Abstract
Background
Connexins are a widespread family of membrane proteins that assemble into hexameric hemichannels, also known as connexons. Connexons regulate membrane permeability in individual cells or couple between adjacent cells to form gap junctions and thereby provide a pathway for regulated intercellular communication. We have now examined the role of connexins in platelets, blood cells that circulate in isolation, but upon tissue injury adhere to each other and the vessel wall to prevent blood loss and facilitate wound repair.
Methods and Results
We report the presence of connexins in platelets, notably connexin37, and that the formation of gap junctions within platelet thrombi is required for the control of clot retraction. Inhibition of connexin function modulated a range of platelet functional responses prior to platelet-platelet contact, and reduced laser induced thrombosis in vivo in mice. Deletion of the Cx37 gene (Gja4) in transgenic mice reduced platelet aggregation, fibrinogen binding, granule secretion and clot retraction indicating an important role for Cx37 hemichannels and gap junctions in platelet thrombus function.
Conclusions
Together, these data demonstrate that platelet gap junctions and hemichannels underpin the control of haemostasis and thrombosis and represent potential therapeutic targets.
Keywords: Connexin37, Gap junction, Haemostasis, Platelets, Thrombosis
Gap junctions are key mediators of intercellular communication, formed from the coupling of two hemichannels (or connexons) on adjacent cells. Each hemichannel results from the oligomerisation of six connexin (Cx) monomers in the cell membrane. Upon docking of two hemichannels on adjacent cells, a pore of approximately 2-3nm in diameter is formed which allows the bidirectional intercellular transport of small molecules of up to 1000Da.1 While gap junctions have recognised roles in intercellular communication in various cell types such as nerve cells,2 oocytes,3 bone marrow stromal cells,4 arterial endothelial cells5 and cardiac myocytes,6 hemichannels also act as conduits for the exchange of molecules with signalling properties between the cytoplasm and extracellular space.7,8 Over 20 connexins have been identified in mammalian cells that are able to form either homomeric or heteromeric hemichannels and gap junctions with a range of conductances and varying mechanisms of channel gating regulation.9,10
Platelets are small, anucleate blood cells derived from their precursors, megakaryocytes, and are vital for haemostasis. Upon blood vessel injury, platelets adhere to exposed subendothelial collagens. Platelet activation then ensues, culminating in aggregation and the formation of a platelet plug, or thrombus, to stem the loss of blood.11 Thrombus formation involves three phases: initiation, extension and perpetuation.12 Initial interactions with collagen are indirect, and mediated via glycoprotein (GP) Ib-V-IX-von Willebrand factor binding which are later stabilised by direct collagen binding to GPVI and integrin α2β1. This enables the platelets to coat the injury site and secrete a range of prothrombotic factors (e.g. ADP, serotonin and fibrinogen).13 Activated platelets also synthesise and release thromboxane A2, an additional secondary agonist for platelet stimulation. During the extension phase, these secreted and synthesised molecules recruit additional platelets to the first layer by stimulating platelets in a paracrine manner.14 At the final perpetuation stage, activation of platelets modulates the affinity of αIIbβ3 which then binds to the bivalent ligand fibrinogen and mediates platelet-platelet aggregation.15 Under some pathological conditions, such as atherosclerotic plaque rupture, platelets are activated inappropriately resulting in the formation of platelet aggregates within the circulation (thrombosis), a principal trigger for heart attack and stroke.16
The connexin hemichannels and gap junctions have been widely studied in various cell types where sustained cell interactions are recognised. Recent reports, however, have indicated the potential for connexins to regulate the functions of some circulating cells (monocytes and T-cells).17-20 While platelets are single circulating cells under normal conditions, upon activation, the formation of a thrombus brings platelets into close proximity for a prolonged period where they function in a coordinated manner. The sustained signalling within the thrombus regulates its stability and subsequent clot retraction, which is important for tissue repair.21 The present study uncovers the role of connexin hemichannels and gap junctions in platelet interactions during thrombus formation and function.
Methods
Detailed methods for transcriptomic analysis, real-time quantitative PCR analysis, human and mouse platelet preparation, aggregation assays, dense granule secretion, immunoblotting, immunohistochemistry, transmission and scanning electron microscopy, calcium flux, flow cytometry, clot retraction and in vivo and in vitro thrombus formation are provided in online-only supplementary data.
FRAP measurements
Fluorescence recovery after photobleaching (FRAP) measurements were made on an Olympus inverted microscope with a confocal laser scanning module (Olympus FluoView1000) as described previously.22 Briefly, human citrated whole blood was incubated with calcein AM (2μg/ml) (Sigma Aldrich) for 20 minutes at 30°C, with exposure to gap junction blockers [100μg/ml 37,43Gap2723; 100μM carbenoxolone or 18β-glycyrrhetinic acid (18β-GA)]; or appropriate control during the final 10 minutes. The blood was then perfused over a collagen (100μg/ml)-coated cover slip in a laminar flow chamber at a shear rate of 1000s−1 and thrombi were allowed to form for 3 minutes. The unbound dye and free blood cells were washed away by perfusing platelet poor plasma for 2 minutes. After confirming the absence of free dye, thrombi were selected via a 60X oil immersion objective lens (UPLSAPO 60XO, NA 1.35, UK) and an 8μm diameter circular region at the centre of the thrombus was exposed to high intensity 488nm laser light for 300 milliseconds to achieve 85% photobleach of fluorescence. Fluorescence images were continuously acquired 8 seconds before and 72 seconds after photobleach. Five thrombi were analysed for each of 4 donors for each of the gap junction blockers. A 40mW multiline argon laser was used for both imaging and photobleach, with outputs of 0.8% and 20% respectively of the 488nm line. Average fluorescence intensities for bleached, non-bleached, and background regions were recorded for each time point. Fluorescence signals were corrected for background and steady loss of signal due to imaging illumination and expressed as F/F0 ratios to normalise fluorescence levels (F) against starting fluorescence (F0). Fluorescence intensities were measured using FluoView software and further analysed using Slidebook5 software (Intelligent Imaging Innovations).
Statistical analysis
The data obtained from FRAP and in vitro thrombus formation experiments in the presence and absence of various gap junction blockers were analysed by general linear model with repeated measures using SPSS (Version 17) statistical package (IBM, USA). The data obtained from clot retraction, aggregation, fibrinogen binding, calcium mobilisation and granule secretions in human samples using gap junction blockers were analysed simultaneously using non-parametric Kruskal-Wallis global statistical method and each inhibited sample was compared with the control using Dunn’s multiple comparison test using GraphPad Prism (Version 5.04) from GraphPad Software. Inc (USA). Data obtained from control and Cx37−/− mice were analysed using non-parametric Mann Whitney test using GraphPad Prism.
