Summary
Objective
Patients with diabetes experience increased cardiovascular complications after cardiac surgery. Hyperglycaemia predicts increased mortality after myocardial infarction and may influence cardiovascular risk in humans. Impaired prosurvival phosphatase and tensin homologue on chromosome 10 (PTEN)-Akt signaling could be an important feature of the diabetic heart rendering it resistant to preconditioning. This study was designed to evaluate for differences and relationships of myocardial PTEN-Akt-related signaling and baseline glycaemic control marker in type 2 diabetic and non-diabetic patients undergoing coronary artery bypass surgery.
Methods
Right atrial biopsies and coronary sinus blood were obtained from 18 type 2 diabetic and 18 non-diabetic patients intraoperatively. Expression and phosphorylation of Akt, eNOS, Bcl-2 and PTEN were evaluated by western blot. Plasma 15-F2t-isoprostane concentrations were evaluated by liquid chromatography-mass spectrometry.
Results
PTEN expression and 15-F2t-isoprostane concentrations were significantly higher in diabetic patients. Increased fasting blood glucose levels correlated with increased coronary sinus plasma 15-F2t-isoprostane concentrations. Increased cardiac 15-F2t-isoprostane generation was highly correlated with myocardial PTEN expression. Bcl-2 expression and eNOS phosphorylation were significantly lower in diabetic compared to non-diabetic patients. Akt phosphorylation tended to be lower in diabetic patients, however this tendency failed to reach statistical significance.
Conclusion
The current results suggest that prosurvival PTEN-Akt signaling is impaired in the diseased diabetic myocardium. Hyperglycaemia and increased oxidative stress may contribute to this phenomenon. These findings strengthen the understanding of the underlying biologic mechanisms of cardiac injury in diabetic patients, which could facilitate development of new treatments to prevent cardiovascular complications in this high-risk population.
Keywords: Type 2 diabetes, Coronary bypass surgery, Ischemia-reperfusion injury, PTEN, Akt signaling
Introduction
Patients with diabetes mellitus are up to five times more likely to develop cardiovascular disease1 and account for nearly thirty percent of patients undergoing cardiac surgery2. Diabetes mellitus is an independent risk factor for increased early and late postoperative morbidity and mortality associated with myocardial injury3. This includes an elevated risk for low cardiac output syndrome that can quadruple the overall mortality rate for coronary artery bypass surgery4.
Major efforts have focused on increasing the myocardial tolerance to ischemia. Unfortunately, the diabetic myocardium is resistant to physical or pharmacologic preconditioning stimulus5. Experimental studies have detected corrupted protective signal transduction pathways and enhanced mitochondrial permeability transition, which could explain the increased susceptibility to injury in ischemia-reperfused diabetic myocardium4, 6. However, the in vivo characteristics that could place patients at risk during surgery have yet to be fully delineated.
Glycaemic control (Fasting blood glucose, FBG) and glycosylated haemoglobin (blood haemoglobin A1c, HbA1c) have been used as markers of diabetic control7, 8. Hyperglycaemia predicts increased mortality after myocardial infarction and may influence cardiovascular risk in humans{Duncan, #46}. More recent interest has focused on the glycaemic control as both marker and mediator of adverse postoperative surgical outcomes{Duncan, #46;Halkos, 2008 #40;Nesto, 2008 #44}. The underlying mechanism of the association is unclear.
The phosphoinositide-3 kinase (PI3K)/Akt prosurvival pathway is central to physical and pharmacologic preconditioning and salvaging ischemia-reperfused myocardium. Once activated, Akt may induce a cytoprotective effect by activating end-effectors including endothelial nitric oxide synthase (eNOS) and anti-apoptotic Bcl-2 to antagonize mitochondrial directed cell death10, 11. The diabetic myocardium appears resistant to the effects of physical preconditioning on Akt activation12, 13. The principle negative regulator of the PI3K/Akt pathway is phosphatase and tensin homologue on chromosome 10 (PTEN)14. Hyperglycaemia induced reactive oxygen or nitrogen species generation could upregulate PTEN antagonism of Akt15, 16.
To date, no therapeutic approach has been demonstrated clinically effective against cardiac injury in the diabetic population. Strengthening our understanding of the underlying biologic mechanisms is a research priority that could facilitate development of new treatments to prevent cardiovascular complications in this high-risk patient population. The aims of our study were 1) to evaluate for differences in the regulation of the PTEN and myocardial Akt-related signaling, and 2) to determine its relationship with baseline biochemical markers of glycaemic control in vivo, in type 2 diabetic and non-diabetic patients presenting for coronary artery bypass surgery.
Subjects and Methods
Patients
This investigation conforms to the principles outlined in the Declaration of Helsinki. Following approval of the University of British Columbia Clinical Research Ethics Board and informed patient consent, 36 patients (18 patients with or without type 2 diabetes) undergoing primary coronary artery bypass graft (CABG) surgery randomized in the PROTECT II study were included. Details of the study have been recently described4.
Study inclusion criteria were: 1) a preoperative systolic blood pressure > 90 mmHg in the absence of inotropic or mechanical support; 2) scheduled for revascularization of two or more coronary vessels. Patients were excluded who: 1) were less than 18 or greater than 80 years old; 2) had co-existing valvular heart disease; 3) had an acute or evolving myocardial infarction; or 4) took nonsteroidal anti-inflammatory drugs, or vitamin C or E within 5 days surgery. Patients stopped taking oral hypoglycemic agents 24 hours before surgery.
