Abstract
Persisting infections are often associated with chronic T cell activation. For certain pathogens, this can lead to T cell exhaustion and survival of what is otherwise a cleared infection. In contrast, for herpesviruses, T cells never eliminate infection once it is established. Instead, effective immunity appears to maintain these pathogens in a state of latency. We used infection with herpes simplex virus (HSV) to examine whether effector-type T cells undergoing chronic stimulation retained functional and proliferative capacity during latency and subsequent reactivation. We found that latency-associated T cells exhibited a polyfunctional phenotype and could secrete a range of effector cytokines. These T cells were also capable of mounting a recall proliferative response on HSV reactivation and could do so repeatedly. Thus, for this latent infection, T cells subjected to chronic antigen stimulation and periodic reactivation retain the ability to respond to local virus challenge.
Introduction
Certain viruses persist as a direct consequence of their ability to inactivate T cells that would otherwise result in their elimination. These include persisting infections by pathogens such as lymphocytic choriomeningitis virus (LCMV) (1, 2)and hepatitis C virus (HCV) (3, 4), both of which can be cleared by an effective T cell response (5, 6). For this class of pathogen, it is thought that chronic or ongoing T cell stimulation is an essential contributor to the inactivation required for virus persistence and ongoing viremia(7, 8). This inactivation takes the form of T cell exhaustion and senescence, where the cells progressively lose various properties associated with full effector function (9). In contrast, other viruses, especially those belonging to the herpesvirus family, do not require compromised immunity for their prolonged survival. Once established, these viruses are never cleared from the infected individual even in the face of effective T cell immunity; instead, they survive by employing various immune evasion strategies (10). In addition, many of these pathogens persist in a state of latency, which is characterised by limited transcriptional activity and little if any virus replication (11). Importantly, effective immunity is actually crucial for maintenance of latency, since for a range of herpesviruses, reactivation from latency is often a major complication associated with generalised immunosuppression (12, 13).
In certain instances, active suppression of herpesvirus reactivation involves direct T cell recognition of persistently infected cells (14). The chronically stimulated T cells involved in virus control are unlikely to be compromised by such events, since their inactivation would result in disease recrudescence. However, while recent reports suggest that T cells associated with latent infection are indeed functional (15-17), there has been no formal demonstration that the cells actually involved in suppressing reactivation are not exhausted by the recognition event or inactivated by the reactivation process. Here, we show that for persisting herpes simplex virus (HSV) infection, T cells involved in latency control remain fully functional and maintain a self-renewing ability despite undergoing chronic stimulation during the latent phase of infection.
Materials and Methods
Mice
C57BL/6 and gBT × B6.CD45.1 (gBT-I.CD45.1) were bred in the Department of Microbiology and Immunology in The University of Melbourne (Melbourne, Australia). The gBT.CD45.1 TCR and gzmBCreERT2/ROSA26EYFP transgenic mice have been described previously (18, 19). gzmBCreERT2/ROSA26EYFP mice received daily injections of tamoxifen (1mg) via i.p. injection as described (18).
Viral infections, ganglia transplantations and adoptive transfer of transgenic CD8+ T cells
Viruses used were the KOS strain of HSV-1 (HSV) and K.L8A with a position 8 alanine mutation in the gB498-505immunodominant determinant derived by recombining HSV.gB-L8A (20) with KOS strain of HSV and selecting for a recombinant that gave equivalent replication and lesions as found with the wild-type virus. Mice were infected with 1×106 PFU of virus via flank scarification as previously described (21). Latently infected ganglia (T8-T12) were transplanted under the kidney capsule of syngeneic recipients as described (17). C57BL/6 mice received 5×104 naïve gBT-I.CD45.1 lymph node cells via intravenous injection.For LCMV infections, mice were infected with 2 × 106 PFU LCMV clone 13 by intravenousinjection.