Results
Presence of connexins in platelets
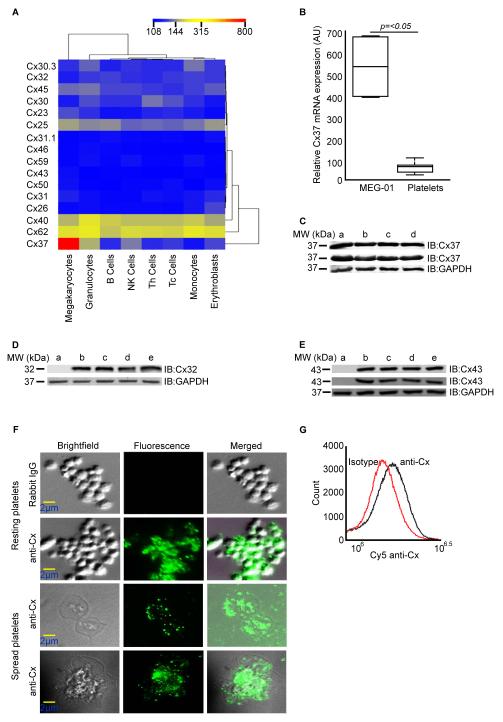
In order to study potential connexin expression in blood cells and megakaryocytes, we analysed transcriptomics data obtained using an Illumina bead chip-based array by the Bloodomics Consortium.24 Varying levels of expression of mRNA for 16 different connexins was found in human megakaryocytes. The transcript for Cx37 was most highly expressed compared to other connexins and in comparison with other human blood cells (Figure 1A). Notable levels of Cx62 and Cx40 mRNAs were also detected in megakaryocytes. Quantitative PCR analysis using mRNA obtained from a highly purified population of human platelets also confirmed the expression of Cx37 (Figure 1B).
Figure 1.
Presence of connexins in megakaryocytes and platelets. A, Connexin mRNA levels were quantified in human megakaryocytes and a range of blood cells by analysis of gene array data. Samples were clustered using the mean normalised intensity values for the probes; 108-low level expression (blue), 315-moderate level expression (yellow) and 800-high level expression (red). B, Expression of Cx37 mRNA levels was quantified in cultured megakaryocyte cell-line, MEG-01 and human platelets using quantitative-PCR, and normalised to ACTB (β-actin) gene expression. Data represent ± standard deviation (S.D), n=10. C, Presence of Cx37 in human platelets was confirmed by immunoblot analysis using two different antibodies obtained from Epitomics and Invitrogen [human endothelial cells (a), and resting (b) or CRP-XL stimulated human platelets for 90 seconds (c) and 5 minutes (d)]. D, Presence of Cx32 and Cx43 (E) in human platelets was confirmed by immunoblot analysis using antibodies obtained from Invitrogen (Cx32 & Cx43) and Epitomics (Cx43) [HeLa cell lysate was used as negative control in blots D and E (lane a). Human liver (D) and heart (E) tissue lysates (b), and resting (c) or CRP-XL stimulated human platelets for 90 seconds (d) and 5 minutes (e)]. GAPDH was used as a loading control. Presence of connexins on the surface of platelets was detected using extracellular loop specific anti-Cx antibody by fluorescence microscopy (F) and flow cytometry (G).
Connexin protein expression was investigated in human platelets. Immunoblot analysis of human platelet lysates along with positive control cell lysate [human endothelial cells (HUVECs)] confirmed the presence of Cx37 (Figure 1C). Stimulation and activation of platelets with collagen-related peptide (CRP-XL) resulted in no change in the levels of Cx37 detected. In addition to Cx37, expression of Cx32 (Figure 1D) and Cx43 (Figure 1E) was also detected in human platelets. The identification of Cx43 in platelets is consistent with the identification of this protein in megakaryocytes.25,26 In accordance with previous reports27 Cx32 and Cx43 were not detected in HeLa cell lysates. It was not possible to assess the expression of Cx62 due to the lack of specific antibodies against this protein.
The presence of connexins on the surface of platelets was confirmed by immunohistochemistry using antibodies which recognise the extracellular regions of Cx32, Cx37, Cx40 and Cx43.28 Washed platelets were allowed to spread on fibrinogen-coated cover slips and immunolabelled with specific antibodies, and analysed using fluorescence microscopy. Connexins were detected on the surface of platelets (Figure 1F), an observation that was confirmed by flow cytometry (Figure 1G).
Gap junctions in platelets
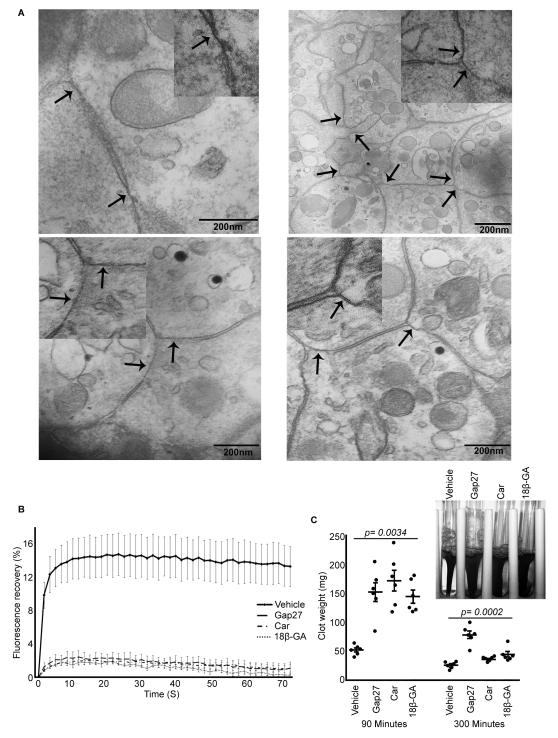
Transmission electron microscopy was used to determine whether gap junction-like structures form between platelets. Washed human platelets were aggregated with thrombin, pelleted, and processed for epoxy resin embedding. The pellet was sectioned (90nm thickness), using an ultra microtome, and stained before analysis by transmission electron microscopy. Electron micrographs show apposite membrane structures between platelets (Figure 2A) typical of gap junction-like structures well established in other cell types.1,7,29
Figure 2.
Gap junctions in platelets. A, Presence of gap junction-like structures between activated platelet membranes was analysed using transmission electron microscopy. Arrows indicate gap junction-like structures similar to other cell types. Inset of figures show enlarged regions. B, FRAP analysis was performed in thrombi formed with calcein-AM dye loaded platelets in the presence or absence of gap junction blockers and fluorescence recovery after photobleaching measured for 72 seconds. Data represent mean ± S.D (n=20 from 4 donors). Data were analysed by general linear model with repeated measures using SPSS statistical package and statistically significant (p=7×10−5) effect of inhibitor treatment was observed. The comparison of time factor (p=4×10−47) and time and inhibitor interactions (p=2×10−86) between samples was also significant. Pair wise comparison between each inhibitor and control was found to be significant (p=<0.05). C, Effect of gap junction blockers on clot retraction was analysed in vitro. Data represent mean ± S.D (n=6). Inset of figure shows a representative image of clot retraction at 90 minutes. The p-values obtained using non-parametric Kruskal-Wallis global test is shown in figure and p-values obtained by Dunn’s multiple comparison test were <0.05 for all the samples at 90 minutes and 37,43Gap27 (for carbenoxolone and 18β-GA, the p-value was >0.05) at 5 hours.
To further explore the existence of connexins and gap junction-mediated intercellular communication between platelets in a thrombus, we used FRAP, which allowed the study of the migration of the fluorescent dye, calcein from one cell to another through gap junction channels in the presence or absence of gap junction blockers following photobleaching of recipient cells.22 Fluorescence recovery was approximately 14% in control-treated thrombi but reduced to 2% in the presence of 37,43Gap27, a connexin mimetic peptide (SRPTEKTIFII) designed to target the extracellular loops of Cx37 and Cx43, and the non-isotype selective connexin inhibitors, carbenoxolone (100μM) or 18β-GA (100μM) (Figure 2B). These observations are consistent with previous FRAP studies of gap junctions between adipose tissue-derived mesenchymal stem cells,30 and provide strong evidence for the involvement of gap junction-mediated intercellular communication between platelets within a thrombus.
