Abstract
Saphenous vein grafts used in coronary artery bypass graft surgery suffer from lower patency rates compared to left internal mammary artery. A number of clinical trials and observational studies have demonstrated a significant benefit of statin treatment on vein graft patency. Aside from their well-known lipid-lowering capacities, statins exert pleiotropic effects by direct inhibition of the mevalonate pathway in the wall of these grafts. This leads to reduced geranylgeranylation of small GTPases such as Rho and Rac. Through this LDL-independent mechanism, statins improve endothelial function and reduce vascular inflammation and oxidative stress, inhibiting also smooth muscle cell proliferation and migration. Although the existing evidence support a beneficial effect of statins on vein grafts biology, more clinical trials focused on the effect of aggressive statin treatment on vein graft patency are required, in order to safely translate this strategy into clinical practice.
Keywords: Coronary Artery Bypass Grafting, Endothelial dysfunction, Pleiotropic Effects, Saphenous Vein Grafts, Statins
Coronary artery bypass grafting (CABG) surgery is still undoubtedly a first line option for the treatment of advanced coronary artery disease (CAD). Although left internal mammary artery (LIMA) is the conduit of choice for revascularization of the left anterior descending coronary artery, the need for more than one grafts makes autologous saphenous vein grafts (SVGs) the most common type of graft in the vast majority of CABG operations. Although clinical outcome post CABG in diabetics and patients with multivessel CAD is better compared to percutaneous coronary intervention (PCI) (1), CABG is not spared complications, both peri- and post-operative, which could threaten the patient’s recuperation (2). SVG use is especially plagued by concerns over long-term patency, with up to 20% of grafts failing in only the first year after surgery (3).
Diverse pharmacological treatment has been extensively used as an adjuvant to CABG, either to minimize complications or improve long-term outcome. In this sense statins, with their extensively discussed lipid-lowering and pleiotropic effects, have been prime candidate drugs for improvement of post-operative outcome in CABG patients. In this review we summarize the clinical data regarding statin use and SVG patency and discuss the specific effects of statins on SVG biology.
Vein graft failure and determining factors
The most common mechanisms mediating SVG failure are acute thrombosis and intimal hyperplasia in the first year after surgery, and atherosclerosis in later stages (4). Apart from the well-known risk factors, such as smoking and hyperlipidemia, the fate of the SVG can be influenced by other determinants such as vessel diameter, surgical technique and others (4). For example, off-pump CABG has suffered from lower long-term SVG patency rates than on-pump (2). Using longitudinal analysis in a population of 50,278 patients, patency rates for the first 1, 5 and 10 years after surgery were 78%, 65%, and 57% for SVGs and 93%, 88%, and 90% for LIMA grafts respectively (3), stressing the fact that SVGs are a rather weak graft, that could potentially be improved by adjuvant pharmaceutical interventions.
Statins and clinical outcome post CABG
During the last decade, statins have been established as mainstay in secondary prevention following cardiovascular events. Initiation of statin treatment improves clinical outcome after an acute coronary syndrome (ACS) (5) and long-term statin administration significantly reduces all-cause mortality in patients with CAD (6).
The potential role of intensive statin therapy during the CABG periprocedural period has been the focus of extensive research. Treatment with fluvastatin 80 mg/day for up to 37 days prior to vascular surgery reduced major adverse cardiovascular events during the post-operative period, with few side effects (7). In addition, preoperative treatment with atorvastatin 20 mg/day for 3-7 days prior-CABG consistently reduced the incidence of paroxysmal atrial fibrillation (8, 9). However, a meta-analysis of 21 randomized statin trials using post-procedural myocardial infarction as primary endpoint revealed a strong protective effect of statins post PCI and non-cardiac procedures but only a trend for CABG, a finding that is possibly due to the smaller number of CABG studies available (10).
In the context of SVG disease, the landmark POST-CABG multi-center randomized trial showed that intensive LDL-lowering treatment with lovastatin ± cholestyramine after CABG (target levels below 85 mg/dl) significantly delays obstructive atherosclerotic changes in SVGs compared to more conservative treatment (target levels below 140 mg/dl) (11). In the same study population, a similar beneficial effect of intensive lipid-lowering treatment was also observed for atherosclerosis progression in the native coronary arteries (12). A more recent randomized trial revealed significant benefits of aggressive statin therapy (atorvastatin 80 mg/day vs. 10 mg/day) on the risk of post-operative complications including the need for coronary revascularization after CABG, with median LDL levels reaching 79 mg/dl in the intensive treatment group (13). Intensive lipid control was also shown to inhibit yellow plaque and thrombus formation in SVGs, as demonstrated by intravascular ultrasound (14). The latest ACC/AHA guidelines for CABG affirm the pivotal role of statins in LDL-lowering therapy for SVG disease prevention and recommend optimal treatment with LDL target levels <100 mg/dl and even <70 mg/dl in high-risk patients (15).
Although statins are traditionally thought to exert their beneficial effects on SVG patency through lipid lowering, there are clinical data suggesting additional mechanisms involved. Analysis of the POST-CABG trial population revealed a significant protective effect of intensive vs. moderate statin treatment on incidence of SVG restenosis, independently of LDL lowering (16). This study was the first proof in a clinical setting for the existence of a dose-dependent effect of statins on SVG patency that is independent of LDL-lowering. The most important studies examining the clinical impact of statins post- CABG, are summarized in the Table.
