reduce to 13 000 per annum, accounting for 34% (95% CI: 29-39%) of MTCT. If maternal HIV incidence during late pregnancy and breastfeeding were reduced by 50% after 2010, and HIV screening were repeated in late pregnancy and at 6-week immunization visits after 2010, the average annual number of MTCT cases over the 2010-15 period would reduce by 28% (95% CI: 25-31%), from 39 000 to 28 000 per annum.
Conclusion
Maternal seroconversion during late pregnancy and breastfeeding contributes significantly to the paediatric HIV burden, and needs greater attention in the planning of PMTCT programmes.
Keywords: HIV/AIDS, vertical transmission, mathematical model, South Africa
Introduction
In 2009, UNAIDS issued a call for the “virtual elimination” of mother-to-child transmission of HIV globally by 2015. This vision has stimulated increased investment in programmes for the prevention of mother-to-child transmission (PMTCT) of HIV. However, most programme focus has been on pregnant women who are identified as HIV-positive during antenatal care. The prevention of transmission from mothers who seroconvert after antenatal screening, in late pregnancy or while breastfeeding, is much more challenging. Acutely-infected women are likely to be at a higher risk of transmitting HIV to their children than chronically-infected women, partly because the high levels of HIV viral load that occur during acute infection are associated with increased risk of perinatal transmission1-3 and transmission through breastfeeding,4, 5 and partly because the maternal immune response during acute infection may not be sufficiently mature to allow significant transfer of protective immunity to the child.6, 7 These women are also more difficult to identify, and are therefore less likely to receive timely antiretroviral prophylaxis and counselling on infant feeding.
Mathematical models of the sexual transmission of HIV have suggested that acute HIV infection could account for a significant proportion of sexual transmission during the early stages of the HIV epidemic,8-10 and allowance for a high level of infectiousness during the first few months after HIV acquisition has thus become standard in modelling the sexual transmission of HIV. However, in modelling vertical transmission of HIV, only a handful of studies have considered maternal seroconversion after the first antenatal visit and the associated high transmission risk during the acute phase of infection.11-13 In the most recent of these studies,12 43% of all vertical transmission in Botswana was estimated to be transmission from mothers who seroconverted after their first antenatal screening visit and before cessation of breastfeeding. Further modelling work is required to assess the significance of transmission from recently-infected mothers in other settings, and to assess the potential impact of interventions to reduce this transmission.
The significance of transmission from recently-infected mothers is likely to depend crucially on factors such as the HIV epidemic stage, the extent of PMTCT programmes, infant feeding practices and the timing of maternal HIV testing in pregnancy. This paper describes a dynamic model that has been developed to assess the effect of these factors. The model has been parameterized using data from South Africa, a country in which PMTCT has been scaled up rapidly over the last decade, where HIV incidence remains high and breastfeeding is common. Our objective is to assess the extent to which recently-infected mothers contribute to total vertical transmission at different stages in the South African epidemic, and to assess the potential impact of programmes to limit this transmission.
Methods
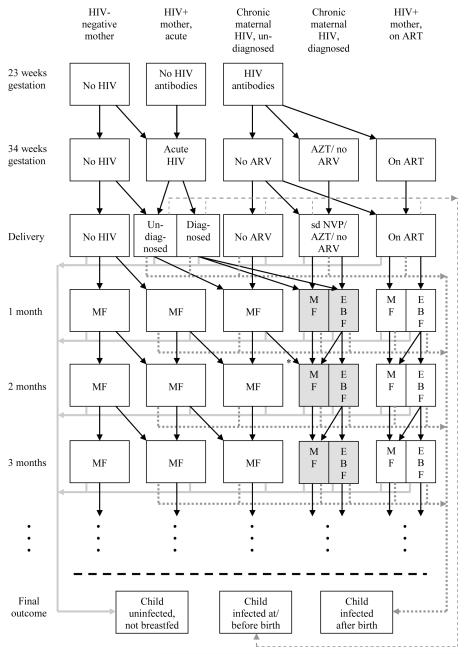
The structure of the model is illustrated in Figure 1. Annual numbers of births to women who are HIV-seronegative at their first antenatal visit, and annual numbers of births to women who are HIV-seropositive at their first antenatal visit, are both estimated from the ASSA2003 AIDS and Demographic model, a model of the South African HIV epidemic that is calibrated to HIV prevalence data collected at first antenatal visits.14, 15 This model is also used to estimate annual HIV incidence rates in pregnant women, and the annual incidence rates and numbers of births are shown in an online appendix (Table 1 of Supplemental Digital Content 1). The first antenatal visit is assumed to occur at 23 weeks gestation16-18 and delivery at 39 weeks,18 on average, so that the average time in which a woman seronegative at her first visit can acquire HIV before delivery is 20 weeks if a 4-week window period is assumed.19 The probability that a pregnant woman seronegative at her first antenatal visit acquires HIV before delivery is therefore calculated as the annual HIV incidence rate in pregnant women multiplied by a factor of 0.38 (20/52). The assumed probability that a woman who acquires HIV in late pregnancy transmits HIV perinatally is based on a review of studies conducted in settings where breastfeeding is rare (summarized in Table 1) and on perinatal transmission rates from HIV-positive South African mothers who reported previously testing negative.20, 21
Figure 1.
Multi-state model of mother-to-child transmission
ART = antiretroviral treatment (long-term treatment for mother’s health); ARV = antiretroviral; AZT = zidovudine; EBF = exclusive breastfeeding; MF = mixed feeding; sd NVP = single-dose nevirapine
Shaded cells represent states in which mothers may administer extended nevirapine prophylaxis to their children. Solid grey lines represent discontinuation (or avoidance) of breastfeeding by mothers of uninfected children, dotted grey lines represent postnatal transmission and dashed grey lines represent mother-to-child transmission at or before delivery. In the default scenario, there is no retesting of mothers, either during late pregnancy or at 6-week immunization visits. However, the model does allow for retesting at 34 weeks gestation, and the model allows for undiagnosed HIV-positive mothers to be diagnosed if screening is conducted at 6- week immunization (*). If women who seroconvert between their first antenatal visit and delivery are diagnosed, it is assumed they may receive short-course ARV prophylaxis, but that they would not be eligible to start ART before delivery. Rates at which women discontinue breastfeeding depend on the age of the child and the mother’s knowledge of her HIV status.
