Abstract
We compared patterns of blood pressure (BP) change between normotensive women, women who developed gestational hypertension or preeclampsia and women who had essential hypertension to examine how distinct these conditions are and whether rates of BP change may help to identify women at risk of hypertensive disorders. We used antenatal clinic BP measurements (median 14 per woman) of 13,016 women from the Avon Longitudinal Study of Parents and Children (ALSPAC) who had a singleton or twin live birth surviving until at least 1 year. Linear spline models were used to describe changes in systolic and diastolic BP in different periods of pregnancy (8-18, 18-30, 30-36 and 36+ weeks gestation). Women who had essential hypertension, and those who developed gestational hypertension or preeclampsia had higher BP at 8 weeks gestation (baseline) compared with normotensive women. The decrease in BP until 18 weeks was smaller in gestational hypertensive compared with normotensive pregnancies. BP rose more rapidly from 18 weeks onwards in gestational hypertensive and preeclamptic pregnancies and from 30 weeks onwards in essential hypertensive compared with normotensive pregnancies. Women who developed preeclampsia had a more rapid increase in BP from 30 weeks onwards than those who developed gestational hypertension or had essential hypertension. Our findings indicate notable patterns of BP change that distinguish women with essential hypertension, gestational hypertension and preeclampsia from each other and from normotensive women, even from early pregnancy. These distinct patterns may be useful for identifying women at risk of developing a hypertensive disorder later in pregnancy.
Keywords: blood pressure, preeclampsia, gestational hypertension, pregnancy, ALSPAC
Introduction
Hypertensive disorders of pregnancy (HDP), consisting of gestational hypertension, preeclampsia and essential hypertension, affect around 10% of pregnancies(1) and are associated with maternal and perinatal mortality(2;3), small for gestational age infants and preterm birth,(1;4-6). Gestational hypertension and preeclampsia are defined using blood pressure (BP) thresholds after 20 weeks gestation, with the additional criteria of proteinuria for preeclampsia.(7)
In normal pregnancy BP initially decreases until 18-20 weeks gestation and then rises until delivery(8-11), and studies have indicated that higher BP pre-pregnancy(12;13), and in the first trimester (14-16) are associated with increased risk of developing gestational hypertension and preeclampsia. The few studies that have assessed BP trajectories across gestation in different HDP groups, suggest the relative change in BP may provide additional indication of HDP risk relative to a single BP measurement. However these studies were limited by their size (mostly N<1000), number of repeat measurements available or outdated definitions of HDP, and did not assess differences in rates of BP change between groups in different periods of pregnancy.(10;17-22) Comparing changes in BP between gestational hypertensive and preeclamptic pregnancies is useful for assessing the distinctness of these conditions, and whether the greater risk associated with preeclampsia is due solely to its additional systemic changes, indicated by proteinuria, or also attributable to the severity of vascular problems, represented by greater increases in BP.
Our study aims to investigate whether patterns of BP change across pregnancy differ between normotensive women, those who have hypertension prior to pregnancy (referred to as ‘essential hypertension’ throughout the paper for ease of reporting), and those who develop gestational hypertension or preeclampsia, in a large prospective cohort of women. Preeclamptic pregnancies have a high risk of medically indicated preterm delivery,(23) particularly if maternal BP rises rapidly in late gestation(24;25). We have therefore modelled BP change jointly with time to delivery to adjust for the shorter gestational period in women who experienced greater rises in BP.
Methods
The Avon Longitudinal Study of Parents and Children (ALSPAC) is a prospective birth cohort study aiming to investigate the health and development of children. The study has been described in full elsewhere(26) and on the website www.bris.ac.uk/alspac. Pregnant women with expected delivery dates between 1st April 1991 and 31st December 1992 living in Avon during their pregnancy were eligible for recruitment. Data has been collected through questionnaires, research clinics and linkage to routine health records. Ethical approval for the study was obtained from the ALSPAC Law and Ethics Committee and from the National Health Service (NHS) local ethics committee. In total 14,541 women were enrolled, 13,796 had singleton or twin pregnancies where the offspring were alive at 1 year and 13,578 of these women had data abstracted from obstetric records. We excluded women who had existing (N=43) or gestational (N=56) diabetes and women who had a gestational period > 44 weeks (N=6), which left 13,016 women with at least one BP measurement available for analysis.
All BP and urine dipstick proteinuria measurements taken routinely as part of antenatal care by midwives, obstetricians or other relevant health professionals, such as general practitioners, were abstracted from obstetric medical records by six trained research midwives. There was no between-midwife variation in mean values of the data abstracted and error rates were consistently <1% in repeated data entry checks. These were single BP measurements taken in the seated position using the appropriate cuff size and diastolic BP (DBP) was measured using Korotkoff phase V. There was a median of 14 (interquartile range of 11 to 16) BP measurements per woman. The gestational age at each visit was derived from the date of measurement and the expected delivery date. A questionnaire administered during pregnancy asked about previous hypertension. Women who reported having previously been diagnosed with hypertension outside of pregnancy and were aged over 16 years at diagnosis were considered to have essential hypertension. For women who did not have essential hypertension, HDP was defined according to International Society for the Study of Hypertension in Pregnancy (ISSHP) criteria(7) with gestational hypertension defined as systolic BP (SBP) ≥ 140 mmHg and/or DBP ≥ 90 mmHg on two occasions after 20 weeks gestation; preeclampsia was defined by the same criteria with proteinuria of 1+ on dipstick testing occurring at the same time as the elevated BP. This produced four mutually exclusive categories of no HDP, gestational hypertension, preeclampsia and essential hypertension. Gestational age at delivery (in weeks) was calculated as the difference between the delivery date and the mother’s reported last menstrual period date, or updated if ultrasound information was available which led to a reassessment of gestation. At the time of recruitment it was not routine clinical practice to perform ultrasound gestational age dating in early pregnancy, and in the data abstracted from the clinical records it was not recorded which few women had a scan or had their gestational age adjusted.