We hypothesised that such communication may help regulate the coordinated response of platelets within the thrombus. Subsequent to the binding of fibrinogen, the integrin αIIbβ3 transduces signals into the cell triggering platelet spreading, and in the latter phase of thrombus formation, clot retraction.31 Such outside-in integrin signalling through αIIbβ3 may be isolated through the measurement of clot retraction in vitro.32 The effect of gap junction blockers on clot retraction was therefore measured. Platelet clots were initiated by the addition of thrombin to PRP in the absence or presence of gap junction blockers (100μg/ml of 37,43Gap27; 100μM of carbenoxolone or 18β-GA), and the retraction rate of the clot was monitored over 5 hours. Initial clot formation (clot weight 15 minutes after addition of thrombin) was not altered in the presence of the gap junction blockers (Supplemental Figure 1). Notably, clot retraction was 3-fold slower in the presence of each gap junction blocker at 90 minutes compared to control-treated samples (Figure 2C). However after 5 hours the clots formed in the presence of carbenoxolone and 18β-GA were retracted similar to control samples, while the rate of clot retraction was still slower with 37,43Gap27. These data suggest that outside-in signalling through αIIbβ3 which controls the coordinated process of clot retraction is influenced by gap junction function.
Gap junction blockers inhibit platelet activation
The effect of 37,43Gap27, carbenoxolone and 18β-GA on platelet aggregation in response to various activators of platelet function was also explored. Different concentrations of each of the gap junction blockers were tested using a GPVI selective ligand, CRP-XL (0.5μg/ml and 1μg/ml) as an agonist and the effect recorded using optical aggregometry. Aggregation induced by a low concentration of CRP-XL was reduced at all concentrations of the gap junction blockers. Inhibition of 40%, 50% and 75% was observed with 37,43Gap27 (100μg/ml), carbenoxolone (100μM) and 18β-GA (100μM) respectively when 0.5μg/ml CRP-XL was used (Figure 3A). Lower levels of inhibition were noted with 1μg/ml CRP-XL (Figure 3B). Aggregation was monitored over an extended period of 10 minutes during which time inhibition was maintained (Supplemental Figure 2A). Similarly, 37,43Gap27, carbenoxolone and 18β-GA mediated inhibition of platelet aggregation to collagen and ADP was also observed (Supplemental Figures 2C and 2D). A scrambled peptide (REKIITSFIPT) was used in platelet aggregation analysis as a control for 37,43Gap27 and showed no inhibitory effects (Supplemental Figure 3).
Figure 3.
Gap junction blockers inhibit platelet activation. Aggregation performed in the presence or absence of gap junction blockers was recorded for 90 seconds following stimulation with CRP-XL [0.5μg/ml (A) and 1μg/ml (B)] or thrombin [0.09U/ml (F) and 0.18U/ml (G)]. V-represents vehicle alone and numbers represent the concentrations of gap junction blockers. Cumulative data represent mean values ± S.D (n=4). The level of aggregation obtained with vehicle was taken as 100%. C, The effect of gap junction blockers on fibrinogen binding was measured by flow cytometry. Data represent mean ± S.D (n=4). The level of fibrinogen binding obtained with vehicle was taken as 100%. Platelets stimulated with CRP-XL (0.5μg/ml) [D] or thrombin (0.09U/ml) [H] in the presence or absence of gap junction blockers were analysed by immunoblotting using anti-phosphotyrosine antibody. E, phosphospecific antibodies against proteins involved in GPVI pathway [1-resting and 2-6 are platelets stimulated with CRP-XL for 90 seconds and treated with vehicle-tyrode (2), 37,43Gap27 (100μg/ml) (3), carbenoxolone (100μM) (4), vehicle-DMSO (5) and 18β-GA (100μM) (6)]. Non-parametric Kruskal-Wallis global (p-value is shown in figure) and Dunn’s multiple comparison tests (p=<0.05) were performed.
Platelet aggregation is dependent on modulation of the conformation of αIIbβ3 through inside-out signalling to increase its affinity for fibrinogen binding.21 Thus, as a marker for inside-out signalling, fibrinogen binding, was measured on the surface of the platelets by flow cytometry. PRP (diluted 100 fold) was stimulated with CRP-XL (1μg/ml) in the absence or presence of gap junction blockers for 90 seconds and fibrinogen binding measured. CRP-XL-stimulated fibrinogen binding was reduced significantly in the presence of gap junction blockers; 30% with 37,43Gap27 (100μg/ml), 60% with carbenoxolone (100μM) and 70% with 18β-GA (100μM) (Figure 3C). Lower concentrations of 37,43Gap27 (10 & 50μg/ml), carbenoxolone (10 & 50μM) and 18β-GA (10 & 50μM) also showed clear reduction in fibrinogen binding (Supplemental Figure 4). This illustrates the involvement of connexins in inside-out integrin signalling in platelets that results in platelet aggregation. Since, this assay was performed using flow cytometry gating on the population of individual platelets, the inhibition of platelet function suggest the potential role for connexin hemichannels in platelets. Functions of hemichannels in various other cell types have been reported, the inhibition of which may be achieved using gap junction blockers such as carbenoxolone, 18β-GA and 37,43Gap27.7,8
Since aggregation was largely inhibited by the gap junction blockers at low concentrations of CRP-XL, the phosphorylation levels of various proteins involved in the GPVI pathway which is stimulated upon binding at collagen or CRP-XL was analysed. Platelet lysates were prepared after stimulation with CRP-XL (0.5μg/ml) in the absence or presence of gap junction blockers (100μg/ml of 37,43Gap27; 100μM of carbenoxolone or 18β-GA). Phosphotyrosine and phospho-specific antibodies against Lyn, LAT, Vav, PLCγ2 and AKT proteins were used to assess phosphorylation status by immunoblot analysis. The total (Figure 3D) and individual protein (Figure 3E) phosphorylation levels were unaffected after treatment with gap junction blockers suggesting that connexins are not involved in the control of receptor proximal events, but instead can modulate the signalling further downstream which may be shared with activation mechanisms stimulated by other agonists. This is consistent with the observed effects of gap junction blockers on the response of platelets to a range of agonists.
The effect of gap junction blockers on platelet activation stimulated by thrombin, which stimulates human platelet activation via the protease activated G-protein coupled receptors, PAR-1 and PAR-433 was measured. Thrombin-stimulated (0.09U/ml and 0.18U/ml) aggregation was also inhibited by each of the gap junction blockers (100μg/ml 37,43Gap27; 100μM carbenoxolone or 18β-GA). Inhibition of 60%, 70% and 80% with 37,43Gap27, carbenoxolone and 18β-GA respectively was recorded when 0.09U/ml thrombin used (Figure 3F) and maintained over 10 minutes (Supplemental Figure 2B). Inhibition was also maintained at lower concentrations of gap junction blockers (Supplemental Figure 5). Inhibition was markedly less pronounced with each gap junction blocker at a higher concentration of thrombin (Figure 3G). As observed with CRP-XL, the levels of thrombin-stimulated total protein tyrosine phosphorylation remained unaffected after treatment with gap junction blockers (Figure 3H). These data suggest that the connexins play a key role in platelet functional responses in both GPVI- and GPCR-stimulated activation pathways, although these do not appear to modulate early kinase mediated signalling events.
Platelet connexins and calcium signalling
Immediately downstream of the early signalling events, elevation of intracellular calcium levels is essential for platelet activation and thus is important in thrombus formation. Calcium mobilisation plays a paramount role in various platelet functions including reorganisation of the actin cytoskeleton necessary for shape change,34 degranulation and integrin αIIbβ3 affinity modulation.35 In platelets, a major central mechanism for elevation of cytosolic Ca2+ is through release from intracellular stores following phospholipase C mediated generation of inositol-1, 4, 5-trisphosphate (IP3) which releases calcium from dense tubular system.36 In addition, platelets express several pathways for Ca2+ influx across the plasma membrane.37 These include store-operated Ca2+ entry through Orai138-40 store-operated Ca2+ channels, ATP-gated P2X141-43 receptors and TRPC644 channels activated by diacylglycerol or phosphatidylinositol 4, 5-bisphosphate depletion.
