Abstract
CD23, the low affinity receptor for IgE, exists in membrane and soluble forms. Soluble CD23 fragments are released from membrane CD23 by the endogenous metalloprotease, ADAM10. When purified tonsil B cells are incubated with IL-4 and anti-CD40 to induce class switching to IgE in vitro, membrane CD23 is up-regulated and soluble CD23 accumulates in the medium prior to IgE synthesis. We have uncoupled the effects of membrane CD23 cleavage and accumulation of soluble CD23 on IgE synthesis in this system. We show that siRNA inhibition of CD23 synthesis or inhibition of membrane CD23 cleavage by an ADAM10 inhibitor, GI254023X, suppress IL-4 and anti-CD40-stimulated IgE synthesis. Addition of a recombinant trimeric soluble CD23, ‘triCD23’, enhances IgE synthesis in this system. This occurs even when endogenous membrane CD23 is protected from cleavage by GI254023X, indicating that IgE synthesis is positively controlled by soluble CD23. We show that triCD23 binds to cells co-expressing membrane IgE and membrane CD21 and caps these proteins on the B cell membrane. Up-regulation of IgE by soluble CD23 occurs after class switch recombination and its effects are isotype-specific. These results suggest that membrane IgE and membrane CD21 co-operate in the soluble CD23-mediated positive regulation of IgE synthesis on cells committed to IgE synthesis. Feedback regulation may occur when the concentration of secreted IgE becomes great enough to allow binding to membrane CD23, thus preventing further release of soluble CD23. We interpret these results with the aid of a model for the up-regulation of IgE by soluble CD23.
Introduction
CD23 is the low-affinity receptor for IgE on B cells. It is initially expressed as a 45 kDa type II membrane protein (mCD23) containing a lectin ‘head’ domain, harboring the IgE binding site, and a C-terminal ‘tail’ in the extracellular sequence [1, 2]. CD23 is assembled into a trimer [3], the predominant form in the B cell membrane, by way of an α-helical coiled-coil stalk that links the three lectin ‘head’ and ‘tail’ domains to their transmembrane and cytoplasmic sequences [4]. CD23 is expressed in two forms, CD23a and CD23b, resulting from alternative transcription initiation sites and differing only by six or seven amino acids in the intracellular N-terminal cytoplasmic sequence [5]. CD23a is expressed on antigen-activated B cells, while CD23b expression is up-regulated by IL-4 in allergic inflammation and is associated with elevated serum IgE [6, 7, 8]. The anti-CD23 mAb, lumiliximab, down-regulates IgE synthesis by human B cells in vitro [9] and reduced human serum IgE levels in a phase I clinical trial in patients with mild-to-moderate persistent allergic asthma [10, 11]. This provides proof of principle that mCD23 is a valid target for therapy. Understanding the regulation of CD23 has the potential to inspire a more cost-effective interventional strategy.
It has long been known that CD23 negatively regulates the synthesis of IgE [12, 13]. The most compelling evidence comes from CD23 knock-out mice, which exhibit greatly increased levels of antigen-specific IgE after immunization [12, 13]. IgE synthesis is also inhibited in human B cells by anti-CD23 antibodies [9, 14, 15] or antigen-IgE complexes which bind to mCD23 [16]. Neither free IgE nor antibody Fab fragments have this inhibitory activity, suggesting that cross-linking of mCD23 is required for the inhibition [15]. These observations suggest that mCD23 may act in a negative feedback mechanism on IgE synthesis.
mCD23 can be cleaved in the stalk region by the endogenous disintegrin and metalloprotease ADAM10, to release a 37 kDa soluble fragment (sCD23) both in vitro [17, 18] and in vivo [18, 19, 20]. After the initial cleavage by ADAM10, sCD23 is susceptible to further cleavage by other proteases into fragments of various sizes (33, 29, 25 and 16 kDa) eliminating additional parts of the stalk and ‘tail’. These fragments lose the ability to independently form trimers but retain the ability to bind IgE, albeit at lower affinity [3]. The 16 kDa fragment, termed derCD23, containing only the lectin domain and ten amino acids of the ‘tail’, results from cleavage by the house dust mite allergen, Der p I [21, 22]. Whether sCD23, resulting from mCD23 cleavage, is involved in the positive regulation of IgE synthesis is not as clear as the negative regulation by mCD23. A number of studies have shown that sCD23 fragments either up- or down-regulate IgE synthesis in human B cells [23, 24], depending on their size and ability to form trimers [9, 25, 26]. The use of selective ADAM10 inhibitors is considered a potential therapy for asthma, based on a recent pre-clinical trial in mice [19]. Thus sCD23, as well as mCD23, may be a promising target for therapy.
Human CD23 binds not only to IgE, but also to CD21 (CR2) [24], CD11b (CR3), CD11c (CR4) [27], the vitronectin receptor (αvβ3) [28] and potentially other, as yet unidentified, proteins. However, IgE and CD21 are the only known ligands on mature B cells, with both binding sites distinct from each other and from the interface between the ‘head’ domains in the CD23 trimer [29]. Prior to the discovery of CD21 as the ‘counter-receptor’ for CD23 on B cells [24, 30], CD21 was already well characterized as the receptor for the C3d fragments of complement that play an important role in the complement cascade and adaptive immunity [31, 32]. In human CD23, the binding site for CD21 resides in the C-terminal ‘tail’ [29]. This ‘tail’ is not present in murine CD23 [33], which may explain why sCD23 expressed in transgenic mice does not up-regulate IgE during immunization, leaving only down-regulation through mCD23 [13, 34, 35]. Soluble CD21, shed from cell membranes, is thought to inhibit IgE synthesis in human B cells by binding to free trimeric sCD23, thereby preventing the binding to membrane CD21 (mCD21) [26, 36]. Antibodies against human CD21 modify IgE synthesis in anti-CD40-stimulated tonsil B cells in an epitope-dependent manner [24, 37]. Hence, mCD21 is implicated in mediating the effects of sCD23 on IgE synthesis.
Due to the multiple forms of CD23, multiple ligands and various activities of the different complexes, the mechanisms involved in IgE regulation by CD23 are still poorly understood. In the present study, we have focused on the positive regulation of IgE synthesis. In 2000, Mayer et al. showed that IL-4 and anti-CD40-stimulated IgE synthesis in human PBMCs can be reduced by the addition of metalloprotease inhibitors [23]. It is uncertain whether this is a direct effect on the B cells or whether it is due to inhibition of mCD23 cleavage and, therefore, sCD23 production. If sCD23 acts on B cells, are the stimulatory signals mediated by mCD21 or an unidentified counter-receptor?
To gain further insight into this question, we have stimulated purified human tonsil B cells with IL-4 and anti-CD40, and used either small interfering RNA (siRNA) to inhibit CD23 synthesis or an ADAM10 inhibitor (GI254023X) [38] to prevent cleavage of mCD23, leading to a reduction in sCD23 levels through two different mechanisms. We have followed the loss of mCD23 from the B cell surface, the appearance of sCD23 in the medium and the expression and secretion of IgE as a function of time for up to 12 days by flow cytometry and ELISA. We have added a recombinant trimeric sCD23, ‘triCD23’, to the ADAM10-inhibited B cells to test its ability to compensate for the reduction of endogenous sCD23. Finally, we have followed the expression of mIgE and mCD21 during the incubation of tonsil B cells with IL-4 and anti-CD40 and examined the effects of triCD23 binding to these ligands by confocal microscopy.
Materials & Methods
Isolation of Human Tonsil B Cells
Following informed written consent, with ethical approval from Guy’s Research Ethics Committee, we obtained human tonsils from donors undergoing routine tonsillectomies. The allergic status of the donor was determined by verbal communication with the parents at the time of consent. Mononuclear cells were separated by density on a Ficoll gradient (GE Healthcare) and B cells isolated using AET-treated SRBCs (TCS Biosciences). B cells were routinely >98% CD20+ and <2% CD3+, as determined by flow cytometry [9].
Small Interfering RNA (siRNA) Transfection
Total B cells were transfected with CD23 siGENOME SMARTpool® siRNA (3μg) (referred to as CD23 siRNA) or ON-TARGETplus Non-targeting siRNA Pool #1 (3μg) (referred to as control siRNA) (Thermo Scientific Dharmacon) using the Amaxa™ Human B cell Nucleofector™ kit and Nucleofector™ II Device (Lonza), according to the manufacturer’s instructions. Transfection efficiency, 30 minutes after transfection, was determined to be 97% using a fluorescent non-functional siRNA (siGLO® Red) (Thermo Scientific Dharmacon). The efficiency of CD23 knockdown was quantified by qPCR.