Type 2 diabetics were defined by an established history and diagnosis of adult-onset diabetes mellitus treated by diet alone or in combination with oral hypoglycemic agents (regardless of insulin use). Insulin use was initiated when adequate glucose control could not be achieved with oral hypoglycemic agents. Clinically, no patients with microvascular complications or evidence of renal dysfunction (creatinine > 135 μmol/l) were enrolled.
All patients received the same anesthetic induction agent (thiopental) and anesthetic maintenance with moderate dose intravenous narcotic, fentanyl and inhalational agent, isoflurane (0.5% to 1% end tidal), prior to atrial biopsy and coronary sinus sampling and subsequent blinded PRO-TECT II experimental intervention according to study protocol4.
Myocardial and coronary sinus blood sampling
Tissue biopsies were collected from the right atrial appendage immediately prior to cardiopulmonary bypass. Biopsies were immediately flash frozen in liquid nitrogen and stored at −80°C for subsequent western blotting analysis. Coronary sinus blood was sampled through a retrograde coronary sinus cannula immediately prior to cardiopulmonary bypass. Blood samples were centrifuged at 1300 g for 10 minutes, and the plasma was stored at −80°C in 1.25 ml aliquots in the presence of 0.005% of butylated hydroxytoluene for subsequent liquid chromatography-mass spectrometry (LC-MS) analysis.
LC-MS analysis
15-F2t-isoprostane analysis was performed using immunoaffinity purification followed by LC-MS analysis. In brief, 500 μl of plasma was pretreated, spiked with 15-F2t-isoprostane-D4 internal standard and applied to an 8-isoprostane affinity column (Cayman Chemical, Ann Arbor, MI, USA) as previously described17. The eluent was dried in a speedvac, and resuspended in water containing 2% acetonitrile and 0.01% NH4OH for subsequent LC-MS analysis. LC was performed using a 12 minute gradient, from 5% to 20% B, with the following mobile phase composition: A) 0.01% NH4OH in water. B) 0.01% NH4OH in acetonitrile. Quantitative analysis was accomplished using an ion trap mass spectrometer (Bruckner Daltonics, Leipzig, Germany), comparing the ratio of the intensity of the signal at m/z = 353.1 and m/z = 357.1.
Western Blotting
Atrial biopsies were homogenized using a tissue grinder in ice-cold radioimmunoprecipitation assay buffer (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) containing 20 mm Tris–HCl (pH 7.4), 150 mm NaCl, 1% Nonider P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 0.004% sodium azide, 1% phenylmethylsulfonyl fluoride, 1% sodium orthovanadate and 1% protease inhibitor cocktail at 4°C. The lysate was centrifuged at 10,000 g at 4°C for 30 minutes to remove the insoluble material. Supernatants were collected. The protein concentration was measured by Bradford protein assay (Bio-Rad Laboratories, Hercules, CA, USA). Then equal amounts of protein (50 μg) from each sample was separated by 12% SDS–PAGE and transferred to nitrocellulose membrane. The membranes were blocked for 1 hour in 5% skim milk and then incubated overnight at 4°C with primary antibody, independently, against the following proteins: PTEN, Akt, phospho-Akt, eNOS, phosphorylated-eNOS, anti-apoptotic Bcl-2, and β-actin (All antibodies purchased from Cell Signaling Technology, Inc., Danvers, MA, USA). Following a 30-minute wash, the membranes were incubated with secondary antibody conjugated to horseradish peroxidase for 1 hour at room temperature. The membranes were then washed for 30 minutes and visualized by enhanced chemiluminescence. Protein expression levels were derived using FluorChem® Q image analyzer (Alpha Innotech, San Leandro, CA, USA), detecting signals from nitrocellulose membranes.
Statistical analysis
All analyses were performed using GraphPad Prism version 4.0 for Mac (GraphPad Software, San Diego California USA, www.graphpad.com). A value of P < 0.05 was considered statistically significant. Differences in categorical variables between diabetic and non-diabetic subjects were compared using Fisher’s exact test (Table 1). All continuous variables were subject to a Shapiro-Wilk normality test and presented as mean and standard deviation (mean ± SD). Normally distributed data derived from diabetic patients was compared with that of non-diabetic counterparts using an unpaired two-tailed t-test with Welch’s correction (Table 1). Non-normally distributed data were similarly compared using a Mann-Whitney test (Figure 1a, c and Figure 2b~h). Correlation analyses were performed using linear regression with a two-tailed Pearson test where the P-value is indicative of a non-zero slope, which is described according to its 95% confidence interval (CI) (Figure 1b, d and Figure 3a, b).
Table 1.
Preoperative patient characteristics
| Characteristic | Non Diabetic (n = 18) | Diabetics (n = 18) | P-value |
|---|---|---|---|
| Gender, male/female | 14/4 | 16/2 | 0.66 |
| Age, year | 62.5 ± 8.5 | 65.7 ± 7.3 | 0.23 |
| Weight, kilogram | 79.57 ± 16.42 | 92.34 ± 14.22 | 0.02 |
| Height, centimeter | 171.3 ± 9.90 | 172.9 ± 5.64 | 0.55 |
| Body surface area, meter2 | 1.92 ± 0.24 | 2.06 ± 0.17 | 0.046 |
| Left ventricular ejection fraction, % | 47.71 ± 10.5 | 56 ± 14.25 | 0.06 |
| Fasting blood glucose level, mmol/L | 6.44 ± 1.42 | 8.1 ± 1.63 | 0.003 |
| Cardiac risk factors, n (%) | |||
| Hypertension | 11 (61) | 15 (83) | 0.26 |
| Current smoker | 5 (28) | 4 (22) | > 0.99 |
| Dyslipidemia | 17 (94) | 17 (94) | > 0.99 |
| Preoperative medication use, n (%) | |||
| β-blocker | 14 (77) | 14 (77) | > 0.99 |
| Calcium-channel blocker | 1 (6) | 9 (50) | 0.007 |
| Angiotensin-converting enzyme inhibitor | 10 (56) | 11 (61) | > 0.99 |
| Digoxin | 1 (6) | 2 (11) | > 0.99 |
| Statin | 18 (100) | 17 (94) | > 0.99 |
Values are presented as mean and standard deviation (mean ± SD), unless otherwise indicated. P-values refer to differences between the groups as determined by the unpaired two-tailed t-test with Welch’s correction for continuous variables or Fisher’s exact test for categorical variables.