Flow cytometry, mAbs, BrdU staining
T cells were recovered from skin, ganglia and ganglia grafts as described (17, 22). The following fluorescently-conjugated antibodies were from BD Pharmingen: anti-CD45.1 (A20), Vα2 (B20.1), anti-CD8α (53-6.7), anti-IFNγ, anti-TNFα, anti-IL-2 and anti-CD107a/b. Anti-Tim-3, anti-Lag3, and anti-CD160 were purchased from eBioscience. Anti-Granzyme B was from Invitrogen and anti-PD-1 was from Biolegend. H-2KbgB(498-505)-phycoerythrin tetramer was generated at the Department of Microbiology and Immunology in The University of Melbourne. For analysis of T cell proliferation, 1.25 mg BrdU was injected i.p. as two injections 12 h apart and mice were analyzed 12 h later. Uptake was detected with a BrdU Flow Kit according to the manufacturers instructions (BD Pharmingen).
Intracellular assays with peptide stimulation
Lytic granule release and cytokine production by gBT-I.CD45.1 T cells was measured by stimulating lymphocytes from pooled ganglia (T8-12 from 2 mice) with 0.1μM gB498-505 peptide in the presence of BrefeldinA, monensin and CD107a/b antibodies. Cells were cultured for 5 h before staining for surface markers. Cells were then fixed using a Cytofix/Cytoperm kit (BD Pharmingen) and stained with intracellular antibodies before analysis by flow cytometry.
Results
HSV-specific T cells in sensory ganglia harbouring latent infection express granzyme B as a consequence of ongoing antigen recognition
Herpes simplex virus (HSV) forms a prototypic latent infection confined to a localised compartment, the sensory ganglia that innervate the site of initial infection, usually skin or mucosal tissue (23). It is known that latent infection is maintained under tight immune control, partly as a resultof the action of CD8+ T cells and their secretion of granzyme B and interferon-γ(IFN- γ) (14, 24, 25). As a consequence, inhibition of virus reactivation likely requires ongoing local T cell stimulation by the persistently infected neurons. To show this is the case, we transferred HSV-specific T cells from the TCR-transgenic mouse, gBT-I, into C57BL/6 mice prior to subjecting the animals to skin infection and examining the T cells in skin and ganglia at the end of the lytic (day 8) and during the latent (after day 20) phase of infection. Figure 1A shows that gBT-I cells in skin, a tissue that clears HSV at the cessation of lytic infection, express low levels of granzyme B during latency. In contrast, those T cells in ganglia harbouring latent HSV infection continued to express granzyme B during the latent phase of infection.
Figure 1. Granzyme B expression by HSV-specific CD8+ T cells in the ganglia is antigen dependent.
Mice were seeded with naïve gBT-I.CD45.1 CD8+ T cells and flank infected with HSV. (A) Granzyme B expression was assessed by flow cytometry on gBT-I.CD45.1 cells isolated from the infected skin flank (left panel) or ganglia (right panel) during the lytic (day 8; black line) or latent (day 30; grey filled line) phases of infection. (B) Mice were seeded with 5 × 104naïve gBT-I T cells (left panel) orwith 5 × 104 memory gBT-I T cells isolated from animals infected with wild-type HSV >30d earlier (right panel). Mice were infected with HSV on the left flank and the HSV gB-mutant virus K.L8A on the right flank (HSV + K.L8A), or either virus alone on a single flank. Shown are numbers of gBT-I cells in the spleen 8-9 days post virus challenge. (C) Mice were infected with HSV on the left flank and K.L8A on the right flank. The number of gBT-I.CD45.1 cells in the ganglia was enumerated by flow cytometry at 20 days (left panel) or 35 days (right panel) following infection.Control (ctrl) was T cells harvested from non-innervating ganglia (T2-6) from K.L8A infected flanks (N.D. at day 20 post infection).Bars represent the mean + SEM with 5-6 mice per group. Statistical analysis was performed using an unpaired Student’s ttest. (D) Representative FACS plots showing granzyme expression by gBT-I.CD45.1 cells in pooled HSV-infected (black line) or K.L8A-infected ganglia (grey filled line)20 days following infection. Data are representative of 5-6 mice per group and 3 independent experiments.