Table 1.
Review of studies assessing HIV transmission from mothers who seroconverted during pregnancy or while breastfeeding, in the absence of antiretroviral prophylaxis
| Study | Location | n | Transmission events |
% |
|---|---|---|---|---|
| Mothers who seroconverted during pregnancy | ||||
| Tovo et al 22 | Italy | 10 | 2 | 20% |
| Rudin et al 23 | Switzerland | 4 | 2 | 50% |
| Hague et al 24 | UK | 9 | 5 | 56% |
| Nielsen-Saines et al 25 | Brazil | 9 | 3 | 33% |
| Roongpisuthipong et al 26 | Thailand | 15 | 2 | 13% |
| Pooled | 47 | 14 | 30%† | |
| Mothers who seroconverted during breastfeeding | ||||
| Colebunders et al 27 | DRC | 3 | 1 | 33% |
| Palasanthiran et al 28 | Australia | 11 | 3 | 27% |
| Hira et al 29 | Zambia | 19 | 3 | 16% |
| Van de Perre et al 30 | Rwanda | 15 | 8 | 53% |
| Embree et al 31 | Kenya | 12 | 5 | 42% |
| Liang et al 32 | China | 106 | 38 | 36% |
| Humphrey et al 33 | Zimbabwe | 334 | -* | 24%* |
| Pooled | 500 | 27%† |
A Kaplan-Meier approach was followed in calculating the cumulative proportion of children infected, and the number of children infected is therefore not shown.
All estimates were pooled using fixed effects models.
Assumptions regarding infant feeding practices in women who are HIV-negative or HIV-positive but undiagnosed are based on the results of the 1998 Demographic and Health Survey.16 87% of these women are assumed to start breastfeeding, and the duration of breastfeeding is modelled using a Weibull distribution with a median of 18 months and a shape parameter of 2. All of these women are assumed to practise mixed feeding, as exclusive breastfeeding (EBF) was rare prior to the introduction of PMTCT programmes.16, 34 Women who were HIV-positive at delivery and who practise mixed feeding are assumed to have a fixed monthly probability of transmitting HIV, h1. Breastfeeding HIV-negative mothers are assumed to acquire HIV at the same rate as pregnant women, and for an average period of 3 months after acquiring HIV, are assumed to have a higher monthly probability of transmitting HIV through mixed feeding, h0. The parameter h0 is estimated from studies of the cumulative HIV transmission risk from breastfeeding mothers who have seroconverted (summarized in Table 1), by noting that this cumulative risk can be expressed as
| (1) |
where μ is the average duration of breastfeeding after seroconversion. Setting μ = 9 months (half of the average duration in the two largest studies32, 33) and setting h0 = 0.16 gives a cumulative transmission risk of 0.28, consistent with the pooled estimate of 0.27 in Table 1.
In each year, a proportion of pregnant women are assumed to receive HIV testing, increasing from 3% in 2000 to 92% in 2010 and subsequent years (see Table 1 of Supplemental Digital Content 1). A fraction of those women testing positive start antiretroviral treatment (ART) if their CD4 count is below 200 (or below 350 following the change in South African guidelines in 201035). Of the remaining women who are diagnosed HIV-positive, a fraction is assumed to receive single-dose nevirapine, and following changes in guidelines in 2008,36 a fraction of women receiving single-dose nevirapine are assumed also to receive short-course zidovudine. Of women who are diagnosed HIV-positive antenatally, 50% are assumed to avoid breastfeeding completely,37, 38 35% practise EBF and 15% practise mixed feeding.39 The monthly probability of postnatal transmission is reduced if the child receives extended nevirapine prophylaxis, if the mother receives ART or if the mother practises EBF. HIV-diagnosed women who practise mixed feeding are assumed to do so for a median of 7 months. HIV-diagnosed women who practise EBF are assumed to do so for a median of 2 months (up to a maximum of 6 months), after which 30% are assumed to discontinue breastfeeding completely and the remainder practise mixed feeding (i.e. continue breastfeeding while introducing complementary feeds), for a median of 7 months.39-41 Following the change in guidelines in 2010,35 a proportion of HIV-diagnosed women who choose to breastfeed are assumed to administer extended nevirapine prophylaxis to their children, with this proportion rising to 80% by 2013. Following the more recent announcement of a phasing out of free provision of formula milk in public clinics, the proportion of HIV-diagnosed women who avoid breastfeeding is assumed to decline from 50% in 2010 to 20% in 2013. Assumptions about vertical transmission rates and the efficacy of PMTCT are summarized in Table 2, and a more detailed description of the model is provided in Supplemental Digital Content 1.
Table 2.
Mother-to-child transmission assumptions
| Parameter | Value (mean) |
Standard deviation* |
Source |
|---|---|---|---|
| % of pregnant HIV+ women with | |||
| CD4 >500 | 36.6% | - | Meta-analysis of |
| CD4 350-500 | 24.5% | - | published studies42 |
| CD4 200-349 | 24.9% | - | |
| CD4 <200 | 14.0% | - | |
| Transmission rate at/before birth, from chronically- infected women with no ARV prophylaxis, with |
|||
| CD4 >500 | 13.4% | - | Meta-analysis of |
| CD4 350-500 | 15.2% | - | published studies42 |
| CD4 200-349 | 25.8% | - | |
| CD4 <200 | 35.0% | - | |
| Transmission rate at/before birth, from acutely- infected women with no ARV prophylaxis |
35.0% | 8.0% | Table 1, Rollins et al20, 21 |
| % of HIV-diagnosed women who receive single-dose nevirapine, if not starting ART |
75.0% | 10.0% | Stringer et al,43 Sherman et al,44 Nkonki et al45 |
| % reduction in perinatal MTCT if mother receives single-dose nevirapine |
40.0% | - | Leroy et al46 |
| % reduction in perinatal MTCT if mother receives short-course zidovudine |
65.0% | - | Sperling et al1 |
| % reduction in perinatal MTCT if mother receives single-dose nevirapine + short-course zidovudine |
80.0% | - | Dabis et al,47 Lallemant et al48 |
| Transmission rate at/before birth, from chronically- infected women starting long-term ART |
2.0% | - | Tonwe-Gold et al,49 Peltier et al,50 Palombi et al,51 Hoffman et al52 |
| Probability of MTCT from chronically- infected mothers, per year of mixed feeding |
14.0% | 2.5% | Meta-analysis,53 adjusted to reflect effect of excluding EBF |
| Probability of MTCT from acutely- infected mothers, per month of mixed feeding |
16.0% | 3.0% | Derived from Table 1 using equation 1 |
| Ratio of postnatal transmission risk per month of EBF to postnatal transmission risk per month of mixed feeding |
50.0% | 15.0% | Coovadia et al,54 Becquet et al55 |
| % reduction in monthly postnatal MTCT risk if child receives extended nevirapine prophylaxis |
60.0% | - | Kumwenda et al,56 Chasela et al,57 SWEN Study Team58 |
| % reduction in monthly postnatal MTCT risk if mother receives long-term ART |
80.0% | - | Taha et al59 |
Standard deviations are shown only for those parameters that are included in the uncertainty analysis.
