Abstract
Rationale: The current management of advanced non–small cell lung cancer (NSCLC) requires differentiation between squamous and nonsquamous subtypes as well as epidermal growth factor receptor (EGFR) mutation status. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is increasingly used for the diagnosis and staging of lung cancer. However, it is unclear whether cytology specimens obtained with EBUS-TBNA are suitable for the subclassification and genotyping of NSCLC.
Objectives: To determine whether cytology specimens obtained from EBUS-TBNA in routine practice are suitable for phenotyping and genotyping of NSCLC.
Methods: Cytological diagnoses from EBUS-TBNA were recorded from 774 patients with known or suspected lung cancer across five centers in the United Kingdom between 2009 and 2011.
Measurements and Main Results: The proportion of patients with a final diagnosis by EBUS-TBNA in whom subtype was classified was 77% (95% confidence interval [CI], 73–80). The rate of NSCLC not otherwise specified (NSCLC-NOS) was significantly reduced in patients who underwent immunohistochemistry (adjusted odds ratio, 0.50; 95% CI, 0.28–0.82; P = 0.016). EGFR mutation analysis was possible in 107 (90%) of the 119 patients in whom mutation analysis was requested. The sensitivity, negative predictive value, and diagnostic accuracy of EBUS-TBNA in patients with NSCLC were 88% (95% CI, 86–91), 72% (95% CI, 66–77), and 91% (95% CI, 89–93), respectively.
Conclusions: This large, multicenter, pragmatic study demonstrates that cytology samples obtained from EBUS-TBNA in routine practice are suitable for subtyping of NSCLC and EGFR mutation analysis and that the use of immunohistochemistry reduces the rate of NSCLC-NOS.
Keywords: endobronchial ultrasound, non–small cell lung cancer, adenocarcinoma, EGFR mutation, NSCLC-NOS
At a Glance Commentary
Scientific Knowledge on the Subject
Subtyping and genotyping of the tumor is central to the modern management of patients with advanced non–small cell lung cancer. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) has emerged as a procedure for the diagnosis and staging of non–small cell cancer. However, it is unclear whether the cytological specimens obtained from EBUS-TBNA are suitable for the subclassification and genotyping of non–small cell lung cancer.
What This Study Adds to the Field
In patients with non–small cell lung cancer, specimens obtained from EBUS-TBNA in routine practice are sufficient to allow subclassification in 77% and genotyping in 90%. The use of immunohistochemistry on EBUS-TBNA samples can reduce the rate of unclassified non–small cell lung cancer.
Traditionally, the pathology of lung cancer has been divided into non–small cell lung cancer (NSCLC) and small cell lung cancer, reflecting the different tumor biology and susceptibility to treatments. In recent years, it has become apparent that subtyping and genotyping helps to guide optimal treatment of advanced NSCLC. Late-phase clinical trials have provided three major observations regarding the efficacy or safety of specific treatments for particular subtypes or genotypes of NSCLC. First, a large, randomized, noninferiority trial of pemetrexed and cisplatin in 1,725 patients with NSCLC (1) demonstrated that pemetrexed is only of benefit in patients with nonsquamous histology, whereas in patients with squamous subtype pemetrexed was inferior to the standard treatment of cisplatin and gemcitabine. This has been reflected in guidance from the National Institute of Health and Clinical Excellence, which recommended pemetrexed as a first-line treatment for patients with adenocarcinoma or large cell carcinoma in September 2009 (2).
A randomized phase II trial of bevacizumab plus carboplatin and paclitaxel versus carboplatin and paclitaxel alone revealed that fatal pulmonary hemorrhage was significantly higher in patients with the squamous subtype of NSCLC (3). Consequently, bevacizumab is contraindicated in patients with squamous cell lung cancer. Third, phase III randomized trials in East Asia have demonstrated that the tyrosine kinase inhibitors only have improved progression-free survival in patients with NSCLC harboring an activating EGFR mutation. In patients without an EGFR mutation, standard chemotherapy may offer superior progression-free survival (4, 5). Further targeted agents in patients with specific cancer genotypes are set to emerge (6).
Coupled with the emergence of personalized therapies for advanced NSCLC has been the rapid expansion of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), which allows sampling of mediastinal and hilar lymphadenopathy under direct vision. The technique uses a 21- or 22-gauge needle and therefore obtains smaller samples than biopsy via mediastinoscopy. EBUS-TBNA was initially developed for the nodal staging of lung cancer. However, it is now commonly used as an initial investigation in patients with suspected NSCLC after computed tomography scan because it may provide a tissue diagnosis and accurate nodal staging in a single investigation (7). However, given the smaller sample size obtained, it is unclear whether aspirates from EBUS-TBNA in routine practice provide sufficient material to allow subtyping and genotyping of NSCLC to guide treatment. We therefore conducted a large, pragmatic, multicenter study to clarify whether samples from EBUS-TBNA were suitable for subtyping of NSCLC and EGFR mutation testing.