Maternal age at delivery and offspring sex were abstracted from obstetric records. Maternal height, pre-pregnancy weight, parity, highest educational qualification and smoking status were obtained from questionnaires administered during early pregnancy. Pre-pregnancy BMI was calculated as weight(kg)/ height(m)2 and categorised as underweight (<18.5kg/m2), normal (18.5-24.9kg/m2), overweight (25.0-29.9kg/m2) and obese (≥30.0kg/m2). Smoking status was classed as “never” for women who did not smoke immediately prior to or during pregnancy, “pre-pregnancy/first trimester” for women who smoked only immediately prior to pregnancy or in the first three months of gestation or “throughout” for women who continued to smoke after the first trimester.
Statistical analysis
We first developed linear spline random effects models for SBP and DBP change across gestation, as previously described(11). To take account of data clustering due to the multiple antenatal visits per woman, multilevel modelling using antenatal visits (level 1) within women (level 2) was used. We randomly sampled one measurement per 2-week period of gestation for each woman, to prevent women with many measurements from having too great an influence on the models, leaving a median of 10 (IQR: 9 to 11) BP measurements per woman. For both SBP and DBP the best-fitting models had knot points (indicating changes in the slope) at 18, 30 and 36 weeks gestation and baseline was set at 8 weeks gestation as there were few measurements prior to this. This produced five individual-level random effects parameters: SBP/DBP at 8 weeks and SBP/DBP change 8-18 weeks, 18-30 weeks, 30-36 weeks and 36 weeks onwards.
Since women who have a greater rise in BP in late gestation are likely to deliver earlier, have fewer BP measurements over pregnancy and therefore less influence in statistical models, the overall average increase in BP in the population would be underestimated by these univariate models. This problem could not be overcome by simply conditioning on gestational age at delivery in our analysis since, if length of gestation is influenced by both BP change and HDP, this would bias our estimate of the association between BP change and HDP (see Figure S1 in Supplemental Material).(27) We therefore combined the random effects spline models for BP with a model for time to delivery to produce a joint model with SBP (or DBP) and time to delivery as outcome variables to adjust for this. Please see Supplemental Material for further information. We included HDP category as a covariate, with normotensive pregnancies as the reference, and adjusted for maternal pre-pregnancy BMI, maternal age, parity, smoking, education and offspring sex (for singleton pregnancies) or twin pregnancy by including these as covariates also. We assessed whether the relationships of maternal characteristics with BP changes across pregnancy differed between HDP categories by testing for interactions between each of the maternal covariates and HDP category.
The correlations between BP changes and time to delivery were derived from the random effects for BP outcomes and the residual error from the time to delivery model (please see Supplemental Material). Model 1 was adjusted for HDP category and maternal covariates. In Model 2 we also adjusted for SBP/DBP at 8 weeks gestation and SBP/DBP change in earlier periods of pregnancy.
To examine whether secondary causes of hypertension or the use of antihypertensive medication affected our results, we repeated analyses excluding 442 women who reported ever having had kidney disease, 41 essential hypertensive women who reported using medication to treat their hypertension and 59 women who reported using antihypertensives, beta blockers or calcium channel blockers (for any reason) regularly, or at any time, during their pregnancy (total excluded = 519). We also completed sensitivity analyses restricting to women who contributed at least 9 BP measurements to the spline models (N=7940) and to women with ≤11 measurements (N=7465).
Statistical analyses were carried out using MLwiN version 2.23 and Stata version 11.2 combined with runmlwin(28).
Results
The characteristics of the full cohort and the subset of women who had complete data on all maternal covariates are shown in Table 1, and by HDP category in Table S1 (see Supplemental Material). The distributions of each of the variables were similar in the full dataset and the subset with complete data. In total 80.0% (N=10,419) of women were normotensive throughout pregnancy, 14.6% (N=1,896) developed gestational hypertension, 2.1% (N=277) developed preeclampsia and 3.3% (N=424) had essential hypertension. The numbers of women who developed gestational hypertension and preeclampsia at different gestational ages are shown in Table S2. Both of these conditions most commonly developed between 35 and 39 weeks gestation. Average BP levels at baseline and average changes in BP per week from univariate models are shown in Table S3.
Table 1. Characteristics of the full dataset and women who had complete data on all covariables.
