Abstract
Background
Individuals with higher blood 25-hydroxy-vitamin D [25(OH)D] levels have a lower risk of developing colorectal cancer (CRC), but the influence of 25(OH)D on mortality after CRC diagnosis is unknown.
Methods
The association between pre-diagnostic 25(OH)D levels and CRC-specific (N=444) and overall mortality (N=541) was prospectively examined among 1,202 participants diagnosed with CRC between 1992-2003 in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Multivariable Cox proportional hazards models were used to calculate hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) according to 25(OH)D quintiles and genetic variation within the VDR and CASR genes. Potential dietary, lifestyle and metabolic effect modifiers were also investigated.
Results
There were 541 deaths, 444 (82%) due to CRC. Mean follow-up was 73 months. In multivariable analysis, higher 25(OH)D levels were associated with a statistically significant reduction in CRC-specific (Ptrend=0.04) and overall mortality (Ptrend=0.01). Participants with 25(OH)D levels in the highest quintile had an adjusted HR of 0.69 (95%CI: 0.50-0.93) for CRC-specific and 0.67 (95%CI: 0.50-0.88) for overall mortality, compared to the lowest quintile. Except for a possible interaction by pre-diagnostic dietary calcium intake (Pinteraction=0.01), no other potential modifying factors related to CRC survival were noted. The VDR (FokI and BsmI) and CASR (rs1801725) genotypes were not associated with survival.
Conclusions
High pre-diagnostic 25(OH)D levels are associated with improved survival of patients with CRC.
Impact
Our findings may stimulate further research directed at investigating the effects of blood vitamin D levels before, at, and after CRC diagnosis on outcomes in CRC patients.
Keywords: vitamin D, colorectal neoplasms, survival, VDR, CASR
INTRODUCTION
Evidence supporting a decreased risk of colorectal cancer (CRC) by higher circulating vitamin D [25(OH)D] levels is strong, particularly from prospective cohort studies in American and European populations (1-8). However, despite very promising findings from cell culture/animal models (9-13), very few observational studies have to date investigated an effect of vitamin D on survival after CRC diagnosis (14-16). Limited existing evidence shows an improvement in CRC-specific and overall survival with higher vitamin D levels, but can be criticized for limited sample size (14, 16) or use of predicted, not actual, post-diagnosis circulating 25(OHD)D levels (15). Some studies have also shown vitamin D levels or genetic variation within the vitamin D pathway to influence survival in other cancers (17-19). However, nothing is known about potential roles of vitamin D-related genes (e.g. vitamin D receptor [VDR] or calcium sensing receptor [CASR]) or other possible effect modifiers in relation to any association of vitamin D with CRC survival. Additionally, it has been proposed that high-dose calcitriol, the hormonally active form of vitamin D, might restore sensitivity to chemotherapy (20); hence, in addition to calcitriol treatment, high vitamin D status prior to diagnosis may provide survival benefits.
Currently, little is known about the effects of pre- and post-diagnostic dietary or lifestyle factors on CRC survival. This is an important point, especially since habits and exposures before cancer diagnosis may affect post-diagnostic lifestyle, and because cancer patients may have a strong interest in making appropriate changes in their diet and lifestyle (21) and could potentially benefit from recommendations on healthy cancer recurrence-preventing diets, supplement use (including vitamin D), and lifestyle factors as part of their treatment, post-treatment recovery and cancer counseling. Such recommendations should be based on carefully conducted randomized clinical trials (RCTs). In this regard, findings from large observational studies can provide guidance for the conduct of future RCTs.
In consideration of these points, we investigated whether 25(OH)D levels determined pre-diagnostically are associated with CRC-specific and overall mortality in patients with CRC diagnosed within the context of a very large European prospective cohort study. We also explored potential modifying factors and whether select genetic polymorphisms in the VDR and CASR genes may influence CRC outcomes.
METHODS
Study Population and Collection of Data
CRC cases in this analysis were participants in a nested case-control study within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort, a large prospective study with over 520,000 participants enrolled in 23 centers in Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and United Kingdom. The methods of the EPIC study have been described in detail elsewhere (22, 23). Between 1992 and 1998, standardized lifestyle and personal history information, anthropometric data, and blood samples were collected from most participants at recruitment. Diet over the previous 1 year was measured at baseline by validated country-specific dietary questionnaires developed to ensure high compliance and better measures of local dietary habits (22). Blood samples are stored at the International Agency for Research on Cancer (IARC, Lyon, France) in −196°C liquid nitrogen for all countries except Denmark (−150°C, nitrogen vapour) and Sweden (−80°C, freezers) where samples are stored locally.
Cancer Incidence Follow-up
Cancer incidence was determined through record linkage with regional cancer registries (Denmark, Italy, the Netherlands, Norway, Spain, Sweden and United Kingdom; complete up to June 2003) or via a combination of methods, including the use of health insurance records, contacts with cancer and pathology registries, and active follow-up through study subjects and their next-of-kin (France, Germany, Naples and Greece; complete up to June 2002).
Vital Status Follow-up
Vital status follow-up (98.5% complete) was collected by record linkage with regional and/or national mortality registries in all countries except France, Germany and Greece, where data are collected through an active follow-up. Censoring dates for complete follow-up were between December 2006 and December 2008 in Denmark, the Netherlands, Spain, the United Kingdom, Sweden, Norway, and Italy. In Germany, Greece, and France follow-up was based on a combination of methods, including health insurance records, cancer and pathology registries, and active follow-up through study subjects and their next-of-kin. In these centres, the end of follow-up was defined as the last known date of contact, or the date of death whichever came first. The last update of endpoint information occurred between December 2006 and June 2010.
Mortality data were coded according to the 10th revision of the International Classification of Diseases, Injuries and Causes of Death (ICD-10). Up to six qualifiers of the cause of death were reviewed. The outcome of interest was assigned based on the underlying cause of death.
Case Ascertainment and Selection
The detailed description of case selection was previously published (1). Briefly, CRC cases were selected among participants (men and women) who developed colon (C18.0-C18.7, according to the ICD-10), rectum (C19-C20), and overlapping/unspecified origin tumors (C18.8 and C18.9). Cancers of the anus were excluded. Colorectal cancer is defined as the combination of the colon and rectal cancers.
Case exclusions included 21 non-adenocarcinoma, 87 due to missing 25(OH)D measurements, and 25 because death certificate and/or autopsy report was the primary source of information for cancer diagnosis giving a final sample size of 1,202 CRC cases (759 colon, 443 rectum). Cases from EPIC collaborating centers in Norway and the Malmo center in Sweden were not included into this analysis because either very few CRC cases were diagnosed after blood donation (Norway), or blood samples were not available for biomarker analyses (Malmo, Sweden) (1). The number of cases for analyses of genetic variation was 1,095 for VDR FokI, 1,103 for VDR BsmI and 1,137 for CASR (Table 1) due to incomplete genotyping data. For analyses of dietary calcium, additional two CRC cases were excluded due to missing nutrient intake values.
Table 1.