Cell Culture
B cells were cultured in 24-well plates (Nunc) at 0.5 × 106 cells/ml in RPMI with penicillin (100IU/ml), streptomycin (100μg/ml), glutamine (2mM) (Invitrogen), 10% FCS (Hyclone, Perbio Biosciences Ltd), transferrin (35μg/ml) and insulin (5μg/ml) (Sigma-Aldrich). Cells were activated with IL-4 (200IU/ml) (R&D Systems) and anti-CD40 antibody (1μg/ml) (G28.5; ATCC) for up to 12 days. Mouse anti-human CD21 mAb (HB5) (Santa Cruz) was added to cells at 0.1, 1 or 10μg/ml. Epitope analysis has shown this clone to inhibit CD23 binding to CD21 [30]. The ADAM10 inhibitor, GI254023X, was purified on a CombiFlash Rf® (Teledyne ISCO) system (Column: RediSep Rf - 4 g Silica, Flow rate: 18 ml/min, Solvent: acetonitrile, tR: 10 min). HPLC analysis at 220 nm confirmed a purity of ≥98 % [38]. Cells were grown for 5 days, to allow the up-regulation of mCD23 and class switch recombination (CSR) to IgE, before addition of the inhibitor (1μM–15μM). Monomeric derCD23 (16145 Da) was made as previously described [29]. lzCD23, made as previously described [9], was modified to produce the more stable trimer, triCD23 (84414 Da) consisting of residues 131-321 of human CD23 prefixed by the trimerization motif (IAAIESK)4 and expressed and refolded from inclusion bodies using the E. coli vector pET151. This additionally provides N-terminal HIS6 and V5 epitope tags and a TEV enzyme cleavage site that has been left un-cleaved in the final product (manuscript in preparation). Recombinant CD23 proteins were dialysed into PBS and sterile-filtered before addition to human B cell cultures. Concentrations were selected to be close to the calculated KD value for trimeric CD23 binding to IgE (10−7M) [9, 29] and used at a weight ratio of 1:3 (triCD23:derCD23) to maintain the same number of mIgE/mCD21 binding sites in each condition.
Quantitative PCR (qPCR)
Total RNA was isolated from cells using RNeasy Mini kits (Qiagen), primed with oligo(dT) and random hexamers and reverse transcribed using Superscript II (Invitrogen). qPCR was performed using TaqMan® MGB gene expression assays (see below) and TaqMan® Universal PCR Master Mix on a 7900HT realtime PCR machine (Applied Biosystems). Gene expression was normalized to an endogenous reference gene (β2-microglobulin) and quantified by ΔΔCt analysis (SDS 2.1 software). GAPDH: Hs02786624_g1; β2microglobulin: 4310886E; CD23: Hs00233627_m1; εGLT: Forward 5′ CTGTCCAGGAACCCGACAGA 3′, Reverse 5′ TGCAGCAGCGGGTCAAG 3′ with MGB probe 6FAM-AG GCACCAAATG-MGB.
Flow Cytometry
B cells were stained for mCD23 expression with mouse anti-human CD23-FITC (1:50) (Dako) and membrane ADAM10 expression with mouse anti-human ADAM10-PE (1:50) (R&D Systems) and incubated on ice in the dark for 45 minutes. For intracellular staining, cells were fixed with 4% paraformaldehyde (Electron Microscopy Sciences) in PBS for 15 minutes, washed and resuspended in permeabilisation buffer (PBS, 0.05% Triton® X-100, 0.5% saponin (Sigma-Aldrich)) for 15 minutes. Goat anti-human IgE-FITC (1:500) (Vector) and monoclonal mouse anti-human IgG-APC (1:50) (Miltenyi Biotec) were added and incubated on ice in the dark for 45 minutes. Collection of flow cytometry data was conducted using a FACSCalibur™ (BD Biosciences), with gating on live cells determined by forward versus side scatter, and events were analyzed using FlowJo software (Treestar).
Immunoglobulin ELISA
Maxisorp plates (Nunc) were coated with polyclonal mouse anti-human IgE (1:7000) (Dako) or polyclonal goat anti-human IgG (1:1000) (AbD Serotec), in pH 9.8 carbonate buffer (distilled water, 0.2M NaCO3, 0.2M NaHCO3), overnight at 4°C. Unbound sites were blocked with 2% milk powder in PBS + 0.05% Tween® 20 (PBS-T) (Sigma-Aldrich) for 1 hour at room temperature. Supernatant samples were then added at appropriate dilutions and plates incubated overnight at 4°C. Human serum IgE (from 800ng/ml) (NIBSC) or IgG (from 200ng/ml) (Sigma-Aldrich) were used to construct standard curves. Binding was detected by mouse anti-human IgE-HRP (1:1000) (Dako) or goat anti-human IgG-HRP (1:1000) (Sigma-Aldrich) in 1% milk powder in PBS-T for 2 hours at 37°C. The color reaction was developed with o-Phenylenediamine (OPD) (Sigma-Aldrich) and analyzed at 492 nm using an automated plate reader (Titertek). Immunoglobulin concentration was calculated from the standard curve using GraphPad Prism 5.03 software (San Diego, USA), with a minimum detectable concentration of ~2ng/ml. IgE secretion (by day 12) ranged from 2ng/ml – 1255ng/ml (mean = 401ng/ml ± 71ng/ml, n=26). IgG secretion (by day 12) ranged from 113ng/ml – 2264ng/ml (mean = 365ng/ml ± 269ng/ml, n=8).
Soluble CD23 ELISA
Human sCD23 EASIA™ ELISA kits (BioSource) were used according to the manufacturer’s instructions. Briefly, supernatants were added to microtiter plates pre-coated with a mixture of monoclonal anti-CD23 antibodies and anti-CD23-HRP was added for 2 hours at room temperature. The color reaction was developed with Tetramethyl benzidine (TMB) and analyzed at 450 nm. The kit recognises the 16, 25, 29 and 37 kDa fragments of sCD23, with a minimum detectable concentration of ~200pg/ml. sCD23 production (by day 12) ranged from 13ng/ml – 102ng/ml (mean = 58ng/ml ± 4ng/ml, n=33).
Confocal Microscopy
Human tonsillar B cells were stimulated for 8 days with IL-4 and anti-CD40, harvested and dead cells removed by density on a Ficoll gradient. 3 × 105 cells were stimulated with either media alone, derCD23 (3μM/48μg/ml), triCD23 (1μM/84μg/ml) or anti-CD21 (10μg/ml) at 37°C for 30 minutes. Cells were fixed with 4% paraformaldehyde, washed (with PBS + 0.05% Triton® X-100), mounted onto poly-L-lysine-coated coverslips and fixed again with 4% paraformaldehyde. Coverslip-mounted cells were stained with goat anti-human IgE-FITC (1:200) (Vector) and monoclonal mouse anti-CD21 (1:100) (HB5 clone) (Santa Cruz) for 1 hour, washed, and secondary anti-mouse-Alexa 594 (1:500) (Molecular Probes, Invitrogen) was added for 45 minutes. The nuclear stain Hoescht 33258 (1:20000) (Molecular Probes, Invitrogen) was added for 10 minutes, cells were washed 3 times and immunofluorescence visualized with an SP2 confocal microscope (Leica).
Statistical Analysis
Flow cytometry and ELISA data are shown relative to control-treated cells (either transfected with control siRNA or stimulated with IL-4 and anti-CD40 alone), to compensate for inter-donor variation. Data from 7 out of 33 donors that failed to respond to IL-4 and anti-CD40, with undetectable levels of IgE expression and secretion by day 12, were excluded. Data are summarized as mean ± SEM. Statistical analysis was performed using ANOVA with Bonferroni correction, unless otherwise stated. A p-value of <0.05 was considered significant (ns = non-significant, * = <0.05, ** = <0.01, *** = <0.001). Significance to control conditions is indicated above data and significance between two conditions is shown between data. Correlation analysis was performed using Spearman’s rank correlation coefficient.