Table 1. Clinical trials involving statin treatment of patients undergoing CABG.
| Study name/Author |
Population | Intervention | Primary End- point |
Outcome |
|---|---|---|---|---|
| POST-CABG trial (1997) [11] |
1351 patients undergoing CABG |
Aggressive vs moderate treatment to lower LDL levels (lovastatin ± cholestyramine) |
Angiographically assessed SVG restenosis |
Aggressive LDL lowering reduces atherosclerotic process of SVGs. |
| White et al. (2001) [12]– Analysis of the POST-CABG trial |
POST-CABG population |
POST-CABG intervention |
Angiographically assessed LCA atherosclerotic progression |
Aggressive LDL lowering reduces atherosclerotic process in native coronary arteries. |
| Domanski et al. (2008) [16]– Analysis of the POST-CABG trial |
POST-CABG population, adjustment for lipid levels |
POST-CABG intervention |
Angiographically assessed SVG restenosis |
Aggressive statin therapy can improve SVG patency independently of its LDL-lowering effects. |
| Song et al. (2008) [8] |
124 patients undergoing elective off- pump CABG |
Atorvastatin 20mg/d vs. placebo for 3 days preoperatively |
Post-operative atrial fibrillation |
Atorvastatin treatment significantly reduced post- operative AF |
| Shah et al. (2008) [13]– Post hoc anaylsis of the TNT trial |
10,001 patients with CAD (4,654 with CABG) |
Atorvastatin 80 mg/d vs. 10mg/d for median 4.9 years |
First major adverse cardiovascular event (MACE) |
Intensive statin therapy reduces MACE incidence and need for repeat revascularization |
| Schouten et al. (2009) [7] |
497 patients undergoing vascular surgery |
Preoperative administration of fluvastatin vs. placebo |
Myocardial Ischemia |
Fluvastatin therapy improves postoperative cardiac outcome. |
| Sun et al. (2011) [9] |
100 patients undergoing elective CABG |
Atorvastatin 20mg/d vs. placebo for 7 days preoperatively |
Post-operative atrial fibrillation |
Atorvastatin administration reduces incidence of post-operative AF |
Abbreviatons: POST-CABG: Post Coronary Artery Bypass Graft, TNT: Treating to New Targets, LDL: Low-Density Lipoprotein; SVG: Saphenous Vein Graft; LCA: Left Coronary Artery; AF: Atrial Fibrillation; CAD: Coronary Artery Disease
The mevalonate pathway: Pleiotropic effects of statins on vein grafts
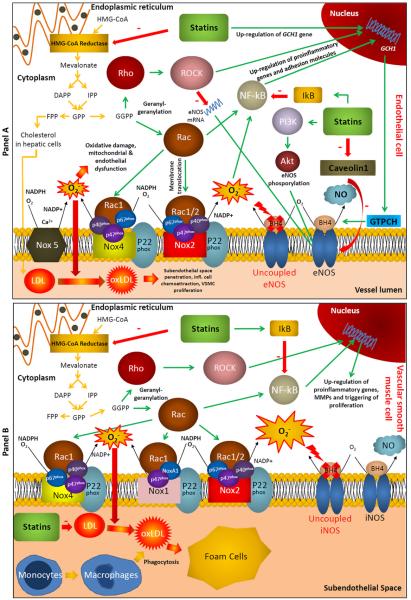
Statins exert their effects through inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase by blocking substrate binding to the active site of the enzyme (17). The NADPH-mediated reduction of HMG-CoA is the rate-limiting step in the mevalonate pathway, which is responsible for endogenous cholesterol biosynthesis in humans. The mevalonate pathway uses acetyl-CoA as basis for synthesis of dimethylallyl pyrophosphate (DMAPP) and isopentenyl pyrophosphate (IPP), themselves precursors to isoprenoids and consecutively squalene and cholesterol synthesis. Thus, by inhibiting the mevalonate pathway, statins lead to reduction in endogenous cholesterol production and LDL levels, because cholesterol is a major component of the LDL molecule (18). Reduced LDL levels result in lower oxidized LDL (oxLDL) and therefore attenuation of the atherosclerotic process (Figure).
Figure.
Panel A. The mevalonate pathway and pleiotropic effects of statins in endothelial cells. By inhibiting HMG-CoA reductase, statins reduce cholesterol biosynthesis in hepatic cells and subsequently circulating LDL. By directly preventing the formation of GGPP in endothelial cells, statins exert direct pleiotropic effects in endothelial cells. More specifically, they inhibit Rac1-mediated NADPH-oxidase activation; they reduce inflammation by preventing NF-kB activation; they ameliorate NO bioavailability by stabilizing eNOS mRNA, increasing vascular BH4, enhancing eNOS phosphorylation at Ser1177 and dissociating eNOS from caveolin-1.
Panel B. The mevalonate pathway and effects of statins on vascular smooth muscle cells. Reduced LDL means less oxLDL accumulation in the subendothelial space and therefore inhibition of foam cell formation. As with endothelial cells, statins exert similar pleiotropic effects in vascular smooth muscle cells, by inhibiting the mevalonate pathway. By preventing protein geranyl-geranylation, statins interfere with Rac1 and prevent the activation of VSMC Nox isoforms, while they reduce vascular inflammation by suppressing NF-kB activation. Moreover, by inhibiting the Rho/ROCK pathway, they attenuate MMP expression and proliferation/migration processes.
In addition, the isoprenoids farnesylpyrophosphate (FPP) and geranyl-geranyl-pyrophosphate (GGPP), downstream intermediates of the mevalonate pathway, are important mediators of protein prenylation, which is a post-translational modification involving addition of isoprene units. Major targets of protein prenylation are the family of small GTPases (Rho, Rac, Ras etc). Geranyl-geranylation of Rho allows lipid anchoring to the membrance and activation of Rho kinases (ROCKs), whereas Rac1 is implicated, among others, in reactive oxygen species (ROS) generation (19). Since the mevalonate pathway is active in vascular cells (endothelial, vascular smooth muscle cells and others), inhibition of the mevalonate pathway by statins also inhibits these processes, explaining a significant portion of what is known as the “pleiotropic effects” of statins (18).
Statins and vein graft failure: Mechanistic insights
Statins and endothelial function of SV grafts
SVG endothelium is especially prone to disruption and dysfunction due to the harvesting process and exposure to arterial blood flow. Endothelial dysfunction, characterized by reduced nitric oxide (NO) bioavailability appears in both arterial and venous endothelium in a similar way (20). Although the role of endothelial dysfunction in atherogenesis is well documented, the vast majority of the literature is focused on the arterial wall, and data on the role of NO bioavailability in veins are limited. However, it is widely accepted that endothelial function is a key feature in SVG homeostasis and that endothelial NO bioavailability in SV grafts is a rational therapeutic target in CABG surgery. Indeed, NO produced by SVGs may play a critical role in short- and long- term grafts patency, as it has vasorelaxant, antithrombotic and anti-inflammatory properties (21). Moreover, long-term maintenance of sufficient NO bioavailability may also exert antiatherogenic effects in these grafts, improving their long-term patency, although this is hard to be tested in clinical studies.