It has recently been shown that granzyme B can be upregulated in a non-specific manner by virus infection (26). To demonstrate that the presence of local infection was not a major driver of non-specific granzyme B expression within the ganglia, we made use of a recombinant virus (K.L8A) that carries a mutation within the immunodominant determinant from the glycoprotein B (gB). The mutant virus did not stimulate HSV-specific gBT-I T cells, which otherwise proliferated and expanded after adoptive transfer and flank skin infection with wild-type HSV (Fig. 1B, left panel). The failure of gBT-I T cells to recognize mutant virus was not limited to just the priming phase of the response and memory gBT-I cells transferred into naïve hosts were also unable to respond following challenge by the mutant virus (Fig. 1B, right panel). Note that both wild-type and K.L8A viruses replicate with equal efficiency within the sensory ganglia and exhibit similar levels of skin disease and peak virus load (Fig. S1).
In mice simultaneously infected with wild-type and mutant virus on opposing flanks, responding gBT-I transgenic T cell populations lodged and then persisted in sensory ganglia infected with either virus (Fig. 1C). Ganglionic gBT-I cell numbers were largely equivalent in wild-type HSVand K.L8A infected ganglia in early latency and divergedas latency progressed,although even at day 35post infection the latter still outnumbered those found in control non-infected ganglia from the same animals.Importantly, activation of gBT-I cells, as measured by granzyme B upregulation, was dependent on specific recognition, with high levels of this effector molecule only found in sensory ganglia harbouring wild-type virus infection (Fig. 1D). Thus, since gB-specific T cells have been implicated in latency control (14), these results argue that granzyme B expression by T cells within sensory ganglia was a marker for recognition of cells persistently infected with HSV.
HSV-specific CD8+ T cells undergoing chronic stimulation during latency show a polyfunctional phenotype and limited expression of inhibitory receptors
Given that the T cell stimulation within the latently infected ganglia is ongoing, and chronic stimulation has been associated with T cell inactivation (1, 2, 27), we next sought to determine whether the T cells persisting in the sensory ganglia retained full functional capabilities. To this end, we examined a number of functional parameters; specifically the secretion of cytokines TNF-α and IFN-γ, and the upregulation of surface expression of CD107, a marker of granule exocytosis. Simultaneous expression of multiple effector molecules has been used as a measure of T cell fitness (28)and their expression is progressively lost as T cells become exhausted from chronic stimulation (9). The extent of this polyfunctionality was assessed during the lytic and latent phases of HSV infection. In addition, T cells were also examined following HSV reactivation, which was initiated by ganglionic excision and transplantation under the kidney capsule of naïve recipients, a method previously shown to initiate this event (17).As can be seen in Figure 2, a high proportion of effector T cells recovered during the lytic phase of infection had multifunctional capabilities. Following a 5-hour stimulation with gB peptide, ~70% of transgenic T cells recovered from acutely infected ganglia were able to produce IFN-γ. Furthermore, the majority of these IFN-γ-expressing T cells were also able to produce TNF-α and expressed CD107.Importantly, the frequency of polyfunctional T cells as measured by these parameters was similar for ganglia harbouring lytic or latent infections and this was also true when it came to IL-2 production (Fig. S2). In addition, T cells that had been subjected to virus reactivationalso retained these capacities. Thus, sustained and repeated exposure to virus antigen in the ganglia did not result in functional inactivation of virus-specific CD8+ T cell responses.
Figure 2. Functionality of HSV-specific CD8+ T cells during lytic and latent phases of infection.