Results
siRNA-induced inhibition of CD23 mRNA, mCD23, sCD23 and IgE secretion
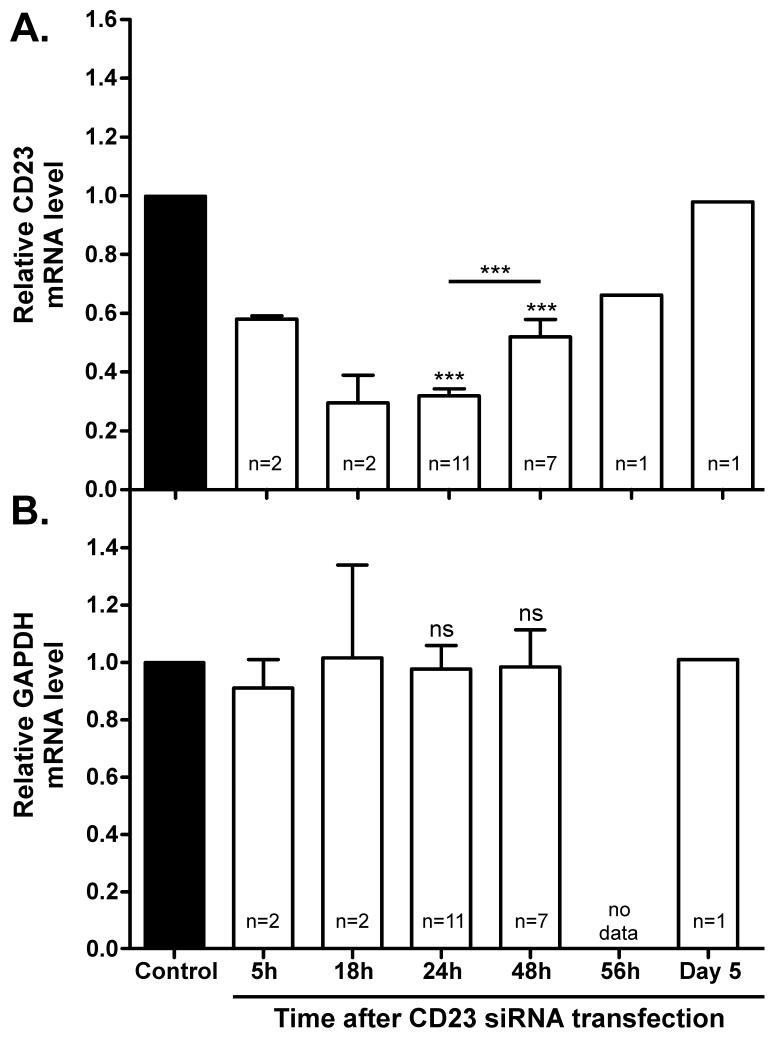
Human tonsillar B cells were transfected with a pool of four siRNA duplexes, directed against CD23 and stimulated with IL-4 and anti-CD40 for up to 12 days. A significant inhibition of CD23 mRNA expression was observed following transfection with CD23 siRNA, compared to control siRNA (Figure 1A), but no effect was seen on the expression of a non-targeted gene, GAPDH (Figure 1B). The maximum knockdown of CD23 (70.0% ± 0.1%) occurred between 18 and 24 hours following transfection, after which CD23 mRNA levels began to recover to that of control siRNA-transfected cells.
Figure 1. siRNA-induced inhibition of CD23 mRNA expression.
Human tonsillar B cells were transfected with either control siRNA (■) or CD23 siRNA (□) and cultured for up to 5 days with IL-4 (200IU/ml) and anti-CD40 (1μg/ml). Cells were harvested at the times indicated and qPCR was performed to quantify mRNA expression levels for (A) CD23 and (B) GAPDH. Expression levels were calculated by ΔΔCt analysis, normalized against the endogenous reference gene β2-microglobulin and expressed relative to control siRNA-transfected cells at each timepoint (n=11).
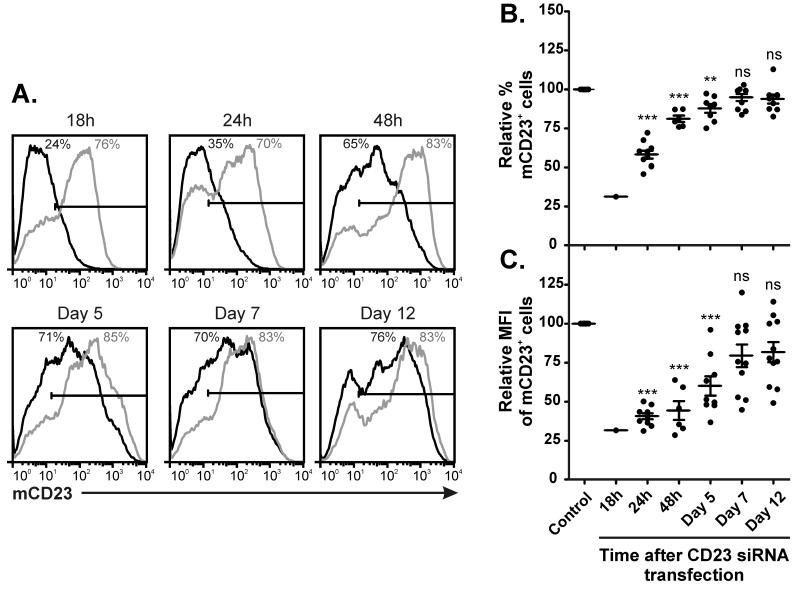
Despite the short-term inhibition of CD23 mRNA shown in Figure 1, mCD23 protein levels were reduced following transfection with CD23 siRNA. Figure 2A shows a representative example of flow cytometric analysis of mCD23 levels, from 18 hours to 12 days following transfection with either control siRNA or CD23 siRNA. In control siRNA-transfected cells, mCD23 expression reached a peak on day 5 (88.4% ± 2.8%, n=8). Figure 2B shows that the percentage of mCD23+ cells was reduced following transfection with CD23 siRNA and this reduction remained statistically significant until day 7, although was largely recovered by 48 hours. The level of mCD23 expression on cells, as measured by the mean fluorescence intensity (MFI), was significantly lower on cells transfected with CD23 siRNA, compared to control siRNA (Figure 2C). This reduction also remained statistically significant until day 7, but the MFI was suppressed to a greater degree than the percentage of mCD23+ cells and took longer to recover.
Figure 2. Reduced mCD23 expression following CD23 siRNA transfection.
(A) Human tonsillar B cells were transfected with either control siRNA (grey line) or CD23 siRNA (black line) and cultured for up to 12 days with IL-4 (200IU/ml) and anti-CD40 (1μg/ml). mCD23 expression was analyzed by flow cytometry and the % of mCD23+ cells (above isotype control as indicated by horizontal marker) are indicated on the histograms (n=1, representative of 11). Multiple donor data, relative to control siRNA-transfected cells at each timepoint, is shown for (B) % mCD23+ cells and (C) CD23+ MFI, at 18 hours to 12 days following transfection (n=11).
sCD23 is produced by cleavage of mCD23, initially by the membrane-bound metalloprotease ADAM10 [18]. sCD23 was first detectable in the supernatant 4 days after transfection with either control siRNA or CD23 siRNA (data not shown). In accordance with the reduced mCD23 levels, sCD23 production decreased significantly following CD23 siRNA transfection (Figure 3A). By day 12, sCD23 levels were, on average, 17.4% (± 5.7%) lower in supernatants from cells transfected with CD23 siRNA, compared to control siRNA. No significant reduction was seen at the earlier timepoints of days 5 and 7. The expression level of ADAM10 was no different between control siRNA and CD23 siRNA-transfected cells at any timepoint during the culture (Figure S1), which reveals that the loss of ADAM10 was not responsible for the decrease in sCD23 levels.
Figure 3. Inhibition of sCD23 production by CD23 siRNA correlates with reduced IgE secretion.
Human tonsillar B cells were transfected with either control siRNA or CD23 siRNA and cultured for up to 12 days with IL-4 (200IU/ml) and anti-CD40 (1μg/ml). (A) Supernatants were harvested on days 5, 7 and 12 and the % inhibition of sCD23 production (●) (n=10) and IgE secretion (○) (n=6) were analyzed by ELISA, relative to control siRNA-transfected cells. (B) Correlation between inhibition of IgE secretion and inhibition of sCD23 production on day 12 (n=6). (C) Supernatants were harvested on day 12 and the % inhibition of IgG secretion was analyzed by ELISA, relative to control siRNA-transfected cells (n=8). (D) qPCR was performed to quantify RNA levels for εGLT, up to 5 days following transfection with either control (■) or CD23 siRNA (□). Expression levels were calculated by ΔΔCt analysis, normalized against the endogenous reference gene β2-microglobulin and expressed relative to control siRNA-transfected cells at 24 hours (n=1, unless indicated otherwise).