Aggressive statin treatment has been associated with improved endothelial function of SVGs in a clinical observational study (22), while in a randomized trial, 4-weeks treatment with simvastatin significantly improved endothelial function evaluated by flow-mediated dilatation compared to ezetimibe, an effect that was independent of LDL-lowering (23). LDL cholesterol is a major determinant of endothelial dysfunction in SVGs (24) and even short-term statin treatment prior CABG leads to a rapid improvement of endothelial function in both arteries and veins (25). However, it had been demonstrated that statins exert a direct effect on endothelial function by directly inhibiting HMG-CoA reductase in the vascular wall, independently of any effects on LDL levels, identifying the mevalonate pathway as a rational, direct therapeutic target for the improvement of SVGs biology (18, 25).
Incubation of isolated endothelial cells from SVGs with cerivastatin significantly increased endothelial nitric oxide synthase (eNOS) expression and subsequently NO bioavailability (26). This is thought to arise from stabilization of eNOS mRNA through statin-mediated inhibition of Rho geranylgeranylation (27). Incubation of human umbilical vein endothelial cells (HUVECs) with simvastatin or fluvastatin, activated the phosphatidylinositol 3-kinase (PI3K)/ protein kinase Akt pathway, leading to enhanced phosphorylation of eNOS and increased activity (28, 29). Similarly, incubation of HUVECs with various statins inhibited H2O2-induced endothelial senescence by increasing eNOS and sirtuin 1 expression (30). Moreover, treatment of apolipoprotein E knock-out mice with rosuvastatin significantly reduced caveolin-1, a molecule that binds to eNOS inhibiting its enzymatic activity (31). Therefore, statin treatment improves both eNOS gene expression and activity, through distinct mechanisms, contributing to increased bioavailability of NO and improved endothelial function.
The ability of eNOS to produce NO is dependent on the binding of its essential co-factor tetrahydrobiopterin (BH4), a process known as “coupling” of the enzyme (32). GTP cyclohydrolase (GTPCH) is the rate-limiting enzyme in BH4 biosynthesis. Incubation of HUVECs with fluvastatin significantly up-regulated GTPCH gene expression and improved NO release (29). Similarly, in streptozotocin-induced diabetic rats, oral atorvastatin increased GTPCH levels and ameliorated eNOS coupling (33).
We have recently shown that 3-day preoperative, high-dose atorvastatin treatment improves endothelial function and vascular redox state, by improving eNOS coupling in both SVG (25) and LIMA grafts (34); this is mediated by increased BH4 bioavailability as a result of up-regulation of GCH1 gene, encoding GTPCH (34). This effect is due to direct inhibition of HMG-CoA reductase within the vascular wall, independently of LDL lowering.
Furthermore, the role of statins in promoting endothelial progenitor cells (EPC) re-endothelialization of SVGs post CABG has been extensively studied. In patients with stable angina, 4-week treatment with atorvastatin 40 mg/day led to significantly elevated levels and functional activity of marrow-derived circulating EPCs (35), an effect mediated by the PI3K/Akt pathway (35). Treatment of EPCs with atorvastatin attenuated homocysteine-induced dysfunction through an AMP-activated protein kinase (AMPK) dependent mechanism (36), while high dose of statin treatment lead to further improvement of EPCs function compared to low dose (37). The Figure illustrates the effects of statins on endothelial cell physiology.
Statins and vasospasm/acute thrombosis of SV grafts
Vasoconstrictor agents such as endothelin-1 (ET1) and angiotensin counteract the vasodilatory effects of NO and have potential implications in VSMC migration/hyperplasia and neointima formation (38). Statins were found to inhibit preproendothelin-1 gene expression through a Rho-mediated mechanism (39). In addition, simvastatin inhibited ET1-mediated contraction in rat aortic rings, an effect that was reversed by mevalonate and mimicked by geranylgeranyl transferase inhibitors (40). A recent study showed that pravastatin attenuates ET1 constrictor response in small rat vessels through enhanced NO release (41). Therefore, statins may contribute to the prevention of grafts’ spasm post CABG.
Statin treatment could potentially affect platelet activation and thrombus formation, which is an important mechanism for acute failure of SVGs. In a porcine model of carotid injury, intravenous lovastatin acutely inhibited platelet aggregation and thrombus formation (42). Treatment of hypercholesterolemic individuals with a statin attenuated thromboxane-dependent platelet activation (43) and modified platelet aggregation by ameliorating their intracellular redox state (44). In this way, statin administration could provide SVGs with additional protection against acute thrombosis, independently of their LDL-lowering effects.
Statins and intimal hyperplasia of SV grafts
Intimal hyperplasia is characterized by migration and proliferation of VSMCs, accumulation of extracellular matrix to the intima and finally plaque formation in the SVGs (45). Treatment of isolated human venous VSMCs with a statin, attenuated the ability of VSMCs to proliferate, through a mevalonate-reversible mechanism (26), and suppressed the formation of vein graft intimal hyperplasia by preventing Rho/ROCK activation specifically in endothelial cells (46). In line to these findings, in an ex-vivo model of human vein VSMC cyclic stretch, statins prevented proliferation by inhibiting the RhoA/ROCK pathway (47). In human venous VSMCs, the same mechanism mediated the simvastatin-induced inhibition of matrix metalloproteinase-9 secretion, an enzyme which is implicated in intimal hyperplasia (48).