In order to assess whether connexins play a role in regulating calcium mobilisation, intracellular calcium levels were measured in fluo4 NW dye loaded platelets (PRP) in the absence or presence of gap junction blockers (100μg/ml 37,43Gap27; 100μM carbenoxolone) for 90 seconds after stimulation with CRP-XL (0.5μg/ml or 1μg/ml) by spectrofluorimetry. Similar to their effects on aggregation, peak calcium levels were reduced by 25% and 40% by 37,43Gap27 and carbenoxolone respectively, following stimulation with CRP-XL (0.5μg/ml) (Figure 4A and 4C) (18β-GA was not used in this assay due to its incompatibility with the dye, fluo4 NW). The inhibition was maintained at lower concentrations of gap junction blockers upon stimulation with 0.5μg/ml CRP-XL (Supplemental Figures 6A and 6B). The inhibitory effects were maintained at the higher concentrations of CRP-XL (Figure 4B and 4D). Similar experiments were performed in the presence of EGTA to block calcium influx and formation of platelet aggregates following platelet stimulation. The overall cytosolic calcium levels were reduced compared to the levels obtained in the absence of EGTA, although the gap junction blockers maintained their ability to inhibit cytosolic calcium elevation (15% by 37,43Gap27 and 25% by carbenoxolone) stimulated by CRP-XL (Figure 4E-4H). The inhibitory effects were still observed at lower concentrations of gap junction blockers upon stimulation with 0.5μg/ml CRP-XL (Supplemental Figures 6C and 6D). These results suggest that connexins predominantly influence release of calcium from intracellular stores in platelets, although they may also control influx of calcium across the plasma membrane. Although the exact mechanism is unknown, it is interesting to note that extracellular Ca2+ levels are known to modulate hemichannel opening in other cell types.7 Therefore, connexins could operate to promote intercellular signalling by diffusion of messengers such as IP3 and Ca2+ between platelets following contact or in enhancing membrane-membrane interactions to promote outside-in signalling as shown for Eph kinases and Ephrins,45 or indeed through sustained hemichannel function within a thrombus.
Figure 4.
Platelet connexins and calcium mobilisation. Calcium mobilisation was measured in Fluo4 NW dye loaded platelets by spectrofluorimetry in the presence or absence of gap junction blockers. Platelets were stimulated with CRP-XL [0.5μg/ml, (A), 1μg/ml (B)] and fluorescence was measured for 90 seconds. Similar experiments were performed in the presence of EGTA (1mM) (E and F). The traces shown here are representative of 4 separate experiments. Data (C, D, G and H) represent mean ± S.D (n=4). The calcium levels obtained with vehicle was taken as 100%. Non-parametric Kruskal-Wallis global (p-value is shown in figure) and Dunn’s multiple comparison tests (p=<0.05) were performed.
Role of connexins in platelet granule secretion
One of the ways in which platelets influence the formation of thrombus is through the release of granule contents. To analyse the involvement of connexins on platelet granule secretion, both α and dense granule secretion was assayed in the absence or presence of gap junction blockers (100μg/ml 37,43Gap27; 100μM carbenoxolone or 18β-GA). α-granule secretion was assessed by measuring the levels of P-selectin exposed on the surface of platelets after stimulation with CRPXL using flow cytometry analysis in washed platelets. CRP-XL (0.5μg/ml)-stimulated α-granule secretion was reduced by 30%, 70% and 75% with 37,43Gap27, carbenoxolone and 18β-GA respectively (Figure 5A). Similar levels of inhibition were seen with higher concentrations of CRP-XL (e.g. 1μg/ml) (Figure 5B). Furthermore, lower concentrations of gap junction blockers inhibited the level of P-selectin exposed on the surface (Supplemental Figures 7A-7D).
Figure 5.
Role of connexins in platelet granule secretion. Granule secretion was measured by stimulating platelets with CRP-XL (0.5μg/ml and 1μg/ml) for 90 seconds. Platelets were stimulated and the level of P-selectin exposed on surface was measured by flow cytometry (A and B). P-selectin exposure was also measured in the presence of EGTA (1mM) (C and D). The level of P-selectin exposure with vehicle was taken as 100%. ATP release in platelets was measured using luminescence-luciferase substrate in the absence (E and F) or presence (G and H) of EGTA (1mM). The level of ATP released in vehicle treated samples was taken as 100%. Data represent mean ± S.D (n=3). Non-parametric Kruskal-Wallis global (p-value is shown in figure) and Dunn’s multiple comparison tests (p=<0.05) were performed.
To determine the role of hemichannels separate from the gap junctions formed during platelet-platelet contact, the effect of the connexin blockers was also assessed on α-granule secretion under conditions that disfavour aggregation [presence of EGTA (1mM), indomethacin (10μM) (to prevent the thromboxane A2 synthesis) and apyrase (2U/ml) (to prevent ATP and ADP activation of platelets)]. This, combined with flow cytometry analysis, enabled their effects on individual platelets to be studied. The maintenance of single platelets was confirmed by microscopy. α-granule secretion was reduced by 30%, 70% and 80% with 37,43Gap27, carbenoxolone and 18β-GA respectively at low concentration of CRP-XL (0.5μg/ml) (Figure 5C), and the inhibition was maintained at a higher concentration of CRP-XL (1μg/ml) (Figure 5D). Similarly, the level of inhibition was clearly observed when low concentrations of gap junction blockers used (Supplemental Figures 7E-7H).
The effect of gap junction blockers on dense granule secretion was assessed by measuring ATP secretion after platelet activation with CRP-XL using a luciferin-luciferase luminescence assay. Platelets were activated with CRP-XL (0.5μg/ml & 1μg/ml) in the absence or presence of gap junction blockers, and both aggregation and ATP secretion were measured simultaneously. ATP secretion was reduced by approximately 20% in the presence of 37,43Gap27 (100μg/ml), and around 60% and 80% in the presence of carbenoxolone (100μM) and 18β-GA (100μM) respectively at 0.5μg/ml of CRP-XL (Figure 5E). The inhibition of dense granule secretion by gap junction blockers was still observed at higher concentration of CRP-XL although the aggregation effects were largely overcome (Figure 5F and 3B).
Similar experiments were performed to assess the effect of gap junction blockers under conditions that disfavour aggregation (as above). ATP secretion was reduced by around 25% in the presence of 37,43Gap27 (100μg/ml) and around 60% and 75% in the presence of carbenoxolone (100μM) and 18β-GA (100μM) respectively (Figure 5G). The inhibitory effects were maintained at higher concentration of CRP-XL (Figure 5H). The ability of gap junction blockers to inhibit granule secretion upon activation and under conditions that disfavour aggregation, indicates that connexins regulate platelet activation even in the absence of gap junction formation.
Effect of gap junction blockers on thrombus formation
Given the presence of connexins on platelets, their ability to form gap junctions and the importance of hemichannels in regulating platelet function, we speculated that connexins might make an important contribution to thrombus formation. Thus, thrombus formation was analysed in vitro by fluorescence microscopy in whole blood under arterial flow conditions in the presence or absence of gap junction blockers. Captured images were analysed by calculating the size and number of thrombi formed, the sum intensity of fluorescence and total thrombus volume. The results indicate that the size, number and volume of thrombi were reduced by all three gap junction blockers (Figure 6A). The sum intensity of thrombi was reduced by approximately 40% (at 10 minutes) with 37,43Gap27 (100μg/ml) and 18β-GA (100μM), whereas, carbenoxolone (100μM) inhibited by approximately 65% (Figure 6B-6E).