| Maternal Characteristic | Full dataset (Total N=13,016) |
Complete data on all variables (N=9,930) |
|---|---|---|
| Gestational age at delivery (weeks) | N=13,016 | |
| median (IQR) | 40 (39, 41) | 40 (39, 41) |
| HDP (%) | N=13,016 | |
| No HDP | 80.05 | 80.26 |
| Gestational hypertension | 14.57 | 14.26 |
| Preeclampsia | 2.13 | 1.95 |
| Essential hypertension | 3.26 | 3.52 |
| Maternal pre-pregnancy BMI (%) | N=10,839 | |
| Underweight | 5.08 | 4.91 |
| Normal | 74.29 | 74.65 |
| Overweight | 15.04 | 14.94 |
| Obese | 5.59 | 5.49 |
| Maternal age (years) (%) | N=13,016 | |
| < 20 | 4.86 | 3.41 |
| 20-24 | 19.48 | 16.73 |
| 25-29 | 38.60 | 39.60 |
| 30-34 | 27.29 | 29.68 |
| 35+ | 9.78 | 10.58 |
| Parity (%) | N=12,097 | |
| Nulliparous | 45.07 | 45.32 |
| Multiparous | 54.93 | 54.68 |
| Smoking (%) | N=12,208 | |
| Never | 66.52 | 69.19 |
| Pre-pregnancy/1st trimester | 13.78 | 13.14 |
| Throughout | 19.70 | 17.66 |
| Education (%) | N=11,674 | |
| CSE/vocational | 30.00 | 27.64 |
| O level | 34.65 | 35.37 |
| A level | 22.42 | 23.33 |
| Degree | 12.93 | 13.66 |
| Pregnancy type (%) | N=13,016 | |
| Male singleton | 50.81 | 50.51 |
| Female singleton | 47.87 | 48.20 |
| Twin | 1.32 | 1.29 |
Abbreviations: BMI body mass index; CSE certificate of secondary education; HDP hypertensive disorder of pregnancy; IQR interquartile range;
Gestational age at delivery ranged from 24 to 44 weeks. Women who had essential hypertension or developed preeclampsia had a shorter average gestational period than normotensive women (Table S4).
Associations of BP change with HDP
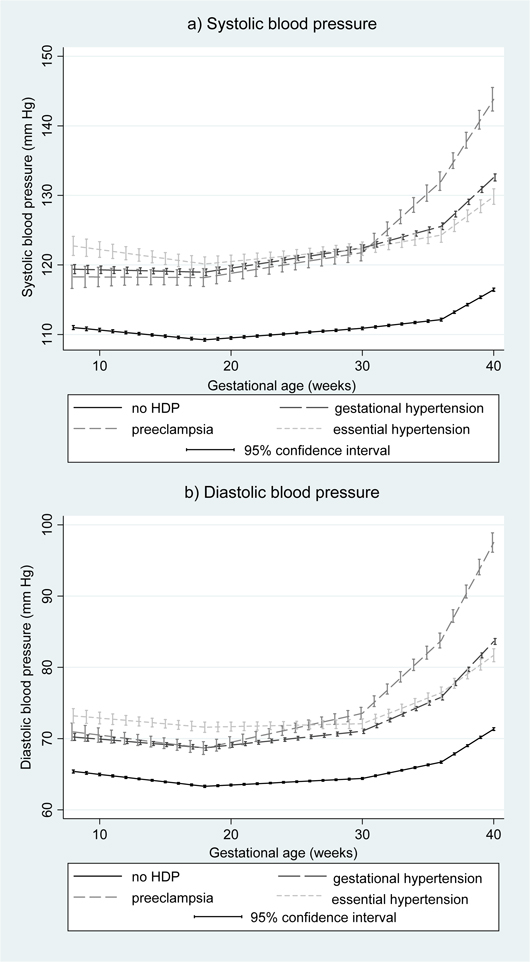
Figure 1 shows the average patterns of BP change across pregnancy by HDP category in the joint models. SBP and DBP were notably higher throughout pregnancy for women who had any of essential hypertension, gestational hypertension or preeclampsia compared with normotensive women. Women who developed gestational hypertension had similar DBP until around 18 weeks gestation and SBP until around 30 weeks gestation to those who developed preeclampsia, after which BP increases were greater in women who developed preeclampsia compared with those who developed gestational hypertension. Women who had essential hypertension had the highest SBP and DBP at 8 weeks, but similar SBP and DBP in late pregnancy to those who developed gestational hypertension.
Figure 1.
Average trajectories of systolic and diastolic blood pressure by hypertensive disorders of pregnancy in the unadjusted joint model (N=13,016)
The mean differences in SBP at 8 weeks and change in SBP in each period of pregnancy associated with gestational hypertension, preeclampsia and essential hypertension compared with no HDP are shown in Table 2. The equivalent associations for DBP are shown in Table 3. In both unadjusted and adjusted models, gestational hypertension (compared with women remaining normotensive) was associated with a higher SBP and DBP at 8 weeks, a smaller decrease in SBP between 8 and 18 weeks, and a greater increase in SBP and DBP between 18 and 30 weeks, between 30 and 36 weeks and after 36 weeks. Preeclampsia (compared with women remaining normotensive) was also associated with a higher SBP and DBP at 8 weeks, and with a greater increase in SBP and DBP between 18 and 30 weeks, 30 and 36 weeks and after 36 weeks. Essential hypertension (compared with women remaining normotensive) was associated with a higher SBP and DBP at 8 weeks, a greater increase in DBP between 30 and 36 weeks gestation and a greater increase in SBP and DBP after 36 weeks.