Selected baseline characteristics of colorectal cancer cases (N=1,202) according to quintile of blood 25(OH)D in the European Prospective Investigation into Cancer and Nutrition (EPIC) study.
| Characteristic | Quintile 1: <36.3 nmol/L (N=242) |
Quintile 2: 36.4-48.6 nmol/L (N=239) |
Blood 25(OH)D Quintile 3: 48.7-60.5 nmol/L (N=241) |
Quintile 4: 60.6-76.8 nmol/L (N=240) |
Quintile 5: >76.8 nmol/L (N=240) |
|---|---|---|---|---|---|
| 25(OH)D, mean(SD), nmol/L | 28.5 (6.0) | 42.6 (3.7) | 54.5 (3.5) | 67.8 (4.4) | 99.3 (25.5) |
| Age at diagnosis, mean(SD), yrs | 62.1 (7.2) | 62.0 (7.1) | 62.5 (7.7) | 62.1 (7.6) | 62.0 (7.1) |
| Women, N (%) | 144 (59.5) | 128 (53.6) | 125 (51.9) | 109 (45.4) | 100 (41.7) |
| Stage of disease, N (%) | |||||
| I | 45 (18.6) | 56 (23.4) | 51 (21.2) | 48 (20.0) | 41 (17.1) |
| II | 52 (21.5) | 43 (18.0) | 37 (15.4) | 50 (20.8) | 59 (24.6) |
| III | 73 (30.2) | 73 (30.5) | 83 (34.4) | 71 (29.6) | 67 (27.9) |
| IV | 33 (13.6) | 29 (12.1) | 23 (9.5) | 19 (7.9) | 24 (10.0) |
| Unknown | 39 (16.1) | 38 (15.9) | 47 (19.5) | 52 (21.7) | 49 (20.4) |
| Grade of differentiation, N (%) | |||||
| Well differentiated | 12 (5.0) | 15 (6.3) | 25 (10.4) | 9 (3.8) | 13 (5.4) |
| Moderately differentiated | 75 (31.0) | 76 (31.8) | 58 (24.1) | 76 (31.7) | 74 (30.8) |
| Poorly differentiated | 14 (5.8) | 16 (6.7) | 23 (9.5) | 15 (6.3) | 13 (5.4) |
| Unknown | 141 (58.3) | 132 (55.2) | 135 (56.0) | 140 (58.3) | 140 (58.3) |
| Location of primary tumor, N (%) | |||||
| Colon | 160 (66.1) | 145 (60.7) | 166 (68.9) | 144 (60.0) | 144 (60.0) |
| Rectum | 82 (33.9) | 94 (39.3) | 75 (31.1) | 96 (40.0) | 96 (40.0) |
| Smoking status, N(%) | |||||
| Never smoker | 95 (39.3) | 102 (42.7) | 102 (42.3) | 100 (41.7) | 92 (38.3) |
| Former smoker | 60 (24.8) | 72 (30.1) | 74 (30.7) | 96 (40.0) | 99 (41.3) |
| Current smoker | 85 (35.1) | 63 (26.4) | 60 (24.9) | 44 (18.3) | 49 (20.4) |
| BMI, mean(SD), kg/m2 | 27.4 (4.9) | 26.9 (4.5) | 26.8 (4.4) | 26.7 (3.9) | 25.8 (3.7) |
| Physical activity, mean(SD), METs | 80.8 (52.8) | 78.7 (45.2) | 89.4 (57.6) | 83.1 (48.5) | 94.9 (58.0) |
| Dietary calcium, mean(SD), mg/d | 964 (412) | 973 (411) | 988 (430) | 1024 (470) | 1051 (403) |
| Season of blood collection | |||||
| Winter | 119 (49.2) | 96 (40.2) | 73 (30.3) | 47 (19.6) | 47 (19.6) |
| Spring | 64 (26.4) | 60 (25.1) | 62 (25.7) | 61 (25.4) | 46 (19.2) |
| Summer | 12 (5.0) | 24 (10.0) | 42 (17.7) | 77 (32.1) | 91 (37.9) |
| Autumn | 47 (19.4) | 59 (24.7) | 64 (26.6) | 55 (22.9) | 56 (23.3) |
| VDR BsmI (rs1544410; 60890G>A), N (%)a | |||||
| bb (GG) | 73 (33.2) | 76 (34.9) | 76 (34.4) | 75 (33.0) | 73 (33.6) |
| bB (GA) | 114 (51.8) | 103 (47.3) | 104 (47.1) | 111 (48.9) | 116 (53.5) |
| BB (AA) | 33 (15.0) | 39 (17.9) | 41 (18.6) | 41 (18.1) | 28 (12.9) |
| VDR FokI (rs2228570; 27823T>C ), N (%)a | |||||
| FF (CC) | 88 (39.6) | 85 (39.7) | 84 (38.7) | 85 (37.4) | 81 (37.7) |
| fF (CT) | 109 (49.1) | 99 (46.3) | 96 (44.2) | 104 (45.8) | 91 (42.3) |
| ff (TT) | 25 (11.3) | 30 (14.0) | 37 (17.1) | 38 (16.7) | 43 (20.0) |
| CASR (rs1801725, G>T ; A986S), N (%)a | |||||
| GG | 168 (73.0) | 167 (74.9) | 162 (71.4) | 177 (76.6) | 171 (75.7) |
| GT | 56 (24.4) | 51 (22.9) | 62 (27.3) | 49 (21.2) | 51 (22.6) |
| TT | 6 (2.6) | 5 (2.2) | 3 (1.3) | 5 (2.2) | 4 (1.8) |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; SD, standard deviation; yrs, years; N, number; BMI, body mass index; METs, metabolic equivalents; VDR, vitamin D receptor; CASR, calcium sensing receptor.
Missing values were not excluded from percentage calculations, thus the sum of percents across sub-groups may not ad up to 100%.
The number of cases for analyses of genetic variation was 1,095 for VDR FokI, 1,103 for VDR BsmI and 1,137 for CASR due to incomplete genotyping data.
The EPIC study was approved by the Ethical Review Board of the IARC and the Institutional Review Board of each participating EPIC center. Written consent was obtained from EPIC participants at enrolment into the study.
Blood 25(OH)D Measurements
Details of 25(OH)D measurements have been published previously (1). Briefly, vitamin D status was quantitatively determined by measuring 25(OH)D in 25 μL of serum (heparin plasma for Swedish samples) using a commercially available enzyme immunoassay kit (OCTEIA™ 25(OH)D Kit, Immuno Diagnostic Systems, Boldon, UK) at the Laboratory for Health Protection Research, National Institute for Public Health and the Environment, the Netherlands. The inter-assay coefficient of variation as determined with two kit control samples was 5.9% at the level of 20.3 nmol/L and 5.4% at the level of 77.4 nmol/L. No significant between-day drift, time shifts or other trends were observed and the percentage of variance attributable to batch-to-batch differences was 4.5%.
Genotyping
Genotyping procedures were described previously (24). Briefly, the VDR (BsmI: rs1544410, 60890G>A; FokI: rs2228570, 27823T>C) and CASR (A986S; rs1801725, G>T) polymorphisms were genotyped by Taqman methodology in 384-well plates read with the Sequence Detection Software on an ABI-Prism7900 instrument, according to the manufacturer’s instructions (Applied Biosystems). Primers and probes were supplied by Applied Biosystems (Assays-by-Design). Each plate included a negative control. Positive controls were duplicated on a separate plate. For all genotypes, the assay success rate was >97% and the internal study duplicate rate was >99%. Failed genotypes were not repeated.