Methods
Patients and EBUS-TBNA Samples
Consecutive patients with suspected NSCLC underwent EBUS-TBNA between January 2009 and March 2011 across five centers in the United Kingdom (University College London Hospital, University Hospital Birmingham, University Hospital of North Tees, Lancashire Teaching Hospital, and Papworth Hospital, Cambridge). The samples obtained at EBUS-TBNA were expelled from the needle using the stylet and placed into liquid fixative for cell-block processing. Needle contents were also flushed with saline into the liquid fixative. The specimen was centrifuged to form a pellet, suspended in agar, fixed in neutral buffered formalin, and processed as a cell block from which a single hematoxylin and eosin (H&E)-stained section was cut. Further sections were cut and used for immunohistochemical staining as required (8).
Pathological and Molecular Techniques
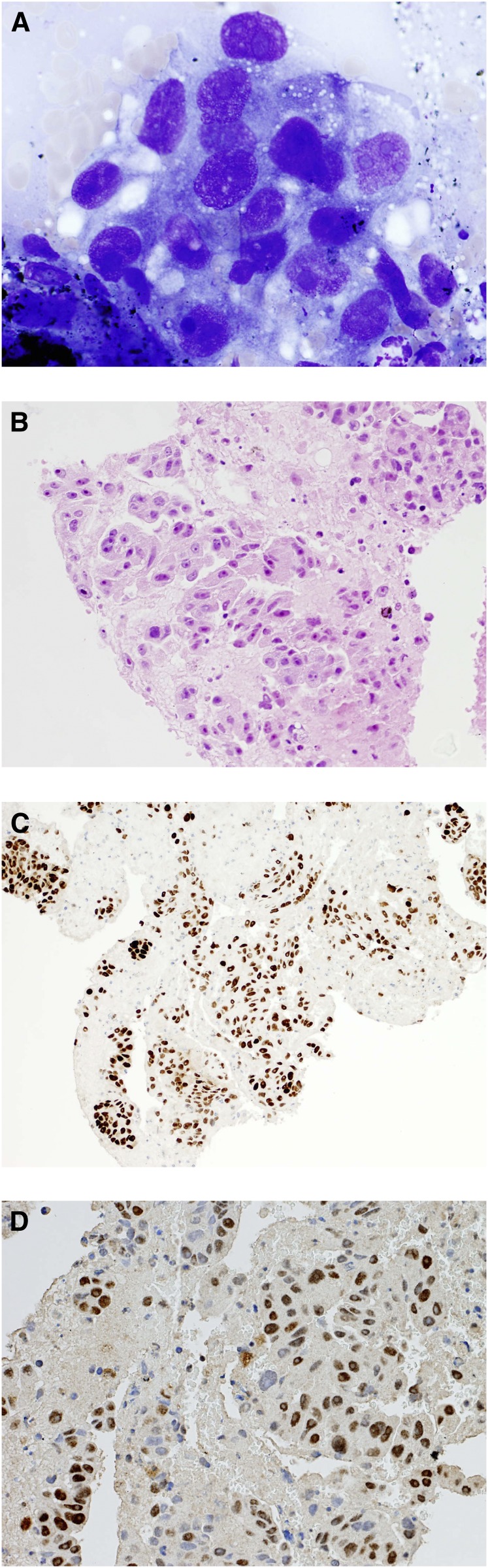
Interpretation of the EBUS-TBNA specimens was performed by the local pathologist, and there was no centralized reporting. Classification of NSCLC was based on morphological appearances (H&E stain), and immunostaining was performed if clinically indicated and if the sample was sufficient (Figures 1A–1D). Antibodies to cytokeratins 5/6 (CK5/6) and p63 were deemed to be consistent with squamous cell carcinoma (9, 10). Antibodies to thyroid transcription factor 1 (TTF-1) were also used because TTF-1 is known to be expressed in approximately 75% of lung adenocarcinomas (11, 12).
Figure 1.
(A) Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) smear demonstrating adenocarcinoma (May-Grunwald Giemsa stain). (B) Cell block obtained from EBUS-TBNA demonstrating adenocarcinoma. (C) Adenocarcinoma from EBUS-TBNA cell block, positive for thyroid transcription factor-1 (TTF-1), confirming lung origin. (D) EBUS-TBNA cell block demonstrating adenocarcinoma to be excision repair cross-complementing group 1 (ERCC1) positive, suggesting resistance to platinum-based chemotherapies.
EGFR mutations were detected using DNA sequencing techniques, and patients were considered to be positive for EGFR mutation if 1 of 29 EGFR mutations was detected by polymerase chain reaction–based assays. Four centers used the commercially available amplification refractory mutation system kit (Qiagen, Valencia, CA), which is able to detect an EGFR mutation in samples that contain 1% tumor. The remaining center used a matrix-assisted laser desorption/ionization mass spectroscopy system for detecting EGFR mutations (MassARRAY; Sequenom, San Diego, CA).