Statins and vascular inflammation in SV grafts
It is well established that atherosclerosis is primarily an inflammatory process involving migration of monocytes from the circulation to the subendothelial space, due to increased levels of proinflammatory cytokines and cell adhesion molecules. LDL is oxidized to ox-LDL which is up-taken by macrophages to form foam cells (Figure) (19). Incubation of human endothelial cells and monocytes with simvastatin reduced chemokine receptor and proinflammatory gene expression through inhibition of protein geranylgeranylation (49). HUVECs treated with simvastatin displayed reduced TNFa-mediated monocyte recruitment (50). In addition, in a model of early SVG arterialization, simvastatin reduced the expression of CD40 and its soluble ligand in a mevalonate-dependent way (51). In apolipoprotein E/LDL-receptor double knock-out mice, oral atorvastatin reduced circulating levels of monocyte-chemoattractant protein 1 (MCP-1), as well as vascular cell adhesion molecule-1 (VCAM-1) and intercellular cell adhesion molecule-1 (ICAM-1) in atherosclerotic lesions (52). In normocholesterolemic humans, treatment with simvastatin attenuated monocyte recruitment through inhibition of CC chemokine receptor 2 (CCR2) (53).
Statins and regulation of redox state in SV grafts
Increased vascular oxidative stress is a major determinant of endothelial dysfunction and atherosclerosis, thus constituting a promising therapeutic target (54). A major cellular enzymatic source of ROS in the vasculature is NADPH-oxidase, which is an enzymatic complex catalyzing the formation of superoxide from molecular oxygen by reducing NADPH (Figure). Different structural isoforms of the enzyme exist, depending on the homologue of the membrane-bound catalytic subunit Nox. Nox2, 4 and 5 are abundant in endothelial cells, whereas Nox1, 2 and 4 can be found in VSMCs (55) (Figure). Nox1, 2 and 4 are activated after translocation of the cytosolic subunits to the membrane through the action of the small GTPase Rac. In animal models, atorvastatin treatment ameliorated vascular redox state (33) and attenuated hypertension (56) by inhibiting the activation of Nox isoforms. We demonstrated that high dose atorvastatin treatment pre-CABG, significantly reduced total and NADPH-oxidase derived vascular superoxide in human SVGs (25). This was mediated by a mevalonate-reversible reduction in Rac1 activation and its associated membrane translocation (25).
Mitochondria-derived ROS are increasingly recognized as an important contributor to vascular oxidative stress, with a significant role in atherosclerosis development (57); statin treatment reduced mitochondrial superoxide generation in SVGs from advanced CAD patients (22).
Statins improve vascular redox state not only through reduction of LDL cholesterol and inhibition of pro-oxidant enzymatic systems but also through enhancement of endogenous antioxidant defences. In hypercholesterolemic rabits, atorvastatin treatment increases erythrocyte and liver glutathione (GSH) levels (58). In both HUVECs and streptozotocin-diabetic mice, statins up-regulated catalase expression (30). Treatment of rat and human VSMCs with simvastatin increased levels of heme-oxygenase 1 (HO-1), an important antioxidant enzyme that mediates some of the anti-inflammatory effects of statins (59). In a randomized trial, simvastatin significantly increased extracellular superoxide dismutase (SOD) activity compared to ezetimibe (23). Similarly, atorvastatin significantly increased endothelial SOD activity in an observational study of heart failure patients (60).
Through their effects on vascular redox state, statins also affect redox-sensitive transcriptional pathways within the vasculature, such as nuclear factor kappa-b (NF-kB) and activator protein 1 (AP1) (Figure). These pathways induce the expression of various proinflammatory, prothrombotic and overall proatherogenic molecules in the vascular wall, such as cytokines, chemokines, adhesion molecules and others (55). NF-kB is activated by oxidative stress and pro-inflammatory stimuli in a GGPP/FPP-dependent way (55). Statins have been shown to inhibit NF-kB activation, both in endothelial cells and VSMCs, by modifying the intracellular redox state and increasing the levels of NF-kB inhibitor IkB (61). In this way, statins could attenuate the atherosclerotic process in SVGs by synergistically affecting both oxidative stress and inflammation.
Therefore, statins exert multiple effects on redox state of SVGs, by improving endogenous antioxidant defences and suppressing the enzymatic sources responsible for ROS generation in the vascular wall. Whether suppression of redox signaling in the wall of these grafts is related with improved patency and better clinical outcome remains to be documented.
Differential effects of lipophilic vs. hydrophilic statins: The concept of “vascular” statins
Uptake of statins from hepatocytes, which are the major sites of LDL production, depends on both active transport and passive diffusion (62). However, this may not be true for non-hepatic cells, where the choice of statin administrated could significantly influence drug levels achieved inside the cells (19). More specifically, lipophilic statins such as atorvastatin and simvastatin (also called “vascular statins”(63)) are more easily diffused across cellular membranes than hydrophilic statins such as rosuvastatin, an effect which could potentially explain some of the observed effects of the aforementioned statins on the vasculature. A comparative study involving ex vivo incubation of primary human VSMCs with various statins found a significant protective effect of lipophilic but not of hydrophilic statins on cell migration and proliferation (64). A very recent review elegantly presents comparisons between different statins on experimental models and clinical studies (65). These data suggest that the type of statin treatment may affect the degree of its vascular pleiotropic effects, but more clinical evidence is required before such a conclusion can be drawn.
Conclusions
Coronary bypass grafting is still considered to be a first line option for the treatment of multi-vessel coronary artery disease, especially in patients with diabetes or impaired left ventricular systolic function (15). Although the left internal mammary artery is the ideal graft routinely used for the revascularization of left anterior descending coronary artery, the presence of multiple stenoses requires the use of additional grafts. Currently, SVGs are routinely used by most surgical teams, but their short- and long-term patency rates are much lower than LIMAs. It is therefore important to identify new pharmaceutical strategies targeting SVGs’ biology that would improve the patency of these grafts.
Statins undoubtedly improve clinical outcome in secondary prevention and especially in patients undergoing coronary revascularization, either by PCI or CABG (7). Since statins exert a number of pleiotropic effects on the vascular wall, beyond lipid lowering, one could hypothesize that statins may have a similar effect on SVGs as well. Indeed, statins reduce vascular oxidative stress in SVGs, improve NO bioavailability and reduce vascular inflammation, all critical components of SVGs failure (19). In addition, statins have systemic antithrombotic and anti-inflammatory effects (49), therefore their administration may prevent acute SVGs failure post CABG.