Covariates
Prognostic factors, known and hypothesized to have an effect on CRC survival, were extracted from the medical records (age at diagnosis, year of diagnosis, tumor stage, grade of differentiation [well/moderately/poorly differentiated, unknown] and location [proximal/distal colon, colon, rectum]), and from baseline questionnaires (age at recruitment, sex, body mass index [BMI], physical activity [METs-hours], dietary calcium intake, smoking status [never, former, and current smokers]). They were considered to be potential confounding variables. Several criteria were used to assess confounding factors: 1) biological plausibility; 2) whether the variable of interest was associated with the outcome and exposure; and 3) whether the hazard ratio of the primary exposure variable substantially changed (by >10%) after adding the potential confounding variable in the model.
Statistical Analyses
Death from CRC was the primary endpoint. Death from any cause was used as a secondary endpoint. Adjusted cumulative incidence curves were used to assess the influence of 25(OH)D on CRC-specific mortality accounting for competing risks (deaths from other causes) (25).
A Cox proportional hazards model stratified by country of cancer diagnosis with time since CRC diagnosis as the time variable was used to calculate hazard ratios (HRs) and 95% confidence intervals (95% CI) of CRC-specific and all-cause mortality, adjusted for age at diagnosis, sex, cancer stage, grade of tumor differentiation, location of tumour, smoking status, body mass index, physical activity, and year of diagnosis. Since available information on tumor stage differed between EPIC centers, a harmonization procedure was used as follows: First, we assigned a broad category for tumor stage (I-IV) based on the TNM staging (N=464), then, if available, based on Dukes classification (N=268), and, finally, based on categories ‘localized/metastatic/metastatic regional/metastatic distant’ provided by study centers (N=255). If several tumor staging classifications were available, a cross-checking for discrepancies was performed. Other covariates including dietary calcium intake, alcohol consumption, education were tested but not included into the final model because they did not meet the criteria for confounders (change in coefficient of interest by >10% after adding the potential confounder).
The proportional hazards assumption was met as assessed by finding the correlation between the Schoenfeld residuals and including a time-dependent covariate in the Cox model. The following exposures of interest were examined: 1) 25(OH)D concentrations (as quintiles, as pre-defined categories [<24, 25-49, 50-74, 75-99, ≥100 nmol/L] (26), and per 24.96 nmol/L [equivalent to 10 ng/mL] increase), and 2) polymorphisms in the VDR and CASR genes.
Subgroup analyses were conducted by categories of biologically plausible effect modifiers. Adjusted HRs and 95%CI were reported for a 24.96 nmol/L increment in 25(OH)D for CRC-specific and overall mortality. Tests of statistical multiplicative interaction between 25(OH)D and relevant factors were assessed by including in the model the cross product of 25(OH)D as a continuous variable and the covariate as a continuous or dichotomous variable, as appropriate.
The effects of the season or month of blood collection on 25(OH)D levels in relation to CRC were assessed by two approaches: 1) adjustment for season of blood collection; 2) standardization of 25(OH)D levels (by the method of Munger et al. (27) and by adding the overall mean of 25(OH)D for all subjects to the residuals derived from a simple regression model fitted to 25(OH)D concentration by month of blood collection).
The effect of incomplete tumor stage information on effect estimates was investigated by several approaches (28): 1) combining all missing values for tumor stage into a single ‘missing’ category (primary analysis); 2) limiting to ‘complete’ records; 3) imputation of missing CRC stage values based on the available information for sex, age at cancer diagnosis, year of diagnosis, vital status, tumour location, and period between cancer diagnosis and death under the missing at random assumption (28) with SAS PROC MI procedure. All statistical tests were two-sided with P-values<0.05 considered statistically significant (SAS software, version 9.2; SAS Institute, Cary/NC).
RESULTS
Patient Characteristics
Among 1,202 eligible CRC cases, there were 541 deaths (from CRC=444, other neoplasms=35, circulatory disease=21, respiratory disease=3, mental disorders=2, infections=1, anemia=1, other causes=8, and missing=26). Mean follow-up was 73 months (standard deviation [SD] = 49months). Vitamin D concentrations were measured on average 46 months (SD=26, range=0.5-138 months) before CRC diagnosis. Selected baseline characteristics of study participants according to quintiles of blood vitamin D levels are listed in Table 1.
Vitamin D and Survival
Higher pre-diagnostic 25(OH)D levels were associated with a statistically significant reduction in CRC-specific and overall mortality after adjusting for multiple prognostic factors(Table 2) and accounting for competing risks of death for CRC-specific mortality (Figure 1). Subjects in the highest (>76.9 nmol/L) vs. lowest 25(OH)D quintile had an adjusted HR of 0.69 (95% CI: 0.50-0.93; Ptrend=0.04) for CRC-specific mortality and 0.67 (95% CI: 0.50-0.88; Ptrend=0.01) for overall mortality. Similar results were obtained in analyses restricted to complete CRC stage records: HRQ5 vs. Q1=0.65 (95%CI: 0.46-0.91; Ptrend=0.03) for CRC-specific and HRQ5 vs. Q1=0.65 (95%CI: 0.48-0.89; Ptrend=0.01) for overall mortality. By tumor location, higher 25(OH)D was associated with reduced mortality for both colon (Ptrend=0.61 for CRC-specific and Ptrend=0.16 for total mortality) and rectal cancers (Ptrend<0.01 for CRC-specific and Ptrend=0.01 for overall mortality; Supplemental Table 1). Though the association was somewhat stronger for rectal cancer (HRQ5 vs. Q1=0.48, 95%CI: 0.29-0.80 for CRC-specific and HRQ5 vs. Q1=0.55, 95%CI: 0.35-0.88 for overall mortality) than for colon cancer (HRQ5 vs. Q1=0.79, 95%CI: 0.53-1.19 for CRC-specific and HRQ5 vs. Q1=0.69, 95%CI: 0.48-1.01 for overall mortality); no statistically significant heterogeneity by tumor location was observed (P-values for heterogeneity were: CRC-specific=0.28 and overall mortality=0.53; Table 3). Considering pre-defined 25(OH)D categories, those with 25(OH)D levels ≥100 nmol/L vs. deficient (<25 nmol/L) had a multivariable adjusted HR of 0.55 (95% CI: 0.32-0.94; Ptrend=0.04) for CRC-specific mortality and 0.53 (95% CI: 0.33-0.87; Ptrend=0.02) for overall mortality (Supplemental Table 2).
Table 2.
Hazard ratios and 95% CIs for CRC-specific and overall mortality according to quintiles of blood 25(OH)D in the European Prospective Investigation into Cancer and Nutrition (EPIC) study (N=1,202).