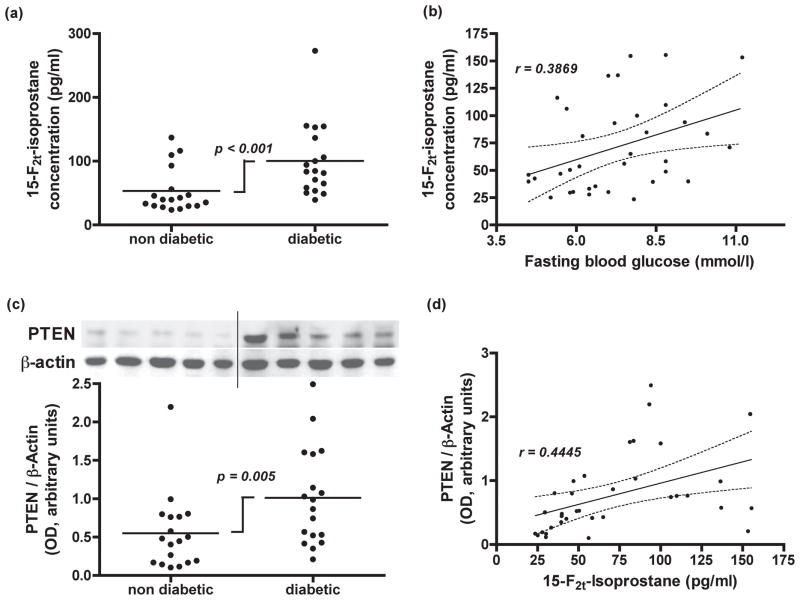
Figure 1.
(a) Dot plot representing plasma free15-F2t-isoprostane concentrations in coronary sinus blood from coronary bypass patients with or without type 2 diabetes. P-values refer to differences between the groups as determined by Mann-Whitney test. (b) Correlation analysis between 15-F2t-isoprostane concentrations and fasting blood glucose levels (slope = 9.06, 95% CI: 1.41 to 16.72; P = 0.02) by using linear regression with a two-tailed Pearson test. (c) Representative western blot for PTEN expression in atrial tissue biopsies taken from patients with or without type 2 diabetes. Dot plot represents PTEN expression normalized to β-actin expression. P-values refer to differences between the groups as determined by Mann-Whitney test. (d) Correlation analysis between atrial tissue PTEN/β-actin expression and coronary sinus plasma free 15-F2t-isoprostane concentrations (slope = 0.007, 95% CI: 0.002 to 0.011; P = 0.008) by linear regression with a two-tailed Pearson test.
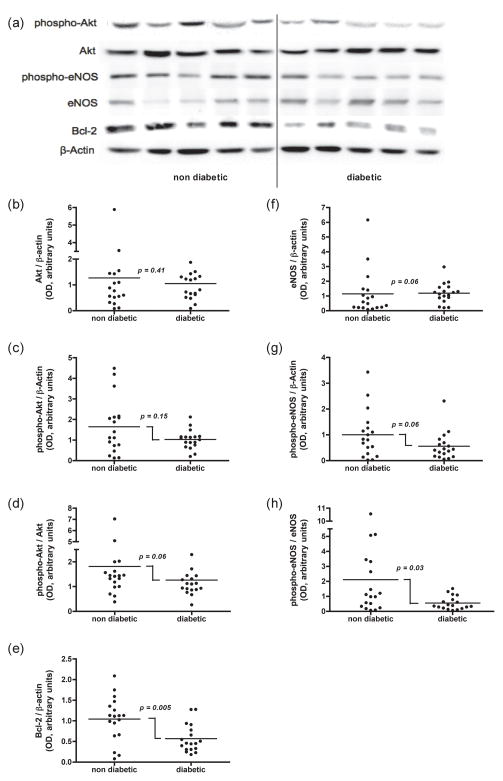
Figure 2.
Western blot comparison of Akt signaling in coronary bypass patients with or without type 2 diabetes. (a) Representative blots for phospho-Akt, Akt, phospho-endothelial nitric oxide (eNOS), eNOS, Bcl-2, and β-actin in non-diabetic and diabetic patients, respectively. Dot plot depictions of (b) Akt/β-actin, (c) Phospho-Akt/β-actin, (d) Phospho-Akt/Akt, (e) Bcl-2/β-actin, (f) eNOS/β-actin, (g) phospho-eNOS/β-actin, (h) phospho-eNOS/eNOS expression in atrial tissue biopsies from patients with or without type 2 diabetes. P-values refer to differences between the groups as determined by Mann-Whitney test.
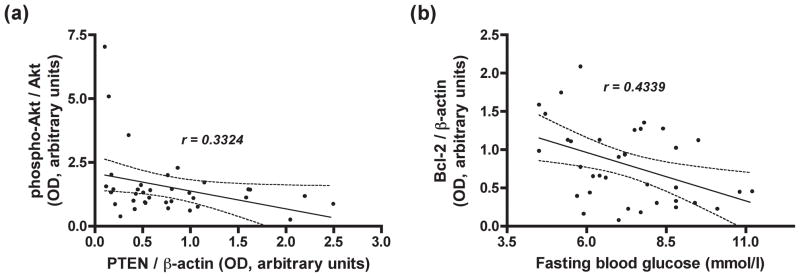
Figure 3.