Mice were seeded with naïve gBT-I.CD45.1 cells and flank infected with HSV. T cells were isolated from the ganglia at day 8(lytic) or day 30 (latent)post infection, or from latent ganglia that had been transplanted beneath the kidney capsule of naïve recipient syngeneic mice (reactivated). T cells subject to transplant-induced reactivation were harvested from ganglia grafts 9 days post transplantation.T cells were stimulated with gB498-505 peptide for 5 hours and thenanalysed for lytic granule release (CD107a/b), IFNγ and TNFα production. Shown are representative FACS profiles of gBT-I.CD45.1 T cells (gated on CD8+ Va2+ CD45.1+ events) co-expressing IFN-γ and TNF-α (left panels) and IFN-γ and CD107 (right panels). Control panels are gBT-I.CD45.1 cells (isolated from latently infected ganglia) incubated without gB498-505 peptide. Frequencies represent means of 6 mice +/− SEM. Data are representative of 3 independent experiments.
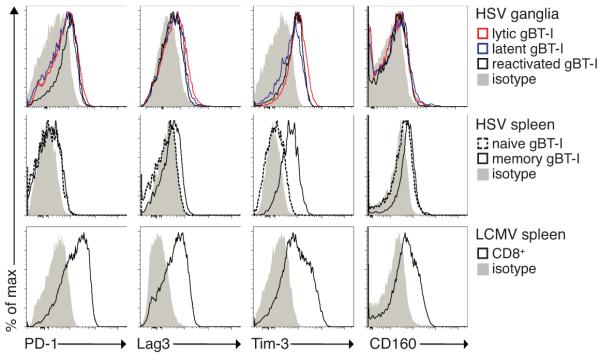
Following prolonged antigenic exposure during chronic infections such as LCMV, virus-specific CD8+ T cells have been shown to upregulate multiple inhibitory receptors, including PD-1, which correlates with T cell exhaustion (29, 30). To determine whether intraganglionic T cells showed an exhausted phenotype, we assessed the expression level of markers associated with this condition, notably PD-1, Lag3, Tim-3 and CD160(29-31), during the lytic, latent and reactivation phases of infection.As shown in Figure 3, T cells in sensory ganglia showed only modest increases in expression of these exhaustion markers compared to memory T cells in the spleen. There was no difference between the respective lyticand latent phases of infection, when T cells are either actively involved in virus clearance or undergoing chronic stimulation. Critically, expression levels were less than those on control exhausted T cell populations from mice chronically infected with LCMV. Overall, these data suggest that T cells found in latent ganglia do not exhibit an overtly exhausted phenotype.
Figure 3. Limited expression of inhibitory receptors by HSV-specific CD8+ T cells in the ganglia.
Mice were seeded with naïve gBT-I.CD45.1 cells and flank infected with HSV. T cells were isolated from the ganglia during lytic (day 8) or latent (day 30) phases of infection, or from T cells reactivated from latency following ganglia transplantation (day 9 post transplantation). Shown is expression of PD-1, Lag3, Tim-3 and CD160 on lytic (red line), latent (blue line) and reactivated (black line) ganglion gBT-I.CD45.1 T cells. Appropriate isotype staining is also shown (grey filled line). Expression of these markers is shown on naïve and memory (d30 post HSV infection) gBT-I.CD45.1 in the spleen (middle panel). As a positive control, C57BL/6 mice were infected with LCMV clone 13 and splenic T cells were isolated at day 14 post infection and stained for inhibitory receptor expression (gated on 45.2+ CD8+ events, lower panel). FACS profiles are representative of 5-8 mice per group.
HSV-specific CD8+ T cells undergoing chronic stimulation during latency can mount a proliferative response on virus reactivation
The results above demonstrated that T cells within HSV-infected ganglia maintained a polyfunctional phenotype and did not bear markers of exhaustion. In addition, we had previously shown that ganglionic T cells underwent proliferation and expansion during HSV reactivation (17). Thus, despite ongoing or chronic stimulation within the sensory ganglia, local T cells appeared fully functional and retained self-renewing capability. However, it remained possible that suchcapabilities were confined to a subpopulation of sequestered cells not involved in active virus recognition. To exclude this, we made use of the gzmBCreERT2/ROSA26EYFP transgenic mice that permit permanent marking of cells expressing granzyme B (18). This marking involves a transgene-driven Crerecombinase that irreversibly induces yellow-fluorescent protein (YFP) expression in cells expressing granzyme B, and only if the latter occurs at the time of administration of the inducing agent, tamoxifen.
