Abstract
Background
Culex tritaeniorhynchus is the primary vector of Japanese encephalitis virus (JEV), a leading cause of encephalitis in Asia. JEV is transmitted in an enzootic cycle involving large wading birds as the reservoirs and swine as amplifying hosts. The development of a JEV vaccine reduced the number of JE cases in regions with comprehensive childhood vaccination programs, such as in Japan and the Republic of Korea. However, the lack of vaccine programs or insufficient coverage of populations in other endemic countries leaves many people susceptible to JEV. The aim of this study was to predict the distribution of Culex tritaeniorhynchus using ecological niche modeling.
Methods/Principal Findings
An ecological niche model was constructed using the Maxent program to map the areas with suitable environmental conditions for the Cx. tritaeniorhynchus vector. Program input consisted of environmental data (temperature, elevation, rainfall) and known locations of vector presence resulting from an extensive literature search and records from MosquitoMap. The statistically significant Maxent model of the estimated probability of Cx. tritaeniorhynchus presence showed that the mean temperatures of the wettest quarter had the greatest impact on the model. Further, the majority of human Japanese encephalitis (JE) cases were located in regions with higher estimated probability of Cx. tritaeniorhynchus presence.
Conclusions/Significance
Our ecological niche model of the estimated probability of Cx. tritaeniorhynchus presence provides a framework for better allocation of vector control resources, particularly in locations where JEV vaccinations are unavailable. Furthermore, this model provides estimates of vector probability that could improve vector surveillance programs and JE control efforts.
Author Summary
Japanese encephalitis virus (JEV) is transmitted predominately by the mosquito, Culex tritaeniorhynchus. The primary reservoirs of the virus are wading birds, with swine serving as amplifying hosts. Despite the development of a JEV vaccine, people remain unvaccinated in endemic countries and are susceptible to JEV infection. The distribution of the JEV vector(s) provides essential information for preventive measures. This study used an ecological niche modeling program to predict the distribution of Cx. tritaeniorhynchus based on collection records and environmental maps (climate, land cover, and elevation). The model showed that the mean temperatures of the wettest quarter had the greatest impact on the model. Of the 25 countries endemic for Japanese encephalitis (JE) endemic countries, seven possessed greater than 50% land area with an estimated high probability of Cx. tritaeniorhynchus presence. Our model provides a useful tool for JEV surveillance programs that focus on vector control strategies.
Introduction
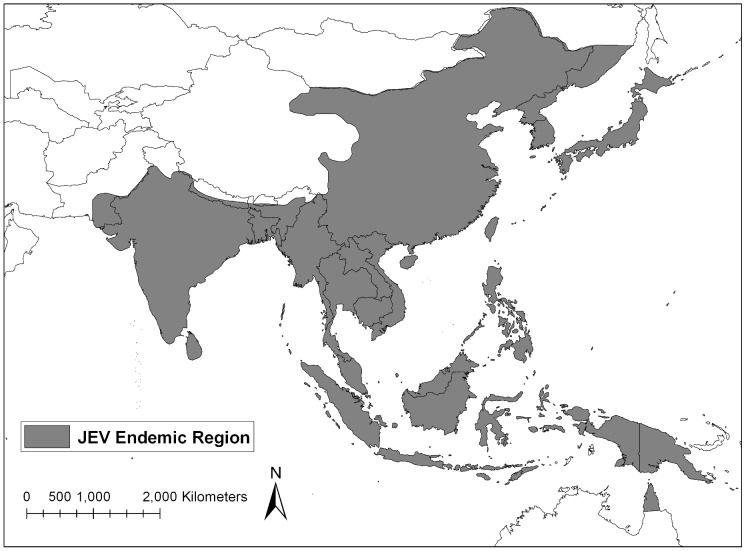
Japanese encephalitis virus (JEV), the causative agent of Japanese encephalitis (JE), is an arbovirus that belongs to the family Flaviviridae and is endemic to Southeast and Northeast Asia, the Pacific Islands, and northern Australia (Figure 1) [1]. The primary vector of JEV is Culex tritaeniorhynchus Giles, but other Culex species (e.g., Culex annulirostris, Culex vishnui Theobald, Culex bitaeniorhynchus Giles, and Culex pipiens Linnaeus) have also been implicated as important viral transmitters [2], [3], [4], [5]. The larval habitat of Cx. tritaeniorhynchus is primarily low lying flooded areas containing grasses and flooded rice paddies, but this species can also be found in urban environments in close proximity to human populations [6]. Within the past 40 years, rice agriculture in JEV endemic countries has increased by 20%, thereby expanding Cx. tritaeniorhynchus habitat and increasing human risk of exposure to vector populations [7].
Figure 1. Japanese encephalitis virus endemic area.
Map adapted from CDC.
Swine, including domestic and feral pigs, serve as amplifying hosts of JEV in endemic areas. The proximity of human populations to pig farms, sties or feral pig populations increases the risks of JEV exposure [8], [9]. Ardeid birds (large wading birds) are an important JEV reservoir and can spread JEV to new regions through their northern migration to breeding and feeding grounds in the spring and southern return in the fall [3]. Additional animals have been identified as host species for JEV, including domesticated animals (chickens, goats, cows, and dogs), as well as bats, flying foxes, ducks, snakes and frogs. However, these are considered dead-end hosts as they infrequently develop sufficient viremias to infect mosquito vectors [10], [11], [12], [13].