Correlation analysis between (a) atrial tissue phospho-Akt/Akt and PTEN/β-actin expression (slope = -0.70, 95% CI: −1.39 to −0.01; P = 0.048), and (b) atrial tissue Bcl-2/β-actin expression and fasting blood glucose levels (slope = −0.13, 95% CI: −0.22 to −0.03; P = 0.008) from 36 patients undergoing by pass surgery. The analyses were performed using linear regression with a two-tailed Pearson test.
Results
Patients
The preoperative characteristics of non-diabetic and diabetic patients are described in Table 1. There were no significant differences between diabetic and non-diabetic groups with respect to gender, age, height, left ventricular ejection fraction, cardiac risk factors (hypertension, current smoker or dyslipidemia), or preoperative medication use (β-blocker, angiotensin-converting enzyme inhibitor, digoxin, or statin). However, weight (P = 0.02), body surface area (P = 0.046) and calcium-channel blocker use (P = 0.007) were higher in diabetic group. Diabetic subjects also had a significant higher fasting blood glucose level (P = 0.003).
A profile of diabetic patients included in the study is listed in Table 2. Diabetic patients had a blood HbA1c level of 6.89 ± 1.16% (range from 4.7% to 9.5%). Diet alone (1 of 18 subjects) or in combination with insulin (3 of 18 subjects) or oral hypoglycemic agents (Metformin, Glyburide, Gliclazide, Rosiglatazone or Thiazolidenedione) were used in diabetic subjects to treat type 2 diabetes.
Table 2.
Profile of diabetic patients included in the study
| No. | Age | Sex | FBG (mmol/l) | HbA1c (%) | Insulin and/or Oral hypoglycaemic or Diet | 15-F2t-isoprostane (pg/ml) | Western blot densitometry analysis
|
|
|---|---|---|---|---|---|---|---|---|
| PTEN/β-actin | Phospho-Akt/Akt | |||||||
| 1 | 74 | M | 7.9 | 6.2 | Metformin | 100.02 | 1.58 | 1.13 |
| 2 | 64 | M | 6.2 | 6 | Metformin | 81.26 | 1.61 | 1.45 |
| 3 | 55 | M | 8.2 | 8.1 | Rosiglatazone | 84.63 | 1.03 | 1.10 |
| 4 | 72 | M | 5.8 | 6.8 | Metformin/Glyburide | 50.35 | 0.53 | 0.91 |
| 5 | 50 | M | 7.2 | 6.3 | Thiazolidenedione | 272.95 | 1.14 | 1.71 |
| 6 | 74 | M | 7 | 7.5 | Metformin | 136.50 | 0.99 | 1.31 |
| 7 | 66 | M | 10.8 | 8.2 | Insulin | 70.90 | 0.87 | 2.29 |
| 8 | 61 | M | 10.1 | na | Metformin | 83.51 | 1.62 | 1.43 |
| 9 | 65 | M | 8.8 | 6.3 | Metformin | 48.85 | 0.52 | 0.94 |
| 10 | 66 | M | 5.7 | 5.5 | Diet | 106.24 | 0.74 | 0.93 |
| 11 | 68 | M | 6.1 | 6.4 | Metformin/Glyburide | 53.55 | 1.08 | 0.77 |
| 12 | 70 | F | 9.4 | 7.1 | Metformin | 94.05 | 2.49 | 0.88 |
| 13 | 72 | M | 7.7 | 6.7 | Metformin/Gliclazide | 154.53 | 2.04 | 0.26 |
| 14 | 71 | M | 7.7 | 6.3 | Insulin/Metformin | 65.03 | 0.43 | 1.27 |
| 15 | 60 | F | 8.4 | 8.7 | Insulin/Metformin | 39.47 | 0.35 | 3.57 |
| 16 | 64 | M | 11.2 | 9.5 | Metformin/Glyburide | 153.20 | 0.21 | 0.86 |
| 17 | 55 | M | 8.8 | 4.7 | Metformin/Glyburide | 58.32 | 0.42 | 0.67 |
| 18 | 76 | M | 8.8 | 6.9 | Metformin | 155.33 | 0.57 | 1.12 |
Abbreviations: FBG, Fasting blood glucose; HbA1c, Haemoglobin A1c, PTEN, phosphatase and tensin homologue on chromosome 10; M, Male; F, Femal; na, not available.
15-F2t-isoprostane levels
In order to investigate whether diabetic patients in the current study had increased reactive oxygen species activity, and to find evidence in support of its relationship with fasting blood glucose levels, we measured plasma free 15-F2t-isoprostane levels in coronary sinus blood. The concentration of 15-F2t-isoprostane in this study was significantly greater in coronary sinus plasma from diabetic patients (100.50 ± 57.26 pg/ml) compared to non-diabetic patients (53.44 ± 35.14 pg/ml) (P < 0.001, Mann-Whitney test; Figure 1a).
Correlation analysis between 15-F2t-isoprostane and fasting blood glucose levels was performed using linear regression. This analysis revealed a significant correlation (P = 0.02, r = 0.3869) with a slope of 9.06 (95% CI: 1.41 to 16.72) (Figure 1b).
PTEN expression
In order to investigate whether diabetic patients presenting for CABG surgery had increased PTEN level, and to find evidence of its relationship with plasma 15-F2t-isoprostane levels, we evaluated PTEN expression by western blot and performed a correlation analysis between PTEN and the 15-F2t-isoprostane levels using linear regression.