Children who acquire HIV are assumed to progress to a state of ART eligibility, after which they may start ART. Rates of progression to ART eligibility and rates of AIDS mortality in ART-eligible children are assumed to depend on age and mode of transmission (perinatal or postnatal), as described elsewhere.42 Numbers of new infections in children and numbers of children in different exposed and infected states are calculated at monthly time steps, starting in 1985. To ensure that the model assumptions regarding MTCT and paediatric HIV survival are plausible, the model is fitted to age-specific paediatric HIV prevalence data from national household surveys conducted in 2005 and 2008,60, 61 using a Bayesian uncertainty analysis approach. Beta prior distributions are specified to represent ranges of uncertainty around key parameters, and the means and standard deviations of these distributions are included in Table 2. Posterior distributions, representing the ranges of model results consistent with both the observed paediatric HIV prevalence data and the ranges of uncertainty around the input parameters, were simulated numerically using Incremental Mixture Importance Sampling.62
Three possible interventions are considered to reduce vertical transmission from mothers who acquire HIV after their first antenatal visit:
Maternal HIV incidence is assumed to reduce by 50% after 2010. A 50% reduction in HIV incidence by 2011 is a target of South Africa’s National Strategic Plan,63 and could potentially be achieved in pregnant and breastfeeding women through intensified condom promotion, partner outreach, microbicides and pre-exposure prophylaxis.
The offer of antenatal screening is assumed to be repeated at 34 weeks gestation in women who tested negative or did not receive testing at their first antenatal visit, from 2010 onwards. This has been recommended in recent PMTCT guidelines,35, 36 but implementation has been limited. It is assumed that 80% of women who previously tested negative would accept the offer of retesting,17 and 50% of women who did not receive testing at their first antenatal visit get tested at 34 weeks.
Mothers and infants are assumed to be screened for HIV at 6-week immunization visits, from 2010 onwards. Although not part of current South African guidelines, this has been proposed by Rollins et al.20 92% of mothers are assumed to attend 6-week immunization visits,16 and 66% of women testing positive are assumed to receive their test results.21 Of those women who are diagnosed HIV-positive, 50% are assumed to discontinue breast feeding immediately (this proportion reducing to 20% by 2013), and those who continue to breastfeed are assumed to do so for a shorter period (median 7 months), with 80% administering nevirapine prophylaxis to their infants.
For ease of reference, we use the term ‘recently-infected mothers’ to refer to women who seroconvert after their first antenatal visit, either during late pregnancy or while breastfeeding.
Results
Age-specific model estimates of HIV prevalence were reasonably consistent with survey estimates, although the model tended to estimate higher HIV prevalence in 2008 than observed in the 2008 survey,42 possibly a reflection of the high rates of test refusal in children.61 Posterior distributions for the MTCT parameters in Table 2 were similar to the prior distributions, except in the case of the probability of MTCT from chronically-infected mothers, per year of mixed feeding (posterior mean of 11.6%, 95% CI: 8.4-15.7%).
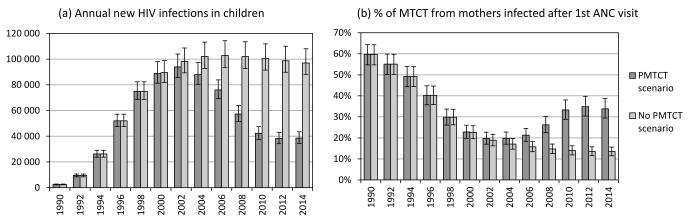
The model estimates that the number of new HIV infections in South African children reached its highest level in 2002, at 94 000 per annum (95% CI: 86 000-104 000), and dropped to 57 000 per annum (95% CI: 51 000-64 000) by 2008. This incidence is 44% lower (95% CI: 39-47%) than the level of paediatric HIV incidence that would have been expected over the same period in the absence of PMTCT (Figure 2a). The number of new HIV infections in children is projected to drop to 39 000 per annum (95% CI: 34 000-43 000) by 2014, following the implementation of the revised 2010 PMTCT guidelines (but not allowing for the additional interventions to reduce transmission from recently-infected mothers). This represents a 60% reduction (95% CI: 57-63%) in paediatric HIV incidence, relative to what would be expected in the absence of PMTCT.
Figure 2.
Comparison of paediatric HIV trends with and without PMTCT
Bars represent means from posterior distributions. Error bars represent 95% confidence intervals.
In 1990, when antenatal HIV prevalence was <1% but HIV incidence was increasing rapidly, an estimated 60% of vertical transmission (95% CI: 55-64%) was from mothers who acquired HIV after their first antenatal visit (Figure 2b). As HIV prevalence in pregnant women increased relative to HIV incidence, the proportion of vertical transmission from recently- infected mothers declined, and would have continued to decline in the absence of PMTCT, to a level of 15% (95% CI: 13-17%) by 2008 (15 000 out of 102 000 infections). However, PMTCT programmes have significantly reduced transmission from mothers who were seropositive at their first antenatal visit, whilst having negligible impact on transmission from recently-infected mothers. As a result, the proportion of vertical transmission from recently- infected mothers has increased since 2002, rising to 26% (95% CI: 22-30%) in 2008 (15 000 out of 57 000 infections). The proportion is projected to increase to 34% (95% CI: 29-39%) by 2014 (13 000 out of 39 000 infections), in the absence of specific interventions to prevent transmission from recently-infected mothers.