To ensure that T cells were marked only during latency, we administered tamoxifen between days 30 and 35 after infection, well after viral antigen presentation in the lymphoid compartment is extinguished (22) and lytic infection has subsided (21). HSV reactivation was initiated at day 40 by means of transplantation-induced reactivation (Fig. 4A), and the reactivating ganglia, isolated from tamoxifen-treated gzmBCreERT2/ROSA26EYFP transgenic mice, contained a population of YFP-expressing T cells (Fig. 4B). Since, the drug was administered between days 30 and 35 after the initiation of infection, this meant that the T cells marked by Cre recombination had recognized infected neurons during latency. Most of the YFP positive cells, but only a minority of non-marked T cells, were virus specific, recognizing the immunodominant determinant from gB as determined by tetramer staining (Fig. 4C). Consequently, the bulk of T cells responding to virus infection during latency appeared specific for this single immunodominant determinant. Importantly, both YFP positive and YFP negative T cells specific for gB had undergone equivalent proliferative bursts after virus reactivation, as assessed by BrdU incorporation (Fig. 4D). Overall, HSV-specific T cells actively involved in recognition of virus infected cells during latency appeared fully capable of proliferation and self-renewal during the process of virus reactivation.
Figure 4. Chronic antigen stimulation does not impair the ability of ganglion-resident T cells to proliferate upon virus reactivation.
gzmBCreERT2/ROSA26EYFP transgenic mice were flank infected with HSV and treated with tamoxifen from day 30 to 35 post infection. Ganglia from these mice were transplanted beneath the kidney capsule of naïve recipient syngeneic mice on day 40 postinfection. BrdU was administered to recipient mice as two i.p. injections 12h apart on day 7 post transplantation. Grafts were recovered on day 8. (A) Schematic depicting the strategy for the analysis of proliferation between YFP+ and YFP−labelled T cells.(B) Representative FACS profiles depicting the frequency of YFP+ CD8+ cells from pooled grafts of BrdU-treated mice and (C) the proportion of gB-tetramerstaining cells of YFP+ CD8+ (black line) and YFP+ CD8− (gray filled line) subsets. (D)BrdU incorporation by YFP+ gB-tetramer+ and YFP− gB-tetramer+ cells. FACS profiles are representative of 5-8 mice per group and 3 independent experiments.
Ganglion-resident T cells respond to successive rounds of reactivation
Certain HSV infected individuals undergo periodic bouts of reactivation, sometimes in a subclinical, continuous cycling pattern (32). The maintenance of functionality, shown above, implies that T cells that initially seed ganglia early in infection should be capable of responding to repeated reactivation episodes. To show that this is the case, gBT-I cells (CD45.1+) were transferred into C57BL/6 (CD45.2+) recipients prior to infection with HSV. After 20 days, by which time latency had been established, ganglia from these mice were subjected to a round of transplantation-induced reactivation as described above. Ganglia were then removed from this primary recipient and subjected to a second round of reactivation at day 15 after primary transplantation, which is well after the day 6 re-establishment of latency in these grafts (17). Note, for secondary reactivation, transplanted ganglia had to be subjected to a short period of in vitro culture (33) to initiate virus replication (Fig. 5A and Fig. S3A). Figure 5B and Supplementary Figure 3B show that ganglion-resident CD45.1+ gBT-I cells, which had already undergone a reactivation episode, expanded again after secondary virus reactivation and ganglia transplantation. Recipients for both primary and secondary transplantation did not contain any CD45.1+ gBT-I cells (Fig. S3C). This argues that the responding transgenic T cells were seeded in the original ganglion donor animals during the initial establishment of latency and were carried over with each successive graft. Thus, not only do T cells retain a proliferative capacity despite chronic antigen stimulation during latency, but they do so after being subjected to repeated episodes of local virus reactivation.