Despite the introduction of an effective vaccine to the public in the mid-1900s, JEV remains the leading cause of viral encephalitis globally [14]. Comprehensive vaccination programs in Japan, Republic of Korea (ROK), Brunei, Australia, and Malaysia have significantly reduced the number of human cases [15]. Rare occurrences of neurological complications associated with the mouse-brain derived JEV vaccine interrupted vaccination programs in some regions, initiating concerns of the reemergence of JEV in an unvaccinated and non-immune population [16], [17]. The prevalence of JE is higher in countries with lower socioeconomic status, when compared to more affluent neighboring countries, indicating the importance of economic and social stability as additional risk factors that impact the transmission and prevalence of JE in non-immune populations [15].
Recent developments in the field of ecological niche modeling and the development of global environmental data sets have resulted in the ability to predict the distribution of vector populations that directly relate to transmission of viruses, parasites, and fungal pathogens and impact on animal and human health. Modeling to estimate the distribution of disease vectors provides useful information in disease-endemic areas, in addition to predicting how anthropogenic changes to the environment will affect disease presence [18], [19], [20], [21].
In the current study, the Maxent ecological niche modeling program was utilized to model the distribution of the primary vector of JEV, Cx. tritaeniorhynchus [22]. The resulting vector habitat suitability map was compared to the reported locations of JE human cases and the current status of established JE vaccination programs by country. Our ecological niche model can be used by public health officials and government agencies in endemic regions to guide implementation of comprehensive vaccination programs, vector control strategies, and public health awareness campaigns.
Methods
Culex tritaeniorhynchus Data Collection
Geographical coordinates of known Cx. tritaeniorhynchus records were identified by performing a literature search in PubMed for all previous field collection studies. When exact geographical coordinates were not provided, locations were approximated by searching for the given city, town, or village using Google Earth software. Further geographical data points for the distribution of Cx. tritaeniorhynchus were obtained through MosquitoMap (http://www.mosquitomap.org/), a database of spatial data points of mosquitoes that is maintained by the Walter Reed Biosystematics Unit, Smithsonian Support Center, Silver Hill, MD. Additional Cx. tritaeniorhynchus collection data were obtained from Force Health Protection and Preventive Medicine, 65th Medical Brigade, Yongsan Army Garrison, ROK.
In previous modeling work in the ROK [20], we found that a large number of collection records in a limited geographical area biased the model. As a result of the large number of collection sites for the ROK (96 unique locations), we reduced the number of records to 23 by deleting all but one randomly selected record per administrative district.
Identification of Japanese Encephalitis (JE) Human Cases
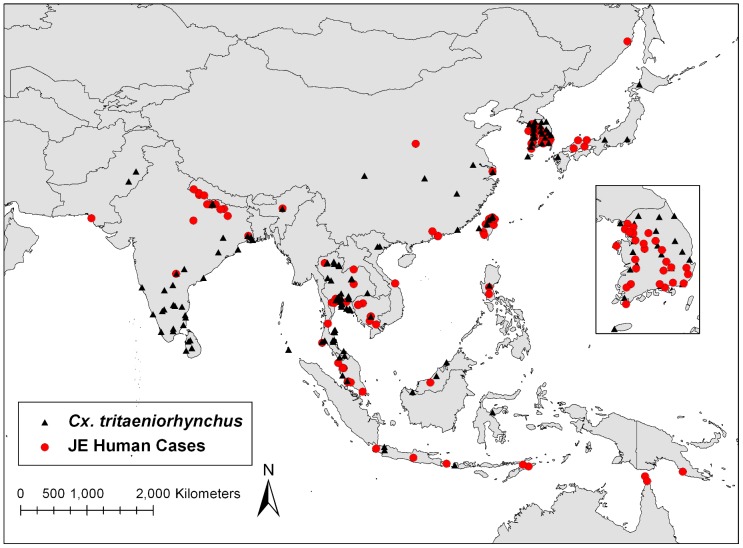
Approximate locations of known human JE cases were determined using locations provided in ProMED mail reports (www.promedmail.org) from 1994 through 2010 (Figure 2). Additional locations of confirmed JE cases were also determined through a PubMed literature search. Exact geographical coordinates were not reported for most documented human cases and were therefore extrapolated using the Google Earth software to obtain the latitude/longitude coordinates of the reported city, town, or village in which JE was documented.
Figure 2. Distribution of known Cx. tritaeniorhynchus locations and documented human cases of JE within endemic region.
Vaccination Programs in JEV Endemic Countries
JEV vaccination program information was obtained from “Japanese Encephalitis Morbidity, Mortality and Disability: Reduction and Control by 2015” published in 2009 by the Program for Appropriate Technology in Health (PATH), Armed Forces Research Institute of Medical Sciences, and BIKEN [15]. Additionally, JEV vaccination programs information was also obtained from the WHO/IVB database [23]. Countries lacking a JEV vaccination program were identified using information in the above mentioned publications and confirmed with additional literature searches. A summary of these data are listed in Table 1.