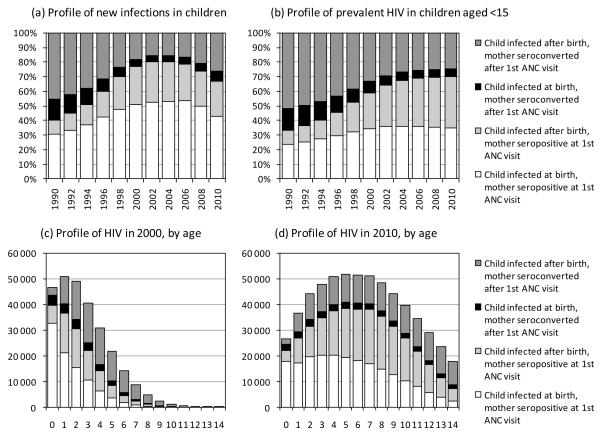
At all stages in the epidemic, most of the vertical transmission from mothers who have acquired HIV after their first antenatal visit is postnatal transmission (Figure 3a), as there is more opportunity for maternal seroconversion during the long breastfeeding period than during the relatively short period between first antenatal visit and delivery. This is in contrast to transmission from mothers who are HIV-positive at their first antenatal visit, which is predominantly perinatal. Because postnatally-infected children are assumed to survive for longer than perinatally-infected children, they account for a greater proportion of prevalent HIV (Figure 3b) than incident HIV (Figure 3a). The proportion of prevalent HIV in children that is attributable to transmission from recently-infected mothers was 30% in 2010 (95% CI: 26-35%). This proportion varies substantially by age, from 18% (95% CI: 13-22%) in infants to 58% (95% CI: 52-65%) in 14-year olds, in 2010 (Figure 3d). In 2000, when the epidemic was less mature, fewer HIV infections occurred in older children, but age-specific proportions of paediatric HIV acquired from recently-infected mothers were higher (Figure 3c).
Figure 3.
Profile of HIV infections in children, according to timing of maternal HIV acquisition and timing of vertical transmission
All proportions and numbers are posterior averages (95% confidence intervals not shown).
Reducing HIV incidence in pregnant and breastfeeding women by 50% would reduce the number of new HIV infections in children, over the 2010-2015 period, by 16.2% (95% CI: 13.9-18.6%). Repeating the offer of HIV testing in late pregnancy would reduce the number of new HIV infections in children by 11.2% (95% CI: 9.5-12.7%), and conducting HIV screening in mothers and infants at 6-week immunization visits would reduce new infections in children by 3.5% (95% CI: 2.7-4.4%). However, the numbers of HIV infections averted per 1000 HIV tests performed in late pregnancy (7.2, 95% CI: 6.4-8.1) and at immunization clinics (1.9, 95% CI: 1.4-2.6) are substantially lower than the number of HIV infections averted per test performed at the first antenatal visit (62.5, 95% CI: 55.7-70.7), and additional screening after the first antenatal visit would therefore be less cost-effective. If all three interventions were introduced together, the average annual number of new paediatric HIV infections over the 2010-2015 period would reduce from 38 000 to 27 000, a reduction of 28% (95% CI: 25-31%). Combined with existing PMTCT interventions, this would represent a reduction in the annual number of new HIV infections of 72% (95% CI: 68-74%) in 2014, relative to what would be expected in the absence of PMTCT.
Discussion
These results suggest that mothers who experience HIV seroconversion during pregnancy and breastfeeding contribute substantially to vertical transmission of HIV. The relative significance of this transmission depends on three factors. Firstly, the contribution is substantially higher during early-stage HIV epidemics, in which HIV incidence rates are high and HIV prevalence rates are still relatively low; the contribution can be expected to decline as antenatal HIV prevalence rises and adult HIV incidence starts to decline. Secondly, the relative contribution is higher the greater the extent of PMTCT programmes, which have been directed almost exclusively at women who are seropositive at their first antenatal visit. Thirdly, the model simulations suggest that the contribution to postnatal transmission is substantially greater than the contribution to perinatal transmission, and the significance of transmission from mothers who seroconvert after their antenatal screening visit is therefore likely to depend on the average duration of breast feeding. The median duration of lactation in South Africa is shorter than in most other African countries,64 and the proportion of vertical transmission from mothers who are recently infected could therefore be higher in other African countries. However, access to PMTCT in other African countries is generally more limited than in South Africa,64 which would imply a lower proportion of vertical transmission from mothers who seroconvert during pregnancy and breastfeeding.
This analysis suggests that “virtual elimination” of mother-to-child transmission of HIV is likely to be challenging, even with optimistic assumptions about the future introduction of interventions to prevent transmission from pregnant women who are initially seronegative. Repeated HIV screening in late pregnancy would have some effect on the identification of recently-infected women, but most of the modelled benefit of this strategy is due to the diagnosis of chronically-infected women who previously refused testing (see section 4 of Supplemental Digital Content 1). The benefits of HIV screening at immunization clinics, if it were introduced in South Africa, would probably be modest, amounting to a less than 5% reduction in current paediatric HIV incidence. The most effective way to limit transmission from seroconverting mothers is to prevent them from acquiring HIV in the first place, but there is uncertainty regarding the ideal means to achieve this. HIV counselling and testing has generally had negligible impact on behaviour in individuals who test HIV-negative,65, 66 and it is therefore debatable whether condom promotion to HIV-negative pregnant women would substantially reduce HIV incidence. HIV counselling and testing of male partners has been suggested as one strategy for limiting maternal HIV incidence,67 but African studies that have attempted to integrate male partners into antenatal HIV testing have typically managed to test only 10-20% of partners.68-70 Promising recent evidence71-73 suggests that tenofovir-based products could provide protection against maternal HIV acquisition, but there is currently a lack of evidence regarding the safety of tenofovir use during pregnancy and lactation.74
A limitation of this analysis is that maternal HIV incidence rates have been estimated from the ASSA2003 model, which calculates HIV incidence in pregnant women as a weighted average of age-specific female incidence rates, where weights are numbers of births to HIV- negative women at each age. This does not allow for the possibility that pregnant women may be biologically or behaviourally different from other women at the same age. Some evidence suggests that women may experience heightened susceptibility to HIV during pregnancy75-77 and during the early postpartum period,78, 79 though other studies have not confirmed this.80 If the actual maternal HIV incidence rates during pregnancy and breastfeeding are higher than estimated by the ASSA2003 model, our model is likely to under-estimate the extent of vertical transmission from mothers who seroconvert during pregnancy and breastfeeding. However, it is also possible that the ASSA2003 model may under-estimate the extent of recent reductions in maternal HIV incidence, due to inadequate allowance for recent increases in condom usage and HIV testing.81 In a sensitivity analysis to explore the effect of either halving or doubling the rate of maternal HIV incidence in 2008, the proportion of mother-to-child transmission from recently-infected mothers changed to 15.4% (95% CI: 13.0-17.9%) and 39.8% (95% CI: 35.2-44.8%) respectively (see section 6 of Supplemental Digital Content 1).