In conclusion, statins provide a particularly useful pharmacological intervention in secondary prevention and improve clinical outcome post-CABG. This is partly due to their beneficial effect on SVGs biology, although more studies are required to explore in details the molecular mechanisms by which statins affect short- and long- term patency in human SVGs. This will identify novel therapeutic targets in these grafts, and may lead to the development of new pharmacological strategies targeting SVG biology in the future.
Glossary
Abbreviatons
- HMG-CoA
3-Hydroxy-3-Methyl-Glutaryl-Coenzyme A
- DAPP
Dimethylallyl Pyrophosphate
- IPP
Isopentenyl-5 Pyrophosphate
- GPP
Geranyl Pyrophosphate
- FPP
Farnesyl Pyrophosphate
- GGPP
Geranylgeranyl Pyrophosphate
- GTP
Guanosine-5′-triphosphate
- NADPH
Nicotinamide Adenine Dinucleotide Phosphate
- BH4
Tetrahydrobiopterin
- O2−
Superoxide
- eNOS
endothelial Nitric Oxide Synthase
- ROCK
Rho Kinase
- PI3K
Phosphoinositide 3-kinase
- Akt
Protein Kinase B
- GTPCH
GTP-Cyclohydrolase
- NO
Nitric Oxide
- NF-kB
Nuclear Factor kappa-B
- AP1
Activator Protein 1
- LDL
Low Density Lipoprotein
- oxLDL
oxidized LDL
- ROS
Reactive Oxygen Species
- VSMC
Vascular Smooth Muscle Cell
- MMP
Matrix Metalloproteinase
References
- 1.Hoffman SN, TenBrook JA, Wolf MP, Pauker SG, Salem DN, Wong JB. A meta-analysis of randomized controlled trials comparing coronary artery bypass graft with percutaneous transluminal coronary angioplasty: one-to eight-year outcomes. J Am Coll Cardiol. 2003;41:1293–304. doi: 10.1016/s0735-1097(03)00157-8. [DOI] [PubMed] [Google Scholar]
- 2.Huffmyer J, Raphael J. The current status of off-pump coronary bypass surgery. Curr Opin Anaesthesiol. 2011;24:64–9. doi: 10.1097/ACO.0b013e328341ccf5. [DOI] [PubMed] [Google Scholar]
- 3.Sabik JF, 3rd, Lytle BW, Blackstone EH, Houghtaling PL, Cosgrove DM. Comparison of saphenous vein and internal thoracic artery graft patency by coronary system. Ann Thorac Surg. 2005;79:544–51. doi: 10.1016/j.athoracsur.2004.07.047. discussion -51. [DOI] [PubMed] [Google Scholar]
- 4.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97:916–31. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 5.Zeymer U, Junger C, Zahn R, Bauer T, Bestehorn K, Senges J, Gitt A. Effects of a secondary prevention combination therapy with an aspirin, an ACE inhibitor and a statin on 1-year mortality of patients with acute myocardial infarction treated with a beta-blocker. Support for a polypill approach. Curr Med Res Opin. 2011;27:1563–70. doi: 10.1185/03007995.2011.590969. [DOI] [PubMed] [Google Scholar]
- 6.Afilalo J, Duque G, Steele R, Jukema JW, de Craen AJ, Eisenberg MJ. Statins for secondary prevention in elderly patients: a hierarchical bayesian meta-analysis. J Am Coll Cardiol. 2008;51:37–45. doi: 10.1016/j.jacc.2007.06.063. [DOI] [PubMed] [Google Scholar]
- 7.Schouten O, Boersma E, Hoeks SE, Benner R, van Urk H, van Sambeek MR, Verhagen HJ, Khan NA, Dunkelgrun M, Bax JJ, Poldermans D. Fluvastatin and perioperative events in patients undergoing vascular surgery. N Engl J Med. 2009;361:980–9. doi: 10.1056/NEJMoa0808207. [DOI] [PubMed] [Google Scholar]
- 8.Song YB, On YK, Kim JH, Shin DH, Kim JS, Sung J, Lee SH, Kim WS, Lee YT. The effects of atorvastatin on the occurrence of postoperative atrial fibrillation after off-pump coronary artery bypass grafting surgery. Am Heart J. 2008;156:373 e9–16. doi: 10.1016/j.ahj.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Sun Y, Ji Q, Mei Y, Wang X, Feng J, Cai J, Chi L. Role of preoperative atorvastatin administration in protection against postoperative atrial fibrillation following conventional coronary artery bypass grafting. Int Heart J. 2011;52:7–11. doi: 10.1536/ihj.52.7. [DOI] [PubMed] [Google Scholar]
- 10.Winchester DE, Wen X, Xie L, Bavry AA. Evidence of pre-procedural statin therapy a meta-analysis of randomized trials. J Am Coll Cardiol. 2010;56:1099–109. doi: 10.1016/j.jacc.2010.04.023. This meta-analysis summarizes some of the clinical trial data available regarding the beneficial effects of statin therapy on post-procedural outcome.
- 11.The Post Coronary Artery Bypass Graft Trial Investigators The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. N Engl J Med. 1997;336:153–62. doi: 10.1056/NEJM199701163360301. [DOI] [PubMed] [Google Scholar]
- 12.White CW, Gobel FL, Campeau L, Knatterud GL, Forman SA, Forrester JS, Geller NL, Herd JA, Hickey A, Hoogwerf BJ, Hunninghake DB, Rosenberg Y, Terrin ML. Effect of an aggressive lipid-lowering strategy on progression of atherosclerosis in the left main coronary artery from patients in the post coronary artery bypass graft trial. Circulation. 2001;104:2660–5. doi: 10.1161/hc4701.099730. [DOI] [PubMed] [Google Scholar]
- 13.Shah SJ, Waters DD, Barter P, Kastelein JJ, Shepherd J, Wenger NK, DeMicco DA, Breazna A, LaRosa JC. Intensive lipid-lowering with atorvastatin for secondary prevention in patients after coronary artery bypass surgery. J Am Coll Cardiol. 2008;51:1938–43. doi: 10.1016/j.jacc.2007.12.054. [DOI] [PubMed] [Google Scholar]
- 14.Hata M, Takayama T, Sezai A, Yoshitake I, Hirayama A, Minami K. Efficacy of aggressive lipid controlling therapy for preventing saphenous vein graft disease. Ann Thorac Surg. 2009;88:1440–4. doi: 10.1016/j.athoracsur.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Eagle KA, Guyton RA, Davidoff R, Edwards FH, Ewy GA, Gardner TJ, Hart JC, Herrmann HC, Hillis LD, Hutter AM, Jr., Lytle BW, Marlow RA, Nugent WC, Orszulak TA, Antman EM, Smith SC, Jr., Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Ornato JP. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery) Circulation. 2004;110:1168–76. doi: 10.1161/01.CIR.0000138790.14877.7D. [DOI] [PubMed] [Google Scholar]
- 16.Domanski M, Tian X, Fleg J, Coady S, Gosen C, Kirby R, Sachdev V, Knatterud G, Braunwald E. Pleiotropic effect of lovastatin, with and without cholestyramine, in the post coronary artery bypass graft (Post CABG) trial. Am J Cardiol. 2008;102:1023–7. doi: 10.1016/j.amjcard.2008.05.053. This study presents data from the POST-CABG trial which prove for the first time the existence of an LDL-independent beneficial effect of statins on SVG patency.