Endpoints and Statistical Analysis
The primary endpoint of the study was the proportion of patients with NSCLC undergoing EBUS-TBNA in whom it was possible to subtype the lung cancer. The coprimary endpoint was the proportion of samples that was suitable for EGFR testing as determined by the local testing center. Using regression analysis, the rate of NSCLC not otherwise specified (NSCLC-NOS) was determined according to age, lymph node location, size, pathological differentiation, and whether immunohistochemistry was performed or not. Covariates demonstrated to be significant at the 10% level were entered into the multivariate model. The unit of analysis was the patient.
Each patient was followed for at least 6 months, and each EBUS-TBNA procedure was classified as a true-positive, true–negative, or false-negative result according to the final diagnosis of malignancy. Standard definitions for the calculation of the sensitivity and negative predictive value of EBUS-TBNA (secondary endpoints) in patients with NSCLC were applied. Proportions were compared using the chi-squared test. All statistical calculations were performed using STATA version 10 (StataCorp, College Station, TX). Ethical approval was not required given the observational nature of the study. Although the study is retrospective, all data were prospectively recorded in each center. Results were fully disclosed to the patients and were discussed in multidisciplinary team meetings to determine the treatment strategies.
Results
Between 2009 and 2011, 774 consecutive patients with known or suspected NSCLC underwent EBUS-TBNA at five centers in the United Kingdom. Four hundred fifty-five (59%) were male, and the median age of patients with NSCLC was 69 years (range, 31–88 years). Baseline characteristics are summarized in Table 1. Two hundred ninety (37%) patients had more than one lymph node sampled, and in total 1,047 lymph nodes were aspirated. The size and location of lymph nodes sampled and the diagnostic yield are shown in Table 2.
TABLE 1.
BASELINE CHARACTERISTICS OF PATIENTS WITH SUSPECTED LUNG CANCER WHO UNDERWENT ENDOBRONCHIAL ULTRASOUND-GUIDED TRANSBRONCHIAL NEEDLE ASPIRATION
| Patient Characteristics | Number |
| Gender | |
| Male | 455 (59%) |
| Female | 319 (41%) |
| Age, yr | |
| <50 | 43 (5%) |
| 50–75 | 540 (70%) |
| >75 | 191 (25%) |
| Ethnicity | |
| Caucasian | 683 (88%) |
| South Asian | 21 (3%) |
| East Asian | 5 (1%) |
| African | 3 (<1%) |
| Caribbean | 2 (<1%) |
| Other | 1 (<1%) |
| Unknown | 59 (8%) |
| Total | 774 |
TABLE 2.
YIELD ACCORDING TO LYMPH NODE STATIONS SAMPLED IN 774 PATIENTS UNDERGOING ENDOBRONCHIAL ULTRASOUND-GUIDED TRANSBRONCHIAL NEEDLE ASPIRATION
| Lymph Node Station | Number of Nodes Sampled | Mean Size of Lymph Node (mm) | Prevalence of NSCLC | Sensitivity | Negative Predictive Value | Diagnostic Accuracy |
| 2R | 13 | 17 | 60% | 83% | 80% | 90% |
| 2L | 3 | 15 | 33% | 100% | 100% | 100% |
| 3P | 3 | 25 | 33% | 100% | 100% | 100% |
| 4R | 282 | 21 | 84% | 90% | 65% | 92% |
| 4L | 113 | 18 | 73% | 80% | 64% | 85% |
| 7 | 436 | 23 | 74% | 90% | 77% | 92% |
| 10R | 104 | 18 | 76% | 87% | 71% | 90% |
| 10L | 46 | 18 | 81% | 90% | 71% | 93% |
| 11R | 41 | 16 | 82% | 93% | 75% | 94% |
| 11L | 6 | 13 | 100% | 50% | 0% | 50% |
| Overall | 1047 | 21 | 77% | 88% | 72% | 91% |
Definition of abbreviation: NSCLC = non–small cell lung cancer.
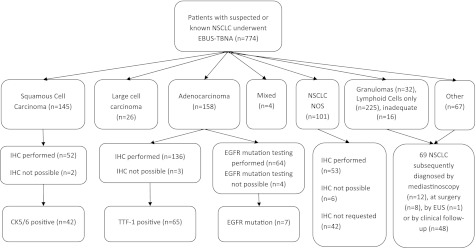
The pathological subtypes of NSCLC diagnosed by EBUS-TBNA are shown in Figure 2. In total, 503 patients had a final diagnosis of NSCLC in intrathoracic lymph nodes. The number of patients with a final diagnosis by EBUS-TBNA of NSCLC-NOS (the primary endpoint) was 101 (23%; 95% confidence interval [CI], 20–27). Two hundred ninety-one patients had their EBUS-TBNA specimens submitted for immunostaining, and this was possible in 280 (96%; 95% CI, 93–98). Of the 101 patients with a diagnosis of NSCLC-NOS by EBUS-TBNA, immunostaining was performed in 53, not done in 42, and not possible in 6 patients. In univariate analysis, there was no association between NSCLC-NOS and age, lymph node size, lymph node location, number of lymph nodes aspirated, and pathological differentiation. However, a significant relationship was seen on univariate and multivariate analysis between immunohistochemistry not performed and the final diagnosis of NSCLC-NOS (Table 3). When immunostaining was possible, the risk of the NSCLC tumor being unclassified was halved in the multivariate analysis (odds ratio, 0.50; 95% CI, 0.28–0.88 P = 0.016).