Figure 5. Ganglion-resident T cells can undergo further expansion following multiple rounds of virus reactivation.
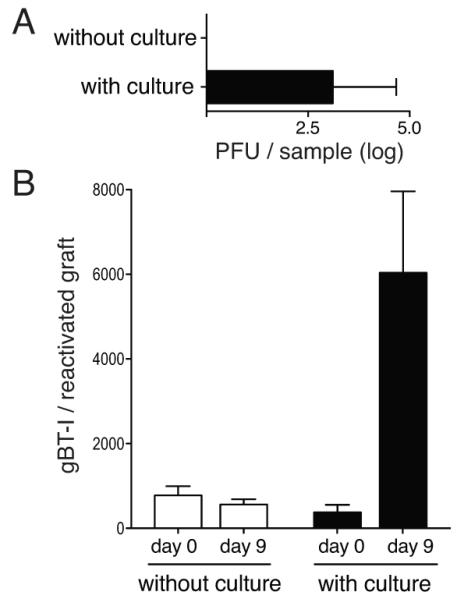
Latent ganglia containing gBT-I.CD45.1 cells were transplanted beneath the kidney capsule of naïve recipient mice. On day 15 posttransplantation the grafts were recovered and either directly grafted into secondary recipients (without culture) or cultivated in vitro for 2 days before being placed under the kidney capsule of secondary naïve recipient mice. (A) Levels of infectious virus isolated from the graft (with or without 2 days of culture) on day 3 post re-transplantation. Bars represent the mean + SEM with 3 mice per group. (B) Number of gBT-I.CD45.1 cells in the grafts (with or without cultivation) on day 0 or day 9 post re-transplantation. Bars represent the mean + SEM with 6-14 mice per group. The data is pooled from 3 independent experiments.
Discussion
Our results demonstrate that T cells involved in control of latency do not appear to be compromised. While the data broadly agree with other reports showing retention of T cell functionality in the latent ganglia (16, 34), they differ frommore recent studies suggesting that HSV can induce exhaustion by an unknown mechanism involving latency-associated transcript(LAT) expression(35, 36). One potential reason for this conflict may have been that only a subset of antigen-specific T cells within the ganglia were actively involved in virus control. Since the majority of T cells within the ganglia do not contact virus infected neurons (37), one could have argued that only the small proportioninvolved in this event were rendered exhausted, with the remainder appearing fully functional. However, by using permanent marking of T cell undergoing activation during latency, we were able to show that cells directly involved in virus controlalso retain full proliferative capacity.
As a consequence, the reason why some but not other experimental systems show evidence for latency-linked T cell exhaustion remains unresolved. One should note that antigen specificity was never strictly demonstrated for the LAT-dependent mechanism(35, 36). The latter is important, since antigen-dependent exhaustion would seem at odds with effective latency control, where persisting T cell functionality would be critical in suppressing virus reactivation. Moreover, HSV latency is restricted to just a few hundred cells (38, 39) and only a fraction of infected neurons show any level of active transcription with little virus replication (40). Given that T cell exhaustion has been attributed, in part, to active viremia and high virus load (7, 8), then the relatively limited nature of the latent HSV infection wouldseem at odds with this phenomenon. Indeed, the extremely low level of antigen expression associated with HSV latency maybe an important contributor to maintenance of T cell functionality.
Separately, when virus loads do increase, as happens during disease recrudescence, we have shown that dendritic cell recruitment leads to productive T cell responses (17). As a consequence, local T cells within the ganglia can undergo multiple rounds of expansion as shown here. Such T cell expansion seen on reactivation would be impossible if the cells were inactivated by chronic stimulation between reactivation episodes. Thus,our results demonstratethat ganglion-resident T cells remain effective at virus control, either during prolonged periods of latency or when HSV repeatedly cycles from quiescent to active phases of infection.