Table 1. Summary of JEV vaccination programs in endemic countries and predicted percentage of land with greater than 25% estimated probability of Cx. tritaeniorhynchus presence based on ecological niche model.
| JEV Endemic Countries | JE vaccination program status1 | Percentage of area >25% estimated probability of Cx. tritaeniorhynchus presence |
| Australia | Administered in endemic areas | 4.6 |
| Bangladesh | No Current Immunization Program | 56.8 |
| Bhutan | No Current Immunization Program | 0 |
| Brunei | No Current Immunization Program | 16.3 |
| Cambodia | No Current Immunization Program | 79.4 |
| China | National Vaccination Program 2010 | 3.6 |
| East Timor | No Current Immunization Program | 68.4 |
| India | Vaccine administered in high risk areas, not integrated into routine immunization program | 19.2 |
| Indonesia | No Current Immunization Program | 14 |
| Japan | National Vaccination Program 2010 | 42.4 |
| Laos | No Current Immunization Program | 19.7 |
| Malaysia | Regional vaccination | 8.4 |
| Myanmar | No Current Immunization Program | 20.5 |
| Nepal | Vaccine introduced in 2006, not widely implemented | 2.8 |
| Democratic People's Republic of Korea | DPRK originated vaccine provided in high risk areas | 21.1 |
| Pakistan | No Current Immunization Program | 0.06 |
| Papua New Guinea | No Current Immunization Program | 11.1 |
| Philippines | No wide scale vaccination program in place , vaccine trial in progress | 46 |
| Republic of Korea | Government mandated mass immunization began in 1971 | 78.8 |
| Russia | No data | 0.01 |
| Singapore | No data | 10.7 |
| Sri Lanka | 18 of 26 districts receive vaccine annually, plans to extend to all districts | 85.2 |
| Taiwan | National vaccination program | 34.2 |
| Thailand | National Vaccination Program 2010 | 80.9 |
| Viet Nam | Vaccine distributed in high risk districts | 61 |
| Western Pacific (Guam, Saipan) | No data | No data |
Obtained from PATH: Japanese Encephalitis Morbidity, Mortality, and Disability: Reduction and Control by 2015 (ref.16) and WHO/IVB database, 193 WHO Member States. Data as of September 2011 (ref. 24).
Environmental Data
One kilometer resolution climate and elevation data were obtained from WorldClim (http://www.worldclim.org/bioclim). The WorldClim organization has processed 50 years of ground-based weather measurements to produce mean monthly minimum and maximum temperatures and precipitation in a grid format at several different resolutions. The data were further processed to produce bioclimatic variables (e.g., mean temperatures of the wettest quarters). For this project, the highest resolution data available from WorldClim (approximately 1 km) were downloaded. In addition to bioclimatic variables, global elevation data obtained from WorldClim was re-sampled to 1-km resolution from NASA's Shuttle Radar Topography Mission (SRTM). Descriptions of the bioclimatic and elevation variables used for this study are listed in Table 2. To better understand the effect of each environmental variable on Cx. tritaeniorhynchus distribution, the values of each environmental layer at each site were extracted using ArcGIS (ESRI, Redlands, California, www.esri.com). This allowed for a comparison to the known environmental and distribution limitations for Cx. tritaeniorhynchus in the literature.
Table 2. Minimum, maximum, mean values and percent contribution of environmental data layers for Cx. tritaeniorhynchus model.
| Variable1 | Description | Min | Max | Mean | Percent Contribution |
| Alt | Altitude (elevation above sea level), m | 0 | 838 | 153.4 | 9.6 |
| Bio01 | Annual mean temperature, °C | 8.2 | 28.9 | 23.3 | 4.4 |
| Bio02 | Mean diurnal range (Mean of monthly (max temp-min temp)), °C | 4.9 | 15 | 9.4 | 4.7 |
| Bio03 | Isothermality [(Bio2/Bio7)*100], °C | 2.2 | 9 | 5.1 | 1.8 |
| Bio04 | Temperature Seasonality (standard deviation * 100), °C | 30.1 | 1031.3 | 366 | 3.3 |
| Bio05 | Max temperature of the warmest month, °C | 25.8 | 42.5 | 33.4 | 1.2 |
| Bio06 | Min temperature of the coldest month, °C | −12.5 | 24.4 | 12.4 | 3.3 |
| Bio07 | Temperature annual range (Bio5-Bio6), °C | 7.2 | 40.7 | 21 | 3.2 |
| Bio08 | Mean temperature of the wettest quarter1, °C | 16.9 | 32.8 | 262 | 21.7 |
| Bio09 | Mean temperature of the driest quarter, °C | −4.9 | 28.7 | 19.5 | 0.3 |
| Bio10 | Mean temperature of the warmest quarter, °C | 20.1 | 34.3 | 27.7 | 0.6 |
| Bio11 | Mean temperature of the coldest quarter, °C | −4.9 | 27.2 | 18.3 | 1 |
| Bio12 | Annual precipitation, mm | 152 | 4005 | 1610.3 | 16.2 |
| Bio13 | Precipitation of the wettest month, mm | 41 | 1011 | 319.4 | 5.9 |
| Bio14 | Precipitation of the driest month, mm | 0 | 233 | 30.8 | 6 |
| Bio15 | Precipitation seasonality (coefficient of variation), mm | 18 | 138 | 74.5 | 5.8 |
| Bio16 | Precipitation of the wettest quarter, mm | 95 | 2455 | 797.4 | 1 |
| Bio17 | Precipitation of the driest quarter, mm | 0 | 786 | 114.9 | 0.7 |
| Bio18 | Precipitation of the warmest quarter, mm | 62 | 1015 | 467.2 | 0.7 |
| Bio19 | Precipitation of the coldest quarter, mm | 11 | 1812 | 260.7 | 8.5 |
A quarter is a period of three months.
