Abstract
Posterior parahippocampal cortex (PHC) supports a range of cognitive functions, in particular scene processing. However, it has recently been suggested that PHC engagement during functional MRI (fMRI) simply reflects the representation of three-dimensional local space. If so, PHC should respond to space in the absence of scenes, geometric layout, objects, or contextual associations. It has also been reported that PHC activation may be influenced by low-level visual properties of stimuli such as spatial frequency. Here we tested whether PHC was responsive to the mere sense of space in highly simplified stimuli, and whether this was affected by their spatial frequency distribution. Participants were scanned using fMRI while viewing depictions of simple three-dimensional space, and matched control stimuli which did not depict a space. Half the stimuli were low-pass filtered to ascertain the impact of spatial frequency. We observed a significant interaction between space and spatial frequency in bilateral PHC. Specifically, stimuli depicting space (more than non-spatial stimuli) engaged right PHC when they featured high spatial frequencies. In contrast, the interaction in left PHC did not show a preferential response to space. We conclude that a simple depiction of three-dimensional space that is devoid of objects, scene layouts or contextual associations is sufficient to robustly engage right PHC, at least when high spatial frequencies are present. We suggest that coding for the presence of space may be a core function of PHC, and could explain its engagement in a range of tasks, including scene processing, where space is always present.
Keywords: parahippocampal cortex, scenes, space, spatial frequency, fMRI
Introduction
An area of posterior parahippocampal cortex (PHC) is known to be particularly responsive when participants view scenes during fMRI scanning [1-4]. Several competing hypotheses have emerged that seek to explain the role PHC plays in scene processing. One prominent theory posits that PHC (or the ‘parahippocampal place area’, PPA) responds to the geometry of scenes embodied in layout-defining features such as walls and other immovable topographical elements [5], although a precise understanding of how this is realised remains elusive [1]. A conflicting account suggests that PHC is not specifically concerned with space or scenes but instead mediates the co-activation of contextually-associated representations [6,7], although this view is disputed [see 3,8,9]. Yet another view of the PHC purports that it responds to low-level properties of visual stimuli, specifically spatial frequency, but with no particular preference for scenes. Rajimehr et al. [10] found stronger PHC response to images with high spatial frequency, although the opposite result of greater PHC responses to low frequencies in natural images has also been reported [11].
Recently, Mullally and Maguire [9] presented novel findings which offered a fresh perspective on the PHC, suggesting it is primarily engaged by any depiction of three-dimensional space. Specifically, they identified space-defining and space ambiguous single objects, where the former consistently evoked a strong sense of the surrounding space while the latter did not, in the absence of any scenes, spatial layout, or context. Visualizing or viewing single space-defining objects compared with space ambiguous objects engaged PHC, despite offering no information about the shape or layout of the space. Moreover, this PHC response was not accounted for by other factors, including contextual associations.
Here we directly tested the Mullally and Maguire [9] hypothesis using novel stimuli that had no complex scene properties, contextual or conceptual cues, geometry or even any objects, but nevertheless promoted a sense of space. If their hypothesis is correct, then viewing these stimuli should be sufficient to engage the PHC during fMRI more than visually similar stimuli that do not provide a sense of space. We also explicitly included spatial frequency as a factor in the design, whilst controlling for contrast and luminance in the stimuli. This afforded us the additional opportunity to examine low- and high-level accounts of PHC function within the same experiment.
Methods
Participants
Nineteen healthy, right-handed participants took part (7 male, mean age 26.68 years, SD 4.95). All had normal or corrected-to-normal vision, and gave informed written consent to participation in accordance with the University College London research ethics committee.
Stimuli
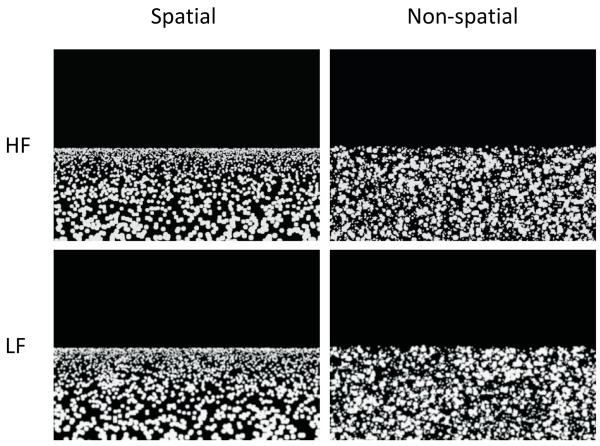
Using a 2×2 factorial design we manipulated the factors of space and spatial frequency (hereafter we refer to spatial frequency as ‘frequency’ to avoid confusion). Stimuli were dot fields (see Figure 1), generated using custom software and fell into four categories: HF Spatial (High Frequency Spatial), LF Spatial (Low Frequency Spatial), HF Non-spatial (High Frequency Non-spatial) and LF Non-spatial (Low Frequency Non-spatial). To add variety to the stimuli, each type of stimulus was produced with 2000, 3000 or 4000 dots, and were of dimensions 835×599 pixels, corresponding to a viewing angle of 9.55×7.14 degrees in the scanner. The upper half of each stimulus was plain black, and the lower half comprised grey-coloured dots (circles) on a black background. All stimuli were presented within a light grey frame on a dark grey background.