Table 2. Mean difference (95% confidence interval) in systolic blood pressure at 8 weeks and change in systolic blood pressure in each period of gestation by hypertensive disorders of pregnancy (N=9,930)*.
| Maternal characteristics | Mean difference in SBP at 8 weeks (mmHg) |
Mean difference in average SBP change (mmHg/week): |
|||
|---|---|---|---|---|---|
| 8-18 weeks | 18-30 weeks | 30-36 weeks | 36+ weeks | ||
| Unadjusted model: | |||||
| No HDP | 0 | 0 | 0 | 0 | 0 |
| Gestational hypertension | 8.189 (7.373, 9.005) | 0.137 ( 0.039, 0.234) | 0.148 (0.089, 0.207) | 0.330 (0.213, 0.447) | 0.707 (0.516, 0.897) |
| Preeclampsia | 7.528 ( 5.541, 9.515) | 0.117 (−0.120, 0.355) | 0.090 (−0.060, 0.240) | 1.475 (1.167, 1.783) | 2.418 (1.818, 3.018) |
| Essential hypertension | 11.046 (9.530, 12.562) | −0.011 (−0.192, 0.171) | 0.005 (−0.108, 0.117) | 0.171 (−0.054, 0.397) | 0.397 (0.017, 0.777) |
| Adjusted model: | |||||
| No HDP | 0 | 0 | 0 | 0 | 0 |
| Gestational hypertension | 7.151 (6.325, 7.978) | 0.107 (0.007, 0.207) | 0.183 (0.122, 0.243) | 0.330 (0.211, 0.450) | 0.744 (0.549, 0.940) |
| Preeclampsia | 6.513 (4.533, 8.493) | 0.072 (−0.167, 0.311) | 0.129 (−0.021, 0.280) | 1.463 (1.154, 1.772) | 2.409 (1.808, 3.011) |
| Essential hypertension | 9.900 (8.389, 11.411) | −0.029 (−0.211, 0.154) | 0.043 (−0.069, 0.156) | 0.182 (−0.045, 0.409) | 0.454 (0.072, 0.836) |
Both models are adjusted for time to delivery; adjusted model is additionally adjusted for maternal pre-pregnancy BMI, age, parity, smoking during pregnancy, education and sex/number of fetuses
In reference category (normotensive women):
For unadjusted model:
mean SBP at 8 weeks (mmHg) = 111.011;
mean SBP change (mmHg/week): 8-18 weeks = -0.164; 18-30 weeks = 0.137; 30-36 weeks = 0.196; 36+ weeks = 1.054
For adjusted model:
mean SBP at 8 weeks (mmHg) = 111.966;
mean SBP change (mmHg/week): 8-18 weeks = -0.188; 18-30 weeks = 0.150; 30-36 weeks = 0.134; 36+ weeks = 1.222
Abbreviations: BMI body mass index; HDP hypertensive disorder of pregnancy; SBP systolic blood pressure;
Table 3. Mean difference (95% confidence interval) in diastolic blood pressure at 8 weeks and change in diastolic blood pressure in each period of gestation by hypertensive disorders of pregnancy (N=9,930)*.
| Maternal characteristics | Mean difference in DBP at 8 weeks (mmHg) |
Mean difference in average DBP change (mmHg/week): |
|||
|---|---|---|---|---|---|
| 8-18 weeks | 18-30 weeks | 30-36 weeks | 36+ weeks | ||
| Unadjusted model: | |||||
| No HDP | 0 | 0 | 0 | 0 | 0 |
| Gestational hypertension | 4.692 (4.096, 5.287) | 0.044 (−0.027, 0.115) | 0.113 (0.069, 0.156) | 0.401 (0.311, 0.491) | 0.805 (0.655, 0.954) |
| Preeclampsia | 5.470 (4.020, 6.920) | −0.007 (−0.180, 0.166) | 0.220 (0.110, 0.331) | 1.406 (1.170, 1.642) | 2.486 (2.017, 2.956) |
| Essential hypertension | 7.562 (6.455, 8.668) | 0.061 (−0.072, 0.193) | −0.055 (−0.137, 0.028) | 0.329 (0.156, 0.502) | 0.274 (−0.023, 0.572) |
| Adjusted model: | |||||
| No HDP | 0 | 0 | 0 | 0 | 0 |
| Gestational hypertension | 3.799 (3.200, 4.398) | 0.038 (−0.035, 0.110) | 0.132 (0.088, 0.177) | 0.370 (0.278, 0.461) | 0.833 (0.680, 0.987) |
| Preeclampsia | 4.674 (3.242, 6.107) | −0.028 (−0.202, 0.145) | 0.239 (0.129, 0.350) | 1.336 (1.099, 1.573) | 2.465 (1.995, 2.935) |
| Essential hypertension | 6.585 (5.490, 7.679) | 0.056 (−0.077, 0.189) | −0.029 (−0.112, 0.054) | 0.312 (0.138, 0.486) | 0.333 (0.035, 0.632) |
Both models are adjusted for time to delivery; adjusted model is additionally adjusted for maternal pre-pregnancy BMI, age, parity, smoking during pregnancy, education and sex/number of fetuses
In reference category (normotensive women):
For unadjusted model:
mean DBP at 8 weeks (mmHg) = 65.393;
mean DBP change (mmHg/week): 8-18 weeks = -0.202; 18-30 weeks = 0.091; 30-36 weeks = 0.375; 36+ weeks = 1.155
For adjusted model:
mean DBP at 8 weeks (mmHg) = 65.494;
mean DBP change (mmHg/week): 8-18 weeks = -0.175; 18-30 weeks = 0.109; 30-36 weeks = 0.463; 36+ weeks = 1.252
Abbreviations: BMI body mass index; DBP diastolic blood pressure; HDP hypertensive disorder of pregnancy
Women who had essential hypertension had higher SBP and DBP at 8 weeks than women who developed gestational hypertension or preeclampsia (all p<0.05) but there was little difference in SBP or DBP at 8 weeks between women who developed gestational hypertension or preeclampsia. Those who developed preeclampsia had a greater increase in DBP between 18 and 30 weeks and in SBP and DBP between 30 and 36 weeks and after 36 weeks than women who developed gestational hypertension or had essential hypertension (all p<0.07). Women who developed gestational hypertension had a greater rise in SBP and DBP between 18 and 30 weeks and in DBP from 36 weeks onwards than those with essential hypertension (all p<0.01), but similar rates of BP change in other periods.