Figure 6.
Effect of gap junction blockers in thrombus formation. DIOC6 labelled human blood was pre-incubated with vehicle or gap junction blockers and perfused over a collagen coated Vena8 BioChip. Images were obtained for every 30 seconds up to 10 minutes and representative images are shown in A. Fluorescence intensity of thrombi obtained in presence of gap junction blockers, 37,43Gap27 (B), carbenoxolone (C) and 18β-GA (D) were compared to vehicle. p-values were calculated by general linear model with repeated measures [p=0.031 (Gap27), 0.007 (Carbenoxolone) and 0.032 (18β-GA)]. The fluorescence intensity obtained at 10 minutes with vehicle was taken as 100% to calculate the % inhibition of gap junction blockers on thrombus formation (E). Data represent mean ± S.D (n=8 from 4 donors). Non-parametric Kruskal-Wallis global (p-value is shown in figure) and Dunn’s multiple comparison tests (p=<0.05) were performed. In vivo thrombosis assay was performed using intravital microscopy as described previously. Representative images of thrombi obtained at different time intervals are shown (F). Arrow indicates the direction of blood flow. Median fluorescence intensity was measured from 24 thrombi (from 4 mice) for each of the control and treated experiments (G).
To assess the impact of gap junctions in vivo, the effect of 37,43Gap27 on arterial thrombosis was measured using a laser injury model in mice.46 37,43Gap27 also inhibited mouse platelet aggregation as observed with human platelets (data not shown, and Figure 3). Following injury, subendothelial collagens are exposed to the blood and thrombus formation is induced at the site of injury. Platelets were labelled with an anti-mouse GPIbβ Alexa fluor-488 antibody. Thrombus formation was monitored over a period of 180 seconds at the site of injury, and the rate and size of thrombus development analysed by calculating the fluorescence intensity. Data analysis was performed for multiple thrombi obtained from 4 control or 37,43Gap27 (100μg/ml of blood)-treated mice. The initial adherence of platelets at the site of injury was relatively unaffected and thus the initial kinetics of thrombus formation was similar in both control and 37,43Gap27 treated animals. Overall thrombus growth, however, were reduced by around 70% in 37,43Gap27-treated mice (Figure 6F and 6G). Together, these data confirm the involvement of connexins in thrombus formation under in vivo conditions.
Characterisation of Cx37 deficient mouse platelets
Since the connexin mimetic peptide, 37,43Gap27 binds Cx37 and Cx43, in order to further assess the specific roles of Cx37 in platelet function, we used a genetic approach and examined the function of platelets from Cx37 knockout (Cx37−/−) mice.47 Developmental defects in Cx37 deficient platelets were excluded through analysis of cellular and subcellular morphology and receptor expression levels. Electron microscopy analysis revealed that the morphology of platelets from Cx37+/+ and Cx37−/− were indistinguishable, with α- and dense granules of normal appearance and frequency (data not shown). Flow cytometry was employed to measure the expression of platelet surface receptors such as αIIbβ3 (Figure 7A), GPVI (Figure 7B) and GPIb (Figure 7C), which were found to be unchanged in Cx37−/− platelets. Similarly the expression level of integrin α2 subunit was confirmed to be normal by immunoblot analysis (Figure 7D). It is possible that deletion of the Cx37 gene (Gja4) may result in compensatory effects on the expression of other platelet connexins. The levels of other platelet connexins such as Cx32 and Cx43 (identified in this study) were also examined by immunoblot analysis. As expected, Cx37 (Figure 7E) was confirmed to be absent in Cx37−/− mice platelets. Cx32 (Figure 7F) and Cx43 (Figure 7G) were detected at similar levels in platelets from Cx37+/+ and Cx37−/− mice.
Figure 7.
Characterisation of Cx37−/− mouse platelets. The expression levels of αIIbβ3 (A), GPVI (B) and GPIbα (C) were analysed by flow cytometry using citrated mouse blood. Data represent mean ± S.D (n=6 Cx37+/+ and Cx37−/− mice). p-values calculated by non-parametric Mann Whitney test are shown in figure (p=>0.05). The expression level of α2 was measured by immunoblot analysis (D) using detection of 14-3-3ζ as a loading control. The expression of Cx37 (E), Cx32 (F) and Cx43 (G) were analysed by immunoblots in Cx37+/+ and Cx37−/− mouse platelets. Blots are representative of 4 separate experiments.
Effect of Cx37 deficiency on murine platelet function
Aggregation assays were performed using washed mouse platelets at the density of 2×108/ml using an optical aggregometer. The aggregation of Cx37−/− platelets was reduced by around 60% at the concentration of 0.5μg/ml CRP-XL (Figure 8A and 8B) in comparison to Cx37+/+ platelets. At the higher concentration of CRP-XL (1μg/ml), the reduction was around 20% (Figure 8C and 8D). These results are consistent with data obtained with human platelets in the presence of the connexin mimetic peptide inhibitor, 37,43Gap27 (Figure 3A and 3B).
Figure 8.
Effect of Cx37 deficiency on mouse platelet function. Aggregation was recorded for 180 seconds following stimulation with CRP-XL [0.5μg/ml (A) and 1μg/ml (C)]. Cumulative data (B and D) represent mean values ± S.D. The level of aggregation obtained with Cx37+/+ was taken as 100%. The level of fibrinogen binding in Cx37+/+ and Cx37−/− was measured by flow cytometry following stimulation with CRP-XL [0.5μg/ml (E) and 1μg/ml (F)]. Data represent mean ± S.D of median fluorescence intensity values. P-selectin exposure upon activation by CRP-XL [0.5μg/ml (G) and 1μg/ml (H)] was measured using citrated mouse blood by flow cytometry. Effect of Cx37 deficiency on clot retraction was analysed in vitro using Cx37−/− mouse platelets (I, image shown on figure was taken at 90 minutes). Data represent mean ± S.D (n=4 Cx37+/+ and Cx37−/− mice) of clot weights measured after 5 hours (J). p-values calculated by non-parametric Mann Whitney test are shown in figure (p=<0.03), (n=4 Cx37+/+ and Cx37−/− mice).
Fibrinogen binding on the surface of platelets (in citrated whole blood) was measured upon activation by CRP-XL. This was also reduced (by approximately 50% at 0.5μg/ml CRP-XL and by 30% at 1μg/ml CRP-XL) in Cx37−/− mice platelets compared to Cx37+/+ platelets (Figure 8E and 8F). In order to assess the role of Cx37 on granule secretion, P-selectin was measured using flow cytometry. Granule secretion was also reduced (by approximately 50% at 0.5μg/ml CRP-XL and 40% at 1μg/ml CRP-XL) in Cx37−/− platelets compared to Cx37+/+ platelets (Figure 8G and 8H).
Given the effects of gap junction blockers on clot retraction, similar assays were also performed using Cx37−/− and Cx37+/+ platelets. The weight of the remaining clot measured (after 5 hours) in Cx37−/− was three times higher than Cx37+/+ platelets indicating the reduced retraction in Cx37−/− platelets [Figure 8I (image shown on figure was taken at 90 minutes) and 8J]. This is consistent with the inhibitory actions of carbenoxolone, 18β-GA and 37,43Gap27 on clot retraction in human PRP (Figure 2C) and point towards a fundamental role for gap junction mediated intercellular communication in the stimulation of platelet thrombus function.