Fig. 1.
Examples of the stimuli. Left column – Spatial stimuli; right column – Non-spatial stimuli; top row – High Spatial Frequency (HF) stimuli; bottom row – Low Spatial Frequency (LF) stimuli. Each stimulus shown here comprises 3000 dots.
Within each stimulus the size of the dots decreased logarithmically from a diameter of 12 pixels to 2 pixels. Non-spatial stimuli had these dots arranged randomly within the lower portion of the image, whereas the spatial stimuli had dots positioned according to an exponential distribution, with fewer smaller dots towards the bottom of the picture. The exact position of the dots in each stimulus was randomized. Low Frequency stimuli were generated by duplicating each stimulus and low-pass filtering using a 5×5 Gaussian convolution kernel (SD 2.0). The strength of filtering was selected to prevent the dots in the stimuli merging to form a single texture. The unfiltered stimuli were termed High Frequency. Fourier analysis with rotational averaging confirmed a substantial reduction in high spatial frequencies (above 10 cycles/degree) for the Low Frequency images. The four sets of stimuli were then matched for mean luminance and contrast using the SHINE toolbox [12]. They were then Gamma-corrected for the MRI scanner’s projector screen using luminance measurements taken along the scanner’s bore, measured using a Minolta CS-100A chromameter. As expected there was a linear increase of luminance with the number of dots in the stimulus. There was also a small but significant increase in luminance for the LF compared to the HF stimuli which was dealt with in the fMRI analyses by including the luminance measurements as a nuisance regressor.
Task and procedure
Stimuli were back-projected onto a screen 73cm from a participant’s eye level in the scanner and reflected into the eyes by an angled mirror attached to the scanner head coil. There were 30 stimuli of each category plus 10% catch trials (total 132 trials per session, two sessions); each stimulus was displayed in the scanner for 2 seconds in pseudo-random order. In the catch trials, 10% of dots within the stimulus disappeared one second after onset. Participants were instructed to press a button whenever they saw dots disappear. Catch trials were later regressed out of the fMRI analyses. Stimulus onsets were jittered with inter-trial intervals (ITIs) selected randomly between 2 and 5 seconds. Stimuli were repeated in a second session in the scanner with catch trials and ITIs randomised. After scanning, participants were debriefed – see Results section.
Scanning parameters
T2*-weighted echo planar images (EPI) with blood oxygen level-dependent (BOLD) contrast were acquired on a 3T whole body MRI scanner (Magnetom TIM Trio, Siemens Healthcare, Erlangen, Germany) operated with the standard RF transmit body coil and 12-channel head receive coil. Whole brain coverage was achieved with the following parameters: 48 oblique axial slices angled at −45° from the axial to coronal plane [13], 2.5mm thickness (inter-slice distance factor 20%), repetition time TR = 3.36s (slice TR = 70ms), excitation flip angle = 90°, echo time TE = 30ms, in-plane resolution 3mm × 3mm, field of view FoV = 192mm × 192mm, 64×64 matrix, phase encoding (PE) in the anterior-posterior direction, 13% oversampling in the PE direction, echo spacing 500μs. The first 6 ‘dummy’ volumes from each session were discarded to allow for T1 equilibration effects. A T1-weighted structural scan was also acquired with 1mm isotropic resolution.
Data analysis
FMRI data were analysed using SPM8 (www.fil.ion.ucl.ac.uk/spm). Spatial pre-processing consisted of realignment and unwarping (using field maps), normalization to the standard EPI template in MNI space using the DARTEL toolbox and spatial smoothing using a Gaussian kernel of 8mm at its full width half maximum. Each trial was modelled from the time of onset of the stimulus to the time it disappeared from the display. Several regressors of no interest were modelled: a parametric regressor for stimulus luminance (see Stimuli), a regressor for the onsets and durations of the catch trials, and regressors for motion and session effects. Subject-specific parameter estimates pertaining to each regressor of interest (betas) were calculated for each voxel. Second level random effects analyses were then run using one-sample t-tests on these parameter estimates (collapsed across sessions). We report the fMRI results at a voxel-level threshold of p<0.001 whole brain uncorrected (minimum cluster size of 5 voxels) for the PHC, as it was our a priori area of interest, and p < 0.05 FWE corrected for elsewhere.