A map of rice growing areas was created by processing GeoCover-LC (Land Cover) data from MDA Information Systems, Inc. (http://www.mdafederal.com/geocover/geocoverlc). GeoCover was created by processing Landsat Thematic Mapper images to create land cover maps for most areas of the world. Each pixel within the GeoCover-LC represents 30 by 30 meters. To convert the image to a resolution that could be used in the Maxent model, ArcGIS was used to count the number of rice pixels within each square kilometer (33 by 33 pixels). Then, a rice percentage was calculated for each square kilometer (number of rice pixels divided by total number of pixels in 1 km) and stored in a final output image.
Ecological Niche Model
The Maxent 3.2.1 modeling program (http://www.cs.princeton.edu/~schapire/maxent/) was utilized to model the distribution of Cx. tritaeniorhynchus based on previously obtained geographical locations. Maxent utilizes a maximum entropy algorithm to analyze values of environmental layers, such as temperature, precipitation, and elevation, at known locations of species occurrence (collection records) to estimate the probable range of the species over a geographic region [22], [24]. This model is based on presence-only data instead of presence/absence data due to the lack of available absence data. Although absence data can be informative for modeling, ecological niche models based on presence-only data are useful in regions with limited collection data [22]. Without absence data, the true probability of presence cannot be modeled. In Maxent, which uses presence only data, the species distribution is output as an estimated probability map [25].
The Maxent program calculates the importance of environmental variables in developing predictive species distribution models by using the jackknife test of variable importance. The jackknife test runs the model 1) once with all variables, 2) dropping out each variable in turn, and 3) with a single variable at a time. Variables are considered import if they produce high training gains when used alone in a model. A variable is also important if the training gain is low when the variable is removed from the model [22].
Maxent utilizes two approaches to validate the accuracy of the model. The first method randomly selects occurrence points to be withheld from the model building to use as testing points. Using multiple definitions, a set of thresholds split the continuous probability values of the model into ‘predicted presence or absence’ categories. Maxent then calculates the p-value based on the null hypothesis that testing points will be predicted as “present” no better than by a random model. The second method calculates the Area Under the Curve (AUC) of the receiver operator characteristic (ROC), a graphical depiction of the sensitivity versus one (1) minus the specificity of the model often used to validate ecological niche models [22], [26]. The AUC indicates whether the model predicts species location better than a random distribution. AUC values of ≤0.5 indicates a random distribution and AUC values >0.9 indicates high reliability of the model [22]. To determine the best combination of environmental data for modeling, the model was run four times using different sets of input layers each time: 1) bioclimatic layers, elevation and rice crop data, 2) bioclimatic layers and elevation data, 3) bioclimatic layers only, and 4) elevation data only.
Results
Ecological Niche Model of Cx. tritaeniorhynchus
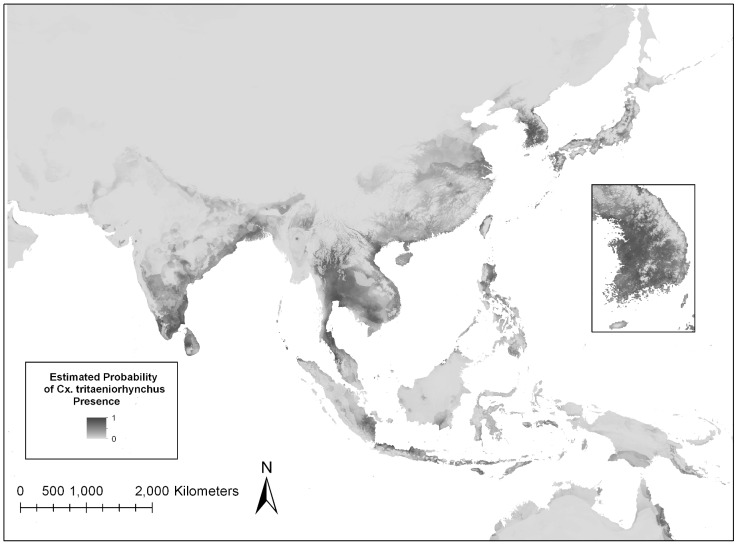
A total of 139 unique sites of documented Cx. tritaeniorhynchus geographical locations were utilized to construct the ecological niche model (Figure 3). Of the 139 total points, 105 (76%) were randomly designated as training points in order to build the model and 34 (24%) points were used to test the model. The model was run four times using different combinations of environmental layers (Table 3). Statistical results indicate that the most accurate model included bioclimatic layers and elevation (Table 3), and therefore this model was used in all subsequent analyses. Statistical evaluation showed the model to have a high accuracy, with the AUC>0.9 and low p-values. The model is available to view or download from www.vectormap.org.
Figure 3. Maxent model estimation of the probability of Cx. tritaeniorhynchus distribution in the JE endemic region.
Darker areas indicate areas that are likely to have suitable habitat for this vector species while lighter areas indicate areas of that are less suitable for the vector.
Table 3. Maxent model accuracy analysis using different sets of environmental data inputs.
| Environmental Data Input | AUC Training Points | AUC Test Points | P-value Minimum Training Presence |
| Bioclimatic and Elevation data | 0.971 | 0.932 | <0.0001 |
| All layers (including rice crop) | 0.968 | 0.919 | <0.0001 |
| Bioclimatic data only | 0.968 | 0.929 | <0.0001 |
| Elevation data only | 0.822 | 0.849 | <0.0001 |
In order to evaluate the contribution of each environmental variable to the model, Maxent utilizes a jackknife test, which indicated that the annual precipitation (bio12) environmental layer is the environmental variable with the highest gain when used in the model by itself. The Maxent program also calculates a percent contribution for each variable in the model. The annual precipitation variable contributed 16.2% of the information used by the model, another indication that it is an important environmental factor for estimating the distribution of Cx. tritaeniorhynchus (Table 2). The mean temperature of the wettest quarter variable (bio08) contributed the highest percentage (21.7%) of the information to the model. Elevation was also an important variable, contributing 9.6% to the model. From the jackknife test, if elevation data were removed from the model, the overall training gain would decrease the most, indicating the elevation variable contained the most unique information of the variables in the Cx. tritaeniorhynchus distribution model.