As shown in Figure 1c, biopsies from diabetic patients had significantly higher PTEN protein expression than those from non-diabetic patients (non-diabetic: 0.55 ± 0.50, diabetic: 1.01 ± 0.64, P = 0.005, Mann-Whitney test). In addition, the linear regression analysis revealed a significant correlation between PTEN/β-actin and 15-F2t-isoprostane levels (P = 0.008, r = 0.4445) with a slope of 0.007 (95% CI: 0.002 to 0.011) (Figure 1d).
Akt expression and phosphorylation
Atrial biopsies from diabetic and non-diabetic patients exhibited similar levels of total Akt protein expression (non-diabetic: 1.27 ± 1.43, diabetic: 1.05 ± 0.66, P = 0.41, Mann-Whitney test; Figure 2a, b). The extent of Akt phosphorylation (phospho-Akt/Akt) tended to be lower in atrial tissue biopsies from diabetic patients (1.26 ± 0.73) than from non-diabetic patients (1.81 ± 1.64), however this tendency was not statistically significant (P = 0.06, Mann-Whitney test; Figure 2a, d).
PTEN is the main negative modulator of PI3K/Akt signaling pathway. In order to find evidence of the relationship of Akt phosphorylation with PTEN expression, we performed a correlation analysis between PTEN and the extent of Akt phosphorylation using linear regression. The linear regression analysis revealed a significant negative correlation between PTEN/β-actin and phospho-Akt/Akt (P = 0.048, r = 0.3324) with a slope of -0.70 (95% CI: −1.39 to −0.01) (Figure 3a).
Expression and activation of Akt targets: Bcl-2 and eNOS
In order to further investigate the extent of Akt signaling, we evaluated the expression and activation of two key downstream signaling intermediates Bcl-2 and eNOS.
The eNOS protein levels tended to be higher in biopsies from diabetic patients (1.18 ± 0.68) than those from non-diabetic patients (1.13 ± 1.54), however this tendency was not statistically significant (P = 0.06, Mann-Whitney test; Figure 2a, f). By contrast, biopsies from diabetic patients had significantly lower extents of eNOS phosphorylation (phospho-eNOS/eNOS) than those from non-diabetic patients (non-diabetic: 2.10 ± 2.67, diabetic: 0.55 ± 0.44, P = 0.03, Mann-Whitney test; Figure 2a, h).
Biopsies from diabetic patients had significantly lower levels of Bcl-2 than those from non-diabetic patients (non-diabetic: 1.04 ± 0.54, diabetic: 0.57 ± 0.34, P = 0.005, Mann-Whitney test; Figure 2a, e). In addition, a linear regression analysis revealed a significant negative correlation between Bcl-2/β-actin and fasting blood glucose levels (P = 0.008, r = 0.4339) with a slope of −0.13 (95% CI: −0.22 to −0.03) (Figure 3b).
Discussion
The aim of the present study was to evaluate for baseline differences and relationships of myocardial PTEN-Akt-related signaling to biochemical makers of glycaemic control in type 2 diabetic and non-diabetic patients undergoing CABG surgery as part of the PRO-TECT II study4. We have made three observations that pertain to patients with type 2 diabetes; 1) increased 15-F2t-isoprostane concentrations in coronary sinus plasma, which was significantly correlated with fasting blood glucose levels; 2) increased myocardial PTEN expression; 3) reduced myocardial Akt signaling, as shown by the combination of decreased eNOS phosphorylation, decreased Bcl-2 expression and a tendency towards lower Akt phosphorylation in the myocardium. These findings strengthen the understanding of the underlying biologic mechanisms of cardiac injury in diabetic patients, which could facilitate development of new treatments to prevent cardiovascular complications in this high-risk patient population.
Cardiovascular diseases are the leading cause of morbidity and mortality in the insulin resistant patient population1. Thirty percent of patients presenting for coronary bypass surgery are insulin resistant2. Diabetes mellitus in general and perioperative hyperglycaemia in particular has been associated with marked increase in postoperative complications including death{Duncan, #46;Nesto, 2008 #44}. The molecular defects in response to type 2 diabetes and hyperglycaemia contributing to poor operative outcomes have not been well defined. Experimental studies suggest that the diabetic heart is resistant to both ischemic and pharmacological preconditioning5, 18. However, the in vivo characteristics that could place patients at risk during surgery have yet to be fully delineated.
Oxidative stress is a hallmark of type 2 diabetes. 15-F2t-isoprostanes are surrogate markers of oxidative stress in type 2 diabetes19, whose levels in coronary sinus blood reflect the amount of reactive oxygen species in coronary circulation. In this study, the baseline level of plasma 15-F2t-isoprostane in coronary sinus blood was significantly higher in patients presenting with type 2 diabetes (Figure 1a). Acute hyperglycaemia stimulates isoprostane generation and decreases plasma antioxidant defenses20, 21. The level of isoprostane is reported to vary with the degree of glucose fluctuation, even in the absence of an increase in HbA1c in healthy patients or those with type 2 diabetes22. This is consistent with our present findings, which demonstrated a significant correlation between fasting blood glucose and coronary15-F2t-isoprostane (Figure 1b).
PTEN is a dual protein lipid phosphatase that dephosphorylates the secondary messenger produced by PI3K, and interrupts the downstream activation of Akt. It plays a significant role in regulating the balance between survival and death in many cell types, including cardiomyocytes. Indeed, PTEN is associated with a reduction in preconditioning efficacy 23, and its inactivation may increase myocardial survival following an ischemic episode and ischemia-reperfusion injury24. Mocanu et al reported an increased PTEN level in the Goto Kakizaki rat heart model and suggested diabetes may be associated with increased PTEN level, at least in the myocardium23. We detected PTEN expression was significantly higher in atrial tissue from patients with type 2 diabetes (Figure 1c), supporting their finding.