Figures S2-S13 show the patterns of SBP and DBP change across pregnancy by each of the maternal risk factors and HDP status, derived from a model including interactions of each of the maternal risk factors with HDP and SBP/DBP at 8 weeks and also with HDP and each of the splines for SBP/DBP change. The figures show that the patterns of association of each of the maternal characteristics with BP changes are similar in each HDP group.
Associations of BP change with time to delivery
Table 4 shows the associations of BP at 8 weeks and changes in BP with time to delivery. Neither SBP or DBP at 8 weeks, nor change in SBP or DBP up to 18 weeks were associated with the length of gestation in any models. However, a greater rise in DBP between 18 and 30 weeks, and in SBP or DBP between 30 and 36 weeks, or from 36 weeks onwards was associated with a shorter gestational period in models adjusted only for HDP category and maternal covariates (Model 1). These associations were stronger when adjusting for baseline BP and previous BP changes (Model 2). The predicted lengths of gestation at two standard deviations above and below the average BP at 8 weeks and changes in BP in each period of gestation are shown in Table S5.
Table 4. Associations of blood pressure at 8 weeks gestation and changes in blood pressure in each period of pregnancy with the time to delivery (N=9,930)*.
| Blood pressure variable | Length of gestation |
|
|---|---|---|
| % increase in gestation | 95% confidence interval | |
| SBP at 8 weeks (mmHg) | ||
| Model 1 | 0.00 | (−0.02, 0.02) |
| SBP change (mmHg/week) | ||
| 8-18 weeks | ||
| Model 1 | −0.20 | (−0.63, 0.20) |
| Model 2 | −0.22 | (−0.65, 0.17) |
| 18-30 weeks | ||
| Model 1 | −0.28 | (−0.73, 0.15) |
| Model 2 | −0.47 | (−0.92, −0.04) |
| 30-36 weeks | ||
| Model 1 | −0.91 | (−1.16, −0.70) |
| Model 2 | −1.17 | (−1.43, −0.95) |
| 36+ weeks | ||
| Model 1 | −0.14 | (−0.26, −0.01) |
| Model 2 | −0.54 | (−0.69, −0.40) |
| DBP at 8 weeks (mmHg) | ||
| Model 1 | 0.1 | (−0.1, 0.4) |
| DBP at 8 weeks (mmHg) | ||
| Model 1 | 0.01 | (−0.02, 0.05) |
| DBP change (mmHg/week) | ||
| 8-18 weeks | ||
| Model 1 | −0.51 | (−1.35, 0.19) |
| Model 2 | −0.55 | (−1.42, 0.18) |
| 18-30 weeks | ||
| Model 1 | −1.29 | (−2.10, −0.62) |
| Model 2 | −1.66 | (−2.42, −1.00) |
| 30-36 weeks | ||
| Model 1 | −1.33 | (−1.62, −1.10) |
| Model 2 | −1.71 | (−2.03, −1.46) |
| 36+ weeks | ||
| Model 1 | −0.16 | (−0.29, −0.03) |
| Model 2 | −0.65 | (−0.79, −0.50) |
Model 1: adjusted for HDP, maternal pre-pregnancy BMI, age, parity, smoking during pregnancy, education and sex/number of fetuses
Model 2: additionally adjusted for SBP/DBP at 8 weeks and SBP/DBP change in earlier periods of gestation
Abbreviations: DBP diastolic blood pressure; SBP systolic blood pressure
When we restricted analyses with length of gestation as the outcome to women who remained normotensive throughout pregnancy (N=7,970), most findings were unchanged except that SBP and DBP change from 36 weeks onwards were associated with length of gestation only in Model 2.
None of the findings were meaningfully changed when we excluded women who reported having kidney disease or taking antihypertensive medication, or women with less than 9 measurements (full results of these sensitivity analyses available from authors). When women who had more than 11 measurements were excluded, findings were similar, except that SBP change between 8 and 18 weeks did not differ between gestational hypertensive and normotensive pregnancies as in the main analysis (adjusted mean difference (95% confidence interval) in SBP change for gestational hypertensive compared with normotensive pregnancies: −0.028 (−0.162, 0.106) mmHg/week). This may be due to the later enrolment time of women who had ≤11 measurements (median 12.3 weeks compared with 9.7 weeks gestation for women with > 11 measurements) or may suggest that this early-pregnancy difference is only apparent in women who develop more severe gestational hypertension.
Discussion
In this large prospective cohort of women, we have shown that women who developed gestational hypertension or preeclampsia had higher BP from very early in pregnancy, a reduced initial decline in SBP to 18 weeks gestation, a faster increase in BP from 18 weeks onwards and then in those destined to develop preeclampsia, a further marked increase in BP from 30 weeks. We also found that women who had essential hypertension had higher BP than all other groups in early pregnancy and a greater rise in BP than normotensive women in late pregnancy, but showed a similar early pregnancy decline in BP to normotensive women.
Of note, BP levels at baseline (8 weeks), although much higher than for women who remained normotensive, were very similar in both preeclamptic women and those with gestational hypertension. This is despite some evidence that the latter two conditions are different from each other, with preeclampsia generally considered a two-stage syndrome, the first stage being poor placentation, and the second being maternal manifestation of hypertension and proteinuria later in pregnancy,(29) whereas gestational hypertension has been described as latent hypertension revealed by pregnancy.(30) There is, however, evidence that gestational hypertension and preeclampsia share many risk factors(31-33) suggesting that there are common mechanisms behind the development of both of these disorders. In addition, women who develop either gestational hypertension or preeclampsia have similarly increased risk of cardiovascular disease in later life(34;35). Although preeclampsia generally carries a greater risk of intrauterine growth restriction and preterm birth than gestational hypertension,(31;33) women who have severe gestational hypertension have greater risk than those with mild preeclampsia.(4) Thus, our results are consistent with other evidence suggesting that there is important overlap between these two conditions.