| 25(OH)D category | Category range, nmol/L |
No. | Event | HR (95% CI) | P trend a |
|---|---|---|---|---|---|
| Colorectal cancer-specific mortality | |||||
| Age-adjustedb | |||||
| Quintile 1 | < 36.3 | 242 | 104 | 1.00 (ref) | 0.02 |
| Quintile 2 | 36.4-48.6 | 239 | 85 | 0.75 (0.56-1.00) | |
| Quintile 3 | 48.7-60.5 | 241 | 95 | 0.90 (0.68-1.19) | |
| Quintile 4 | 60.6-76.8 | 240 | 78 | 0.68 (0.51-0.92) | |
| Quintile 5 | > 76.8 | 240 | 82 | 0.69 (0.52-0.92) | |
| Multivariablec | |||||
| Quintile 1 | < 36.3 | 242 | 104 | 1.00 (ref) | 0.04 |
| Quintile 2 | 36.4-48.6 | 239 | 85 | 0.76 (0.56-1.02) | |
| Quintile 3 | 48.7-60.5 | 241 | 95 | 0.93 (0.69-1.24) | |
| Quintile 4 | 60.6-76.8 | 240 | 78 | 0.78 (0.58-1.06) | |
| Quintile 5 | > 76.8 | 240 | 82 | 0.69 (0.50-0.93) | |
| Overall mortality | |||||
| Age-adjustedb | |||||
| Quintile 1 | < 36.3 | 242 | 128 | 1.00 (ref) | <0.01 |
| Quintile 2 | 36.4-48.6 | 239 | 108 | 0.79 (0.61-1.03) | |
| Quintile 3 | 48.7-60.5 | 241 | 117 | 0.89 (0.69-1.15) | |
| Quintile 4 | 60.6-76.8 | 240 | 95 | 0.68 (0.52-0.90) | |
| Quintile 5 | > 76.8 | 240 | 93 | 0.65 (0.49-0.84) | |
| Multivariablec | |||||
| Quintile 1 | < 36.3 | 242 | 128 | 1.00 (ref) | <0.01 |
| Quintile 2 | 36.4-48.6 | 239 | 108 | 0.82 (0.63-1.07) | |
| Quintile 3 | 48.7-60.5 | 241 | 117 | 0.91 (0.70-1.18) | |
| Quintile 4 | 60.6-76.8 | 240 | 95 | 0.78 (0.59-1.03) | |
| Quintile 5 | > 76.8 | 240 | 93 | 0.67 (0.50-0.88) | |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; No., number; HR, hazard ratio; 95% CI, 95% confidence interval; ref., referent category.
Ptrend was calculated using the median value of each 25(OH)D category as a continuous variable, adjusted for variables in the corresponding models.
HRs, 95% CIs, and P-values are adjusted for age at diagnosis (in years as a continuous variable) and stratified by country of residence.
Multivariable HRs, 95% CIs, and P-values are adjusted for age at diagnosis (in years as a continuous variable), sex (men or women), cancer stage (I to IV, unknown), grade of tumor differentiation (well differentiated, moderately differentiated, poorly differentiated, or unknown), location of primary tumour (colon or rectum), smoking status (current, former, never smoker or unknown), body mass index (BMI) (in kg/m2 as a continuous variable), physical activity (in METs as a continuous variable), season of blood collection (winter, spring, summer and autumn) and year of diagnosis (as a continuous variable), and stratified by country of residence.
Figure 1.
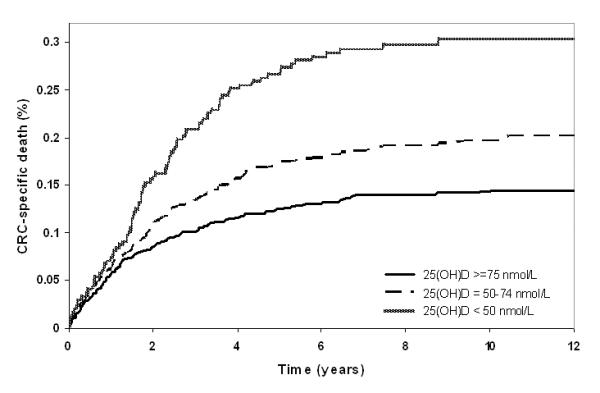
Adjusted cumulative incidence curve of colorectal cancer-specific mortality by pre-defined levels of pre-diagnostic 25(OH)D (<50, deficient; 50-74, insufficient; ≥50 nmol/L, sufficient vitamin D status, on the basis of proposed levels of vitamin D deficiency/insufficiency).
Table 3.
Adjusted hazard ratios and 95% CIs for an increment of 24.96 nmol/L (equivalent to 10 ng/mL) of 25(OH)D for colorectal cancer-specific and overall mortality across strata of potential effect modifiers.*
| Risk factor | Colorectal cancer-specific mortality | Overall mortality | ||
|---|---|---|---|---|
|
|
||||
| Multivariablea HR (95% CI) |
P for interaction or trend | Multivariablea HR (95% CI) |
P for interaction or trend | |
| All participants | 0.92 (0.85-1.00) | 0.07 b | 0.91 (0.84-0.99) | 0.03 b |
| Sensitivity analyses | ||||
| Participants with complete CRC stage data | 0.93 (0.84-1.02) | 0.11 b | 0.92 (0.84-1.01) | 0.08 b |
| All participants with imputed CRC stage datac | 0.93 (0.85-1.02) d | 0.15 d | 0.92 (0.84-1.00) d | 0.05 d |
| Follow-upe | ||||
| ≥ 2 yrs | 0.90 (0.81-1.00) | 0.06 b | 0.89 (0.81-0.98) | 0.02 b |
| ≥ 3 yrs | 0.92 (0.81-1.04) | 0.16 b | 0.92 (0.83-1.03) | 0.15 b |
| ≥ 5 yrs | 0.88 (0.73-1.06) | 0.17 b | 0.90 (0.76-1.06) | 0.21 b |
| Sex | ||||
| Women | 0.87 (0.76-1.00) | 0.26 | 0.86 (0.76-0.99) | 0.29 |
| Men | 0.97 (0.86-1.09) | 0.94 (0.84-1.04) | ||
| Age at diagnosis, yrs | ||||
| < 62.4 | 0.86 (0.76-0.99) | 0.38 | 0.89 (0.79-1.01) | 0.80 |
| ≥ 62.4 | 0.96 (0.85-1.08) | 0.90 (0.81-1.01) | ||
| Anatomical site | ||||
| Colon | 0.99 (0.88-1.11) | 0.28 | 0.95 (0.86-1.06) | 0.53 |
| Rectum | 0.81 (0.69-0.95) | 0.85 (0.73-0.98) | ||
| Colon sub-sitef | ||||
| Proximal | 0.91 (0.77-1.09) | 0.72 | 0.94 (0.80-1.10) | 0.57 |
| Distal | 1.05 (0.86-1.29) | 0.95 (0.79-1.15) | ||
| Stageg | ||||
| I and II | 0.78 (0.61-1.00) | 0.25 | 0.80 (0.66-0.98) | 0.23 |
| III and IV | 0.97 (0.87-1.08) | 0.96 (0.87-1.06) | ||
| Year of diagnosis | ||||
| 1993-1999 | 0.89 (0.77-1.03) | 0.53 | 0.84 (0.73-0.96) | 0.24 |
| 1999-2004 | 0.95 (0.85-1.07) | 0.96 (0.86-1.07) | ||
| Season of blood collectionh | ||||
| Summer/autumn | 0.97 (0.84-1.11) | 0.23 | 0.96 (0.85-1.09) | 0.14 |
| Winter/spring | 0.89 (0.78-1.01) | 0.87 (0.77-0.98) | ||
| Season of diagnosish | ||||
| Summer/autumn | 0.92 (0.80-1.06) | 0.71 | 0.89 (0.78-1.01) | 0.44 |
| Winter/spring | 0.92 (0.82-1.04) | 0.92 (0.82-1.03) | ||
| Dietary calcium, mg/df | ||||
| < 928 | 1.00 (0.89-1.13) | 0.01 | 0.99 (0.89-1.11) | 0.01 |
| ≥ 928 | 0.85 (0.74-0.97) | 0.84 (0.74-0.94) | ||
| Smoking status | ||||
| Never smoker | 0.86 (0.73-1.02) | 0.13 | 0.87 (0.74-1.02) | 0.24 |
| Former smoker | 0.99 (0.86-1.13) | 0.97 (0.86-1.10) | ||
| Current smoker | 0.74 (0.61-0.91) | 0.80 (0.67-0.95) | ||
| BMI, kg/m2 | ||||
| < 25 | 0.87 (0.74-1.01) | 0.53 | 0.86 (0.75-0.99) | 0.31 |
| 25-29.9 | 0.96 (0.85-1.09) | 0.94 (0.84-1.06) | ||
| ≥ 30 | 0.96 (0.74-1.24) | 1.00 (0.80-1.26) | ||
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; No., number; HR, hazard ratio; 95% CI, 95% confidence interval; yrs, years; mg/d, milligram per day; BMI, body mass index.