A more general limitation is the lack of information regarding several key MTCT parameters. It is possible that feeding practices of HIV-negative and undiagnosed HIV-positive mothers may have changed since the time of the 1998 DHS, but there is little evidence to show this.61,82 There is also little information on feeding practices in women who have been diagnosed HIV-positive and counselled on infant feeding. Since the introduction of the new PMTCT guidelines in 2008 and 2010, there has been little data on the extent to which the changes recommended in these guidelines have been implemented, and the model projections beyond 2008 therefore need to be treated with caution. A recent South African survey found that perinatal HIV transmission rates by 6 weeks were reduced to 3.5% by 2010,83 and this implies a higher level of PMTCT coverage than assumed in our model. Since higher PMTCT coverage implies proportionately greater contributions to vertical transmission from recently- infected mothers, our model may therefore under-estimate the proportion of vertical transmission in 2010 that was from recently-infected mothers. There is also uncertainty regarding the extent to which HIV prevalence data and PMTCT data collected from women attending public antenatal clinics can be generalized to the whole population of pregnant women, some of whom seek private antenatal care or do not seek antenatal care at all. However, the proportion of pregnant women who access public sector antenatal care in South Africa is around 82%,16 and any bias due to the exclusion of other women is therefore likely to be small.
As efforts to eliminate vertical transmission intensify, it will become increasingly important to focus on transmission from recently-infected mothers. Projections that do not take this transmission into account are likely to understate paediatric HIV incidence and prevalence substantially, with implications for the estimation of paediatric ART need and AIDS mortality. Policymakers will need to seek creative solutions to this problem and will need to move towards greater integration of adult and paediatric HIV prevention if this challenge is to be addressed effectively.
Supplementary Material
Acknowledgements
Leigh Johnson thanks the South African Medical Research Council and the William and Flora Hewlett Foundation for funding.
Footnotes
Funded in part by the South African Medical Research Council and the William and Flora Hewlett Foundation; no conclicts to declare
List of supplemental digital content
‘Suppl material.doc’
Description: Assumptions regarding mother-to-child transmission of HIV and paediatric HIV survival, additional results and sensitivity analyses
Presented at the 5th South African AIDS Conference, Durban, South Africa, 7-10 June 2011.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sperling RS, Shapiro DE, Coombs RW, et al. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. N Engl J Med. 1996;335:1621–9. doi: 10.1056/NEJM199611283352201. [DOI] [PubMed] [Google Scholar]
- 2.Farquhar C, Mbori-Ngacha D, Overbaugh J, et al. Illness during pregnancy and bacterial vaginosis are associated with in-utero HIV-1 transmission. AIDS. 2010;24:153–5. doi: 10.1097/QAD.0b013e32832326d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taha TE, Kumwenda NI, Hoover DR, et al. Nevirapine and zidovudine at birth to reduce perinatal transmission of HIV in an African setting: a randomized controlled trial. JAMA. 2004;292:202–9. doi: 10.1001/jama.292.2.202. [DOI] [PubMed] [Google Scholar]
- 4.Rousseau CM, Nduati RW, Richardson BA, et al. Association of levels of HIV-1- infected breast milk cells and risk of mother-to-child transmission. J Infect Dis. 2004;190:1880–8. doi: 10.1086/425076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pillay K, Coutsoudis A, York D, et al. Cell-free virus in breast milk of HIV-1-seropositive women. J Acquir Immun Defic Syndr. 2000;24:330–6. doi: 10.1097/00126334-200008010-00006. [DOI] [PubMed] [Google Scholar]
- 6.Barin F, Jourdain G, Brunet S, et al. Revisiting the role of neutralizing antibodies in mother-to-child transmission of HIV-1. J Infect Dis. 2006;193:1504–11. doi: 10.1086/503778. [DOI] [PubMed] [Google Scholar]
- 7.Van de Perre P, Simonon A, Hitimana DG, et al. Infective and anti-infective properties of breastmilk from HIV-1-infected women. Lancet. 1993;341:914–8. doi: 10.1016/0140-6736(93)91210-d. [DOI] [PubMed] [Google Scholar]
- 8.Koopman JS, Jacquez JA, Welch GW, et al. The role of early HIV infection in the spread of HIV through populations. J Acquir Immun Defic Syndr. 1997;14:249–258. doi: 10.1097/00042560-199703010-00009. [DOI] [PubMed] [Google Scholar]
- 9.Hyman JM, Li J, Stanley EA. The differential infectivity and staged progression models for the transmission of HIV. Math Biosci. 1999;155:77–109. doi: 10.1016/s0025-5564(98)10057-3. [DOI] [PubMed] [Google Scholar]
- 10.Abu-Raddad LJ, Longini IM., Jr. No HIV stage is dominant in driving the HIV epidemic in sub-Saharan Africa. AIDS. 2008;22:1055–61. doi: 10.1097/QAD.0b013e3282f8af84. [DOI] [PubMed] [Google Scholar]
- 11.Dube S, Boily MC, Mugurungi O, et al. Estimating vertically acquired HIV infections and the impact of the prevention of mother-to-child transmission program in Zimbabwe: insights from decision analysis models. J Acquir Immun Defic Syndr. 2008;48:72–81. doi: 10.1097/QAI.0b013e31816bcdbb. [DOI] [PubMed] [Google Scholar]