Figure 2.
Flowchart of patients. CK = cytokeratin; EBUS-TBNA = endobronchial ultrasound-guided transbronchial needle aspiration; EGFR = epidermal growth factor receptor; EUS = endoscopic ultrasound; IHC = immunohistochemistry; NOS = not otherwise specified; NSCLC = non–small cell lung cancer; TTF-1, thyroid transcription factor 1.
TABLE 3.
UNIVARIATE AND MULTIVARIATE ANALYSES OF FACTORS TO PREDICT NON–SMALL CELL LUNG CANCER NOT OTHERWISE SPECIFIED IN PATIENTS UNDERGOING ENDOBRONCHIAL ULTRASOUND-GUIDED TRANSBRONCHIAL NEEDLE ASPIRATION
| Covariate | Unadjusted OR of NSCLC-NOS (95% CI) | Univariate P Value | Adjusted OR of NSCLC-NOS (95% CI) | Multivariate* P Value |
| Age | 0.99 (0.97–1.01) | 0.53 | ||
| Lymph node location (mediastinal vs. hilar) | 0.64 (0.34–1.19) | 0.159 | ||
| Lymph node size | 1.0 (0.96–1.05) | 0.92 | ||
| Pathological differentiation | 1.66 (0.92–3.00) | 0.09 | 1.44 (0.79–2.62) | 0.24 |
| Immunohistochemistry performed | 0.47 (0.27–0.82) | 0.008 | 0.50 (0.28–0.88) | 0.016 |
Definition of abbreviations: CI = confidence interval; NSCLC-NOS = non–small cell lung cancer not otherwise specified; OR = odds ratio.
On the basis of univariate results, only pathological differentiation and performance of immunohistochemistry were included in the multivariate model. Performing immunohistochemistry significantly reduced the odds of obtaining a diagnosis of NSCLC-NOS.
Five hundred two patients had lymph nodes aspirated that were greater than 1 cm in short axis. Of these, 291 had NSCLC diagnosed by EBUS-TBNA, and the number of patients diagnosed with NSCLC–NOS was 55 (19%; 95% CI, 15–24%) in this subgroup. In the 88 patients with recorded lymph node size less than or equal to 1 cm in short axis, the prevalence of malignancy was 54% (48 patients), and six patients were diagnosed with NSCLC-NOS (26%; 95% CI, 12–47). Lymph node size was not recorded in 180 patients. There was no statistically significant difference (P = 0.41) in the NOS-NSCLC rate in nodes greater or less than 1 cm in short axis. Five hundred ninety-two patients had sampling with a 22-gauge needle, whereas a 21-gauge needle was used in 107 patients and was associated with NSCLC-NOS rates of 27% (95% CI, 23– 32) and 11% (95% CI, 5–21%), respectively (P = 0.006). Needle size was not recorded in 75 patients.
One hundred nineteen (27%) patients who had NSCLC diagnosed by EBUS-TBNA had EGFR mutation analysis requested on the routinely obtained sample. Of these, 68 (57%) were adenocarcinoma, 19 (16%) had squamous cell carcinoma, 10 (8%) had large cell carcinoma, and 22 (18%) had NSCLC-NOS. EGFR mutation analysis was possible (the coprimary endpoint) in 107 (90%; 95% CI, 82–94) cases, and 7 (6%) patients with EGFR mutations were identified. Of the seven patients who had an EGFR mutation, all were Caucasian and had adenocarcinoma. The median age of these patients was 58 years (range, 53–71 years), and five patients (71%) were female. Four out of the seven EBUS-TBNA samples that expressed an EGFR mutation stained for TTF-1.
In the overall cohort of 774 patients, EBUS had a sensitivity of 88% (95% CI, 86–91), a negative predictive value of 72% (95% CI, 66–77), and a diagnostic accuracy of 91% (95% CI, 89–93). Of the 69 false-negative EBUS-TBNA procedures, 62 patients had lymphoid cells only aspirated and subsequent surgery, mediastinoscopy, or clinical follow-up confirmed malignancy (Figure 2). Seven patients with inadequate EBUS-TBNA samples were shown to harbor malignancy in the mediastinal lymph nodes. None of the 32 specimens in which granulomas only were found at EBUS-TBNA was proven to be false negative.
The sensitivity from aspiration of hilar lymph nodes (stations 10 and 11) was 88% (95% CI, 79–94) and no different from the sensitivity from mediastinal lymph nodes (88%; 95% CI, 85–91). The median size of hilar lymph nodes was 15 mm (range, 7–40). Sensitivity in patients with lymph nodes 1 cm or less in short-axis was 65% (95% CI, 50–77) and was significantly lower than the sensitivity of 91% (95% CI, 88–93; P < 0.0001) in patients with nodes greater than 1 cm. There was no interaction between lymph node location and size.