Subgroup analyses were conducted by time between blood collection and cancer diagnosis, sex, age at diagnosis (median-dichotomized; <62, ≥62 years), location of tumor (colon vs. rectum; and within the colon, proximal vs. distal), cancer stage (I-II vs. III-IV), season of diagnosis (winter/spring vs. summer/autumn), year of diagnosis (median-dichotomized; <1999, ≥1999), pre-diagnostic BMI (WHO categories: <25, normal; 25-29, overweight; ≥30 kg/m2, obese), smoking status (current, former, never), and dietary calcium intake (median-dichotomized; <928, ≥928 mg/d). In interaction analyses, adjusted HRs and 95% CI for an increment of 24.96 nmol/L of 25(OH)D levels for CRC-specific and overall mortality were reported. Tests of statistical interaction between 25(OH)D and relevant factors were assessed by including in the model the cross product of 25(OH)D levels as a continuous variable and the covariate as a continuous or dichotomous variable, as appropriate.
Multivariable HRs, 95% CIs, and P-values are adjusted for age at diagnosis (in years as a continuous variable), sex (men or women), cancer stage (I to IV, unknown), grade of tumor differentiation (well differentiated, moderately differentiated, poorly differentiated, or unknown), location of primary tumour (colon or rectum), smoking status (current, former, never smoker or unknown), body mass index (BMI) (in kg/m2 as a continuous variable), physical activity (in METs as a continuous variable), season of blood collection (winter, spring, summer and autumn) and year of diagnosis (as a continuous variable), and stratified by country of residence. In the stratified models, the stratifying variable was not adjusted for. P value for interaction is presented unless otherwise indicated.
Ptrend.
Missing stage data were imputed using the algorithm described in the Statistical section.
Combined HR estimate and 95% CI; P value from a t-test for the hypothesis that the parameter is equal to its null value.
Multivariable model including all CRC cases with time interval between blood collection and cancer diagnosis (follow-up) of more than 2, 3, and 5 years.
Only colon tumors with known locations were included. Unspecified (N=63) and overlapping lesion of colon (N=9) tumors were excluded.
Missing data were not included in the analyses.
Summer/autumn period included June, July, August, September, October, and November; winter/spring period included December, January, February, March, April, and May.
In the analyses by pre-defined categories, participants with high dietary calcium intake (≥928 mg/d) and high pre-diagnostic vitamin D levels (>100 nmol/L) had an adjusted HR of 0.24 (95%CI, 0.11-0.54; Ptrend= 0.012) for CRC-specific mortality and 0.26 (95%CI=0.13-0.53; Ptrend=0.002) for overall mortality compared to participants with the lowest 25(OH)D levels (<25 nmol/L). Whereas among participants with low calcium intake, the corresponding HRs were 0.86 (95%CI=0.41-1.82; Ptrend=0.877) for CRC-specific and 0.92 (95%CI=0.46-1.86; Ptrend=0.733) for overall mortality.
Sensitivity analyses showed: 1) reduction of CRC-specific and overall mortality with increasing 25(OH)D levels after exclusion of cases diagnosed within 2, 3 and 5 years of blood collection; 2) similar results for analyses restricted to complete CRC stage records or imputation of missing stage values (Table 3). The effect estimates were stronger among patients with stage I/II disease (HRs for CRC-specific=0.78, 95%CI: 0.61-1.00 and overall mortality=0.80, 95%CI: 0.66-0.98; Table 3). Adjustment or standardization of 25(OH)D by month of blood collection did not substantially change the effect estimates (data not shown). No significant heterogeneity by geographical region (Northern/Central/Southern Europe) was observed (P=0.702).
Interactions with Other Predictors of Mortality
The examination of possible interactions across strata of other factors related to CRC survival and recurrence showed that the inverse association between 25(OH)D and CRC-specific and overall mortality remained unchanged across most subcategories. One potential interaction was observed with dietary calcium intake (P=0.01), such that participants with high baseline dietary calcium intake ( ≥928 mg/d) had decreased CRC-specific and overall mortality with increasing levels of vitamin D, but not participants with low calcium intake (<928 mg/d) (Table 3). In the analyses by pre-defined categories, participants with high dietary calcium intake (≥928 mg/d) and high pre-diagnosis vitamin D levels (>100 nmol/L) had an adjusted HR of 0.24 (95% CI, 0.11-0.54; Ptrend=0.01) for CRC-specific mortality and 0.26 (95% CI, 0.13-0.53; Ptrend<0.01) for overall mortality compared to participants with the lowest 25(OH)D levels (<25 nmol/L). Whereas among participants with low calcium intake, the corresponding HRs were 0.86 (95% CI, 0.41-1.82; Ptrend=0.88) for CRC-specific and 0.92 (95% CI, 0.46-1.86; Ptrend=0.73) for overall mortality.
VDR and CASR Genetic Polymorphisms and Survival
VDR BsmI or FokI polymorphisms were not associated with CRC-specific (Table 4) or overall mortality (data not shown). No survival difference was observed by CASR (rs1801725) genotype except for a potential protective effect of having at least one T allele (age-, sex- and stage-adjusted HR for GT/TT vs. GG was 0.86, 95% CI: 0.68-1.08; P=0.19). Similarly null results were obtained in analyses stratified by tumor location and median-dichotomized 25(OH)D levels (data not shown).
Table 4.
The association of VDR and CASR genotypes with colorectal cancer-specific mortality in the European Prospective Investigation into Cancer and Nutrition (EPIC) study.
| Age-adjusteda | Multivariableb | |||
|---|---|---|---|---|
|
|
||||
| No. | Even t |
HR (95% CI) | HR (95% CI) | |
| VDR BsmI (rs1544410) | ||||
| bb (GG) | 373 | 132 | 1.00 (ref) | 1.00 (ref) |
| bB (GA) | 548 | 202 | 1.07 (0.86-1.33) | 1.01 (0.81- 1.27) |
| BB (AA) | 182 | 62 | 0.94 (0.69-1.27) | 1.18 (0.87- 1.61) |
| P trend c | 0.76 | 0.42 | ||
| VDR FokI (rs2228570) | ||||
| FF (CC) | 423 | 162 | 1.00 (ref) | 1.00 (ref) |
| fF (CT) | 499 | 178 | 0.90 (0.73-1.12) | 0.96 (0.77- 1.19) |
| ff (TT) | 173 | 59 | 0.81 (0.60-1.10) | 0.94 (0.70- 1.28) |
| P trend c | 0.27 | 0.90 | ||
| CASR (rs1801725) | ||||
| GG | 845 | 321 | 1.00 (ref) | 1.00 (ref) |
| GT | 269 | 90 | 0.88 (0.70-1.12) | 0.93 (0.73- 1.17) |
| TT | 23 | 5 | 0.53 (0.22-1.28) | 0.47 (0.19- 1.14) |
| P trend c | 0.08 | 0.11 | ||
Abbreviations: No., number; HR, hazard ratio; 95% CI, 95% confidence interval; VDR, vitamin D receptor; ref., referent category; CaSR, calcium sensing receptor.