Our finding that BP, and in particular DBP, rose more steeply from 18 weeks gestation onwards in women who developed preeclampsia compared with those who developed gestational hypertension or had essential hypertension, is striking. Previous analyses of rhythm adjusted mean BP in 202 women at risk of HDP also demonstrated equivalent BP in early pregnancy and then later discordance, with higher BP in preeclampsia than gestational hypertension.(21) However a study using clinic BP measurements (N~8000) found that women who developed preeclampsia had lower BP in the first trimester than women who developed gestational hypertension, which may be due to the slightly later timing of measurement (median gestational age 13.2 weeks) than our 8 weeks baseline. In agreement with our findings, they also reported that preeclamptic women had a steeper rise in BP across pregnancy compared with gestational hypertensive women.(22) Another study (N=212) reported that women who developed preeclampsia had higher rhythm adjusted mean BP in late pregnancy than women who had essential hypertension or developed gestational hypertension.(20) Our observed early rise in BP in preeclampsia is consistent with histological studies of impaired trophoblast invasion of myometrial arterial segments and thereby a necessity to increase baseline maternal placental perfusion(36) as evidenced by the increase in DBP.
Given the similarities in risk factors between gestational hypertension and preeclampsia, the discordance in BP trajectory and presence of proteinuria which would define preeclampsia, may simply be reflecting the extent of impaired placentation and an individual’s renal threshold for manifesting proteinuria. Consistent with this approximately 50% of women who manifest mild hypertension before 35 weeks gestation develop preeclampsia later in pregnancy.(29) Furthermore, even if gestational hypertension and preeclampsia are influenced by different biological mechanisms then our results suggest that equivalent levels of early pregnancy BP predispose to both, but that the biological changes related to preeclampsia produce more extreme increases in BP than those related to gestational hypertension.
The relationship we found between a greater rise in BP after 18 weeks and a shorter time to delivery is in agreement with the study by Zhang et al, which investigated BP change in 8,325 nulliparous women from six countries. They found that women who had an early preterm birth (<34 weeks) and those who had a late preterm birth (34-36 weeks) had a greater rise in SBP and DBP between 12-19 weeks and 30-34 weeks gestation compared with women who delivered at term. Associations with spontaneous preterm birth were attenuated compared with those with all preterm births, but remained strong.(37) We were unable to restrict to spontaneous deliveries as we did not have this data available. However, we found that associations of DBP change from 18 weeks onwards and SBP change from 30 weeks onwards with length of gestation remained strong when restricting to women with normotensive pregnancies. The mechanism underlying the relationship between BP change and time to delivery is not clear, but the hypothalamic pituitary adrenal axis regulates both BP and maternal cortisol concentrations which are negatively associated with gestational age at birth.(38)
The strengths of our study are its large size, many repeated measurements of BP which allowed HDP to be classified accurately, and the ability to model BP change and time to delivery in parallel. Limitations of our study include the use of routinely collected clinic measurements of BP. These are likely to be subject to a high degree of variability over the course of the day and between observers as the method of measurement was not standardised between clinics. However, this reflects what is currently used in clinical practice to detect gestational hypertension and preeclampsia and thus our results are applicable to this setting. We would not expect this to introduce bias into our findings, only random measurement error. Over 90% of women in this study attended one of two hospitals and when we repeated the analysis for each of these separately results were similar. Since measurements of BP prior to pregnancy were unavailable we have used self-report of previous hypertension to classify essential hypertension. The higher level of BP in early pregnancy which we found in this group compared to all other HDP groups does however support this self-report. Although we have named this group “essential hypertensives”, this is likely to include a small number of women who had secondary hypertension as we did not have information on all secondary causes. We were unable to distinguish between medically indicated induced and spontaneous deliveries; we have attempted to eliminate medically indicated deliveries related to high BP by repeating analyses in normotensive women, but cannot exclude induced deliveries which were related to other conditions.
Perspectives
Our findings suggest that, as well as early pregnancy BP, the rate of increase in BP after 18 weeks may provide an early indication of those women most at risk of developing gestational hypertension or preeclampsia, before the BP threshold used for diagnosis of HDP is crossed. Furthermore, women who developed gestational hypertension had a smaller decrease in SBP up to 18 weeks, compared to normotensive women, meaning that careful planned monitoring of BP change in those with high levels at their booking clinic may help earlier identification of women at risk of developing either gestational hypertension or preeclampsia.
Supplementary Material
Acknowledgements
We are extremely grateful to all of the families who took part in this study, the midwives for their help in recruiting them and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
Funding:
This work was supported by the UK Wellcome Trust [grant number WT087997MA] and US National Institutes of Health [grant number R01 DK077659]. Core support for ALSPAC is provided by the UK Medical Research Council, the Wellcome Trust and the University of Bristol. The UK Medical Research Council provides funding for the MRC Centre for Causal Analyses in Translational Epidemiology [grant number G0600705]. AF is funded by a UK MRC research fellowship.