Discussion
In the intact circulation platelets normally circulate as individual discoid bodies, but show a marked ability to adhere to each other to form a thrombus via the support of the integrin αIIbβ3 and the adhesion proteins fibrinogen and von Willebrand factor.11 Recent studies45 have indicated that sustained signalling is required to maintain thrombus stability, although little is known of how this is mediated. The contraction of the clot then ensues, a coordinated response driven by platelet integrins,21 that results in the shrinking of the thrombus and the drawing together of wound edges. Given that platelet function may therefore be acutely regulated while in isolation in the plasma, and in concert within a thrombus, the possibility was explored that connexin hemichannels and gap junctions may be present in platelets and regulate the different phases of platelet function.
We report the expression of several members of the connexin family in human megakaryocytes and platelets, where Cx37 expression was found to be notably abundant. Consistent with the formation and function of gap junctions within the thrombus, dye transfer between cells was observed, and blocked by pharmacological connexin-blocking agents, including the Cx37 and Cx43 selective mimetic peptide, 37,43Gap27. The retraction of platelet-rich clots was also reduced in the presence of these agents, strongly supporting the notion that gap junction-dependent intercellular signalling within a thrombus is required for synchronised platelet function.
Connexin blocking agents were found to inhibit a range of platelet functions, including aggregation, α-granule secretion and fibrinogen binding, suggesting that prior to the association of platelets within a thrombus, hemichannels are also of importance for platelet function. While calcium signalling was diminished in the presence of gap junction blockers, it is unclear whether the mode of hemichannel function may be through ion channel functionality (e.g. by cooperation with Orai1,38-40 TRPC644 and P2X141-43) or through interaction with other components of the platelet cell machinery. As platelets become activated, the formation of initial micro-thrombi, leading to macro-thrombi may result in complex combinations of hemichannel and gap junction function, although the effect of gap junction blockers on platelets treated under conditions that disfavour platelet-platelet aggregation, strongly suggest initial platelet reactivity is modulated through hemichannels, and later events within the thrombus governed by gap junctions. The potent inhibition of both thrombus formation in vitro using human blood under arterial flow conditions and thrombosis in mice by gap junction blockers, suggest that gap junctions may offer new avenues for therapeutic intervention.
Our detailed characterisation of platelet function using three unrelated gap junction blockers in parallel point strongly to an activatory role for connexins in platelets. Due to the inability to truly inhibit the action of specific connexins, it is possible that this represents the compound actions of several connexins, each of which may exhibit different roles and regulation as hetero- or homomeric connexons in the context of platelet or platelet thrombi. To begin to dissect the roles of connexins, and particularly to focus on the family member most notably expressed (at transcript level) in megakaryocytes, we explored the function of Cx37−/− mouse platelets. Cx37−/− platelets were confirmed to lack Cx37 protein, however no abnormalities in platelet morphology or in their receptor expression levels were observed in comparison to Cx37+/+ platelets. Further functional characterisation of Cx37−/− platelets revealed clear inhibition in platelet function compared to control platelets. These results are consistent with the data obtained using human platelets in the presence of various gap junction blockers. Our dose response experiments using multiple approaches in human platelets along with detailed characterisation (including confirmation of genotype: lack of expressed protein) of Cx37−/− mouse platelets confirm Cx37 to positively influence platelet function. It is important to note that while these experiments reveal positive role for Cx37 in platelet function, the potential contributions of other connexins identified in this study in the regulation of platelets (positive or negative) cannot be excluded.
It is interesting to reflect that recent studies implicate Cx37 on macrophages in inflammatory responses that lead to atherogenesis in susceptible mice.19 Indeed, a single nucleotide polymorphism (C1019T) within the human Cx37 gene (GJA4) has been reported to be a potential prognostic marker for atherosclerosis and myocardial infarction.48,49 Given the established role of platelet-monocyte adhesion and the initiation or progression of atherosclerosis, it is tempting to speculate that the role of platelet connexin function may extend to platelet-immune cell intercellular signalling in a range of pathological conditions.
Prior to this study, Angelillo-Scherrer et al50 also reported the expression of Cx37 (and no other connexins) in platelets where it was proposed to be involved in the regulation of platelet function. The prominent expression of Cx37 in human and mouse platelets is indeed consistent with our findings. In contrast to our work, however, they concluded that this connexin serves to inhibit platelet function. The reasons for this discrepancy are not entirely clear. We can conclude that platelets possess a range of connexin family members and therefore the situation may be more complex than originally suspected, although in some cases similar experimental approaches were employed. Moreover, in the above study, increased human platelet reactivity was reported using a single inhibitor, 18α-GA, which we have found to be inappropriate for analysis of platelet function due to its ability to activate platelet function in the absence of agonists (Supplemental Figure 8). Indeed, in some aggregation data presented (in the previous study) concentrations of agonists were used that in themselves did not cause aggregation in many donors, calling into question whether increased responses in the presence of 18α-GA represented increased platelet sensitivity or were due to non-specific effects of the agent. To overcome this, in the present study a range of pharmacological agents were used that in the absence of a platelet agonist to do not cause platelet activation, allowing the study of connexin function in the presence of physiologically relevant levels of platelet activating factors. The substantial variability in response in the platelets from different donors in the previously published study was also a concern, and therefore we have presented cumulative data with appropriate statistical analysis.
Angelillo-Scherrer et al50 also reported increased aggregation responses using the platelets from Cx37−/− mice. We have scrutinised the methodologies used in both studies, since the experiments reported in the present study were obtained using the same mouse line. It is possible that differences in anticoagulants used may underlie apparent discrepancies in reactivity, although ambiguity in the methodology previously presented makes this difficult to confirm. It is interesting to note that given the high agonist concentrations used in the study of Angelillo-Scherrer et al50 (e.g. collagen at 5μg/ml) very slow aggregation responses are observed particularly for analysis in platelet rich plasma, with no aggregation apparent until 100 seconds after stimulation. Furthermore, this is reversible, a characteristic of weak stimulation where secretion has not been stimulated.
The analysis of haemostasis and thrombosis in Cx37−/− mice may present a useful insight into the function of this protein and potentially other connexins in platelets in vivo. Indeed, Angelillo-Scherrer et al report a reduction in bleeding time and increased thromboembolism, although the effects of systemic deficiency of Cx37 on endothelial cell/blood vessel susceptibility to injury have not been established. It may also be pertinent to note that Cx37 plays an important roles in lung function51,52 that may present further confounding issues in the thromboembolism model. Our analysis of thrombosis in vivo indicates that upon the general inhibition of connexin function thrombi formed are less stable. It is therefore possible that thrombi formed in Cx37−/− mice are less stable compared to the Cx37+/+ mice, and more susceptible to embolisation resulting in reduced the survival time. It is likely that cell (platelet) specific deletion of Cx37 and the other connexins detected in platelets will be necessary to completely unravel these inconsistencies between the studies, and to examine potential differential roles of different platelet connexins.
We conclude that our study provides detailed evidence for a fundamental role of connexins in platelet function. The connexins, particularly Cx37, contribute to the early phases of platelet activation through formation of hemichannels. In addition, they drive thrombus formation and subsequent clot retraction following gap junction formation. Further research is required to establish the molecular nature of the signalling mechanisms through which platelet connexins exert their effects. It is possible that they contribute to calcium signalling, although additional possibilities suggested in other cell systems,53 such as the dilution of cGMP levels between platelets within a thrombus, and consequent reduction in inhibitory signalling, may also contribute to the effects. Finally, the identification and function of connexin hemichannels and gap junctions in platelets may represent potential targets for novel anti-platelet therapies.
Supplementary Material
Clinical perspective.