Since transfection with CD23 siRNA successfully resulted in reduced sCD23 levels by day 12, we next investigated the association between reduced sCD23 production and IgE synthesis at this timepoint. Secreted IgE (sIgE) was first detectable in the supernatant 5 days after stimulation with IL-4 and anti-CD40 (data not shown). Figure 3A also shows the relative levels of sIgE 5, 7 and 12 days after transfection with control siRNA or CD23 siRNA. Of the ten donors analyzed for sCD23 production, four donors did not produce any detectable sIgE by day 12 and so were excluded from the analysis. CD23 siRNA-transfected cells secreted 47.4% (± 9.5%) less IgE than control siRNA-transfected cells (Figure 3A). The extent to which sIgE levels were inhibited was not related to the amount of IgE the cells secreted. Figure 3B correlates the level of inhibition of sCD23 production with the level of inhibition of IgE secretion. The positive correlation between sCD23 and sIgE is statistically significant (r=0.94, p=0.0167), as determined by Spearman’s rank correlation coefficient (n=6). Importantly, CD23 siRNA transfection led to no significant changes in the levels of IgG secretion by day 12 (Figure 3C). From day 7 onwards, following transfection with CD23 siRNA, mCD23 levels had returned to those of control siRNA-transfected cells (Figures 2B and 2C). Thus any CD23-mediated differences in IgE secretion between days 7 and 12 can be attributed to sCD23, rather than mCD23.
Figure 3D shows qPCR analysis of ε germline transcripts (εGLT), an early marker of CSR to IgE. No significant differences in εGLT levels were detected between control siRNA and CD23 siRNA-transfected cells at either 24 or 48 hours following transfection, despite the inhibition of IgE secretion shown in Figure 3A. Membrane and intracellular staining for IgE and IgG, analyzed by flow cytometry, showed no significant changes in the percentage of IgE+ or IgG+ cells between control siRNA and CD23 siRNA-transfected cells at days 7 or 12 (Figure S2). In addition, there were no differences in general cell viability (live cell gating determined by forward versus side scatter) or maturation (CD38 expression) between control siRNA and CD23 siRNA-transfected cells (data not shown).
ADAM10 inhibition with GI254023X reduces mCD23 shedding, sCD23 release and IgE secretion
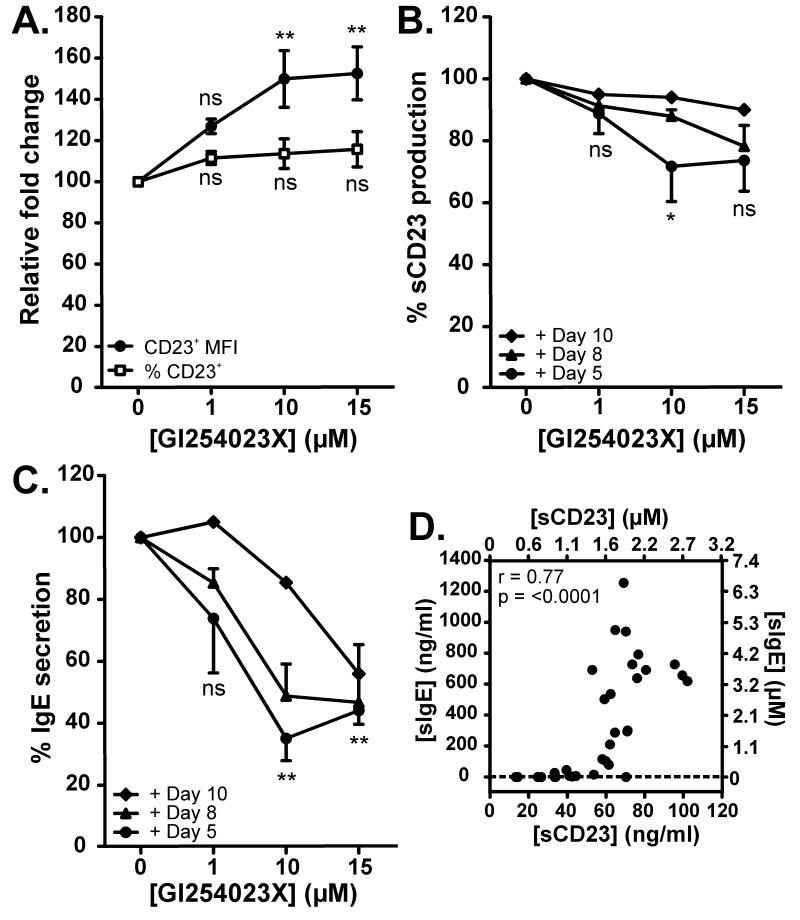
Since CD23 siRNA transfection reduced sCD23 through the early reduction of mCD23 expression, a different approach was taken that would reduce sCD23 levels through preventing mCD23 shedding, thus maintaining mCD23 levels. To achieve this, we used the ADAM10 inhibitor GI254023X. Human tonsillar B cells were stimulated with IL-4 and anti-CD40 for 5 days, after which GI254023X was added at 1μM–15μM. The addition of GI254023X did not lead to a significant increase in the percentage of CD23+ cells, although a significant dose-dependent accumulation of mCD23 on the surface of CD23+ cells (as measured by MFI) was observed by day 12 (Figure 4A). With the addition of 10μM GI254023X, this was accompanied by a significant reduction in sCD23 production (28.3% ± 9.9%) (Figure 4B) and a significant reduction in IgE secretion (64.9% ± 7.2%) (Figure 4C), with no effect on IgG secretion (data not shown).
Figure 4. ADAM10 inhibition with GI254023X reduces mCD23 shedding, sCD23 release and IgE secretion.
Human tonsillar B cells were cultured for 12 days with IL-4 (200IU/ml) and anti-CD40 (1μg/ml) in the absence or presence of varying concentrations of GI254023X, added on day 5 unless otherwise stated. (A) mCD23 expression was analyzed by flow cytometry on day 12 and the fold change plotted for the % of mCD23+ cells (□) and CD23+ MFI (●), relative to cells cultured with IL-4/anti-CD40 alone (n=6). GI254023X was added on day 5 (●) (n=6), day 8 (▲) (n=2) or day 10 (◆) (n=1) and supernatants were analyzed on day 12 for (B) sCD23 production and (C) IgE secretion, relative to cells cultured with IL-4/anti-CD40 alone. (D) Correlation between sCD23 and sIgE levels after 12 days (n=33). Values are shown in ng/ml on the bottom x-axis and left y-axis and in μM on the top x-axis and right y-axis (1μM 190 kDa sIgE = 190ng/ml, 1μM 37 kDa sCD23 = 37ng/ml).
To assess the mechanism of action, GI254023X was added at day 8 or day 10, in addition to day 5, and sCD23 and sIgE levels were determined at day 12. Figures 4B and 4C show that when the inhibitor was added progressively later in the incubation period, the levels of both sCD23 and sIgE were higher, due to the shorter time period in which GI254023X could inhibit mCD23 shedding. However, inhibition of sCD23 production and IgE secretion could still be achieved even when GI254023X was added as late as day 10 in the incubation period. In further support of this association, Figure 4D shows the relationship between sCD23 and sIgE levels, measured in the supernatant following 12 days stimulation with IL-4 and anti-CD40. The sCD23 concentrations are indicated in ng/ml, and also in μM, to facilitate comparison with the known KD values of interaction with IgE and CD21 (see the Discussion). The positive correlation between sCD23 and sIgE is statistically significant (r=0.77, p=<0.0001), as determined by Spearman’s rank correlation coefficient (n=33). There appear to be two phases in the relationship, separated by a threshold concentration of sCD23 (60ng/ml/1.6μM), above which a steep rise in IgE secretion was observed.