The values of each environmental variable at each recorded location of occurrence were extracted using ArcGIS (Table 1). For example, the known locations for Cx. tritaeniorhynchus used in the model fell within 0 and 838 meters of elevation. This is consistent with the published reports that Cx. tritaeniorhynchus is rarely collected above 1,000 meters [27], [28].
Human JE Cases and Vector Presence Estimation
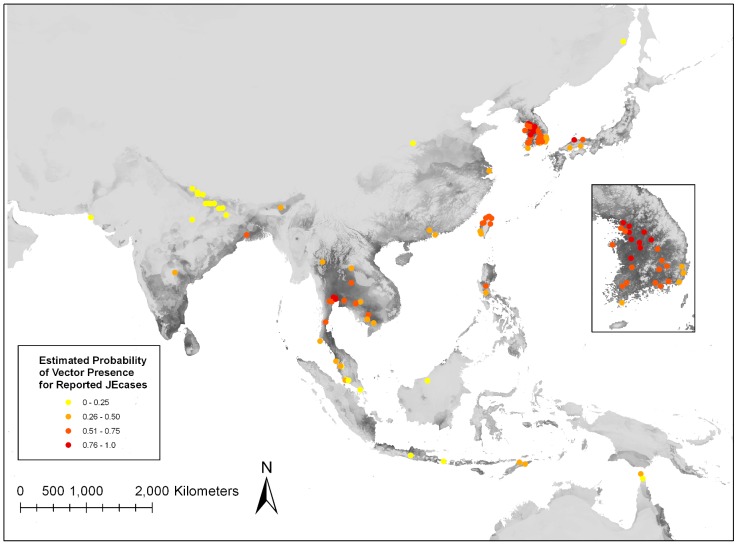
Ninety-six reported JE case locations were identified in endemic regions (Figure 2). ArcGIS analysis categorized human JE cases based on the estimated probability of Cx. tritaeniorhynchus presence (Figure 4). Human JE cases were identified at locations with a range of estimated probability of vector presence, including regions with 25% or less estimated probability. However, the majority (>75%) of human JE cases were reported from regions with greater than 25% estimated probability of Cx. tritaeniorhynchus presence. Limited availability of location data of human JE cases greatly impacts any associations between areas of high estimated vector probability and disease. For instance, the lack of human JE cases in other regions of estimated high probability of vector presence could be due to lack of reporting, improper diagnosis, or due to successful prevention strategies.
Figure 4. Human JE cases categorized by color based on the estimated probability of Cx. tritaeniorhynchus presence.
Cx. tritaeniorhynchus Presence Estimation Per Country
ArcGIS analysis determined the approximate percentage of each country with >25% probability of Cx. tritaeniorhynchus presence based on the Maxent model (Table 1). Of the 25 endemic countries, seven possessed >50% of their land area with a higher probability of Cx. tritaeniorhynchus presence. Three countries (Bhutan, Pakistan, and Russia) possessed <1% of their total country area with a 25% probability of Cx. tritaeniorhynchus presence.
Discussion
In this study, a statistically significant ecological niche model for Cx. tritaeniorhynchus was developed using mosquito presence records, climate, and elevation variables. Locations of human cases of JE generally fell within the higher probability areas of Cx. tritaeniorhynchus (Figure 4). Regions of estimated high probability of Cx. tritaeniorhynchus presence (Figure 3) are representative of preferred environments, based on temperature, precipitation and elevation where Cx. tritaeniorhynchus habitats occur. This model serves as a tool to fill in knowledge gaps regarding Cx. tritaeniorhynchus and can be utilized by health care professionals and policy officials in endemic regions to help guide the development and implementation of disease mitigating strategies in endemic regions.
The Maxent program identifies important environmental variables that are major contributors to the vector distribution model. Based on the jackknife test of variable importance, the annual precipitation (bio12) is an important contributor to the model. Additionally, the mean precipitation of the wettest quarters (bio08) and elevation also contributed greatly to the model for distribution of Cx. tritaeniorhynchus (Table 2). Previous studies that aimed to identify favorable ecological conditions of mosquitoes found that the optimal temperature of JEV vectors is between 22.8 and 34.5°C [29]. The importance of temperature during the wet season in the model is attributed to temperatures and flooded habitats that are optimal for larval development and adult survival. Locations in which the temperatures do not fall into the optimal range during the rainy season may therefore experience fewer mosquitoes, despite harboring the appropriate habitat. Temperature also plays a role in disease transmission rates, as higher temperatures increase the rates of virus replication and dissemination, while decreasing the time from mosquito infection to transmission of the virus to animal and human hosts [30].