Chronic pathological reactive oxygen species production induces PTEN expression14, 15. In our patient series, increased fasting blood glucose levels correlated with increased levels of 15-F2t-isoprostane in coronary sinus blood (Figure 1b). Increased cardiac 15-F2t-isoprostane generation was highly correlated with PTEN in human diabetic myocardium (Figure 1d), indicative of isoprostane’s potential role in its generation.
The activation of Akt signaling is central to cardioprotective signaling pathways that reduce myocardial ischemia-reperfusion injury11. Accordingly, ischemic and pharmacological pre- and post-conditioning strategies exert their cardioprotective effects by recruiting the Akt signaling pathway10. To protect the diabetic myocardium, it appears necessary to sufficiently recruit these preconditioning stimuli. The resistance of the diabetic myocardium to preconditioning implies that a higher signaling threshold and a critical level of Akt phosphorylation must be attained to mediate myocardial protection. Indeed, Tsang et al reported a reduced level of Akt phosphorylation in a Goto-Kakizaki rat type 2 diabetic model12. By contrast, Huisamen reported that Akt was hyperphosphorylated in a Zucker obese rat model, but under-phosphorylated in a streptozotocin-induced type 2 diabetic rat model25. In the current study, the extent of Akt phosphorylation tended to be lower in the diabetic group, but this trend failed to reach statistical significance (P = 0.06; Figure 2a, d). This tendency is in agreement with other clinical studies reporting significant reductions in Akt phosphorylation among diabetic patients13, 26. The preoperative use of insulin, an Akt activator, may have confounded our results since this trend becomes significant in subanalysis of patients treated with oral hypoglycemic agents alone (see Table 2; P = 0.01 for phospho/total Akt if excluding patients who received preoperative insulin, data not shown).
Phosphorylation of eNOS and upregulation of Bcl-2 represent two important pro-survival end-effectors downstream of Akt signaling. Akt directly activates eNOS by phosphorylation at Serine-1177 and Serine-615, and induces Bcl-2 protein expression through cyclic AMP-response element-binding protein and nuclear transcription factor-κB 27, 28. Our data show that both the extent of eNOS phosphorylation, and the expression of Bcl-2 expression are significantly lower in atrial tissue from diabetic patients. The combination of lower eNOS phosphorylation and Bcl-2 expression may further reflect impaired Akt signaling.
We observed a trend of increased total eNOS expression in patients with type 2 diabetes (Figure 1a, f). Since the increased total eNOS expression was accompanied by a significantly lower phospho-eNOS/eNOS ratio (Figure 1a, h), our results may reflect a compensation mechanism whereby reduced eNOS activation induces eNOS expression in insulin resistance. We acknowledge that these results contrast with several previous reports26, and further investigation is warranted.
We examined for a potential association between PTEN and Akt phosphorylation in our study. Our results revealed a significant negative correlation between PTEN and the extent of Akt phosphorylation (Figure 3a), suggesting that the decrease in the level of Akt phosphorylation results from an increased level of PTEN in myocardial tissue from insulin resistant patients.
Sulphonylureas, biguanides, thiazolidinediones and meglitinides have antioxidant effects29. Metformin treatment has been associated with nitric oxide generation in response to increases in AMP-activated protein kinase mediated activation of eNOS30. Thiazolidinediones are insulin sensitizers that improve the actions of insulin in endothelium. Despite recent reports of the cardioprotective properties of the acute administration of metformin in the setting of myocardial ischemia-reperfusion30, we observed increased baseline levels of PTEN and reductions in baseline Akt and eNOS in patients on chronic therapy, a pattern consistent with underlying insulin resistance. In our clinical practice, we do not substitute insulin therapy when withholding oral hypoglycemic therapies prior to surgery. Therefore, these baseline derangements may explain the increased resistance to ischemic preconditioning stimuli previously described in ex vivo atrial tissues from patients with type 2 diabetes as previously described by Sivaraman et al13. These investigators detected reduced levels of Akt activation in human diabetic myocardium, but levels of PTEN, an upstream regulator (reactive oxygen species), and putative downstream effectors of Akt were not measured, as in our study.
Previous study identified the relationship of blood glucose and HgmA1c to downregulation of vascular antiapoptotic Bcl2 in skin31. We were able to identify such a direct correlation in human diabetic myocardium in our study (Figure 1b). Given the importance of Bcl2 in the prevention of mitochondrial directed cell death, this finding may be indicative of an underlying mitochondrial defect in diabetic cardiac tissue, rendering it susceptible to ischemia-reperfusion injury.
Study limitation
This study describes baseline characteristics. The observational nature reflects association and cannot test for, or directly imply, causation. Inter-patient biologic variability, while not unexpected, might influence and explain overlapping results. Detailed analysis of the potential confounding effects of preoperative drug therapies on our findings in blood and tissue is beyond the scope of this study. Protein expression in the atrium tissue is a surrogate measure of activity and expression in ventricular tissue. However, previous authors have reported a lack of significant differences between chambers, and have established the use of atrial biopsies in ex vivo experiments32.