- 12.Lu L, Legwaila K, Motswere C, et al. HIV incidence in pregnancy and the first post-partum year and implications for PMTCT programs, Francistown, Botswana, 2008 [Abstract 91]; Presented at: 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 8-11 Feb 2009. [Google Scholar]
- 13.Soorapanth S, Sansom S, Bulterys M, et al. Cost-effectiveness of HIV rescreening during late pregnancy to prevent mother-to-child HIV transmission in South Africa and other resource-limited settings. J Acquir Immun Defic Syndr. 2006;42:213–21. doi: 10.1097/01.qai.0000214812.72916.bc. [DOI] [PubMed] [Google Scholar]
- 14.Dorrington RE, Johnson LF, Bradshaw D, et al. The Demographic Impact of HIV/AIDS in South Africa. National and Provincial Indicators for 2006. Cape Town; Centre for Actuarial Research, South African Medical Research Council and Actuarial Society of South Africa; 2006; Available at: http://www.commerce.uct.ac.za/care. [Google Scholar]
- 15.Johnson LF, Dorrington RE. Modelling the demographic impact of HIV/AIDS in South Africa and the likely impact of interventions. Demographic Res. 2006;14:541–74. [Google Scholar]
- 16.Department of Health South Africa Demographic and Health Survey 1998. Full Report. 1999 [Google Scholar]
- 17.Moodley D, Esterhuizen TM, Pather T, et al. High HIV incidence during pregnancy: compelling reason for repeat HIV testing. AIDS. 2009;23:1255–9. doi: 10.1097/QAD.0b013e32832a5934. [DOI] [PubMed] [Google Scholar]
- 18.Jackson DJ, Chopra M, Doherty TM, et al. Operational effectiveness and 36 week HIV-free survival in the South African programme to prevent mother-to-child transmission of HIV-1. AIDS. 2007;21:509–516. doi: 10.1097/QAD.0b013e32801424d2. [DOI] [PubMed] [Google Scholar]
- 19.Lindbäck S, Thorstensson R, Karlsson A, et al. Diagnosis of primary HIV-1 infection and duration of follow-up after HIV exposure. AIDS. 2000;14:2333–2339. doi: 10.1097/00002030-200010200-00014. [DOI] [PubMed] [Google Scholar]
- 20.Rollins N, Little K, Mzolo S, et al. Surveillance of mother-to-child transmission prevention programmes at immunization clinics: the case for universal screening. AIDS. 2007;21:1341–7. doi: 10.1097/QAD.0b013e32814db7d4. [DOI] [PubMed] [Google Scholar]
- 21.Rollins N, Mzolo S, Moodley T, et al. Universal HIV testing of infants at immunization clinics: an acceptable and feasible approach for early infant diagnosis in high HIV prevalence settings. AIDS. 2009;23:1851–7. doi: 10.1097/QAD.0b013e32832d84fd. [DOI] [PubMed] [Google Scholar]
- 22.Tovo PA, Palomba E, Gabiano C, et al. Human immunodeficiency virus type 1 (HIV- 1) seroconversion during pregnancy does not increase the risk of perinatal transmission. Br J Obstet Gynaecol. 1991;98:940–2. doi: 10.1111/j.1471-0528.1991.tb13520.x. [DOI] [PubMed] [Google Scholar]
- 23.Rudin C, Lauper U, Biedermann K. HIV seroconversion during pregnancy [Abstract W.C.3247]; Presented at: 7th International AIDS Conference; Florence, Italy. 16-21 June 1991. [Google Scholar]
- 24.Hague RA, Mok JY, Johnstone FD, et al. Maternal factors in HIV transmission. Int J STD AIDS. 1993;4:142–6. doi: 10.1177/095646249300400304. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen-Saines K, Melo M, Varella I, et al. Primary HIV-1 infection during pregnancy: high rate of HIV-1 MTCT in a cohort of patients in southern Brazil. Retrovirology. 2008;5(Suppl 1):O1. [Google Scholar]
- 26.Roongpisuthipong A, Siriwasin W, Simonds RJ, et al. HIV seroconversion during pregnancy and risk for mother-to-infant transmission. J Acquir Immun Defic Syndr. 2001;26:348–51. doi: 10.1097/00126334-200104010-00011. [DOI] [PubMed] [Google Scholar]
- 27.Colebunders R, Kapita B, Nekwei W, et al. Breastfeeding and transmission of HIV. Lancet. 1988;2:1487. doi: 10.1016/s0140-6736(88)90957-9. [DOI] [PubMed] [Google Scholar]
- 28.Palasanthiran P, Ziegler JB, Stewart GJ, et al. Breast-feeding during primary maternal human immunodeficiency virus infection and risk of transmission from mother to infant. J Infect Dis. 1993;167:441–4. doi: 10.1093/infdis/167.2.441. [DOI] [PubMed] [Google Scholar]
- 29.Hira SK, Mangrola UG, Mwale C, et al. Apparent vertical transmission of human immunodeficiency virus type 1 by breast-feeding in Zambia. J Pediatr. 1990;117:421–4. doi: 10.1016/s0022-3476(05)81084-4. [DOI] [PubMed] [Google Scholar]
- 30.Van de Perre P, Simonon A, Msellati P, et al. Postnatal transmission of human immunodeficiency virus type 1 from mother to infant. N Engl J Med. 1991;325:593–8. doi: 10.1056/NEJM199108293250901. [DOI] [PubMed] [Google Scholar]
- 31.Embree JE, Njenga S, Datta P, et al. Risk factors for postnatal mother-child transmission of HIV-1. AIDS. 2000;14:2535–41. doi: 10.1097/00002030-200011100-00016. [DOI] [PubMed] [Google Scholar]
- 32.Liang K, Gui X, Zhang YZ, et al. A case series of 104 women infected with HIV-1 via blood transfusion postnatally: high rate of HIV-1 transmission to infants through breast- feeding. J Infect Dis. 2009;200:682–6. doi: 10.1086/605123. [DOI] [PubMed] [Google Scholar]
- 33.Humphrey JH, Marinda E, Mutasa K, et al. Mother to child transmission of HIV among Zimbabwean women who seroconverted postnatally: prospective cohort study. BMJ. 2010;341:c6580. doi: 10.1136/bmj.c6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bland RM, Rollins NC, Coutsoudis A, et al. Breastfeeding practices in an area of high HIV prevalence in rural South Africa. Acta Paediatr. 2002;91:704–11. [Google Scholar]
- 35.Department of Health Clinical Guidelines: PMTCT (Prevention of Mother-to-Child Transmission) 2010 Available at: http://www.doh.gov.za/docs/facts-f.html.