Footnotes
Conflict of interest:
None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- (1).Roberts CL, Algert CS, Morris JM, Ford JB, Henderson-Smart DJ. Hypertensive disorders in pregnancy: a population-based study. Med J Australia. 2005;182:332–335. doi: 10.5694/j.1326-5377.2005.tb06730.x. [DOI] [PubMed] [Google Scholar]
- (2).The Confidential Enquiry into Maternal and Child Health (CEMACH) In: Saving Mothers’ Lives: Reviewing Maternal Deaths to Make Motherhood Safer-2003-2005. Lewis G, editor. The Seventh Report on Confidential Enquiries into Maternal Deaths in the United Kingdom; 2007. [Google Scholar]
- (3).Steegers EA, von DP, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- (4).Buchbinder A, Sibai BM, Caritis S, MacPherson C, Hauth J, Lindheimer MD, Klebanoff M, VanDorsten P, Landon M, Paul R, Miodovnik M, Meis P, Thurnau G. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186:66–71. doi: 10.1067/mob.2002.120080. [DOI] [PubMed] [Google Scholar]
- (5).Heard AR, Dekker GA, Chan A, Jacobs DJ, Vreeburg SA, Priest KR. Hypertension during pregnancy in South Australia, Part 1: Pregnancy outcomes. Aust NZ J Obstet Gyn. 2004;44:404–409. doi: 10.1111/j.1479-828X.2004.00267.x. [DOI] [PubMed] [Google Scholar]
- (6).Ananth CV, Peedicayil A, Savitz DA. Effect of Hypertensive Diseases in Pregnancy on Birth-Weight, Gestational Duration, and Small-For-Gestational-Age Births. Epidemiology. 1995;6:391–395. doi: 10.1097/00001648-199507000-00011. [DOI] [PubMed] [Google Scholar]
- (7).Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: Statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP) Hypertens Pregnancy. 2001;20:IX–XIV. doi: 10.1081/PRG-100104165. [DOI] [PubMed] [Google Scholar]
- (8).MacGillivray I, Rose GA, Rowe B. Blood Pressure Survey in Pregnancy. Clin Sci. 1969;37:395–407. [PubMed] [Google Scholar]
- (9).Friedman EA, Neff RK. Pregnancy, outcome as related to hypertension, edema, and proteinuria. Perspect Nephrol Hypertens. 1976;5:13–22. [PubMed] [Google Scholar]
- (10).Moutquin JM, Rainville C, Giroux L, Raynauld P, Amyot G, Bilodeau R, Pelland N. A prospective study of blood pressure in pregnancy: prediction of preeclampsia. Am J Obstet Gynecol. 1985;151:191–196. doi: 10.1016/0002-9378(85)90010-9. [DOI] [PubMed] [Google Scholar]
- (11).Macdonald-Wallis C, Tilling K, Fraser A, Nelson SM, Lawlor DA. Established pre-eclampsia risk factors are related to patterns of blood pressure change in normal term pregnancy: Findings from the Avon Longitudinal Study of Parents and Children (ALSPAC) J Hypertens. 2011;29:1703–1711. doi: 10.1097/HJH.0b013e328349eec6. [DOI] [PubMed] [Google Scholar]
- (12).Rang S, Wolf H, von Montfrans GA, Karemaker JM. Serial assessment of cardiovascular control shows early signs of developing pre-eclampsia. J Hypertens. 2004;22:369–376. doi: 10.1097/00004872-200402000-00022. [DOI] [PubMed] [Google Scholar]
- (13).Magnussen EB, Vatten LJ, Lund-Nilsen TI, Salvesen KA, Davey Smith G, Romundstad PR. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: population based cohort study. BMJ. 2007;335:978–981. doi: 10.1136/bmj.39366.416817.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Cnossen JS, Vollebregt KC, de Vrieze N, ter Riet G, Mol BWJ, Franx A, Khan KS, van der Post JAM. Accuracy of mean arterial pressure and blood pressure measurements in predicting pre-eclampsia: systematic review and meta-analysis. BMJ. 2008;336:1117–1120. doi: 10.1136/bmj.39540.522049.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Akolekar R, Syngelaki A, Sarquis R, Zvanca M, Nicolaides KH. Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11-13 weeks. Prenatal Diagnosis. 2011;31:66–74. doi: 10.1002/pd.2660. [DOI] [PubMed] [Google Scholar]
- (16).Poon LCY, Kametas NA, Valencia C, Chelemen T, Nicolaides KH. Hypertensive Disorders in Pregnancy: Screening by Systolic Diastolic and Mean Arterial Pressure at 11-13 Weeks. Hypertens Pregnancy. 2011;30:93–107. doi: 10.3109/10641955.2010.484086. [DOI] [PubMed] [Google Scholar]
- (17).Reiss RE, Oshaughnessy RW, Quilligan TJ, Zuspan FP. Retrospective Comparison of Blood-Pressure Course During Preeclamptic and Matched Control Pregnancies. Am J Obstet Gynecol. 1987;156:894–898. doi: 10.1016/0002-9378(87)90347-4. [DOI] [PubMed] [Google Scholar]
- (18).Easterling TR, Benedetti TJ, Schmucker BC, Millard SP. Maternal Hemodynamics in Normal and Preeclamptic Pregnancies - A Longitudinal-Study. Obstet Gynecol. 1990;76:1061–1069. [PubMed] [Google Scholar]
- (19).Smith AJ, Walters WA, Buckley NA, Gallagher L, Mason A, Mcpherson J. Hypertensive and Normal-Pregnancy - A Longitudinal-Study of Blood-Pressure, Distensibility of Dorsal Hand Veins and the Ratio of the Stable Metabolites of Thromboxane A(2) and Prostacyclin in Plasma. Brit J Obstet Gynaec. 1995;102:900–906. doi: 10.1111/j.1471-0528.1995.tb10879.x. [DOI] [PubMed] [Google Scholar]