Upon blood vessel injury, platelets adhere to exposed subendothelial collagens and are activated, triggering thrombus formation in order to prevent bleeding. Inappropriate activation of platelets under pathological conditions such as atherosclerosis results in thrombosis which may lead to heart attack or stroke. Connexins are membrane proteins that assemble into channels (hemichannels or connexons) in the plasma membrane of selected cells facilitating communication between the cytoplasm and external environments. Docking of connexons on neighbouring cells also results in gap junctions which mediate direct intercellular communication. Hence, hemichannels and gap junctions coordinate and synchronise the functions of various tissues and organs for example the heart. In the present study, we report the presence of multiple connexins in platelets. The results of this study indicate that gap junctions form between platelets in thrombi and conduct intercellular signals that promote retraction of blood clots, an important step in wound repair. Connexins were also found to regulate the activation of isolated platelets, pointing towards the importance of hemichannels on platelets prior to thrombus formation. Consistent with this, the deletion of the Cx37 gene in mice resulted in reduced clot retraction and platelet activation. Inhibition of platelet connexins using pharmacological agents diminished thrombus formation in vitro in human blood and thrombotic responses in mice. Together, this study provides evidence for a fundamental role of connexin hemichannels and gap junctions in the activation of platelets and the regulation of thrombus function, and suggests that connexins may represent potential avenues for the development of novel antithrombotic agents.
Acknowledgments
We would like thank Prof Willem H Ouwehand and Dr Nicholas A Watkins for providing the gene array data. We are grateful to Prof William Howard Evans and Dr Michael Fry for advice and critical evaluation during the preparation of this manuscript. We thank John Kanady for his support during clot retraction assays. We are grateful for the AZCC/ARL-Division of Biotechnology Cytometry Core facility at University of Arizona for their support with flow cytometry analysis.
Funding Sources: This work was supported by the British Heart Foundation (grant numbers: RG/05/007, PG/08/100/26125, PG/05/014 and PG/09/094), Medical Research Council (grant number: G0701399), Wellcome Trust, UK (grant number: 082338/Z/07/Z), BBSRC studentship, Leicester NIHR Biomedical Research Unit in Cardiovascular Disease, Glenfield Hospital, Leicester, UK and NIH (grant number: HL64232).
Footnotes
Journal Subject Codes: [152] Ion channels/membrane transport; [172] Arterial thrombosis; [92] Platelets; [177] Secretion; [178] Aggregation; [181] Signal transduction; [186] Platelet function inhibitors
Conflict of Interest Disclosures: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goodenough DA, Paul DL. Gap junctions. Cold Spring Harb Perspect Biol. 2009;1:a002576. doi: 10.1101/cshperspect.a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruzzone R DR. Structure and functions of gap junctions in the developing brain. Cell Tissue Res. 2006;326:239–248. doi: 10.1007/s00441-006-0287-0. [DOI] [PubMed] [Google Scholar]
- 3.Kidder GM, Mhawi AA. Gap junctions and ovarian folliculogenesis. Reproduction. 2002;123:613–620. doi: 10.1530/rep.0.1230613. [DOI] [PubMed] [Google Scholar]
- 4.Dorshkind K, Green L, Godwin A, Fletcher WH. Connexin-43-type gap junctions mediate communication between bone marrow stromal cells. Blood. 1993;82:38–45. [PubMed] [Google Scholar]
- 5.Figueroa XF, Duling BR. Gap junctions in the control of vascular function. Antioxid Redox Signal. 2009;11:251–266. doi: 10.1089/ars.2008.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohr S. Role of gap junctions in the propagation of the cardiac action potential. Cardiovasc Res. 2004;62:309–322. doi: 10.1016/j.cardiores.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 7.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 8.John S, Cesario D, Weiss JN. Gap junctional hemichannels in the heart. Acta Physiol Scand. 2003;179:23–31. doi: 10.1046/j.1365-201X.2003.01197.x. [DOI] [PubMed] [Google Scholar]
- 9.Falk MM, Buehler LK, Kumar NM, Gilula NB. Cell-free synthesis and assembly of connexins into functional gap junction membrane channels. EMBO J. 1997;16:2703–2716. doi: 10.1093/emboj/16.10.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segretain D, Falk MM. Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochim Biophys Acta. 2004;1662:3–21. doi: 10.1016/j.bbamem.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Gibbins JM. Platelet adhesion signalling and the regulation of thrombus formation. J Cell Sci. 2004;117:3415–3425. doi: 10.1242/jcs.01325. [DOI] [PubMed] [Google Scholar]
- 12.Brass LF. Thrombin and platelet activation. Chest. 2003;124:18S–25S. doi: 10.1378/chest.124.3_suppl.18s. [DOI] [PubMed] [Google Scholar]
- 13.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 14.Ruggeri ZM. Mechanisms initiating platelet thrombus formation. Thromb Haemost. 1997;78:611–616. [PubMed] [Google Scholar]
- 15.Shattil SJ, Kashiwagi H, Pampori N. Integrin signaling: the platelet paradigm. Blood. 1998;91:2645–2657. [PubMed] [Google Scholar]
- 16.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 17.Bermudez-Fajardo A, Yliharsila M, Evans WH, Newby AC, Oviedo-Orta E. CD4+ T lymphocyte subsets express connexin 43 and establish gap junction channel communication with macrophages in vitro. J Leukoc Biol. 2007;82:608–612. doi: 10.1189/jlb.0307134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oviedo-Orta E, Perreau M, Evans WH, Potolicchio I. Control of the proliferation of activated CD4+ T cells by connexins. J Leukoc Biol. 2010;88:79–86. doi: 10.1189/jlb.0909613. [DOI] [PubMed] [Google Scholar]
- 19.Wong CW, Christen T, Roth I, Chadjichristos CE, Derouette JP, Foglia BF, Chanson M, Goodenough DA, Kwak BR. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nat Med. 2006;12:950–954. doi: 10.1038/nm1441. [DOI] [PubMed] [Google Scholar]
- 20.Derouette JP, Wong C, Burnier L, Morel S, Sutter E, Galan K, Brisset AC, Roth I, Chadjichristos CE, Kwak BR. Molecular role of Cx37 in advanced atherosclerosis: a micro-array study. Atherosclerosis. 2009;206:69–76. doi: 10.1016/j.atherosclerosis.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Calderwood DA. Integrin activation. J Cell Sci. 2004;117:657–666. doi: 10.1242/jcs.01014. [DOI] [PubMed] [Google Scholar]
- 22.Lalo U, Allsopp RC, Mahaut-Smith MP, Evans RJ. P2X1 receptor mobility and trafficking; regulation by receptor insertion and activation. J Neurochem. 2010;113:1177–1187. doi: 10.1111/j.1471-4159.2010.06730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans WH, Leybaert L. Mimetic peptides as blockers of connexin channel-facilitated intercellular communication. Cell Commun Adhes. 2007;14:265–273. doi: 10.1080/15419060801891034. [DOI] [PubMed] [Google Scholar]