One patient’s EBUS procedure resulted in death. The patient was a 48-year-old male who presented with stage IV adenocarcinoma of the lung. The EBUS-TBNA procedure was uncomplicated, and the patient was discharged home after the procedure with normal vital observations. Twenty-four hours later, the patient was admitted to hospital with clinical features of severe pneumonia and sepsis. Group A Streptococcus was isolated from blood cultures and from a throat swab. The patient deteriorated from sepsis and respiratory failure and died within 48 hours of admission. The scenario was attributed to the carriage of organisms by the EBUS scope from the pharynx into the lungs. No other complications were reported.
Discussion
Although the sophistication of patient selection for treatment has increased, the size of lung cancer samples to obtain that information has reduced. The challenge for the pathologist and lung cancer multidisciplinary team is to optimize diagnostic specimens and staging while supplying sufficient information to guide oncological therapy. Because at least 75% of patients have inoperable disease, the information to guide treatment algorithms must be obtained from small histology or cytology specimens.
EBUS-TBNA is an important investigation for the diagnosis of mediastinal and hilar lymphadenopathy in patients with lung cancer. It has been recommended as an initial investigation by the National Institute of Health and Clinical Excellence in patients with enlarged mediastinal lymph nodes because it may provide an inoperable disease stage and a pathological diagnosis in a single investigation (2). This large, multicenter, pragmatic implementation study demonstrates that routine samples from EBUS-TBNA provide sufficient information to allow subtyping in 77% and EGFR mutation testing in 90% of patients with NSCLC.
The proportion of patients with NSCLC for whom the final diagnosis using EBUS-TBNA specimens was NSCLC NOS was 23%. This is consistent with data from alternative biopsy techniques. An analysis of the California Cancer Registry of 175,298 patients diagnosed with lung cancer between 1989 and 2006 demonstrated a NSCLC-NOS rate of 22.1% (13). The rate of NSCLC-NOS was higher in the patients who had a cytological diagnosis alone (37%). The UK National Lung Cancer Audit published data on 26,731 patients diagnosed with NSCLC in England and Wales in 2010 (14). These patients underwent diagnosis and staging of lung cancer in a real-world setting, and the audit demonstrated an overall NSCLC-NOS rate of 24.4%. This highlights that EBUS-TBNA may be as good as other sample acquisition techniques for subtyping and that there will always be a proportion of patients with NSCLC in whom further subtyping is not possible due to lack of differentiation. EBUS-TBNA is also able to sample central parenchymal lung lesions that would otherwise not be accessible without a more invasive approach (15). Therefore, increased application of EBUS-TBNA may improve the rate of histological confirmation in patients with NSCLC, which stands at a mean of 72% in the UK National Lung Cancer Audit (14).
Previous studies have shown that samples from cytology are valid when compared with subsequent larger samples. Indeed, the morphologic features that distinguish squamous cell carcinoma (predominantly keratinized cytoplasm and intercellular bridges) from adenocarcinoma (mucin vacuoles and gland formation) span less than the 250-μm inner diameter of a 25-gauge fine needle (16). In a recent retrospective study of 48 patients (17), cell block samples from EBUS-TBNA were compared with histological specimens obtained by alternative procedures such as bronchoscopy and CT-guided biopsy. All subtypes diagnosed by EBUS-TBNA were validated by histological samples. When immunohistochemistry was performed on cell blocks, there were six cases diagnosed as NSCLC-NOS on EBUS-TBNA samples, which were diagnosed with a specific cell type on alternative histological samples (three adenocarcinomas, two squamous cell carcinomas, and one large cell undifferentiated carcinoma). A further study of 101 individuals demonstrated a 93% concordance between small biopsy and cytology specimens (18). As in this study, a lack of supporting immunohistochemistry contributed to unclassified cytology cases. In another report, 158 (85%) cases of NSCLC were typed by cytology and 28 (15%) were classified as NSCLC-NOS (19). Using histological specimens from the same patients, 183 (98%) cases were subtyped by histology, and only 3 (2%) cases were classified as NSCLC-NOS. There was 88% concordance between cytological and histological typing. The available data therefore confirm that cytological specimens are reliable for subtyping, with no false-positive results from cytological subtyping observed, and that use of immunohistochemistry can reduce the NSCLC-NOS rate.
Immunohistochemistry profiles do not feature in the diagnostic criteria for squamous cell or adenocarcinoma in the current WHO classification of NSCLC, which is based on resected surgical specimens (20). However, when morphological criteria are unable to distinguish subtypes in smaller samples, a panel of antibodies, including TTF-1, p63, and CK5/6, as well as a mucin stain has been recommended to minimize the proportion of NSCLC tumors that remain unclassified and to make the key distinction between squamous and nonsquamous subtypes (21). The current study shows that samples obtained by EBUS-TBNA are suitable for this approach from any accessible lymph node station and even when sampling lymph nodes less than 1 cm in size.