Effect of exogenous sCD23 fragments on IgE secretion
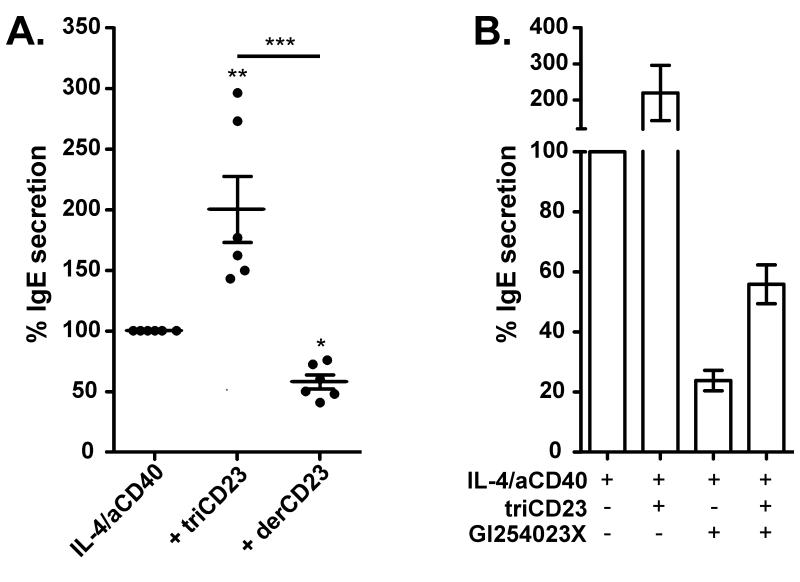
We have previously shown that oligomeric sCD23 (lzCD23) above a certain threshold concentration up-regulates, whereas monomeric sCD23 (derCD23) down-regulates, IgE production from human tonsillar B cells [9]. The IgE-potentiating ability of the more stable triCD23 was assessed by culturing B cells with IL-4, anti-CD40 and triCD23 (1μM) (added at day 0) for 12 days. Although not a naturally occurring fragment, triCD23 has been specifically designed to mimic the trimeric state of endogenous sCD23 when it is first cleaved off the membrane in allergic tissues. Western blot analysis showed triCD23 did not degrade into smaller fragments during the 12 days in culture (data not shown). Analysis by ELISA on day 12 showed a 200.0% (± 27.2%) increase in IgE secretion from cells cultured with triCD23 compared to IL-4 and anti-CD40 alone, whereas cells cultured with monomeric derCD23 produced significantly less IgE (Figure 5A). These effects were isotype-specific, as IgG secretion remained unaffected, and flow cytometric analysis showed triCD23 had no effect on the percentage of IgE+ cells (Figure S3), suggesting that triCD23 preferentially promotes expression of the secreted form of IgE.
Figure 5. Effect of exogenous sCD23 fragments on IgE secretion.
(A) Human tonsillar B cells were cultured for 12 days with IL-4 (200IU/ml) and anti-CD40 (1μg/ml) alone, + triCD23 (1μM/84μg/ml) or + derCD23 (3μM/48μg/ml). Supernatants were analyzed on day 12 for IgE secretion, relative to cells cultured with IL-4/anti-CD40 alone (n=6). (B) Human tonsillar B cells were cultured for 12 days with IL-4/anti-CD40 alone, + triCD23 (1μM), + GI254023X (5μM) or + triCD23 & GI254023X. Supernatants were analyzed on day 12 for IgE secretion, relative to cells cultured with IL-4/anti-CD40 alone (n=2).
The ability of triCD23 to rescue the inhibition of sCD23 production and IgE secretion caused by addition of the ADAM10 inhibitor (GI254023X) was then investigated. As shown in Figure 5B, IgE secretion increased 2.3-fold when GI254023X (5μM) was co-cultured with triCD23 (1μM), compared to GI254023X alone.
Trimeric soluble CD23 co-localises mIgE and mCD21
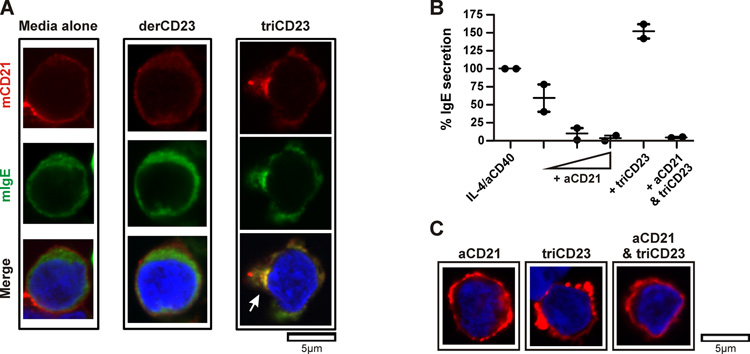
Having established here that sCD23 was involved in the positive regulation of IgE, we next sought to identify the mechanism by which this may occur. Confocal microscopy was utilized to test the binding of triCD23 to mIgE and mCD21 and to visualize the surface dynamics of these complexes. Human tonsillar B cells were cultured with IL-4 and anti-CD40 for 8 days, to ensure sufficient time for the dual expression of mIgE and mCD21 (Figure S4) and then stimulated for 30 minutes in the presence of media alone, derCD23 or triCD23. The left and middle columns of Figure 6A show the uniform distribution of mIgE and mCD21 on the surface of B cells incubated with either media alone or monomeric derCD23, respectively. The right column of Figure 6A shows the redistribution and co-localisation of mIgE and mCD21, following incubation with triCD23. Several distinct microclusters formed and particularly strong capping of mIgE and CD21 is indicated on the merged image in the lower right panel of Figure 6A.
Figure 6. triCD23 co-localises both mIgE and mCD21 to up-regulate IgE secretion.
(A) Human tonsillar B cells were cultured for 8 days with IL-4 (200IU/ml) and anti-CD40 (1μg/ml) and stimulated for 30 minutes with media alone, derCD23 (3μM/48μg/ml) or triCD23 (1μM/84μg/ml). Cells were stained with mouse anti-CD21, plus secondary anti-mouse-Alexa 594 (red), anti-IgE-FITC (green), and the nuclear stain Hoescht (blue). Cells were visualized by confocal microscopy and images show a single field of view, with the bottom panel showing a three-color overlay. The white arrow indicates strong capping of mIgE with mCD21 (n=1, representative of 3). (B) Human tonsillar B cells were cultured for 12 days with IL-4/anti-CD40 alone, + anti-CD21 (0.1, 1 or 10μg/ml), + triCD23 (1μM) or + aCD21 (10μg/ml) & triCD23. IgE secretion was analysed by ELISA on day 12, relative to cells cultured with IL-4/anti-CD40 alone (n=2). (C) Cells were cultured as described in (A) and stimulated with anti-CD21 (10μg/ml), triCD23 (1μM) or anti-CD21 & triCD23. Cells were stained for mCD21 (red) and the nucleus (blue) and visualized by confocal microscopy (n=1).
To further investigate the role of mCD21 in the sCD23-mediated regulation of IgE synthesis, tonsil B cells were cultured with increasing concentrations of an anti-CD21 mAb (HB5 clone) for 12 days. Figure 6B shows the addition of anti-CD21 resulted in a dose-dependent decrease in IgE secretion. This was accompanied by an increase in mCD23 expression and a decrease in sCD23 production (data not shown). When cells were cultured with a combination of anti-CD21 (10μg/ml) and triCD23 (1μM), the IgE-stimulating effects of triCD23 were blocked. Figure 6C shows confocal microscopy analysis of mCD21 expression. Total B cells were cultured with IL-4 and anti-CD40 for 8 days and then stimulated for 30 minutes in the presence of anti-CD21, triCD23 or anti-CD21 and triCD23. Distinct microclusters of mCD21 formed on the surface of cells stimulated with either anti-CD21 or triCD23. In the cells pre-treated with anti-CD21 before stimulation with triCD23, the distribution of mCD21 was more uniform. Together, these data support the hypothesis shown in Figure 7, whereby trimeric sCD23 molecules can bind both mIgE and mCD21 to stimulate IgE synthesis.
Figure 7. Proposed mechanism of IgE up-regulation by trimeric soluble CD23.
In this model, membrane CD23 (mCD23) is cleaved by ADAM10 to release trimeric soluble CD23 (sCD23), which co-ligates both membrane IgE (mIgE) and membrane CD21 (mCD21) on the surface of IgE-committed B cells to up-regulate IgE synthesis, triggering the onset of allergic symptoms.
Discussion
The cytokines IL-4 and IL-13 induce the CSR to all immunoglobulin isotypes downstream from Cμ in the heavy-chain locus on human chromosome 14, but can be replaced by other cytokines for switching to isotypes other than IgE. IL-4 up-regulates CD23, so that the expression of CD23 and IgE are inextricably linked [25]. The role of CD23 in the regulation of IgE in the human system appears to be more complex than in the mouse [2].
Negative regulation of IgE synthesis through mCD23 occurs in murine B cells in vivo [13, 39] and in human B cells in vitro [15]. In human B cells, this requires the cross-linking of mCD23, e.g. by antibodies or antigen-IgE complexes. In the present study, we focused on a suggested mechanism by which sCD23 positively regulates IgE synthesis exclusively in human B cells [9, 29]. In analyzing this mechanism, it is necessary to consider that there are multiple forms of sCD23, several potential ligands and various complexes with different affinities and topological constraints associated with binding to their ligands in solution and particularly on cells.
sCD23 is released from cells by the action of an endogenous metalloprotease, identified as ADAM10 [17, 18], and accumulates in the supernatant of B cells following IL-4 and anti-CD40-stimulated CSR to IgE [23, 40, 41]. Here we have used CD23 siRNA and an ADAM10 inhibitor (GI254023X) to confirm that mCD23 expression and cleavage to release sCD23 is required for the enhancement of IgE synthesis in primary human B cells.