- 36.Department of Health Policy and Guidelines for the Implementation of the PMTCT programme. 2008 Available at: http://www.doh.gov.za/docs/index.html.
- 37.Moodley D, Moodley J, Coovadia H, et al. A multicenter randomized controlled trial of nevirapine versus a combination of zidovudine and lamivudine to reduce intrapartum and early postpartum mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 2003;187:725–35. doi: 10.1086/367898. [DOI] [PubMed] [Google Scholar]
- 38.Doherty T, Besser M, Donohue S, et al. An Evaluation of the Prevention of Mother- to-child Transmission (PMTCT) of HIV Initiative in South Africa: Lessons and Key Recommendations. Health Systems Trust; Durban: 2003. Available at: http://www.hst.org.za/sites/default/files/pmtct_national.pdf. [Google Scholar]
- 39.Coutsoudis A, Pillay K, Kuhn L, et al. Method of feeding and transmission of HIV-1 from mothers to children by 15 months of age: prospective cohort study from Durban, South Africa. AIDS. 2001;15:379–87. doi: 10.1097/00002030-200102160-00011. [DOI] [PubMed] [Google Scholar]
- 40.Goga AE, Van Wyk B, Doherty T, et al. Operational effectiveness of guidelines on complete breast-feeding cessation to reduce mother-to-child transmission of HIV: results from a prospective observational cohort study at routine prevention of mother-to-child transmission sites, South Africa. J Acquir Immun Defic Syndr. 2009;50:521–8. doi: 10.1097/qai.0b013e3181990620. [DOI] [PubMed] [Google Scholar]
- 41.Doherty T, Chopra M, Jackson D, et al. Effectiveness of the WHO/UNICEF guidelines on infant feeding for HIV-positive women: results from a prospective cohort study in South Africa. AIDS. 2007;21:1791–7. doi: 10.1097/QAD.0b013e32827b1462. [DOI] [PubMed] [Google Scholar]
- 42.Johnson LF. A model of paediatric HIV in South Africa. Centre for Infectious Disease Epidemiology and Research; University of Cape Town: 2010. Available at: http://webdav.uct.ac.za/depts/epi/publications/documents/Paediatric_HIV_modelling5.pdf. [Google Scholar]
- 43.Stringer EM, Ekouevi DK, Coetzee D, et al. Coverage of nevirapine-based services to prevent mother-to-child HIV transmission in 4 African countries. JAMA. 2010;304:293–302. doi: 10.1001/jama.2010.990. [DOI] [PubMed] [Google Scholar]
- 44.Sherman GG, Jones SA, Coovadia AH, et al. PMTCT from research to reality - results from a routine service. S Afr Med J. 2004;94:289–92. [PubMed] [Google Scholar]
- 45.Nkonki LL, Doherty TM, Hill Z, et al. Missed opportunities for participation in prevention of mother to child transmission programmes: simplicity of nevirapine does not necessarily lead to optimal uptake, a qualitative study. AIDS Res Ther. 2007;4:27. doi: 10.1186/1742-6405-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leroy V, Sakarovitch C, Cortina-Borja M, et al. Is there a difference in the efficacy of peripartum antiretroviral regimens in reducing mother-to-child transmission of HIV in Africa? AIDS. 2005;19:1865–75. doi: 10.1097/01.aids.0000188423.02786.55. [DOI] [PubMed] [Google Scholar]
- 47.Dabis F, Bequet L, Ekouevi DK, et al. Field efficacy of zidovudine, lamivudine and single-dose nevirapine to prevent peripartum HIV transmission. AIDS. 2005;19:309–18. [PMC free article] [PubMed] [Google Scholar]
- 48.Lallemant M, Jourdain G, Le Coeur S, et al. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med. 2004;351:217–28. doi: 10.1056/NEJMoa033500. [DOI] [PubMed] [Google Scholar]
- 49.Tonwe-Gold B, Ekouevi DK, Viho I, et al. Antiretroviral treatment and prevention of peripartum and postnatal HIV transmission in West Africa: evaluation of a two-tiered approach. PLoS Med. 2007;4:e257. doi: 10.1371/journal.pmed.0040257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peltier CA, Ndayisaba GF, Lepage P, et al. Breastfeeding with maternal antiretroviral therapy or formula feeding to prevent HIV postnatal mother-to-child transmission in Rwanda. AIDS. 2009;23:2415–23. doi: 10.1097/QAD.0b013e32832ec20d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palombi L, Marazzi MC, Voetberg A, et al. Treatment acceleration program and the experience of the DREAM program in prevention of mother-to-child transmission of HIV. AIDS. 2007;21(Suppl 4):S65–71. doi: 10.1097/01.aids.0000279708.09180.f5. [DOI] [PubMed] [Google Scholar]
- 52.Hoffman RM, Black V, Technau K, et al. Effects of highly active antiretroviral therapy duration and regimen on risk for mother-to-child transmission of HIV in Johannesburg, South Africa. J Acquir Immun Defic Syndr. 2010;54:35–41. doi: 10.1097/QAI.0b013e3181cf9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breastfeeding and HIV International Transmission Study Group Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154–2166. doi: 10.1086/420834. [DOI] [PubMed] [Google Scholar]
- 54.Coovadia HM, Rollins NC, Bland RM, et al. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet. 2007;369:1107–16. doi: 10.1016/S0140-6736(07)60283-9. [DOI] [PubMed] [Google Scholar]
- 55.Becquet R, Bland R, Leroy V, et al. Duration, pattern of breastfeeding and postnatal transmission of HIV: pooled analysis of individual data from West and South African cohorts. PLoS One. 2009;4:e7397. doi: 10.1371/journal.pone.0007397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumwenda NI, Hoover DR, Mofenson LM, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359:119–29. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 57.Chasela CS, Hudgens MG, Jamieson DJ, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–81. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Six Week Extended-dose Nevirapine Study Team Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet. 2008;372:300–13. doi: 10.1016/S0140-6736(08)61114-9. [DOI] [PubMed] [Google Scholar]
- 59.Taha TE, Kumwenda J, Cole SR, et al. Postnatal HIV-1 transmission after cessation of infant extended antiretroviral prophylaxis and effect of maternal highly active antiretroviral therapy. J Infect Dis. 2009;200:1490–7. doi: 10.1086/644598. [DOI] [PubMed] [Google Scholar]
- 60.Shisana O, Rehle T, Simbayi LC, et al. South African National HIV Prevalence, HIV Incidence, Behaviours and Communication Survey, 2005. HSRC Press; Cape Town: 2005. Available at: http://www.hsrcpress.ac.za. [Google Scholar]
- 61.Shisana O, Simbayi LC, Rehle T, et al. South African National HIV Prevalence, Incidence, Behaviour and Communication Survey, 2008. The Health of Our Children. 2010 Available at: http://www.hsrc.ac.za/Research_Publication-21767.phtml.