The EGFR-tyrosine kinase inhibitors erlotinib and gefitinib have become established as first-line treatments for patients with advanced lung cancer that harbor an EGFR mutation. Current European Society of Medical Oncology guidelines recommend that all never- or former light smokers (<15 pack years) or patients with nonsquamous histology should be tested for EGFR mutation status regardless of performance status (22). Cytological samples in alcohol-based fixatives may preserve nucleic acids better than formalin (23), and molecular profiling of cytology samples has been shown to be reliable when compared with histological samples from the same patient (24). In this study, EGFR mutation testing was requested in 119 patients, and the test was possible and deemed reliable in 107 (90%) cases. In the remaining cases, there was insufficient tumor sample to perform the investigation. Previous studies have assessed the utility of EBUS-TBNA samples for EGFR testing with variable results. In one study, EGFR mutation testing was possible in 27 out of 35 patients (77%) undergoing EUS-FNA or EBUS-TBNA (25). Another study of 36 patients in Spain undergoing EBUS-TBNA suggested EGFR mutation analysis was feasible in 26 (72%) cases (26). Billah demonstrated that 96% of specimens from EBUS-TBNA in a cancer center were able to undergo EGFR mutation testing (27). Similarly high rates of reliable EGFR mutation testing of EBUS-TBNA samples have been observed by Nakajima and colleagues (28, 29). A recent study, in which cell blocks were prepared from 128 lung cancer cytology specimens, demonstrated that molecular analysis was possible in 98% of specimens (30).
It is widely accepted that NSCLC may contain areas of mixed adenocarcinoma, large cell and squamous cell carcinoma. Up to 25% of small cell carcinomas are thought to contain areas of NSCLC differentiation (31). This pathological heterogeneity implies that smaller cytological samples may not be representative of the entire lesion. Another potential area of controversy in NSCLC is that of genetic tumor heterogeneity. Conflicting evidence exists. Three studies comparing EGFR mutation status in primary tumor and local lymph node metastases demonstrated significant discrepancies between the sites (32–34). However, a recent study showed that when highly sensitive techniques for mutation detection were used, no discordant mutation patterns were detected among 77 paired primary and metastatic tumors (35). These authors suggested that weak EGFR mutation signals in an area without EGFR amplification may not reach the threshold of detection because of the mixture with normal cells resulting in pseudoheterogeneity. The authors concluded that true genetic heterogeneity is rare (35). This latter view would support EGFR mutation status being assessed in the most accessible tissue only rather than multiple sites being sampled.
This study confirms the high yield from EBUS-TBNA of detecting malignancy in intrathoracic lymph nodes in a real world setting. A sensitivity of 88% in 774 patients was observed, which is similar to a sensitivity of 93% observed in a meta-analysis of 1,299 patients (36). This study contains the first reported death attributed to EBUS-TBNA. The patient was likely immunosuppressed due to widely metastatic malignancy and succumbed to sepsis within 72 hours of the procedure. Sepsis may be attributed to the process of introducing pharyngeal microorganisms into the lower respiratory tract. The large number of patients included in this study renders subgroup analyses powerful. EBUS-TBNA of lymph nodes less than 1 cm has a significantly lower sensitivity than when the procedure is performed in nodes larger than 1 cm. This may be due to the increased technical difficulty of sampling smaller lymph nodes. However, when small lymph nodes were sampled successfully (regardless of lymph node location), the samples were suitable for NSCLC subtyping and EGFR mutation analysis.
There are several limitations of this study. Pathological samples in this study did not undergo central review; however, this reflects the pragmatic nature of the study and results in strong external validity. The centers included in the study carry out a high volume of EBUS-TBNA procedures with experienced operators and pathologists. Despite the observational design of this study, data were collected prospectively in each center. A final issue is that not all negative EBUS-TBNA cases underwent mediastinoscopy. However, all patients underwent at least 6 months of clinical follow-up to allow a clinical diagnosis to be made.
Recent guidance has suggested a novel algorithm for the diagnosis of adenocarcinoma in small biopsies and cytological samples (37). In patients with positive cytology and classic morphology for adenocarcinoma or squamous cell carcinoma, no further markers are required, and those with adenocarcinoma can be submitted directly for EGFR mutation testing. Samples that are classified as NSCLC-NOS on morphology are recommended to undergo a panel of immunohistochemistry that includes one squamous cell carcinoma marker and one adenocarcinoma marker with or without mucin staining. If the NSCLC tumor remains unclassified, then molecular analysis is recommended. This multicenter study clearly demonstrates that samples from EBUS-TBNA obtained in routine practice are suitable for entry into this new diagnostic algorithm and provides further impetus for the use of EBUS-TBNA as an initial diagnostic procedure in patients with suspected lung cancer.