Short-term inhibition of CD23 mRNA with siRNA led to a significant reduction in the frequency and MFI of CD23+ cells until day 7 following transfection. By day 12, but not at the earlier timepoints of day 5 and 7, CD23 siRNA-transfected cells showed significantly reduced sCD23 production, which correlated with reduced IgE secretion, implicating sCD23 in the up-regulation of human IgE synthesis (Figures 1-3).
To further investigate the role of sCD23, ADAM10 was inhibited using GI254023X. It has been previously been shown that inhibition of ADAM10 in vivo [20, 42], and the addition of GI254023X to human tonsil B cells [18], results in decreased sCD23 production. However, subsequent effects on IgE were not analyzed. In this study, addition of GI254023X resulted in increased mCD23 expression, reduced sCD23 production and reduced IgE secretion. Inhibition of sCD23 production and IgE secretion could still achieved when GI254023X was added progressively later in the incubation period, albeit to lesser extents, indicating that the ADAM10 inhibitor is regulating secretion of IgE by a post-switch event (Figure 4). Since mCD23 is not cleaved, but is actually elevated in these conditions, the observed inhibition of IgE synthesis firmly points to positive regulation mediated through sCD23. De-repression of negative signalling through mCD23 does not appear to play a major role. When sIgE binds to mCD23, it blocks metalloprotease cleavage and the release of sCD23 [16]. However, this is not a complication in the present system as the sIgE concentrations in the medium (<10−10M) are well below the KD of the IgE-mCD23 interaction. As in previous work with alternative matrix metalloprotease inhibitors and IL-4-stimulated human peripheral blood lymphocytes [23], we conclude that sCD23 is required to maintain IgE synthesis in human B cells.
We confirmed the findings from previous studies [9, 23, 26] that addition of recombinant trimeric sCD23 (triCD23) to primary human B cells enhances IgE synthesis (Figure 5A). We now also show that when B cells are incubated with GI254023X in the presence of triCD23, a relief from GI254023X-mediated IgE suppression is observed (Figure 5B), confirming the positive regulation of IgE synthesis by sCD23. However, the level of IgE synthesis was not fully restored to that of cells cultured only with triCD23. This might be explained by off-target effects of the ADAM10 inhibitor. Further studies are required to investigate this possibility.
mIgE and mCD21 are the prime candidates for mediating the stimulatory effects of sCD23. We show here that mCD21 expression declines in the first few days of incubation with IL-4 and anti-CD40 and reaches a plateau on day 5 (Figure S4). Loss of mCD21 is probably due to the shedding of soluble fragments [43] and further shedding may be prevented by the binding of sCD23 to mCD21 and mIgE. Although the concentrations of endogenous sCD23 (Figure 4D) are far lower than the KD for the 1:1 interaction with mCD21 or mIgE (KD = 10−6 and 10−8M, respectively) [9, 29], the avidity effect of binding of three sCD23 ‘heads’ as a trimer to multiple mCD21 and/or mIgE molecules may dramatically enhance binding affinity at the cell surface. Prior binding of sCD23 to mIgE, the stronger ligand, may enable the recruitment of mCD21 into a tri-molecular complex. The CD23 binding site for the two N-terminal domains of CD21 lies in the C-terminal ‘tail’, and is sufficiently distant from the IgE binding site to allow the simultaneous binding of both ligands in solution [29]. Indeed, the observed capping of mIgE and mCD21 on B cells stimulated with triCD23 (Figure 6A) reveals that there are likewise no topological constraints to prevent a trimeric CD23 molecule from co-ligating mIgE and mCD21 and forming the predicted multi-molecular network on the cell surface [29]. In cells where CD23 binding to mCD21 is blocked with the addition of an anti-CD21 mAb, triCD23 is no longer able to increase IgE secretion (Figure 6B).
Figure 4D reveals that there is a remarkable relationship between the concentration of sCD23 and sIgE after incubation of tonsil B cells with IL-4 and anti-CD40. There appears to only be a slight increase in sIgE at low sCD23 concentrations, up to 60ng/ml (1.6μM) (concentrations calculated for the 37 kDa fragment). However, above this threshold we observed a steep rise in sIgE with increasing concentrations of sCD23. The relationship shown in Figure 4D may reflect the avidity of sCD23 in the tri-molecular complexes with mIgE and mCD21 at the surface of the fluid B cell membrane, as this curve exhibits cooperativity.
Earlier work has shown that the incubation of PBMCs with sCD23 stimulates ‘ongoing’ IgE synthesis, rather than increasing CSR to IgE, which would require stimulation by IL-4 (or IL-13) [41]. In our system, with IL-4 and anti-CD40 stimulation, we observed no difference in the expression of εGLT, an early marker for CSR, in CD23 siRNA-, compared to control siRNA-transfected cells (Figure 3D). Neither inhibition of sCD23 through siRNA transfection nor addition of recombinant triCD23 altered the proportion of IgE+ or IgG+ cells when measured by flow cytometry (Figures S2 and S3B). Additional experiments with tonsil B cells have shown CSR to occur in the first few days of the incubations (Hobson et al., unpublished). However, recombinant sCD23 can still increase IgE synthesis when added as late as day 9 in the incubations (Jutton et al., unpublished). It has also been shown that the addition of a metalloprotease inhibitor terminates incremental IgE synthesis after CSR has occurred (Figure 4C) [23]. Taken together, these experiments indicate an isotype-specific role for sCD23 in promoting IgE synthesis through a post-switch mechanism.
Fearon and Carter showed that co-ligation of antigen-specific IgM and the CD19-CD21-TAPA complex on naïve B cells by antigen, covalently linked to the C3d fragment of complement, stimulates B cell proliferation in the immune response [31]. The effect of a blocking antibody against CD21 in vivo demonstrated the importance of this mechanism for a robust T cell-dependent immune response [44]. The mechanism operates by synergistic signalling through IgM and CD21 to augment the expression of the B cell survival factors, Bcl-XL and Bcl-2, respectively [45]. CD23 expressed in a fibroblast cell line can mimic the activity of the antigen-C3d complexes in lowering the threshold of B cell proliferation by an anti-IgM (surrogate antigen) [46]. However, it is not known whether mIgE can mimic mIgM in this mechanism. The cytoplasmic sequence of IgE, required for survival, differs from that of IgM (and other isotypes) [47], although mIgE is associated with the same signal transduction proteins (the α and β subunits) as other isotypes [48]. Evidently, mIgE has some capacity for signalling, but nothing is known about the signal transduction pathways.
It is informative to consider the sequence of events after placing the B cells into culture with IL-4 and anti-CD40. At first, sCD23 can only bind to mCD21, which may elicit a proliferative response [49]. When IgE is expressed on the membrane, sCD23 appears to sequester the mIgE and mCD21 in raft-like structures (Figure 6A) [29], which resemble the fate of cross-linked IgM-CD19-CD21-TAPA complexes in complement-enhanced IgM BCR activation [31, 50]. Whether formation of a mIgE-sCD23-mCD21 complex would lead to similar functional consequences remains to be investigated.
In this study, two experimental approaches were taken to reduce sCD23 production in primary human B cells. Both techniques culminated in reduced sCD23 production and reduced IgE secretion, albeit through different actions on mCD23 (reduced expression or inhibition of cleavage). Each approach has its limitations, but they are different from each other, and the combined results conclusively demonstrate that sCD23 stimulates IgE synthesis in human B cells.
Our results suggest that sCD23 may be an active partner, rather than an innocent bystander, in regulating IgE synthesis and therefore a promising therapeutic target for allergic disease. Two current strategies, the anti-CD23 mAb lumiliximab, which showed efficacy in lowering IgE levels in asthmatic patients [10, 11], and metalloprotease inhibitors, already tested in mice [19], are aimed primarily at mCD23. Our results, and those from others [9, 23, 26, 41, 51], should encourage the rational design of inhibitors of sCD23 binding to its ligands for the treatment of allergy and asthma.
Supplementary Material
Acknowledgements
We thank the staff at the Evelina Children’s Hospital, Guy’s and St. Thomas’ National Health Service Foundation Trust, for their help with the collection of tonsils. We thank R.J.B. and C.W. of the MRC & Asthma UK Protein Production Facility for providing the anti-CD40 mAb and for the design of triCD23.