- (20).Benedetto C, Zonca M, Marozio L, Dolci C, Carandente F, Massobrio M. Blood pressure patterns in normal pregnancy and in pregnancy-induced hypertension, preeclampsia, and chronic hypertension. Obstet Gynecol. 1996;88:503–510. doi: 10.1016/0029-7844(96)00217-7. [DOI] [PubMed] [Google Scholar]
- (21).Hermida RC, Ayala DE, Mojon A, Fernandez JR, Alonso I, Silva I, Ucieda R, Iglesias M. Blood pressure patterns in normal pregnancy, gestational hypertension, and preeclampsia. Hypertension. 2000;36:149–158. doi: 10.1161/01.hyp.36.2.149. [DOI] [PubMed] [Google Scholar]
- (22).Gaillard R, Bakker R, Willemsen SP, Hofman A, Steegers EA, Jaddoe VW. Blood pressure tracking during pregnancy and the risk of gestational hypertensive disorders: The Generation R Study. Eur Heart J. 2011;32:3088–3097. doi: 10.1093/eurheartj/ehr275. [DOI] [PubMed] [Google Scholar]
- (23).Ananth CV, Vintzileos AM. Maternal-fetal conditions necessitating a medical intervention resulting in preterm birth. Am J Obstet Gynecol. 2006;195:1557–1563. doi: 10.1016/j.ajog.2006.05.021. [DOI] [PubMed] [Google Scholar]
- (24).National Institute for Health and Clinical Excellence Hypertension in Pregnancy: NICE Clinical Guideline CG107. 2010 http://guidance.nice.org.uk/CG107.
- (25).Gifford RW, August PA, Cunningham G, Green LA, Lindheimer MD, McNellis D, Roberts JM, Sibai BM, Taler SJ. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- (26).Golding J, Pembrey M, Jones R. ALSPAC-The Avon Longitudinal Study of Parents and Children - I. Study methodology. Paediatr Perinat Ep. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- (27).Greenland S. Quantifying biases in causal models: Classical confounding vs collider-stratification bias. Epidemiology. 2003;14:300–306. [PubMed] [Google Scholar]
- (28).Leckie G, Charlton C. runmlwin: Stata module for fitting multilevel models in the MLwiN software package. University of Bristol; Centre for Multilevel Modelling: 2011. [Google Scholar]
- (29).Barton JR, O‘Brien JM, Bergauer NK, Jacques DL, Sibai BM. Mild gestational hypertension remote from term: Progression and outcome. Am J Obstet Gynecol. 2001;184:979–983. doi: 10.1067/mob.2001.112905. [DOI] [PubMed] [Google Scholar]
- (30).Chesley LC. Hypertension in pregnancy: definitions, familial factor, and remote prognosis. Kidney Int. 1980;18:234–240. doi: 10.1038/ki.1980.131. [DOI] [PubMed] [Google Scholar]
- (31).Geelhoed JJM, Fraser A, Tilling K, Benfield L, Davey Smith G, Sattar N, Nelson SM, Lawlor DA. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures: the Avon Longitudinal Study of Parents and Children. Circulation. 2010;122:1192–1199. doi: 10.1161/CIRCULATIONAHA.110.936674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Salonen Ros H, Cnattingius S, Lipworth L. Comparison of risk factors for preeclampsia and gestational hypertension in a population-based cohort study. Am J Epidemiol. 1998;147:1062–1070. doi: 10.1093/oxfordjournals.aje.a009400. [DOI] [PubMed] [Google Scholar]
- (33).Villar J, Carroli G, Wojdyla D, Abalos E, Giordano D, Ba'aqeel H, Farnot U, Bergsjo P, Bakketeig L, Lumbiganon P, Campodonico L, Al-Mazrou Y, Lindheimer M, Kramer M. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am J Obstet Gynecol. 2006;194:921–931. doi: 10.1016/j.ajog.2005.10.813. [DOI] [PubMed] [Google Scholar]
- (34).Himmelmann A, Svensson A, Hansson L. Relation of Maternal Blood-Pressure During Pregnancy to Birth-Weight and Blood-Pressure in Children - the Hypertension in Pregnancy Offspring Study. J Intern Med. 1994;235:347–352. doi: 10.1111/j.1365-2796.1994.tb01085.x. [DOI] [PubMed] [Google Scholar]
- (35).Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, Smith WCS. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326:845–849. doi: 10.1136/bmj.326.7394.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Pijnenborg R, Brosens I. Deep trophoblast invasion and spiral artery remodelling. In: Pijnenborg R, Brosens I, Romero R, editors. Placental bed disorders: basic science and its translation to obstetrics. Cambridge University Press; Cambridge: 2010. pp. 97–107. [Google Scholar]
- (37).Zhang J, Villar J, Sun W, Merialdi M, Abdel-Aleem H, Mathai M, Ali M, Yu KF, Zavaleta N, Purwar M, Ngoc NTN, Campodonico L, Landoulsi S, Lindheimer M, Carroli G. Blood pressure dynamics during pregnancy and spontaneous preterm birth. Am J Obstet Gynecol. 2007;197:e1–e6. doi: 10.1016/j.ajog.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Giurgescu C. Are Maternal Cortisol Levels Related to Preterm Birth? J Obstet Gynecol Neonatal Nurs. 2009;38:377–390. doi: 10.1111/j.1552-6909.2009.01034.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.