- 62.Raftery AE, Bao L. Estimating and projecting trends in HIV/AIDS generalized epidemics using Incremental Mixture Importance Sampling. Biometrics. 2010;66:1162–73. doi: 10.1111/j.1541-0420.2010.01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Department of Health HIV and AIDS and STI Strategic Plan for South Africa, 2007-2011. 2007 Available at: http://www.doh.gov.za/docs/misc/stratplan-f.html.
- 64.Mahy M, Stover J, Kiragu K, et al. What will it take to achieve virtual elimination of mother-to-child transmission of HIV? An assessment of current progress and future needs. Sex Transm Infect. 2010;86(Suppl 2):ii48–55. doi: 10.1136/sti.2010.045989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weinhardt LS, Carey MP, Johnson BT, et al. Effects of HIV counseling and testing on sexual risk behavior: a meta-analytic review of published research, 1985-1997. Am J Public Health. 1999;89:1397–405. doi: 10.2105/ajph.89.9.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Denison JA, O’Reilly KR, Schmid GP, et al. HIV voluntary counseling and testing and behavioral risk reduction in developing countries: a meta-analysis, 1990-2005. AIDS Behav. 2008;12:363–73. doi: 10.1007/s10461-007-9349-x. [DOI] [PubMed] [Google Scholar]
- 67.Musiime V, Ssali F, Kizito H, et al. Need for review of prevention of mother-to-child transmission practice especially in discordant couples: a case of mother-to-child transmission of HIV during breast feeding by a mother who tested HIV negative antenatally. AIDS. 2007;21:1658–9. doi: 10.1097/QAD.0b013e32826fb7d8. [DOI] [PubMed] [Google Scholar]
- 68.Aluisio A, Richardson BA, Bosire R, et al. Male antenatal attendance and HIV testing are associated with decreased infant HIV infection and increased HIV-free survival. J Acquir Immun Defic Syndr. 2011;56:76–82. doi: 10.1097/QAI.0b013e3181fdb4c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Msuya SE, Mbizvo EM, Hussain A, et al. Low male partner participation in antenatal HIV counselling and testing in northern Tanzania: implications for preventive programs. AIDS Care. 2008;20:700–9. doi: 10.1080/09540120701687059. [DOI] [PubMed] [Google Scholar]
- 70.Katz DA, Kiarie JN, John-Stewart GC, et al. Male perspectives on incorporating men into antenatal HIV counseling and testing. PLoS One. 2009;4:e7602. doi: 10.1371/journal.pone.0007602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baeten J, Celum C. Antiretroviral pre-exposure prophylaxis for HIV-1 prevention among heterosexual African men and women: the Partners PrEP Study [Abstract MOAX0106]; Presented at: 6th International AIDS Society Conference; Rome, Italy. 17-20 July 2011. [Google Scholar]
- 73.Thigpen MC, Kebaabetswe PM, Smith DK, et al. Daily oral antiretroviral use for the prevention of HIV infection in heterosexually active young adults in Botswana: results from the TDF2 study [Abstract WELBC01]; Presented at: 6th International AIDS Society Conference; Rome, Italy. 17-20 July 2011. [Google Scholar]
- 74.Foster C, Lyall H, Olmscheid B, et al. Tenofovir disoproxil fumarate in pregnancy and prevention of mother-to-child transmission of HIV-1: is it time to move on from zidovudine? HIV Med. 2009;10:397–406. doi: 10.1111/j.1468-1293.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- 75.Gray RH, Li X, Kigozi G, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366:1182–1188. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- 76.Taha TE, Hoover DR, Dallabetta GA, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS. 1998;12:1699–706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 77.Mugo NR, Heffron R, Donnell D, et al. Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS. 2011;25:1887–95. doi: 10.1097/QAD.0b013e32834a9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leroy V, Van de Perre P, Lepage P, et al. Seroincidence of HIV-1 infection in African women of reproductive age: a prospective cohort study in Kigali, Rwanda, 1988-1992. AIDS. 1994;8:983–6. doi: 10.1097/00002030-199407000-00017. [DOI] [PubMed] [Google Scholar]
- 79.Munjoma MW, Mhlanga FG, Mapingure MP, et al. The incidence of HIV among women recruited during late pregnancy and followed up for six years after childbirth in Zimbabwe. BMC Public Health. 2010;10:668. doi: 10.1186/1471-2458-10-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morrison CS, Wang J, Van Der Pol B, et al. Pregnancy and the risk of HIV-1 acquisition among women in Uganda and Zimbabwe. AIDS. 2007;21:1027–34. doi: 10.1097/QAD.0b013e3280f00fc4. [DOI] [PubMed] [Google Scholar]
- 81.Rehle TM, Hallett TB, Shisana O, et al. A decline in new HIV infections in South Africa: estimating HIV incidence from three national HIV surveys in 2002, 2005 and 2008. PLoS One. 2010;5:e11094. doi: 10.1371/journal.pone.0011094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Department of Health . South Africa Demographic and Health Survey 2003: Preliminary Report. Pretoria: 2004. Available at: http://www.doh.gov.za/docs/ [Google Scholar]
- 83.Goga A, Lombard C, Dinh T, et al. Impact of the national prevention of mother-to-child transmission (PMTCT) programme on mother-to-child transmission of HIV (MTCT), South Africa, 2011 [Abstract 675]; Presented at: 5th South African AIDS Conference; Durban, South Africa. 7-10 June 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.