- 17.Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292:1160–4. doi: 10.1126/science.1059344. [DOI] [PubMed] [Google Scholar]
- 18.Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol Med. 2008;14:37–44. doi: 10.1016/j.molmed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Q, Liao JK. Statins and cardiovascular diseases: from cholesterol lowering to pleiotropy. Curr Pharm Des. 2009;15:467–78. doi: 10.2174/138161209787315684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antoniades C, Mussa S, Shirodaria C, Lee J, Diesch J, Taggart DP, Channon KM, Leeson P. Relation of preoperative radial artery flow-mediated dilatation to nitric oxide bioavailability in radial artery grafts used in off-pump coronary artery bypass grafting. Am J Cardiol. 2009;103:216–20. doi: 10.1016/j.amjcard.2008.08.056. [DOI] [PubMed] [Google Scholar]
- 21.Tousoulis D, Antoniades C, Koumallos N, Marinou K, Stefanadi E, Latsios G, Stefanadis C. Novel therapies targeting vascular endothelium. Endothelium: journal of endothelial cell research. 2006;13:411–21. doi: 10.1080/10623320601061714. [DOI] [PubMed] [Google Scholar]
- 22.Delles C, Dymott JA, Neisius U, Rocchiccioli JP, Bryce GJ, Moreno MU, Carty DM, Berg GA, Hamilton CA, Dominiczak AF. Reduced LDL-cholesterol levels in patients with coronary artery disease are paralleled by improved endothelial function: An observational study in patients from 2003 and 2007. Atherosclerosis. 2010;211:271–7. doi: 10.1016/j.atherosclerosis.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landmesser U, Bahlmann F, Mueller M, Spiekermann S, Kirchhoff N, Schulz S, Manes C, Fischer D, de Groot K, Fliser D, Fauler G, Marz W, Drexler H. Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation. 2005;111:2356–63. doi: 10.1161/01.CIR.0000164260.82417.3F. [DOI] [PubMed] [Google Scholar]
- 24.Al-Benna S, Hamilton CA, McClure JD, Rogers PN, Berg GA, Ford I, Delles C, Dominiczak AF. Low-density lipoprotein cholesterol determines oxidative stress and endothelial dysfunction in saphenous veins from patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:218–23. doi: 10.1161/01.ATV.0000193626.22269.45. [DOI] [PubMed] [Google Scholar]
- 25.Antoniades C, Bakogiannis C, Tousoulis D, Reilly S, Zhang MH, Paschalis A, Antonopoulos AS, Demosthenous M, Miliou A, Psarros C, Marinou K, Sfyras N, Economopoulos G, Casadei B, Channon KM, Stefanadis C. Preoperative atorvastatin treatment in CABG patients rapidly improves vein graft redox state by inhibition of Rac1 and NADPH-oxidase activity. Circulation. 2010;122:S66–73. doi: 10.1161/CIRCULATIONAHA.109.927376. This is the first study demonstrating a mevalonate-reversible inhibition of Rac1 and NADPH-oxidase by statins in human SVGs, in an LDL-independent way.
- 26.Yang Z, Kozai T, van der Loo B, Viswambharan H, Lachat M, Turina MI, Malinski T, Luscher TF. HMG-CoA reductase inhibition improves endothelial cell function and inhibits smooth muscle cell proliferation in human saphenous veins. J Am Coll Cardiol. 2000;36:1691–7. doi: 10.1016/s0735-1097(00)00924-4. [DOI] [PubMed] [Google Scholar]
- 27.Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273:24266–71. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 28.Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–10. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aoki C, Nakano A, Tanaka S, Yanagi K, Ohta S, Jojima T, Kasai K, Takekawa H, Hirata K, Hattori Y. Fluvastatin upregulates endothelial nitric oxide synthase activity via enhancement of its phosphorylation and expression and via an increase in tetrahydrobiopterin in vascular endothelial cells. International journal of cardiology. 2010 doi: 10.1016/j.ijcard.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 30.Ota H, Eto M, Kano MR, Kahyo T, Setou M, Ogawa S, Iijima K, Akishita M, Ouchi Y. Induction of endothelial nitric oxide synthase, SIRT1, and catalase by statins inhibits endothelial senescence through the Akt pathway. Arterioscler Thromb Vasc Biol. 2010;30:2205–11. doi: 10.1161/ATVBAHA.110.210500. [DOI] [PubMed] [Google Scholar]
- 31.Pelat M, Dessy C, Massion P, Desager JP, Feron O, Balligand JL. Rosuvastatin decreases caveolin-1 and improves nitric oxide-dependent heart rate and blood pressure variability in apolipoprotein E−/− mice in vivo. Circulation. 2003;107:2480–6. doi: 10.1161/01.CIR.0000065601.83526.3E. [DOI] [PubMed] [Google Scholar]
- 32.Antoniades C, Shirodaria C, Crabtree M, Rinze R, Alp N, Cunnington C, Diesch J, Tousoulis D, Stefanadis C, Leeson P, Ratnatunga C, Pillai R, Channon KM. Altered plasma versus vascular biopterins in human atherosclerosis reveal relationships between endothelial nitric oxide synthase coupling, endothelial function, and inflammation. Circulation. 2007;116:2851–9. doi: 10.1161/CIRCULATIONAHA.107.704155. [DOI] [PubMed] [Google Scholar]
- 33.Wenzel P, Daiber A, Oelze M, Brandt M, Closs E, Xu J, Thum T, Bauersachs J, Ertl G, Zou MH, Forstermann U, Munzel T. Mechanisms underlying recoupling of eNOS by HMG-CoA reductase inhibition in a rat model of streptozotocin-induced diabetes mellitus. Atherosclerosis. 2008;198:65–76. doi: 10.1016/j.atherosclerosis.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antoniades C, Bakogiannis C, Leeson P, Guzik TJ, Zhang MH, Tousoulis D, Antonopoulos AS, Demosthenous M, Marinou K, Hale A, Paschalis A, Psarros C, Triantafyllou C, Bendall J, Casadei B, Stefanadis C, Channon KM. Rapid, Direct Effects of Statin Treatment on Arterial Redox State and Nitric Oxide Bioavailability in Human Atherosclerosis via Tetrahydrobiopterin-Mediated Endothelial Nitric Oxide Synthase Coupling. Circulation. 2011;124:335–45. doi: 10.1161/CIRCULATIONAHA.110.985150. This study shows for the first time in humans a statin-mediated improvement in eNOS coupling, through upregulation of the GTPCH gene and increased BH4 bioavailability, independently of LDL-lowering.