PTEN negatively regulates insulin signaling via action on Akt. Suppression of PTEN has been shown to restore glycaemic control33. PTEN is modulated during myocardial ischemia and reperfusion and its loss is involved in the induction of the ischemic preconditioning stimulus, via the transient upregulation of Akt at reperfusion34. Chronic Akt activation may be maladaptive and associated with cardiac dysfunction and failure, but its acute enhancement is associated with cyto- and cardioprotection, and improved contractility35. Based on our findings, the inhibition of PTEN or activation of Akt signaling to enhance insulin signaling, simultaneously targeting perioperative glycometrics and cardiac tissues, may prove to be an effective treatment alternative for the cardioprotection of surgical patients presenting with type 2 diabetes. The response of myocardial PTEN and Akt signaling to an intravenous anesthetic with antioxidant potential that inhibits mitochondrial permeability transition or pharmacologic preconditioning with volatile anesthesia is currently being evaluated in patients with type 2 diabetes presenting for cardiac surgery (the PRO-TECT II Study)4. The clinical efficacy of such an approach could improve outcomes and prognosis. This has yet to be determined.
Acknowledgments
This work was supported by Canadian Institutes of Health Research Operating Grant #82757. Koen Raedschelders and Yu Hui are supported by Canada Graduate Scholarships from the Canadian Institutes of Health Research. The authors thank Dr. Kumar for facilitating our use of FluorChem® Q image analyzer in their laboratory, without which, completion of this project would not have been possible. We acknowledge the collaborative support of the cardiac surgeons and anesthesiologists at our center.
Footnotes
Disclosures
We declare that we have no conflict of interest.
References
- 1.Brown JR, Edwards FH, O’Connor GT, Ross CS, Furnary AP. The diabetic disadvantage: historical outcomes measures in diabetic patients undergoing cardiac surgery -- the pre-intravenous insulin era. Semin Thorac Cardiovasc Surg. 2006;18:281–288. doi: 10.1053/j.semtcvs.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Scrutinio D, Giannuzzi P. Comorbidity in patients undergoing coronary artery bypass graft surgery: impact on outcome and implications for cardiac rehabilitation. Eur J Cardiovasc Prev Rehabil. 2008;15:379–385. doi: 10.1097/HJR.0b013e3282fd5c6f. [DOI] [PubMed] [Google Scholar]
- 3.Leavitt BJ. The effects of diabetes mellitus on coronary artery bypass graft surgery. Curr Diab Rep. 2007;7:20–24. doi: 10.1007/s11892-007-0005-7. [DOI] [PubMed] [Google Scholar]
- 4.Ansley DM, Raedschelders K, Chen DDY, Choi PT. Rationale, design and baseline characteristics of the PRO-TECT II study: PROpofol CardioproTECTion for Type II diabetics: a randomized, controlled trial of high-dose propofol versus isoflurane preconditioning in patients undergoing on-pump coronary artery bypass graft surgery. Contemporary clinical trials. 2009;30:380–385. doi: 10.1016/j.cct.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Huffmyer J, Raphael J. Physiology and pharmacology of myocardial preconditioning and postconditioning. Semin Cardiothorac Vasc Anesth. 2009;13:5–18. doi: 10.1177/1089253208330709. [DOI] [PubMed] [Google Scholar]
- 6.Kehl F, Krolikowski JG, Mraovic B, Pagel PS, Warltier DC, Kersten JR. Hyperglycemia prevents isoflurane-induced preconditioning against myocardial infarction. Anesthesiology. 2002;96:183–188. doi: 10.1097/00000542-200201000-00032. [DOI] [PubMed] [Google Scholar]
- 7.Halkos ME, Puskas JD, Lattouf OM, Kilgo P, Kerendi F, Song HK, Guyton RA, Thourani VH. Elevated preoperative hemoglobin A1c level is predictive of adverse events after coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2008;136:631–640. doi: 10.1016/j.jtcvs.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 8.Nesto RW, Lago RM. Glucose: a biomarker in acute myocardial infarction ready for prime time? Circulation. 2008;117:990–992. doi: 10.1161/CIRCULATIONAHA.107.757450. [DOI] [PubMed] [Google Scholar]
- 9.Duncan AE, Abd-Elsayed A, Maheshwari A, Xu M, Soltesz E, Koch CG. Role of intraoperative and postoperative blood glucose concentrations in predicting outcomes after cardiac surgery. Anesthesiology. 2010;112:860–871. doi: 10.1097/ALN.0b013e3181d3d4b4. [DOI] [PubMed] [Google Scholar]
- 10.Hausenloy DJ, Yellon DM. Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail Rev. 2007;12:217–234. doi: 10.1007/s10741-007-9026-1. [DOI] [PubMed] [Google Scholar]
- 11.Mullonkal CJ, Toledo-Pereyra LH. Akt in ischemia and reperfusion. J of Investigative Surgery. 2007;20:195–203. doi: 10.1080/08941930701366471. [DOI] [PubMed] [Google Scholar]
- 12.Tsang A, Hausenloy DJ, Mocanu MM, Carr RD, Yellon DM. Preconditioning the diabetic heart: the importance of Akt phosphorylation. Diabetes. 2005;54:2360–2364. doi: 10.2337/diabetes.54.8.2360. [DOI] [PubMed] [Google Scholar]