Supplementary Material
Acknowledgments
We thank Dr. Thomas Gebhardt (The University of Melbourne) for comments and helpful suggestions.
Footnotes
This work was supported by grants from the Australian Research Council and the National Health and Medical Research Council.
References
- 1.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechner F, Gruener NH, Urbani S, Uggeri J, Santantonio T, Kammer AR, Cerny A, Phillips R, Ferrari C, Pape GR, Klenerman P. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur. J. Immunol. 2000;30:2479–2487. doi: 10.1002/1521-4141(200009)30:9<2479::AID-IMMU2479>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 4.Gruener NH, Lechner F, Jung MC, Diepolder H, Gerlach T, Lauer G, Walker B, Sullivan J, Phillips R, Pape GR, Klenerman P. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J. Virol. 2001;75:5550–5558. doi: 10.1128/JVI.75.12.5550-5558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamieson BD, Butler LD, Ahmed R. Effective clearance of a persistent viral infection requires cooperation between virus-specific Lyt2+ T cells and nonspecific bone marrow-derived cells. J. Virol. 1987;61:3930–3937. doi: 10.1128/jvi.61.12.3930-3937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klenerman P, Hill A. T cells and viral persistence: lessons from diverse infections. Nat. Immunol. 2005;6:873–879. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- 8.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr. Opin. Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gewurz BE, Vyas JM, Ploegh HL. Herpesvirus evasion of T-cell immunity. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K, editors. Human Herpesviruses: Biology, Therapy and immunoprophylaxis. Cambridge University Press; Cambridge: 2007. Chapter 62. [PubMed] [Google Scholar]
- 11.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 12.Meyers JD. Infection in bone marrow transplant recipients. Am. J. Med. 1986;81:27–38. doi: 10.1016/0002-9343(86)90511-5. [DOI] [PubMed] [Google Scholar]
- 13.Patel R, Paya CV. Infections in solid-organ transplant recipients. Clin. Microbiol. Rev. 1997;10:86–124. doi: 10.1128/cmr.10.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bannard O, Kraman M, Fearon DT. Cutting edge: Virus-specific CD8+ T cell clones and the maintenance of replicative function during a persistent viral infection. J. Immunol. 2010;185:7141–7145. doi: 10.4049/jimmunol.1002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank GM, Lepisto AJ, Freeman ML, Sheridan BS, Cherpes TL, Hendricks RL. Early CD4+ T cell help prevents partial CD8+ T cell exhaustion and promotes maintenance of herpes simplex virus 1 latency. J. Immunol. 2010;184:277–286. doi: 10.4049/jimmunol.0902373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 18.Bannard O, Kraman M, Fearon DT. Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science. 2009;323:505–509. doi: 10.1126/science.1166831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mueller SN, Heath W, McLain JD, Carbone FR, Jones CM. Characterization of two TCR transgenic mouse lines specific for herpes simplex virus. Immunol. Cell Biol. 2002;80:156–163. doi: 10.1046/j.1440-1711.2002.01071.x. [DOI] [PubMed] [Google Scholar]
- 20.Stock AT, Jones CM, Heath WR, Carbone FR. CTL response compensation for the loss of an immunodominant class I-restricted HSV-1 determinant. Immunol. Cell Biol. 2006;84:543–550. doi: 10.1111/j.1440-1711.2006.01469.x. [DOI] [PubMed] [Google Scholar]
- 21.van Lint A, Ayers M, Brooks AG, Coles RM, Heath WR, Carbone FR. Herpes simplex virus-specific CD8+ T cells can clear established lytic infections from skin and nerves and can partially limit the early spread of virus after cutaneous inoculation. J. Immunol. 2004;172:392–397. doi: 10.4049/jimmunol.172.1.392. [DOI] [PubMed] [Google Scholar]