Supplementary Material
Footnotes
Supported by grant G0800465/1 from the UK Medical Research Council (N.N., S.M.J.). This study was partly undertaken at UCLH/UCL, which received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centres funding scheme (N.N., S.M.J.). This study was also partly undertaken at Papworth Hospital, Cambridge, which is funded by the Department of Health’s NIHR Biomedical Research Centres funding Scheme (R.C.R., D.M.R.) and the Cambridge Experimental Cancer Medicines Centre and by the NIHR Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London (A.G.N.).
Originally Published in Press as DOI: 10.1164/rccm.201202-0294OC on April 12, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543–3551 [DOI] [PubMed] [Google Scholar]
- 2.National Institute of Health and Clinical Excellence Pemetrexed for the first-line treatment of non-small-cell lung cancer [Internet; accessed 2012 Jan 13]. Available from: www.nice.org.uk/TA181
- 3.Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF III, Gaudreault J, Damico LA, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004;22:2184–2191 [DOI] [PubMed] [Google Scholar]
- 4.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–957 [DOI] [PubMed] [Google Scholar]
- 5.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735–742 [DOI] [PubMed] [Google Scholar]
- 6.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Janne PA, Costa DB, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navani N, Spiro SG, Janes SM. Mediastinal staging of NSCLC with endoscopic and endobronchial ultrasound. Nat Rev Clin Oncol 2009;6:278–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace WA, Monaghan HM, Salter DM, Gibbons MA, Skwarski KM. Endobronchial ultrasound-guided fine-needle aspiration and liquid-based thin-layer cytology. J Clin Pathol 2007;60:388–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufmann O, Fietze E, Mengs J, Dietel M. Value of p63 and cytokeratin 5/6 as immunohistochemical markers for the differential diagnosis of poorly differentiated and undifferentiated carcinomas. Am J Clin Pathol 2001;116:823–830 [DOI] [PubMed] [Google Scholar]
- 10.Khayyata S, Yun S, Pasha T, Jian B, McGrath C, Yu G, Gupta P, Baloch Z. Value of P63 and CK5/6 in distinguishing squamous cell carcinoma from adenocarcinoma in lung fine-needle aspiration specimens. Diagn Cytopathol 2009;37:178–183 [DOI] [PubMed] [Google Scholar]
- 11.Yatabe Y, Mitsudomi T, Takahashi T. TTF-1 expression in pulmonary adenocarcinomas. Am J Surg Pathol 2002;26:767–773 [DOI] [PubMed] [Google Scholar]
- 12.Stenhouse G, Fyfe N, King G, Chapman A, Kerr KM. Thyroid transcription factor 1 in pulmonary adenocarcinoma. J Clin Pathol 2004;57:383–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ou SH, Zell JA. Carcinoma NOS is a common histologic diagnosis and is increasing in proportion among non-small cell lung cancer histologies. J Thorac Oncol 2009;4:1202–1211 [DOI] [PubMed] [Google Scholar]
- 14.NHS Information Centre National lung cancer audit [Internet; accessed 2012 Jan 13]. Available from: http://www.ic.nhs.uk/webfiles/Services/NCASP/audits%20and%20reports/NHSIC_National_Lung_Cancer_Audit_2010_V1.0.pdf
- 15.Tournoy KG, Rintoul RC, van Meerbeeck JP, Carroll NR, Praet M, Buttery RC, van Kralingen KW, Rabe KF, Annema JT. EBUS-TBNA for the diagnosis of central parenchymal lung lesions not visible at routine bronchoscopy. Lung Cancer 2009;63:45–49 [DOI] [PubMed] [Google Scholar]
- 16.Fischer AH, Cibas ES, Howell LP, Kurian EM, Laucirica R, Moriarty AT, Renshaw AA, Zakowski MF, Young NA. Role of cytology in the management of non-small-cell lung cancer. J Clin Oncol 2011;29:3331–3332 [DOI] [PubMed] [Google Scholar]
- 17.Wallace WA, Rassl DM. Accuracy of cell typing in nonsmall cell lung cancer by EBUS/EUS-FNA cytological samples. Eur Respir J 2011;38:911–917 [DOI] [PubMed] [Google Scholar]
- 18.Sigel CS, Moreira AL, Travis WD, Zakowski MF, Thornton RH, Riely GJ, Rekhtman N. Subtyping of non-small cell lung carcinoma: a comparison of small biopsy and cytology specimens. J Thorac Oncol 2011;6:1849–1856 [DOI] [PubMed] [Google Scholar]
- 19.Nizzoli R, Tiseo M, Gelsomino F, Bartolotti M, Majori M, Ferrari L, De Filippo M, Rindi G, Silini EM, Guazzi A, et al. Accuracy of fine needle aspiration cytology in the pathological typing of non-small cell lung cancer. J Thorac Oncol 2011;6:489–493 [DOI] [PubMed] [Google Scholar]
- 20.Travis WD. BEM-HH-KealE. WHO classification: pathology and genetics: tumours of the lung, pleura, thymus, and heart. Lyon, France: IARC Press; 2004 [Google Scholar]