Results
Behavioural
Behavioural data were not collected for one participant during scanning due to a technical problem. Performance on the incidental vigilance task during scanning did not differ significantly between the conditions, and there was no interaction between space and spatial frequency (mean hit rate 0.84, SD 0.28; all F(1, 17) ≤ 2.87, p ≥ 0.11). Reaction times for the vigilance task also did not differ between conditions, with no interaction (mean 597.78ms, SD 94.4; all F(1, 17) ≤ 2.5, p ≥ 0.13).
After scanning, participants were shown all the stimuli again. To gauge whether participants experienced a sense of space they were asked for each stimulus: “Do you see a space?” (yes or no). Participants’ agreement with our space/non-space distinction was high for each stimulus category (all means above 90%). These space/non-space designations by the participants were used in the construction of regressors for the fMRI analysis.
fMRI
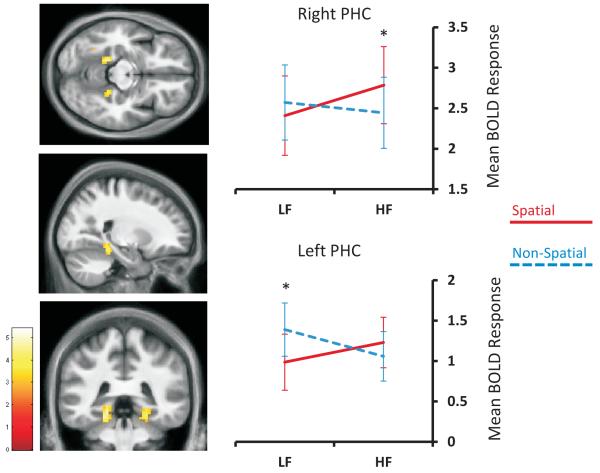
No brain regions were more active for the main effect of Spatial > Non-spatial stimuli. This is explained by a significant interaction between the factors of space and frequency that was specific to bilateral parahippocampal cortex (left PHC: −18, −39, −12; Z=3.67; right PHC 24, −39, −15; Z=3.45; see Figure 2). No other brain regions emerged from this interaction analysis.
Fig. 2.
The interaction of space and spatial frequency in parahippocampal cortex. Left column: activations are displayed on sections of the average structural MRI scan of the participants (at p<0.005 uncorrected for display purposes). The colour bar, lower left, indicates the Z-scores associated with each voxel. Right column: LF=low spatial frequency stimuli; HF=high spatial frequency stimuli; PHC=parahippocampal cortex. Error bars represent +/−1 standard error of the mean; *p < 0.05.
To unpack this result we analysed the components of the interaction (simple effects) separately using region of interest analyses that were defined orthogonally to our main results. Peak locations for parahippocampal cortex were determined using the Neurosynth meta-analysis tool (http://neurosynth.org/; search term ‘PPA’; resulting in left PHC peak: −24, −42, −9; right PHC peak: 27, −43, −10). The mean responses for left and right PHC were extracted using the MarsBaR toolbox from a sphere of radius 4mm positioned at the Neurosynth-identified peaks in each participant’s first-level data. These were then averaged across participants to give a mean response for each cluster in each condition. Analyses were small-volume corrected using a threshold of p<0.05 FWE-corrected for multiple comparisons. Having examined all of the simple effects for frequency and for space, we report below only those that reached statistical significance.
Considering the high frequency stimuli, we found significantly greater activation for spatial compared to non-spatial stimuli in right PHC (Z=2.51, p=0.038). This suggests that the right PHC was driven by spatial more than non-spatial stimuli specifically within the high frequency domain (see Figure 2), as there was no significant difference in activity between spatial and non-spatial stimuli in the low frequency domain (Z=2.12, p=0.083). Interestingly, the interaction in left PHC was driven by the combination of an increased response to non-spatial stimuli and a decreased response to spatial stimuli within the low frequency domain (Z=3.04, p=0.008, see Figure 2). The left PHC had a slight preference for spatial compared to non-spatial stimuli in the high frequency domain, although this was not statistically significant (Z=1.43, p=0.231).
Discussion
We found a significant interaction between space and spatial frequency that was specific to bilateral parahippocampal cortex. Analysis of this interaction demonstrated that, in line with the hypothesis of Mullally and Maguire [9], simple depictions of three-dimensional space were sufficient to engage PHC, particularly on the right. The highly simplified stimuli we employed meant that objects, object-in-place binding, scenes, scene layouts/geometry, schema, and contextual associations were precluded from driving this effect. Although a previous study reported PHC activation in response to photographs of empty rooms [5], in contrast our stimuli contained no room or scene contexts and were free of layout-defining features (e.g. walls, corners).