- 22.Stock AT, Jones CM, Heath WR, Carbone FR. Rapid recruitment and activation of CD8+ T cells after herpes simplex virus type 1 skin infection. Immunol. Cell Biol. 2011;89:143–148. doi: 10.1038/icb.2010.66. [DOI] [PubMed] [Google Scholar]
- 23.Roizman B, Knipe DM, Whitley R. Herpes Simplex Viruses. In: Knipe DM, Howley PM, editors. Fields Virology, Fifth Edition. 2007 ed Lippincott, Williams and Wilkins; Philadelphia, PA: 2007. pp. 2501–2601. [Google Scholar]
- 24.Cantin EM, Hinton DR, Chen J, Openshaw H. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J. Virol. 1995;69:4898–4905. doi: 10.1128/jvi.69.8.4898-4905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohlmeier JE, Cookenham T, Roberts AD, Miller SC, Woodland DL. Type I interferons regulate cytolytic activity of memory CD8+ T cells in the lung airways during respiratory virus challenge. Immunity. 2010;33:96–105. doi: 10.1016/j.immuni.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rocha B, Grandien A, Freitas AA. Anergy and exhaustion are independent mechanisms of peripheral T cell tolerance. J. Exp. Med. 1995;181:993–1003. doi: 10.1084/jem.181.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 29.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 30.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. U. S. A. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wald A, Corey L, Cone R, Hobson A, Davis G, Zeh J. Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J. Clin. Invest. 1997;99:1092–1097. doi: 10.1172/JCI119237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevens JG, Cook ML. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971;173:843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- 34.Sheridan BS, Khanna KM, Frank GM, Hendricks RL. Latent virus influences the generation and maintenance of CD8+ T cell memory. J. Immunol. 2006;177:8356–8364. doi: 10.4049/jimmunol.177.12.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen SJ, Hamrah P, Gate D, Mott KR, Mantopoulos D, Zheng L, Town T, Jones C, von Andrian UH, Freeman GJ, Sharpe AH, Benmohamed L, Ahmed R, Wechsler SL, Ghiasi H. The role of LAT in increased CD8+ T cell exhaustion in trigeminal ganglia of mice latently infected with herpes simplex virus 1. J. Virol. 2011;85:4184–4197. doi: 10.1128/JVI.02290-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chentoufi AA, Kritzer E, Tran MV, Dasgupta G, Lim CH, Yu DC, Afifi RE, Jiang X, Carpenter D, Osorio N, Hsiang C, Nesburn AB, Wechsler SL, Benmohamed L. The herpes simplex virus 1 latency-associated transcript promotes functional exhaustion of virus-specific CD8+ T cells in latently infected trigeminal ganglia: a novel immune evasion mechanism. J. Virol. 2011;85:9127–9138. doi: 10.1128/JVI.00587-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Held K, Junker A, Dornmair K, Meinl E, Sinicina I, Brandt T, Theil D, Derfuss T. Expression of herpes simplex virus 1-encoded microRNAs in human trigeminal ganglia and their relation to local T-cell infiltrates. J. Virol. 2011;85:9680–9685. doi: 10.1128/JVI.00874-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sawtell NM. Comprehensive quantification of herpes simplex virus latency at the single-cell level. J. Virol. 1997;71:5423–5431. doi: 10.1128/jvi.71.7.5423-5431.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wakim LM, Jones CM, Gebhardt T, Preston CM, Carbone FR. CD8+ T-cell attenuation of cutaneous herpes simplex virus infection reduces the average viral copy number of the ensuing latent infection. Immunol. Cell Biol. 2008;86:666–675. doi: 10.1038/icb.2008.47. [DOI] [PubMed] [Google Scholar]
- 40.Feldman LT, Ellison AR, Voytek CC, Yang L, Krause P, Margolis TP. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99:978–983. doi: 10.1073/pnas.022301899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.