Footnotes
A.C. is a Medical Research Council (MRC) & Asthma UK funded PhD student as part of the MRC & Asthma UK Centre for Allergic Mechanisms of Asthma. M.J. was funded by an MRC CASE PhD Studentship with Novartis. The study was supported by Wellcome Trust Programme Grant 076343. We further acknowledge financial support from the UK Department of Health via the National Institute for Health Research (NIHR) Comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
aCD21, anti-CD21; aCD40, anti-CD40; ADAM, A Disintegrin and Metalloprotease; CSR, Class Switch Recombination; derCD23, 16 kDa fragment of soluble CD23 resulting from cleavage by Der p I; εGLT, Epsilon Germline Transcript; m, Membrane; MFI, Mean Fluorescence Intensity; PBS-T, PBS + 0.05% Tween® 20; qPCR, Quantitative PCR; s, Soluble; sIgE, Secreted IgE; siRNA, Small Interfering RNA; triCD23, Recombinant Trimeric CD23.
For non-standard abbreviations used 3 or more times, see footnoteiv.
References
- 1.Spiegelberg HL. Fc epsilon R2/CD23: its discovery and possible functions. Monogr Allergy. 1991;29:1–8. [PubMed] [Google Scholar]
- 2.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nature Reviews Immunology. 2008;8(3):205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 3.Beavil RL, Graber P, Aubonney N, Bonnefoy JY, Gould HJ. CD23/Fc epsilon RII and its soluble fragments can form oligomers on the cell surface and in solution. Immunology. 1995;84(2):202–206. [PMC free article] [PubMed] [Google Scholar]
- 4.Beavil AJ, Edmeades RL, Gould HJ, Sutton BJ. Alpha-helical coiled-coil stalks in the low-affinity receptor for IgE (Fc epsilon RII/CD23) and related C-type lectins. Proc Natl Acad Sci U S A. 1992;89(2):753–757. doi: 10.1073/pnas.89.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokota A, Kikutani H, Tanaka T, Sato R, Barsumian EL, Suemura M, Kishimoto T. Two species of human Fc epsilon receptor II (Fc epsilon RII/CD23): tissue-specific and IL-4-specific regulation of gene expression. Cell. 1988;55(4):611–618. doi: 10.1016/0092-8674(88)90219-x. [DOI] [PubMed] [Google Scholar]
- 6.Lorenzo GD, Mansueto P, Melluso M, Morici G, Cigna D, Candore G, Caruso C. Serum levels of total IgE and soluble CD23 in bronchial asthma. Mediators Inflamm. 1996;5(1):43–46. doi: 10.1155/S0962935196000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Lorenzo G, Drago A, Pellitteri ME, Candore G, Colombo A, Potestio M, Di Salvo A, Mansueto S, Caruso C. Serum levels of soluble CD23 in patients with asthma or rhinitis monosensitive to Parietaria. Its relation to total serum IgE levels and eosinophil cationic protein during and out of the pollen season. Allergy Asthma Proc. 1999;20(2):119–125. doi: 10.2500/108854199778612590. [DOI] [PubMed] [Google Scholar]
- 8.Yanagihara Y, Sarfati M, Marsh D, Nutman T, Delespesse G. Serum levels of IgE-binding factor (soluble CD23) in diseases associated with elevated IgE. Clin Exp Allergy. 1990;20(4):395–401. doi: 10.1111/j.1365-2222.1990.tb02800.x. [DOI] [PubMed] [Google Scholar]
- 9.McCloskey N, Hunt J, Beavil RL, Jutton MR, Grundy GJ, Girardi E, Fabiane SM, Fear DJ, Conrad DH, Sutton BJ, Gould HJ. Soluble CD23 monomers inhibit and oligomers stimulate IGE synthesis in human B cells. J Biol Chem. 2007;282(33):24083–24091. doi: 10.1074/jbc.M703195200. [DOI] [PubMed] [Google Scholar]
- 10.Rosenwasser LJ, Busse WW, Lizambri RG, Olejnik TA, Totoritis MC. Allergic asthma and an anti-CD23 mAb (IDEC-152): results of a phase I, single-dose, dose-escalating clinical trial. J Allergy Clin Immunol. 2003;112(3):563–570. doi: 10.1016/s0091-6749(03)01861-x. [DOI] [PubMed] [Google Scholar]
- 11.Rosenwasser LJ, Meng J. Anti-CD23. Clin Rev Allergy Immunol. 2005;29(1):61–72. doi: 10.1385/CRIAI:29:1:061. [DOI] [PubMed] [Google Scholar]
- 12.Cho SW, Kilmon MA, Studer EJ, van der Putten H, Conrad DH. B cell activation and Ig, especially IgE, production is inhibited by high CD23 levels in vivo and in vitro. Cell Immunol. 1997;180(1):36–46. doi: 10.1006/cimm.1997.1174. [DOI] [PubMed] [Google Scholar]
- 13.Yu P, Kosco-Vilbois M, Richards M, Kohler G, Lamers MC. Negative feedback regulation of IgE synthesis by murine CD23. Nature. 1994;369(6483):753–756. doi: 10.1038/369753a0. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura T, Kloetzer WS, Brams P, Hariharan K, Chamat S, Cao X, LaBarre MJ, Chinn PC, Morena RA, Shestowsky WS, Li YP, Chen A, Reff ME. In vitro IgE inhibition in B cells by anti-CD23 monoclonal antibodies is functionally dependent on the immunoglobulin Fc domain. Int J Immunopharmacol. 2000;22(2):131–141. doi: 10.1016/s0192-0561(99)00068-5. [DOI] [PubMed] [Google Scholar]
- 15.Sherr E, Macy E, Kimata H, Gilly M, Saxon A. Binding the low affinity Fc epsilon R on B cells suppresses ongoing human IgE synthesis. J Immunol. 1989;142(2):481–489. [PubMed] [Google Scholar]
- 16.Conrad DH, Ford JW, Sturgill JL, Gibb DR. CD23: an overlooked regulator of allergic disease. Curr Allergy Asthma Rep. 2007;7(5):331–337. doi: 10.1007/s11882-007-0050-y. [DOI] [PubMed] [Google Scholar]
- 17.Lemieux GA, Blumenkron F, Yeung N, Zhou P, Williams J, Grammer AC, Petrovich R, Lipsky PE, Moss ML, Werb Z. The low affinity IgE receptor (CD23) is cleaved by the metalloproteinase ADAM10. J Biol Chem. 2007;282(20):14836–14844. doi: 10.1074/jbc.M608414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weskamp G, Ford JW, Sturgill J, Martin S, Docherty AJ, Swendeman S, Broadway N, Hartmann D, Saftig P, Umland S, Sehara-Fujisawa A, Black RA, Ludwig A, Becherer JD, Conrad DH, Blobel CP. ADAM10 is a principal ‘sheddase’ of the low-affinity immunoglobulin E receptor CD23. Nat Immunol. 2006;7(12):1293–1298. doi: 10.1038/ni1399. [DOI] [PubMed] [Google Scholar]
- 19.Mathews JA, Ford J, Norton S, Kang D, Dellinger A, Gibb DR, Ford AQ, Massay H, Kepley CL, Scherle P, Keegan AD, Conrad DH. A potential new target for asthma therapy: A Disintegrin and Metalloprotease 10 (ADAM10) involvement in murine experimental asthma. Allergy. 2011;66(9):1193–1200. doi: 10.1111/j.1398-9995.2011.02614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibb DR, El Shikh M, Kang DJ, Rowe WJ, El Sayed R, Cichy J, Yagita H, Tew JG, Dempsey PJ, Crawford HC, Conrad DH. ADAM10 is essential for Notch2-dependent marginal zone B cell development and CD23 cleavage in vivo. Journal of Experimental Medicine. 2010;207(3):623–635. doi: 10.1084/jem.20091990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gough L, Schulz O, Sewell HF, Shakib F. The cysteine protease activity of the major dust mite allergen Der p 1 selectively enhances the immunoglobulin E antibody response. J Exp Med. 1999;190(12):1897–1902. doi: 10.1084/jem.190.12.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz O, Sutton BJ, Beavil RL, Shi J, Sewell HF, Gould HJ, Laing P, Shakib F. Cleavage of the low-affinity receptor for human IgE (CD23) by a mite cysteine protease: nature of the cleaved fragment in relation to the structure and function of CD23. Eur J Immunol. 1997;27(3):584–588. doi: 10.1002/eji.1830270303. [DOI] [PubMed] [Google Scholar]