Interestingly, the preference for spatial stimuli only pertained when they were of high spatial frequency. Blurred (low frequency) stimuli, even when depicting a space, did not engage the right PHC to the same degree as high frequency spatial stimuli. Given that participants identified which stimuli were spatial with high accuracy in all conditions, this finding is unlikely to be explained by the effectiveness of the spatial depiction in the stimuli. It has been suggested that there may be an evolutionary advantage for PHC to be selectively tuned for high spatial frequencies [10], for instance facilitating the detection of food/predators in visually complex environments. Our data now show that this effect is not dependent on complex content within the space, suggesting that high frequency spatial tuning may be fundamental to the operating mechanism of the (right) PHC.
In contrast to right PHC, there was a lack of spatial selectivity in the left, with a significant preference for non-spatial stimuli in the low frequency condition. This result differs from previous studies. Peyrin et al. [11] reported low frequency sensitivity in right PHC, and not left as we found here. In addition, Rajimehr et al. [10] reported a stronger response to non-spatial stimuli when they contained only high spatial frequencies, whereas our non-spatial stimuli triggered a stronger response when they contained only lower spatial frequencies. Differences between the methodologies of the three studies likely contributed to the disparities. For example, Rajimehr et al. (2011)’s spatial frequency filtering was more extreme, removing all low spatial frequencies, producing stimuli akin to line drawings. We purposely kept our filtering more subtle, as we considered the resulting differences in contrast and colour to be potentially confounding effects. The reason for our result in left PHC is not clear. Future studies will be needed to further elucidate the PHC laterality difference that we have uncovered here. Nevertheless, our findings highlight the potential influence of low-level visual properties on higher-level functions within PHC, and emphasise the need to control for such factors in future PHC-focussed experiments.
We conclude that a simple depiction of three-dimensional space that is devoid of objects, scene layouts or contextual associations is sufficient to robustly engage right PHC, at least when high spatial frequencies are present. We suggest that coding for the presence of space may be a core function of PHC, and could explain its engagement in a range of tasks, including scene processing, spatial navigation, autobiographical memory and thinking about the future, where space is always present [14,15].
Acknowledgments
We thank Nikolaus Weiskopf, David Bradbury, Janice Glensman and Eric Featherstone for technical assistance.
This work was funded by the Wellcome Trust (EAM, SLM, DSS) and the Brain Research Trust (PZ).
Footnotes
Conflicts of interest: There are no conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Epstein RA. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cog Sci. 2008;12:388–96. doi: 10.1016/j.tics.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epstein RA. Cognitive neuroscience: scene layout from vision and touch. Curr Biol. 2011;21:R437–8. doi: 10.1016/j.cub.2011.04.037. [DOI] [PubMed] [Google Scholar]
- 3.Henderson JM, Larson CL, Zhu DC. Full scenes produce more activation than close-up scenes and scene-diagnostic objects in parahippocampal and retrosplenial cortex: an fMRI study. Brain Cogn. 2008;66:40–9. doi: 10.1016/j.bandc.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Park S, Chun MM. Different roles of the parahippocampal place area (PPA) and retrosplenial cortex (RSC) in panoramic scene perception. NeuroImage. 2009;47:1747–1756. doi: 10.1016/j.neuroimage.2009.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein RA, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- 6.Bar M. Visual objects in context. Nat Rev Neurosci. 2004;5:617–29. doi: 10.1038/nrn1476. [DOI] [PubMed] [Google Scholar]
- 7.Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–58. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- 8.Epstein RA, Ward EJ. How reliable are visual context effects in the parahippocampal place area? Cereb Cortex. 2010;20:294–303. doi: 10.1093/cercor/bhp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mullally SL, Maguire EA. A new role for the parahippocampal cortex in representing space. J Neurosci. 2011;31:7441–9. doi: 10.1523/JNEUROSCI.0267-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajimehr R, Devaney KJ, Bilenko NY, Young JC, Tootell RBH. The “Parahippocampal Place Area” Responds Preferentially to High Spatial Frequencies in Humans and Monkeys. PLoS Biol. 2011;9:e1000608. doi: 10.1371/journal.pbio.1000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peyrin C, Baciu M, Segebarth C, Marendaz C. Cerebral regions and hemispheric specialization for processing spatial frequencies during natural scene recognition. An event-related fMRI study. NeuroImage. 2004;23:698–707. doi: 10.1016/j.neuroimage.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Willenbockel V, Sadr J, Fiset D, Horne GO, Gosselin F, Tanaka JW. Controlling low-level image properties: the SHINE toolbox. Beh Res Methods. 2010;42:671–84. doi: 10.3758/BRM.42.3.671. [DOI] [PubMed] [Google Scholar]
- 13.Weiskopf N, Hutton C, Josephs O, Deichmann R. Optimal EPI parameters for reduction of susceptibility-induced BOLD sensitivity losses: a whole-brain analysis at 3 T and 1.5 T. NeuroImage. 2006;33:493–504. doi: 10.1016/j.neuroimage.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 14.Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]