Sampling bias is an issue that affects the accuracy of the model as the model was developed using existing data from the literature and VectorMap. Therefore, some regions have not been sampled in the study area and some have been oversampled. Cx. tritaeniorhynchus data for China were very limited (Figure 2), which may mean that some potential environmental conditions of Cx. tritaeniorhynchus were not represented in the model, in particular, the cooler Northeast region of China. Because modeling was limited to Cx. tritaeniorhynchus, there is a potential that for some regions, other primary or secondary vectors, i.e., Cx. annulirostris, Cx. bitaeniorhynchus, and Cx. vishnui, may predominate and maintain transmission of JEV in these areas. Collection records of Cx. tritaeniorhynchus were obtained spanning many decades and at different times during the year, furthering the impact of sampling bias on our model. In addition, the density of Cx. tritaeniorhynchus was not collected in this study and is an important limitation as vector abundance plays a crucial role in disease transmission. Further collection studies are therefore needed to determine the abundance of vector species in addition to presence in endemic regions.
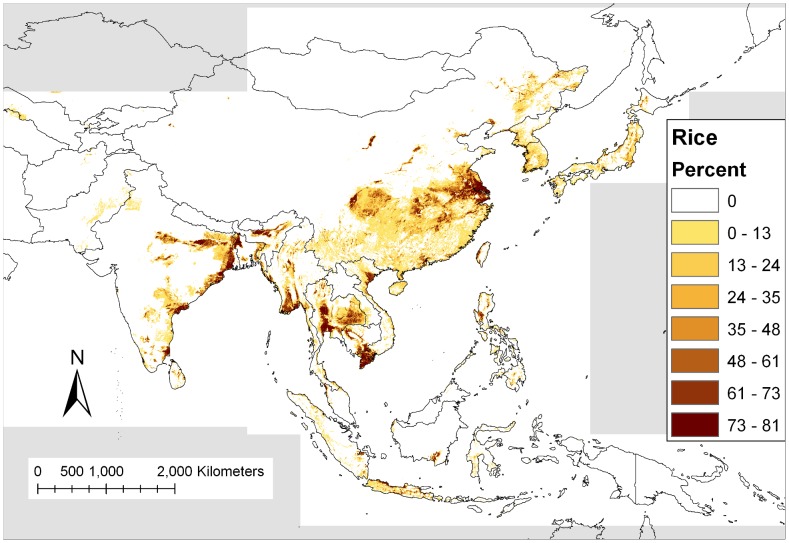
Low-lying flooded areas containing grasses, including rice paddies, are the primary larval habitats for Cx. tritaeniorhynchus. An increase in the amount of flooded rice field habitat has shown to be positively correlated with increases in adult populations of Cx. tritaeniorhynchus in the ROK [31]. Although the rice map derived from the GeoCover Land Cover map (Figure 5) does generally match the predicted occurrence of Cx. tritaeniorhynchus, there are some areas where the model predicts the presence of the mosquito, yet no rice crops were mapped. For some areas, rice may not have been identified correctly on the satellite images, since agricultural areas were limited or were adjacent to other predominant habitats. For example, rice is produced in Nepal [32], but no rice fields were identified by GeoCover in Nepal, since the identification of small rice fields in mountainous areas on satellite images can be difficult. Alternatively, this shows that environments other than rice fields are suitable habitat for Cx. tritaeniorhynchus.
Figure 5. Percent of 30 meter pixels classified as rice land cover within 1 one square kilometer derived from the GeoCover Land Cover product.
Gray areas indicate no data.
The predicted probability of Cx. tritaeniorhynchus presence values were used to determine the percentage of a country at high risk (greater than 25% probability) for vector presence (Table 1). Many Asian countries have high percentages of their total land area with a >25% probability for the presence of Cx. tritaeniorhynchus. Cambodia, the ROK, Sri Lanka, and Thailand have over 75% of their land area with a >25% probability of Cx. tritaeniorhynchus presence. Countries demonstrating >50% of their total land area and with Cx. tritaeniorhynchus occurrence >25% probability includes: Bangladesh, East Timor, and Vietnam. However, some countries may have small areas of vector habitat close to large populations that can result in outbreaks despite low percentage estimated probability in the region overall. Further, this analysis does not take into account country size or vector abundance, which would also impact disease transmission. Although additional factors contribute to JE disease risks, the distribution of the vector populations within a country is a valuable data set when considering the necessity of vaccination and other health risk reduction programs.
Human JE cases were categorized based on the estimated probability of vector presence at the reported location (Figure 4). Interestingly, a portion of human cases were reported from regions with 25% or less estimated probability of Cx. tritaeniorhynchus presence. JE cases not falling within high probability pixels could have been acquired in nearby locations. Even for precisely located case data, the high resolution of the model (one kilometer pixels) increases the likelihood that the predicted Cx. tritaeniorhynchus location does not match disease acquisition location as many people travel more than a kilometer in the course of a typical day. Alternatively, additional factors other than the presence of Cx. tritaeniorhynchus may be important when determining the risk of disease. For instance, other JEV vectors may dominant in these regions estimated with low Cx. tritaeniorhynchus presence. JE is one of many febrile illnesses that affect human populations in Asia. Difficulties arise in diagnosis of JE in patients based on symptoms alone that range from mild to very severe, with laboratory tests required for confirmation. Obtaining geographical data of where human cases were acquired is made difficult due to lack of confident diagnoses, patient travel history, and spatial data. The lack of precision of the reported case locations may also contribute to lower numbers of JE cases falling within high probability Cx. tritaeniorhynchus pixels. Identification of human JE cases in this study is extremely limited and does not represent all human cases in JE endemic regions. In many cases, only a village or city name was given for reported cases. A previous study to model the distribution of Cx. tritaeniorhynchus to predict JE in the Republic of Korea found human cases to occur in areas of high estimated probability of vector presence [20]. This study, however, utilized intensive vector collection methods and JE case data were obtained from the Korea Centers for Disease Control resulting in an overall more extensive and accurate model. This illustrates the need for increased surveillance of vector and human JE cases in order to generate more accurate risk models for JE. In order to evaluate the impact of vector presence on the risk of JE in humans, comprehensive efforts to identify specific locations of both symptomatic and asymptomatic JE cases across endemic regions are needed.