- 23.Mayer RJ, Bolognese BJ, Al-Mahdi N, Cook RM, Flamberg PL, Hansbury MJ, Khandekar S, Appelbaum E, Faller A, Marshall LA. Inhibition of CD23 processing correlates with inhibition of IL-4-stimulated IgE production in human PBL and hu-PBL-reconstituted SCID mice. Clin Exp Allergy. 2000;30(5):719–727. doi: 10.1046/j.1365-2222.2000.00812.x. [DOI] [PubMed] [Google Scholar]
- 24.Aubry JP, Pochon S, Graber P, Jansen KU, Bonnefoy JY. CD21 is a ligand for CD23 and regulates IgE production. Nature. 1992;358(6386):505–507. doi: 10.1038/358505a0. [DOI] [PubMed] [Google Scholar]
- 25.Delespesse G, Sarfati M, Wu CY, Fournier S, Letellier M. The low-affinity receptor for IgE. Immunol Rev. 1992;125:77–97. doi: 10.1111/j.1600-065x.1992.tb00626.x. [DOI] [PubMed] [Google Scholar]
- 26.Bowles SL, Jaeger C, Ferrara C, Fingeroth J, Van De Venter M, Oosthuizen V. Comparative binding of soluble fragments (derCD23, sCD23, and exCD23) of recombinant human CD23 to CD21 (SCR 1-2) and native IgE, and their effect on IgE regulation. Cell Immunol. 2011 doi: 10.1016/j.cellimm.2011.08.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Bajorath J, Aruffo A. Structure-based modeling of the ligand binding domain of the human cell surface receptor CD23 and comparison of two independently derived molecular models. Protein Sci. 1996;5(2):240–247. doi: 10.1002/pro.5560050207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermann P, Armant M, Brown E, Rubio M, Ishihara H, Ulrich D, Caspary RG, Lindberg FP, Armitage R, Maliszewski C, Delespesse G, Sarfati M. The vitronectin receptor and its associated CD47 molecule mediates proinflammatory cytokine synthesis in human monocytes by interaction with soluble CD23. J Cell Biol. 1999;144(4):767–775. doi: 10.1083/jcb.144.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hibbert RG, Teriete P, Grundy GJ, Beavil RL, Reljic R, Holers VM, Hannan JP, Sutton BJ, Gould HJ, McDonnell JM. The structure of human CD23 and its interactions with IgE and CD21. J Exp Med. 2005;202(6):751–760. doi: 10.1084/jem.20050811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aubry JP, Pochon S, Gauchat JF, Nueda-Marin A, Holers VM, Graber P, Siegfried C, Bonnefoy JY. CD23 interacts with a new functional extracytoplasmic domain involving N-linked oligosaccharides on CD21. J Immunol. 1994;152(12):5806–5813. [PubMed] [Google Scholar]
- 31.Fearon DT, Carter RH. The CD19/CR2/TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annu Rev Immunol. 1995;13:127–149. doi: 10.1146/annurev.iy.13.040195.001015. [DOI] [PubMed] [Google Scholar]
- 32.Cherukuri A, Cheng PC, Pierce SK. The role of the CD19/CD21 complex in B cell processing and presentation of complement-tagged antigens. J Immunol. 2001;167(1):163–172. doi: 10.4049/jimmunol.167.1.163. [DOI] [PubMed] [Google Scholar]
- 33.Dierks SE, Bartlett WC, Edmeades RL, Gould HJ, Rao M, Conrad DH. The oligomeric nature of the murine Fc epsilon RII/CD23. Implications for function. J Immunol. 1993;150(6):2372–2382. [PubMed] [Google Scholar]
- 34.Texido G, Eibel H, Le Gros G, van der Putten H. Transgene CD23 expression on lymphoid cells modulates IgE and IgG1 responses. J Immunol. 1994;153(7):3028–3042. [PubMed] [Google Scholar]
- 35.Lamers MC, Yu P. Regulation of IgE synthesis. Lessons from the study of IgE transgenic and CD23-deficient mice. Immunol Rev. 1995;148:71–95. doi: 10.1111/j.1600-065x.1995.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 36.Fremeaux-Bacchi V, Fischer E, Lecoanet-Henchoz S, Mani JC, Bonnefoy JY, Kazatchkine MD. Soluble CD21 (sCD21) forms biologically active complexes with CD23: sCD21 is present in normal plasma as a complex with trimeric CD23 and inhibits soluble CD23-induced IgE synthesis by B cells. Int Immunol. 1998;10(10):1459–1466. doi: 10.1093/intimm/10.10.1459. [DOI] [PubMed] [Google Scholar]
- 37.Henchoz S, Gauchat JF, Aubry JP, Graber P, Pochon S, Bonnefoy JY. Stimulation of human IgE production by a subset of anti-CD21 monoclonal antibodies: requirement of a co-signal to modulate epsilon transcripts. Immunology. 1994;81(2):285–290. [PMC free article] [PubMed] [Google Scholar]
- 38.Hoettecke N, Ludwig A, Foro S, Schmidt B. Improved synthesis of ADAM10 inhibitor GI254023X. Neurodegener Dis. 2010;7(4):232–238. doi: 10.1159/000267865. [DOI] [PubMed] [Google Scholar]
- 39.Cheng LE, Wang ZE, Locksley RM. Murine B cells regulate serum IgE levels in a CD23-dependent manner. J Immunol. 2010;185(9):5040–5047. doi: 10.4049/jimmunol.1001900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christie G, Barton A, Bolognese B, Buckle DR, Cook RM, Hansbury MJ, Harper GP, Marshall LA, McCord ME, Moulder K, Murdock PR, Seal SM, Spackman VM, Weston BJ, Mayer RJ. IgE secretion is attenuated by an inhibitor of proteolytic processing of CD23 (Fc epsilonRII) Eur J Immunol. 1997;27(12):3228–3235. doi: 10.1002/eji.1830271221. [DOI] [PubMed] [Google Scholar]
- 41.Saxon A, Ke Z, Bahati L, Stevens RH. Soluble CD23 containing B cell supernatants induce IgE from peripheral blood B-lymphocytes and costimulate with interleukin-4 in induction of IgE. J Allergy Clin Immunol. 1990;86(3 Pt 1):333–344. doi: 10.1016/s0091-6749(05)80096-x. [DOI] [PubMed] [Google Scholar]
- 42.Sturgill JL, Mathews J, Scherle P, Conrad DH. Glutamate signaling through the kainate receptor enhances human immunoglobulin production. J Neuroimmunol. 2011 doi: 10.1016/j.jneuroim.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fremeaux-Bacchi V, Bernard I, Maillet F, Mani JC, Fontaine M, Bonnefoy JY, Kazatchkine MD, Fischer E. Human lymphocytes shed a soluble form of CD21 (the C3dg/Epstein-Barr virus receptor, CR2) that binds iC3b and CD23. Eur J Immunol. 1996;26(7):1497–1503. doi: 10.1002/eji.1830260714. [DOI] [PubMed] [Google Scholar]
- 44.Hebell T, Ahearn JM, Fearon DT. Suppression of the immune response by a soluble complement receptor of B lymphocytes. Science. 1991;254(5028):102–105. doi: 10.1126/science.1718035. [DOI] [PubMed] [Google Scholar]
- 45.Roberts T, Snow EC. Cutting edge: recruitment of the CD19/CD21 coreceptor to B cell antigen receptor is required for antigen-mediated expression of Bcl-2 by resting and cycling hen egg lysozyme transgenic B cells. J Immunol. 1999;162(8):4377–4380. [PubMed] [Google Scholar]
- 46.Reljic R, Cosentino G, Gould HJ. Function of CD23 in the response of human B cells to antigen. Eur J Immunol. 1997;27(2):572–575. doi: 10.1002/eji.1830270232. [DOI] [PubMed] [Google Scholar]
- 47.Achatz G, Nitschke L, Lamers MC. Effect of transmembrane and cytoplasmic domains of IgE on the IgE response. Science. 1997;276(5311):409–411. doi: 10.1126/science.276.5311.409. [DOI] [PubMed] [Google Scholar]
- 48.Venkitaraman AR, Williams GT, Dariavach P, Neuberger MS. The B-cell antigen receptor of the five immunoglobulin classes. Nature. 1991;352(6338):777–781. doi: 10.1038/352777a0. [DOI] [PubMed] [Google Scholar]
- 49.Bohnsack JF, Cooper NR. CR2 ligands modulate human B cell activation. J Immunol. 1988;141(8):2569–2576. [PubMed] [Google Scholar]
- 50.Pierce SK. Lipid rafts and B-cell activation. Nat Rev Immunol. 2002;2(2):96–105. doi: 10.1038/nri726. [DOI] [PubMed] [Google Scholar]
- 51.Sarfati M, Delespesse G. Possible role of human lymphocyte receptor for IgE (CD23) or its soluble fragments in the in vitro synthesis of human IgE. J Immunol. 1988;141(7):2195–2199. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.