- 24.Watkins NA, Gusnanto A, de Bono B, De S, Miranda-Saavedra D, Hardie DL, Angenent WG, Attwood AP, Ellis PD, Erber W, Foad NS, Garner SF, Isacke CM, Jolley J, Koch K, Macaulay IC, Morley SL, Rendon A, Rice KM, Taylor N, Thijssen-Timmer DC, Tijssen MR, van der Schoot CE, Wernisch L, Winzer T, Dudbridge F, Buckley CD, Langford CF, Teichmann S, Gottgens B, Ouwehand WH. A HaemAtlas: characterizing gene expression in differentiated human blood cells. Blood. 2009;113:e1–9. doi: 10.1182/blood-2008-06-162958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciovacco WA, Goldberg CG, Taylor AF, Lemieux JM, Horowitz MC, Donahue HJ, Kacena MA. The role of gap junctions in megakaryocyte-mediated osteoblast proliferation and differentiation. Bone. 2009;44:80–86. doi: 10.1016/j.bone.2008.08.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krenacs T, Rosendaal M. Connexin43 gap junctions in normal, regenerating, and cultured mouse bone marrow and in human leukemias: their possible involvement in blood formation. Am J Pathol. 1998;152:993–1004. [PMC free article] [PubMed] [Google Scholar]
- 27.Ihara A, Muramatsu T, Shimono M. Expression of connexin 32 and 43 in developing rat submandibular salivary glands. Arch Oral Biol. 2000;45:227–235. doi: 10.1016/s0003-9969(99)00128-4. [DOI] [PubMed] [Google Scholar]
- 28.Rahman S, Evans WH. Topography of connexin32 in rat liver gap junctions. Evidence for an intramolecular disulphide linkage connecting the two extracellular peptide loops. J Cell Sci. 1991;100:567–578. doi: 10.1242/jcs.100.3.567. [DOI] [PubMed] [Google Scholar]
- 29.Huttner I, Boutet M, More RH. Gap junctions in arterial endothelium. J Cell Biol. 1973;57:247–252. doi: 10.1083/jcb.57.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sauer H, Sharifpanah F, Hatry M, Steffen P, Bartsch C, Heller R, Padmasekar M, Howaldt HP, Bein G, Wartenberg M. NOS inhibition synchronizes calcium oscillations in human adipose tissue-derived mesenchymal stem cells by increasing gap-junctional coupling. J Cell Physiol. 2011;226:1642–1650. doi: 10.1002/jcp.22495. [DOI] [PubMed] [Google Scholar]
- 31.Phillips DR, Nannizzi-Alaimo L, Prasad KS. Beta3 tyrosine phosphorylation in alphaIIbbeta3 (platelet membrane GP IIb-IIIa) outside-in integrin signaling. Thromb Haemost. 2001;86:246–258. [PubMed] [Google Scholar]
- 32.Gong H, Shen B, Flevaris P, Chow C, Lam SC, Voyno-Yasenetskaya TA, Kozasa T, Du X. G protein subunit Galpha13 binds to integrin alphaIIbbeta3 and mediates integrin “outside-in” signaling. Science. 2010;327:340–343. doi: 10.1126/science.1174779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 34.Hathaway DR, Adelstein RS. Human platelet myosin light chain kinase requires the calcium-binding protein calmodulin for activity. Proc Natl Acad Sci U S A. 1979;76:1653–1657. doi: 10.1073/pnas.76.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shattil SJ, Brass LF. Induction of the fibrinogen receptor on human platelets by intracellular mediators. J Biol Chem. 1987;262:992–1000. [PubMed] [Google Scholar]
- 36.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 37.Varga-Szabo D, Braun A, Nieswandt B. Calcium signaling in platelets. J Thromb Haemost. 2009;7:1057–1066. doi: 10.1111/j.1538-7836.2009.03455.x. [DOI] [PubMed] [Google Scholar]
- 38.Braun A, Varga-Szabo D, Kleinschnitz C, Pleines I, Bender M, Austinat M, Bosl M, Stoll G, Nieswandt B. Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation. Blood. 2009;113:2056–2063. doi: 10.1182/blood-2008-07-171611. [DOI] [PubMed] [Google Scholar]
- 39.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 40.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacKenzie AB, Mahaut-Smith MP, Sage SO. Activation of receptor-operated cation channels via P2X1 not P2T purinoceptors in human platelets. J Biol Chem. 1996;271:2879–2881. doi: 10.1074/jbc.271.6.2879. [DOI] [PubMed] [Google Scholar]
- 42.Fung CY, Cendana C, Farndale RW, Mahaut-Smith MP. Primary and secondary agonists can use P2X(1) receptors as a major pathway to increase intracellular Ca(2+) in the human platelet. J Thromb Haemost. 2007;5:910–917. doi: 10.1111/j.1538-7836.2007.02525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tolhurst G, Vial C, Leon C, Gachet C, Evans RJ, Mahaut-Smith MP. Interplay between P2Y(1), P2Y(12), and P2X(1) receptors in the activation of megakaryocyte cation influx currents by ADP: evidence that the primary megakaryocyte represents a fully functional model of platelet P2 receptor signaling. Blood. 2005;106:1644–1651. doi: 10.1182/blood-2005-02-0725. [DOI] [PubMed] [Google Scholar]
- 44.Hassock SR, Zhu MX, Trost C, Flockerzi V, Authi KS. Expression and role of TRPC proteins in human platelets: evidence that TRPC6 forms the store-independent calcium entry channel. Blood. 2002;100:2801–2811. doi: 10.1182/blood-2002-03-0723. [DOI] [PubMed] [Google Scholar]
- 45.Prevost N, Woulfe DS, Jiang H, Stalker TJ, Marchese P, Ruggeri ZM, Brass LF. Eph kinases and ephrins support thrombus growth and stability by regulating integrin outside-in signaling in platelets. Proc Natl Acad Sci U S A. 2005;102:9820–9825. doi: 10.1073/pnas.0404065102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat Med. 2002;8:1175–1181. doi: 10.1038/nm782. [DOI] [PubMed] [Google Scholar]
- 47.Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. Nature. 1997;385:525–529. doi: 10.1038/385525a0. [DOI] [PubMed] [Google Scholar]
- 48.Yamada Y, Izawa H, Ichihara S, Takatsu F, Ishihara H, Hirayama H, Sone T, Tanaka M, Yokota M. Prediction of the risk of myocardial infarction from polymorphisms in candidate genes. N Engl J Med. 2002;347:1916–1923. doi: 10.1056/NEJMoa021445. [DOI] [PubMed] [Google Scholar]
- 49.Incalcaterra E, Caruso M, Balistreri CR, Candore G, Lo Presti R, Hoffmann E, Caimi G. Role of genetic polymorphisms in myocardial infarction at young age. Clin Hemorheol Microcirc. 2010;46:291–298. doi: 10.3233/CH-2010-1353. [DOI] [PubMed] [Google Scholar]
- 50.Angelillo-Scherrer A, Fontana P, Burnier L, Roth I, Sugamele R, Brisset A, Morel S, Nolli S, Sutter E, Chassot A, Capron C, Borgel D, Saller F, Chanson M, Kwak BR. Connexin37 Limits Thrombus Propensity by Downregulating Platelet Reactivity. Circulation. 2011;124:930–939. doi: 10.1161/CIRCULATIONAHA.110.015479. [DOI] [PubMed] [Google Scholar]
- 51.Abraham V, Chou ML, DeBolt KM, Koval M. Phenotypic control of gap junctional communication by cultured alveolar epithelial cells. Am J Physiol. 1999;276:L825–834. doi: 10.1152/ajplung.1999.276.5.L825. [DOI] [PubMed] [Google Scholar]
- 52.Abraham V, Chou ML, George P, Pooler P, Zaman A, Savani RC, Koval M. Heterocellular gap junctional communication between alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1085–1093. doi: 10.1152/ajplung.2001.280.6.L1085. [DOI] [PubMed] [Google Scholar]
- 53.Norris RP, Freudzon M, Nikolaev VO, Jaffe LA. Epidermal growth factor receptor kinase activity is required for gap junction closure and for part of the decrease in ovarian follicle cGMP in response to LH. Reproduction. 2010;140:655–662. doi: 10.1530/REP-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.