- 21.Nicholson AG, Gonzalez D, Shah P, Pynegar MJ, Deshmukh M, Rice A, Popat S. Refining the diagnosis and EGFR status of non-small cell lung carcinoma in biopsy and cytologic material, using a panel of mucin staining, TTF-1, cytokeratin 5/6, and P63, and EGFR mutation analysis. J Thorac Oncol 2010;5:436–441 [DOI] [PubMed] [Google Scholar]
- 22.Felip E, Gridelli C, Baas P, Rosell R, Stahel R. Metastatic non-small-cell lung cancer: consensus on pathology and molecular tests, first-line, second-line, and third-line therapy: 1st ESMO Consensus Conference in Lung Cancer; Lugano 2010. Ann Oncol 2011;22:1507–1519 [DOI] [PubMed] [Google Scholar]
- 23.Vincek V, Nassiri M, Nadji M, Morales AR. A tissue fixative that protects macromolecules (DNA, RNA, and protein) and histomorphology in clinical samples. Lab Invest 2003;83:1427–1435 [DOI] [PubMed] [Google Scholar]
- 24.van Eijk R, Licht J, Schrumpf M, Talebian YM, Ruano D, Forte GI, Nederlof PM, Veselic M, Rabe KF, Annema JT, et al. Rapid KRAS, EGFR, BRAF and PIK3CA mutation analysis of fine needle aspirates from non-small-cell lung cancer using allele-specific qPCR. PLoS ONE 2011;6:e17791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuurbiers OC, Looijen-Salamon MG, Ligtenberg MJ, van der Heijden HF. A brief retrospective report on the feasibility of epidermal growth factor receptor and KRAS mutation analysis in transesophageal ultrasound- and endobronchial ultrasound-guided fine needle cytological aspirates. J Thorac Oncol 2010;5:1664–1667 [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Olive I, Monso E, Andreo F, Sanz-Santos J, Taron M, Molina-Vila MA, Llatjos M, Castella E, Moran T, Bertran-Alamillo J, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for identifying EGFR mutations. Eur Respir J 2010;35:391–395 [DOI] [PubMed] [Google Scholar]
- 27.Billah S, Stewart J, Staerkel G, Chen S, Gong Y, Guo M. EGFR and KRAS mutations in lung carcinoma: molecular testing by using cytology specimens. Cancer Cytopathol 2011;119:111–117 [DOI] [PubMed] [Google Scholar]
- 28.Nakajima T, Yasufuku K, Suzuki M, Hiroshima K, Kubo R, Mohammed S, Miyagi Y, Matsukuma S, Sekine Y, Fujisawa T. Assessment of epidermal growth factor receptor mutation by endobronchial ultrasound-guided transbronchial needle aspiration. Chest 2007;132:597–602 [DOI] [PubMed] [Google Scholar]
- 29.Nakajima T, Yasufuku K, Nakagawara A, Kimura H, Yoshino I. Multigene mutation analysis of metastatic lymph nodes in non-small cell lung cancer diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration. Chest 2011;140:1319–1324 [DOI] [PubMed] [Google Scholar]
- 30.Rekhtman N, Brandt SM, Sigel CS, Friedlander MA, Riely GJ, Travis WD, Zakowski MF, Moreira AL. Suitability of thoracic cytology for new therapeutic paradigms in non-small cell lung carcinoma: high accuracy of tumor subtyping and feasibility of EGFR and KRAS molecular testing. J Thorac Oncol 2011;6:451–458 [DOI] [PubMed] [Google Scholar]
- 31.Anraku M, Waddell TK. Surgery for small-cell lung cancer. Semin Thorac Cardiovasc Surg 2006;18:211–216 [DOI] [PubMed] [Google Scholar]
- 32.Sun L, Zhang Q, Luan H, Zhan Z, Wang C, Sun B. Comparison of KRAS and EGFR gene status between primary non-small cell lung cancer and local lymph node metastases: implications for clinical practice. J Exp Clin Cancer Res 2011;30:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park S, Holmes-Tisch AJ, Cho EY, Shim YM, Kim J, Kim HS, Lee J, Park YH, Ahn JS, Park K, et al. Discordance of molecular biomarkers associated with epidermal growth factor receptor pathway between primary tumors and lymph node metastasis in non-small cell lung cancer. J Thorac Oncol 2009;4:809–815 [DOI] [PubMed] [Google Scholar]
- 34.Schmid K, Oehl N, Wrba F, Pirker R, Pirker C, Filipits M. EGFR/KRAS/BRAF mutations in primary lung adenocarcinomas and corresponding locoregional lymph node metastases. Clin Cancer Res 2009;15:4554–4560 [DOI] [PubMed] [Google Scholar]
- 35.Yatabe Y, Matsuo K, Mitsudomi T. Heterogeneous distribution of EGFR mutations is extremely rare in lung adenocarcinoma. J Clin Oncol 2011;29:2972–2977 [DOI] [PubMed] [Google Scholar]
- 36.Gu P, Zhao YZ, Jiang LY, Zhang W, Xin Y, Han BH. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009;45:1389–1396 [DOI] [PubMed] [Google Scholar]
- 37.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ, Van Schil PE, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.