- 35.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rutten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–7. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jia F, Wu C, Chen Z, Lu G. AMP-activated protein kinase inhibits homocysteine-induced dysfunction and apoptosis in endothelial progenitor cells. Cardiovasc Drugs Ther. 2011;25:21–9. doi: 10.1007/s10557-010-6277-1. [DOI] [PubMed] [Google Scholar]
- 37.Hibbert B, Ma X, Pourdjabbar A, Simard T, Rayner K, Sun J, Chen YX, Filion L, O’Brien ER. Pre-procedural atorvastatin mobilizes endothelial progenitor cells: clues to the salutary effects of statins on healing of stented human arteries. PLoS One. 2011;6:e16413. doi: 10.1371/journal.pone.0016413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Z, Krasnici N, Luscher TF. Endothelin-1 potentiates human smooth muscle cell growth to PDGF: effects of ETA and ETB receptor blockade. Circulation. 1999;100:5–8. doi: 10.1161/01.cir.100.1.5. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez-Perera O, Perez-Sala D, Soria E, Lamas S. Involvement of Rho GTPases in the transcriptional inhibition of preproendothelin-1 gene expression by simvastatin in vascular endothelial cells. Circ Res. 2000;87:616–22. doi: 10.1161/01.res.87.7.616. [DOI] [PubMed] [Google Scholar]
- 40.Mraiche F, Cena J, Das D, Vollrath B. Effects of statins on vascular function of endothelin-1. Br J Pharmacol. 2005;144:715–26. doi: 10.1038/sj.bjp.0706114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghaffari N, Ball C, Kennedy JA, Stafford I, Beltrame JF. Acute modulation of vasoconstrictor responses by pravastatin in small vessels. Circ J. 2011;75:1506–14. doi: 10.1253/circj.cj-10-0954. [DOI] [PubMed] [Google Scholar]
- 42.Obi C, Wysokinski W, Karnicki K, Owen WG, McBane RD., 2nd Inhibition of platelet-rich arterial thrombus in vivo: acute antithrombotic effect of intravenous HMG-CoA reductase therapy. Arterioscler Thromb Vasc Biol. 2009;29:1271–6. doi: 10.1161/ATVBAHA.109.190884. [DOI] [PubMed] [Google Scholar]
- 43.Puccetti L, Santilli F, Pasqui AL, Lattanzio S, Liani R, Ciani F, Ferrante E, Ciabattoni G, Scarpini F, Ghezzi A, Auteri A, Davi G. Effects of atorvastatin and rosuvastatin on thromboxane-dependent platelet activation and oxidative stress in hypercholesterolemia. Atherosclerosis. 2011;214:122–8. doi: 10.1016/j.atherosclerosis.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Haramaki N, Ikeda H, Takenaka K, Katoh A, Sugano R, Yamagishi S, Matsuoka H, Imaizumi T. Fluvastatin alters platelet aggregability in patients with hypercholesterolemia: possible improvement of intraplatelet redox imbalance via HMG-CoA reductase. Arterioscler Thromb Vasc Biol. 2007;27:1471–7. doi: 10.1161/ATVBAHA.106.128793. [DOI] [PubMed] [Google Scholar]
- 45.Sugimoto M, Yamanouchi D, Komori K. Therapeutic approach against intimal hyperplasia of vein grafts through endothelial nitric oxide synthase/nitric oxide (eNOS/NO) and the Rho/Rho-kinase pathway. Surg Today. 2009;39:459–65. doi: 10.1007/s00595-008-3912-6. [DOI] [PubMed] [Google Scholar]
- 46.Yamanouchi D, Banno H, Nakayama M, Sugimoto M, Fujita H, Kobayashi M, Kuwano H, Komori K. Hydrophilic statin suppresses vein graft intimal hyperplasia via endothelial cell-tropic Rho-kinase inhibition. J Vasc Surg. 2005;42:757–64. doi: 10.1016/j.jvs.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 47.Kozai T, Eto M, Yang Z, Shimokawa H, Luscher TF. Statins prevent pulsatile stretch-induced proliferation of human saphenous vein smooth muscle cells via inhibition of Rho/Rho-kinase pathway. Cardiovasc Res. 2005;68:475–82. doi: 10.1016/j.cardiores.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Turner NA, O’Regan DJ, Ball SG, Porter KE. Simvastatin inhibits MMP-9 secretion from human saphenous vein smooth muscle cells by inhibiting the RhoA/ROCK pathway and reducing MMP-9 mRNA levels. FASEB J. 2005;19:804–6. doi: 10.1096/fj.04-2852fje. [DOI] [PubMed] [Google Scholar]