- 13.Sivaraman V, Hausenloy DJ, Wynne AM, Yellon DM. Preconditioning the diabetic human myocardium. J Cell Mol Med. 2010;14:1740–1746. doi: 10.1111/j.1582-4934.2009.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang KH, Lemke G, Kim JW. The PI3K-PTEN tug-of-war, oxidative stress and retinal degeneration. Trends in Molecular Medicine. 2009;15:191–198. doi: 10.1016/j.molmed.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song P, Wu Y, Xu J, Xie Z, Dong Y, Zhang M, Zou MH. Reactive nitrogen species induced by hyperglycemia suppresses Akt signaling and triggers apoptosis by upregulating phosphatase PTEN (phosphatase and tensin homologue deleted on chromosome 10) in an LKB1-dependent manner. Circulation. 2007;116:1585–1595. doi: 10.1161/CIRCULATIONAHA.107.716498. [DOI] [PubMed] [Google Scholar]
- 16.Seo JH, Ahn Y, Lee SR, Yeol Yeo C, Chung Hur K. The major target of the endogenously generated reactive oxygen species in response to insulin stimulation is phosphatase and tensin homolog and not phosphoinositide-3 kinase (PI-3 kinase) in the PI-3 kinase/Akt pathway. Mol Biol Cell. 2005;16:348–357. doi: 10.1091/mbc.E04-05-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sircar D, Subbaiah PV. Isoprostane measurement in plasma and urine by liquid chromatography-mass spectrometry with one-step sample preparation. Clin Chem. 2007;53:251–258. doi: 10.1373/clinchem.2006.074989. [DOI] [PubMed] [Google Scholar]
- 18.Gross ER, Hsu AK, Gross GJ. Diabetes abolishes morphine-induced cardioprotection via multiple pathways upstream of glycogen synthase kinase-3beta. Diabetes. 2007;56:127–136. doi: 10.2337/db06-0907. [DOI] [PubMed] [Google Scholar]
- 19.Kaviarasan S, Muniandy S, Qvist R, Ismail IS. F(2)-isoprostanes as novel biomarkers for type 2 diabetes: a review. J Clin Biochem Nutr. 2009;45:1–8. doi: 10.3164/jcbn.08-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faure P, Polge C, Monneret D, Favier A, Halimi S. Plasma 15-F2t isoprostane concentrations are increased during acute fructose loading in type 2 diabetes. Diabetes Metab. 2008;34:148–154. doi: 10.1016/j.diabet.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Sampson MJ, Gopaul N, Davies IR, Hughes DA, Carrier MJ. Plasma F2 isoprostanes: direct evidence of increased free radical damage during acute hyperglycemia in type 2 diabetes. Diabetes Care. 2002;25:537–541. doi: 10.2337/diacare.25.3.537. [DOI] [PubMed] [Google Scholar]
- 22.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 23.Mocanu MM, Field DC, Yellon DM. A potential role for PTEN in the diabetic heart. Cardiovascular drugs and therapy/sponsored by the International Society of Cardiovascular Pharmacotherapy. 2006;20:319–321. doi: 10.1007/s10557-006-8876-4. [DOI] [PubMed] [Google Scholar]
- 24.Ruan H, Li J, Ren S, Gao J, Li G, Kim R, Wu H, Wang Y. Inducible and cardiac specific PTEN inactivation protects ischemia/reperfusion injury. J Mol Cell Cardiol. 2009;46:193–200. doi: 10.1016/j.yjmcc.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 25.Huisamen B. Protein kinase B in the diabetic heart. Mol Cell Biochem. 2003;249:31–38. [PubMed] [Google Scholar]
- 26.Sasso FC, Torella D, Carbonara O, Ellison GM, Torella M, Scardone M, Marra C, Nasti R, Marfella R, Cozzolino D, Indolfi C, Cotrufo M, Torella R, Salvatore T. Increased vascular endothelial growth factor expression but impaired vascular endothelial growth factor receptor signaling in the myocardium of type 2 diabetic patients with chronic coronary heart disease. J Am Coll Cardiol. 2005;46:827–834. doi: 10.1016/j.jacc.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Wang B, Shravah J, Luo H, Raedschelders K, Chen D, Ansley D. Propofol protects against hydrogen peroxide-induced injury in cardiac H9c2 cells via Akt activation and Bcl-2 up-regulation. Biochem Biophys Res Commun. 2009;389:105–111. doi: 10.1016/j.bbrc.2009.08.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275:10761–10766. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- 29.Giannini S, Serio M, Galli A. Pleiotropic effects of thiazolidinediones: taking a look beyond antidiabetic activity. J Endocrinol Invest. 2004;27:982–991. doi: 10.1007/BF03347546. [DOI] [PubMed] [Google Scholar]
- 30.Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57:696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- 31.Hasnan J, Yusof MI, Damitri TD, Faridah AR, Adenan AS, Norbaini TH. Relationship between apoptotic markers (Bax and Bcl-2) and biochemical markers in type 2 diabetes mellitus. Singapore Med J. 2010;51:50–55. [PubMed] [Google Scholar]
- 32.Valen G, Paulsson G, Bennet AM, Hansson GK, Vaage J. Gene expression of inflammatory mediators in different chambers of the human heart. Ann Thorac Surg. 2000;70:562–567. doi: 10.1016/s0003-4975(00)01507-1. [DOI] [PubMed] [Google Scholar]
- 33.Butler M, McKay RA, Popoff IJ, Gaarde WA, Witchell D, Murray SF, Dean NM, Bhanot S, Monia BP. Specific inhibition of PTEN expression reverses hyperglycemia in diabetic mice. Diabetes. 2002;51:1028–1034. doi: 10.2337/diabetes.51.4.1028. [DOI] [PubMed] [Google Scholar]
- 34.Cai Z, Semenza GL. PTEN activity is modulated during ischemia and reperfusion: involvement in the induction and decay of preconditioning. Circ Res. 2005;97:1351–1359. doi: 10.1161/01.RES.0000195656.52760.30. [DOI] [PubMed] [Google Scholar]
- 35.Matsui T, Rosenzweig A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt. J Mol Cell Cardiol. 2005;38:63–71. doi: 10.1016/j.yjmcc.2004.11.005. [DOI] [PubMed] [Google Scholar]