HRs, 95% CIs, and P-values are adjusted for age at diagnosis (in years as a continuous variable) and stratified by country of residence.
Multivariable HRs, 95% CIs, and P-values are adjusted for age at diagnosis (in years as a continuous variable), sex (men or women), cancer stage (I to IV, unknown). Further adjustment for other covariates did not change estimates substantially.
Ptrend from the log-additive model.
DISCUSSION
Among participants with CRC, higher pre-diagnostic blood 25(OH)D levels were associated with a significant reduction in CRC-specific and overall mortality. A potential interaction by pre-diagnostic dietary calcium intake was observed, which deserves further investigation. No statistically significant differences in survival were found by genetic variation in the VDR or CASR genes.
Several prospective epidemiologic studies (3-8, 29) including from this cohort (1) have consistently found an inverse association between higher pre-diagnostic 25(OH)D levels and CRC risk. Similar to the results for CRC incidence, higher vitamin D levels have been suggested to be inversely associated with CRC-specific and overall mortality among persons diagnosed with CRC in a small number of studies (14-16). Findings from the Nurses’ Health Study and the Health Professionals Follow-up Study have shown an association between either higher pre-diagnostic 25(OH)D levels(14) or higher predicted post-diagnosis 25(OH)D score (15) and improvement in CRC-specific and overall survival. However, one study (14) was limited by its relatively small sample size and the other (15) by its use of predicted, not actual, post-diagnosis vitamin D levels . Another study from Japan has suggested that higher 25(OH)D levels at surgery are associated with a better survival (16), but it is also limited by small sample size. Our findings are in line with these results supporting the favorable influence of higher pre-diagnostic vitamin D status on the outcomes in patients with CRC.
There is strong experimental evidence supporting the protective effects of vitamin D against CRC development and progression. Proposed mechanisms for these effects involve bile acid catabolism, direct effects on cell proliferation, differentiation, apoptosis, growth factor signaling, immunomodulation, and reduction of invasiveness and angiogenesis (30, 31). Vitamin D appears to be able to act directly on the colorectal mucosa, which has been shown to express vitamin D receptor (VDR) and key vitamin D metabolizing enzymes (32-34), suggesting local production of the active vitamin D hormone (1,25-(OH)-vitamin D) from its main circulating form 25(OH)D. The latter is known to be the best indicator of vitamin D status integrating dietary and supplemental intakes and ultraviolet light exposure (35). It is biologically plausible that vitamin D actions may be modified by calcium intake since vitamin D is a major regulator of calcium homeostasis. Very few studies primarily of colorectal adenomas have shown that either both agents act together to reduce the risk or recurrence of adenomas (36-38) or that the inverse association with 25(OH)D is observed mostly among those with low calcium intake (39). Previous studies considering 25(OH)D-CRC survival association did not observe any interactions with dietary calcium intake (14, 15). However, in the present study we found a suggestion that the reduction in mortality with increasing levels of circulating 25(OH)D may be limited to participants with higher dietary calcium intake. If verified in other studies, calcium supplementation in combination with vitamin D may be potentially useful for improved survival in CRC patients.
Vitamin D acts at least in part through binding with the VDR resulting in an activation of more than 200 vitamin D–responsive genes that are involved in various signaling pathways(40). Though numerous single nucleotide polymorphisms (SNPs) have been discovered in the human VDR gene, only relatively few that are thought to be functionally important have been studied in relation to CRC, and found to be only weakly associated with CRC risk(41-43). No relevant studies on colorectal cancer survival were published. As to survival after other cancer diagnosis, the VDR haplotype (G-T-C,Cdx2-FokI-BsmI) and SNPs related to the lowest VDR expression or function (FokI, Cdx2) were associated with worse survival in patients with advanced non-small-cell lung (17, 18) and epithelial ovarian (44) cancers. The present findings showed no association of the FokI and BsmI genotypes with survival after CRC diagnosis and did not highlight any interactions with circulating 25(OH)D levels. The CASR A986S polymorphism, which may affect the receptor’s function, has been shown to be associated with variation in serum calcium (45). Nevertheless, the present study did not observe any statistically significant associations of the CASR A986S genotypes with CRC survival. But, there was a suggestion that the TT genotype may be associated with a reduction in CRC-specific and overall mortality.
The strengths of our study include its prospective design (though not originally established to investigate cancer prognosis), large size, detailed data on potential confounders, high follow-up rate, geographically diverse European populations, and pre-diagnostic measurement of circulating 25(OH)D level, the best indicator of bodily vitamin D status integrating dietary and supplemental vitamin D intakes and vitamin D internally produced from ultraviolet B exposure to the skin. A key limitation is the use of a single measurement of 25(OH)D taken on average 46 months before cancer diagnosis as a marker of vitamin D exposure, which may not reflect vitamin D status at the time of or after CRC diagnosis. To address this, we performed additional analyses by the time period between blood collection and cancer diagnosis, showing little change in the association between 25(OH)D and mortality. In addition, we assessed any effects of the season or month of blood collection using various approaches that indicated no substantial influence of the seasonal variation in vitamin D status on our results. Another limitation is the unavailability of data on CRC treatment. An assumption may be that CRC treatment may not differ substantially by European country. Nevertheless, to account for this, our analyses were conducted stratified by country of CRC diagnosis and adjusted for year of diagnosis to account for possible changes in the CRC treatment during the period under study. To estimate the effect of missing CRC stage data we have used several approaches (28), all of which have demonstrated the robustness of effect estimates against uncertainties in CRC stage classification. Though the study was the largest compared to other prospective studies on the same topic, it may still be limited for investigation of possible 25(OH)D-diet/lifestyle/gene interactions. It is also possible that higher 25(OH)D levels are acting as a proxy for healthy lifestyle (e.g., high physical activity, lower BMI, healthier diet), which may independently influence CRC survival. However, our results were adjusted for pre-diagnostic BMI and physical activity, though the latter might have been a subject to the measurement error and misclassification in the EPIC study (46). An additional potential limitation which is inherent to all observational studies is the presence of possible residual confounding. However, in our multivariate models a large number of potentially important for CRC survival confounding variables were considered. It is also important to note that cancer survivors are very likely to make lifestyle changes including initiation of vitamin and mineral supplement use after diagnosis (21, 47). Thus, although studies using pre-diagnostic levels of vitamin D might be limited, they are still contributing to the overall evidence that vitamin D may improve survival after CRC diagnosis.
This large and comprehensive study, based on the EPIC cohort has shown that higher blood vitamin D levels before CRC diagnosis are associated with reduction in CRC-specific and overall mortality. Further prognostic studies among cancer patients are needed to determine whether 25(OH)D levels at diagnosis and post-diagnosis correlate with those measured prior to diagnosis, and influence all-cause and disease-specific survival among CRC patients.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Rene Lambert for advice on staging classification of CRC, C. Biessy and B. Hemon for their assistance in database preparation, L. Marie Dit Asse for statistical advice on competing risk analysis, and J. Cremers and P. Beekhof for their excellent laboratory assistance in the blood vitamin D measurement. The work by Dr. V. Fedirko reported in this paper was undertaken during her tenure of a postdoctoral fellowship at the International Agency for Research on Cancer.