- 49.Veillard NR, Braunersreuther V, Arnaud C, Burger F, Pelli G, Steffens S, Mach F. Simvastatin modulates chemokine and chemokine receptor expression by geranylgeranyl isoprenoid pathway in human endothelial cells and macrophages. Atherosclerosis. 2006;188:51–8. doi: 10.1016/j.atherosclerosis.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 50.Cicha I, Beronov K, Ramirez EL, Osterode K, Goppelt-Struebe M, Raaz D, Yilmaz A, Daniel WG, Garlichs CD. Shear stress preconditioning modulates endothelial susceptibility to circulating TNF-alpha and monocytic cell recruitment in a simplified model of arterial bifurcations. Atherosclerosis. 2009;207:93–102. doi: 10.1016/j.atherosclerosis.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 51.Chello M, Spadaccio C, Anselmi A, Patti G, Lusini M, Di Sciascio G, Covino E. Simvastatin reduces CD40 expression in an experimental model of early arterialization of saphenous vein graft. J Surg Res. 2006;136:302–8. doi: 10.1016/j.jss.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 52.Nachtigal P, Pospisilova N, Jamborova G, Pospechova K, Solichova D, Andrys C, Zdansky P, Micuda S, Semecky V. Atorvastatin has hypolipidemic and anti-inflammatory effects in apoE/LDL receptor-double-knockout mice. Life Sci. 2008;82:708–17. doi: 10.1016/j.lfs.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 53.Han KH, Ryu J, Hong KH, Ko J, Pak YK, Kim JB, Park SW, Kim JJ. HMG-CoA reductase inhibition reduces monocyte CC chemokine receptor 2 expression and monocyte chemoattractant protein-1-mediated monocyte recruitment in vivo. Circulation. 2005;111:1439–47. doi: 10.1161/01.CIR.0000158484.18024.1F. [DOI] [PubMed] [Google Scholar]
- 54.Munzel T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J. 2010;31:2741–8. doi: 10.1093/eurheartj/ehq396. [DOI] [PubMed] [Google Scholar]
- 55.Antoniades C, Antonopoulos AS, Bendall JK, Channon KM. Targeting redox signaling in the vascular wall: from basic science to clinical practice. Curr Pharm Des. 2009;15:329–42. doi: 10.2174/138161209787354230. [DOI] [PubMed] [Google Scholar]
- 56.Cui W, Matsuno K, Iwata K, Ibi M, Katsuyama M, Kakehi T, Sasaki M, Ikami K, Zhu K, Yabe-Nishimura C. NADPH oxidase isoforms and anti-hypertensive effects of atorvastatin demonstrated in two animal models. Journal of pharmacological sciences. 2009;111:260–8. doi: 10.1254/jphs.09148fp. [DOI] [PubMed] [Google Scholar]
- 57.Mercer JR, Cheng KK, Figg N, Gorenne I, Mahmoudi M, Griffin J, Vidal-Puig A, Logan A, Murphy MP, Bennett M. DNA damage links mitochondrial dysfunction to atherosclerosis and the metabolic syndrome. Circ Res. 2010;107:1021–31. doi: 10.1161/CIRCRESAHA.110.218966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aydin S, Uzun H, Sozer V, Altug T. Effects of atorvastatin therapy on protein oxidation and oxidative DNA damage in hypercholesterolemic rabbits. Pharmacol Res. 2009;59:242–7. doi: 10.1016/j.phrs.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 59.Lee TS, Chang CC, Zhu Y, Shyy JY. Simvastatin induces heme oxygenase-1: a novel mechanism of vessel protection. Circulation. 2004;110:1296–302. doi: 10.1161/01.CIR.0000140694.67251.9C. [DOI] [PubMed] [Google Scholar]
- 60.Castro PF, Miranda R, Verdejo HE, Greig D, Gabrielli LA, Alcaino H, Chiong M, Bustos C, Garcia L, Mellado R, Vukasovic JL, Godoy I, Lavandero S. Pleiotropic effects of atorvastatin in heart failure: role in oxidative stress, inflammation, endothelial function, and exercise capacity. J Heart Lung Transplant. 2008;27:435–41. doi: 10.1016/j.healun.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 61.Ortego M, Bustos C, Hernandez-Presa MA, Tunon J, Diaz C, Hernandez G, Egido J. Atorvastatin reduces NF-kappaB activation and chemokine expression in vascular smooth muscle cells and mononuclear cells. Atherosclerosis. 1999;147:253–61. doi: 10.1016/s0021-9150(99)00193-8. [DOI] [PubMed] [Google Scholar]
- 62.Bailey KM, Romaine SP, Jackson BM, Farrin AJ, Efthymiou M, Barth JH, Copeland J, McCormack T, Whitehead A, Flather MD, Samani NJ, Nixon J, Hall AS, Balmforth AJ. Hepatic metabolism and transporter gene variants enhance response to rosuvastatin in patients with acute myocardial infarction: the GEOSTAT-1 Study. Circ Cardiovasc Genet. 2010;3:276–85. doi: 10.1161/CIRCGENETICS.109.898502. [DOI] [PubMed] [Google Scholar]
- 63.Tomizawa A, Hattori Y, Suzuki K, Okayasu T, Kase H, Satoh H, Kasai K. Effects of statins on vascular endothelial function in hypercholesterolemic patients with type 2 diabetes mellitus: fluvastatin vs. rosuvastatin. International journal of cardiology. 2010;144:108–9. doi: 10.1016/j.ijcard.2008.12.146. [DOI] [PubMed] [Google Scholar]
- 64.Turner NA, Midgley L, O’Regan DJ, Porter KE. Comparison of the efficacies of five different statins on inhibition of human saphenous vein smooth muscle cell proliferation and invasion. J Cardiovasc Pharmacol. 2007;50:458–61. doi: 10.1097/FJC.0b013e318123767f. [DOI] [PubMed] [Google Scholar]
- 65.Koh KK, Sakuma I, Quon MJ. Differential metabolic effects of distinct statins. Atherosclerosis. 2011;215:1–8. doi: 10.1016/j.atherosclerosis.2010.10.036. [DOI] [PubMed] [Google Scholar]