Ecological niche modeling inherently possesses limitations in that it makes predictions based solely on environmental variables that impact larval development and adult survival. Other important factors that influence vector distributions include: vector control strategies, public health campaigns, socioeconomic status, human population densities, anthropogenic changes to land (creation of vector habitat), vector species competition, and predator influences on their potential distribution and population densities. Further, the use of WorldClim data may underestimate or ignore environmental variables that occur during a short time period or transient habitat suitable for the vector to survive. Incorporation of these variables will undoubtedly increase the validity of the model. These factors are also important to take into consideration when implementing mosquito control initiatives and vaccination campaigns.
The reemergence of JEV remains possible due to multiple factors. Increases in the pig farming industry, modification and expansion of arable lands for wetland rice farming, and a fraction of the population unvaccinated/non-immune, in combination with optimal climatic conditions, contribute to the potential for periodic outbreaks of JE as the one observed in the ROK [9]. Genotype analyses of circulating JEV strains identified the reemergence of genotype V, which was unseen in Asia for over 50 years [33], [34]. The identification of emerging/reemerging JEV strains is important for vaccine development and the implementation of effective vaccination programs. Increased surveillance in areas with known vector populations and additional risk factors, such as reservoir and amplifying hosts, will aid in the identification of circulating JEV strains as well as strains that are emerging in novel human and vector populations. Understanding the vector distribution is a key step to effectively understanding JEV risks and also to preventing additional outbreaks of JE in endemic countries.
Footnotes
The authors have declared that no competing interests exist.
This project was funded by the Armed Forces Health Surveillance Center, Division of Global Emerging Infections Surveillance & Response System (GEIS) Operations (http://afhsc.mil/geis), grant: C0648_12_MH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hills SL, Nett RJ, Fischer M. Brunette GW, editor. CDC Health Information for International Travel 2012. 2011. Infectious Diseases Related to Travel: Japanese encephalitis: Oxford University Press.
- 2.Bhattacharya S, Chakraborty SK, Chakraborty S, Ghosh KK, Palit A, et al. Density of Culex vishnui and appearance of JE antibody in sentinel chicks and wild birds in relation to Japanese encephalitis cases. Trop Geogr Med. 1986;38:46–50. [PubMed] [Google Scholar]
- 3.Buescher EL, Scherer WF, Rosenberg MZ, Gresser I, Hardy JL, et al. Ecologic studies of Japanese encephalitis virus in Japan. II. Mosquito infection. Am J Trop Med Hyg. 1959;8:651–664. doi: 10.4269/ajtmh.1959.8.651. [DOI] [PubMed] [Google Scholar]
- 4.Ritchie SA, Phillips D, Broom A, Mackenzie J, Poidinger M, et al. Isolation of Japanese encephalitis virus from Culex annulirostris in Australia. Am J Trop Med Hyg. 1997;56:80–84. doi: 10.4269/ajtmh.1997.56.80. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee K, Deshmukh PK, Ilkal MA, Dhanda V. Transmission of Japanese encephalitis virus by Culex bitaeniorhynchus Giles. Indian J Med Res. 1978;67:889–893. [PubMed] [Google Scholar]
- 6.Reuben R. Studies on the mosquitoes of North Arcot District, Madras State, India. 5. Breeding places of the Culex vishnui group of species. J Med Entomol. 1971;8:363–366. doi: 10.1093/jmedent/8.4.363. [DOI] [PubMed] [Google Scholar]
- 7.Keiser J, Maltese MF, Erlanger TE, Bos R, Tanner M, et al. Effect of irrigated rice agriculture on Japanese encephalitis, including challenges and opportunities for integrated vector management. Acta Trop. 2005;95:40–57. doi: 10.1016/j.actatropica.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Scherer WF, Moyer JT, Izumi T, Gresser I, Mc CJ. Ecologic studies of Japanese encephalitis virus in Japan. VI. Swine infection. Am J Trop Med Hyg. 1959;8:698–706. doi: 10.4269/ajtmh.1959.8.698. [DOI] [PubMed] [Google Scholar]
- 9.Kim HC, Klein TA, Takhampunya R, Evans BP, Mingmongkolchai S, et al. Japanese encephalitis virus in culicine mosquitoes (Diptera: Culicidae) collected at Daeseongdong, a village in the demilitarized zone of the Republic of Korea. Journal of Medical Entomology. 2011;48:1250–1256. doi: 10.1603/me11091. [DOI] [PubMed] [Google Scholar]
- 10.Ting SH, Tan HC, Wong WK, Ng ML, Chan SH, et al. Seroepidemiology of neutralizing antibodies to Japanese encephalitis virus in Singapore: continued transmission despite abolishment of pig farming? Acta Trop. 2004;92:187–191. doi: 10.1016/j.actatropica.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Wang JL, Pan XL, Zhang HL, Fu SH, Wang HY, et al. Japanese encephalitis viruses from bats in Yunnan, China. Emerg Infect Dis. 2009;15:939–942. doi: 10.3201/eid1506.081525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Hurk AF, Smith CS, Field HE, Smith IL, Northill JA, et al. Transmission of Japanese Encephalitis virus from the black flying fox, Pteropus alecto, to Culex annulirostris mosquitoes, despite the absence of detectable viremia. Am J Trop Med Hyg. 2009;81:457–462. [PubMed] [Google Scholar]
- 13.Pfeffer M, Dobler G. Emergence of zoonotic arboviruses by animal trade and migration. Parasit Vectors. 2010;3:35. doi: 10.1186/1756-3305-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai TF. New initiatives for the control of Japanese encephalitis by vaccination: Minutes of a WHO/CVI meeting, Bangkok, Thailand, 13–15 October 1998. Vaccine. 2000;18,(Suppl 2):1–25. doi: 10.1016/s0264-410x(00)00037-2. [DOI] [PubMed] [Google Scholar]
- 15.PATH, WHO. 2009. Japanese Encephalitis Morbidity, Mortality, and Disability: Reduction and Control by 2015.