Grant Support This work was supported by the World Cancer Research Fund (WCRF), London, UK (grant number 2005/12). The EPIC study was supported by “Europe Against Cancer” Programme of the European Commission (SANCO); Ligue contre le Cancer; Institut Gustave Roussy; Mutuelle Générale de l’Education Nationale; Institut National de la Santé et de la Recherche Médicale (INSERM); German Cancer Aid; German Cancer Research Center; German Federal Ministry of Education and Research; Danish Cancer Society; Health Research Fund (FIS) of the Spanish Ministry of Health; the CIBER en Epidemiología y Salud Pública (CIBERESP), Spain; ISCIII RETIC (RD06/0020); Spanish Regional Governments of Andalusia, Asturias, Basque Country, Murcia (No 6236) and Navarra and the Catalan Institute of Oncology; Cancer Research UK; Medical Research Council, UK; the Hellenic Health Foundation, the Stavros Niarchos Foundation and the Hellenic Ministry of Health; Italian Association for Research on Cancer; Italian National Research Council; Compagnia di San Paolo; Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (The Netherlands); Swedish Cancer Society; Swedish Scientific Council; Regional Governments of Skane and Vasterbotten, Sweden; and Nordforsk centre of excellence programme HELGA.
Role of funding sources The funding sources had no influence on the design of the study; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the paper for publication.
Footnotes
Conflict of interest: None declared.
References
- 1.Jenab M, Bueno-de-Mesquita HB, Ferrari P, van Duijnhoven FJ, Norat T, Pischon T, et al. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations:a nested case-control study. BMJ. 2010;340:b5500. doi: 10.1136/bmj.b5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu K, Feskanich D, Fuchs CS, Willett WC, Hollis BW, Giovannucci EL. A nested case control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J Natl Cancer Inst. 2007;99(14):1120–9. doi: 10.1093/jnci/djm038. [DOI] [PubMed] [Google Scholar]
- 3.Feskanich D, Ma J, Fuchs CS, Kirkner GJ, Hankinson SE, Hollis BW, et al. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2004;13:1502–1508. [PubMed] [Google Scholar]
- 4.Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugane S. Plasma vitamin D and risk of colorectal cancer: the Japan Public Health Center-Based Prospective Study. Br J Cancer. 2007;97(3):446–51. doi: 10.1038/sj.bjc.6603892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garland CF, Comstock GW, Garland FC, Helsing KJ, Shaw EK, Gorham ED. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989;2:1176–1178. doi: 10.1016/s0140-6736(89)91789-3. [DOI] [PubMed] [Google Scholar]
- 6.Tangrea J, Helzlsouer K, Pietinen P, Taylor P, Hollis B, Virtamo J, et al. Serum levels of vitamin D metabolites and the subsequent risk of colon and rectal cancer in Finnish men. Cancer Causes Control. 1997;8:615–625. doi: 10.1023/a:1018450531136. [DOI] [PubMed] [Google Scholar]
- 7.Braun MM, Helzlsouer KJ, Hollis BW, Comstock GW. Colon cancer and serum vitamin D metabolite levels 10-17 years prior to diagnosis. Am J Epidemiol. 1995;142:608–611. doi: 10.1093/oxfordjournals.aje.a117682. [DOI] [PubMed] [Google Scholar]
- 8.Woolcott CG, Wilkens LR, Nomura AM, Horst RL, Goodman MT, Murphy SP, et al. Plasma 25-hydroxyvitamin D levels and the risk of colorectal cancer: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 19(1):130–4. doi: 10.1158/1055-9965.EPI-09-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans SR, Shchepotin EI, Young H, Rochon J, Uskokovic M, Shchepotin IB. 1,25-dihydroxyvitamin D3 synthetic analogs inhibit spontaneous metastases in a 1,2-dimethylhydrazine-induced colon carcinogenesis model. Int J Oncol. 2000;16(6):1249–54. doi: 10.3892/ijo.16.6.1249. [DOI] [PubMed] [Google Scholar]
- 10.Giuliano AR, Franceschi RT, Wood RJ. Characterization of the vitamin D receptor from the Caco-2 human colon carcinoma cell line: effect of cellular differentiation. Arch Biochem Biophys. 1991;285(2):261–9. doi: 10.1016/0003-9861(91)90358-p. [DOI] [PubMed] [Google Scholar]
- 11.Iseki K, Tatsuta M, Uehara H, Iishi H, Yano H, Sakai N, et al. Inhibition of angiogenesis as a mechanism for inhibition by 1alpha-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 of colon carcinogenesis induced by azoxymethane in Wistar rats. Int J Cancer. 1999;81(5):730–3. doi: 10.1002/(sici)1097-0215(19990531)81:5<730::aid-ijc11>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 12.Shabahang M, Buras RR, Davoodi F, Schumaker LM, Nauta RJ, Uskokovic MR, et al. Growth inhibition of HT-29 human colon cancer cells by analogues of 1,25-dihydroxyvitamin D3. Cancer Res. 1994;54(15):4057–64. [PubMed] [Google Scholar]
- 13.Zhao X, Feldman D. Regulation of vitamin D receptor abundance and responsiveness during differentiation of HT-29 human colon cancer cells. Endocrinology. 1993;132(4):1808–14. doi: 10.1210/endo.132.4.8384998. [DOI] [PubMed] [Google Scholar]
- 14.Ng K, Meyerhardt JA, Wu K, Feskanich D, Hollis BW, Giovannucci EL, et al. Circulating 25-hydroxyvitamin d levels and survival in patients with colorectal cancer. J Clin Oncol. 2008;26(18):2984–91. doi: 10.1200/JCO.2007.15.1027. [DOI] [PubMed] [Google Scholar]
- 15.Ng K, Wolpin BM, Meyerhardt JA, Wu K, Chan AT, Hollis BW, et al. Prospective study of predictors of vitamin D status and survival in patients with colorectal cancer. Br J Cancer. 2009;101(6):916–23. doi: 10.1038/sj.bjc.6605262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mezawa H, Sugiura T, Watanabe M, Norizoe C, Takahashi D, Shimojima A, et al. Serum vitamin D levels and survival of patients with colorectal cancer: post-hoc analysis of a prospective cohort study. BMC Cancer. 2010;10:347. doi: 10.1186/1471-2407-10-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heist RS, Zhou W, Wang Z, Liu G, Neuberg D, Su L, et al. Circulating 25-hydroxyvitamin D, VDR polymorphisms, and survival in advanced non-small-cell lung cancer. J Clin Oncol. 2008;26(34):5596–602. doi: 10.1200/JCO.2008.18.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou W, Heist RS, Liu G, Neuberg DS, Asomaning K, Su L, et al. Polymorphisms of vitamin D receptor and survival in early-stage non-small cell lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2239–45. doi: 10.1158/1055-9965.EPI-06-0023. [DOI] [PubMed] [Google Scholar]
- 19.Tretli S, Hernes E, Berg JP, Hestvik UE, Robsahm TE. Association between serum 25(OH)D and death from prostate cancer. Br J Cancer. 2009;100(3):450–4. doi: 10.1038/sj.bjc.6604865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrioli R, Pascucci A, Francini E, Marsili S, Sciandivasci A, De Rubertis G, et al. Weekly high-dose calcitriol and docetaxel in patients with metastatic hormone-refractory prostate cancer previously exposed to docetaxel. BJU Int. 2007;100(4):775–9. doi: 10.1111/j.1464-410X.2007.07019.x. [DOI] [PubMed] [Google Scholar]
- 21.Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, Bowen DJ. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. J Am Diet Assoc. 2003;103(3):323–8. doi: 10.1053/jada.2003.50045. [DOI] [PubMed] [Google Scholar]
- 22.Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5(6B):1113–24. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 23.Bingham S, Riboli E. Diet and cancer--the European Prospective Investigation into Cancer and Nutrition. Nat Rev Cancer. 2004;4(3):206–15. doi: 10.1038/nrc1298. [DOI] [PubMed] [Google Scholar]
- 24.Jenab M, McKay J, Bueno-de-Mesquita HB, van Duijnhoven FJ, Ferrari P, Slimani N, et al. Vitamin D receptor and calcium sensing receptor polymorphisms and the risk of colorectal cancer in European populations. Cancer Epidemiol Biomarkers Prev. 2009;18(9):2485–91. doi: 10.1158/1055-9965.EPI-09-0319. [DOI] [PubMed] [Google Scholar]
- 25.Rosthoj S, Andersen PK, Abildstrom SZ. SAS macros for estimation of the cumulative incidence functions based on a Cox regression model for competing risks survival data. Comput Methods Programs Biomed. 2004;74(1):69–75. doi: 10.1016/S0169-2607(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 26.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96(7) doi: 10.1210/jc.2011-0385. doi:10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 27.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832–8. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 28.Nur U, Shack LG, Rachet B, Carpenter JR, Coleman MP. Modelling relative survival in the presence of incomplete data: a tutorial. Int J Epidemiol. 2010;39(1):118–28. doi: 10.1093/ije/dyp309. [DOI] [PubMed] [Google Scholar]
- 29.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O’Sullivan MJ, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354(7):684–96. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 30.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer. 2003;3(8):601–14. doi: 10.1038/nrc1144. [DOI] [PubMed] [Google Scholar]
- 31.Bostick RM, Goodman M, Sidelnikov E. Calcium and vitamin D. In: Potter JD, Lindor NM, editors. Genetics of Colorectal Cancer. Springer Science + Business Media, LLC; New York, NY: 2009. pp. 277–296. [Google Scholar]
- 32.Matusiak D, Murillo G, Carroll RE, Mehta RG, Benya RV. Expression of vitamin D receptor and 25-hydroxyvitamin D3-1{alpha}-hydroxylase in normal and malignant human colon. Cancer Epidemiol Biomarkers Prev. 2005;14(10):2370–2376. doi: 10.1158/1055-9965.EPI-05-0257. [DOI] [PubMed] [Google Scholar]
- 33.Tangpricha V, Flanagan JN, Whitlatch LW, Tseng CC, Chen TC, Holt PR, et al. 25-hydroxyvitamin D-1alpha-hydroxylase in normal and malignant colon tissue. Lancet. 2001;357(9269):1673–4. doi: 10.1016/S0140-6736(00)04831-5. [DOI] [PubMed] [Google Scholar]
- 34.Matusiak D, Benya RV. CYP27A1 and CYP24 expression as a function of malignant transformation in the colon. J Histochem Cytochem. 2007;55(12):1257–64. doi: 10.1369/jhc.7A7286.2007. [DOI] [PubMed] [Google Scholar]
- 35.Prentice A, Goldberg GR, Schoenmakers I. Vitamin D across the lifecycle: physiology and biomarkers. Am J Clin Nutr. 2008;88(2):500S–506S. doi: 10.1093/ajcn/88.2.500S. [DOI] [PubMed] [Google Scholar]
- 36.Grau MV, Baron JA, Sandler RS, Haile RW, Beach ML, Church TR, et al. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst. 2003;95:1765–1771. doi: 10.1093/jnci/djg110. [DOI] [PubMed] [Google Scholar]
- 37.Peters U, McGlynn KA, Chatterjee N, Gunter E, Garcia-Closas M, Rothman N, et al. Vitamin D, calcium, and vitamin D receptor polymorphism in colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2001;10:1267–1274. [PubMed] [Google Scholar]
- 38.Oh K, Willett WC, Wu K, Fuchs CS, Giovannucci EL. Calcium and vitamin D intakes in relation to risk of distal colorectal adenoma in women. Am J Epidemiol. 2007;165:1178–1186. doi: 10.1093/aje/kwm026. [DOI] [PubMed] [Google Scholar]
- 39.Levine AJ, Harper JM, Ervin CM, Chen YH, Harmon E, Xue S, et al. Serum 25-hydroxyvitamin D, dietary calcium intake, and distal colorectal adenoma risk. Nutr Cancer. 2001;39:35–41. doi: 10.1207/S15327914nc391_5. [DOI] [PubMed] [Google Scholar]
- 40.Ebert R, Schutze N, Adamski J, Jakob F. Vitamin D signaling is modulated on multiple levels in health and disease. Mol Cell Endocrinol. 2006;248:149–159. doi: 10.1016/j.mce.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 41.McCullough ML, Bostick RM, Mayo TL. Vitamin D gene pathway polymorphisms and risk of colorectal, breast, and prostate cancer. Annu Rev Nutr. 2009;29:111–32. doi: 10.1146/annurev-nutr-080508-141248. [DOI] [PubMed] [Google Scholar]
- 42.Touvier M, Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, et al. Meta-analyses of vitamin D intake, 25-hydroxyvitamin D status, vitamin D receptor polymorphisms and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2011 doi: 10.1158/1055-9965.EPI-10-1141. [DOI] [PubMed] [Google Scholar]
- 43.Jenab M, McKay J, Bueno-de-Mesquita HB, van Duijnhoven FJ, Ferrari P, Slimani N, et al. Vitamin D receptor and calcium sensing receptor polymorphisms and the risk of colorectal cancer in European populations. Cancer Epidemiol Biomarkers Prev. 2009;18(9):2485–91. doi: 10.1158/1055-9965.EPI-09-0319. [DOI] [PubMed] [Google Scholar]
- 44.Tamez S, Norizoe C, Ochiai K, Takahashi D, Shimojima A, Tsutsumi Y, et al. Vitamin D receptor polymorphisms and prognosis of patients with epithelial ovarian cancer. Br J Cancer. 2009 doi: 10.1038/sj.bjc.6605414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kapur K, Johnson T, Beckmann ND, Sehmi J, Tanaka T, Kutalik Z, et al. Genome-wide meta-analysis for serum calcium identifies significantly associated SNPs near the calcium-sensing receptor (CASR) gene. PLoS Genet. 2010;6(7):e1001035. doi: 10.1371/journal.pgen.1001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedenreich C, Cust A, Lahmann PH, Steindorf K, Boutron-Ruault MC, Clavel-Chapelon F, et al. Physical activity and risk of endometrial cancer: the European prospective investigation into cancer and nutrition. Int J Cancer. 2007;121(2):347–55. doi: 10.1002/ijc.22676. [DOI] [PubMed] [Google Scholar]
- 47.Velicer CM, Ulrich CM. Vitamin and mineral supplement use among US adults after cancer diagnosis: a systematic review. J Clin Oncol. 2008;26(4):665–73. doi: 10.1200/JCO.2007.13.5905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