- 16.Plesner AM, Arlien-Soborg P, Herning M. Neurological complications to vaccination against Japanese encephalitis. Eur J Neurol. 1998;5:479–485. doi: 10.1046/j.1468-1331.1998.550479.x. [DOI] [PubMed] [Google Scholar]
- 17.Konishi E, Shoda M, Yamamoto S, Arai S, Tanaka-Taya K, et al. Natural infection with Japanese encephalitis virus among inhabitants of Japan: a nationwide survey of antibodies against nonstructural 1 protein. Vaccine. 2006;24:3054–3056. doi: 10.1016/j.vaccine.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez C, Wang O, Strutz SE, Gonzalez-Salazar C, Sanchez-Cordero V, et al. Climate change and risk of leishmaniasis in north america: predictions from ecological niche models of vector and reservoir species. PLoS Negl Trop Dis. 2010;4:e585. doi: 10.1371/journal.pntd.0000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mak S, Morshed M, Henry B. Ecological niche modeling of lyme disease in British Columbia, Canada. J Med Entomol. 2010;47:99–105. doi: 10.1603/033.047.0114. [DOI] [PubMed] [Google Scholar]
- 20.Masuoka P, Klein TA, Kim HC, Claborn DM, Achee N, et al. Modeling the distribution of Culex tritaeniorhynchus to predict Japanese encephalitis distribution in the Republic of Korea. Geospat Health. 2010;5:45–57. doi: 10.4081/gh.2010.186. [DOI] [PubMed] [Google Scholar]
- 21.Masuoka PM, Burke R, Colaccico M, Razuri H, Hill D, et al. Predicted geographic ranges for North American sylvatic Trichinella species. J Parasitol. 2009;95:829–837. doi: 10.1645/GE-1952.1. [DOI] [PubMed] [Google Scholar]
- 22.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecological Modelling. 2006;190:231–259. [Google Scholar]
- 23.WHO/IVB. 2011. Countries Using JE Vaccine in National Immunization Schedule, 2010.
- 24.Phillips SJ, Dudik M, Schapire RE. A maximum entropy approach to species distribution modeling. AMC Press; 2004. pp. 655–662. [Google Scholar]
- 25.Li W, Guo Q, Elkan C. Can we model the probability of presence of species without absence data? Ecography. 2011;34:1096–1105. [Google Scholar]
- 26.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 27.Pandey B, Yamamoto A, Morita K, Kurosawa Y, Rai S, et al. Serodiagnosis of Japanese encephalitis among Nepalese patients by the particle agglutination assay. Epidemiol Infect. 2003;131:881–885. doi: 10.1017/s0950268803008835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peiris JS, Amerasinghe FP, Arunagiri CK, Perera LP, Karunaratne SH, et al. Japanese encephalitis in Sri Lanka: comparison of vector and virus ecology in different agro-climatic areas. Trans R Soc Trop Med Hyg. 1993;87:541–548. doi: 10.1016/0035-9203(93)90080-a. [DOI] [PubMed] [Google Scholar]
- 29.Murty US, Rao MS, Arunachalam N. The effects of climatic factors on the distribution and abundance of Japanese encephalitis vectors in Kurnool district of Andhra Pradesh, India. J Vector Borne Dis. 2010;47:26–32. [PubMed] [Google Scholar]
- 30.Takahashi M. The effects of environmental and physiological conditions of Culex tritaeniorhynchus on the pattern of transmission of Japanese encephalitis virus. J Med Entomol. 1976;13:275–284. doi: 10.1093/jmedent/13.3.275. [DOI] [PubMed] [Google Scholar]
- 31.Richards EE, Masuoka P, Brett-Major D, Smith M, Klein TA, et al. The relationship between mosquito abundance and rice field density in the Republic of Korea. Int J Health Geogr. 2010;9:32. doi: 10.1186/1476-072X-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joshi KD, Witcombe JR. The impact of participatory plant breeding (PPB) on landrace diversity: A case study for high-altitude rice in Nepal. Euphytica. 2003;134:117–125. [Google Scholar]
- 33.Li MH, Fu SH, Chen WX, Wang HY, Guo YH, et al. Genotype v Japanese encephalitis virus is emerging. PLoS Negl Trop Dis. 2011;5:e1231. doi: 10.1371/journal.pntd.0001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takhampunya R, Kim HC, Tippayachai B, Kengluecha A, Klein TA, et al. Emergence of Japanese encephalitis virus genotype V in the Republic of Korea. Virol J. 2011;8:449. doi: 10.1186/1743-422X-8-449. [DOI] [PMC free article] [PubMed] [Google Scholar]